Abstract
Puberty is a period characterized by brain reorganization that contributes to the development of neural and behavioral responses to gonadal steroids. A single injection of the bacterial endotoxin, lipopolysaccharide (LPS), during the pubertal period decreases sexual receptivity in response to ovarian hormones in adulthood. Because chronic estradiol treatment alleviates depression-like symptoms in ovariectomized adult mice, we investigated the effect of pubertal LPS treatment on estradiol’s antidepressant effects. We hypothesized that pubertal LPS treatment would decrease the antidepressant-like effect of estradiol in adult ovariectomized female mice, as it decreases other behavioral responses to ovarian hormones. As expected, chronic estradiol treatment decreased depression-like behavior, as measured by the duration of immobility, in saline-treated mice from two different strains, as well as in mice treated with LPS in adulthood. In contrast, in mice treated pubertally with LPS, estradiol strikingly increased the duration of immobility. No difference in body weight and in locomotion was found among the groups, suggesting that the differences in depression-like behavior were not due to differences in body weight or locomotor activity between LPS-treated and control mice. These results suggest that exposure to an immune challenge during the pubertal period alters the responsiveness of depression-like behavior to estradiol.
Keywords: Lipopolysaccharide, Depression, Estradiol, Puberty, Females, Forced Swim test, Tail Suspension test, Stress, Immune Challenge
1. Introduction
Puberty is a period of brain remodeling by sex steroid hormones, as animals develop reproductive maturity (Levitt, 2003;Schulz et al., 2009;Sisk and Foster, 2004). In mice and humans, long-term exposure to stressors during the pubertal period is associated with a variety of physiological changes (Laviola et al., 2002;Young and Altemus, 2004;Goel and Bale, 2007). Exposure to stressors during the pubertal period can have long-term negative effects on the reproductive capacity of female mice (Paris et al., 1973). Similarly, inbred C57BL/6 female mice exposed to shipping stress or to an immune challenge (lipopolysaccharide; LPS), but not other stressors, during the pubertal period display reduced hormone-induced sexual receptivity in adulthood contrasted with mice stressed earlier or later in development (Laroche et al., 2009b;Laroche et al., 2009a). These effects are seen in the outbred CD1 mouse strain as well (Ismail et al., 2011), albeit at a slightly later age.
Although not an indicator of full reproductive maturity, vaginal opening of CD1 mice in our laboratory occurs at approximately 30 days old, contrasted with approximately 25 days old in C57BL/6 mice (Ismail and Blaustein, unpublished observation). Laroche and colleagues (Laroche et al., 2009b;Laroche et al., 2009a) reported that in C57BL/6 mice, the effects of LPS and shipping on adult behavioral response to estradiol and progesterone started around 4 weeks old and were most robust at five to six weeks old, and mice shipped or injected with LPS later than six weeks old were insensitive to the long-term effects of the stressors on response to ovarian hormones. In contrast, in CD1 mice, the effects of shipping and an immune challenge at puberty resulted in decreased responsiveness to estradiol and progesterone in adulthood in females shipped at six weeks old, but not in those shipped younger, and also in mice shipped at eight weeks old, but not in those shipped at ten weeks old (Ismail et al., 2011). Together, these findings suggest that exposure to particular stressors during puberty reduces behavioral responsiveness to estradiol and progesterone in adulthood and that the vulnerable pubertal period extends until at least eight weeks old in the CD1 strain.
Estradiol also plays a prominent role in behaviors related to mental health. For example, female rats (Diaz-Veliz et al., 1997) and mice (Galeeva and Tuohimaa, 2001) display reduced anxiety-like behavior during proestrus and estrus, but increased levels during diestrus. Moreover, ovariectomy increases, and hormone replacement decreases, these behaviors (Diaz-Veliz et al., 1989). Interestingly, mice treated with LPS during puberty do not respond to hormonal treatment with the expected decrease in anxiety-like behavior (Olesen et al., 2011). Likewise, estradiol modulates the expression of depression-like behaviors (Okada et al., 1997;Rachman et al., 1998;Dalla et al., 2004). Tests that are currently used to examine depression-like symptoms in mouse models are the tail suspension (Trullas, Jackson & Skolnick, 1989) and forced swim tests (Porsolt, Le Pichon & Jalfre, 1977; Porsolt, Anton, Blavet & Jalfre, 1978). In both of these tests, the duration of immobile behavior is thought to represent a state of behavioral despair. Ovariectomy increases and subchronic or chronic estradiol replacement decreases the duration of immobility in these tests (Okada et al., 1997;Bernardi et al., 1989). To our knowledge, the effects of ovarian hormones on another test for depression, the sucrose preference test for anhedonia, have not been systematically studied. A study on postpartum depression was unclear regarding the effects of gonadal hormones on this test (Green et al., 2009).
The objective of the first experiment was to determine whether an immune challenge during the pubertal period decreases behavioral responsiveness of depression–like behaviors to chronic estradiol treatment in inbred C57BL/6 (Experiment 1). In order to determine if this effect is generalizable to other strains of mouse, we also tested the effect of the immune challenge on behavioral responsiveness to chronic estradiol treatment in the outbred CD1 strain of mice (Experiment 2). We hypothesized that, as shown previously, chronic estradiol treatment in adulthood would have an antidepressant-like effect, and that pubertal LPS treatment would reduce the effects of chronic estradiol treatment in both strains of mice.
2. Experimental Procedures
2.1 Animals
Sixty-four C57BL/6 (n of 8 × 2 ages (pubertal and adult) × 2 treatments (saline and LPS) × 2 hormone capsules (oil and estradiol)) and 64 CD1 (n of 8 × 2 ages (pubertal and adult) × 2 treatments (saline and LPS) × 2 hormone capsules (oil and estradiol)) female mice were purchased from Charles River Laboratories (Kingston, NY) and housed in an all-female colony room under controlled temperature (24 ± 2°C) and reversed 14-h light: 10-h dark cycle (lights off at 10:00am). Mice were housed in groups of four in polycarbonate cages with ad libitum access to food (Teklad 2014), Harlan Laboratories, Madison, WI, phytoestrogen-reduced diet) and water supplied in glass water bottles. Cages were lined with a combination of wood shavings and CareFRESH (International Absorbents, Inc., Ferndale, WA) bedding, and a fresh Nestlet (Ancare Corp., Baltimore, NY) was placed in each cage when they were changed. Testing and handling occurred during the dark phase of the illumination cycle under dim red light. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst.
2.2 Bacterial endotoxin, lipopolysaccharide (LPS)
Thirty-two 6-week or 8-week old C57Bl/6 mice and 32 6-week or 10-week old CD1 mice were injected at the end of the light cycle with either sterile saline or 1.5mg LPS/kg body weight (obtained from Escherichia coli serotype O26:B6; no. L3755; Sigma Chemical Co., St. Louis, MO) dissolved at a concentration of 0.1mg/ml in sterile saline. Mice were returned to their home cage immediately after injection. Sickness behavior was scored as described below, and animals were weighed 24 and 48 hours following treatment to assess the effect of LPS treatment on body weight loss. The dose of LPS was chosen based on previous work (Laroche et al., 2009a) and on pilot work in which it caused only mild sickness lasting less than 48 hours in C57BL/6 mice under our housing conditions.
2.3 Sickness behavior
Sickness behavior was scored 30 min, 4, 24 and 48 hours following treatment by two observers, who were blind to the treatment conditions. Animals were evaluated with respect to the number of symptoms displayed (one symptom = score of 1; two symptoms = score of 2, three symptoms = score of 3) (Gibb et al., 2008). The symptoms examined were lethargy (diminished locomotion), huddling (curled body posture), and ptosis (drooping eyelids). This rating procedure correlates with other methods of sickness scoring, such as scoring the severity of each symptom independently on a four-point scale (Gandhi et al., 2007). Mice were also weighed 24 and 48 hours following treatment to examine changes in body weight.
2.4 Long-term changes in body weight
Long-term body weight changes were examined in a separate cohort of 48 C57Bl/6 mice that were part of a larger telemetry study examining changes in body temperature following exposure to pubertal immune challenge (data not shown here). A large number of mice were used for each treatment group (24 mice per group: 6 experimental and 18 cage mates) in the telemetry study, since body temperature measurement could only be recorded in one mouse per cage of four mice. Long-term changes in body weight following saline or LPS exposure was assessed in these mice by weighing them once a week for a total of fifteen weeks.
2.5 Ovariectomy and capsule implantation
Five weeks following saline or LPS treatment, 64 C57Bl/6 and 64 CD1 mice were ovariectomized and implanted with a capsule containing estradiol or oil vehicle. Mice were anesthetized with isoflurane (3% at one l/min, inhaled). Incisions were made to the ventral skin and the muscle layer to locate the uterine horns and ovaries. Once located, the uterine horns were tied with absorbable suture, and the ovaries were removed. The muscle layer was sutured, and the skin incision was closed with wound clips. Then, an incision was made to the dorsal skin layer of the neck, and the mice were implanted with either a 2.5cm long Silastic© implant (1.57mm I.D. × 3.18mm O.D) filled with 50 µg of 17β-estradiol dissolved in 25 µl of sesame oil or a control implant filled only with oil vehicle. This estradiol implant yields a high physiological concentration of estradiol in the plasma (85–120 pg/ml) (Kudwa et al., 2009). The incision was closed with wound clips. Mice were placed on a heating pad to recover until they were fully awake. They were then returned to their home cage with an additional water bottle containing a 3 % solution of Children’s Tylenol (160 mg of acetaminophen/ 5 ml dissolved in tap water) for 48 hours. Behavioral testing for depression-like symptoms began one week later.
2.6 Behavioral testing
2.6.1 Forced Swim Test
Sixty-four C57Bl/6 and 64 CD1 mice underwent the forced swim test. This 5-min test was conducted with experimenters that were blind to treatment groups. The duration of the test was chosen based on reports that mice quickly adopt an immobile posture (Porsolt et al., 1978;Dalla et al., 2004). Mice were placed in a four-liter glass cylinder containing three liters of water at 24 ± 2°C. The duration (in seconds) of struggling (attempts to climb the wall of the cylinder), swimming (active circular swimming) and floating (absence of movement or small movements of posterior paws that do not result in displacement) were recorded. At the end of the test, mice were removed from the cylinder and placed in a recovery cage on a heating pad until they were dry, and then they were returned to their home cages. To confirm the results, the test was repeated one week later.
2.6.2 Tail Suspension Test
One week following the last forced swim test, the sixty-four C57Bl/6 and 64 CD1 mice underwent the tail suspension test. This test also consists a five minute session. Mice were suspended 60 cm above the ground by adhesive tape placed within 1cm from the tip of the tail. The duration of immobility (absence of any body movement) was recorded. Mice were returned to their home cage upon termination of the testing. To confirm the results, the test was repeated one week later.
2.6.3 Sucrose Preference Test
One week following the last locomotion test, the same sixty-four C57Bl/6 and 64 CD1 mice underwent the forced swim test. Mice were given a free choice between two graduated bottles, one containing 0.8% sucrose solution and another with tap water over 48 hours. To prevent habituation to drinking side preference, the position of bottles was changed after 24 hours. Mice were not food or water deprived prior to testing. Liquid consumption was measured daily to calculate the preference ratio: (volume of sucrose consumed/total volume of liquid consumed) × 100.
Locomotor Activity
Photocell-beamed Chambers
Locomotor activity was measured in Plexiglas cages (28.5 × 28.5 × 20cm) containing two levels of photocell beams placed at 2 and 7 cm from the bottom of the cage (Med Associates, St-Albans, VT, USA) to record both horizontal (locomotion) and vertical (rearing) behaviors. The same 64 C57Bl/6 and 64 CD1 mice underwent testing for locomotor activity. Mice were brought into the test room at least two hours prior to testing to habituate to the new environment during the dark phase of their light: dark cycle. They were also placed in the chambers for a five minute habituation period. Distance (cm) and velocity (cm/sec) of locomotion were recorded during a 10-minute session, after which, mice were returned to their home cage.
Open-Field Arena
During the dark phase of their light cycle, locomotor activity was also evaluated by placing mice in the corner of an open-field arena (60 × 60 × 32cm) for five minutes. Using lines mapped out in the bottom of the open-field, the number of lines crossed was recorded. Before testing, mice were habituated to the arena for five minutes.
EXPERIMENT 1: The effect of pubertal LPS treatment on the antidepressant-like effects of estradiol in adult, female, inbred C57BL/6 mice
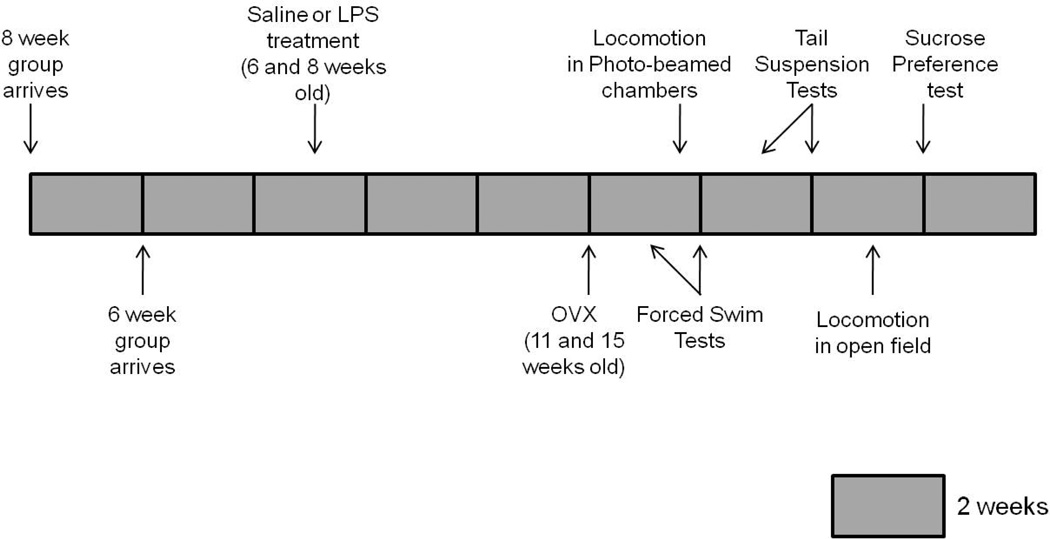
Thirty-two C57BL/6 female mice were shipped at three weeks old from Charles River Laboratories (negative control group). Two weeks later, another set of 32 C57BL/6 female mice was shipped at three weeks old from Charles River Laboratories (experimental group). Three weeks later, when mice in the experimental group were six weeks old, and mice in the negative control group were eight weeks old, all mice were injected either with LPS or saline. The group injected at eight weeks old is the negative control group because, in previous studies, immune challenge (Laroche et al., 2009a) or shipping (Laroche et al., 2009b) at that age did not result in a decrease in behavioral responsiveness to estradiol and progesterone in adulthood. Sixty-four C57Bl/6 mice were ovariectomized four weeks later and implanted subcutaneously with either an estradiol-filled or a vehicle oil-filled Silastic© capsule. Beginning one week later, mice were tested for depression-like behavior using forced swim, tail suspension and the sucrose preference tests. Locomotion activity was examined using photocell-beamed chambers and an open field arena. To investigate whether the differences in the duration of immobility were due to long-term changes in body weight, a separate cohort of 48 female mice, that were part of a large telemetry study, as mentioned above, were shipped at three weeks old from Charles River Laboratories. Three weeks later, mice were injected either with saline or with LPS. Mice were weighed once a week for 15 weeks. Figure 1 depicts the experimental timeline.
Figure 1.
Graphical schematic of the experimental design for Experiment 1.
EXPERIMENT 2: The effect of pubertal LPS treatment on the antidepressant-like effects of estradiol in adult, female outbred CD1 mice to examine if the results of Experiment 1 can be extended to another strain of mice
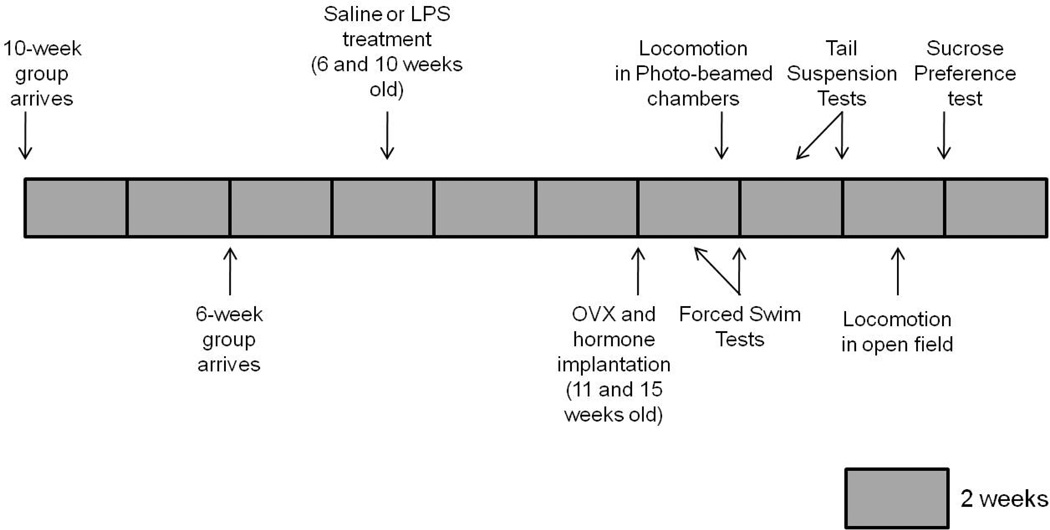
Thirty-two CD1 female mice were shipped at three weeks old from Charles River Laboratories (negative control group). Four weeks later, another set of 32 CD1 female mice was shipped at three weeks old from Charles River Laboratories (experimental group). Three weeks later, when mice in the experimental group were six weeks old, and mice in the negative control group were ten weeks old, all mice were injected either with saline or with LPS. As in Experiment 1, the latter group is the negative control group based on the findings that exposure to LPS at ten weeks old did not result in a decrease in behavioral responsiveness to estradiol and progesterone in adulthood (Ismail et al., 2011). Sixty-four CD1 mice were ovariectomized five weeks later and implanted subcutaneously with either an estradiol or oil-vehicle-filled Silastic© capsule. Following one week of recovery, testing for depression-like behavior began using forced swim, tail suspension and the sucrose preference tests. Locomotor activity was examined as in Experiment 1. Figure 2 depicts the experimental timeline.
Figure 2.
Graphical schematic of the experimental design for Experiment 2.
Statistical Analysis
Three-way repeated measures analyses of variance (ANOVAs) were used to examine sickness 0.5, 4, 24 and 48 hours following saline or LPS treatment and the percent change in body weight from baseline (on the day of the treatment). Three-way ANOVAs were used to examine the effect of age, hormone and treatment on the duration of immobility during the forced swim, tail suspension and to analyze the results from the locomotion tests, and post hoc tests (least significant difference, LSD) were used to assess pairwise contrasts when appropriate. To examine long-term changes in body weight due to pubertal LPS treatment, a repeated measures ANOVA was followed by appropriate post-hoc tests. All analyses were run in SPSS Inc. statistical package 11.5 (Chicago, Illinois). Outliers were eliminated using the Boxplot method (Reimann et al., 2005). The criterion for statistical reliability was set to p < 0.05.
Results
EXPERIMENT 1: The effect of pubertal LPS treatment on the antidepressant-like effects of estradiol in inbred C57BL/6 mice
Sickness behavior
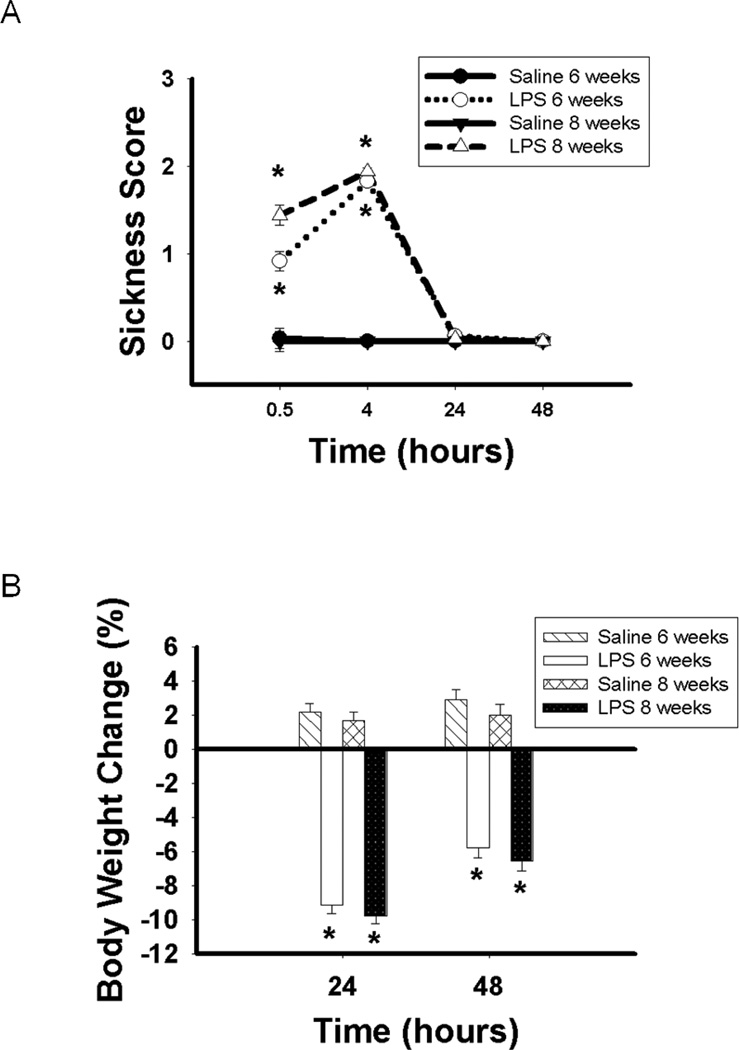
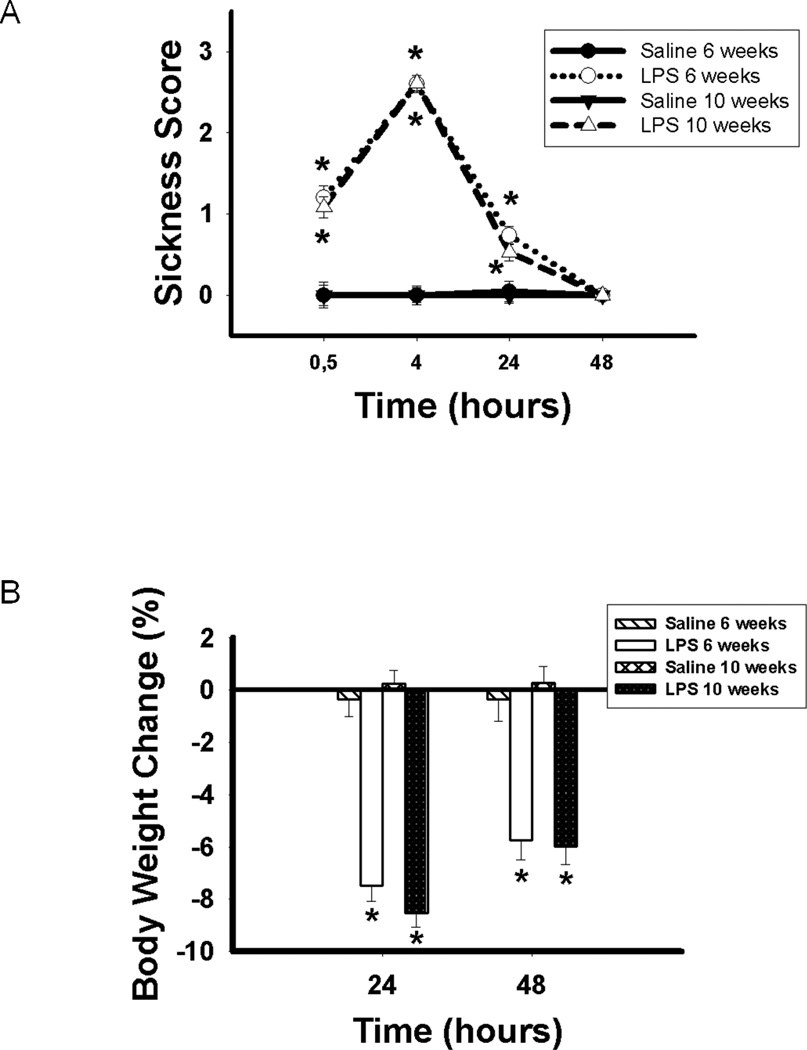
Statistical analyses revealed significant time×treatment (F(3, 180) = 217.37; p < 0.05), time × age (F(3, 180) = 3.74; p < 0.05), treatment × age (F(1, 60) = 5.75; p < 0.05) and time × treatment × age (F(3, 180) = 4.83; p < 0.05) interactions. Pairwise comparisons indicated that mice treated with LPS at six or eight weeks of age displayed significantly more sickness symptoms than mice treated with saline at 30 min (p < 0.001 and p < 0.001, respectively) and 4 hours (p < 0.001 and p < 0.001, respectively) following treatment. Moreover, mice treated with LPS at eight weeks old displayed significantly more sickness symptoms than those treated with LPS at six weeks old 30 min following treatment (p < 0.005). No sickness symptoms were displayed 24 hours following treatment (Figure 3A).
Figure 3.
A) Sickness score (Mean ± SEM) and B) percent body weight (Mean ± SEM) change in C57Bl/6 mice treated with either saline or LPS at 6 or 8 weeks old. Significantly greater than saline controls: *p < 0.05.
Body weight
As expected, statistical analyses revealed a significant time × treatment (F(1, 59) = 31.10; p < 0.001) interaction. Pairwise comparisons indicated that mice treated with LPS at six or eight weeks of age displayed significantly greater body weight loss than mice treated with saline 24 hours (p < 0.001 and p < 0.001, respectively) and 48 hours (p < 0.001 and p < 0.001, respectively) following treatment (Figure 3B).
Long-term Changes in body weight
Statistical analysis revealed a significant time×treatment interaction (F(14, 546) = 9.00, p < 0.05). Pairwise comparisons revealed that LPS-treated mice lost significantly more weight at six weeks of age (p < 0.05) following treatment and gained significantly more weight at eight (p < 0.05), nine (p < 0.05) and ten (p < 0.05) weeks of age compared to saline-treated mice. There was no significant difference in body weight change as of 11 weeks of age between LPS- and saline-treated mice (data not shown). The body weight of mice treated with saline or with LPS prior to treatment (saline group: 16.98g; LPS group: 16.80g) or nine weeks later at the end of testing did not differ (saline group: 23.04g; LPS group: 23.20g). These findings suggest that the differences in the duration of immobility in the forced swim and tail suspension tests were not due to long-term changes in body weight.
Forced Swim Test
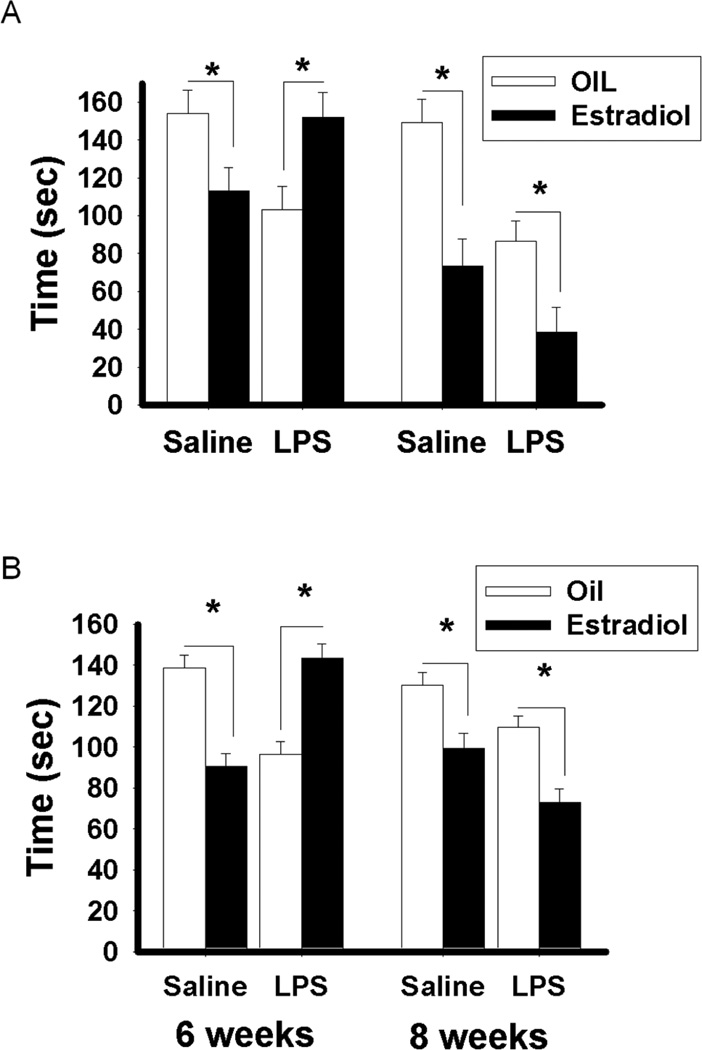
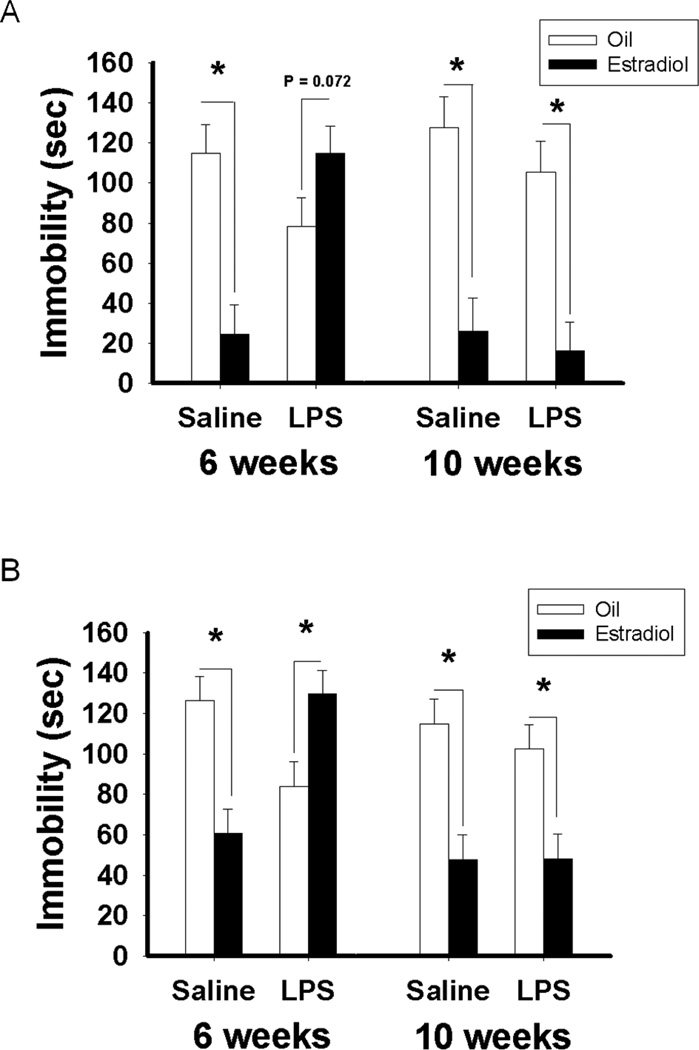
Overall statistical analyses revealed significant treatment × age (F(1, 54) = 5.76; p < 0.05), treatment × hormone (F(1, 54) = 10.97; p < 0.05) and age × hormone (F(1, 54) = 13.66; p = 0.001) interactions. In mice treated with saline at six weeks of age, estradiol treatment in adulthood significantly reduced the duration of immobility compared to oil treatment (p < 0.05). Estradiol treatment also significantly decreased the duration of immobility compared to oil treatment in the negative control group, in mice treated with either saline or LPS at eight weeks of age (p < 0.001, p < 0.01, respectively). In contrast, in mice treated with LPS at six weeks of age, estradiol treatment in adulthood had the opposite effect actually increasing the duration of immobility compared to oil treatment (p < 0.05) (Figure 4A). The same pattern of results was obtained when the test was repeated a week later (data not shown).
Figure 4.
Duration of immobility (Mean ± SEM) during A) the forced swim test and B) the tail suspension test in C57Bl/6 mice treated with saline or LPS at six or eight weeks old and treated either with estradiol or oil vehicle in adulthood. Significant difference between mice treated with estradiol and those treated with oil-vehicle: *p < 0.05.
Tail suspension test
Similarly to results from the forced swim test, statistical analyses revealed significant treatment × age (F(1, 54) = 9.89; p < 0.005), treatment × hormone (F(1, 54) = 30.23; p < 0.001), age × hormone (F(1, 54) = 17.52; p < 0.001), and treatment × age × hormone (F(1, 54) = 31.47; p < 0.001) interactions. In mice treated with saline at six weeks of age, estradiol treatment significantly reduced the duration of immobility compared to oil treatment in adulthood (p < 0.001). However, in mice treated with LPS at six weeks of age, estradiol treatment significantly increased the duration of immobility compared to oil treatment (p < 0.001). In comparison, estradiol treatment significantly decreased the duration of immobility compared to oil treatment in mice treated with saline or with LPS at eight weeks of age (p < 0.001, p < 0.001, respectively) (Figure 4B). The same pattern of results was obtained when the test was repeated a week later (data not shown).
Sucrose Preference Test
There was no significant difference between the groups. Statistical analysis revealed no significant main effect or interaction (data not shown). The percentage of sucrose consumed by mice treated with saline or LPS at six weeks of age and implanted, in adulthood, with a capsule containing either estradiol or oil vehicle did not differ at the end of the 48-hour period (saline-oil: Mean = 80.37, SEM = 4.42; saline-estradiol: Mean = 81.47, SEM = 3.83; LPS-oil: Mean = 77.28, SEM = 3.83; LPS-estradiol: Mean = 83.36, SEM = 4.42). Similar to mice treated during puberty at 6 weeks old, the percentage of sucrose consumed by mice treated with saline or LPS at eight weeks of age and implanted, in adulthood, with a capsule containing either estradiol or oil vehicle did not differ at the end of the 48-hour period (saline-oil: Mean = 77.17, SEM = 3.83; saline-estradiol: Mean = 68.68, SEM = 4.42; LPS-oil: Mean = 83.70, SEM = 3.83; LPS-estradiol: Mean = 81.03, SEM = 4.42).
Locomotor activity
There was a no significant differences in the distance travelled, the velocity of travel and rearing n photocell-beamed chambers or in the number of line crossings and time spent in the center in the open field between saline- or LPS-treated females at 6 or 8 weeks of age treated either with the oil vehicle or with estradiol during the first week of testing (data not shown).
EXPERIMENT 2: The effect of pubertal LPS treatment on the antidepressant-like effects of estradiol in outbred CD1 mice
Sickness behavior
Statistical analyses revealed a significant time × treatment (F(3, 183) = 126.64; p < 0.001) interaction. No sickness symptoms were displayed 48 hours following treatment. Pairwise comparisons indicated that mice treated with LPS at six or ten weeks of age displayed significantly more sickness symptoms than mice treated with saline 30 min (p < 0.001 and p < 0.001, respectively), 4 hours (p < 0.001 and p < 0.001, respectively) and 24 hours (p < 0.001 and p < 0.001, respectively) following treatment (Figure 5A).
Figure 5.
A) Sickness score (Mean ± SEM) and B) percent body weight (Mean ± SEM) change in CD1 mice treated with either saline or LPS at six or ten weeks old. Significantly greater than saline controls: *p < 0.05.
Body weight
As in Experiment 1, statistical analyses revealed a significant time × treatment (F(1, 61) = 18.32; p < 0.001) interaction. Pairwise comparisons indicated that mice treated with LPS at six or eight weeks of age displayed significantly greater body weight loss than mice treated with saline 24 hours (p < 0.001 and p < 0.001, respectively) and 48 hours (p < 0.001 and p < 0.001, respectively) following treatment (Figure 5B).
Forced Swim Test
There was significant treatment × age (F(1, 53) = 4.12; p < 0.05), treatment × hormone (F(1,53) = 10.85; p < 0.005), age × hormone (F(1, 53) = 10.59; p < 0.005), and treatment × age × hormone (F(1, 53) = 7.31; p < 0.01) interactions. Pairwise comparisons indicated that, in mice treated with saline at six weeks of age (control group), estradiol treatment in adulthood reduced the duration of immobility compared to oil treatment (p < 0.001). However, in mice treated with LPS at six weeks of age, estradiol treatment was ineffective at decreasing the duration of immobility. In these mice, estradiol treatment tended to increase the duration of immobility compared to oil treatment (p = 0.072). In comparison, estradiol treatment significantly decreased the duration of immobility compared to oil treatment in mice treated with saline or with LPS at ten weeks of age (p < 0.001, p < 0.01, respectively) (Figure 6A). The same pattern of results was obtained when the test was repeated a week later (data not shown).
Figure 6.
Duration of immobility (Mean ± SEM) during A) the forced swim test and B) the tail suspension test in CD1 mice treated with saline or LPS at six or ten weeks old and treated either with estradiol or oil vehicle in adulthood. Significant difference between mice treated with estradiol and those treated with oil-vehicle: *p < 0.05.
Tail suspension test
Similar to results from the forced swim test, statistical analyses revealed significant treatment × hormone (F(1, 57) = 16.21; p < 0.001) and treatment × age × hormone (F(1, 57) = 12.72; p < 0.001) interactions. Pairwise comparisons indicated that, in mice treated with saline at six weeks of age, estradiol treatment significantly reduced the duration of immobility compared to oil treatment in adulthood (p < 0.001). However, in mice treated with LPS at six weeks of age, estradiol significantly increased the duration of immobility compared to oil treatment (p < 0.005). In comparison, estradiol treatment significantly decreased the duration of immobility compared to oil treatment in mice treated with saline or with LPS at ten weeks of age (p = 0.001, p < 0.005, respectively) (Figure 6B). The same pattern of results was obtained when the test was repeated a week later (data not shown).
Sucrose Preference Test
There was no significant difference between the oil- and estradiol-treated mice. Statistical analysis revealed a significant treatment × age interaction (F(1, 23) = 7.30, p < 0.05) and a tendency towards a time of measurement (24 or 48 hours) × age interaction (F(1, 23) = 3.52, p = 0.073) (Data not shown). The percentage of sucrose consumed by mice treated with saline or LPS at six weeks of age and implanted, in adulthood, with a capsule containing either estradiol or oil vehicle did not differ at the end of the 48-hour period (saline-oil: Mean = 67.63, SEM = 5.00; saline-estradiol: Mean = 63.15, SEM = 5.00; LPS-oil: Mean = 59.39, SEM = 5.00; LPSestradiol: Mean = 65.78, SEM = 5.00). Similar to mice treated during puberty at 6 weeks old, the percentage of sucrose consumed by mice treated with saline or LPS at ten weeks of age and implanted, in adulthood, with a capsule containing either estradiol or oil vehicle did not differ at the end of the 48-hour period (saline-oil: Mean = 50.21, SEM = 5.00; saline-estradiol: Mean = 60.36, SEM = 5.00; LPS-oil: Mean = 62.72, SEM = 5.00; LPS-estradiol: Mean = 60.24, SEM = 5.77).
Locomotor activity
There was a no significant differences in the distance travelled, the velocity of travel and rearing in photocell-beamed chambers or in the number of line crossings and time spent in the center in the open field between saline- or LPS-treated females at six or ten weeks of age treated either with the oil vehicle or with estradiol during the first week of testing (data not shown).
Discussion
Exposure to stressors during the pubertal period has lasting effects on the behavioral responsiveness to ovarian hormones in adulthood. For example, exposure to either shipping or LPS at that time reduces sexual receptivity in response to estradiol and progesterone in adults of two strains of mice (Laroche et al., 2009a;Laroche et al., 2009b;Ismail et al., 2011). Ovarian hormones also play a prominent role on mental health; for example estradiol has an antidepressant action in humans (Schmidt and Rubinow, 2009) and rodents (Okada et al., 1997;Rachman et al., 1998;Dalla et al., 2004).
Previously, researchers have shown that ovariectomy prolongs immobility duration in adult rats and chronic estradiol treatment via either a Silastic capsule or an osmotic minipump ameliorates the behavioral change (Okada et al., 1997;Dalla et al., 2004). Therefore, in the current study, we hypothesized that in C57Bl/6 and CD1 female mice treated pubertally with LPS, chronic estradiol treatment would be less effective at reducing depression-like behavior in adulthood. In contrast to control mice, in which estradiol had antidepressant-like effects, estradiol actually increased depression-like behavior in mice treated with LPS at six weeks old. These results indicate that pubertal immune challenge not only results in failure of estradiol to decrease depressive-like symptoms; rather it results in a reversal of the behavioral response to estradiol. While the mechanism underlying this effect remains to be investigated, it is possible that the alteration in behavioral responsiveness to estradiol is due to changes in estrogen receptor expression. Recently, we showed that pubertal exposure to a shipping stressor causes changes in estrogen receptor-α expression in adulthood (Ismail et al., 2011). Nonetheless, the findings of the current study extend to depression-like behaviors, those endpoints of estradiol action which are influenced by pubertal immune challenge.
In this study, we examined the effect of pubertal LPS treatment on the responsiveness to chronic estradiol treatment in mice treated during puberty or adulthood with either saline or LPS and ovariectomized and implanted with a capsule containing either estradiol or oil vehicle. All females were ovariectomized in adulthood for two reasons. First, it was necessary to control for any possible differences in endogenous estradiol levels between mice treated pubertally with saline or with LPS. Second, it was important to control for the effects of other ovarian hormones on depression-like behavior. Therefore, to specifically examine the effect of pubertal LPS treatment on the responsiveness to chronic estradiol treatment, the ovariectomized females were implanted with a capsule containing either estradiol or oil vehicle.
It is interesting to note that pubertal LPS treatment also decreases the duration of immobility in the forced swim and tail suspension tests, suggesting an organizational influence of early LPS exposure in an antidepressant-like direction. Although the mechanism underlying this effect remains to be investigated, the LPS-induced decrease in depression-like behavior in pubertal mice might contribute to the paradoxical interaction between pubertal LPS exposure and behavioral responsiveness to estradiol in adulthood. This could explain the fact that pubertal LPS treatment reverses the effects of estradiol, whereas with other behaviors that we have studied (e.g., anxiety-like behavior (Olesen et al., 2011), sexual behavior (Laroche et al., 2009a,2009b, Ismail et al., 2011) and cognitive function (Ismail et al., in preparation), LPS decreases or eliminates the effects of estradiol or estradiol and progesterone.
It is unlikely that the difference in depression-like behavior between mice treated with LPS during the pubertal period or in adulthood is due to a differential response to the immune challenge. There was only a small increase in sickness symptoms 30 min following treatment in C57Bl/6 mice treated with LPS at eight weeks old contrasted to those treated at six weeks old, a difference not maintained at other time points; both groups recovered by 48 hours. Consistent with other findings (Olesen et al., 2011), there also was no major difference in sickness behavior in CD1 mice treated with LPS at six or ten weeks old. Furthermore, there was no difference in LPS-induced body weight loss between mice treated at six and ten weeks old. To our knowledge, there is also no report in the literature suggesting differences in immune response between pubertal and adult mice following LPS treatment
It is also unlikely that the difference in depression-like behavior is due to a difference in body weight between saline- and LPS-treated mice. The results of Experiment 1 revealed a significant weight loss at six weeks of age in LPS-treated mice compared to saline control mice. Then, between eight and ten weeks of age, LPS-treated mice gained significantly more weight compared to saline-treated mice. However, these differences were not maintained after ten weeks of age and both groups continued to show the same change in body weight in subsequent weeks.
As others have done (Dalla, et al., 2004; Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, et al., 2008), we exposed mice to the forced swim and tail suspension tests twice in each experiment to confirm the results obtained in the first test. Since we obtained the expected effect of estradiol in the forced swim and tail suspension tests on both series of tests, these findings indicates that the first test did not have a negative effect on the results of the second test.
LPS treatment induces short-lasting depression-like behavior starting about 24 to 48 hours following treatment (Yirmiya, 1996;Dantzer et al., 2008;Anisman et al., 2005;Painsipp et al., 2011) and ending less than 28 days later in group-housed adult CD1 mice. In contrast, in group-housed, adult C57Bl/6 female mice, LPS treatment decreases depression-like behavior 24 hours following treatment and increases it 28 days later, suggesting a long-term effect of the immune challenge on depression-like behavior in this strain of mice (Painsipp et al., 2011). In the current study, we did not note any strain difference in depression-like behavior. In fact, the depression-like responses were similar in both experiments, with C57Bl/6 mice and CD-1 mice.
Although we examined the effect of pubertal or adult LPS treatment on the behavioral responsiveness of depression-like behavior in inbred C57Bl/6 and CD-1 mice, the goal of this experiment was not to do a strain-differences experiment; rather, it was to determine if the antidepressive effect was idiosyncratic to a particular strain of mice. This study shows that pubertal LPS treatment alters the responsiveness of depression-like behavior to estradiol treatment in adulthood in two disparate strains of mice.
While the duration of immobility during the forced swim and tail suspension tests is thought to represent a state of behavioral despair and is known to be modulated by circulating estradiol levels (Okada et al., 1997;Dalla et al., 2004), the consumption of sucrose during the sucrose preference test is said to be indicative of the anhedonia symptom of depression. To our knowledge, it is unclear whether ovarian hormones have an effect on the sucrose preference test. In the current study, while the duration of immobility during the forced swim and tail suspension tests was altered by LPS and estradiol treatments, there was no difference in sucrose consumption during the sucrose preference test, suggesting that different mechanisms modulate the behavioral despair and anhedonia symptoms of depression. It should also be noted that the sucrose preference test was conducted at the end of the experiment and about six weeks following capsule implantation. Therefore, the lack of hormone effect on the sucrose preference test could be due to the capsule becoming inactive.
In order to maintain a constant time interval across groups between the treatment and ovariectomy, the animals differed in age at the time of testing. However, because the animals were ovariectomized in adulthood, it is unlikely that the age at testing was responsible for the behavioral differences observed. In fact, in earlier work, C57BL/6 mice shipped at six weeks old and ovariectomized either one or seven weeks later displayed reduced behavioral responsiveness to estradiol and progesterone (Laroche et al., 2009b), suggesting that age at ovariectomy or testing is not responsible for the behavioral differences among groups.
The two forms of estrogen receptors (ERs), ER-α and ER-β (White et al., 1987;Lubahn et al., 1993;Kuiper et al., 1996;Merchenthaler et al., 2004), are widely distributed in the brain, and sometimes are co-expressed in neurons (Shughrue et al., 1997;Greco et al., 2001). These receptors share either antagonistic, synergistic or sequential relationships depending on the brain circuits or behaviors examined (Rissman, 2008). Although exposure to a shipping stressor during the pubertal period decreases ER-α expression in the some brain areas (Ismail et al., 2011), the effects of an immune challenge on ER expression are not known. The antidepressant effect of estradiol is thought to be mediated predominantly by ER-β (Osterlund, 2010). Therefore, the inability of estradiol to decrease depression-like behavior in mice treated pubertally with LPS would suggest an alteration in ER-β expression in relevant brain regions, like the hippocampus, an area importantly associated with depression and the action of anti-depressants (Darnaudery et al., 2007;Fuchs et al., 2004;Santarelli et al., 2003). This remains to be investigated in a future experiment.
Taken together, these findings in the present study demonstrate that pubertal immune challenge alters the behavioral responsiveness to the antidepressant effects of estradiol in both inbred and outbred strains of mice. In the current experiments, estradiol treatment did not only fail to decrease depression-like behaviors; rather, it increases these behaviors in mice treated with LPS during puberty. These results collectively support the idea that the effect of altered responsiveness to ovarian hormones following exposure to stressors during puberty extends to numerous estradiol-dependent behaviors and it is not idiosyncratic to one or two behaviors.
Highlights.
Estradiol increases depression-like behavior in mice treated with LPS during puberty.
Pubertal immune challenge alters the behavioral responsiveness to estradiol.
Pubertal LPS treatment does not cause lasting changes in body weight and locomotion.
Acknowledgements
We would like to thank Dr. Kristin Olesen for feedback and discussions and Peter Garas, Ryan Rogan, Sarah Servattalab, Alex Zaltsman and Nassim Bouchentouf for technical assistance.
This work was supported by NIH grant MH 093854, by the National Science Foundation under grant number 1050179, and an Isis grant from the Society for Women's Health Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- Bernardi M, Vergoni AV, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–1068. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur J Neurosci. 2004;20:217–228. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudery M, Louvart H, Defrance L, Leonhardt M, Morley-Fletcher S, Gruber SH, Galietta G, Mathe AA, Maccari S. Impact of an intense stress on ethanol consumption in female rats characterized by their pre-stress preference: modulation by prenatal stress. Brain Res. 2007;1131:181–186. doi: 10.1016/j.brainres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Alarcon T, Espinoza C, Dussaubat N, Mora S. Ketanserin and anxiety levels: influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacol Biochem Behav. 1997;58:637–642. doi: 10.1016/s0091-3057(97)90004-6. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Soto V, Dussaubat N, Mora S. Influence of the estrous cycle, ovariectomy and estradiol replacement upon the acquisition of conditioned avoidance responses in rats. Physiol Behav. 1989;46:397–401. doi: 10.1016/0031-9384(89)90010-3. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S481–S490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Galeeva A, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–47. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav Immun. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun. 2008;22:573–589. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Identifying early behavioral and molecular markers of future stress sensitivity. Endocrinology. 2007;148:4585–4591. doi: 10.1210/en.2007-0479. [DOI] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Green AD, Barr AM, Galea LA. Role of estradiol withdrawal in 'anhedonic' sucrose consumption: a model of postpartum depression. Physiol Behav. 2009;97:259–265. doi: 10.1016/j.physbeh.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–571. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda SI, Rissman EF. Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol Behav. 2009;97:146–150. doi: 10.1016/j.physbeh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009a;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009b;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Morley-Fletcher S, Terranova ML. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res. 2002;130:117–125. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn J Pharmacol. 1997;73:93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Ismail N, Merchasin ED, Blaustein JD. Long-term alteration of anxiolytic effects of ovarian hormones in female mice by a peripubertal immune challenge. Horm Behav. 2011;60:318–326. doi: 10.1016/j.yhbeh.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochim Biophys Acta. 2010;1800:1136–1144. doi: 10.1016/j.bbagen.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Kofer MJ, Sinner F, Holzer P. Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS One. 2011;6:e20719. doi: 10.1371/journal.pone.0020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris A, Kelly P, Ramaley JA. Effects on short-term stress upon fertility. II. After puberty. Fertil Steril. 1973;24:546–552. doi: 10.1016/s0015-0282(16)39796-5. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998;95:13941– 13946. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann C, Filzmoser P, Garrett RG. Background and threshold: critical comparison of methods of determination. Sci Total Environ. 2005;346:1–16. doi: 10.1016/j.scitotenv.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- White R, Lees JA, Needham M, Ham J, Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]