Abstract
Purpose
Community-acquired pneumonia (CAP) is the most common infection leading to hospitalization in the U.S. The objective of this study was to evaluate management practices for inpatient CAP in relation to IDSA/ATS guidelines to identify opportunities for antibiotic and health care resource stewardship.
Methods
This was a retrospective cohort study of adults hospitalized for CAP at a single institution from April 15, 2008 – May 31, 2009.
Results
Of 209 cases, 166 (79%) were admitted to a medical ward and 43 (21%) to the intensive care unit (ICU). 61 (29%) cases were candidates for outpatient therapy per IDSA/ATS guidance with a CURB-65 score of 0 or 1 and absence of hypoxemia. 110 sputum cultures were ordered; however, an evaluable sample was obtained in 49 (45%) cases, median time from antibiotic initiation to specimen collection was 11 (IQR 6–19) hours, and a potential pathogen was identified in only 18 (16%). Blood cultures were routinely obtained for both non-ICU (81%) and ICU (95%) cases, but 15 of 36 (42%) positive cultures were false-positive results. The most common antibiotic regimen was ceftriaxone plus azithromycin (182, 87% cases). Discordant with IDSA/ATS recommendations, oral step-down therapy consisted of a new antibiotic class in 120 (66%), most commonly levofloxacin (101, 55%). Treatment durations were typically longer than suggested with a median of 10 (IQR 8 – 12) days.
Conclusions
In this cohort of patients hospitalized for CAP, management was frequently inconsistent with IDSA/ATS guideline recommendations revealing potential targets to reduce unnecessary antibiotic and health care resource utilization.
Keywords: pneumonia, community-acquired pneumonia, guidelines, Infectious Diseases Society of America, antimicrobial stewardship, Streptococcus pneumoniae, fluoroquinolones
Introduction
Community-acquired pneumonia (CAP) is the most common infection leading to hospitalization in the United States [1] and accounts for a substantial amount of antibiotic use. Ensuring optimal antibiotic prescribing for common infections is of fundamental importance to antibiotic stewardship efforts aimed at conserving currently licensed agents for as long as possible. Furthermore, attention to effective use of health care resources for common medical conditions such as inpatient CAP may help control rising health care costs while maintaining a high quality of care [2].
Adherence to treatment recommendations in the 2007 Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline for the management of CAP has been associated with reduced mortality and length of hospital stay [3]. In addition, local guidelines and other antimicrobial stewardship interventions have successfully improved the care of patients hospitalized with CAP [4–7]. However, before such interventions can be developed and implemented at a given institution, an in-depth understanding of management practices in relation to national guideline recommendations and quality measures is essential. We previously published an evaluation of antibiotic prescribing practices in cases of outpatient CAP demonstrating frequent discordance with treatment suggested in the 2007 IDSA/ATS guideline [8]. The objective of this study was to perform a systematic and comprehensive assessment of management practices for patients hospitalized with CAP in relation to IDSA/ATS guideline recommendations in order to identify institutional targets for better antibiotic and health care resource stewardship.
Methods
Study setting and population
Denver Health is a vertically-integrated public safety net institution. Patients can access care at multiple sites including a 477-bed teaching hospital, emergency department, urgent care center, community health centers, sub-specialty clinics, and the public health department [9]. These sites are linked by a computerized health information system. Patients hospitalized with CAP are managed in medical wards (non-ICU cases) or an intensive care unit (ICU). Non-ICU cases are managed by either a housestaff teaching service or a hospitalist non-teaching service. The ICU is a closed-management academic model staffed by five critical care physicians.
Study design
We performed a retrospective cohort study of consecutive adults at least 18 years old hospitalized with CAP from April 15, 2008 through May 31, 2009. Eligible patients were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) criteria proposed by the Centers for Medicare and Medicaid Services (CMS) for inpatient pneumonia quality measures [10]. Manual chart review was then performed to identify cases meeting the study definition of CAP. Cases were excluded that did not meet this definition or that involved transfer from another facility, leaving against medical advice, presence of a co-existing infection requiring antibiotic therapy, age >89 years, incorrect ICD-9-CM code, missing records, not principal diagnosis, prisoner status, pregnancy, Pneumocystis jirovecii pneumonia, or mycobacterial infection. For patients with multiple hospitalizations at Denver Health for pneumonia during the study period, only the initial episode was reviewed.
Demographics, selected clinical characteristics, laboratory data, inpatient antibiotic administration, and selected clinical outcomes were extracted electronically via our health care data warehouse. Using a standardized data collection instrument, we performed manual chart abstraction for variables not available through the warehouse and to validate electronically-obtained data (T.J., S.S., L.C.). All clinical encounters occurring within 30 days after hospital discharge were reviewed to determine clinical outcomes. Prior to the study, the three abstractors performed chart review on ten pilot cases; discrepancies were reviewed and reconciled in order to decrease variability in the review process. A sample of abstraction forms was compared with the final database to ensure accuracy of data entry. The study was reviewed by the Colorado Multiple Institutional Review Board and deemed to represent quality-improvement work.
Study definitions
Community-acquired pneumonia was defined as: 1) a new infiltrate by chest radiograph or computed tomography (CT) within 48 hours of hospital admission, 2) signs or symptoms of pneumonia not thought to be due to an alternative etiology, and 3) not hospitalized within 90 days, resident of a nursing facility, dialysis-dependent, or having received parenteral antibiotic therapy, chemotherapy, or wound care within 30 days [11, 12]. Comorbid conditions were defined by provider diagnosis in the medical record. Severity of illness was determined with the CURB-65 score (confusion, uremia, respiratory rate, low blood pressure, age 65 years or greater) as previously described [13]. Prolonged antibiotic therapy was defined as 10 or more days of treatment.
Use of chest CT was classified as early when obtained within 1 day of hospital admission. An expectorated sputum for Gram stain and culture was classified as an adequate sample when microscopy revealed <10 epithelial cells and >25 polymorphonuclear cells per high power field. The time to sputum culture was defined as the interval from the first dose of antibiotic to the first sample collected. Relevant pathogens were considered to be microorganisms known to cause pneumonia cultured from blood or pleural fluid, present in >105 colonies per mL by quantitative bronchoalveolar lavage (BAL) culture, or pure growth from an expectorated sputum or qualitative BAL specimen.
Clinical failure was a composite endpoint of any of the following during the hospitalization or 30-day follow-up period: 1) treatment failure, defined as a change in antibiotic therapy due to worsening signs or symptoms of infection or lack of clinical improvement, 2) in-hospital mortality, 3) recurrence, defined as signs or symptoms of infection after completion of therapy requiring re-initiation of antibiotics, 4) re-hospitalization due to pulmonary infection, or 5) death during the follow-up period.
Data Analysis
Given expected differences in the evaluation, treatment, and outcomes of cases depending on severity of illness, analyses were performed both for the total cohort and stratified by non-ICU cases and ICU cases. Descriptive statistics were used to summarize demographic and clinical characteristics, use of microbiological and imaging studies, antibiotic treatment, and clinical outcomes. We performed multiple logistic regression using stepwise selection to assess factors associated with prolonged antibiotic therapy (≥10 days). To do this, all potential factors were included in a full model for univariate analysis regardless of P value. The Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were subsequently used to evaluate the goodness-of-fit of potential adjusted models. Variables were included in the final adjusted model based on goodness-of-fit, potential confounders, and collinearity with other factors. A P value of <.05 was considered to be statistically significant. We used SAS Version 9.2 (SAS Institute, Cary, NC) for data analysis.
Results
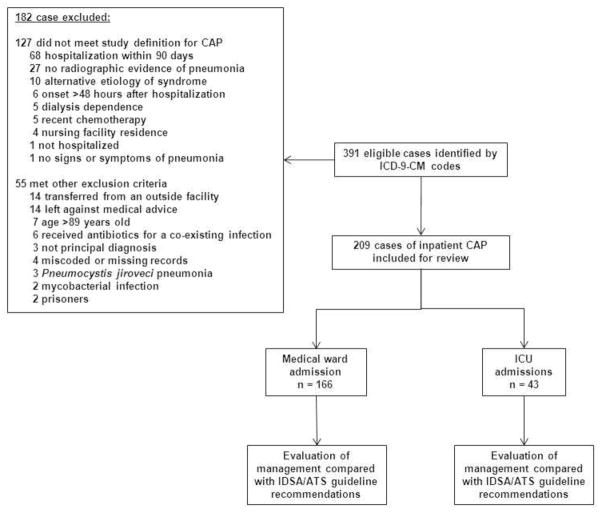
391 eligible cases were identified with the initial ICD-9-CM search. 182 cases were excluded for reasons shown in Figure 1. Of the 209 cases included for analysis, 166 (79%) were non-ICU cases and 43 (21%) were ICU cases. 110 of the non-ICU cases were treated by housestaff teaching services while the other 56 by hospitalist non-teaching services. The most common comorbidities and risk factors for pneumonia included current smoking (108, 52%), alcohol abuse (54, 26%), diabetes mellitus (46, 22%), chronic obstructive pulmonary disease (35, 17%), and asthma (30, 14%) (Table 1). The CURB-65 score was 0 or 1 in the majority of cases (137, 66%); 61 (29%) had a score of 0 or 1 and absence of a new oxygen requirement.
Figure 1.
Study schematic.
Table 1.
Demographics and clinical characteristics
| Non-ICU cases n = 166 | ICU cases n = 43 | Total n = 209 | |
|---|---|---|---|
| Age, mean ± standard deviation | 53 ± 15 | 52 ± 15 | 53 ± 15 |
| Male | 91 (55) | 26 (60) | 117 (56) |
| Foreign-born | 33 (20) | 6 (14) | 39 (19) |
| Comorbid conditions | |||
| Current smoking | 85 (51) | 23 (53) | 108 (52) |
| Alcohol abuse | 41 (25) | 13 (30) | 54 (26) |
| Diabetes mellitus | 31 (19) | 15 (35) | 46 (22) |
| COPD | 30 (18) | 5 (12) | 35 (17) |
| Asthma | 26 (16) | 4 (9) | 30 (14) |
| Prior pneumonia | 24 (14) | 4 (9) | 28 (13) |
| Cardiovascular diseasea | 20 (12) | 6 (14) | 26 (12) |
| HIV infection | 11 (7) | 1 (2) | 12 (6) |
| Cirrhosis | 7 (4) | 3 (7) | 10 (5) |
| Injection drug use | 5 (3) | 3 (7) | 8 (4) |
| Failed outpatient antibiotic therapy | 17 (10) | 0 (0) | 17 (8) |
| CURB-65 score | |||
| 0 | 55 (33) | 4 (9) | 59 (28) |
| 1 | 67 (40) | 11 (26) | 78 (37) |
| 2 | 32 (19) | 12 (28) | 44 (21) |
| 3 | 11 (7) | 11 (26) | 22 (11) |
| 4 | 1 (1) | 5 (12) | 6 (3) |
| New oxygen requirementb | 87 (52) | 30 (70) | 117 (56) |
| CURB-65 score 0 or 1 AND absence of new oxygen requirement | 58 (35) | 3 (7) | 61 (29) |
| Hypotension requiring aggressive | 16 (10) | 20 (47) | 36 (17) |
| fluid resuscitation | |||
| Mechanical ventilation | 2 (1) | 13 (30) | 15 (7) |
| Septic shock | 2 (1) | 13 (30) | 15 (7) |
| Multilobar infiltrate | 68 (41) | 22 (51) | 90 (43) |
| Bacteremia | 9 (5) | 12 (28) | 21 (10) |
Data presented as n (%), unless otherwise noted.
COPD, chronic obstructive pulmonary disease
coronary artery disease or congestive heart failure
pulse oximetry measurement less than 90% on room air or at baseline oxygen requirement for those on chronic supplemental oxygen
Chest CT scan was performed in 99 (47%) cases (Table 2); 59 (60%) of these were pulmonary embolism protocol studies. Most were obtained early in the course of hospitalization (90/99, 91%) and when an infiltrate was present by plain film (54, 55%). Blood cultures were ordered routinely for both non-ICU (81%) and ICU (95%) cases; however, 15 of 36 (42%) positive cultures were false-positive results including coagulase-negative staphylococci (13), bacillus species, not anthracis (1), and enterococcus species (1). Streptococcus pneumoniae was the infecting pathogen in 17 of 21 (81%) cases with true bacteremia. Culture of an expectorated sputum was ordered in 110 (53%) cases; however, an adequate specimen was obtained in only 49 (45%) of such cases. The median time to collection was 11 hours (interquartile range [IQR] 6 – 19), and a pathogen was identified in only 18 (16%). In total, a pathogen was identified by any microbiological test in 36 (17%) of 209 cases, most commonly S. pneumoniae (25, 12%). Microbiological testing was less likely to yield a pathogen in non-ICU (17, 10%) compared with ICU cases (19, 44%) (p<.001).
Table 2.
Use of imaging and microbiological studies
| Non-ICU cases n = 166 | ICU cases n = 43 | Total n = 209 | |
|---|---|---|---|
| Chest radiograph | 163 (98) | 43 (100) | 206 (99) |
| Infiltrate present | 104 (64) | 31 (72) | 135 (66) |
| Possible infiltrate | 40 (25) | 12 (28) | 52 (25) |
| No infiltrate | 19 (12) | 0 | 19 (9) |
| Chest CT | 78 (47) | 21 (49) | 99 (47) |
| Early | 70 (90) | 20 (95) | 90 (91) |
| Late | 8 (10) | 1 (5) | 9 (9) |
| Infiltrate present by radiograph | 39 (50) | 15 (71) | 54 (55) |
| Possible infiltrate by radiograph | 17 (22) | 6 (29) | 23 (23) |
| Blood cultures obtained | 135 (81) | 41 (95) | 176 (84) |
| False-positive culture | 12 (9) | 3 (7) | 15 (9) |
| True bacteremia | 9 (5) | 12 (28) | 21 (12) |
| Sputum culture ordered | 84 (51) | 26 (60) | 110 (53) |
| Adequate sample obtained | 32 (38) | 17 (65) | 49 (45) |
| Time to specimen, median hours (IQR)a | 12 (6–20) | 9 (5–17) | 11 (6–19) |
| Relevant microorganism identified | 9 (11) | 9 (35) | 18 (16) |
| Bronchoalveolar lavage | 7 (4) | 11 (26) | 18 (9) |
| Relevant microorganism identified | 2 (29) | 9 (82) | 11 (61) |
| Pleural fluid | 12 (7) | 6 (14) | 18 (9) |
| Relevant microorganism identified | 2 (17) | 4 (67) | 6 (33) |
| Microorganisms identified | 17 (10) | 19 (44) | 36 (17) |
| Streptococcus pneumoniae | 11 | 14 | 25 |
| Methicillin-susceptible S. aureus | 2 | 1 | 3 |
| Methicillin-resistant S. aureus | 1 | 2 | 3 |
| Neisseria meningiditis | 0 | 1 | 1 |
| Streptococcus milleri and anaerobes | 0 | 1 | 1 |
| Moraxella catarhallis | 1 | 0 | 1 |
| Haemophilus influenzae | 1 | 0 | 1 |
| Mycoplasma pneumoniae | 1 | 0 | 1 |
IQR, interquartile range
time from initial antibiotic dose to specimen collection
The most frequently prescribed antibiotic regimen during the hospital stay was ceftriaxone plus azithromycin, administered in 147 (89%) non-ICU cases and 35 (81%) ICU cases (Table 3). Use of anti-pseudomonal beta-lactam agents and vancomycin was more common in ICU cases (p<.001 for both). In total, 53 (25%) patients received at least one dose of vancomycin (median duration 2 days [IQR 1 – 4]). Of the 182 cases treated with ceftriaxone plus azithromycin during the hospitalization, 120 (66%) were discharged on a new drug class, most commonly levofloxacin (101, 55%). The median duration of therapy was 10 days for both non-ICU (IQR 8 – 14) and ICU (IQR 8 – 12) cases. Therapy was prolonged (≥10 days) in 117 (56%) cases. In multivariate logistic regression, bacteremia was the only factor independently associated with prolonged therapy (odds ratio [OR] 12.9, 95% confidence interval [CI] 1.1 – 157.9) while discharge with azithromycin monotherapy (OR 0.02, 95%CI 0.001 – 0.2) and cardiovascular disease (OR 0.3, 95%CI 0.1 – 0.8) were inversely associated with prolonged therapy (Table 4). A summary of management practices compared with IDSA/ATS guideline recommendations is provided in Table 5.
Table 3.
Antibiotic therapy
| Non-ICU cases n = 166 | ICU cases n = 43 | Total n = 209 | |
|---|---|---|---|
| Inpatient antibiotic therapy, at least 1 dose | |||
| Ceftriaxone plus azithromycin | 147 (89) | 35 (81) | 182 (87) |
| Levofloxacin | 55 (33) | 11 (26) | 66 (32) |
| Vancomycina | 31 (19) | 22 (51) | 53 (25) |
| Anti-pseudomonal beta-lactamb | 23 (14) | 16 (37) | 39 (19) |
| Other beta-lactamc | 12 (7) | 5 (12) | 17 (8) |
| Trimethoprim-sulfamethoxazole | 8 (5) | 3 (7) | 11 (5) |
| Clindamycin | 6 (4) | 4 (9) | 10 (5) |
| Doxycycline | 5 (3) | 2 (5) | 7 (3) |
| Ceftriaxoned | 1 (1) | 5 (12) | 6 (3) |
| Linezolid | 3 (2) | 2 (5) | 5 (2) |
| Azithromycine | 3 (2) | 0 | 3 (1) |
| Aminoglycoside | 0 | 1 (2) | 1 (0.5) |
| Duration inpatient therapy, median days (IQR) | 3 (3–5) | 6 (4–9) | 4 (3–6) |
| Discharge therapy | |||
| Levofloxacin | 99 (60) | 20 (47) | 119 (57) |
| Azithromycin | 29 (17) | 3 (7) | 32 (15) |
| Amoxicillin-clavulanate | 18 (11) | 2 (5) | 20 (10) |
| Doxycycline | 13 (8) | 6 (14) | 19 (9) |
| Oral cephalosporin | 7 (4) | 0 | 7 (3) |
| Other beta-lactam | 2 (1) | 2 (5) | 4 (2) |
| Other | 3 (2) | 1 (2) | 4 (2) |
| Duration outpatient therapy, median days (IQR) | 6 (4–7) | 5 (3–7) | 6 (4–7) |
| Inpatient ceftriaxone plus azithromycin and discharged on new drug class (n = 182) | 97 (66) | 23 (66) | 120 (66) |
| Levofloxacin | 84 (57) | 17 (49) | 101 (55) |
| Doxycycline | 13 (9) | 6 (17) | 19 (10) |
| Total duration therapy, median days (IQR) | 10 (8–14) | 10 (8–12) | 10 (8–12) |
IQR, interquartile range
median (IQR) treatment duration of 2 (1–5) days in non-ICU cases and 2 (1–4) in ICU cases
piperacillin-tazobactam, cefepime, or imipenem-cilastatin
penicillin, amoxicillin, amoxicillin-clavulanate
without azithromycin,
without ceftriaxone
Table 4.
Final multiple logistic regression model of factors associated with prolonged antibiotic therapy (≥10 days)
| Odds Ratio (95% confidence interval) | P | |
|---|---|---|
| COPD | 0.6 (0.3 – 1.3) | 0.19 |
| Cardiovascular diseasea | 0.3 (0.1 – 0.8) | 0.02 |
| Hypotension | 3.0 (0.9 – 9.8) | 0.07 |
| Bacteremia | 12.9 (1.1 – 157.9) | 0.045 |
| Azithromycin monotherapy at discharge | 0.02 (0.001 – 0.2) | 0.001 |
coronary artery disease or congestive heart failure
Table 5.
Summary of management practices in relation to IDSA/ATS guideline recommendations.
| IDSA Recommendation | Study cohort | Assessment | Strategy to improve antibiotic and health care resource utilization |
|---|---|---|---|
| Use severity of illness score (e.g., CURB-65) to assist in decision to admit | 66% had CURB-65 score of 0 or 1; 27% had score of 0 or 1 and absence of hypoxemia | Frequent hospitalization of low-risk patients | Emergency department provider education and implementation of CURB- 65 score as adjunct in site of care decision |
| Perform sputum gram stain and culture only if good-quality specimen can be obtained and processed appropriately; obtain prior to antibiotic therapy | When ordered, adequate sputum sample obtained in 45% of cases and yield of pathogen in 16% Median time to sputum collection 11 hours after antibiotic initiation | Low-yield test due to inadequate samples and time delay | Target use to ICU cases and those with risk of multi-drug resistant pathogen. Engage nursing, respiratory therapy, and microbiology laboratory to improve sample collection, processing, and timing. |
| Obtain pretreatment blood cultures in selected cases | Blood cultures obtained in 81% of non-ICU cases; 12 of 21 (57%) positive results were false-positive | Blood cultures obtained routinely rather than selectively in non-ICU cases with a high proportion of false-positive results | Limit blood cultures in non-ICU to those with risk for multi-drug resistant pathogens. Engage nursing and phlebotomy to reduce blood culture contamination rate. |
| Empirically treat with a respiratory fluoroquinolone or a β-lactam plus macrolide in non-ICU cases | >90% of non-ICU cases treated with guideline-recommended therapy | Excellent adherence to empiric antibiotic selection guidance | Promote ongoing adherence to guideline- concordant empiric therapy |
| For CA-MRSA infection, add vancomycin or linezolid | MRSA cultured from respiratory specimens in 3 cases; 53 (25%) treated with vancomycin | Empiric therapy against MRSA common | Educate providers regarding local incidence of CA-MRSA pneumonia and provide specific guidance on when to consider empiric MRSA therapy |
| When switching to oral antibiotics, either the same agent as the intravenous antibiotic or the same drug class should be used* | 66% of patients initially treated with ceftriaxone and azithromycin discharged on a new drug class, most often a fluoroquinolone | Unnecessary exposure to a third drug class and contribution to fluoroquinolone overuse | Provide institution-specific recommendations for oral step-down therapy consistent with IDSA/ATS guidance |
| Treat until afebrile and clinically stable (5 days minimum). Most patients become clinically stable in 3–7 days, so longer durations rarely necessary | Duration of therapy ≥10 days in 56% of cases | Prolonged treatment durations the norm in both non-ICU and ICU cases | Promote short-course therapy (5–7 days) in uncomplicated, clinically responding cases. |
macrolide alone suggested for those treated with intravenous β-lactam plus macrolide combination
In total, clinical failure occurred in 19 (9%) cases and was somewhat less frequent in non-ICU (7%) compared with ICU (16%) cases (p = .07) (Table 6.). In-hospital failure events (treatment failure or death) tended to be more common than adverse outcomes after discharge (rehospitalization due to pulmonary infection, recurrence, or death within 30 days).
Table 6.
Clinical outcomes
| Non-ICU cases n = 166 | ICU cases n = 43 | Total n = 209 | |
|---|---|---|---|
| Clinical failure | 12 (7) | 7 (16) | 19 (9) |
| Inhospital mortality | 1 (8) | 2 (29) | 3 (16) |
| Treatment failure | 8 (67) | 4 (57) | 12 (63) |
| Recurrence | 3 (25) | 0 | 3 (16) |
| Re-hospitalization due to pulmonary infection | 2 (1) | 1 (2) | 3 (1) |
| Death within 30 days after discharge | 0 | 0 | 0 |
| Floor to ICU transfer after 24 hours | 6 (1) | -- | 6/166 (1) |
| Rehospitalization within 30 days | 10 (6) | 2 (5) | 12 (6) |
| Length of hospital stay, median days (IQR) | 3 (2–4) | 5 (3–9) | 3 (2–5) |
ICU, intensive care unit; IQR, interquartile range
Discussion
Community-acquired pneumonia is the most common infection leading to hospitalization in the United States. In this cohort of inpatients with CAP, local clinical practice differed from IDSA/ATS guideline recommendations with respect to the decision to hospitalize, microbiological testing, oral antibiotic selection, and duration of therapy.
There is a large body of literature describing the diagnosis and treatment of inpatient CAP, and previous studies have identified opportunities and interventions to improve antibiotic prescribing and use of health care resources [4–7, 14–17]. Our study is unique in that it provides a model for a global assessment of management practices in comparison with national guideline recommendations allowing individual hospitals to identify aspects of care that may warrant dedication of resources for quality improvement.
The IDSA/ATS guideline recommends the use of a severity-of-illness score such as CURB-65 to assist in the decision to hospitalize or treat as an outpatient [11]. Patients with a CURB-65 score of 0 or 1 have a low risk of adverse outcomes and are generally recommended for outpatient therapy [13]. This severity of illness indicator was not routinely used in our emergency department during the study period, and the majority of patients hospitalized with CAP had a score or 0 or 1. Moreover, nearly 30% had both a low CURB-65 score and absence of a new oxygen requirement, a marker of severity of illness that the CURB-65 score does not take into account [18]. Although social factors and/or comorbid conditions likely influenced the decision to hospitalize in some cases [19], admission may have been avoided in at least a subset of patients by using the CURB-65 score as an adjunct in the decision-making process.
The observation that CT scans were performed in close to half of cases and nearly always within 1 day of admission is noteworthy, and similar frequent use of chest CT has been described previously [20]. Although the role of CT in the initial evaluation of CAP has not been clearly defined, plain films remain the standard of care for the diagnosis [21]. CT may be useful to evaluate for an alternative etiology when the diagnosis remains in question (e.g., pulmonary embolus) or a specific complication of CAP (e.g., loculated pleural effusion). However, in our cohort, evidence of pneumonia was present by plain film in most cases where a CT was obtained. Furthermore, the early use of CT (most commonly in the emergency department) is suggestive of a use for diagnostic purposes rather than evaluation for complications. This study was not designed to assess the appropriateness of CT use, but given the expense and growing body of evidence of long-term risk of malignancy associated with radiation exposure from CT [22], this is an area that requires further study. In the meantime, efforts should focus on ensuring an appropriate indication for chest CT in the setting of CAP to improve patient safety and control costs.
The utility of blood cultures for patients hospitalized with CAP is controversial [23, 24]. The IDSA/ATS guideline advocates for their use in critically ill patients and those with certain clinical factors or comorbidities. CMS requires blood cultures for ICU cases but has made them optional for non-ICU cases [10]. In our cohort, blood cultures were obtained in the majority of all admissions; the rate of 81% we observed in non-ICU cases was more consistent with routine use rather than targeted use as suggested in the guideline. We demonstrated an unintended consequence of such routine use of blood cultures in the non-ICU setting as 12 of 21 (57%) positive cultures were false-positive results. Since false-positive blood cultures lead to unnecessary antibiotic therapy, increased length of stay, and expense [25, 26], this potential for misinformation is an important consideration in developing local strategies for use of blood cultures in CAP.
The utility of Gram stain and culture of expectorated sputum is also controversial [27]. Sputum cultures were ordered in over half of cases; however, similar to a prior study [17], a sample was not able to be obtained or was not adequate for evaluation the majority of the time. Furthermore, time delays in specimen collection contributed to a particularly low yield of pathogens for this test, especially in non-ICU cases. To summarize, we observed routine use of blood cultures in non-ICU patients, frequent false-positive blood cultures, and poor yield of expectorated sputum cultures due to inadequate samples and time delays. Although we certainly recognize the importance and value of obtaining high-quality microbiological samples for culture in the appropriate clinical setting, our findings reveal several areas where use of these resources could be improved.
We identified 3 common prescribing practices for inpatient CAP that likely contribute to unnecessary antibiotic exposure: 1) frequent use of vancomycin, 2) transition to a new drug class for oral step-down therapy, and 3) use of a longer treatment duration than is likely necessary according to the IDSA/ATS guideline. First, we were surprised to find that one quarter of patients were treated with vancomycin. Although some use was associated with false-positive blood cultures (prior to speciation of coagulase-negative staphylococci), most cases did not involve positive blood cultures suggesting that vancomycin was added due to concern of methicillin-resistant Staphylococcus aureus (MRSA) pneumonia. However, MRSA was identified in only 3 patients (1.4%), a finding consistent with a recent epidemiological study of CAP in the United States [28]. Clinical features associated with MRSA pneumonia described in the study by Moran and colleagues [28] may be useful to provide specific guidance regarding when to empirically cover for MRSA and reduce what appears to be “vancomycin creep” into CAP therapy.
The IDSA/ATS guideline recommends that when converting to oral therapy, either the same agent or the same drug class should be used, and that switching to a different class due to high bioavailability (such as a fluoroquinolone) is generally not indicated. When an intravenous β-lactam/macrolide combination is initially used, step-down therapy with an oral macrolide alone is suggested [11]. Inconsistent with this guidance, we found that two thirds of patients initially treated with ceftriaxone plus azithromycin were transitioned to a new antibiotic class upon discharge, most often a respiratory fluoroquinolone. Given the association between fluoroquinolone exposure and both antimicrobial resistance [29–31] and severe Clostridium difficile infection [32–34], this practice may put patients at unnecessary risk. In addition, since fluoroquinolones have activity against Mycobacterium tuberculosis, their use for CAP has resulted in delayed diagnosis and resistance in cases of tuberculosis [35, 36]. For these reasons, we agree with those who have advocated for more selective use of fluoroquinolones in CAP [35, 37, 38] and believe that preventing the switch to this drug class for oral step-down therapy is an appropriate initial step to decrease use.
A number of studies have demonstrated that treatment courses of fewer than 7 days are effective for inpatient CAP [39]. In one study of patients hospitalized with moderate to severe CAP, of whom 12% were bacteremic, 3 days of therapy for was as efficacious as 8 days [14]. However, we demonstrated that 10 – 14 day courses (or longer) were prescribed in well over half of cases. By multivariate analysis, there was a notable absence of clinical factors positively associated with prolonged treatment (with the exception of bacteremia) reflecting the widespread nature of this prescribing practice. Of note, total and risk-adjusted antibiotic use at our hospital has been among the lowest in studies of academic hospitals [40, 41] suggesting that these prolonged treatment durations are not unique to our institution. At least one other institution has described similar practice [5]. The growing body of evidence regarding the effectiveness of short-course therapy as well as the risk of C. difficile infection and other adverse events associated with longer courses of therapy [32, 42] suggest an important opportunity to reduce antibiotic exposure for CAP while improving patient safety. Currently, we advocate 5-day treatment courses for clinically responding patients. We agree with others who have suggested duration of therapy as a potential future CMS performance measure [5].
This study has several limitations. First, it was performed at a single institution, and the results may not be generalizable. As a U.S hospital, we used the IDSA/ATS guideline for reference which has importance differences with respect to diagnostic and treatment recommendations as compared with European guidance [43]. Second, this was a retrospective study. Clinical failure events occurring after hospital discharge may have been missed. To minimize the potential for reviewer bias associated with this study design, we captured data electronically when possible. Our study was largely descriptive and focused on objective hospital data such as use of diagnostic tests and antibiotics; we therefore doubt reviewer bias had a significant impact on the results. Third, our microbiological data underrepresent organisms such as Mycoplasma, Chlamydia, and Legionella species where testing is not routinely performed and microaerophilic streptococci and anaerobic organisms that can cause pneumonia [44] but are identified as normal oral flora by sputum culture. Conversely, growth of Staphylococcus aureus or gram-negative organisms in sputum culture often represent false-positive results [45]. Fourth, we did not assess the appropriateness of the decision to hospitalize, use of imaging studies or microbiological tests, and antibiotic therapy for individual cases. Therefore, we cannot conclude with certainty that the use of any of these resources was unnecessary. Last, the sample size precluded analysis of factors associated with adverse outcomes.
In summary, a detailed assessment of management practices for inpatient CAP compared with IDSA/ATS guideline recommendations revealed multiple opportunities for antibiotic and health care resource stewardship through reducing hospitalizations of low-risk patients, more appropriate use of microbiological tests, preventing antibiotic class-switching when transitioning to oral therapy, and shortening the duration of therapy. This model of a needs assessment could be used by other hospitals and antimicrobial stewardship programs to identify focus areas for quality improvement. A subsequent intervention involving a local guideline, peer champion advocacy, information technology, and clinician education could reduce antibiotic exposure, decrease health care costs, and improve patient safety.
Acknowledgments
This work was supported by the Department of Patient Safety and Quality, Denver Health Medical Center. Dr. Jenkins was supported by the National Institute of Allergy and Infectious Diseases (1K23AI099082-01A1).
Footnotes
Financial disclosure: Michael Hanley owns stock in Pfizer and GlaxoSmithKline. All other authors, no conflicts.
References
- 1.Hall MJ, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010;(29):1–20. 24. [PubMed] [Google Scholar]
- 2.Bodenheimer T, Fernandez A. High and rising health care costs. Part 4: can costs be controlled while preserving quality? Ann Intern Med. 2005;143(1):26–31. doi: 10.7326/0003-4819-143-1-200507050-00007. [DOI] [PubMed] [Google Scholar]
- 3.Arnold FW, et al. Improving outcomes in elderly patients with community-acquired pneumonia by adhering to national guidelines: Community-Acquired Pneumonia Organization International cohort study results. Archives of Internal Medicine. 2009;169(16):1515–24. doi: 10.1001/archinternmed.2009.265. [DOI] [PubMed] [Google Scholar]
- 4.Carratala J, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Archives of Internal Medicine. 2012;172(12):922–8. doi: 10.1001/archinternmed.2012.1690. [DOI] [PubMed] [Google Scholar]
- 5.Avdic E, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2012;54(11):1581–7. doi: 10.1093/cid/cis242. [DOI] [PubMed] [Google Scholar]
- 6.Mansouri MD, et al. Impact of an antibiotic restriction program on antibiotic utilization in the treatment of community-acquired pneumonia in a Veterans Affairs Medical Center. Infection. 2011;39(1):53–8. doi: 10.1007/s15010-010-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrie TJ, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283(6):749–55. doi: 10.1001/jama.283.6.749. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins TC, et al. Risk factors for drug-resistant Streptococcus pneumoniae and antibiotic prescribing practices in outpatient community-acquired pneumonia. Academic Emergency Medicine: official journal of the Society for Academic Emergency Medicine. 2012;19(6):703–6. doi: 10.1111/j.1553-2712.2012.01365.x. [DOI] [PubMed] [Google Scholar]
- 9.Gabow P, Eisert S, Wright R. Denver Health: a model for the integration of a public hospital and community health centers. Ann Intern Med. 2003;138(2):143–9. doi: 10.7326/0003-4819-138-2-200301210-00016. [DOI] [PubMed] [Google Scholar]
- 10. [accessed September 4, 2012];The Joint Commission Specifications Manual for National Hospital Inpatient Quality Measures. 4.1 Available at: http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx. [Google Scholar]
- 11.Mandell LA, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;4(4 Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 13.Lim WS, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.el Moussaoui R, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332(7554):1355. doi: 10.1136/bmj.332.7554.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe C, et al. Guideline-concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–31. doi: 10.1001/archinternmed.2009.259. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez JA, et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community-acquired pneumonia. Arch Intern Med. 1999;159(20):2449–54. doi: 10.1001/archinte.159.20.2449. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Vazquez E, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med. 2004;164(16):1807–11. doi: 10.1001/archinte.164.16.1807. [DOI] [PubMed] [Google Scholar]
- 18.Sanz F, et al. Hypoxemia adds to the CURB-65 pneumonia severity score in hospitalized patients with mild pneumonia. Respir Care. 2011;56(5):612–8. doi: 10.4187/respcare.00853. [DOI] [PubMed] [Google Scholar]
- 19.Arnold FW, et al. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–4. doi: 10.1378/chest.124.1.121. [DOI] [PubMed] [Google Scholar]
- 20.Beall DP, et al. Utilization of computed tomography in patients hospitalized with community-acquired pneumonia. Md Med J. 1998;47(4):182–7. [PubMed] [Google Scholar]
- 21.Katz DS, Leung AN. Radiology of pneumonia. Clin Chest Med. 1999;20(3):549–62. doi: 10.1016/s0272-5231(05)70235-5. [DOI] [PubMed] [Google Scholar]
- 22.Smith-Bindman R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afshar N, et al. Blood cultures for community-acquired pneumonia: are they worthy of two quality measures? A systematic review. J Hosp Med. 2009;4(2):112–23. doi: 10.1002/jhm.382. [DOI] [PubMed] [Google Scholar]
- 24.Walls RM, Resnick J. The Joint Commission on Accreditation of Healthcare Organizations and Center for Medicare and Medicaid Services community-acquired pneumonia initiative: what went wrong? Ann Emerg Med. 2005;46(5):409–11. doi: 10.1016/j.annemergmed.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Metersky ML, et al. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–7. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 26.Alahmadi YM, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 77(3):233–6. doi: 10.1016/j.jhin.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Plouffe JF, McNally C, File TM., Jr Value of noninvasive studies in community-acquired pneumonia. Infect Dis Clin North Am. 1998;12(3):689–99. ix. doi: 10.1016/s0891-5520(05)70205-1. [DOI] [PubMed] [Google Scholar]
- 28.Moran GJ, et al. Prevalence of methicillin-resistant staphylococcus aureus as an etiology of community-acquired pneumonia. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2012;54(8):1126–33. doi: 10.1093/cid/cis022. [DOI] [PubMed] [Google Scholar]
- 29.Johnson L, et al. Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates. Am J Med. 2008;121(10):876–84. doi: 10.1016/j.amjmed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 30.MacDougall C, et al. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin Infect Dis. 2005;41(4):435–40. doi: 10.1086/432056. [DOI] [PubMed] [Google Scholar]
- 31.Adam HJ, et al. Association between fluoroquinolone usage and a dramatic rise in ciprofloxacin-resistant Streptococcus pneumoniae in Canada, 1997–2006. Int J Antimicrob Agents. 2009;34(1):82–5. doi: 10.1016/j.ijantimicag.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Stevens V, et al. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53(1):42–8. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 33.Kallen AJ, et al. Complete restriction of fluoroquinolone use to control an outbreak of Clostridium difficile infection at a community hospital. Infect Control Hosp Epidemiol. 2009;30(3):264–72. doi: 10.1086/595694. [DOI] [PubMed] [Google Scholar]
- 34.Pepin J, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–60. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 35.Bernardo J, Yew WW. How are we creating fluoroquinolone-resistant tuberculosis? Am J Respir Crit Care Med. 2009;180(4):288–9. doi: 10.1164/rccm.200906-0863ED. [DOI] [PubMed] [Google Scholar]
- 36.Chen TC, et al. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: a systematic review and meta-analysis. Int J Infect Dis. 2011;15(3):e211–6. doi: 10.1016/j.ijid.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Zervos M, et al. Comparative efficacies and tolerabilities of intravenous azithromycin plus ceftriaxone and intravenous levofloxacin with step-down oral therapy for hospitalized patients with moderate to severe community-acquired pneumonia. Treat Respir Med. 2004;3(5):329–36. doi: 10.2165/00151829-200403050-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wong-Beringer A, et al. An antimicrobial stewardship program with a focus on reducing fluoroquinolone overuse. Pharmacotherapy. 2009;29(6):736–43. doi: 10.1592/phco.29.6.736. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52(10):1232–40. doi: 10.1093/cid/cir063. [DOI] [PubMed] [Google Scholar]
- 40.Pakyz AL, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168(20):2254–60. doi: 10.1001/archinte.168.20.2254. [DOI] [PubMed] [Google Scholar]
- 41.Polk RE, et al. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2011;53(11):1100–10. doi: 10.1093/cid/cir672. [DOI] [PubMed] [Google Scholar]
- 42.Norrby SR. Short-term treatment of uncomplicated lower urinary tract infections in women. Rev Infect Dis. 1990;12(3):458–67. doi: 10.1093/clinids/12.3.458. [DOI] [PubMed] [Google Scholar]
- 43.Woodhead M, et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17(Suppl 6):E1–59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartlett JG, Rosenblatt JE, Finegold SM. Percutaneous transtracheal aspiration in the diagnosis of anaerobic pulmonary infection. Ann Intern Med. 1973;79(4):535–40. doi: 10.7326/0003-4819-79-4-535. [DOI] [PubMed] [Google Scholar]
- 45.Ries K, Levison ME, Kaye D. Transtracheal aspiration in pulmonary infection. Arch Intern Med. 1974;133(3):453–8. [PubMed] [Google Scholar]