Abstract
The mycobactericidal properties of macrophages include the delivery of bacteria to a hydrolytic lysosome enriched in bactericidal Ubiquitin-derived peptides (Ub-peptides). To better understand interactions of ubiquitin-derived peptides with mycobacteria, we further characterized the structure and function of the bactericidal Ub-peptide Ub2. We found that Ub2 adopts a β-sheet conformation in the context of sodium dodecyl sulfate (SDS) micelles and phospholipid (POPC:POPG, 1:1) vesicles that was dependent upon the primary sequence of the peptide. Point mutations in Ub2 that reduced the net charge of the peptide decreased Ub2 bactericidal activity. We investigated Ub-peptide function in the context of model membranes and intact bacteria. Differential scanning calorimetry analysis demonstrated that Ub2 inserts into and perturbs model phospholipid vesicles. In addition, we demonstrate that Ub2 disrupts the integrity of the mycobacterial membrane, equilibrates the transmembrane potential and localizes within both the mycobacterial membrane and cytoplasm of treated bacteria. Finally, we identified additional bactericidal Ub-peptides and characterized their activity and structure. This study provides new insight into the mycobactericidal mechanisms of Ub-peptides.
Mycobacterium tuberculosis is an intracellular pathogen that infects one third of the world population with 2 million TB-related deaths reported annually (1). In the macrophage, M. tuberculosis survives by arresting phagosome maturation and blocking fusion of the bacteria-containing vacuole with the lysosome. However, in activated or autophagic macrophages, mycobacteria are delivered to the lysosome and killed (2–4). We found that ubiquitin-derived peptides (Ub-peptides) contribute to the mycobactericidal activity of the lysosome, and subsequently showed that immunodepletion of ubiquitin from the lysosomal extract results in reduced bactericidal activity (3, 5). While full-length ubiquitin lacks bactericidal activity, Ub-peptides obtained in vitro via digestion of ubiquitin by cathepsin proteases effectively kill mycobacteria. Combined, these data suggest that ubiquitylated proteins are delivered to the lysosomal compartment, where cathepsin proteases release Ub-peptides that possess antimicrobial activity (6).
Our subsequent efforts have focused on characterizing the mycobactericidal activity of Ub-peptides. Many of these studies use the mycobactericidal ubiquitin-derived peptide Ub2 (STLHLVLRLRGG). Using kinetic fluorometric assays, we previously showed that Ub2 targets and reduces mycobacterial membrane integrity (5). Based upon these results, we hypothesized that Ub-peptides act similarly to other characterized host antimicrobial peptides that are key components of the host innate immune response. These peptides are thought to target and damage the microbial membrane, although some may possess additional bactericidal activities (7, 8). Biophysical studies on antimicrobial peptides using model membranes have revealed diverse structures and suggest several potential mechanisms for membrane permeabilization. For example, the antimicrobial peptide LL-37 adopts an alpha helical conformation and likely disrupts bacterial membranes by initially “carpeting” the surface of the membrane, then inserting at the polar/nonpolar interface of the membrane. In support of this model, differential scanning calorimetry (DSC) analysis demonstrated that LL-37 interacts with and alters thermodynamic properties of model lipid bilayers (9). To further understand the mechanism by which Ub-peptides function, we used biochemical approaches to define the Ub2 structure-function relationship and identify biologically-relevant Ub-peptides. We found that the primary sequence of Ub2 is vital to Ub2 secondary structure, membrane-targeting ability and bactericidal activity. In addition, we demonstrate that Ub2 localizes within the mycobacterial membrane and cytoplasm. Finally, additional bactericidal Ub-peptides were identified and their bactericidal activities and structures characterized.
Experimental procedures
Maintenance of bacterial cultures
M. smegmatis mc2155 and M. bovis BCG (Pasteur) were maintained in Middlebrook 7H9 medium (Difco) or on Middlebrook 7H11 agar (Difco) plates supplemented with ADS. Bacillus megaterium 899 was obtained from John Helmann at Cornell University. Escherichia coli DH5α and B. megaterium were routinely cultured in Luria broth and LB agar plates.
Antimicrobial peptides
Ub-peptides were synthesized by GenScript (Piscataway, NJ) with > 90% purity. Lyophilized peptide aliquots were stored at −80 °C. Peptides were resuspended in deionized H2O (dH2O) at 1 mM and used immediately. LL-37 was purchased from Anaspec (Fremont, CA).
Circular dichroism
Circular dichroism (CD) spectra of Ub-peptides were recorded on a 215 CD Spectrometer (Aviv Instruments Inc., NJ). The lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidyl-choline (POPG) were purchased from Avanti Polar Lipids, Inc. (Alabaster, NY). POPC and POPG were mixed at a 1:1 molar ratio and dried under a stream of nitrogen. POPC:POPG was resuspended in 10 mM Tris HCl, pH 7.4 and sonicated 45 minutes at 40 °C to form vesicles. Ub-peptides were added at a final concentration of 100 µM in dH2O to 100 mM sodium dodecyl sulfate (SDS) or 470 µM POPC:POPG (1:1). CD measurements were taken with a 0.1 cm path length QS cuvette (Hellma Analytics). Spectra were recorded at 4 °C from 260 to 190 nm, measuring in 0.5 nm steps. The average of triplicate recordings was graphed.
Bactericidal assays
To determine the antimicrobial activity of Ub-peptides against M. smegmatis, B. megaterium, and E. coli, bacteria were normalized to 5 × 105 CFU/mL in 7H9 medium or Luria broth and treated with two-fold dilutions of peptide overnight at 37 °C. The number of surviving bacteria was determined by plating serial dilutions on 7H11 or LB agar plates. MICs were calculated and are defined as the minimal concentration resulting in ≥90% decreased bacterial viability.
Differential scanning calorimetry
The lipids 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dimyristol-sn-glycero-3-[phospho-rac-(1-glycerol)] (DMPG), and 1,2-dipalmitoleoyl-sn-glycero-3-phophoethanolamine (DiPoPE) were purchased from Avanti Polar Lipids, Inc. DMPC and DMPG were mixed in a 1:1 molar ratio in glass tubes and solvent dried from lipids under a stream of nitrogen. DMPC:DMPG was resuspended to a concentration of 2.5 mg/mL in buffer containing 20 mM HEPES, 100 mM NaCl at pH 7.0 in the presence or absence of peptide. DMPC:DMPG samples were heated to 50 °C in a water bath for 2 minutes, followed by 30 seconds of vortexing. Heating and vortexing of samples was repeated a total of three times to form multilamellar lipid vesicles. Samples were degassed under vacuum for 15 minutes after cooling to room temperature. Samples were loaded into an Applied Thermodynamics N-DSC II at 10 °C, heated in the cell to 40 °C, and cooled back to 10 °C before scanning. Samples were scanned from 5 – 40 °C at a rate of 1°C/minute and a buffer scan was subtracted for baseline correction. We measured the main phase transition temperature of lipids using Cpcalc v2.2 from Calorimetry Sciences Corporation.
For DiPoPE samples, lipids were dried in glass tubes under a stream of nitrogen and resuspended to a concentration of 10 mg/mL in buffer containing 10 mM Tris, 100 mM NaCl, 1 mM EDTA, 0.002% NaN3 at pH 7.4 in the presence or absence of peptide. Multilamellar lipid vesicles were prepared and degassed as previously, but with a 60 °C water bath. Samples were loaded into the cell at 25 °C, heated to 60 °C, and cooled to 10 °C before scanning. Samples were scanned twice from 10 – 60 °C at a rate of 1 °C/minute and a buffer scan subtracted for baseline correction of data. We measured the Lα to H‖ phase transition temperature as described above.
Measurement of ΔΨ equilibration in mycobacteria
Equilibration of the transmembrane potential (ΔΨ) was assessed by labeling M. smegmatis mc2155 and M. bovis BCG with 3,3’-diethyloxacarbocyanine iodide (DiOC2) (Molecular Probes, Eugene, OR) in PBS supplemented with 50 mM K2SO4, pH 7.2. Mycobacteria were washed with buffer, stained with 30 µM DiOC2 in buffer for 5 minutes, washed, and resuspended in buffer at OD600=0.2. Cells were treated in triplicate with dH2O, DMSO, 50 µM Ub2, or 50 µM carbonyl cyanide m-chlorophenylhydrazone (CCCP) as a control for ΔΨ dissipation. Fluorescence emission for DiOC2 was measured using a Gemini EM fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) at 612 and 538 nm using the excitation wavelength 485 nm after a 30 minute incubation. The DiOC2 red / green fluorescence ratio was calculated by dividing the fluorescence intensity at λ = 612 nm by λ = 538 nm.
Evaluation of membrane leakage by diffusion of vesicle-encapsulated fluorophore
The ability of Ub2 and Ub2 point substitution peptides to disrupt model bacterial membranes was determined by the encapsulation of carboxyfluorescein in POPC:POPG (1:1) vesicles according to previously established methods with some modification (10). Lipids were hydrated at a concentration of 10 mM in buffer containing 10 mM Tris pH 7.4, 0.1 mM EDTA, and 50 mM carboxyfluorescein. The encapsulation of fluorophore in large unilamellar vesicles (LUVs) was performed by 20 minutes sonication in a Branson 2510 sonicator followed by snap-freezing in a dry ice ethanol bath, which was repeated a total of four times. Vesicles were separated from non-encapsulated dye by passage through a Sephadex column (11). The filtered vesicles containing carboxyfluorescein were diluted to 50 µM lipid with 10 mM Tris pH 7.4, 0.1 mM EDTA, and 100 mM KCl. The diluted vesicles were treated with 10 µM peptide and equilibration of fluorescence monitored using a Gemini EM fluorescence microplate reader. After 30 minutes, the fluorescence of each sample was recorded and the fluorescence of dH2O treated vesicles subtracted. The release of dye from vesicles by peptides was compared to vesicles treated with 0.01% Triton-X-100, which dissolves vesicles and releases the encapsulated dye.
Subcellular fractionation and Western blot analysis
M. smegmatis mc2155 was treated with 50 µM Ub2-biotin or buffer control for 1 hour before harvesting bacterial cultures. To determine the localization of Ub2, subcellular fractions were obtained by differential centrifugation (12). Briefly, cells were washed with PBS and resuspended at an OD600=10 in PBS containing protease inhibitors (Complete Protease Inhibitor Cocktail, Roche), then broken by bead beating with 0.1 mm glass beads for 1 minute. Unbroken cells were pelleted at 3,000 × g and the supernatant retained as total lysate. The total lysate was ultracentrifuged at 27,000 × g for 1 hour at 4°C. The pellet was saved as the cell wall. The supernatant was centrifuged at 100,000 × g for 1 hour at 4°C to separate the cytoplasm (supernatant) from the cell membrane (pellet). Equivalent amounts of membrane and cytoplasmic fractions were applied to nitrocellulose using a slot blotter. Western analysis was performed using monoclonal antibody against biotin (Abcam Inc.), polyclonal antibody against the membrane protein Ag85, and monoclonal antibody IT-42 against the cytoplasmic protein KatG. Antibody against Ag85 and KatG were obtained from BEI Resources (Manassas, VA). HRP-conjugated secondary antibody was used at a 1:10,000 dilution.
High performance liquid chromatography (HPLC) and mass spectrometry analysis
HPLC fractionation and mass spectrometry analysis were performed to identify biologically relevant Ub-peptides. 2 mg of purified Ubiquitin (Sigma) was digested by cathepsin L protease (Biomol). Digested ubiquitin was separated by HPLC on a C18 reverse phase column using an acetonitrile gradient. Each fraction was evaluated by bactericidal assays as described above. Peptides in the fractions that resulted in over 90% reduction in M. smegmatis viability were identified by mass spectrometry.
CMFDA assay to measure the pH of the bacterial cytoplasm
Membrane integrity was assessed by labeling M. smegmatis mc2155 with 5’-chloromethylfluoroscein diacetate (CMFDA) (Molecular Probes, Eugene, OR) in PBS, pH 7.2. Bacteria were washed twice in PBS, pH 7 before being resuspended at OD600=1.5 in PBS, pH 5.5 and transferred to a 96-well microplate. Fluorescence emission was measured at 520 nm using the excitation wavelengths of 450 nm and 490 nm in a SpectraMax Gemini EM fluorescent plate reader for 10 minutes (Molecular Devices, Sunnyvale, CA). Ub-peptides were used at 100 µM. Conversion of the excitation ratio to pH was achieved through polynomial regression of a standard curve generated by treating CMFDA-labeled bacteria with 10 µM nigericin in buffers of known pH. The assay was performed in triplicate, and the average pH of the bacterial cytoplasm after nigericin and Ub-peptide treatment is presented graphically.
Results
Ub2 adopts a β-sheet structure in membrane mimicking conditions
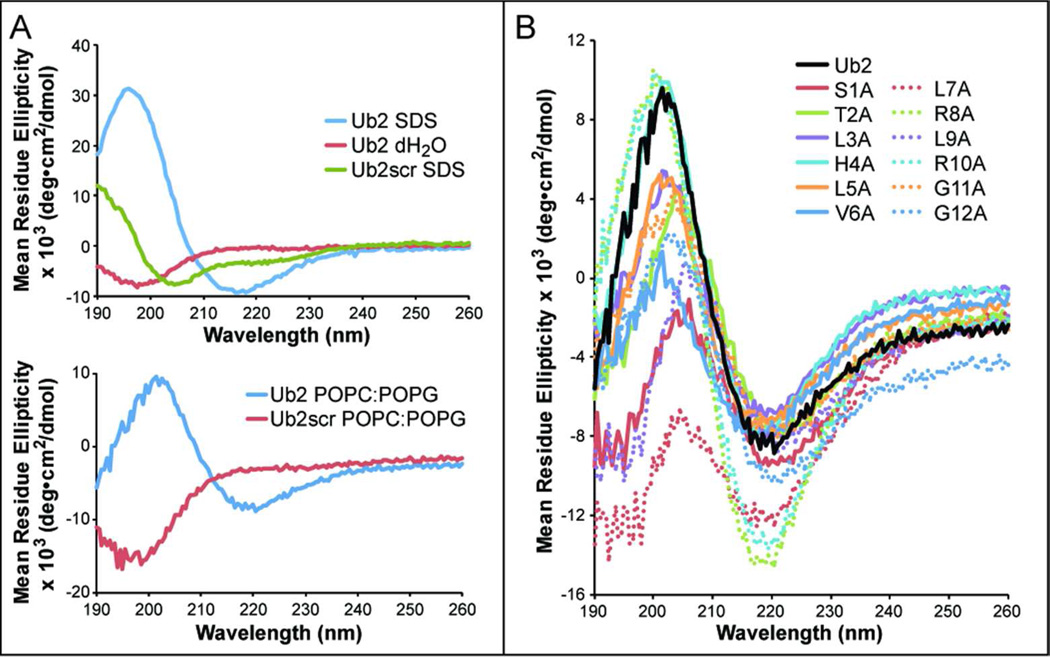
Host antimicrobial peptides adopt a number of conformations including α-helices, relaxed coils, and anti-parallel β-sheets. To determine the secondary structure of the antimicrobial peptide Ub2, we performed circular dichroism (CD). Initial CD measurements indicated Ub2 was largely unstructured in aqueous solution (Figure 1A). It is possible that Ub2 is primarily disordered in the aqueous environment, but adopts a stable conformation upon interaction with the bacterial membrane. This context-dependent shift in conformation would be consistent with that observed in other antimicrobial peptides. Notably, histone-derived antimicrobial peptides lacked structure in aqueous solution, but acquire a stable structure in the presence of membrane mimetic conditions (13). We therefore performed CD of Ub-peptides in the presence of membrane mimetics. In the presence of SDS micelles or POPC:POPG vesicles, the Ub2 peptide produced a negative peak at 220 nm and a positive peak at 197 nm, consistent with a β-sheet secondary structure (Figure 1A).
Figure 1. Ub2 adopts a β-sheet structure in membrane mimetics that depends on primary amino acid sequence.
A) Circular dichroism (CD) spectra of 100 µM Ub2 (STLHLVLRLRGG) or Ub2scr (RLGRLVSLHTLG) were measured in the presence and in absence of 100 mM SDS (top panel) or 470 µM POPC:POPG vesicles (bottom panel). B) CD spectra of 100 µM Ub2 point substitution peptides in the presence of 470 µM POPC:POPG vesicles were indicative of β-sheet structure.
Ub2 function and structure depends on the primary amino acid sequence
To determine whether the primary sequence of Ub2 was important for Ub2 function or structure, we generated the Ub2scr peptide (RLGRLVSLHTLG) that has a scrambled amino acid sequence relative to Ub2 (STLHLVLRLRGG). Ub2scr lacked bactericidal activity against M. smegmatis mc2155 (Table 1). To determine whether it still adopted a β-sheet in the context of a membrane-mimetic, we performed CD on the Ub2scr peptide in the presence of SDS or POPC:POPG. Unlike Ub2, Ub2scr did not adopt a β-sheet structure and instead appeared slightly α-helical in SDS or unordered in POPC:POPG (Figure 1A). These data suggested that the primary amino acid sequence of Ub2 is important for both bactericidal activity and secondary structure.
Table 1.
MICs of Ub2 and Ub2 point substituted peptides against M. smegmatis, B. megaterium and E. coli
| MIC (µM) | ||||
|---|---|---|---|---|
| Peptide | Sequence (Charge) | M. smegmatis | B. megaterium | E. coli |
| Ub2 | STLHLVLRLRGG (+3) | 50 | 5 | 10 |
| Ub2scr | RLGRLVSLHTLG (+3) | > 400 | >100 | >100 |
| Ub2S1A | ATLHLVLRLRGG (+3) | 25 | 5 | 10 |
| Ub2T2A | SALHLVLRLRGG (+3) | 50 | 50 | >100 |
| Ub2L3A | STAHLVLRLRGG (+3) | 100 | 50 | >100 |
| Ub2H4A | STLALVLRLRGG (+2) | > 400 | 10 | 10 |
| Ub2L5A | STLHAVLRLRGG (+3) | 50 | 50 | 5 |
| Ub2V6A | STLHLALRLRGG (+3) | 25 | 50 | >100 |
| Ub2L7A | STLHLVARLRGG (+3) | 100 | 50 | >100 |
| Ub2R8A | STLHLVLALRGG (+2) | > 400 | 100 | >100 |
| Ub2L9A | STLHLVLRARGG (+3) | 50 | 50 | 50 |
| Ub2R10A | STLHLVLRLAGG (+2) | > 400 | >100 | >100 |
| Ub2G11A | STLHLVLRLRAG (+3) | > 400 | 50 | >100 |
| Ub2G12A | STLHLVLRLRGA (+3) | > 400 | 50 | >100 |
To further define the important residues for Ub2 structure and function, we performed scanning alanine mutagenesis on the Ub2 peptide. Twelve Ub2 point substituted peptides were synthesized and bactericidal assays were performed to identify key amino acid residues for Ub2 function. Point substitutions that reduced the positive charge of Ub2 (Ub2H4A, Ub2R8A and Ub2R10A) and peptides with altered C-terminal residues (Ub2G11A and Ub2G12A) were not bactericidal against M. smegmatis (Table 1). The Ub2S1A and Ub2V6A peptides had reduced MICs against M. smegmatis compared to Ub2. Ub2L3A and Ub2L7A had higher MIC values than Ub2 but still retained activity against M. smegmatis. We also determined the MICs of the Ub2 peptides against the Gram-negative species E. coli and the Gram-positive species B. megaterium (Table 1). Consistent with our results with M. smegmatis, we found that the Ub2scr lacked activity against E. coli and B. megaterium. Ub2R8A, Ub2R10A, Ub2G11A and Ub2G12A also had reduced bactericidal activity against both species. However, Ub2H4A retained much of its activity against E. coli and B. megaterium, suggesting that a charged residue at this location of the peptide is more important for function against mycobacteria than other species. To determine whether specific amino acid residues were important for Ub2 structure, CD spectrometry was performed to using POPC:POPG vesicles. All Ub2 point substitution peptides retained a β-sheet structure, though the total β-sheet composition presented by alanine substituted peptides (especially S1A, L7A, L9A) is not identical to Ub2 (Figure 1B). These results indicate that Ub2 structure does not depend on a single residue and that peptide structure alone does not confer bactericidal activity. However, peptide activity does depend on the charge of the peptide, suggesting that electrostatic interactions occur between the peptide and the bacterial membrane.
Ub2 affects membrane structure and integrity
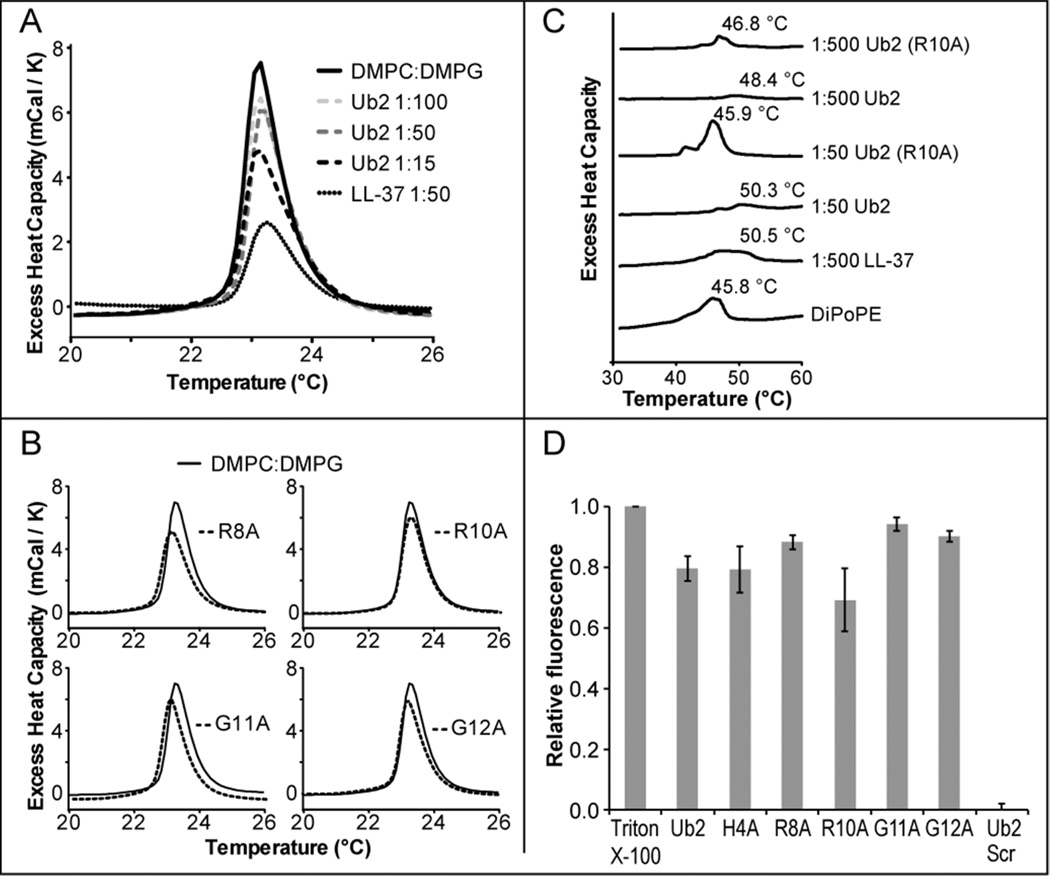
Our working model is that Ub2 peptides target and damage the inner bacterial membrane. Differential scanning calorimetry (DSC) can be used to demonstrate interactions between antimicrobial peptides and lipids in terms of changes in the thermodynamic phase transition of model lipids. To determine if Ub2 function involves perturbation of membrane properties, we performed DSC on multilamellar vesicles composed of DMPC:DMPG (1:1) or DiPoPE. As a control, we included the antimicrobial peptide LL-37 that is known to reduce the enthalpy (ΔH) of the gel lamellar (Lβ) to fluid lamellar (Lα) phase transition of DMPC:DMPG vesicles (14). We observed a sharp peak for lipid alone at 23.3 °C whose amplitude was reduced upon addition of increasing concentrations of Ub2. This result indicates that Ub2 reduces the enthalpy of the Lβ-Lα phase transition (Figure 2A). While Ub2 incorporation perturbed the shape of the main phase transition temperature peak, higher concentrations of Ub2 were required to achieve this than for LL-37. DSC was also used to determine if the non-bactericidal Ub2 point substitution peptides were able to target membranes and perturb their thermodynamic properties. Reduced enthalpy upon treatment of DMPC:DMPG vesicles with Ub2R8A, Ub2R10A, Ub2G11A and Ub2G12A in DMPC:DMPG membranes indicates that these peptides also incorporate and reduce the enthalpy of the phase transition (Figure 2B).
Figure 2. Perturbation of membrane properties by Ub2 and point substitution peptides.
A) Phase transition temperatures of DMPC:DMPG (1:1) multilamellar vesicles in the presence and absence of Ub2. Ub2 was added to DMPC:DMPG (1:1) at the indicated peptide-to-lipid proportions. Addition of LL-37 or Ub2, perturbs the main phase transition peak height, suggesting insertion into the vesicles. B) Ub2 point substitution peptides were added to DMPC:DMPG (1:1) at a peptide-to-lipid ratio of 1:50. The addition of non-bactericidal Ub2 point substitution peptides reduced the enthalpy of the phase transition temperature. C) Phase transition temperatures of DiPoPE in the presence and absence of Ub-peptides. The addition of LL-37 or Ub2 increases the temperature required for phase transition of DiPoPE suggesting the peptides prevent the Lα to H‖ transition. D) Relief of carboxyfluorescein self-quenching by disruption of dye encapsulation in large unilamellar vesicles of POPC:POPG (1:1). Carboxyfluorescein-containing POPC:POPG (1:1) vesicles were incubated in the presence of either 10 µM Ub-peptides or 0.01% Triton-X. Relative fluorescence is defined as the fluorescence of peptide-treated samples relative to detergent-released carboxyfluorescein. The average and +/− standard deviations from three independent assays are shown.
We also examined the ability of Ub2 to insert into DiPoPE liposomes by DSC. DiPoPE undergoes the fluid lamellar (Lα) to inverted hexagonal (H‖) phase transition temperature at 45.8 °C. Addition of Ub2 restricts the transition to H‖ and induces positive curvature strain (Figure 2C). However, we also noted that a 10-fold greater amount of Ub2 was required to achieve shift in transition temperature equivalent to LL-37. These data obtained with model lipid bilayers suggest that in the context of a membrane, Ub2 incorporates into the hydrophobic core of the membrane and perturbs them. Using the DiPoPE liposomes, we again investigated the membrane targeting properties of a non-bactericidal Ub2 point substituted peptide. We found that Ub2R10A peptide did not raise the phase transition temperature to the same extent as equivalent amounts of Ub2. This result suggests that in the context of these membrane conditions, the Ub2R10A is less able to insert and induce curvature in DiPoPE membranes.
To determine whether Ub-peptide treatment results in increased permeability of membranes, we performed a dye leakage assay. POPC:POPG (1:1) liposomes containing carboxyfluorescein at a self-quenching concentration were prepared. Relief of carboxyfluorescein self-quenching upon treatment with Ub2 and inactive Ub2 point substitution peptides was measured and expressed as fluorescence relative to vesicles lysed with detergent. Addition of the Ub2scr peptide resulted in no dye leakage and was statistically equivalent to dH2O treated vesicles. Addition of Ub2 and non-bactericidal Ub2 point substitution peptides resulted in increased fluorescence consistent with release of carboxyfluorescein (Figure 2D). These data suggest that structured, biologically-inactive Ub-peptides are still able to interact with model membranes, but in the context of a bacteria are either deficient in reaching or effectively interacting with the bacterial membrane.
Ub2 equilibrates ΔΨ across mycobacterial membranes
Our previous work demonstrated that treatment of mycobacteria with Ub-peptides results in the equilibration of the external and internal pH (5). To further understand the consequences of Ub-peptide damage, we performed an additional fluorescent assay using the fluorophore DiOC2 to measure the transmembrane potential (ΔΨ) of mycobacteria upon Ub2 treatment. This assay utilizes the charge-sensitive properties of DiOC2 whose red/green fluorescence emission ratio is greater when the dye is associated with the negatively charged internal bacterial membrane. Dissipation of ΔΨ results in lower red/green emission ratios. The intact ΔΨ of control M. smegmatis mc2155 treated with either DMSO or dH2O yielded emission ratios of 0.103 ± 0.002 and 0.099 ± 0.001, respectively (Table 2). Following a 30-minute treatment with 50 µM CCCP or 50 µM Ub2 the emission ratio was reduced to 0.039 ± 0.002 and 0.048 ± 0.001, respectively. This reduction of the red/green emission ratio upon Ub2 treatment indicates a loss of ΔΨ across the membrane. We found the same result for M. bovis BCG, where we measured intact ΔΨ for DMSO (0.112 ± 0.001) and dH2O (0.109 ±0.003) treated cells and reduced ΔΨ with 30 minutes CCCP (0.086 ± 0.003) and Ub2 (0.042 ± 0.002) treatment. Combined our data demonstrate that Ub2 treatment not only results in the loss of ΔpH, but also eliminates the ΔΨ in fast- and slow-growing mycobacteria.
Table 2.
Effect of Ub2 on the mycobacterial transmembrane potential
| DiOC2 red / green fluorescence ratio | ||||
|---|---|---|---|---|
| Strain | DMSO | CCCP | dH2O | Ub2 |
| M. smegmatis | 0.103±0.002 | 0.039±0.002 | 0.099±0.001 | 0.048±0.001 |
| M. bovis | 0.112±0.001 | 0.086±0.003 | 0.109±0.003 | 0.042±0.002 |
Ub-peptides localize to the mycobacterial membrane and cytoplasm
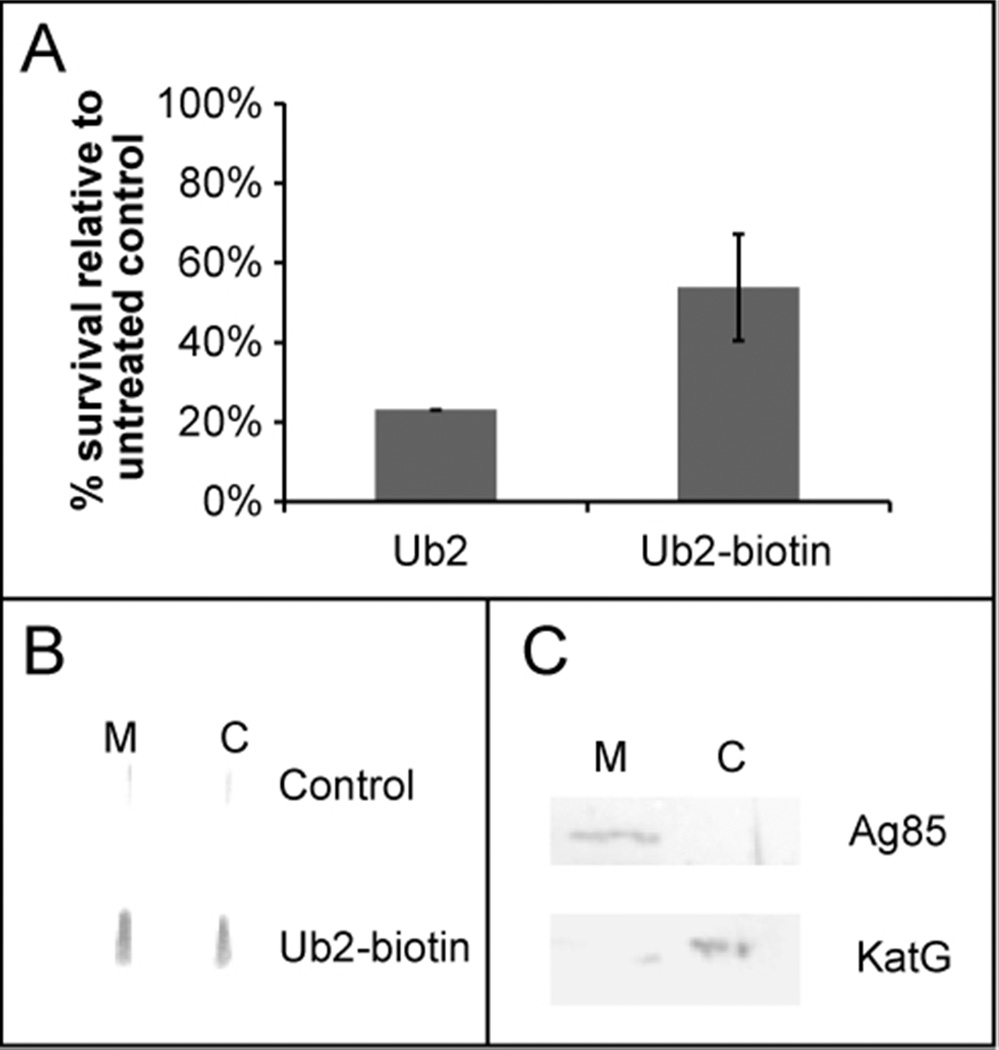
After insertion in the bacterial membrane, some host antimicrobial peptides translocate into the cytoplasm where they are proposed to have additional targets. Our previous membrane integrity studies suggested that Ub-peptides localize to the bacterial membrane. To verify this localization and determine if Ub2 also accesses the cytoplasm, we performed subcellular localization studies. A biotinylated Ub2 peptide was synthesized and the bactericidal activity was tested. The bactericidal activity of Ub2-biotin was similar to that of Ub2 (Figure 3A). Upon subcellular fractionation and Western blot analysis we found Ub2-biotin in both the membrane and cytoplasmic fractions (Figure 3B). As a control for the purity of our fractions, we performed Western analysis using antibody against known membrane and cytoplasmic proteins. This analysis ruled out significant contamination of our fractions and supports localization of Ub2-biotin to both the membrane and cytoplasm (Figure 3C).
Figure 3. Subcellular localization of Ub2-biotin.
A) M. smegmatis mc2155 was sub-cultured to 5×105 cfu/mL in 7H9 medium containing 50 µM Ub2 or Ub2-biotin. Following overnight treatment, the number of viable bacteria was determined by plating serial dilutions, and the average of three replicates is shown. B) M. smegmatis mc2155 was treated with 50 µM Ub2-biotin or buffer control. Bacteria were harvested, membrane (M) and cytoplasmic (C) fractions obtained and applied to nitrocellulose using a slot blotter. Western analysis was performed with monoclonal antibody against biotin. C) To assess the purity of the subcellular fractionation protocol, membrane and cytoplasmic fractions were resolved on a 12% SDS-PAGE gel and Western analysis performed using antibodies against the Ag85 membrane protein and the cytoplasmic protein KatG.
Biologically relevant Ub-peptides were isolated
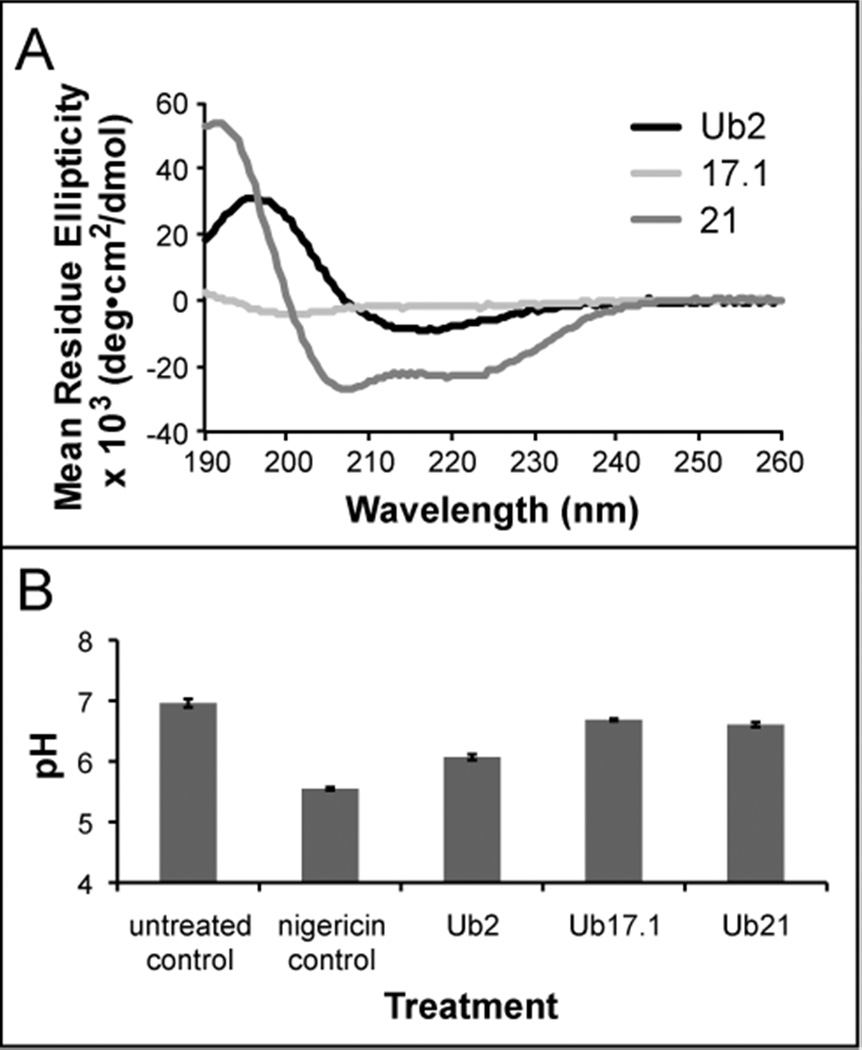
We investigated the existence of other biologically relevant Ub-peptides, as many diverse Ub-peptides can be generated as a result of cathepsin proteolysis of ubiquitylated proteins. Full-length ubiquitin was digested with cathepsin L protease, and the resulting Ub-peptides were separated by HPLC. Each fraction was collected and tested for bactericidal activity (data not shown). Mass spectrometry identified the Ub-peptides present in each of the bactericidal fractions. Multiple peptides were identified in some bactericidal fractions, for example Ub17.1 and Ub17.2. To confirm bactericidal activity of the novel Ub-peptides, synthetic peptides were tested against M. smegmatis, B. megaterium, and E. coli. Ub17.1 (KTLTGKTITLE) and Ub21 (EVEPSDTIENVKAKIQ) exhibited bactericidal activity against M. smegmatis and E. coli comparable to Ub2 (Table 3). To determine if the β-sheet structure is a shared property of bactericidal Ub-peptides, we performed CD spectroscopy. CD spectra measurements of Ub17.1 were consistent with unordered secondary structure, whereas Ub21 exhibited CD spectra measurements associated with α-helices (Figure 4A).
Table 3.
MICs of biologically-relevant Ub-peptides
| MIC (µM) | ||||
|---|---|---|---|---|
| Peptide | Sequence |
M. smegmatis |
B. megaterium |
E. coli |
| 17.1 | KTLTGKTITLE | 50 | >100 | 10 |
| 21 | EVEPSDTIENVKAKIQ | 50 | >100 | 10 |
Figure 4. Secondary structure and activity of biologically relevant peptides.
A) Secondary structures of bactericidal Ub-peptides Ub17.1 and Ub21 were determined by CD spectrometry. Peptides were analyzed at 100 µM in the presence of 100 mM SDS. B) CMFDA-labeled M.smegmatis was resuspended in PBS, pH 5.5 and treated with nigericin or 100 µM Ub-peptide. Fluorescence emission at 520 nm was measured at excitation wavelengths of 450 nm and 490 nm, and the excitation ratio converted to pH by regression. The average and +/− standard deviations from three assays are shown.
We previously showed that Ub2 reduces the ΔpH of the M. smegmatis membrane integrity using an assay based upon the pH- sensitive fluorophore carboxyfluorescein (CMFDA) (5). CMFDA is a membrane-permeable probe, which is processed into a membrane- impermeable form after uptake. The fluorescence emission of fluorescein at 520 nm is pH-sensitive upon excitation at 490 nm, but is pH-insensitive when excited at 450 nm. CMFDA-labeled M. smegmatis were resuspended in a pH 5.5 buffer and treated with Ub-peptides. Membrane integrity is evaluated by the shift in fluorescence upon exposure of the intracellular fluorophore to the extracellular pH. To determine whether Ub17.1 and Ub21 possessed membrane-damaging properties, we performed the CMFDA fluorimetric assay on M. smegmatis. While there was a drop observed in the cytoplasmic pH following treatment with Ub17.1 and Ub21, membrane integrity was not compromised to the same extent as upon treatment with Ub2 (Figure 4B). These results suggests the mechanism of action or efficiency of these peptides differs from Ub2. It is possible that these peptides either disrupt the membrane transiently or require more time than Ub2 to cause equivalent membrane damage.
Discussion
Our previous studies showed Ubiquitin-derived peptides (Ub-peptides) contribute to the lysosomal killing of M. tuberculosis (3). This novel finding greatly impacts our knowledge of the host antimicrobial repertoire and the immune control of mycobacterial infections. To better understand the bactericidal mechanism of Ub-peptides, we further characterized the structure and function of the model Ub-peptide Ub2 and identified two novel biologically-relevant Ub-peptides. Using circular dichroism, we found that Ub2 adopts a β-sheet structure in the context of membrane-mimetics SDS micelles and POPG:POPC (1:1) liposomes. When the amino acids of Ub2 are scrambled, the peptide loses ordered structure and bactericidal function. This is not without precedent as previous work demonstrated that some but not all Bac2A scrambled peptides lose bactericidal activity (15).
We next determined amino acids important for Ub2 bactericidal activity and structure using scanning alanine mutagenesis. Ub2H4A, Ub2R8A, Ub2R10A, Ub2G11A, and Ub2G12A were not bactericidal against M. smegmatis, indicating that the crucial residues of Ub2 for bactericidal activity are charged or positioned at the C-terminal end of the peptide. The importance of positive charge for bactericidal function of Ub2 is consistent with the proposed model for cationic antimicrobial peptides that are thought to initially interact with bacterial membranes via electrostatic interactions. In addition, DSC analysis of non-bactericidal peptides in the context of DiPoPE liposomes demonstrated that Ub2R10A was unable to stabilize positive curvature as robustly as Ub2, suggesting that the loss of charge results in a peptide that fails to establish the same stable lipid-peptide interactions as Ub2. The peptides with reduced positive charge remain structured and able to disrupt artificial membranes as assessed by membrane leakage, so it is possible that these peptides lack bactericidal activity because they either have poor access to the bacterial membrane or are unable to stably insert. Alternatively, our in vitro studies may not provide the correct lipid environment for discerning differences in activity between Ub2 and alanine substituted peptides. The inner membrane of mycobacteria is compositionally similar to other species of bacteria (16), and Ub2 is bactericidal against multiple species. Therefore, we used phospholipids that are commonly used to model bacterial membranes, but acknowledge that others have demonstrated that lipid composition can significantly impact peptide function (17). Ub2G11A, and Ub2G12A also lacked bactericidal activity, but the underlying reason for this phenotype is less clear. The requirement of glycine for function at these positions suggests the necessity of flexibility or tight packing at the C-terminus. It is possible that the C-terminal glycines are required for formation of a cap, which folds back to make contacts and stabilize the peptide in a β-sheet structure. A C-terminal glycine appears to stabilize the α-helical structure of pleurocidin and enhance bactericidal activity (18).
Based upon our previous work and the data presented in this report, we propose a model whereby a key attribute of Ub2 is its ability to interact with and disrupt the mycobacterial membrane. Using fluorimetric assays on intact mycobacteria, we showed previously that treatment with Ub2 results in the equilibration of the external and internal pH (5), and we report here the loss of transmembrane potential. These studies are complemented with differential scanning calorimetry and dye encapsulation assays performed on model liposomes. Similar to the well-characterized antimicrobial peptide LL-37, Ub2 reduced the phase transition of DMPC:DMPG liposomes and increased the Lα to H‖ phase transition temperature of DiPoPE liposomes. We did note that LL-37 was more robust in these assays and that ten-fold higher levels of Ub2 were required to achieve the same effect. Using POPC:POPG liposomes preloaded with carboxyfluorescein, we found that Ub2 treatment disrupts the membrane sufficiently to result in dye leakage and de-quenching that is measured in terms of increased fluorescence. We confirmed the membrane-targeting and insertion ability of Ub2 relative to mycobacteria by performing subcellular fractionation of M. smegmatis upon treatment with a biotinylated Ub2. Combined, our data demonstrate that Ub2 inserts into membrane environments including bacterial membranes and disrupts them to the extent that internal contents are released. Loss of membrane integrity in harsh environments such as the lysosome likely exposes the bacterium to adverse conditions including additional bactericidal molecules.
Several mechanisms are proposed for antimicrobial peptide incorporation into the bacterial membrane and access to the cytoplasm (7, 8). Peptides are thought to initially interact with the membrane electrostatically, inserting parallel to the membrane. This interaction may itself result in transient or sustained disruption of the membrane by interfering with lipid organization and/or packing. In the “carpet” model, the antimicrobial peptide coats the bacterial membrane surface to the extent that the peptides collapse inward, destroying the cytoplasmic membrane (19). In the pore-forming model, a high local concentration of peptide results in reorganization of the peptide perpendicular to the membrane and the formation of pores where the peptide forms the channel in a manner similar to the staves of a barrel (20, 21). In the case of short peptides like Ub2 that are unable to span the membrane, the micellar aggregate channel model is a more likely mechanism. This model proposes that high local concentrations of peptide induces the cytoplasmic membrane to form micellar-like structures that effectively generate channels in the membrane (22–24). Our subcellular localization data indicate that Ub2 not only inserts into the bacterial membrane, but also reaches the cytoplasm. Other antimicrobial peptides that possess additional targets including macromolecule biosynthesis have been identified and their activities characterized (25–27). In light of our data that non-bactericidal Ub2 point substituted peptides retained membrane targeting capacity, we have not ruled out metabolic processes or bacterial enzymes as the secondary targets of Ub-peptides.
The lysosome is a highly toxic environment characterized by low pH, and enriched in hydrolytic enzymes. Recently, the mycobactericidal contribution of antimicrobial peptides generated in the lysosome was revealed (3, 5, 28). Cathepsin cleavage of proteins generates random peptides, and its possible that the lysosome contains multiple bactericidal Ub-peptides. Additional bactericidal Ub-peptides were identified from in vitro digest of ubiquitin. Ub17.1 and Ub21 have lower net charges than Ub2 and exhibited unordered and alpha-helical secondary structures, respectively. The Ub-peptides Ub17.1 and Ub21, showed little to no disruption of the mycobacterial membrane using the CMFDA assay. These results suggest Ub17.1 and Ub21 function in a distinct manner from Ub2, perhaps by targeting the membrane in a less disruptive manner. Therefore, it is likely that the lysosomal milieu contains a mixture of Ub-peptides that possess a range of structures and activities. Further characterization of these peptides may reveal mycobactericidal properties that can be enhanced or utilized to target mycobacteria and mycobacterial infections.
Acknowledgements
We thank Ujwal Shinde for helpful insight and discussion and the OHSU Proteomics core facility for technical assistance.
Funding source Statement: This work was supported by National Institutes of Health award AI087840 and a Collins Medical Trust award to G.E.P.
Abbreviations
- Ub-peptides
Ubiquitin-derived peptides
- SDS
sodium dodecyl sulfate
- DSC
differential scanning calorimetry
- CD
circular dichroism
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol
- POPG
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidyl-choline
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DMPG
1,2-dimyristol-sn-glycero-3-[phospho-rac-(1-glycerol)]
- DiPoPE
1,2-dipalmitoleoyl-sn-glycero-3-phophoethanolamine
- ΔΨ
transmembrane potential
- DiOC2
3,3’-diethyloxacarbocyanine iodide
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- LUVs
large unilamellar vesicles
- CMFDA
5’-chloromethylfluoroscein diacetate
References
- 1.WHO. Fact Sheet No. 104. who.int. 2010 Sep 28;
- 2.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium- containing phagosomes in murine macrophages. J. Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- 3.Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 5.Purdy GE, Niederweis M, Russell DG. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol. Microbiol. 2009;73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purdy GE. Taking Out TB-Lysosomal Trafficking and Mycobactericidal Ubiquitin-Derived Peptides. Front Microbiol. 2011;2:7. doi: 10.3389/fmicb.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 8.Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 9.Henzler-Wildman KA, Lee D-K, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 10.Yandek LE, Pokorny A, Florén A, Knoelke K, Langel ü, Almeida PFF. Mechanism of the Cell-Penetrating Peptide Transportan 10 Permeation of Lipid Bilayers. Biophysical Journal. 2007;92:2434–2444. doi: 10.1529/biophysj.106.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry DW, White JC, Goldman ID. Rapid separation of low molecular weight solutes from liposomes without dilution. Anal. Biochem. 1978;90:809–815. doi: 10.1016/0003-2697(78)90172-0. [DOI] [PubMed] [Google Scholar]
- 12.Mawuenyega KG, Forst CV, Dobos KM, Belisle JT, Chen J, Bradbury EM, Bradbury ARM, Chen X. Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol. Biol. Cell. 2005;16:396–404. doi: 10.1091/mbc.E04-04-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsao HS, Spinella SA, Lee AT, Elmore DE. Design of novel histone-derived antimicrobial peptides. Peptides. 2009;30:2168–2173. doi: 10.1016/j.peptides.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Perturbation of the Hydrophobic Core of Lipid Bilayers by the Human Antimicrobial Peptide LL-37. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]
- 15.Hilpert K, Elliott MR, Volkmer-Engert R, Henklein P, Donini O, Zhou Q, Winkler DFH, Hancock REW. Sequence requirements and an optimization strategy for short antimicrobial peptides. Chem. Biol. 2006;13:1101–1107. doi: 10.1016/j.chembiol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 17.Cheng JTJ, Hale JD, Elliott M, Hancock REW, Straus SK. The importance of bacterial membrane composition in the structure and function of aurein 2.2 and selected variants. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2011;1808:622–633. doi: 10.1016/j.bbamem.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Bryksa BC, MacDonald LD, Patrzykat A, Douglas SE, Mattatall NR. A C-terminal glycine suppresses production of pleurocidin as a fusion peptide in Escherichia coli. Protein Expr. Purif. 2006;45:88–98. doi: 10.1016/j.pep.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47:451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Huang HW. Action of antimicrobial peptides: two-state model. Biochemistry. 2000;39:8347–8352. doi: 10.1021/bi000946l. [DOI] [PubMed] [Google Scholar]
- 21.Huang HW. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochim. Biophys. Acta. 2006;1758:1292–1302. doi: 10.1016/j.bbamem.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 23.Powers J-PS, Hancock REW. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Wu M, Maier E, Benz R, Hancock RE. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 25.Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC. Structure- activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8245–8250. doi: 10.1073/pnas.150518097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subbalakshmi C, Sitaram N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiology Letters. 1998;160:91–96. doi: 10.1111/j.1574-6968.1998.tb12896.x. [DOI] [PubMed] [Google Scholar]
- 28.Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, Virgin HW, Kyei GB, Johansen T, Vergne I, Deretic V. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]