Abstract
Hepatitis C virus (HCV) is a major cause of chronic hepatitis and hepatocellular carcinoma worldwide. Due to shared transmission routes, the prevalence of HCV is especially high among individuals infected with HIV. HIV uninfected individuals spontaneously clear HCV approximately 30% of the time, while the rate of control in HIV infected individuals who subsequently acquire HCV is substantially lower. In addition, complications of HCV are more frequent in those with HIV infection, making liver disease the leading cause of non-AIDS-related death in HIV infected individuals. This review summarizes recent advances in understanding the role of the innate and adaptive immune responses to HCV in those with and without HIV. Further defining the interaction between hepatitis C and the host immune system will potentially reveal insights into HCV pathogenesis and the host’s ability to prevent persistent infection, as well as direct the development of vaccines.
Keywords: Hepatitis C, HCV, viral hepatitis, hepatitis, Immunity, innate immune response, Interferons, IFN, cellular immunity, adaptive immune response, neutralizing antibodies, chronic infection, acute infection, liver disease, spontaneous clearance, treatment response, SVR, vaccination, HIV, HIV-HCV coinfection, coinfection, T cell response, B cell response, interleukin 18, interleukin 28B, virus
INTRODUCTION
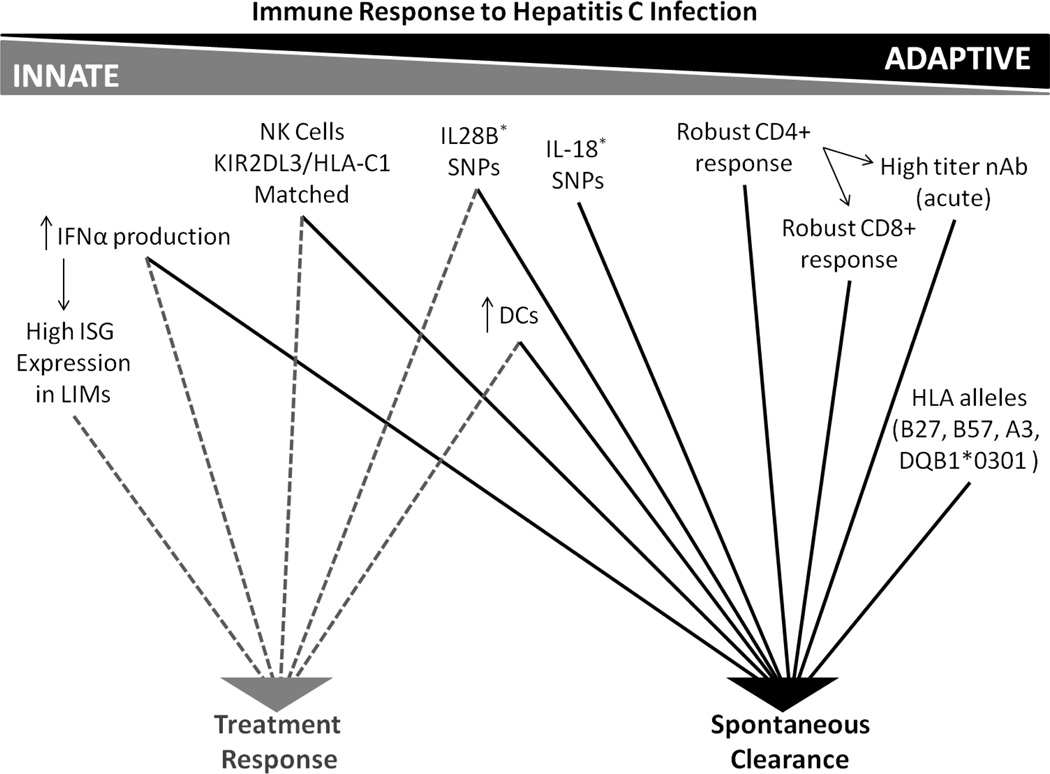
Worldwide, it is estimated that more than 170 million individuals are infected with Hepatitis C Virus (HCV) [1]. Following an acute infection period that is generally asymptomatic, spontaneous clearance of HCV occurs in approximately 30% of HIV uninfected patients [2]. The remaining 70% progress to chronic infection, a subset of whom will develop cirrhosis and hepatocellular carcinoma [3, 4]. Due to shared transmission routes, the prevalence of HCV is especially high among individuals infected with HIV. In the United States and Europe, approximately 16% of HIV-infected persons are co-infected with HCV [5]. Spontaneous clearance rates are lower and complications of HCV more frequent in those with HIV infection, making liver disease the leading cause of non-AIDS-related death in HIV infected individuals [6, 7]. HCV is known only to infect humans and chimpanzees. The virus replicates continuously in hepatocytes, establishing an intrahepatic infection that generally results in alanine aminotransferase (ALT) elevation within 8 weeks after initial exposure. HCV-specific antibodies (Ab) and T cell responses become detectable in the same delayed time frame [2, 3]. Studies have demonstrated that robust innate and adaptive immune responses are necessary for viral clearance [3, 4] (Figure 1).
Figure 1.
Immune Response to Hepatitis C Infection
INNATE IMMUNITY
A hallmark of the innate response to HCV in the liver is the immediate induction of interferons (IFNs), and cytokines. The release of IFNs creates an antiviral state in uninfected cells and inhibits the replication of HCV in infected cells. The release of IFNs and cytokines both inhibits further cellular infection and activates effector cells of the immune system, linking the innate and adaptive arms [3].
Actions of Interferons
The initial response to HCV infection includes the production of type I IFNs in the liver. Activation of toll-like receptor 3 (TLR3) signaling and retinoic acid-inducible gene-I (RIG-I) initiates release of IFN-β, followed later by α. The secreted IFN-β binds to the IFNα/β receptor of neighboring cells, activating the Jak/Stat pathway and inducing an antiviral state. Activation of the Jak/Stat pathway leads to the induction of interferon stimulated genes (ISGs), which degrade viral RNA and amplify the overall IFN response. Specifically, the induction of the ISG IRF7 stimulates IFN subtype diversification and permits the production of type I IFN-α. The newly produced IFN is thought to induce an antiviral state within the liver, potentially contributing to clearance of the virus [3, 4]. The antiviral effect of IFN-α on HCV is borne out by the fact that pegylated interferon-alpha (PEG-IFN alpha) with ribavirin (RBV) has been the mainstay of HCV therapy. However, HCV has evolved mechanisms to prevent activation of both TLR signaling and RIG-I sensing [8]. The HCV serine protease NS3/4A processes HCV nonstructural proteins from the precursor HCV polyprotein, but its protease activity also cleaves interferon-beta promoter stimulator 1 (IPS-1), an adaptor involved in RIG-I-mediated antiviral activity. In addition, NS3/4A has been shown to cleave the Toll/interleukin-1 receptor/resistance domain-containing adaptor-inducing IFN (TRIF) protein, the signaling adaptor molecule for TLR3 [9]. Thus NS3/4A prevents TLR3- and RIG-I-mediated antiviral signaling. The ability of HCV to impair type-I IFN pathway signaling may mean that other innate pathways, such as the type III IFN pathway, are important in effective HCV control.
The type I IFN responses have been more extensively studied, however recent studies suggest that type III IFNs, consisting of IL28A/B and IL29 (lambda 2, 3 and 1, respectively) are also important. Similar to type I IFNS, type III IFNs are upregulated in response to activation of RIG-I and TLR3, and signal through the Jak/Stat pathway [10]. Although both types of IFN induce ISGs and have similar antiviral effects, they bind to different receptors with distinct cellular distribution patterns, indicating divergent biological roles [10]. Single nucleotide polymorphisms (SNPs) rs12979860 C/T and rs8099917 T/G located on chromosome 19 in the region of the IL-28B gene have been associated with both spontaneous clearance and response to PEG-IFN alpha/RBV therapy in individuals infected with HCV. Genotyping of rs12979860 C/T, which is located 3 kb upstream of the IL-28B gene, in HCV cohorts comprised of individuals who spontaneously cleared the virus or had persistent infection showed that the C/C genotype strongly enhanced resolution of HCV infection [2]. In addition, the rs12979860 C/C was associated with a favorable response to PEG-IFN alpha/RBV treatment in a genome-wide association study of 1,137 patients. The study showed a two- to threefold greater rate of sustained viral response (SVR) in association with the C/C genotype versus the C/T and T/T genotypes of the rs12979860 SNP [11]. Three smaller studies subsequently reported similarly increased PEG-IFN alpha/RBV response rates in patients with the T/T genotype (versus G/T or G/G) of another SNP, rs8099917, which is located 8.9 kb downstream from IL-28B in the intergenic region between the IL-28A and IL-28B genes [12–14].
ISG expression pattern in the liver has also been shown to be a predictor of response to therapy. In chimpanzees infected with HCV, highly elevated hepatic ISG expression was associated with no further induction of ISGs and no antiviral efficacy following IFN-alpha treatment [15]. Studies in humans have also reported that the ISG expression pattern within liver infiltrating monocytes (LIM) and hepatocytes is key to predicting outcome. High ISG expression in LIM predicted treatment response, whereas high ISG expression in hepatocytes predicted treatment failure [16]. A later study demonstrated that IL28B genotype and ISG expression independently predict IFN responsiveness and suggested that subjects with the unfavorable IL28B genotype had aberrant baseline induction of innate immune response pathways, resulting in impaired virologic response [17]. Understanding the effect of type III IFNs on HCV infection is also of great interest. Recent research suggests type III IFNs may be the predominant antiviral response to HCV infection in primary human hepatocytes (PHH) and in chimpanzees [18, 19]. PHHs showed an enhanced ability to produce type III IFNs and limited ability to produce type I IFN’s in response to HCV infection. Type III IFN induction caused significant upregulation of ISGs and blocked type I IFNs from acting upon the PHH. The significant induction of ISGs and desensitization of PHH to type I IFNs may explain why high expression of ISGs in hepatocytes contribute to nonresponse to IFN treatment [16, 18, 19]. Exactly how the favorable IL28B genotype and induction of different IFNs contribute to innate immune responses is unknown, but the association of the IL28B gene region and ISG induction with spontaneous and treatment-associated control of HCV suggests an important role for innate immunity in general and perhaps interferon lambda specifically in control of HCV infection.
Actions of Cellular Innate Immunity
In addition to the rapid induction of IFN, the cells of the innate immune system contribute to control of HCV infection. Key immune cells populate the liver and circulate in the periphery. During an acute infection, natural killer (NK) and NK T cell activation leads to interferon-gamma (IFN-γ) production and cytotoxic killing of infected hepatocytes [20–22]. It has been hypothesized that this IFN-γ mediated viral clearance is more efficient than that of T cell mediate clearance [21]. Interestingly, the killing ability of NK cells may be controlled by host genetic factors. Patients with homozygosity of a particular KIR receptor (KIR2DL3) on NK cells and matched HLA type (C1) ligand for that KIR are more likely to spontaneously resolve infection or achieve SVR under treatment, suggesting genetic factors affect NK-mediated control of HCV [21, 22].
The production of IFN-γ by NK and NK T cells leads to the activation of Kupffer cells and the release of proinflammatory molecules. Important proinflammatory molecules include TNF-α, galectin-9, a key regulator in inflammatory pathways within the liver, and interleukin-18 (IL-18), which is primarily produced by macrophages and may be the earliest observed immune response to the virus [23, 24]. The IL-18 response is characterized by a four-fold rise of the cytokine in the plasma on average upon infection, coinciding with detection of HCV RNA in the blood and prior to or at the time of ALT elevation in serum [24]. The initial peak in IL-18 levels begins to decline within a couple months of infection regardless of infection outcome. IL-18 levels return to baseline levels if the virus is cleared. If the virus persists, IL-18 levels decline from the peak, but remain elevated at two- to three- fold higher than levels observed prior to infection [24]. The role of IL-18 in HCV infection is unclear. SNPs rs1946518 -607C/A and rs187238 -137G/C found in the IL-18 promoter have been associated with spontaneous clearance in African-American injection drug users (IDUs) [25]. An additional SNP (rs3750912) in the coding region of the IL-18 binding protein (IL-18BP) gene was found to be associated with clearance of HCV in European ancestry and African-Americans and may play a role in HCV clearance by effecting the activity or production of IL-18 [26]. The role of these proinflammatory molecules in HCV clearance is unclear. However, these molecules are known to promote tissue inflammation, to enhance control of viral replication, and to induce the maturation of monocyte-derived dendritic cells (DCs) [23]. DCs help bridge the innate and adaptive arms of immunity. Plasmacytoid DCs (pDCs) are thought to be the major source of IFN-α driving the innate response, while myeloid DCs (mDCs) activate the adaptive immune response by migrating to lymphoid tissue to prime naïve T cells [27, 28]. The role of DCs during infection is incompletely understood. The frequency of DCs circulating in peripheral blood may be predictive of infection outcome and their function may be impaired in HCV infection; however, other studies show normal DC function in HCV infection and clinical immunosuppression is not observed prior to developing advanced liver disease as might be expected with global DC dysfunction. [29–33].
Continuous activation of the immune system has detrimental effects on the surrounding tissues and the body’s ability to fight infection. In chronic HCV, the number of NK and NK T cells present in the liver significantly declines and the remaining cells lose the ability to properly kill and clear infected cells [20, 21]. The constant presence of proinflammatory may lead to progressive liver injury, cirrhosis, and hepatocellular carinoma [23]. Dysregulation of innate immune system function by HCV is not entirely understood and requires further research.
HIV-HCV Coinfection and Innate Immunity
The innate immune system is further challenged when an individual is coinfected with HCV and HIV. The protective effects of the immune system present in monoinfection are lost or altered with the immune dysregulation HIV brings in coinfection [34, 35]. In contrast to the uniformly beneficial effects of IL28B CC genotype in HCV monoinfected individuals, the IL28B protective genotype is associated with decreased survival in antiretroviral treated HIV and HIV-HCV coninfected patients and the unfavorable genotypes (CT/TT) have been associated with survival [36]. As with HCV monoinfected individuals, the impact of the IL28B genotype on infection outcome is not fully understood. In addition, HIV infection alters innate immune cell function. Macrophages are susceptible to HIV infection in the absence of HCV infection. It was recently reported that type III IFNs induced by HCV can prevent HIV infection of macrophages, potentially altering macrophage responses to HIV [37, 38]. HIV has also been reported to infect DCs and reduce their numbers, which may contribute to poor immune responses in coinfected patients [39]. Chronic HIV-1 infection also results in disruption of NK cell function with altered NK receptor expression and aberrant or decreased cytokine secretion [40]. In general, the presence of both infections limits the ability of the innate immune system to fight infection.
ADAPTIVE IMMUNITY
A unique characteristic of the adaptive immune response to HCV infection is the delayed onset of specific antibody (Ab) and T cell responses [41–43]. Because general immunosuppression is not observed in HCV infected patients before the development of end stage liver disease, an HCV-specific deficiency in priming naïve B and T cells has been suggested as a potential mechanism to explain this delay [4].
Actions of Humoral Immunity
The antibody response to acute HCV infection is primarily restricted to IgG1, of low titer, and delayed in appearance [41, 42]. A specific neutralizing Ab (nAb) response to HCV can take eight to twenty weeks to develop [4, 42]. The ability of agammglobulinemic patients to control infection [44] and the documented cases of HCV clearance prior to the development of nAbs have lead investigators to question if the humoral immune response is required for clearance. Early research indicated that high-titer and crossreactive nAbs are rarely detectable in acute infection and develop late into chronic infection [43, 45]. However, another study using individualized pseudoparticles expressing sequential envelope sequences to measure neutralization by autologous sera demonstrated that Abs neutralizing earlier sequence variants were detected at earlier time points than Abs neutralizing later variants, indicating clearance and evolution of viral variants in response to pressure from nAbs. [46]. This study also showed that high-titer nAbs peaked at the time of viral clearance in all spontaneous resolvers, whereas chronically evolving subjects displayed low-titer or absent nAbs throughout early acute infection. A more recent study provided support for potential clinical relevance of nAbs by demonstrating that a subject who had been chronically infected for two years spontaneously cleared infection after the development of a strong nAb response and reversal of the T cell exhaustion phenotype [47]. Both studies offer evidence that the humoral immune response is involved, and may be necessary, in the resolution of HCV infection.
Studies investigating B cell responses in HCV infected individuals have yet to provide a clear understanding of the impact of HCV infection on B cell development. Initial research suggested that there were no observable difference in numbers or function between B cells from HCV infected individuals and healthy controls [48]. However, more recent studies have demonstrated that the majority of patients with chronic HCV infections carry a substantial proportion of activated B cells [49–51]. The importance of this difference is not yet known and these studies do not agree on other aspects of B cell function and phenotype in HCV such as the distribution of B cells in naïve and memory subsets. Nevertheless, altered B cell function and development have been implicated clearly in extrahepatic disorders associated with HCV infection. The most common disorder is mixed cryoglobulimia (MC) [52]. Recent studies have indicated that MC is triggered by clonal expansion of B cells, apoptosis of naïve B cells, and an increase in size of the immature B cell subset in order to maintain homeostasis [52]. This shift in B cell homeostasis has also been shown to disrupt tolerance, posing a potential risk for the development of autoimmune disease [53]. Epidemiological studies have clearly demonstrated a correlation between chronic HCV infection and occurrence of B-cell non-Hodgkin's lymphomas. Although the mechanisms through which HCV infection induces B-cell lymphoproliferation have not been completely elucidated, possibilities include indirect activation, chronic stimulation, direct infection and mutation of important genes within HCV-specific B cells [54]. Studying these disorders in the setting of HCV infection might provide insight into B cell development as well as HCV pathogenesis.
Actions of T cell Immunity
T cell responses to HCV develop five to twelve weeks after infection [4, 43, 55–57]. This delay is observed regardless of outcome and has been attributed to impaired T cell priming [55, 58]. Though the delayed response of T cells is thought to have little effect on outcome, the quality of CD4+ and CD8+ T cell response generated has been shown to significantly influence resolution of infection [55–57]. The detection of fully functional HCV-specific CD4+ T cells during acute infection is associated with subsequent clearance, suggesting that CD4+ help is necessary for maintaining viral control [56, 57]. Development of a robust CD8+ T cell response is also important in the outcome of infection. There is a temporal association between the detection of HCV-specific CD8+ T cell responses and viral clearance, indicating that virus-specific CD8+ T cells are important in controlling viremia [56, 59]. The CD8+ T cell repertoire is developed early in acute infection and requires simultaneous CD4+ T cell help to maintain responses [43, 57–60]. Without this help, the majority of individuals will progress to a chronic infection, where CD8+ responses decline and are not replaced [43, 61]. Incomplete control of HCV replication by CD8+ T cells is associated in chimpanzees and humans with emergence of viral escape mutations in class I major histocompatibility complex-restricted epitopes and persistence of HCV [60, 62]. Recent studies have reported strong associations linking protective HLA alleles (Class I B27, B57, A3, and Class II DQB1*0301) with spontaneous clearance of infection, further supporting a role of T cells in control since HLA molecules present antigen to T cells [63–65].
Even with the development of CD4+ and CD8+ T cell responses, a subject may still progress to chronic infection. A recent report suggested that transition to chronic infection is not the result of a failure to mount a broad CD4+ T cell response, but is caused by a secondary mechanism that leads to the collapse of the HCV-specific CD4+ T cell response [66]. These results suggest that HCV persistence is not the result of a failure to prime HCV-specific CD4+ T cells, but instead that CD4+ T cell responses undergo rapid exhaustion and deletion in the majority of patients. When CD8+ T cells are still detectable, they exhibit an exhausted phenotype characterized in part by high PD-1+ on T cells specific for intact HCV epitopes, low CD127, and high Tim-3+ surface expression [67, 68]. While T cell exhaustion has been considered to be irreversible without therapeutic manipulation, a recent study reported reversal of T cell exhaustion in conjunction with development of nAbs in a patient after two years of infection [47]. In sum, CD4+ T cell help is lost and CD8+ T cells decline with progression to chronic infection remaining cells either recognizing epitopes no longer present due to viral mutation or expressing high levels of molecules like PD-1 that inhibit T cell function when specific for epitopes that remain intact..
HIV-HCV Coinfection and Adaptive Immunity
As with innate immunity, HIV coinfection alters the adaptive immune system response to HCV. A hallmark of HIV monoinfection is the depletion of CD4+ T cells and generation of immunosupression [34, 35]. It has been demonstrated that this depletion of CD4+ T cells has a detrimental effect on the generation of HCV-specific Abs and leads to a loss of previously generated immunity against HCV [70]. This impairment of antibody generation may be due to the fact that HIV infection induces increased turnover of B cells leading to an increased frequency of short-lived plasmablasts, activated and exhausted B cells, immature transitional B cells and fewer memory B cells [34]. The immune dysregulation associated with HIV infection results in the lower rates of spontaneous control of HCV infection as well as more liver disease progression.
PROPHYLACTIC VACCINATION AND COINFECTION
Continuing advances in chemotherapeutic regimens are promising for effecting cures of HCV and may reduce transmission. However, they are unlikely to control HCV given that therapy for HCV does not provide immunity against subsequent infection and access t o therapy is limited for many of those infected. IDUs and high-risk groups such as health-care providers and men who have sex with men are at risk for new infections and will continue to be at increased risk of infection despite better regimens for HCV treatment. HCV transmission is likely to persist in areas with limited access to antiviral drugs and poor needle injection and blood product hygiene. It has been estimated that fewer than 50% of HCV-infected persons are diagnosed in most developed countries [72] and he proportion of patients who access and complete treatment remains low. The numbers of patients aware of and accessing care are substantially lower in less developed countries. For these reasons as well as the cost of therapy, HCV therapy may not have a significant impact on the disease in many parts of the world and may have minimal impact in blocking the spread of infection within the human population. Therefore, development of a vaccine to prevent chronic HCV infection, if not to prevent infection altogether, is essential for control of HCV disease.
Development of a HCV vaccine will have to overcome many challenges seen in development of an HIV vaccine, including the inability to safely use live-attenuated or killed virus vaccines, heterogeneity of the virus, and the capacity to evade the host immune response. However, multiple studies have demonstrated that previously infected individuals who control their initial infections and become reinfected are substantially less likely than people infected for the first time to develop persistent infection [73–75]. The duration and maximum level of viremia during subsequent episodes of reinfection were significantly decreased compared with those of the primary infection in the same subjects [73–74]. In contrast to the decline in HCV-specific T cells seen in chronic infection, one study showed that reinfection was associated with a significant increase in the breadth of T cell responses. This study also showed more frequent detection of cross-reactive nAbs in reinfected subjects who control infection than is typically seen in those who progress to chronic infection. A study in chimpanzees also showed that rapid resolution of viremia after HCV rechallenge temporally coincided with massive expansion of the dominant memory T cells [76]. HCV reinfection is associated with a reduction in the magnitude and duration of viremia versus initial infection, broadening of cellular immune responses, and generation of cross-reactive humoral responses, all of which are consistent with development of adaptive immunity that is not sterilizing but protects against chronic disease.
Current prophylactic strategies include vaccines that develop HCV-specific CD4+ and/or CD8+ T cell response, HCV nAbs, or a combination of T cell response and nAb response [77–82]. There has been a movement toward preventing chronic infection rather than aiming for sterilizing immunity with a vaccine. The design of prophylactic and therapeutic vaccines has proven challenging, but the first clinical trial of a prophylactic vaccine in individuals at risk for HCV infection began in 2012 with the aim of preventing chronic infection. Lessons from HCV and HIV vaccine attempts as well as the current vaccine trial regardless of outcome could be useful in the development of vaccines against both diseases.
CONCLUSION
Deciphering the immune response to HCV remains a challenging task. Knowledge of how to combat the virus will improve as research unravels the actions of the immune system required for resolution of infection (Figure 1). Previous research has revealed multiple factors that determine the immune response to HCV infection and that co-infection with HIV alters the immune response, leading to more frequent progression to chronic infection and development of liver disease. The transition from an initial innate to a specific adaptive response is a key point of immune regulation in infection and the intersection of those two systems may control progression to chronic infection instead of resolution. Chronic HCV mono-infection and co-infection with HIV have become a public health epidemic. Though the spread of disease may be controlled through treatment, until the underlying immunology behind chronic infection is understood, the generation of an effective HCV vaccine based on correlates of protective immunity may remain a challenge.
Footnotes
Disclosure: No potential conflicts of interest relevant to this article were reported.
Reference List
- 1.World Health Organization. Hepatitis C: global prevalence. Wkly Epidemiol Rec. 1997:341–348.
- 2.Thomas DL, Thio CL, Martin MP, et al. Genetic Variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li K, Lemon S. Innate immune responses in hepatitis C virus infection. Semin Immunopathol. 2012 doi: 10.1007/s00281-012-0332-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman KE, Rouster SD, Chung R, et al. Hepatitis C prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 6.The Centers for Disease Control: HIV and Viral Hepatitis. [Accessed October 2012]; Available at http://www.cdc.gov/hiv/resources/factsheets/hepatitis.htm.
- 7.Bica I, McGovern B, Dhar R, et al. Increasing Mortality Due to End-Stage Liver Disease in Patients with Human Immunodeficiency Virus Infection. Clin Infect Dis. 2001;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 8.Gale M, Jr, Foy EM. Evasion of intracellular host defense by hepatitis C virus. Nature. 2005;436(7053):939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 9.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102(8):2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balagopal A, Thomas DL, Thio CL, et al. IL28B and the Control of Hepatitis C Virus Infection. Gastroenterology. 2012;139(6):1865–1876. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. Early in a series of landmark papers demonstrating that patients infected with HCV who had one of two variants in the IL28B locus (rs12979860 CC or rs8099917 TT) were significantly more likely to respond to PEG-IFN-α /ribavirin therapy and to spontaneously resolve acute HCV infection than patients with other IL28B variants. Hundreds of subsequent studies have examined the role of IL28B polymorphisms in HCV infection and treatment and a test for the IL28B variants is in use to guide clinical decision making.
- 12.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 13.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 14.Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–1345. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 15.Lanford RE, Guerra B, Bigger CB, et al. Lack of Response to exogenous interferon-alpha in the liver of chimpanzees chronically infected with hepatitis C virus. Hepatology. 2007;46(4):999–1008. doi: 10.1002/hep.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGilvray I, Feld JJ, Chen L, et al. Hepatic cell-type specific gene expression better predicts HCV treatment outcome than IL28B genotype. Gastroenterology. 2012;142(4):1122–1131. doi: 10.1053/j.gastro.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Naggie S, Osinusi A, Katsounas A, et al. Dysregulation of innate immunity in hepatitis C virus genotype 1 IL28B-unfavorable genotype patients: Impaired viral kinetics and therapeutic response. Hepatology. 2012;56(2):444–454. doi: 10.1002/hep.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas E, Gonzalez VD, Qisheng L, et al. HCV Infection Induces a Unique Hepatic Innate Immune Response Associated With Robust Production of Type III Interferons. Gastroenterology. 2011;142:978–988. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park H, Serti E, Eke O, et al. IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection. Hepatology. 2012 doi: 10.1002/hep.25897. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahlenstiel G, Titerence RH, Koh C, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138(1):325–335. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amadei B, Urbani S, Cazaly A, et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeromski J, Mozer-Lisewska I, Kaczmarek M, et al. NK cells prevalence, subsets and function in viral hepatitis C. Arch Immunol Ther Exp (Warsz) 2011;59:449–455. doi: 10.1007/s00005-011-0145-y. [DOI] [PubMed] [Google Scholar]
- 23.Mengshol JA, Golden-Mason L, Arikawa T, et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS ONE. 2010;5(3):e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chattergoon M, Levine JS, Latanich R, et al. High plasma interleukin-18 levels mark the acute phase of hepatitis C virus infection. J Infect Dis. 2011;204(11):1730–1740. doi: 10.1093/infdis/jir642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An P, Thio CL, Kirk G, et al. Regulatory polymorphisms in the interleukin-18 promoter are associated with hepatitis C virus clearance. J Infect Dis. 2008;198(8):1159–1165. doi: 10.1086/592047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosbruger TL, Duggal P, Goedert JJ, et al. Large-scale candidate gene analysis of spontaneous clearance of hepatitis C virus. J Infect Dis. 2010;201(9):1371–1380. doi: 10.1086/651606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takahashi K, Shinichi A, Wieland S, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. PNAS. 2012;107(16):7431–7436. doi: 10.1073/pnas.1002301107. The HCV NS3/4A protease blocks the innate host response in infected hepatocyte cell lines in vitro, but HCV induces a strong type 1 interferon response in the infected liver so the source of that interferon was unclear. This paper demonstrated that HCV-infected cells induce an interferon response in pDCs by a mechanism that requires active viral replication, direct cell-cell contact, and Toll-like receptor 7 signaling. These findings highlight a potential source for interferon in acute HCV infection that could result in HCV inhibition in vivo.
- 28.Zhang S, et al. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of HCV-infected cells and induction of IFNα. Hepatology. 2012 doi: 10.1002/hep.25827. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrella A, Atripaldi L, Bellopede P, et al. Flow cytometry assay of myeloid dendritic cells (mDCs) in peripheral blood during acute hepatitis C: possible pathogenetic mechanisms. World Journal of Gastroenterology. 2006;12(7):1105–1109. doi: 10.3748/wjg.v12.i7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancharou AY, Titov LP, DuBuske LM. Altered phenotype and function of monocyte-derived dendritic cells in acute hepatitis C and chronic hepatitis C. J Allergy Clin Immunol. 2009;123(2):S221. [Google Scholar]
- 31.Ulsenheimer A, Gerlach JT, Jung MC, et al. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology. 2005;41(3):643–651. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 32.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97(10):3171–3176. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 33.Barnes E, Salio M, Cerundolo V, et al. Monocyte derived dendritic cells retain their functional capacity in patients following infection with hepatitis C virus. J Viral Hepat. 2008;15(3):219–228. doi: 10.1111/j.1365-2893.2007.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim A, Chung R. Coinfection with HIV-1 and HCV--a one-two punch. Gastroenterology. 2009;137(3):795–814. doi: 10.1053/j.gastro.2009.06.040. An excellent review of the mechanisms used by HIV and HCV to evade immune system control, epidemiologic associations that render coinfection common, and the pathogenesis of accelerated liver disease in patients with HIV-1/HCV coinfection.
- 35.Lambotin M, Barth H, Moog C, et al. Challenges for HCV vaccine development in HIV-HCV coinfection. Expert Review of Vaccines. 2012;11(7):791–804. doi: 10.1586/erv.12.52. [DOI] [PubMed] [Google Scholar]
- 36.Parczewski M, Bander D, Leszczyszyn-Pynka M, et al. IL28B CC Genotype Is Associated with Higher All-Cause Mortality in HIV-Infected Patients. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/AID.2011.0354. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Hou W, Wang X, Ye L, et al. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol. 2009;83(8):3834–3842. doi: 10.1128/JVI.01773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu MQ, Zhou DJ, Wang X, et al. IFN-λ3 inhibits HIV infection of macrophages through the JAK-STAT pathway. PLoS ONE. 2012;7(4):e35902. doi: 10.1371/journal.pone.0035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambotin M, Raghuraman S, Stoll-Keller F, et al. A look behind closed doors: interaction of persistent viruses with dendritic cells. Nat Rev Microbiol. 2010;8(5):350–360. doi: 10.1038/nrmicro2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iannello A, Debbeche O, Samarani S, et al. Antiviral NK cell responses in HIV infection: II. viral strategies for evasion and lessons for immunotherapy and vaccination. J Leukoc Biol. 2008;84:27–49. doi: 10.1189/jlb.0907649. [DOI] [PubMed] [Google Scholar]
- 41.Chen M, Sallberg M, Sonnerborg A, et al. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116(1):135–143. doi: 10.1016/s0016-5085(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 42.Netski DM, Mosburger T, Depla E, et al. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41(5):667–675. doi: 10.1086/432478. [DOI] [PubMed] [Google Scholar]
- 43.Cox AL, Mosbruger T, Lauer GM, et al. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42(1):104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christie JM, Healey CJ, Watson J, et al. Clinical outcome of hypogammaglobulinaemic patients following outbreak of acute hepatitis C: 2 year follow up. Clin Exp Immunol. 1997;110(1):4–8. doi: 10.1046/j.1365-2249.1997.5081412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Logvinoff C, Major ME, Oldach D, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. PNAS. 2004;101(27):10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dowd KA, Netski DM, Wang XH, et al. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136(7):2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raghuraman S, Park H, Osburn W, et al. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis. 2012;205:763–771. doi: 10.1093/infdis/jir835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fournillier A, Freida D, Defrance T, et al. Analysis of B-lymphocyte differentiation in patients infected with hepatitis C virus. J Med Virol. 2004;72(4):566–574. doi: 10.1002/jmv.20039. [DOI] [PubMed] [Google Scholar]
- 49.Oliviero B, Cerino A, Varchetta S, et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55(1):53–60. doi: 10.1016/j.jhep.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Racanelli V, Frassanito MA, Leone P, Galiano M, De Re V, Silvestris F, et al. Antibody production and in vitro behavior of CD27-defined B-cell subsets: persistent hepatitis C virus infection changes the rules. J Virol. 2006;80:3923–3934. doi: 10.1128/JVI.80.8.3923-3934.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosa D, Saletti G, De Gregorio E, et al. Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holz LE, Yoon JC, Raghuraman S, et al. B-cell Homeostasis in Chronic Hepatitis C Virus-Related Mixed Cryoglobulinemia is Maintained Through Naïve B-cell Apoptosis. Hepatology. 2012 doi: 10.1002/hep.25821. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roughan J, Reardon K, Cogburn K, et al. Chronic hepatitis C virus infection breaks tolerance and drives polyclonal expansion of autoreactive B cells. Clin Vaccine Immunol. 2012;19(7):1027–1037. doi: 10.1128/CVI.00194-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forghieri F, Luppi M, Barozzi P, et al. Pathogenetic Mechanisms of Hepatitis C Virus-Induced B-Cell Lymphomagenesis. Clin Dev Immunol. 2012;2012:1–9. doi: 10.1155/2012/807351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker CM. In: Adaptive Immunity to Heptitis C Virus, Advances in Virus Research. 78. Maramorosch K, Shatkin AJ, Murphy FA, editors. Academic Press; 2010. pp. 43–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neumann-Haefelin C, Thimme R. Success and failure of virus-specific T cell responses in hepatitis C virus infection. Dig Dis. 2011;29(4):416–422. doi: 10.1159/000329807. [DOI] [PubMed] [Google Scholar]
- 57.Klenerman P, Thimme R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut. 2012;61:1226–1234. doi: 10.1136/gutjnl-2011-300620. [DOI] [PubMed] [Google Scholar]
- 58.Shin EC, Park SH, Demino M, et al. Delayed Induction, Not Impaired Recruitment of Specific CD8(+) T Cells, Causes the Late Onset of Acute Hepatitis C. Gastroenterology. 2011;141(2):686–695. doi: 10.1053/j.gastro.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197(12):1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302(5645):659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 61.Moroshima C, Di Bisceglie AM, Rothman AL, et al. Antigen-specific T lymphocyte proliferation decreases over time in advanced chronic hepatitis C. J Viral Hepat. 2012;19(6):404–413. doi: 10.1111/j.1365-2893.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox AL, Mosbruger T, Mao Q, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201(11):1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dazert E, Neumann-Haefelin C, Bressanelli S, et al. Loss of viral fitness and cross-recognition by CD8 + T cells limit HCV escape from a protective HLA-B27 – restricted human immune response. J Clin Invest. 2009;119:376–386. doi: 10.1172/JCI36587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kunholm MH, Kavacs A, Gao X, et al. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology. 2010;51(5):1514–1522. doi: 10.1002/hep.23515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim AY, Kuntzen T, Timm J, et al. Spontaneous control of HCV is associated with expression of HLA-B 57 and preservation of targeted epitopes. Gastroenterology. 2011;140(2):686–696. doi: 10.1053/j.gastro.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulze zur Wiesch J, Ciuffreda D, Lewis-Ximenez L, et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J Exp Med. 2012;209(1):61–75. doi: 10.1084/jem.20100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rutebemberwa A, Ray SC, Astemborski J, et al. High-Programmed Death-1 Levels on Hepatitis C Virus-Specific T Cells during Acute Infection are Associated with Viral Persistence and Require Preservation of Cognate Antigen during Chronice Infection. J Immunol. 2008;181:8215–8225. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bengsch B, Seigel B, Ruhl M, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathogens. 2010;6(6) doi: 10.1371/journal.ppat.1000947. e1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMahan RH, Golden-Mason L, Nishimure MI, et al. Tim-3 expression on PD-1 + HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120(12):4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Netski D, Mosbruger TJ, Astemborski J, et al. CD4+ T cell-dependent reduction in hepatitis C virus-specific humoral immune responses after HIV infection. J Infect Dis. 2007;195(6):857–863. doi: 10.1086/511826. [DOI] [PubMed] [Google Scholar]
- 71.Noursadeghi M, Katz DR, Miller RF. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect Dis. 2006;6:794–804. doi: 10.1016/S1473-3099(06)70656-9. [DOI] [PubMed] [Google Scholar]
- 72.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Osburn W, Fisher B, Dowd K, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138(1):315–324. doi: 10.1053/j.gastro.2009.09.017. This paper demonstrated that HCV reinfection is associated with a reduction in the magnitude and duration of viremia versus the initial infection with associated broadening of the cellular immune response and generation of cross-reactive humoral responses. These findings are consistent with the development of adaptive immune responses that protect against persistent disease in the absence of sterilizing immunity. The fact that protective immunity is possible highlights the plausibility of a preventive vaccine since sterilizing immunity may not be required.
- 74.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 75.Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult drug users: a prospective study of incident infection, resolution and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramalingam RK, Meyer-Olson D, Shoukry NH, et al. Kinetic analysis by real-time PCR of hepatitis C virus (HCV)-specific T cells in peripheral blood and liver after challenge with HCV. J Virol. 2008;82(21):10487–10492. doi: 10.1128/JVI.00588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Folgori A, Capone S, Ruggeri L, et al. A T-cell HC V vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12(2):190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 78. Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4(115):115ra1.. doi: 10.1126/scitranslmed.3003155. This paper describes the use of non-human primate adenoviral vector systems encoding HCV antigens to generate robust T cell responses to HCV. These novel vectors are highly immunogenic, appear safe, and antibodies directed against human adenoviruses are minimally cross reactive against them. This vaccine platform forms the foundation of the only prophylactic HCV vaccine trial performed to date in at-risk individuals.
- 79.Meunier JC, Gottwein JM, Houghton M, et al. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J Infect Dis. 2011;204(8):1186–1190. doi: 10.1093/infdis/jir511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drane D, Maraskovsky E, Gibson R, et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: a Phase I study in healthy volunteers. Human Vaccines. 2009;5(3):151–157. doi: 10.4161/hv.5.3.6614. [DOI] [PubMed] [Google Scholar]
- 81.Garrone P, Fluckiger AC, Mangeot PE, et al. A prime–boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med. 2011;3(94):94ra71. doi: 10.1126/scitranslmed.3002330. [DOI] [PubMed] [Google Scholar]
- 82.Lin Y, Kwon T, Polo J, et al. Induction of broad CD4+ and CD8+ T-cell responses and cross-neutralizing antibodies against hepatitis C virus by vaccination with Th1-adjuvanted polypeptides followed by defective alphaviral particles expressing envelope glycoproteins gpE1 and gpE2 and nonstructural proteins 3, 4, and 5. J Virol. 2008;82(15):7492–7503. doi: 10.1128/JVI.02743-07. [DOI] [PMC free article] [PubMed] [Google Scholar]