Abstract
Objective
TBI contributes to morbidity in children and boys are disproportionately represented. Autoregulation is impaired more in male compared to female piglets after TBI through sex dependent upregulation of the spasmogen ET-1 and ERK mitogen activated protein kinase (MAPK, a family of 3 kinases, ERK, p38 and JNK). Elevation of mean arterial pressure (MAP) leading to increased cerebral perfusion pressure (CPP) via phenylephrine (Phe) improves impairment of autoregulation after TBI in female but not male piglets through modulation of ET-1 and ERK MAPK upregulation, blocked in females, but aggravated in males. We hypothesized that pressor choice to elevate CPP is important in improving cerebral hemodynamics after TBI and that dopamine (DA) will prevent impairment of autoregulation in both male and female piglets through blockade of ET-1 and ERK MAPK.
Design
Prospective, randomized animal study.
Setting
University laboratory.
Subjects
Newborn (1–5 day old) pigs.
Interventions
CPP and pial artery diameter were determined before and after lateral fluid percussion brain injury was produced in piglets equipped with a closed cranial window. DA (15 μg/Kg/min iv) was administered 30 min post FPI. CSF ERK MAPK was determined by ELISA.
Measurements and Main Results
DA increased CPP equivalently in both sexes and prevented sex dependent reductions in pial artery diameter after FPI. Loss of pial artery dilation during hypotension was greater in male compared to female piglets after FPI, but DA prevented such impairment equivalently in both sexes post injury. ET-1 and ERK MAPK release was greater in male compared to female piglets after FPI, but DA also blocked their upregulation equivalently in male and female piglets after FPI.
Conclusions
These data indicate that DA is protective of autoregulation after FPI in both sexes. These observations advocate for the consideration of development of sex based therapies for treatment of hemodynamic sequalae of pediatric TBI.
Keywords: cerebral autoregulation, endothelin, signal transduction, traumatic brain injury, dopamine, cerebral perfusion pressure
Introduction
Hypotension and low cerebral perfusion pressure (CPP) are associated with poor outcomes after traumatic brain injury (TBI) in children (1). After TBI, boys of all ages and children under 4 years have particularly devastating outcomes (2). Cerebral autoregulation is often impaired after TBI (3) and with concomitant hypotension, cerebral ischemia may ensue, leading to poor outcome.
Since ethical considerations constrain mechanistic studies in children with TBI, we have used an established porcine model of fluid percussion injury (FPI) that mimics TBI to corroborate clinical observations regarding cerebral autoregulation and hypotension after TBI (4). Like humans, piglets have gyrencephalic brains, are sensitive to FPI, and newborn and juvenile pigs mimic young (< 4 years) and older (> 4 years) children. Autoregulation is impaired to a greater extent in newborn compared to juvenile pigs (5), which parallels that observed clinically (3,6). Upregulation of the spasmogen endothelin-1 (ET-1) is greater and contributes to the more robust impairment of autoregulation in younger compared to older pigs (5). More recently, we have observed that ET-1 is upregulated more in male compared to female piglets after FPI, contributory to sex dependent greater impairment of autoregulation in male versus female piglets (7).
Current 2003 Pediatric Guidelines recommend maintaining CPP above 40 mm Hg (8). Despite these therapeutic targets, there are no guidelines regarding how this should be achieved other than therapies to lower intracranial pressure (ICP) by using mannitol or hypertonic saline (9), the latter which may be desirable because of the added benefit of increasing CPP beyond what would be expected due to the drop in ICP (10). In addition to decreasing ICP, vasopressors to elevate mean arterial pressure (MAP) are commonly used to preserve CPP by normalizing BP in TBI patients with hypotension. However, CPP-directed therapy has remained somewhat controversial because it has been observed to either have no effect or in fact worsen outcome (11). Additionally, CPP has been considered to be a poor surrogate for cerebral blood flow (CBF) (12) since regional or local CBF may be markedly reduced even if CPP is normal (11). Since ICP monitors are not universally used to guide CPP therapy, especially in young children, clinicians often rely on MAP to estimate CBF. Three vasopressors commonly used to elevate MAP are phenylephrine (Phe), norepinephrine (NE) and dopamine (DA) (13). We recently observed that Phe prevented in female but exacerbated in male piglets the impairment of cerebral autoregulation after FPI (14). While Phe blunted ET-1 and extra cellular signal-regulated kinase (ERK) MAPK upregulation in female piglets after FPI, there was an unanticipated and unwanted Phe-mediated aggravation of ET-1 and ERK MAPK upregulation in male piglets post injury (7). The latter compounded the already greater release of ET-1 and ERK MAPK in males compared to females after FPI and appeared to contribute to the sex dependent impairment of autoregulation (7).
In the present study, we speculated that pressor choice to elevate CPP is important in improving cerebral hemodynamics after TBI. Phe is a selective alpha-1 agonist, while DA stimulates dopamanergic and beta adrenoceptors at low dose and activates alpha-1 and alpha-2 adrenoceptors at higher doses. In intracerebral vessels, although alpha adrenoceptor density is low, alpha-1 and alpha-2 receptors mediate vasoconstriction. We hypothesized that a pressor with a different spectrum of receptor activation than Phe in yielding elevated CPP, such as DA, will prevent impairment of autoregulation in both male and female piglets through equivalent blockade of ET-1 and ERK MAPK upregulation post insult.
Methods
Closed cranial window technique and TBI
Newborn pigs (1–5 days, 1.0–1.4 Kg) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were anesthetized with isoflurane (1–2 MAC). Anesthesia was maintained with a-chloralose (30–50 mg/ kg. supplemented with 5 mg / kg/h i.v.). A catheter was inserted into a femoral artery to monitor blood pressure and to sample for blood gas tensions and pH. Drugs to maintain anesthesia were administered through a second catheter placed in a femoral vein. The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37° – 39° C, monitored rectally.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consisted of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to CSF, of the following composition (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 37° C and had the following chemistry: pH 7.33, pCO2 46 mm Hg, and pO2 43 mm Hg, which was similar to that of endogenous CSF. Pial arterial vessel diameter was measured with a microscope, a camera, a video output screen and a video microscaler. An Integra Camino monitor was used to measure ICP.
CBF was measured in the cerebral cortex using radioactively labeled microspheres (14). Briefly, neutron activated microspheres (Biopal; 15μm diameter; 300,000–800,000 spheres) were injected into the left ventricle. After each experiment, the pig was sacrificed and the brain removed and weighed. The energy from each nuclide was separated by differential spectroscopy. Aliquots of the actual microsphere solutions injected were used for overlap calculations. The count in each milliliter per minute of blood flow was determined by dividing the counts in the reference withdrawal by the rate of reference withdrawal. Thus blood flow can be calculated as Q = C × R × CR−1, where Q is brain blood flow (in ml/min), C is counts per minute (cpm) in the tissue sample, R is the rate of withdrawal of reference blood sample (in ml/min), and CR is the total counts in the reference blood sample. CBF so determined reflect flow to the cerebral cortex both ipsilateral and contralateral to the injury site.
The method used to induce brain FPI has been described previously (15). A device designed by the Medical College of Virginia was used. A small opening was made in the parietal skull contralateral to the cranial window. A metal shaft was sealed into the opening on top of intact dura and fluid coupled to the brain injury device, eg the shaft was connected to the transducer housing, which was in turn connected to the fluid percussion device. The device itself consisted of an acrylic plastic cylindrical reservoir 60 cm long, 4.5 cm in diameter, and 0.5 cm thick. One end of the device was connected to the transducer housing, whereas the other end had an acrylic plastic piston mounted on O-rings. The exposed end of the piston was covered with a rubber pad. The entire system was filled with 0.9 % saline. The percussion device was supported by two brackets mounted on a platform. FPI was induced by striking the piston with a 4.8 kg pendulum. The intensity of the injury (usually 1.9–2.3 atm. with a constant duration of 19–23 ms) was controlled by varying the height from which the pendulum was allowed to fall. The pressure pulse of the injury was recorded on a storage oscilloscope triggered photoelectrically by the fall of the pendulum. The amplitude of the pressure pulse was used to determine the intensity of the injury.
Protocol
Pial small arteries (resting diameter, 120–160 μm) were examined to determine the effects of FPI. Typically, 2–3 ml of artificial CSF were flushed through the window over a 30s period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μl of the total cranial window volume of 500 μl was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
Seven experimental groups were studied (all n=5): (1) sham control, (2) sham control treated with dopamine (DA, 15 μg/Kg/min, iv), (3) FPI, pre-treated with vehicle, (4) FPI pre-treated with DA, (5) FPI post-treated with vehicle, (6) FPI post-treated with DA and (7) FPI post-treated with the ERK MAPK antagonist U 0126 (1 mg/kg iv). Hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 ml blood/Kg to induce moderate or severe hypotension (decreases in MAP of 25 and 45%, respectively. Such decreases in blood pressure were maintained constant for 10 min by titration of additional blood withdrawal or blood reinfusion. The vehicle for all agents was 0.9% saline, except for the MAPK inhibitor, which used dimethyl sulfoxide (100 μl) diluted with 9.9 ml 0.9% saline. In sham control animals, responses to hypotension (moderate, severe) and papaverine (10−8, 10−6 M) were obtained initially and then again 1h later in the presence of the agent vehicle. In drug pre-treated FPI animals, drugs were administered 30 min before FPI and the responses to hypotension and papaverine obtained at 1h after injury. In drug post-treated animals, drugs were administered 30 min after FPI and responses to hypotension and papaverine and CSF samples collected at 1h post insult. The order of agonist administration was randomized within animal drug treatment groups. Papaverine was used as a negative control in that its vasodilator response is known to be unchanged after FPI (14).
ELISA and RIA
Commercially available enzyme-linked immunosorbent assay (ELISA) Kits were used to quantity CSF ERK MAPK (Assay Designs) concentration, while radioimmunoassay (RIA) kits were used to quantify ET-1 (Phoenix Pharmaceuticals). Phosphorylated ERK MAPK enzyme values were normalized to total form and then expressed as percent of the control condition.
Statistical analysis
Pial artery diameter, CSF ERK MAPK and ET-1 values were analyzed using ANOVA for repeated measures. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. An α level of p<0.05 was considered significant in all statistical tests. Values are represented as mean ± SEM of the absolute value or as percentage changes from control value.
Results
DA increases CPP equivalently in both sexes after FPI
One hour after FPI, ICP was higher in the male than the female piglet at equivalent insult levels (1.9 ± 0.1 and 2.0 ± 0.1 atm) (Fig 1A). ICP values during combined moderate (25% decrease in MAP) or severe (45% decrease in MAP) hypotension and FPI were also greater in the male compared to the female (Fig 1A). DA (15 μg/Kg/min iv) pre-treatment (30 min before FPI) and post-treatment (30 min after FPI) blunted FPI associated increases in ICP at 1h post injury in male and female piglets (Fig 1A). During combined hypotension and FPI, DA blunted further increases in ICP equivalently in males and females (Fig 1A). CPP was lower at 1h post injury in the male than the female (Fig 1B). Similarly, during combined hypotension and FPI, CPP was also lower more in the male than the female (Fig 1B). DA increased CPP equivalently in both sexes after FPI and FPI + hypotension (Fig 1B). DA produced comparable increases in MAP in male and female piglets (Fig1C).
Figure 1.
A. ICP (mm Hg) and B. CPP (mm Hg) during normotension, moderate and severe hypotension in sham, FPI, and FPI + DA (15 μg/Kg/min iv) pre-treatment and post-treatment (30 min before or 30 min after injury) male and female pigs, n=5. C. MAP (mm Hg) during DA pre- and post-treatment in male and female pigs, n=5. *p<0.05 compared with corresponding sham value. +p<0.05 compared with corresponding normotension value #p<0.05 compared with corresponding nontreateded FPI value &p<0.05 compared with corresponding female value.
DA prevents sex dependent reductions in pial artery diameter and CBF after FPI
FPI produced greater reductions in pial artery diameter and blood flow in the parietal cortex in male compared to female pigs (Fig 2,3), similar to previous observations (13). DA pre and post-treatment blunted FPI-induced reductions in pial artery diameter and CBF similarly in the female and male (Fig 2,3).
Figure 2.
Influence of FPI on pial artery diameter in vehicle (FPI) and DA (15 μg/Kg/min iv) 30 min pre or 30 min post FPI in newborn male and female pigs, n=5. *p<0.05 compared with corresponding FPI vehicle value +p<0.05 compared with corresponding female FPI value.
Figure 3.
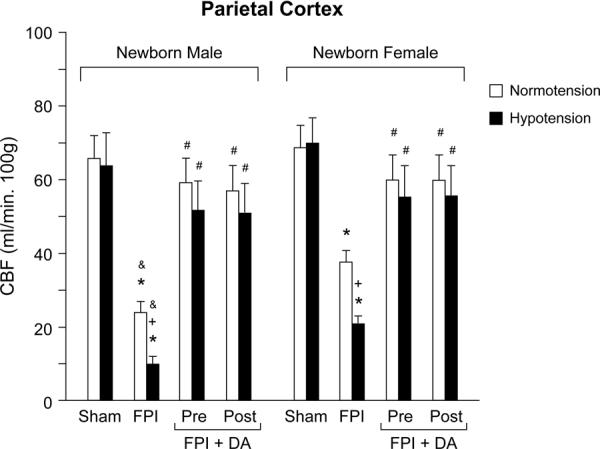
CBF (ml/min .100g) in the parietal cortex during normotension and severe hypotension (hypotension) in sham, FPI, and FPI + DA (15 μg/Kg/min iv) pre and post-treated newborn male and female pigs, n=3–5. *p<0.05 compared with corresponding sham value +p<0.05 compared with corresponding normotension value #p<0.05 compared with corresponding FPI non-treated value &p<0.05 compared with corresponding female value.
DA prevents loss of CBF autoregulation and pial artery dilation during hypotension in both sexes after FPI
CBF in the parietal cortex was unchanged during hypotension prior to FPI, supportive of intact cerebral autoregulation pre-insult (Fig 3). After FPI, CBF was lower in the male than in the female (Fig 3). CBF was reduced further during moderate and severe hypotension, but such reductions were greater in the male than the female (Fig 3). In contrast, DA (15 μg/kg/min iv; pre- and post- treatment) prevented loss of cerebral autoregulation during hypotension after FPI in the female pig (Fig 3). Similar protection of cerebral autoregulation in the male was observed with DA administration 30 min before or after FPI (Fig 3). These data indicate that DA prevents loss of CBF autoregulation during hypotension after FPI equivalently in both sexes.
Moderate and severe hypotension (24 ± 1 and 45 ± 2% decrease in MAP, respectively) produced reproducible increases in pial artery diameter under sham control conditions. Prior to FPI, hypotensive pial artery dilation was significantly less in male than female piglets (Fig 4), similar to that recently published (14). Within 1 hr of FPI, hypotensive pial artery dilation was impaired in both sexes, but the degree of impairment was greater in the male compared to the female piglet (Fig 4). Treatment with DA under sham control conditions had no effect on hypotensive pial artery dilation (Fig 4). However, pre- or post-treatment with DA fully restored the pial artery dilation in response to hypotension to that observed under sham control conditions in both sexes (Fig 4). Papaverine induced pial artery dilation was unchanged by FPI and DA (Fig 5). Further, pial artery dilation to papaverine was unchanged by DA in the absence of FPI (Fig 5).
Figure 4.

Influence of moderate and severe hypotension on pial artery diameter in newborn male (A) and female (B) pigs before injury (sham control), before injury treated with DA (15 μg/Kg/min iv), 1 h after FPI, 1h after FPI treated 30 min prior to injury with DA, and 1h after FPI treated with DA 30 min after injury, n=5. *p<0.05 compared with corresponding sham value +p<0.05 compared with corresponding FPI nontreated value #p<0.05 compared with corresponding female value.
Figure 5.

Influence of papaverine (10−8, 10−6 M) on pial artery diameter in newborn male and female pigs before injury (sham control), before injury treated with DA (15 μg/Kg/min iv), 1 h after FPI, 1h after FPI treated 30 min prior to injury with DA, and 1h after FPI treated with DA 30 min after injury, n=5.
DA blocks ET-1 and ERK MAPK upregulation equivalently in both sexes after FPI, contributing to protection of cerebral autoregulation during hypotension
CSF ET-1 and ERK MAPK concentrations were elevated more in the male than the female piglet within 1h after equivalent levels of FPI (1.9 ± 0.1 and 2.0 ± 0.1 atm) (Figs 6,7). DA (15 μg/kg/min iv) administered either 30 min before or 30 min after FPI blocked ET-1 and ERK MAPK release equivalently in male and female piglets after FPI (Figs 6,7). U 0126 (1 mg/kg iv), a putative ERK MAPK antagonist, blocked ERK MAPK upregulation in male and female piglets after FPI (Fig 7), similar to prior studies (14), supportive of its efficacy as an ERK MAPK antagonist. U 0126 has been shown to protect against hypoperfusion and loss of autoregulation during hypotension after FPI (16,17), thereby suggesting that ERK MAPK release post insult contributes to impaired cerebral hemodynamics. Similarly, BQ 123 has also been observed to block ET-1 induced pial artery vasoconstriction, supportive of its efficacy as an ET-1 antagonist (18), while preventing impairment of pial artery dilation during hypotension after FPI (5).
Figure 6.

Influence of FPI on CSF ET-1 in the absence and presence of DA pre- and post-treatment, n = 5. *p<0.05 compared with sham control (0)value +p<0.05 compared with corresponding no DA value #p<0.05 compared with corresponding female value.
Figure 7.

Phosphorylation of ERK MAPK in CSF prior to FPI (0 time), as a function of time after FPI (h) in pigs treated with vehicle (FPI), DA (15 μg/Kg/min iv) pre- or post-treatment (30 min) + FPI, or U0126 (1 mg/kg iv) + FPI, n=5. Data expressed as percent of control by ELISA determination of phospho ERK MAPK and total ERK MAPK isoforms and subsequent normalization to total form. A: newborn male, B: newborn female. *p<0.05 compared with corresponding 0 time value + p<0.05 compared with corresponding vehicle treated value #p<0.05 compared with corresponding female value
Blood Chemistry
Blood chemistry values were collected before and after all experiments. There were no statistically significant differences in pH, pCO2, or pO2 between sham control, FPI, and FPI and drug treated animals.
Discussion
An important new finding of translational relevance in this study is that choice of systemic pressor to elevate CPP influences cerebral hemodynamic outcome as a function of sex in the setting of TBI. In the present and in a series of recent prior studies, we have used a widely accepted clinical critical care pathway for treatment of TBI, elevation of MAP to limit hypoperfusion, to inform the study design of our basic science piglet model of TBI. While several different pressors can be used, the pressor of choice often may be Phe due to its longer duration of action and peak elevation of MAP (6). However, when used in our piglet TBI model, we were surprised to observe that while Phe is protective of cerebral hemodynamics, particularly for autoregulation, in female piglets, it aggravates cerebrovascular dysregulation in male piglets post injury (14). Because of this perplexing observation, we hypothesized in the present study that pressor choice may influence outcome. Indeed, we obtained provocative data indicating that this may, in fact, be the case in that another pressor, DA, produced the opposite outcome, equivalent cerebrohemodynamic protection in both male and female piglets in the setting of TBI. Additional experiments were then designed to utilize the advantage of use of a basic science animal mimic of the clinical situation to ask mechanistically driven questions with the intent of understanding why two pressors could produce such divergent cerebrohemodynamic outcomes.
In the first study of this series, we observed that ERK MAPK was elevated after FPI more in the male compared to the female pig and that Phe aggravated ERK MAPK upregulation in males but abrogated such elevation in female pigs (14). ERK MAPK upregulation is known to contribute to hypoperfusion after FPI (17) while the ERK MAPK antagonist U 0126 prevents impairment of cerebral autoregulation after FPI (14) and its aggravation by Phe in the male post insult (14).
Since years earlier we had noted a series of observations suggestive that ET-1 could elevate CSF ERK MAPK in the setting of FPI (19), we next demonstrated that the ET-1 antagonist BQ 123 blocked elevation of CSF ERK MAPK and the aggravation of such elevation by Phe after FPI (7). Co-administered BQ 123 with Phe also prevented impairment of autoregulatory pial artery dilation during hypotension after FPI, supportive of the intermediary role for ET-1 in sex dependent Phe-mediated hemodynamic dysregulation. In contrast, papaverine induced pial artery dilation was unchanged after FPI and Phe in male and female piglets, indicating the specificity of the Phe effect. BQ 123 has been shown to be an efficacious ET-1 antagonist in the piglet cerebral circulation (18).
An interesting observation in the present study, confirmatory of another recent publication (7), relates to the finding that the increase in the CSF concentration of the spasmogen ET-1 is greater in males compared with females after equivalent FPI. Previously, we had observed that ET-1 was released after FPI and that it contributed to impaired autoregulation during hypotension more in younger compared to older pigs (5). What was uninvestigated at that time was whether release was similarly different as a function of sex. Autoregulation during hypotension, similarly impaired more in the male than the female after FPI (14,16,20), paralleled the sex dependent release of this spasmogen, suggesting that ET-1 may be an important contributor to sex based differences in dysregulation in the setting of TBI. Elevation of MAP with Phe blunted ET-1 upregulation in the female piglet, but aggravated release of this spasmogen in the male piglet after FPI (7). In contrast, DA completely blocked ET-1 upregulation equivalently in male and female piglets after FPI. Taken together, choice of pressor to elevate MAP achieves differential cerebrohemodynamic outcome mechanistically due to sex specific modulatory effects of the pressor on ET-1 upregulation, thereby influencing subsequent release of ERK MAPK.
Several somewhat paradoxical observations were also noted in the present study. Release of a spasmogen like ET-1 that produces greater reductions in pial artery diameter in males than females should result in a larger decreased cerebral blood volume and decreased ICP in males compared to females. However, males in the current studies were observed to demonstrate higher ICP compared to females after FPI. Blunting abnormal arterial diameter increases during hypotension would also be expected to increase ICP through increased blood volume, but ICP was actually lower with the administration of DA. The results of these studies are also inconsistent with the observations in human studies where DA increased ICP (27). We speculate that the relative constrictor/dilator ratio in the cerebral circulation, in fact, determines the ultimate disposition of ICP, and not just the mere presence of a spasmogen. The role of constrictor/dilator ratios in determining outcome after FPI will be addressed in future studies.
Activation of K+ channels, particularly ATP and calcium sensitive (Katp and Kca) channels, increases K+ efflux, produces hyperpolarization of vascular muscle, and is an important mechanism for cerebral vasodilation, including hypotension (21). Cerebrovasodilation mediated by K channel agonists and autoregulation during hypotension were observed to be impaired more in males than females after FPI (14,16,20,22). Administration of an endogenous K channel agonist, adrenomedullin, prevents sex dependent impairment of autoregulation post insult through blockade of the upregulation of ERK MAPK (16,20). ET-1 also contributes to blunted K channel agonist mediated dilation after FPI via release of activated oxygen (O2−) (19).
Recent studies were designed to elucidate the sequential relationships between ET-1, ERK MAPK, activated oxygen, and K channels in impairment of cerebral autoregulation, and its aggravation by Phe, as a function of sex in the setting of FPI (7). In a prior study, we observed that generation of activated oxygen on the brain in a concentration roughly similar to that observed endogenously after FPI blocked pial artery dilation in response to Katp and Kca channel agonists, which was prevented by the ERK MAPK antagonist U 0126 (23), indicating that superoxide was upstream of ERK MAPK. More recently, we showed that the ET-1 antagonist BQ 123 blunted while the free radical scavenger SODCAT blocked ERK MAPK release after FPI, supportive of the sequential pathway wherein FPI releases ET-1 to increase superoxide which in turn releases ERK MAPK (7). More ET-1, activated oxygen, and ERK MAPK are released in males compared to females to sequentially impair K channel mediated cerebrovasodilation, contributing to impaired autoregulation during hypotension after TBI (7,22). Systemic pressor support with Phe exacerbates dysregulation via aggravation of the sequential impairment of K channel mediated cerebrovasodilation in males but abrogates such impairment and is protective in females after TBI in females but not males (7,22). Whether DA protection of K channel agonist mediated cerebrovasodilation after FPI can serve as an explanation for equivalent prevention of impairment of cerebral autoregulation in male and female pigs post insult is an intriguing question for investigation in future studies.
Others have investigated the cerebrohemodynamic effects of DA in the setting of TBI in basic science animal models as well as in patients. In a rodent impact acceleration model of rapidly increasing ICP, DA restored CBF through elevation of CPP (24). However, despite the restoration of CBF, ICP and edema formation were not improved by DA (24). In a second rodent TBI model, cortical contusion, DA also increased tissue water content (24). In an adult swine model of FPI, CPP directed therapy with combined Phe and DA improved brain oxygenation and maintained cerebrovascular CO2 reactivity while decreasing brain edema (25). When comparing DA to NE in head-injured patients, NE was observed to produce more significant and predictable increases in TCD flow velocity than DA (26), while DA increased ICP more for the same MAP compared to NE (27). No significant differences between NE and DA on cerebral oxygenation or metabolism either at baseline or following a CPP intervention were observed in head injured patients (28). However, similar to the TCD flow velocity study, the response to a CPP intervention with DA on oxygenation seemed to be more variable than that with NE (28). DA, then, could be viewed as a pressor whose use in CPP intervention could lead to an unpredictable outcome. When comparing the therapeutic use of Phe, DA, and NE in head injured patients, Phe was associated with higher MAP and CPP compared to DA and NE (13).
An experimental caveat of the present study is the lack of brain edema determination. In our prior studies widespread cortical damage and hemorrhage along with loss of neurons in CA1 and CA3 hippocampus were observed (17, 29). FPI is a mixed focal/diffuse model of TBI which evidences only minimal cerebral contusion at the injury site. In the context of prior observations using Phe as the means to increase MAP in piglet FPI, data from the present study support the hypothesis that pressor choice is important in determining cerebrohemodynamic outcome after TBI. These data advocate for the consideration of development of sex based therapies for treatment of hemodynamic sequalae of pediatric TBI.
Acknowledgments
Sources of Financial Support: This research was supported by HD 57355 (WMA) from the National Institutes of Health.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Català-Temprano A, Claret Teruel G, Cambra Lasaosa FJ, et al. Intracranial pressure and cerebral perfusion pressure as risk factors in children with traumatic brain injury. J Neurosurg. 2007;106(6 Suppl):463–466. doi: 10.3171/ped.2007.106.6.463. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Freeman SS, Udomphorn Y, Armstead WM, et al. Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology. 2008;108:588–595. doi: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- 4.Armstead WM. Age dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation. 2000;7:225–235. [PubMed] [Google Scholar]
- 5.Armstead WM. Role of endothelin-1 in age dependent cerebrovascular hypotensive responses after brain injury. Am J Physiol. 1999;277:H1884–1894. doi: 10.1152/ajpheart.1999.277.5.H1884. [DOI] [PubMed] [Google Scholar]
- 6.Digennaro JL, Mack CD, Malakouti A, et al. Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci. 2011;32:420–430. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstead WM, Riley J, Vavilala MS. TBI sex dependently upregulates ET-1 to impair autoregulation which is aggravated by phenylephrine in males but is abrogated in females. J Neurotrauma. doi: 10.1089/neu.2011.2248. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adelson PD, Bratton SL, Carney NA, et al. American Association for Surgery of Trauma; Child Neurology Society; International Society for Pediatric Neurosurgery; International Trauma Anesthesia and Critical Care Society; Society of Critical Care Medicine; World Federation of Pediatric Intensive and Critical Care Societies. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;4(3 Suppl):S12–18. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- 9.Bratton SL, Chestnut RM, Ghajar J, et al. Brain Trauma Foundation: American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S7–13. S59–64. [Google Scholar]
- 10.Keenan HT, Nocera M, Bratton SL. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatr Crit Care Med. 2005;6(5):537–541. doi: 10.1097/01.PCC.0000164638.44600.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coles JP, Steiner A, Johnston AJ, et al. Does induced hypertension reduce cerebral ischemia within traumatized human brain? Brain 2004. 2004;127:2479–2490. doi: 10.1093/brain/awh268. [DOI] [PubMed] [Google Scholar]
- 12.Cremer OL, van Dijk GW, van Wensen E, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med. 2005;33(10):2207–2213. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- 13.Sookplung P, Siriussawakul A, Malakouti A, et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. NeuroCrit Care. 2011;15:46–54. doi: 10.1007/s12028-010-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstead WM, Kiessling JW, Kofke WA, et al. Impaired cerebral blood flow autoregulation during post traumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by ERK MAPK upregulation. Crit Care Med. 2010;38:1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstead WM. Brain injury impairs ATP-sensitive K channel function in piglet cerebral arteries. Stroke. 1997;28:2273–2280. doi: 10.1161/01.str.28.11.2273. [DOI] [PubMed] [Google Scholar]
- 16.Armstead WM, Kiessling JW, Bdeir K, et al. Adrenomedullin prevents sex dependent impairment of cerebal autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J Neurotrauma. 2010;27:391–402. doi: 10.1089/neu.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstead WM, Cines DB, Bdeir K, et al. uPA modulates the age dependent effect of brain injury on cerebral hemodynamics through LRP and ERK MAPK. J Cereb Blood Flow Metab. 2009;29:524–533. doi: 10.1038/jcbfm.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstead WM, Raghupathi R. Endothelin and the neurovascular unit in pediatric traumatic brain injury. Neurological Research. 2011;33:127–132. doi: 10.1179/016164111X12881719352138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstead WM. Age and cerebral circulation. Pathophysiology. 2005;12:5–15. doi: 10.1016/j.pathophys.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Armstead WM, Vavilala MS. Adrenomedullin reduces gender dependent loss of hypotensive cerebrovasodilation after newborn brain injury through activation of ATP-dependent K channels. J Cereb Blood Flow Metab. 2007;27:1702–1709. doi: 10.1038/sj.jcbfm.9600473. [DOI] [PubMed] [Google Scholar]
- 21.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 22.Armstead WM, Kiessling JW, Riley J, et al. Phenylephrine infusion prevents impairment of ATP and Calcium sensitive K channel mediated cerebrovasodilation after brain injury in female but aggravates impairment in male piglets through modulation of ERK MAPK upregulation. J Neurotrauma. 2011;28:105–111. doi: 10.1089/neu.2010.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstead WM. Superoxide generation links protein kinase C activation to impaired ATP-sensitive K+ channel function after brain injury. Stroke. 1999;30:153–159. doi: 10.1161/01.str.30.1.153. [DOI] [PubMed] [Google Scholar]
- 24.Beaumont A, Hayasaki K, Marmarou A, et al. Contrasting effects of dopamine therapy in experimental brain injury. J Neurotrauma. 2001;18:1359–1372. doi: 10.1089/08977150152725650. [DOI] [PubMed] [Google Scholar]
- 25.Malhotra AK, Schweitzer JB, Fabian TC, et al. Cerebral perfusion pressure directed therapy following traumatic brain injury and hypotension in swine. J Neurotrauma. 2003;20:827–839. doi: 10.1089/089771503322385764. [DOI] [PubMed] [Google Scholar]
- 26.Steiner LA, Johnston AJ, Czosnyka M, et al. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head injured patients. Crit Care Med. 2004;32:1049–1054. doi: 10.1097/01.ccm.0000120054.32845.a6. [DOI] [PubMed] [Google Scholar]
- 27.Ract C, Vigue B. Comparison of the cerebral effects of dopamine and norepinephrine in severely head-injured patients. Intensive Care Med. 2001;27:101–106. doi: 10.1007/s001340000754. [DOI] [PubMed] [Google Scholar]
- 28.Johnston AJ, Steiner LA, Chatfield DA, et al. Effect of cerebral perfusion pressure augmentation with dopamine and norepinephrine on global and focal brain oxygenation after traumatic brain injury. Intensive Care Med. 2004;30:791–797. doi: 10.1007/s00134-003-2155-7. [DOI] [PubMed] [Google Scholar]
- 29.Armstead WM, Nassar T, Akkawi S, et al. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nature Neuroscience. 2006;9:1150–1155. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]




