Abstract
Rationale
Functional interactions between limbic regions that process emotions and frontal networks that guide response functions provide a substrate for emotional cues to influence behavior. Stimulation of postsynaptic α2 adrenoceptors enhances the function of prefrontal regions in these networks. However, the impact of this stimulation on the emotional biasing of behavior has not been established.
Objectives
This study tested the effect of the postsynaptic α2 adrenoceptor agonist guanfacine on the emotional biasing of response execution and inhibition in prefrontal cortex.
Methods
Fifteen healthy young adults were scanned twice with functional magnetic resonance imaging while performing a face emotion go/no-go task following counterbalanced administration of single doses of oral guanfacine (1 mg) and placebo in a double-blind, crossover design.
Results
Lower perceptual sensitivity and less response bias for sad faces resulted in fewer correct responses compared to happy and neutral faces, but had no effect on correct inhibitions. Guanfacine increased the sensitivity and bias selectively for sad faces, resulting in response accuracy comparable to happy and neutral faces, and reversed the valence-dependent variation in response-related activation in left dorsolateral prefrontal cortex (DLPFC), resulting in enhanced activation for response execution cued by sad faces relative to happy and neutral faces, in line with other frontoparietal regions.
Conclusions
These results provide evidence that guanfacine stimulation of postsynaptic α2 adrenoceptors moderates DLPFC activation associated with the emotional biasing of response execution processes. The findings have implications for the α2 adrenoceptor agonist treatment of attention-deficit hyperactivity disorder (ADHD).
Keywords: Guanfacine, Response execution, Response inhibition, Emotion, fMRI, Go/No-go task, Prefrontal cortex
Introduction
Socially appropriate behavior requires the encoding of context-dependent emotional cues to guide decisions about which actions to perform and which actions to suppress (Haberman and Whitney 2007). The facial expressions and body gestures that convey these emotional cues in social contexts have also been found to influence multiple domains of cognitive function (Phelps and LeDoux 2005), including response selection, execution, and inhibition (Maxwell et al. 2005; Schulz et al. 2007). Thus, the positive affect (Otta et al. 1994) and approach behavior (Johansson and Ronnberg 1996) elicited by facial expressions of happiness resulted in faster responses that were more difficult to suppress (Hare et al. 2005; Schulz et al. 2007), while expressionless (neutral) faces reduced the accuracy of responses to happy and sad faces (Schulz et al. 2009; Lewis et al. 2008). Identifying the neural mechanisms that support the emotional biasing reflected in response functions has important implications for addressing the problems with impulsivity and emotion regulation that characterize a wide array of psychiatric disorders (Lewis et al. 2008; Murphy et al. 1999; Musser et al. 2011; Silbersweig et al. 2007; Walcott and Landau 2004).
Functional interactions between limbic structures that process affective cues and prefrontal cortex regions that guide behavior provide a putative cortical entry point for emotion to bias response functions (Dolan, 2007). The amygdala is a core component of a limbic network for rapidly detecting and encoding the intensity and valence of emotionally salient stimuli (Dolan 2007; LeDoux 1998), and sends extensive projections to orbital aspects of inferior frontal gyrus (Petrides and Pandya 2002). The inferior frontal gyrus pars orbitalis integrates this emotional information with contextual input from inferotemporal cortex to compute the behavioral significance of cue stimuli (Sakagami and Pan 2007), with separate sets of neurons coding for cues that signal behavioral execution and inhibition (Sakagami et al. 2001). In turn, dorsolateral prefrontal cortex (DLPFC) integrates the pars orbitalis output and visuomotor input from parietal association cortices to exert goal-directed control by biasing neural activity in task-related sensorimotor regions (Egner and Hirsch 2005; Miller and Cohen 2001) and releasing frontal operculum from inhibitory control (Stevens et al. 2007, 2009). Right frontal operculum is purportedly a neural effector for both goal-directed hand actions (Iacoboni and Wilson 2006) and the inhibition of such actions (Garavan et al. 2006; Xue et al. 2008), and has been shown to display both context- and valence-dependent variations in activation during response inhibition (Schulz et al. 2009).
Prefrontal cortex function is intricately modulated by ascending noradrenergic projections from the pontine nucleus locus coeruleus (Arnsten and Li 2005). Noradrenaline released from these projections acts at postsynaptic α2 adrenergic receptors to suppress spontaneous activity (Wang et al. 2011) and increase evoked firing of pyramidal neurons (Carr et al. 2007; Gamo et al. 2010), thereby enhancing the response gain of prefrontal neuronal ensembles to task-relevant inputs (Aston-Jones and Cohen 2005). This increase in response gain has been shown to boost the cue-evoked activation of DLPFC (Clerkin et al. 2009), and may contribute to improvements in response inhibition and increases in frontal opercular activation produced by the noradrenaline reuptake-inhibitor atomoxetine (Chamberlain et al. 2006; 2009). These α2 adrenoceptor actions offer a mechanism to enhance the top-down control of the emotional biasing of response functions using α2 adrenoceptor agonists already approved for the treatment of attention-deficit/hyperactivity disorder (ADHD) (Sallee and Eaton 2010).
This study tested the impact of postsynaptic α2 adrenoceptor stimulation on the emotional biasing of response functions in healthy adults using event-related functional magnetic resonance imaging (fMRI) together with a pharmacological challenge with the α2 adrenoceptor agonist guanfacine. The adults were scanned twice while performing a face emotion go/no-go task following single oral doses of guanfacine and placebo in a double-blind, counterbalanced design. It was predicted that guanfacine stimulation of postsynaptic α2A adrenoceptors would be associated with activation gains in inferior frontal gyrus pars orbitalis, DLPFC, and frontal operculum, which reflect enhanced cognitive control to overcome the reported emotional biasing of response functions, and would result in improved accuracy of responses to sad faces and inhibition of responses to happy faces compared to placebo.
Methods
Participants
Fifteen right-handed adults (8 females) with a mean (± SEM) age of 25.7 ± 1.2 years (range = 21 – 35 years) were recruited via university and medical center campus postings for the study. All participants provided written informed consent for participation after a complete description of the study was provided to them. Participants were compensated for their time. The study was approved by the Institutional Review Boards of Queens College and The Mount Sinai School of Medicine.
Subjects were screened for contraindications to study participation with physical and mental status exams, and ratings on the Beck Anxiety Inventory (BAI) (Beck et al. 1988), Beck Depression Inventory – II (BDI-II) (Steer et al. 1999), and Conners Adult ADHD Rating Scale (CAARS) (Conners et al. 1999). Full Scale IQ was estimated with the Matrix Reasoning and Vocabulary subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999). A total score ≥ 15 on the BDI-II or the BAI, a T-score 1 SD above the mean (i.e., > 60) on the CAARS Total ADHD Symptoms index, and an estimated IQ < 80 were exclusionary for the study. Mean BDI-II and BAI total scores were both 1.4 ± 0.5, mean CAARS Total ADHD Symptoms T-score was 42.9 ± 2.5, and mean estimated IQ was 113.7 ± 9.6.
General Experimental Design
Participants completed fMRI scans on two separate days, with a mean of 7.0 ± 2.5 days between scans. Blood pressure was measured and 1 mg oral guanfacine or placebo was administered 90 minutes prior to the scans in a counterbalanced, double-blind design. The 1 mg dose of guanfacine was chosen to minimize sedation in the scanner and for its primarily postsynaptic binding profile (Arnsten et al. 1988; Engberg and Eriksson 1991). Participants completed a training session that tested simple face perception and presented the face emotion go/no-go task. Blood pressure was measured at the end of each scan session.
Face emotion go/no-go task
The face emotion go/no-go task has previously been described (Schulz et al. 2009). The task consisted of six 252-s blocks that each began and ended with a 30-s central fixation-cross. Each block contained 72 (75%) go cues and 24 (25%) no-go cues, yielding a total of 432 go cues and 144 no-go cues across the task. Participants had to respond rapidly with the right index finger to “go” cues and withhold responses to “no-go” cues. Stimuli were presented in the center of the screen for 500 ms with an interstimulus interval that was pseudorandomized from 1250 to 1750 ms (mean per block = 1500 ms). Face stimuli consisted of gray-scaled happy, sad, and neutral facial expressions from 18 individuals (9 female, 9 male) from the MacBrain Face Stimulus Set (Tottenham et al. 2009; available at www.macbrain.org). The images were normalized for size and luminance, morphed to exclude hair, and cropped into a black square, which was presented against a black background. Alternating the valence of the face stimuli used as trial cues resulted in six blocks with the following trials: (1) happy go / sad no-go; (2) sad go / neutral no-go; (3) neutral go / happy no-go; (4) happy go / neutral no-go; (5) sad go / happy no-go; and (6) neutral go / sad no-go. Trial order was determined by counterbalancing across all conditions in the task (e.g., trial type, facial expression, face ethnicity, face gender, face) to ensure that each trial type followed every other trial type equally often.
fMRI image acquisition
All participants were scanned on the same 3.0 Tesla Siemens Allegra (Siemens, Erlangen, Germany) head-dedicated MRI scanner. Functional T2*-weighted images depicting the blood oxygenation level-dependent (BOLD) were obtained every 3 s (TR = 3) using gradient-echo echo-planar imaging. Each functional image comprised a brain volume of 42 axial slices with an in-plane resolution of 3.75 × 3.75 mm and a thickness of 2.5 mm with a gap of 0.825 mm. The matrix size was 64 × 64 and the field of view was 210 mm. The TR was a trade-off for whole-brain coverage with thinner slices that minimized distortions and increased sensitivity in regions of interest (e.g., inferior frontal gyrus pars orbitalis). The participants each completed 6 runs of 252 seconds on two separate days. A high-resolution T2-weighted anatomical image was also acquired at the same 42 slice locations with a turbo spin-echo pulse sequence. All images were acquired with slices positioned parallel to the intercommisural line.
Behavioral analysis
Percent correct responses on go trials served as the measure of response execution, while percent correct inhibitions on no-go trials was the primary measure of response inhibition. Reaction time (RT) was also calculated for go trials. The signal detection variables d-prime (d′) and criterion (c) were computed to measure discriminability and response bias, respectively (Macmillan and Creelman 2005). The variables d′ and c were calculated from the hit and false alarm rates and thus provided pooled measures of performance on both go and no-go trials. Higher d′ values indicate greater discriminability, while negative c values indicate a bias to respond (as opposed to a bias to inhibit), with larger negative values indicating greater bias (Stanislaw and Todorov 1999).
The effects of guanfacine and emotion on performance were tested with repeated measures analyses of variance (ANOVA) with face emotion (happy vs. sad vs. neutral) and drug (guanfacine vs. placebo) as within-subject factors. Separate ANOVA with drug (guanfacine vs. placebo) and time (pre-scan vs. post-scan) as within-subject factors were used to test the effect of guanfacine on blood pressure. Statistical significance was set at the 0.05 level for these analyses. All probabilities were based on two-tailed tests. Partial eta squared (ηp2) values were calculated to estimate the size of the guanfacine and emotion effects on behavioral performance.
fMRI analysis
Pre-processing and analyses of the fMRI data were conducted using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). Each participant’s placebo and guanfacine time series were separately corrected for the staggered acquisition of slices during echo-planar imaging and realigned to the first volume in each time series to correct for inter-scan motion. Next, the placebo and guanfacine time series were co-registered to their respective T2-weighted anatomical images and then to each other. The time series were subsequently spatially normalized to the Montreal Neurological Institute template using normalization parameters estimated from the first T2-weighted image and were then resampled with a 2 × 2 × 2 mm voxel size. Finally, the time series were spatially smoothed with an 8-mm full width at half-maximum isotropic Gaussian kernel.
Event-related analyses were conducted individually for each participant using a general linear model (GLM) to determine the relationship between the observed event-related BOLD signals and regressors that represented expected neural responses to trial events. Regressors were created by convolving a train of delta functions that represented the individual trial events with the default SPM basis function, which consisted of a synthetic hemodynamic response function (HRF) (Friston et al. 1998). Twelve regressors were entered into the GLM, representing the 2 trial types of interest (correct no-go event vs. correct go event) × 3 face emotions (happy vs. sad vs. neutral) × 2 drugs (guanfacine vs. placebo). Go and no-go errors and six motion parameters were entered as covariates of no interest in the GLM (Johnstone et al. 2006). Contrasting the parameter estimates for each regressor versus baseline resulted in 12 contrast maps that each represented the specific BOLD response to a single interaction effect (e.g., happy no-go trials in the guanfacine condition).
The 12 contrast maps for each participant were entered into a second-level group analysis that used a factorial ANOVA model with trial type, face emotion, and drug as within-subjects factors. This statistical model enabled us to test all possible two-way and three-way interactions. The resultant voxel-wise statistical maps were thresholded for significance using a cluster-size algorithm that protects against false-positive results (Hayasaka et al. 2004). The height (intensity) threshold of each activated voxel was set at a p value of 0.005 and the extent (cluster) threshold was fixed at κ > 100 contiguous voxels. A Monte Carlo simulation that accounted for image resolution and smoothing parameters established that a cluster extent of 100 contiguous resampled voxels (2 mm3) corrected for multiple voxel comparisons at p < 0.01. The simulation is described in Slotnick and Schacter (2004).
Results
Blood pressure
Guanfacine reduced blood pressure during the scan compared to placebo (see Supplementary Table 1). Repeated measures ANOVA revealed a significant main effect of time [F (1,15) = 8.43, p = 0.012] and drug × time interaction [F (1,15) = 5.67, p = 0.032] on pulse rate, and a significant drug × time interaction on systolic blood pressure [F (1,15) = 7.17, p = 0.018], but not diastolic blood pressure (p = 0.88). Reductions in systolic blood pressure over the scan session were only seen for guanfacine, while decreases in pulse rate were seen for both conditions, but were greater for guanfacine than placebo. There were no significant main effects of drug (all p > 0.10).
Behavioral performance
Guanfacine moderated the effect of face emotion on the accuracy of response execution on the go/no-go task, but had no effect on response inhibition (Table 1). Repeated measures ANOVAs revealed a significant main effect of face emotion [F (2, 28) = 9.95, p = 0.001, ηp2 = 0.42] and face emotion × drug interaction [F (2, 28) = 14.53, p < 0.001, ηp2 = 0.51] on the percentage of correct responses on go trials, but not on the percentage of correct inhibitions on no-go trials (face emotion, p = 0.79, ηp2 < 0.001; face emotion × drug, p = 0.83, ηp2 = 0.16). Similar effects were found for the signal detection measures d’ [face emotion, F (2, 28) = 6.24, p = 0.006, ηp2 = 0.32; face emotion × drug, F (2, 28) = 10.99, p < 0.001, ηp2 = 0.46] and c [face emotion, F (2, 28) = 6.31, p = 0.006, ηp2 = 0.33; face emotion × drug, F (2, 28) = 5.64, p = 0.009, ηp2 = 0.30]. Post-hoc Bonferroni tests revealed that the accuracy of responses was lower, and consequently, perceptual sensitivity and response bias were reduced for sad faces only in the placebo condition (Figure 1). There was also a significant effect of face emotion on RT on go trials [Table 1; F (2, 28) = 9.02, p = 0.001, ηp2 = 0.39]. There were no significant main effects of drug (all p > 0.10).
Table 1.
Dependent measures of emotional go/no-go task performance
| Variable | Placebo | Guanfacine | ||||
|---|---|---|---|---|---|---|
| Happy | Sad | Neutral | Happy | Sad | Neutral | |
| No-go trials | ||||||
| Correct inhibitions (%) | 90.9 (2.4) | 90.6 (1.5) | 90.2 (1.8) | 89.9 (1.1) | 89.9 (1.4) | 90.5 (2.2) |
| Go trials | ||||||
| Correct responses (%)a | 96.7 (1.1) | 88.9 (2.6) | 94.9 (1.6) | 95.7 (1.9) | 96.0 (1.5) | 95.0 (1.86) |
| RT (ms)b | 485 (27) | 501 (26) | 512 (39) | 478 (20) | 499 (22) | 521 (23) |
| Signal detection | ||||||
| D-prime (d′)a | 3.7 (0.3) | 2.8 (0.1) | 3.5 (0.2) | 3.6 (0.2) | 3.6 (0.2) | 3.5 (0.2) |
| Criterion (c)a | −0.3 (0.1) | −0.1 (0.1) | −0.3 (0.1) | −0.4 (0.1) | −0.3 (0.1) | −0.3 (0.1) |
Values are presented as mean (standard error of the mean). RT reaction time
sad < happy = neutral for placebo but happy = sad = neutral for guanfacine, p < 0.05
happy < sad < neutral, p < 0.05
Fig. 1.
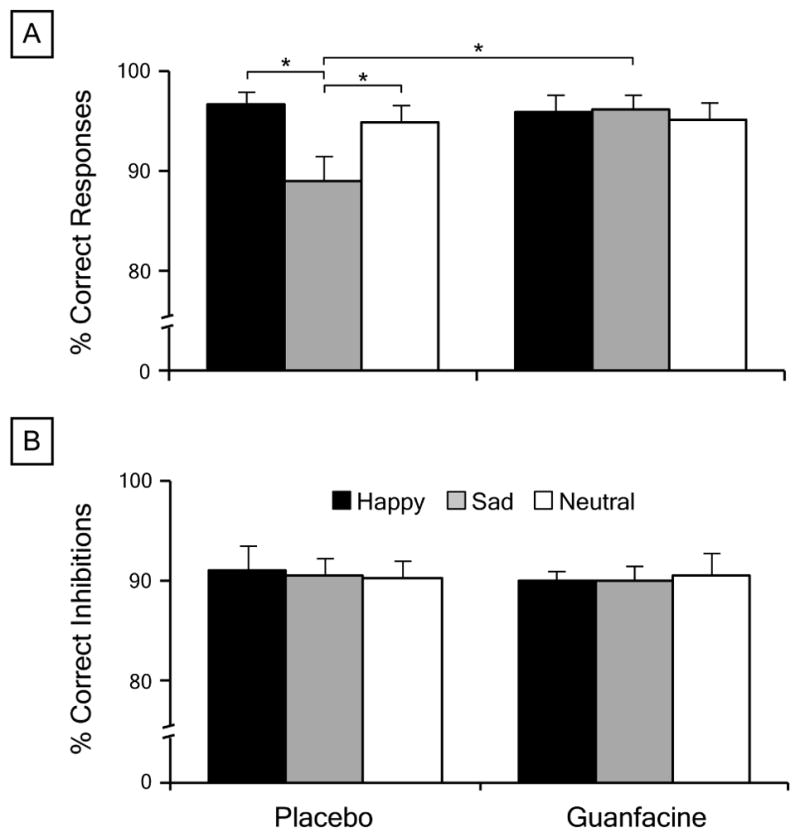
Effects of face emotion and drug on go/no-go task performance. a Mean percentage of correct responses on go trials cued by happy, sad, and neutral faces following 1 mg guanfacine and placebo. b Mean percentage of correct inhibitions on no-go trials cued by happy, sad, and neutral faces following guanfacine and placebo. Error bars indicate standard error of the mean. * p < 0.01
fMRI responses
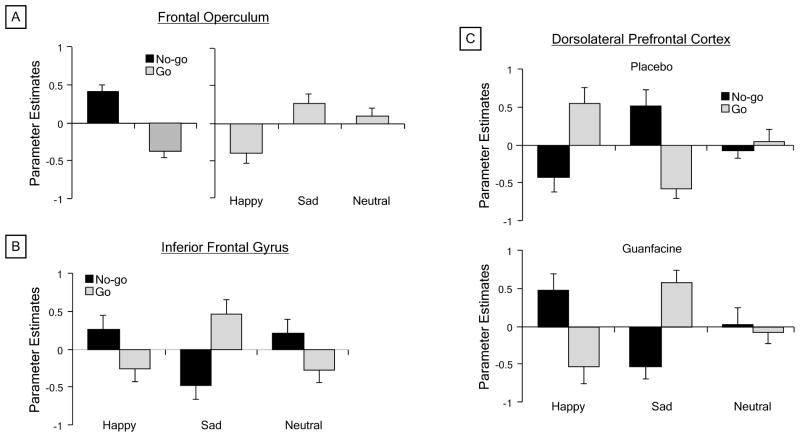
Face emotion influenced neural activation for response execution and inhibition in prefrontal cortex and other regions. The factorial ANOVA identified significant main effects of trial type in two independent brain networks that have been linked to response execution and inhibition (Table 2). Primary motor, striatal, and cerebellar regions showed greater activation for correct go trials than correct no-go trials, while greater activation for no-go than go trials was seen in frontal, parietal, and temporal regions, including left DLPFC and right frontal operculum extending superiorly to DLPFC. The latter region completely overlapped a separate cluster of activation in right frontal operculum that demonstrated a significant main effect of face emotion (Fig. 2). Post-hoc comparisons revealed that activation in this frontal opercular cluster was greater for sad faces than neutral faces, which in turn was greater than the deactivation for happy faces (Fig. 3a). Further, significant trial type × face emotion interactions were seen in right inferior frontal gyrus pars orbitalis, left primary motor cortex, and several parietal regions (Table 2). Plotting the inferior frontal activation revealed that sad faces evoked activation for go trials versus deactivation for no-go trials, but happy and neutral faces evoked activation for no-go trials versus deactivation for go trials (Fig. 3b). This pattern of activation was conserved across the motor and parietal clusters.
Table 2.
Regional activation during the emotional go/no-go task that showed significant main effects for Trial (go vs. no-go), Face Emotion (happy vs. sad vs. neutral), and Drug (guanfacine vs. placebo)
| Brain region | BA | MNI coordinates
|
No. of voxels | F value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Trial: go > no-go | ||||||
| Left primary motor cortex | 4 | −32 | −26 | 60 | 2,016 | 55.16 |
| Right cerebellum | --- | 20 | −50 | −22 | 1,358 | 50.51 |
| Right cerebellum | --- | 14 | −58 | −50 | 229 | 22.13 |
| Left pulvinar | --- | −18 | −22 | 12 | 2,895 | 33.91 |
| Right caudate nucleus | --- | 18 | 26 | 2 | 103 | 15.34 |
| Left caudate nucleus | --- | −10 | 22 | 2 | 242 | 13.72 |
| Trial: no-go > go | ||||||
| Right frontal operculum / DLPFC | 44/46 | 44 | 8 | 32 | 2,489 | 48.87 |
| Left dorsolateral prefrontal cortex | 46 | −50 | 26 | 28 | 643 | 28.49 |
| Right dorsal anterior cingulate cortex | 32 | 8 | 8 | 48 | 374 | 17.31 |
| Right superior parietal lobule | 7 | 20 | −72 | 38 | 475 | 16.59 |
| Right fusiform gyrus | 37 | 40 | −44 | −18 | 6,669 | 45.86 |
| Left fusiform gyrus | 37 | −40 | −52 | −12 | 2,689 | 45.51 |
| Face Emotion: sad > neutral > happy | ||||||
| Right frontal operculum | 44 | 46 | 12 | 28 | 405 | 9.07 |
| Drug: guan > placebo | ||||||
| Bilateral perigenual cingulate cortex | 32 | 2 | 50 | 4 | 1,133 | 23.92 |
| Right temporoparietal cortical junction | 40 | 44 | −26 | 24 | 397 | 20.39 |
| Right cuneus | 18 | 4 | −86 | 24 | 397 | 14.47 |
| Left inferior occipital gyrus | 18 | 14 | −96 | −14 | 150 | 12.35 |
| Right posterior insula cortex | --- | 42 | −12 | 14 | 602 | 15.97 |
| Left posterior insula cortex | --- | −38 | −20 | 14 | 187 | 10.90 |
| Trial × Face Emotiona | ||||||
| Right inferior frontal gyrus | 47 | 48 | 42 | −8 | 130 | 10.65 |
| Left primary motor cortex | 4 | −48 | −8 | 28 | 158 | 9.21 |
| Right posterior cingulate cortex | 31 | 6 | −36 | 44 | 519 | 11.98 |
| Left inferior parietal lobule | 40 | −42 | −42 | 44 | 524 | 9.32 |
| Right superior parietal lobule | 7 | 40 | −52 | 48 | 1,792 | 14.81 |
| Trial × Drugb | ||||||
| Right posterior cingulate cortex | 31 | 8 | −60 | 8 | 415 | 17.42 |
| Left thalamus | --- | −8 | −14 | 0 | 221 | 14.41 |
| Trial × Face Emotion × Drugc | ||||||
| Left dorsolateral prefrontal cortex | 9 | −50 | 22 | 34 | 216 | 8.96 |
F values and x, y, and z coordinates refer to the peak voxel of activation within each cluster. All regions were significant at p < 0.005, extent threshold corrected for multiple voxel comparisons at p < 0.01. There were no significant Emotion × Drug interaction effects.
BA Brodmann area, DLPFC dorsolateral prefrontal cortex, MNI Montreal Neurological Institute
nogo > go for sad but go > no-go for happy and neutral
nogo > go for placebo but go > no-go for guanfacine
(nogo > go for sad but go > no-go for happy and neutral) for placebo but (go > no-go for sad but no-go > go for happy and neutral) for guanfacine
Fig. 2.
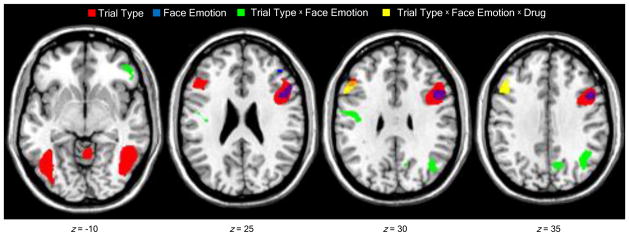
Brain regions that showed effects of trial type (go vs. no-go), face emotion (happy vs. sad vs. neutral), and drug (guanfacine vs. placebo) on task-related activation. Axial images depict significant main effects of trial type and face emotion in right frontal operculum, trial type × face emotion interaction in right inferior frontal gyrus pars orbitalis, and trial type × face emotion × drug interaction in left dorsolateral prefrontal cortex. Figures thresholded at p < 0.005 (cluster corrected for multiple voxel comparisons > 100 contiguous voxels). Montreal Neurological Institute z-coordinates indicate the distance (in mm) from the intercommissural line. Right side of image corresponds to the right side of the brain.
Fig. 3.
Effects of trial type (go vs. no-go), face emotion (happy vs. sad vs. neutral), and drug (guanfacine vs. placebo) on task-related activation illustrated for regions of interest in prefrontal cortex. Mean parameter estimates of task-related activation were plotted for the significant: a main effects of Trial Type and Face Emotion in right frontal operculum; b Trial type × face emotion interaction in right inferior frontal gyrus pars orbitalis; and c Trial type × face emotion × drug interaction in left dorsolateral prefrontal cortex. Error bars indicate standard error of the mean
Guanfacine moderated the effect of face emotion on neural activation for response execution and inhibition selectively in left DLPFC (Table 2). The ANOVA identified a trial type × face emotion × drug interaction in a cluster that partially overlapped with the DLPFC region that showed an effect of trial type (Fig. 2). Guanfacine reversed the valence-dependent pattern of activation for go and no-go trials seen in this cluster following placebo. Thus, sad faces evoked activation for no-go trials in the placebo condition and for go trials in the guanfacine condition, whereas happy faces, and to a lesser extent neutral faces, elicited activation for go trials following placebo and for no-go trials following guanfacine (Fig. 3c). In addition, significant main effects of drug and trial type × drug interactions were found in disparate regions that are not typically engaged for go/no-go tasks (Table 2).
Discussion
The current results provide evidence that the α2 adrenoceptor agonist guanfacine moderates left DLPFC activation associated with the emotional biasing of response execution in healthy adults. Left DLPFC is part of a wider frontoparietal network that is specialized to use emotional and contextual information to guide response execution and inhibition. Frontal opercular, inferior frontal, DLPFC, and parietal areas of this network all showed variations in activation as a function of face valence that were comparable to that seen in a previous study (Schulz et al. 2009). Guanfacine moderated the effect of face emotion on this activation selectively at the level of DLPFC, by reversing the valence-dependent pattern of task-related activation, which resulted in activation for response execution and deactivation for response inhibition cued by sad faces relative to happy and neutral faces. These guanfacine actions were associated with selective increases in the relatively low sensitivity and response bias for sad faces seen in the placebo condition, which principally reflected improvements in response execution. In contrast, neither face valence nor guanfacine had an effect on response inhibition. These results offer clues about the emotional biasing of motor functions and the manipulation of this bias by α2 adrenoceptor stimulation.
The present behavioral findings provide clear evidence that emotional cues bias response execution processes on the go/no-go task. The negative values for the signal detection measure c in the current study indicate a bias to respond rather than inhibit for all three face emotions, with less response bias for sad than happy and neutral faces (Stanislaw and Todorov 1999). The differences in response bias across the face emotions were mainly due to fewer correct responses to sad faces, which is partially consistent with our previous report of less accurate responses to both sad and happy faces (Schulz et al. 2009). Moreover, the enhanced response bias and perceptual sensitivity for sad faces following guanfacine reflected an increase in the percentage of correct responses to sad faces. However, unlike previous studies, our finding of faster responses to happy faces did not seem to reflect a broader emotional bias that interfered with the inhibition of responses to happy faces on no-go trials (i.e., fewer correct inhibitions) (Hare et al. 2005; Schulz et al. 2007). Rather, both findings may reflect the inclusion of neutral faces as trial cues. Neutral expressions tend to be mistakenly evaluated as sad faces (Lee et al. 2008; Russell and Fehr 1987), which may have been further compounded by the use of faces with closed mouths (Calvo and Nummenmaa 2008). These difficulties with face discrimination could account for both the poorer accuracy for sad faces and the slower responses to neutral and sad faces than happy faces in this study. The selective effect of guanfacine on the emotional biasing of accuracy for sad faces confirms that these effects were task-specific rather than a general consequence of the medication.
The selective impact of guanfacine on the valence- and task-dependent activation of left DLPFC is consistent with the model of this region as the apex of a frontoparietal network for the context-dependent control of goal-directed behavior (Fuster 2002; Miller and Cohen 2001). Left DLPFC receives limbic and inferotemporal input indirectly via inferior frontal gyrus pars orbitalis (Petrides and Pandya 2002) and represents this input as distinct patterns of neural activation (Fuster 2002; Miller and Cohen 2001). These context representations form the neural basis for DLPFC to exert top-down control over sensorimotor processors that directly support task performance (Sakagami and Pan 2007; Silton et al. 2010), by amplifying responses to task-relevant signals (Egner and Hirsch 2005), particularly in the presence of competing response options, such as for sad faces in the current study (Hester et al. 2004). The guanfacine reversal of the DLPFC function for response to sad faces, from deactivation to activation relative to responses cued by happy and neutral faces, may thus reflect increases in top-down control to overcome the difficulties with responding to sad faces.
The gain in DLPFC activation associated with improvement in response execution cued by sad faces is consistent with the well-described neural actions of guanfacine. Guanfacine stimulation of postsynaptic α2 adrenoceptors suppresses an inward cation current (Wang et al. 2007), which raises the excitability of pyramidal neurons (Carr et al. 2007) and strengthens local DLPFC recurrent networks that support top-down attention control (Wang et al. 2007). These α2 adrenoceptor actions have been shown to increase neuronal firing in DLPFC for the preferred direction in a working memory task in monkeys (Avery et al. 2000; Gamo et al. 2010). Similar mechanisms may have improved response execution for sad faces in the current study.
The valence- and task-dependent activation of the inferior frontal gyrus pars orbitalis seen in the current study provides support for the model of this region as a cortical entry point through which emotional cues influence response functions (Sakagami and Pan 2007). The pars orbitalis purportedly computes the behavioral significance of stimuli by integrating information about the target with affective input from amygdala (Petrides and Pandya 2002) and contextual input from inferotemporal cortex (Ungerleider et al. 1989), and is unique among prefrontal areas in that it contains separate populations of neurons that fire selectively for sensory cues that signal behavioral execution and inhibition (Sakagami et al. 2001). The interactive effect of face valence and trial type on pars orbitalis activation in the current study is consistent with the integration of emotional and contextual cues. The pars orbitalis may influence motor functions through projections to DLPFC (Miyachi et al. 2005) and dense connections with the frontal operculum (Petrides and Pandya 2002).
The frontal operculum on the non-dominant side has been implicated in complex sensory guided motor acts (Iacoboni and Wilson 2006). The current finding of greater frontal operculum activation for no-go relative to go trials is consistent with meta-analyses that have implicated the region as a neural effector for response inhibition (Garavan et al. 2006; Simmonds et al. 2008). The overlapping region of operculum that independently displayed greater activation for sad faces than neutral and happy faces may thus have reflected differences in the difficulty of inhibiting responses to sad faces (Schulz et al. 2009). The lack of an interaction between face valence and trial type points to an exclusive role for frontal operculum in response inhibition. The fact that guanfacine did not influence activation in frontal operculum may explain the lack of effect of the α2 adrenoceptor agonist on response inhibition in this study and others (e.g., Muller et al. 2005). Gains in frontal opercular activation have previously been associated with improvements in response inhibition in healthy adults (Chamberlain et al 2009).
Limitations
The attribution of the behavioral and neural changes in the current study to the biochemical effects of guanfacine is mitigated by the difficulties with measuring local drug actions in humans. Plasma measures of guanfacine and its metabolites that would have strengthened claims of causality were purposely not obtained to minimize participant risk and burden. However, the depressant effect that guanfacine had on blood pressure recordings in this study confirm that the medication, which was originally developed as an antihypertensive, had the desired biochemical effects, at least peripherally.
The absence of face valence effects in amygdala and other limbic regions in this study was unexpected given the previous reports of such activation using emotional go/no-go tasks (Schulz et al. 2009; Goldstein et al. 2007; Hare et al. 2005). This lack of amygdala activation may reflect the difficulty with successfully imaging this subcortical region (Merboldt et al. 2001) and/or the focus on prefrontal cortex in this study, and the use of an analytic approach that was optimized to detect effects in this large region of interest. The extent or cluster threshold (> 100 voxels) that was needed to correct for the multiple voxel comparisons may have been too large to detect effects in the relatively small amygdala. It must also be noted that the relatively small sample size in this study may have limited the statistical power to detect more subtle effects of guanfacine, especially on the behavioral measures of response execution and inhibition.
Clinical Implications
The current findings have potential implications for the α2 adrenoceptor agonist treatment of psychiatric disorders characterized by problems with impulsivity and emotion regulation. The specific effect of guanfacine on the response bias for sad faces suggests an application for α2 adrenoceptor agonists in the treatment of the mood-congruent biases that characterize major depression (Blaney 1986). However, α2 adrenoceptor agonists have no reported antidepressant properties, and successful antidepressant treatment does not seem to involve alterations of adrenoceptor function (Charney et al. 1984; Price et al. 1986). The lack of antidepressant effects may in part reflect the selective action of guanfacine on executive functions mediated by DLPFC (Jakala et al. 1999), rather than on the limbic affective mechanisms that have been implicated in both mood-congruent biases (Elliott et al. 2000, 2002) and the pathophysiology of major depression (Elliott et al. 2011).
The guanfacine modulation of DLPFC activation associated with emotional biasing of response execution may offer a possible mechanism to address the emotional reactivity and dysregulation that are common to ADHD (Musser et al. 2011; Walcott and Landau 2004). Clinical experience and the few available studies suggest that these emotion regulation problems are not necessarily well served by the psychostimulant medications used to treat ADHD (Manos et al. 2011; Pelham et al. 1991). The finding that guanfacine enhanced response-related DLPFC activation to increase the reduced bias and sensitivity for sad faces suggests that the medication may improve emotion regulation and reduce the extraneous influence of emotion on response functions in individuals with noradrenergic dysfunction. Suboptimal postsynaptic α2A adrenoceptor regulation of DLPFC function has been implicated in the pathophysiology of attention-deficit/hyperactivity disorder (ADHD)(Brennan and Arnsten 2008) and is a promising target for pharmacological treatments for the disorder (Arnsten et al. 2007). Our results support further investigation of the use of guanfacine to treat affect-related regulatory problems in individuals with ADHD.
Conclusions
The present results demonstrate that the α2 adrenoceptor agonist guanfacine moderates left DLPFC activation associated with the emotional biasing of response execution in healthy adults. Guanfacine inverted the trial- and valence-dependent pattern of DLPFC activation, and thereby increased left DLPFC activation for responses to sad faces relative to happy and neutral faces. These guanfacine actions were associated with improvements in the poor accuracy of responses to sad faces relative to happy and neutral faces in the placebo condition, but had no effect on behavioral measures of response inhibition. The selective action of guanfacine on control networks centered in DLPFC may offer a possible mechanism to address the emotional reactivity and regulation deficits commonly seen in patients with ADHD.
Supplementary Material
Acknowledgments
This research was supported by Grant No. MH070892 to KPS from the National Institute of Mental Health and by a pilot grant to KPS from the Mount Sinai School of Medicine General Clinical Research Center, which is funded by grant MO1RR00071 from the National Center for Research Resources, a component of the National Institutes of Health. Development of the MacBrain Face Stimulus Set (NimStim) was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at nimtottenham@ucla.edu for more information concerning the stimulus set. Thanks to our volunteers for participating in this study and to Hanna Oltarzewska and Frank Macaluso for their invaluable support in data acquisition.
This research was supported by National Institutes of Health (NIH) Grant K01MH070892 and Grant MO1RR00071 from the National Center for Research Resources, a component of the NIH. Dr. Newcorn is a recipient of research grants from Eli Lilly, Ortho-McNeil-Janssen and Shire; and is or has been an advisor/consultant for Alcobra, Biobehavioral Diagnostics, Eli Lilly, Ortho-McNeil-Janssen, and Shire.
Footnotes
Financial disclosures Dr. Newcorn is a recipient of grants for research support from Eli Lilly & Co., Ortho-McNeil-Janssen, and Shire and is or has been an advisor/consultant for Alcobra, Eli Lilly & Co., NEOS, Ortho-McNeil-Janssen, Shire, and BioBehavioral Diagnostics Company. No other authors have financial interests or potential conflicts of interest to declare.
Contributor Information
Kurt P. Schulz, Email: kurt.schulz@mssm.edu, Department of Psychiatry, The Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1230, New York, NY 10029, USA, Tel: +1 212 241 6623, Fax: +1 212 659 8986
Suzanne M. Clerkin, Department of Psychiatry, The Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1230, New York, NY 10029, USA
Jin Fan, Department of Psychiatry, The Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1230, New York, NY 10029, USA. Department of Psychology, Queens College of the City University of New York, Flushing, NY 11367, USA.
Jeffrey M. Halperin, Department of Psychiatry, The Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1230, New York, NY 10029, USA. Department of Psychology, Queens College of the City University of New York, Flushing, NY 11367, USA
Jeffrey H. Newcorn, Department of Psychiatry, The Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1230, New York, NY 10029, USA
References
- Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Scahill L, Findling RL. alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. Journal of child and adolescent psychopharmacology. 2007;17:393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Blaney PH. Affect and memory: a review. Psychological bulletin. 1986;99:229–246. [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Annals of the New York Academy of Sciences. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Nummenmaa L. Detection of emotional faces: salient physical features guide effective visual search. J Exp Psychol Gen. 2008;137:471–494. doi: 10.1037/a0012771. [DOI] [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, Lavin A. alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol. 2007;584:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Sternberg DE. The effect of mianserin on alpha-2 adrenergic receptor function in depressed patients. The British journal of psychiatry : the journal of mental science. 1984;144:407–416. doi: 10.1192/bjp.144.4.407. [DOI] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Halperin JM, Newcorn JH, Ivanov I, Tang CY, Fan J. Guanfacine potentiates the activation of prefrontal cortex evoked by warning signals. Biol Psychiatry. 2009;66:307–312. doi: 10.1016/j.biopsych.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow MA. Conners’ Adult ADHD Rating Scales (CAARS) Multi-Health Systems, Inc; New York: 1999. [Google Scholar]
- Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of general psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Elliott R, Zahn R, Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg G, Eriksson E. Effects of alpha 2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ)-treated rats. Naunyn-Schmiedeberg’s archives of pharmacology. 1991;343:472–477. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. Journal of neurocytology. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, Yang Y, Thomas K, Levy K, Silverman M, Clarkin J, Posner M, Kernberg O, Stern E, Silbersweig D. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36:1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Haberman J, Whitney D. Rapid extraction of mean emotion and gender from sets of faces. Curr Biol. 2007;17:R751–753. doi: 10.1016/j.cub.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hester R, Murphy K, Garavan H. Beyond common resources: the cortical basis for resolving task interference. Neuroimage. 2004;23:202–212. doi: 10.1016/j.neuroimage.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Wilson SM. Beyond a single area: motor control and language within a neural architecture encompassing Broca’s area. Cortex. 2006;42:503–506. doi: 10.1016/s0010-9452(08)70387-3. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Johansson K, Ronnberg J. Speech gestures and facial expression in speechreading. Scand J Psychol. 1996;37:132–139. doi: 10.1111/j.1467-9450.1996.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum Brain Mapp. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain: the mysterious underpinnings of emotional life. Touchstone; New York: 1998. [Google Scholar]
- Lee E, Kang JI, Park IH, Kim JJ, An SK. Is a neutral face really evaluated as being emotionally neutral? Psychiatry Res. 2008;157:77–85. doi: 10.1016/j.psychres.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Granic I, Lamm C, Zelazo PD, Stieben J, Todd RM, Moadab I, Pepler D. Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Dev Psychopathol. 2008;20:913–939. doi: 10.1017/S0954579408000448. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. 2. Lawrence Erlbaum Associates, Inc; Mahwah, New Jersey: 2005. [Google Scholar]
- Manos MJ, Brams M, Childress AC, Findling RL, Lopez FA, Jensen PS. Changes in emotions related to medication used to treat ADHD. Part I: literature review. J Atten Disord. 2011;15:101–112. doi: 10.1177/1087054710381230. [DOI] [PubMed] [Google Scholar]
- Maxwell JS, Shackman AJ, Davidson RJ. Unattended facial expressions asymmetrically bias the concurrent processing of nonemotional information. J Cogn Neurosci. 2005;17:1386–1395. doi: 10.1162/0898929054985437. [DOI] [PubMed] [Google Scholar]
- Merboldt KD, Fransson P, Bruhn H, Frahm J. Functional MRI of the human amygdala? Neuroimage. 2001;14:253–257. doi: 10.1006/nimg.2001.0802. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Lu X, Inoue S, Iwasaki T, Koike S, Nambu A, Takada M. Organization of multisynaptic inputs from prefrontal cortex to primary motor cortex as revealed by retrograde transneuronal transport of rabies virus. J Neurosci. 2005;25:2547–2556. doi: 10.1523/JNEUROSCI.4186-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Clark L, Lam ML, Moore RM, Murphy CL, Richmond NK, Sandhu RS, Wilkins IA, Menon DK, Sahakian BJ, Robbins TW. Lack of effects of guanfacine on executive and memory functions in healthy male volunteers. Psychopharmacology (Berl) 2005;182:205–213. doi: 10.1007/s00213-005-0078-4. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, Nigg JT. Emotion Regulation via the Autonomic Nervous System in Children with Attention-Deficit/Hyperactivity Disorder (ADHD) J Abnorm Child Psychol. 2011;39:841–852. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otta E, Lira BB, Delevati NM, Cesar OP, Pires CS. The effect of smiling and of head tilting on person perception. J Psychol. 1994;128:323–331. doi: 10.1080/00223980.1994.9712736. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Milich R, Cummings EM, Murphy DA, Schaughency EA, Greiner AR. Effects of background anger, provocation, and methylphenidate on emotional arousal and aggressive responding in attention-deficit hyperactivity disordered boys with and without concurrent aggressiveness. Journal of abnormal child psychology. 1991;19:407–426. doi: 10.1007/BF00919086. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Price LH, Charney DS, Heninger GR. Effects of trazodone treatment on alpha-2 adrenoceptor function in depressed patients. Psychopharmacology. 1986;89:38–44. doi: 10.1007/BF00175186. [DOI] [PubMed] [Google Scholar]
- Russell JA, Fehr B. Relativity in the perception of emotion in facial expressions. J Exp Psychol Gen. 1987;116:223–237. [Google Scholar]
- Sakagami M, Pan X. Functional role of the ventrolateral prefrontal cortex in decision making. Curr Opin Neurobiol. 2007;17:228–233. doi: 10.1016/j.conb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O. A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. J Neurosci. 2001;21:4801–4808. doi: 10.1523/JNEUROSCI.21-13-04801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Eaton K. Guanfacine extended-release for attention-deficit/hyperactivity disorder (ADHD) Expert Opin Pharmacother. 2010;11:2549–2556. doi: 10.1517/14656566.2010.517523. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Hum Brain Mapp. 2009;30:2821–2833. doi: 10.1002/hbm.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM. Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Arch Clin Neuropsychol. 2007;22:151–160. doi: 10.1016/j.acn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Brendel G, Pan H, Beutel M, Pavony MT, Epstein J, Lenzenweger MF, Thomas KM, Posner MI, Stern E. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Spielberg JM, Edgar JC, Sass SM, Stewart JL, Sutton BP, Banich MT, Miller GA. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior research methods, instruments, & computers : a journal of the Psychonomic Society, Inc. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, Beck AT. Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J Clin Psychol. 1999;55:117–128. doi: 10.1002/(sici)1097-4679(199901)55:1<117::aid-jclp12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during error commission. Hum Brain Mapp. 2009;30:24–37. doi: 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76:473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Walcott CM, Landau S. The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol. 2004;33:772–782. doi: 10.1207/s15374424jccp3304_12. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu J, Gui ZH, Ali U, Fan LL, Hou C, Wang T, Chen L, Li Q. alpha(2)-Adrenoceptor regulates the spontaneous and the GABA/glutamate modulated firing activity of the rat medial prefrontal cortex pyramidal neurons. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.