Abstract
A novel strategy for affinity-based surface modification of the conducting polymer, polypyrrole, (PPy), has been developed. A 12-amino acid peptide (THRTSTLDYFVI, hereafter denoted T59) was previously identified via the phage display technique. This peptide non-covalently binds to the chlorine-doped conducting polymer polypyrrole (PPyCl). Studies have previously shown that conductive polymers have promising application in neural electrodes, sensors, and for improving regeneration and healing of peripheral nerves and other tissues. Thus, the strong and specific attachment of bio-active molecules to the surface of PPy using the T59 affinity peptide is an exciting new approach to enhance the bioactivity of electrically active materials for various biomedical applications. We demonstrate this by using T59 as a tether to modify PPyCl with the laminin fragment IKVAV to enhance cell interactions, as well as with the so-called stealth molecule poly(ethylene glycol; PEG) to decrease cell interactions. Using these two modification strategies, we were able to control cell attachment and neurite extension on the PPy surface, which is critical for different applications (i.e., the goal for tissue regeneration is to enhance cell interactions, whereas the goal for electrode and sensor applications is to reduce glial cell interactions and thus decrease scarring). Significantly, the conductivity of the PPyCl surface was unaffected by this surface modification technique, which is not the case with other methods that have been explored to surface modify conducting polymers. Finally, using subcutaneous implants, we confirmed that the PPyCl treated with the T59 peptide did not react in vivo differently than untreated PPyCl.
Keywords: ((Conducting Polymer, Surface Modification, Tissue Engineering, Functional Coatings, Neural Electrodes))
Introduction
The conducting polymer, poly(pyrrole), PPy, has many potential biomedical applications, ranging from a coating on neural electrodes, as a material in biosensing and drug delivery, and as an electrically active platform for tissue engineering1–8. Literature suggests that the chloride doped form, PPyCl, is a well tolerated biomaterial3,6,8–10. It is known that electrical stimulation with implanted metallic electrets improves regeneration of sensory and motor axons11–14. Electrical stimulation has been shown to affect numerous cell types, including endothelial cells 10,15, the rat derived, neuron-like PC12 cell line6, dissociated dorsal root ganglia16, hippocampal neurons17, and is moreover capable of inducing mesenchymal stem cells to differentiate down an osteogenic path18. The presentation of chemical and biochemical cues in the presence of a non-metallic, electrically-active substrate will allow the coupling of these two cues for cell signaling. In this work we discuss a surface modification strategy that uses an affinity peptide to bind non-covalently to PPyCl. This peptide sequence was identified using directed evolution based on the phage display technique19. Briefly, this approach uses a library of 1014 phage, with a variable domain on the coat of the phage to select for binding against a desired binding target. This technique was originally applied to traditional biological systems, seeking antigens to target known biological receptors and recognition sequences for important biomolecules, however this approach has more recently been reported to be applicable to synthetic molecules and inorganic materials20,21. The peptide that was selected to bind to PPyCl is composed of 12-amino acid residues, THRTSTLDYFVI, and was isolated by iterative panning against PPyCl; we will subsequently refer to the peptide as T59 for convenience.
Our primary interest in this approach is as a tissue engineering platform with a combination of biochemical and electrical cues. However, we anticipate that the affinity based surface modification approach will also be applicable to neural electrodes and sensors using PPy. Such electrodes have received attention in the literature but are often limited by problems of signal fidelity degradation over time1,2,22,23. Recording high-fidelity signals is dependent on intimate contact between the electrode and the environment around electrically active cells. Protein layers and encapsulating cells can form transport barriers to the ions involved in signaling and result in signal degradation over time. Increased surface area is one approach at solving this problem and conducting polymers have been utilized in this effort. There are examples in literature of numerous approaches; synthesizing PPy scaffolds in hydrogels surrounding recording electrodes24, depositing PPy around micron and submicron sized beads to achieve complex geometries25, and similarly by depositing PPy around electro-spun fibers26, all seeking to reduce impedance through greater surface area.
Alternatively, increasing or decreasing particular biological interactions is another strategy which has been investigated to improve material performance. Radical insertion chemistries17, monomer modification27, and dopant modification2 have all been attempted; however, these processing methods may render the materials less conductive, therefore increasing their impedances. This balancing act illustrates both the advantages and challenges of conducting polymers.
The T59 peptide offers an alternative methodology for achieving controlled surface chemistry without chemical modification of the PPy surface, thus decreasing the impact on electrical activity. The addition of poly(ethylene glycol), PEG, or other masking molecules through our affinity based technique may reduce the amount of protein adsorbed and consequently the adsorption of cells resulting in better signal from recording electrodes. T59 was conjugated to PEG which is widely used to render surfaces less susceptible to both protein and cell adhesion28. Alternatively, improved biological interaction gives this approach great potential to improve PPy as a tissue engineering scaffold. To illustrate this, T59 was conjugated to the IKVAV peptide sequence. IKVAV is found in the extracellular matrix protein, laminin, which functions to promote cell adhesion and neurite extension in some neurons and neuron-like cells29. Laminin is an important molecule in peripheral neural regeneration; as a major component of the basal lamina, regenerating cells are programmed to recognize this cue. It is therefore logical to include this functionality in biomimetic scaffold design29.
Experimental
Conductivity Measurement
PPyCl films were electrochemically deposited on ITO (Delta Technologies) slides for 90 seconds with a uniform surface area of 2.5 cm × 2.5 cm. ITO slides were used as the working electrode in a three electrode cell. A platinum mesh was used as the counter electrode and a saturated calomel electrode was used as a reference. The reaction solution contained 0.1 M pyrrole (Sigma) and 0.25 M NaCl (Sigma) in deionized (DI) water. Electrochemical synthesis was performed by application of a potential difference of 720 mV for 30 seconds using a CH Instruments 620 potentiostat. Following polymerization the slides were rinsed with DI water, soaked for 30 minutes in 70% EtOH (Sigma) in DI water, then rinsed three more times with DI water.
These films were then cut into 1.0 cm × 2.5 cm strips and incubated 12 hours at room temperature in 10 mM PBS (7.4 pH phosphate buffered saline) or 100 μM T59 in 10 mM PBS. Samples were rinsed with DI water and dried overnight under vacuum and fixed to a glass slide. The four point probe technique for material conductivity was used to compare the conductivity of PPyCl films. The conductivity was calculated based on the method previously published by Runyan in 197530.
Laminin Fragment Study
PPyCl films were electrochemically deposited on ITO coated slides in 1 cm radius circular wells, according to the procedure previously described for the conductivity study described earlier. These wells were filled with 300 μL of the experimental surface treatment. The films were treated with 100 μM of each of the following conditions: (1) the T59 peptide, (2) laminin A Chain (IKVAV) obtained from American Peptide, (3) T59-IKVAV (THRTSTLDYFVIGGGGGQAASIKVAV) obtained from American Peptide, (4) the whole protein laminin incubated at 5 mg/mL (Trevigen), and (5) DI water. The wells were then rinsed a minimum of three times with an excess of PBS.
PC12 cells were obtained from ATCC, and were added to the wells at a concentration of 40,000 cells per cm2, in a low serum media formulation F12k (Sigma), 1% fetal bovine serum (FBS) (Sigma) by volume. Cells were primed with NGF (nerve growth factor) (Invitrogen) for 3 days prior to use in standard medium formulation ( F12k, 15% FBS, 2.5% horse serum (Sigma)). Cells were passaged onto the treated PPyCl films and cultured for 48 hours, in standard conditions of 5% CO, 37°C
Electrical Stimulation Studies
Electrical stimulation samples were cultured identically to the procedures listed above for the laminin study, with the exception that the wells included two silver wires in contact with the culture medium to act as counter and reference electrodes, while the PPyCl film served as the working electrode. The cells were allowed to attach for 24 hours before the application of 100 mV for 2 hours in potentiostat mode. These cells were then allowed to incubate for an additional 24 hours, before being fixed, stained, and analyzed.
Cell Analysis
To fix the cells, the medium was aspirated and replaced with 4% paraformaldehyde for 30 minutes at 37° C. The cells were then stained with DAPI (Molecular Probes) and phalloidin-Alexa 488 (Molecular Probes). The stained cells were then mounted and imaged with an inverted phase-contrast and fluorescence microscope (IX-70, Olympus), using a color CCD video camera (Optronics MagnaFire, model S60800). Six images were taken from each well, and each condition was replicated three times on two separate days. Images were then analyzed with ImageJ software (NIH) to quantify the number of cells, the number of neurites expressed, and the length of the neurites observed.
T59-PEG Study
The T59 peptide prepared with a glycine/serine spacer and C-terminal cystine, or T59-GGGSSC obtained from American Peptide, was reacted with mPEG-Maleimide, molecular weight 2,000 Da, (Laysan Bio) to generate a T59-PEG conjugate. The lyophilized T59-GGGSSC was dissolved in thiol-free PBS at pH 7.2 at 5 mg/mL and added dropwise to a solution of mPEG-Maleimide at 100 mg/mL of thiol-free PBS at pH 7.2. The T59-PEG conjugate was purified using a BioRad gel filtration column and re-concentrated with 3,000 molecular weight cut off membrane filtration cartridge (Millipore). PPyCl films were prepared according to the same protocol as the earlier conductivity studies. Films were incubated for 2 hours at room temperature with PBS, 100 μg/mL T59-PEG conjugate, 50 μg/mL T59-GGGSSC, or 50 μg/mL PEG (MW 2,000).
Cortical astrocytes were isolated and cultured according to published protocol31. Cells were seeded at 10,000 cells/cm2 on the prepared PPyCl substrates, and cultured for 4 hours in low serum medium (DMEM, 1% FBS by volume, Sigma); the medium was then exchanged with standard medium (DMEM, 10% FBS) and cultured for one day. A group of samples were imaged at this point. Remaining samples had the MTS reagent (Sigma) added to the medium and were incubated for an additional 4 hours. 100 μL of the medium was removed from each sample and transferred to a 96 well plate. The absorbance at 490 nm was observed with a Flx800 spectrophotometer (BioTek).
In Vivo Study
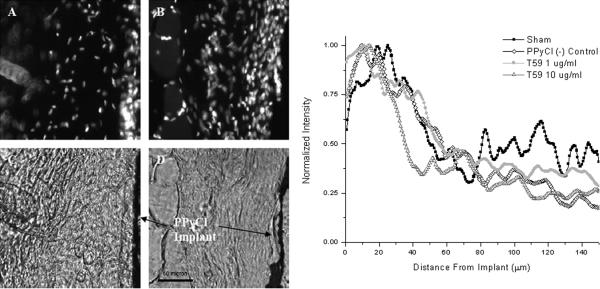
This study was approved by the IACUC at the University of Texas at Austin. NIH guidelines for the care and use of laboratory animals (NIH Publication #85–23 Rev. 1985) have been observed. PPyCl implants were produced by first electrochemically depositing PPyCl on ITO slides as described in the conductivity studies. To provide mechanical support, the PPyCl films were backed with 1 mm PLGA (85:15) (Polysciences) which was drop cast on the PPyCl film; the composite films were cut to a final size of 1 cm2. Implants were placed subcutaneously in the backs of 2 month old male Sprague Dawley rats. Each animal had three implants and one sham surgery: a PBS-treated PPyCl implant and two PPyCl implants incubated in the T59 peptide, one at 1 and the other at 10 μg/mL. The sham surgery consisted of the ~1cm incision and blunt dissection of the subcutaneous pouch into which the implant would placed, then the wound was closed with three to five stitches. The implants were distributed in a square pattern with each implant at a corner separated by ~4 cm. After 14 days the animals were euthanized and the implants removed. Tissues were fixed with 4% paraformaldehyde (Sigma) then cryo-sectioned at 20 microns in thickness and stained with the DAPI (Molecular Probes) nuclear stain. The implants were clearly visible in the unstained bright-field micrographs. DAPI stain typically showed a higher density of nuclei at the interface with the PPyCl implant associated with the foreign body response. Stained images were acquired at a uniform exposure time of 20 ms. The images were post-processed to align the implants in a vertical direction to facilitate intensity mapping, which was conducted by averaging fluorescence intensity as a function of perpendicular distance from the implant. Images used for this analysis were also cropped to 100 μm by 100 μm sections adjacent to the implant. For reference, the profile of cells surrounding the PPyCl implants is shown in Figure 5e, normalized for each section, and plotted as a function of distance from the implant surface. The values from each of three sections per animal for each of the four animals were averaged together to provide a quantitative representation of the nuclear profile.
Figure 5.
Representative sections of subcutaneous PPyCl implants. Panels (A) and (C) show PPyCl implant treated with 10 μg/mL T59 while panels (B) and (D) show an untreated control implant. (A) and (B) show the DAPI staining of the sections while (C) and (D) show phase micrographs of PPyCl implants allowing for clear identification of the implant. (E) Normalized intensity profiles of DAPI nuclear stain versus distance from the PPyCl implant. Data was taken from 20 μm thick sections.
2. Results and Discussion
Conductivity of Peptide-Modified Surface
Conductivity is the most distinguishing feature of PPy as a material. We have tested the conductivity of T59-treated PPyCl films with the four point probe technique described by Runyan30. In Figure 1 we compare the conductivity of PPyCl films freshly synthesized with those incubated for 12 hours in 10 mM PBS or in 100 μM T59 in 10 mM PBS. The conductivity was reduced after incubation in both PBS and 100 μM T59. This is expected because of de-doping and oxidation of the polymer in aqueous solution. However, there is no statistical difference between the PBS-treated film and the T59-treated film, which suggests that the binding of T59 to PPy does not impair its electroactive properties.
Figure 1.
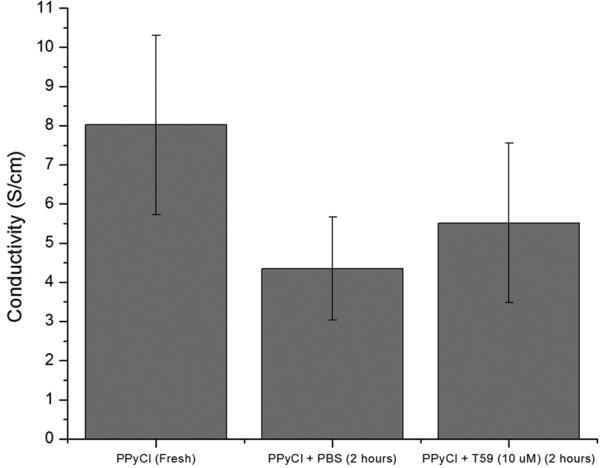
Four point probe conductivity measurements for a freshly synthesized PPyCl film, and films incubated in PBS and T59 at 10 μm in PBS; n=3.
It should be noted however, that the envisioned application of this work will likely require large molecule conjugation using T59, which may introduce a transport barrier to the ions involved in neuronal signal recording (weakening the signal) or in reduction of conductivity over time due to de-doping of the polymer (potentially stabilizing conductivity). So, while we note that the bulk conductivity of the PPyCl material is not affected by exposure to T59, 4-point probe technique measures bulk conductivity and we cannot comment about these potential effects. In any case, the retention of conductivity upon treatment with T59 is a significant improvement over other methods for surface modification of PPy17,32.
Cell Response to T59-Laminin Fragment Treated Surfaces
PPyCl films were treated with T59 peptide which had been conjugated to the cell adhesive and neurite promoting sequence IKVAV33. The full sequence of this peptide is THRTSTLDYFVIGGGGGQAASIKVAV, which we refer to as T59-IKVAV. To control for non-specific adhesion and the signaling effects of the IKVAV sequence we used two negative controls: the original T59 sequence and the native source of IKVAV, the laminin A-chain residues 2091–2108, CSRRRKQAASIKVAVSADR, referred to here as IKVAV. Finally, the intact laminin protein was non-specifically adsorbed to PPyCl as a positive control. Laminin was used as a positive control because this is the native ECM protein from which the IKVAV sequence was derived, and thus represents the optimum conditions for neuron interactions. We should note that our ultimate goal of using T59-IKVAV is an attempt to improve binding of cells to PPy, and to as closely mimic laminin as possible. We do not use laminin to modify PPy because the non-specifically adsorbed laminin would eventually be displaced over time in the body. Our goal is to demonstrate that we can use a higher affinity binding interaction (via T59) to approximate and approach binding that is provided with the intact, natural ECM molecule. In contrast, we could in theory use T59-laminin to provide a strong interaction of the laminin with PPy and to promote cell adhesion, but this is not currently practical because it is cost prohibitive for synthesis.
PC12 cells34 reversibly differentiate into neuron like cells that extend neurites (projections from the cell body of a neuron, that are either axons or dendrites) when exposed NGF. Cells were cultured for two days at 37° C and 5% CO2. Cells were then fixed with paraformaldehyde and stained with phalloidin and DAPI. We analyzed attachment, neurite number and neurite length. Figure 2(a) shows the results for the average number of attached cells on the modified PPyCl surfaces. The number of adhered cells on T59-IKVAV was statistically greater than for the negative controls and significantly lower than for the positive control laminin (ANOVA cut off α ≤ 0.05). The results confirm that we have successfully rendered the PPyCl surface more cell adhesive with the T59-IKVAV surface modification.
Figure 2.
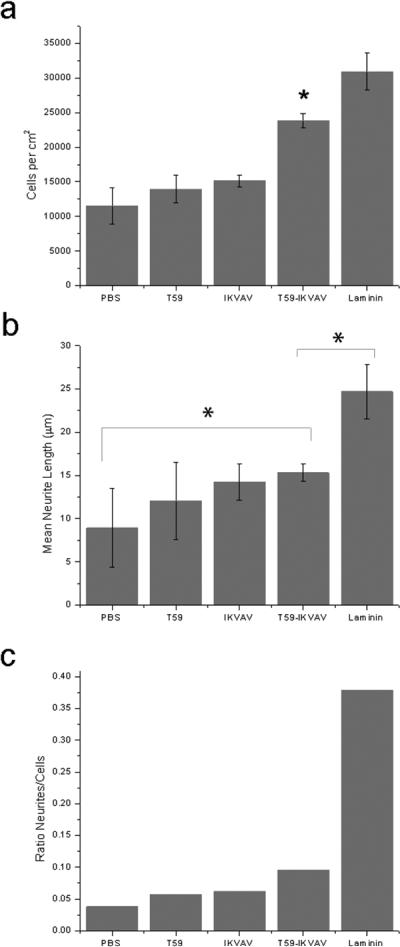
(a). PC12 cell density on PPyCl films treated with PBS, T59, IKVAV peptide, T59-IKVAV, and laminin after 48 hours in culture. ANOVA was performed; the T59-IKVAV treated film had a significantly greater number of cells attached relative to the negative controls and significantly fewer cells than the positive control, laminin. (b). Mean neurite lengths of PC12 cells on treated surfaces, ANOVA was performed; T59-IKVAV treated films had significantly longer neurites than the untreated films, and significantly shorter neurites than the laminin-treated films, but the neurite lengths for the T59 and IKVAV negative controls although shorter, were not statistically significant. (c.) Ratio of neurites to number of attached PC12 cells. (*Denotes statistical significance with an acceptance condition α≤0.05)
Figures 2(b) and 2(c) illustrate the average neurite length and ratio of neurites per attached cell on each treated material. The average neurite length on T59-IKVAV treated films was significantly longer than was the neurite length on the PBS treated films. Neurite lengths on films treated with T59-IKVAV were also greater than for the other two negative controls, T59 and IKVAV, but this result is not statistically significant. As expected, the positive control laminin promoted significantly longer neurites than all other conditions. The T59-IKVAV treatment generated more neurites per cell than any of the negative controls. Again, the laminin positive control produced more neurites per cell than T59-IKVAV. This is anticipated as IKVAV is one of several cell adhesive and neurite promoting sequences from laminin. The ratio of observed neurites per attached cell cannot distinguish between a single neurite on many cells or many neurites on a single cell, but is intended as a broad measure of neurite formation scaled to cell density. Both of these measures show that the T59-IKVAV surface treatment is a significant improvement over an untreated film in terms of cell adhesion and neuritogenesis. Although not a statistically significant difference from the other two negative controls we did observe more and longer neurites on T59-IKVAV treated films than on films treated with T59 or IKVAV individually.
We have not directly measured the surface concentration of the T59-IKVAV bound to PPy. We have instead chosen an incubation concentration above the saturation limit such that we can predict that the concentration will be in the upper limit of 1.27 +/− 0.42 femtomoles/cm2. Primary neuronal cell types have reported differing critical surface concentrations of IKVAV of 3 picomoles/cm2 35 and 8 picomoles/cm2 36 in order to induce cellular response to IKVAV. However, using PC12 cells, responses to surface concentrations as low as 8–150 femtomoles/cm2 have been reported37. Our findings suggest that T59-IKVAV can promote cellular response in PC12 cells at similar concentrations.
An important observation about cell attachment is that the maturation of focal adhesions requires the clustering of integrins38,39. This observation suggests a possible mechanism for the improved cellular attachment on the T59-IKVAV treated substrate. While we have not stained for focal adhesions in this work, future work may be focused on this hypothesis.
Cell Response to Electrically-Stimulated T59-Laminin Fragment Treated Surfaces
The affinity surface modification strategy allows us to combine the electrical properties of PPyCl (which are not impacted with this approach) with the biological properties imparted by the T59-IKVAV molecule. Similar to the laminin fragment study, PC12 cells were cultured on PPyCl films treated with either: PBS, 100 μM T59-IKVAV, or 5 mg/mL laminin. Cells were cultured for 24 hours at 37° C and 5% CO2 and then subjected to 100 mV for 2 hours in which the PPy film acted as the working electrode. Following electrical stimulation the media was exchanged and the cells were cultured for an additional 2 days. Cells were then fixed and stained using the same protocol as described in the previous section.
Cells cultured on the T59-IKVAV treated film exhibited a statistically significant increase in attachment relative to an untreated film, as seen in Figure 3(a). Interestingly, the data show that there was no statistically significant difference in cell attachment between the T59-IKVAV and the positive control laminin on the stimulated films. Figures 3(b) and 3(c) show a statistically relevant increase in neurite length on the T59-IKVAV treated surfaces versus the unmodified surface following electrical stimulation; as well as a greater number of neurites per attached cell. The T59-IKVAV again underperformed the laminin positive control in terms of both average neurite length and number of neurites observed.
Figure 3.
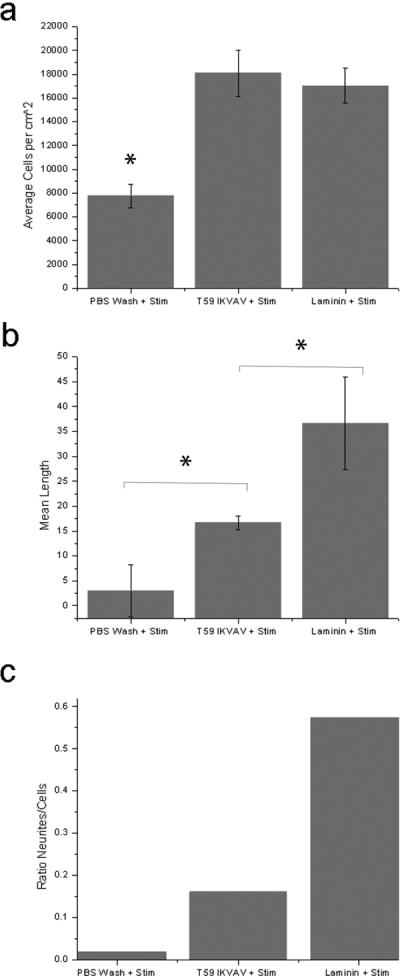
(a). PC12 cell density on PPyCl films treated with PBS, T59-IKVAV, or laminin after electrical stimulation of 100 mV, for 2 hours. ANOVA was performed; the T59-IKVAV treated films had significantly more cells than the untreated films, but were not different from the positive control, laminin. (b.) Mean neurite length, ANOVA was performed T59-IKVAV condition was statistically different from both the untreated surface and the laminin-treated positive control. (c.) Ratio of neurites to the number of attached PC12 cells. (*Denotes statistical significance with an acceptance condition α≤0.05)
It has been previously shown that PC12 cells respond to electrical stimulation on PPyCl by extending longer neurites6. With this result, we show that our T59-IKVAV can be used as a surface modification technique in conjunction with electrical stimulation to generate more neurites per cell. This experiment also is consistent with the previously reported benefits of electrical stimulation on neurite length of PC12 cells on PPyCl6, even if the observed increase in neurite length is not statistically significant in the T59-IKVAV result. Parallel to this earlier experiment, which used high serum conditions with electrical stimulation, we observe an increase in the average neurite length on laminin treated films by more than 40%. Our work with bare PPy films, in a low serum medium, compares poorly with this result, suggesting again how critical the role of protein absorption is to cell attachment and neurite extension40.
Comparing neurite length on electrically stimulated films to the unstimulated films, we observe that films treated with T59-IKVAV had more cells attached and increased neurite lengths. When comparing the fraction of cells expressing neurites however, the positive effect of electrical stimulation is very clear. In both the case of the T59-IKVAV treated surface and the laminin treated surface there were approximately 50% more neurites per cell in the presence of electrical stimulation. Electrical stimulation in conjunction with a properly modified surface promotes the formation of neurites and thus shows great promise as a future avenue for neural regeneration strategies.
T59-Poly(Ethylene Glycol) Modified PPyCl
The T59-GGGSSC peptide was reacted with a poly(ethylene-glycol)-maleimide, using a standard conjugation coupling of a thiol with a maleimide41, and will be referred to as T59-PEG. The molecular weight of the PEG chain was 2,000 Da. This molecular weight of PEG was chosen because it was most similar in molecular weight to the IKVAV (GGGGGQAASIKVAV) conjugate which was used. This is useful in approximating a similar surface coverage and binding behavior, or at least in reducing the effect of excluded volume as much as possible. Cortical astrocytes were used as the model cell for this study. These cells are a major component of the glial cell population in the central nervous system, and are among the cells that interfere with chronic neural recording electrodes. The PPyCl films used for this experiment were prepared identically to those used for the laminin fragment studies, and were incubated for 2 hours with either PBS, 100 μg/mL T59-PEG (~22 μM), 50 μg/mL T59 (~28 μM), or 50 μg/mL PEG (~23 μM). Astrocytes were then seeded on the surfaces at 10,000 cells per square centimeter and cultured for 24 hours in standard culture conditions.
Phase micrographs of two PPyCl surfaces seeded with 10,000 cells per square centimeter are shown in Figure 4. In 4(a) we see an untreated PPyCl surface with a population of attached astrocytes in their star-shaped, spread morphology. This is in contrast to 4(b) in which we see a T59-PEG treated film, with fewer attached cells, predominately in a rounded morphology, reflecting poorly attached astrocytes. To quantify the cell population on the modified surfaces, we performed the MTS cell viability assay on the cell populations after 24 hours in culture. The T59-PEG treated surface exhibited a statistically significant decrease in cell population relative to all control surfaces as shown in Figure 4(c).
Figure 4.
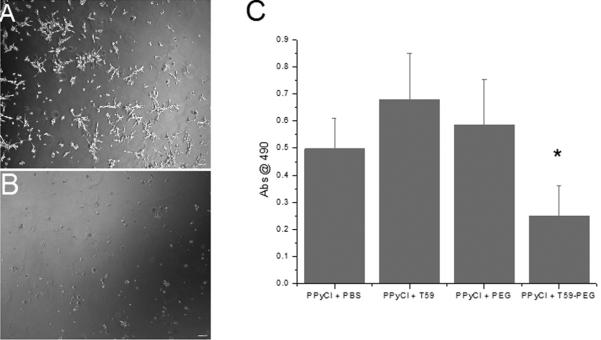
Representative images of cortical astrocytes seeded on (A) PPyCl and (B) PPyCl treated with T59-PEG at 28 hours after seeding. Note the rounded, unattached morphology of the astrocytes on the PPyCl treated with T59-PEG. (C) MTS assay results showing viability of astrocytes on PPyCl after being treated with T59-PEG or controls. T59-PEG treated film had a statistically significant reduction in astrocyte population (*Denotes statistical significance with an acceptance condition α≤0.05)
Poly(ethylene glycol) surface modification is a common strategy to render surfaces less susceptible to cell and protein adhesion. This is a clear demonstration of utilizing T59 to present negative cell adhesion cues. We suggest that the T59 affinity peptide technique can be used to engineer non-cell-adhesive/non-metallic electrodes.
In Vivo Study
In this study we have compared subcutaneous PPyCl implants, which have been treated with the T59 peptide at two concentrations, or were incubated in PBS for an equivalent time of 2 hours. We present a quantitative analysis based on cell nuclear density, taken from micrographs of 20 μm tissue sections. Figure 5(c) shows tissue treated with 10 μg/mL T59 whereas 5(d) shows an untreated PPyCl implant. Figures 5(a) and 5(b) are fluorescence micrographs corresponding to the same tissue section and magnification as 5(c)and 5(d),_stained with DAPI to highlight cell nuclei. Nuclear density was measured as intensity, which was numerically averaged in the vertical direction as a function of distance from the implant as seen in Figure 5e. There is a marked increase in cell nuclei, expected as part of the foreign body response and is typical of implanted materials. Importantly, the maximum intensity of nuclear stain is not statistically different for the implants both treated and untreated with T59.
These results suggest and are consistent with previous studies showing that PPy is a well-tolerated biomaterial3,8,9. We did not seek to repeat these general biocompatibility studies, rather we looked to compare the tissue response to PPy with and without T59. It appears that PPy/T59 is similarly well tolerated in vivo. We did not anticipate any negative effects from the introduction of the T59 peptide in vivo as the T59 sequence has no putative conserved domains with any known protein (NCBI BLAST search of the sequence, THRTSTLDYFVI, at blast.ncbi.nlm.nih.gov/). In this study we present results which we believe lay the foundation for the use of the T59 peptide in vivo, and for use in modifying PPy for various biomedical applications.
Conclusions
We have demonstrated the ability to use a novel peptide (T59) isolated using phage display to successfully modify the surface of the conducting polymer, PPy. By conjugating both the cell adhesive sequence IKVAV, and the non cell-adhesive polymer PEG, we illustrate the diversity of potential application. In the case of the T59 peptide conjugated to IKVAV, we observed that the neuron like PC12 cells exhibited improved attachment and longer and more neurites, indicating successful surface modification. We were also able to passivate the surface and prevent adhesion of activated cortical astrocytes with the surface treatment of T59 conjugated to PEG. Importantly, the T59 peptide treatment of the surface did not reduce the bulk conductivity of PPy. Furthermore, we have shown that there was no significant change in response to a subcutaneous implant of PPy with the addition of the T59 sequence. These results highlight the promise of this and other molecular recognition based techniques to modify material surfaces.
References
- 1.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56(2):261–72. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24(5):777–87. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 3.George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005;26(17):3511–9. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Liao W, Randall BA, Alba NA, Cui XT. Conducting polymer-based impedimetric aptamer biosensor for in situ detection. Anal Bioanal Chem. 2008;392(5):861–4. doi: 10.1007/s00216-008-2354-8. [DOI] [PubMed] [Google Scholar]
- 5.Olayo R, Rios C, Salgado-Ceballos H, Cruz GJ, Morales J, Olayo MG, Alcaraz-Zubeldia M, Alvarez AL, Mondragon R, Morales A, et al. Tissue spinal cord response in rats after implants of polypyrrole and polyethylene glycol obtained by plasma. J Mater Sci Mater Med. 2008;19(2):817–26. doi: 10.1007/s10856-007-3080-z. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci U S A. 1997;94(17):8948–53. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvakumaran J, Keddie JL, Ewins DJ, Hughes MP. Protein adsorption on materials for recording sites on implantable microelectrodes. J Mater Sci Mater Med. 2008;19(1):143–51. doi: 10.1007/s10856-007-3110-x. [DOI] [PubMed] [Google Scholar]
- 8.Williams RLaDPJ. A preliminary assessment of poly(pyrrole) in nerve guide studies. Journal of Materials Science: Materials in Medicine. 1994;5:429–433. [Google Scholar]
- 9.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, Yao J, Chen F, Chen G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2004;68(3):411–22. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 10.Wong JY, Langer R, Ingber DE. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc Natl Acad Sci U S A. 1994;91(8):3201–4. doi: 10.1073/pnas.91.8.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12(12):4381–90. [PubMed] [Google Scholar]
- 12.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20(7):2602–8. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005;194(1):221–9. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205(2):347–59. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Garner B, Georgevich A, Hodgson AJ, Liu L, Wallace GG. Polypyrrole-heparin composites as stimulus-responsive substrates for endothelial cell growth. J Biomed Mater Res. 1999;44(2):121–9. doi: 10.1002/(sici)1097-4636(199902)44:2<121::aid-jbm1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Song HK, Toste B, Ahmann K, Hoffman-Kim D, Palmore GT. Micropatterns of positive guidance cues anchored to polypyrrole doped with polyglutamic acid: a new platform for characterizing neurite extension in complex environments. Biomaterials. 2006;27(3):473–84. doi: 10.1016/j.biomaterials.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81(1):135–49. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castano H, O'Rear EA, McFetridge PS, Sikavitsas VI. Polypyrrole thin films formed by admicellar polymerization support the osteogenic differentiation of mesenchymal stem cells. Macromol Biosci. 2004;4(8):785–94. doi: 10.1002/mabi.200300123. [DOI] [PubMed] [Google Scholar]
- 19.Sanghvi AB, Miller KP, Belcher AM, Schmidt CE. Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nat Mater. 2005;4(6):496–502. doi: 10.1038/nmat1397. [DOI] [PubMed] [Google Scholar]
- 20.Sarikaya M, Tamerler C, Jen AK, Schulten K, Baneyx F. Molecular biomimetics: nanotechnology through biology. Nat Mater. 2003;2(9):577–85. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 21.Tamerler C, Sarikaya M. Molecular biomimetics: utilizing nature's molecular ways in practical engineering. Acta Biomater. 2007;3(3):289–99. doi: 10.1016/j.actbio.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Abidian MR, Martin DC. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials. 2008;29(9):1273–83. doi: 10.1016/j.biomaterials.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008;29(24–25):3393–9. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Yoon SG, Lee YM, Kim HC, Kim SI. Electrical behavior of polymer hydrogel composed of poly(vinyl alcohol)-hyaluronic acid in solution. Biosens Bioelectron. 2004;19(6):531–6. doi: 10.1016/s0956-5663(03)00277-x. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Lipkin K, Martin DC. Electrochemical fabrication of conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) nanofibrils on microfabricated neural prosthetic devices. J Biomater Sci Polym Ed. 2007;18(8):1075–89. doi: 10.1163/156856207781494359. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30(26):4325–35. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JW, Serna F, Nickels J, Schmidt CE. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules. 2006;7(6):1692–5. doi: 10.1021/bm060220q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai NP, Hubbell JA. Solution technique to incorporate polyethylene oxide and other water-soluble polymers into surfaces of polymeric biomaterials. Biomaterials. 1991;12(2):144–53. doi: 10.1016/0142-9612(91)90193-e. [DOI] [PubMed] [Google Scholar]
- 29.Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK, Yamada Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264(27):16174–82. [PubMed] [Google Scholar]
- 30.Runyan WR. Semiconductor Measurements and Instrumentation. McGraw-Hill; New York: 1975. [Google Scholar]
- 31.Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1(5):2406–15. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- 32.Gomez N, Lu Y, Chen S, Schmidt CE. Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials. 2007;28(2):271–84. doi: 10.1016/j.biomaterials.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 33.Yamada Y, Kleinman HK. Functional domains of cell adhesion molecules. Curr Opin Cell Biol. 1992;4(5):819–23. doi: 10.1016/0955-0674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 34.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73(7):2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thid D, Bally M, Holm K, Chessari S, Tosatti S, Textor M, Gold J. Issues of ligand accessibility and mobility in initial cell attachment. Langmuir. 2007;23(23):11693–704. doi: 10.1021/la701159u. [DOI] [PubMed] [Google Scholar]
- 36.Thid D, Holm K, Eriksson PS, Ekeroth J, Kasemo B, Gold J. Supported phospholipid bilayers as a platform for neural progenitor cell culture. J Biomed Mater Res A. 2008;84(4):940–53. doi: 10.1002/jbm.a.31358. [DOI] [PubMed] [Google Scholar]
- 37.Tong YW, Shoichet MS. Peptide surface modification of poly(tetrafluoroethylene-co-hexafluoropropylene) enhances its interaction with central nervous system neurons. J Biomed Mater Res. 1998;42(1):85–95. doi: 10.1002/(sici)1097-4636(199810)42:1<85::aid-jbm11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 38.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(Pt 10):1677–86. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267(5199):883–5. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 40.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22(10):1055–64. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 41.Hermanson GT. Bioconjugate Chemistry. 1996 Bioconjugate Chemistry. [Google Scholar]