Abstract
Here, we introduce “tethered capsule endomicroscopy,” that involves swallowing an optomechanically-engineered pill that captures cross-sectional, 30 μm (lateral) × 7 μm (axial) resolution, microscopic images of the gut wall as it travels through the digestive tract. Results in human subjects show that this technique rapidly provides three-dimensional, microstructural images of the upper gastrointestinal tract in a simple and painless procedure, opening up new opportunities for screening for internal diseases.
Diseases of the gastrointestinal (GI) tract are commonly diagnosed by endoscopy, where a flexible video-imaging probe is advanced through a natural orifice into the luminal digestive organs. If an abnormal region is identified, endoscopic biopsy forceps are used to extract a small amount of tissue from the suspect area. The biopsy is then processed and reviewed under a microscope by a pathologist who renders the final diagnosis. It is estimated that approximately 15 million such biopsies are excised and analyzed every year in the United States 1.
While endoscopy has significantly improved health outcomes, it has certain inefficiencies that limit its impact. For most endoscopic procedures, subjects are sedated, requiring a specialized setting, equipment, and medical staff to monitor for adverse reactions. Transnasal endoscopy, which has been gaining in popularity in some endoscopy communities2, does not require sedation, yet necessitates a trained endoscopist to conduct the procedure. Endoscopy is therefore time-consuming and costly, making population-based screening for most conditions difficult to justify. Some GI diseases involve microscopic features that manifest below the tissue surface. Because video imaging only provides information about the superficial mucosal structures, these features cannot be seen with conventional endoscopy or even higher resolution forms of endoscopy such as high-definition magnification endoscopy3. For these cases, the physician must randomly biopsy multiple portions of the organ with the hope of sampling the correct spot, which, unfortunately, is often missed.
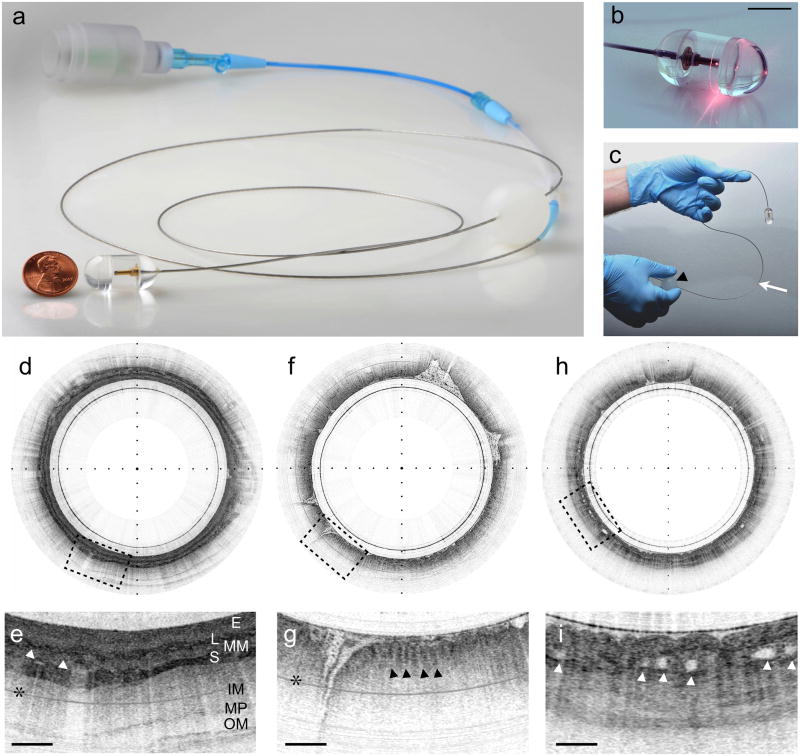
We have created a novel, tethered optomechanical pill 4 (Figs. 1a–c, Supplementary Fig. 1) that captures three-dimensional microscopic images of the digestive organs after it has been swallowed. The tethered capsule employs optical frequency domain (OFDI) imaging5 technology to provide cross-sectional architectural morphologic data that has previously been shown to enable the diagnosis of Barrett’s and high-grade neoplastic changes in the esophagus6–8. The capsule portion of the device is connected to a thin, string-like tether (Figs. 1a–c, Supplementary Fig. 1) that allows the operator to control the position of the capsule in the GI tract 9, effectuates a circumferential scan of the miniature focusing optics in the pill, and transceives light to and from the capsule. Once swallowed, the luminal organs constrict around the pill and gradually push it down the GI tract under the natural propulsion force of peristalsis. Once the capsule has reached the distal-most region of interest, it is pulled back using the tether, again while imaging. During its transit, multiple 30 μm (lateral) × 7 μm (axial) resolution OFDI cross-sections of the luminal organ (Figs. 1d, f, h and Supplementary Movies 1, 2) are acquired enabling the visualization of normal squamous mucosa (Figs. 1e, 2c), stomach (Figs. 1g, 2a), and Barrett’s epithelia (Figs. 1i, 2b). Sequential cross-sections may also be compiled to reconstruct a three-dimensional microscopic representation of the entire luminal organ (Figs. 2d–f and Supplementary Movie 3). After the procedure, the capsule is withdrawn through the mouth and disinfected for reuse. In a study of 13 subjects (normal volunteers (n=7), volunteers with known Barrett’s esophagus (n=6)), we found that the mean transit time for imaging ~15 cm length of esophagus was only 58 sec. For four imaging passes (two up and two down), resulting in four complete datasets, the entire procedure lasted an average of approximately 6 minutes (6 min 18 sec) from capsule insertion to extraction. There were no complications of tethered capsule endomicroscopy. Following the procedure, the majority (12/13) of the subjects reported that they would prefer tethered capsule endomicroscopy to conventional endoscopy.
Figure 1. Tethered capsule endomicroscopy.
(a) Photograph of the entire tethered capsule endomicroscopy device, showing the capsule portion adjacent to a penny for scale. (b) Close-up, time-integrated photograph of the tethered capsule endomicroscope, transmitting red light as the internal optics rotate. (c) The tether (arrow) is very flexible and a plastic ball, attached to the tether (arrowhead), facilitates manipulation of the device. (d) Tethered capsule endomicroscopy image of the normal esophagus, obtained from a normal volunteer in vivo. (e) 3x expanded view of (d) demonstrates the normal esophageal wall architectural morphology, including the squamous epithelium (E), muscularis mucosa (MM), lamina propria (L), submucosa (S), containing blood vessels (arrowheads), inner and outer muscularis (IM) and (OM), and myenteric plexus (MP). (f) Tethered capsule endomicroscopy cross-sectional image of the stomach, obtained from a normal volunteer in vivo, with a 3x expanded view in (g) displaying characteristic glandular “pits” (arrowheads). (h) Image obtained from a patient with histopathologically-confirmed Barrett’s esophagus in vivo. (i) The 3x-magnified view of (h) shows an irregular luminal surface, heterogeneous backscattering, and glands within the mucosa (arrowheads). Tick marks in (d, f, h) - 1 mm; scale bars in (e, g, i) denote 0.5 mm. * multiple reflection artifact.
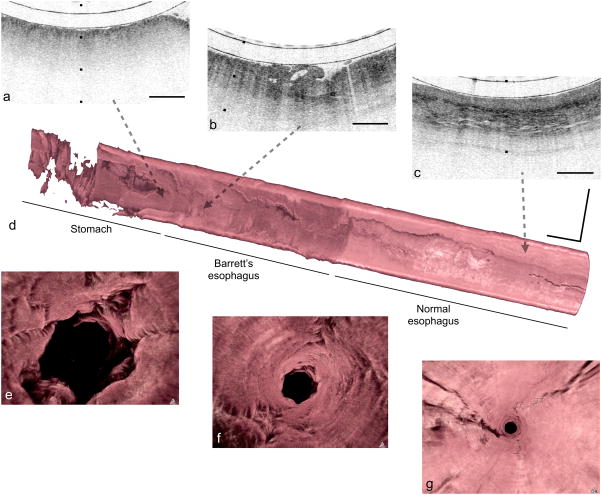
Figure 2. Tethered capsule endomicroscopy data from a patient with a diagnosis of Barrett’s esophagus and high-grade dysplasia/intramucosal carcinoma.
Portion of a cross-sectional tethered capsule microscopy image of stomach (a), Barrett’s with architectural atypia suggestive of high-grade dysplasia (b), and squamous mucosa (c) can be seen at the distal, mid, and proximal ends of the esophagus, respectively. (d) A three-dimensional representation of the tethered capsule endomicroscopy dataset shows a 4 cm segment of Barrett’s esophagus with multiple raised plaques/nodules, one of which corresponds to the features shown in (b). (e–g) Three-dimensional flythrough views of the stomach, Barrett’s segment, and squamous mucosa, respectively, demonstrating a clear difference between the superficial appearance of the rugal folds of the stomach, the crypt pattern of Barrett’s esophagus, and the smooth surface of the squamous mucosa. Tick marks and scale bars in (a–c) represent 1 mm. Scale bars in (d) represent 1 cm.
Tethered capsule endomicroscopy has the opportunity to open up new possibilities for medical screening and diagnosis of GI tract organs. Because these images are obtained from singly scattered light rather than the multiply scattered color reflectance of endoscopy, they carry architectural microscopic image information that is spatially correlated with histopathology from corresponding locations6–8. The device furthermore acquires three-dimensional microscopic image data from large segments of luminal tissues, enabling the comprehensive assessment of subsurface microstructures that are not evident and can be missed by endoscopy. Since the tethered endomicroscopy pill traverses the GI tract without visual guidance, the training required to conduct the procedure is minimal. This and the brevity and ease with which the procedure is performed will enable internal microscopic imaging in almost any health care setting, including in the primary care physician’s office. In addition, because the device can be retrieved and disinfected, tethered capsule endomicroscopy could be inexpensive 9, making it feasible to screen large populations for upper digestive diseases. A somewhat surprising finding of this first foray into capsule endomicroscopy was the degree to which the esophagus remained close to the outer surface of the pill in a manner such that high quality images were obtained (an average of 94.5% of all frames). This result indicates that other in vivo endomicroscopy technologies, such as confocal microscopy10,11, could also be effective when implemented using a capsule. Looking towards the future, the full potential of capsule endomicroscopy will truly come to light through the implementation of tether-free pills, the addition of video imaging for guidance and the incorporation of externally-controlled locomotion 12,13 and concomitant biopsy and therapy 14 functionalities.
METHODS
OFDI technology
OFDI is a cross-sectional, interferometric microscopic imaging technique that records light reflected as a function of depth within tissue 15, information that may be used to accurately render pathologic diagnoses in digestive tract tissues such as the esophagus 6. The OFDI system utilized in this study illuminated tissue using near-infrared (NIR) wavelengths sweeping from 1250–1380 nm. We acquired circumferential, cross-sectional images at 20 frames second−1 using a total of 2048 axial (depth) scans per image. Axial resolution was 7 μm in tissue (estimated refractive index n=1.4) and the sensitivity was ~110 dB. During the procedure, we recorded all raw data in real time and also displayed subsampled versions of the images in real time. Immediately following the imaging session, we reconstructed the images at full resolution (2900 × 2900 pixels) and displayed them using an inverse gray scale lookup table. We automatically aligned frames and rotationally registered them using cross-correlation in ImageJ. We removed signals from the capsule’s inner and outer surfaces prior to three-dimensional volume rendering (Osirix 4.0). We measured the percentage of frames where the capsule was in proximity to the esophagus by dividing the number of frames in which the esophageal wall was clearly visible for greater than 50% of its circumference by the total number of frames.
Tethered capsule endomicroscope device
The capsule comprises a 12.8 mm (diameter) × 24.8 mm (length) transparent, cylindrical shell bounded by hemispherical end caps (Supplementary Fig. 1). The shell encloses miniature optics that redirect focused (FWHM diameter 30 μm) light outside the capsule. The capsule is connected to a flexible, 0.96 mm diameter sheath, which serves as a tether9 (Supplementary Fig. 1). The sheath encloses a driveshaft and an optical fiber; the fiber transmits light to and receives light from the miniature optics inside the capsule. The driveshaft conveys rotational torque from the system’s optical rotary junction to the capsule’s optics (Supplementary Fig. 1). Circumferential, cross-sectional images are acquired as the rotary junction and thus the optical beam in the capsule continuously spins. Three-dimensional images are obtained while acquiring cross-sectional images as the tethered capsule moves up and down the digestive tract.
Imaging procedure
Healthy, unsedated volunteers were enrolled in the study. Subjects were asked to swallow the capsule endomicroscope and then take a sip of water. While the operator held the tether, the capsule was gently allowed to descend through the esophagus to the stomach. The distance between the capsule and the incisors was recorded using 5-cm-spaced marks on the tether. We visualized images in real time to determine when the capsule had reached the stomach. Once in the stomach, we gradually pulled back the pill up through the esophagus to the mouth while imaging. We performed a total of four imaging passes (two up and two down) in each subject. Following imaging, we removed the tethered capsule and disinfected it for reuse in accordance with the standard procedure for the disinfection of GI endoscopes (submersion in Cidex OPA for 12 minutes). Immediately after the pill was withdrawn, we asked each subject whether or not procedure was preferable to endoscopy. The Partners IRB approved the study (Protocol #2011P002619).
Supplementary Material
Supplementary Figure 1. (a) Overview of the tethered capsule endomicroscopy device. The capsule is attached to a tether. A polypropylene (PP) ball handle is affixed to the tether 60 cm from the capsule. The tether terminates at the rotary junction, which is connected to the imaging console and display. Together, the ball and tether allow control of the pill’s location and provide a means for extracting the capsule from the subject when the procedure is complete. (b) Expanded schematic of the capsule. The tether is comprised of a sheath surrounding a driveshaft that encloses an optical fiber. Within the capsule, the driveshaft is connected to a steel tube that contains the miniature imaging optics, including a ferrule, spacer, GRIN lens, and 45-degree reflecting prism. Proximal rotational motion of the driveshaft and fiber at the rotary junction is transduced to the distal steel tube and optics in the capsule, causing the focused beam to rotate around the outer circumference of the capsule. The Teflon washer acts as a bearing to reduce friction between the steel tube and the proximal end cap.
Supplementary Movie 1. Tethered capsule endomicroscopy fall-through dataset from a normal volunteer, obtained in vivo. During this video, the device traveled from the esophagus to the stomach from 30 – 50 cm from the incisors. Frames are displayed using an inverse gray scale lookup table. Tick marks-1 mm.
Tethered capsule endomicroscopy pull-back dataset from a patient with known Barrett’s esophagus, obtained in vivo. During this video, the capsule traveled from the stomach to the esophagus from 50- 35 cm from the incisors. Frames are displayed using an inverse gray scale lookup table. Tick marks-1 mm.
Three-dimensional fly through movie, rendered from the Supplementary Movie 2 dataset. The fly through starts at the proximal aspect of the esophagus, where the surface of the squamous mucosa is relatively smooth and has a higher OFDI signal. At approximately the midpoint of the scan, the perspective enters the Barrett’s mucosa, which has a rough, irregular mucosal surface and a lower OFDI signal. At the end of the fly through movie, the view changes to stomach, where the mucosal surface is smoother than that of the Barrett’s segment and rugal folds can be seen.
Acknowledgments
The authors would like to thank Bill Puricelli and Milen Shishkov for their valuable assistance. This work was supported in part by NIH R01DK091923 and R01CA103769. We used REDCap electronic data capture tools hosted at Massachusetts General Hospital to collect and manage study data.
Footnotes
AUTHOR CONTRIBUTIONS
Device design: MG, RC, KG, MS, BB, MR, GT
Study design: JS, MG, MR, LK, GT
Conduct of the study: JS, NN, LK, KG, MG, GT
Data processing: GT, MG, KG
Manuscript writing: GT, MG
Manuscript review and editing: All authors
References
- 1.Cullen KA, Hall MJ, Golosinskiy A. Natl Health Stat Report. 2009:1–25. [PubMed] [Google Scholar]
- 2.Maffei M, Dumonceau JM. Swiss Med Wkly. 2008;138:658–664. doi: 10.4414/smw.2008.12220. [DOI] [PubMed] [Google Scholar]
- 3.Wasielica-Berger J, Baniukiewicz A, Wroblewski E, Chwiesko A, Dabrowski A. Dig Dis Sci. 2011;56:1987–1995. doi: 10.1007/s10620-010-1551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seibel EJ, et al. IEEE Transactions on Biomedical Engineering. 2008;55:1032–1042. doi: 10.1109/TBME.2008.915680. [DOI] [PubMed] [Google Scholar]
- 5.Yun SH, et al. Nat Med. 2006;12:1429–1433. doi: 10.1038/nm1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans JA, et al. Gastrointest Endosc. 2007;65:50–56. doi: 10.1016/j.gie.2006.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JA, et al. Clin Gastroenterol Hepatol. 2006;4:38–43. doi: 10.1053/S1542-3565(05)00746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poneros JM, et al. Gastroenterology. 2001;120:7–12. doi: 10.1053/gast.2001.20911. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez FC, Shaukat MS, Young MA, Johnson DA, Akins R. Gastrointest Endosc. 2005;61:741–746. doi: 10.1016/s0016-5107(05)00322-6. [DOI] [PubMed] [Google Scholar]
- 10.Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Gastrointest Endosc Clin N Am. 2005;15:715–731. doi: 10.1016/j.giec.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Wallace MB, Fockens P. Gastroenterology. 2009;136:1509–1513. doi: 10.1053/j.gastro.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Quirini M, et al. Gastrointest Endosc. 2008;67:1153–1158. doi: 10.1016/j.gie.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 13.Kim B, Lee MG, Lee YP, Kim Y, Lee G. Sensors and Actuators A: Physical. 2006;125:429–437. [Google Scholar]
- 14.Vakoc BJ, Tearney GJ, Bouma BE. J Biomed Opt. 2007;12:020501. doi: 10.1117/1.2714027. [DOI] [PubMed] [Google Scholar]
- 15.Yun S, Tearney G, de Boer J, Iftimia N, Bouma B. Opt Express. 2003;11:2953–2963. doi: 10.1364/oe.11.002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (a) Overview of the tethered capsule endomicroscopy device. The capsule is attached to a tether. A polypropylene (PP) ball handle is affixed to the tether 60 cm from the capsule. The tether terminates at the rotary junction, which is connected to the imaging console and display. Together, the ball and tether allow control of the pill’s location and provide a means for extracting the capsule from the subject when the procedure is complete. (b) Expanded schematic of the capsule. The tether is comprised of a sheath surrounding a driveshaft that encloses an optical fiber. Within the capsule, the driveshaft is connected to a steel tube that contains the miniature imaging optics, including a ferrule, spacer, GRIN lens, and 45-degree reflecting prism. Proximal rotational motion of the driveshaft and fiber at the rotary junction is transduced to the distal steel tube and optics in the capsule, causing the focused beam to rotate around the outer circumference of the capsule. The Teflon washer acts as a bearing to reduce friction between the steel tube and the proximal end cap.
Supplementary Movie 1. Tethered capsule endomicroscopy fall-through dataset from a normal volunteer, obtained in vivo. During this video, the device traveled from the esophagus to the stomach from 30 – 50 cm from the incisors. Frames are displayed using an inverse gray scale lookup table. Tick marks-1 mm.
Tethered capsule endomicroscopy pull-back dataset from a patient with known Barrett’s esophagus, obtained in vivo. During this video, the capsule traveled from the stomach to the esophagus from 50- 35 cm from the incisors. Frames are displayed using an inverse gray scale lookup table. Tick marks-1 mm.
Three-dimensional fly through movie, rendered from the Supplementary Movie 2 dataset. The fly through starts at the proximal aspect of the esophagus, where the surface of the squamous mucosa is relatively smooth and has a higher OFDI signal. At approximately the midpoint of the scan, the perspective enters the Barrett’s mucosa, which has a rough, irregular mucosal surface and a lower OFDI signal. At the end of the fly through movie, the view changes to stomach, where the mucosal surface is smoother than that of the Barrett’s segment and rugal folds can be seen.