Abstract
Throughout their lives all cells constantly experience and respond to various mechanical forces. These frequently originate externally but can also arise internally as a result of the contractile actin cytoskeleton. Mechanical forces trigger multiple signaling pathways. Several converge and result in the activation of the GTPase RhoA. In this review we focus on the pathways by which mechanical force leads to RhoA regulation, especially when force is transmitted via cell adhesion molecules that mediate either cell-matrix or cell-cell interactions. We discuss both the upstream signaling events that lead to activation of RhoA, as well as the downstream consequences of this pathway. These include not only cytoskeletal reorganization and, in a positive feedback loop, increased myosin-generated contraction, but also profound effects on gene expression and differentiation.
Introduction
All cells are exposed to mechanical forces and to a greater or lesser degree respond to these forces. In the vertebrate body, cells experience different types of force according to their tissue location. For example, endothelial cells lining blood vessels, as well as epithelial cells lining certain ducts or cavities, experience mechanical force from the passage of fluid over the cell surface. Cells in the skeletal system (bone and cartilage) but also many other cells are exposed to compression. Throughout most tissues, cells experience varying degrees of tension, which can arise from external forces or from within the cell as a result of actomyosin contractility. It is important to note, however, that the very high tensional forces experienced by some tissues, such as tendons and ligaments, are usually transmitted by extracellular matrix (ECM) components such as collagen fibers and the cells within these tissues are shielded from the tension by the ECM (1). Some forces on cells may be cyclical as experienced by cells in contact with the blood circulation, or as a result of rhythmic activities such as breathing or walking, whereas other cells experience sustained force for varying periods of time.
Experiments exploring how cells respond to different types of mechanical force go back a long way. For example, in early experiments stretching cells was shown to stimulate their proliferation (2). Stretching of myotube cultures induced responses equivalent to muscle hypertrophy (3). The growth cones of elongating neurites were found to exert mechanical force (4) and to respond to externally applied forces (5). Similarly, fibroblasts and other cells were observed to generate tractional forces on the underlying substratum(6) and to be able to harness these forces to orient collagen fibers (7). Application of mechanical tension to migrating cells in culture using a microneedle inhibited extension perpendicular to the axis of tension but allowed or even promoted extension that was parallel with the force (8).
Although research in the field of mechanotransduction has been active for many years, much of it was focused on systems, tissues and cells that are very overtly affected by mechanical stimuli, such as vascular endothelial cells and vascular smooth muscle exposed to flow and/or stretch, or osteoblasts that experience compressive forces. However, during the past decade there has been an explosion of interest in the more universal responses of cells to mechanical forces and progress is occurring rapidly. Whether the forces are applied exogenously on cells or are generated endogenously, they are usually transmitted to the ECM or to neighboring cells via cell adhesion molecules. Consequently, considerable interest has been directed at understanding the signaling pathways that are initiated in response to mechanical forces that are applied to adhesion molecules (9). Multiple signaling pathways have been identified, including tyrosine kinases, ion channels and GTPases (10). One of the pathways that appears to be involved in many cells responding to mechanical force involves activation of Rho family GTPases, particularly RhoA. In this review we will focus primarily on the signaling pathways that lead to activation of RhoA in response to mechanical force and we will discuss the consequences of this pathway. The reader is directed to recent comprehensive reviews for information about mechanotransduction in various contexts (11–16).
The Rho pathway
In contrast to most plant cells that have rigid cell walls, the mechanical properties of animal cells are critically dependent on their cytoskeletons, consisting of microtubules, actin microfilaments, various types of intermediate filaments and also septins (17). All of these filament systems may contribute to the mechanical properties of animal cells, although with respect to how cells respond to exogenously applied forces most attention has been directed toward the actin cytoskeleton. When actin filaments are highly crosslinked they can give rise to a relatively rigid cell cortex. However, this can be rapidly remodeled to allow cell protrusion and changes in cell shape. The polymerization of actin filaments drives many types of cell extension. In conjunction with myosin, actin filaments can generate contractile forces, exerting traction on the surrounding matrix or on other cells and contributing to major changes in cell morphology. The interaction of myosin with actin not only contributes to the response of cells to exogenously applied forces but is responsible for generating endogenous forces within cells.
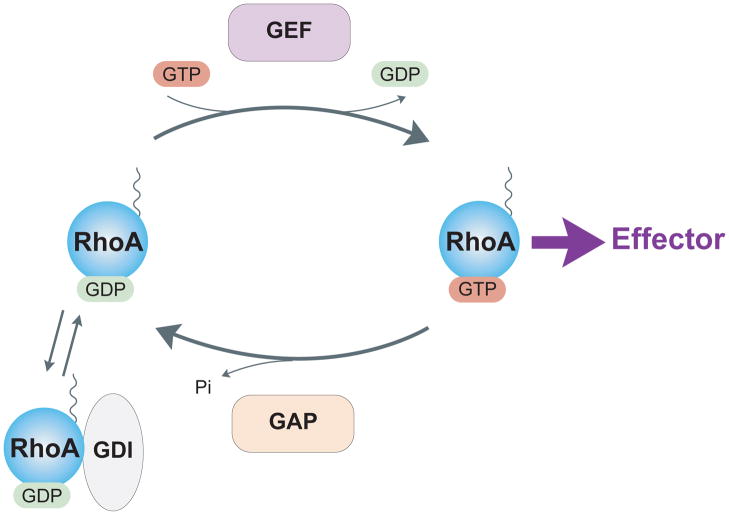
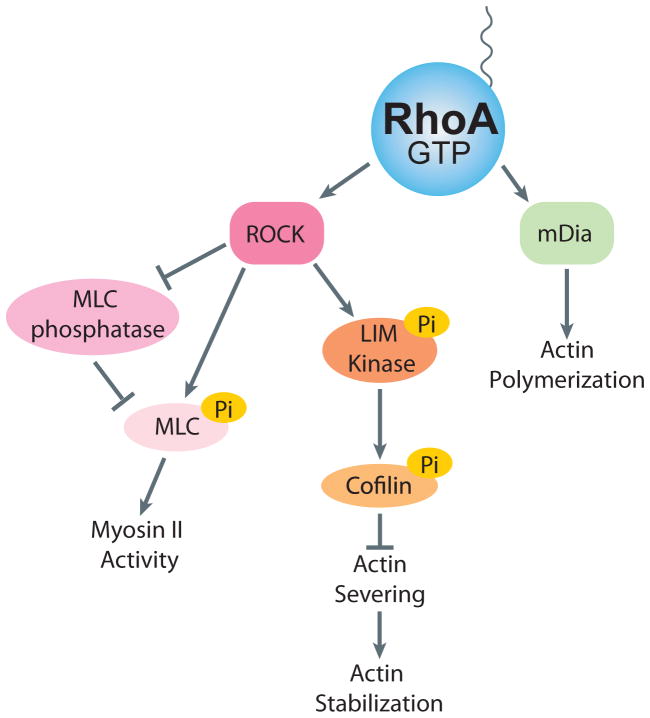
The Rho family of GTPases are key regulators of the actin cytoskeleton. The mammalian genome encodes approximately 20 Rho GTPases, although the three ubiquitous ones, RhoA, Rac1 and Cdc42, are the most studied and each has distinct effects on the actin cytoskeleton(18, 19). In the context of mechanotransduction, most effort has been directed at determining the role of RhoA, which is the focus of this review. In large part, this reflects the fact that RhoA regulates the activity of myosin II and consequently is responsible for much of the intracellular tension and force that is generated within cells (20). RhoA cycles between an inactive GDP state and an active GTP state (Figure 1). Three classes of proteins regulate this cycle: guanine nucleotide-exchange factors (GEFs), GTPase-activating proteins (GAPs) and guanine nucleotide-dissociation inhibitors (GDIs)(21). GEFs activate Rho proteins by catalyzing the exchange of GDP for GTP(22) and GAPs stimulate the intrinsic GTPase activity, leading to the return to the inactive state(23). The inactive pool of RhoA is maintained in the cytosol by association with GDI(24) and it is in the active GTP-bound conformation that RhoA interacts with its effectors and performs its functions (Figure 2). With respect to regulating the activity of myosin II, the critical effector is Rho kinase (ROCK), which exists in two isoforms, ROCK1 and ROCK2. Both isoforms promote myosin II activity by elevating the phosphorylation of the regulatory myosin light chain (MLC). This occurs both directly by phosphorylation of the regulatory MLC (25) and indirectly by phosphorylation and consequent inhibition of the MLC phosphatase (26). The phosphorylation of the MLC promotes assembly of myosin II into bipolar filaments and enhances the ATPase activity of myosin II. Together these effects increase the contractile force generated by myosin II on actin filaments. ROCK also phosphorylates and activates another kinase, LIM kinase, which in turn phosphorylates and inhibits the actin-severing protein cofilin (27). By inhibiting cofilin’s actin severing activity, this increases the stability of actin filaments. Active RhoA also promotes actin filament polymerization. This occurs by RhoA binding a different effector, mDia1, which is member of the formin family of actin nucleating factors (28) (Figure 2).
Figure 1. RhoA cycle.
Like most G proteins, RhoA cycles between an inactive GDP-bound form and an active GTP-bound form. Activation is mediated by guanine nucleotide exchange factors (GEFs) that catalyze exchange of GDP for GTP. GTPase activating proteins (GAPs) inactivate RhoA by stimulating intrinsic GTPase activity. GDI sequesters inactive GDP-bound RhoA in the cytoplasm.
Figure 2. RhoA Effector Signaling.
Activated RhoA interacts with effector proteins to lead to actomyosin contracity and actin stablization. ROCK signals by MLC phosphorylation to increase myosin II activity and LIM Kinase to increase actin stabilization. mDia nucleates actin polymerization.
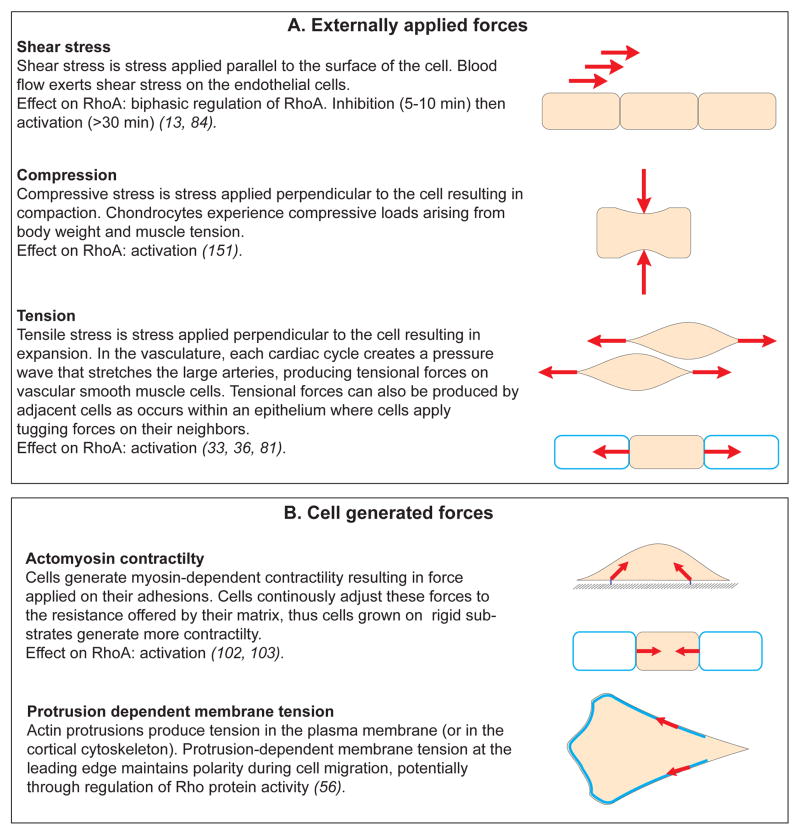
In the context of mechanical signals, one can distinguish two types of forces experienced by cells: (1) forces which are externally applied to the cells, such as the shear stress exerted by blood flow on the surface of endothelial cells, or (2) forces which are generated by the cell itself with its cytoskeleton(29, 30) (Figure 3). Despite the apparent differences between these two signals, applied forces and cell-generated forces share some similarities in their transduction modalities and seem to regulate the same molecular mechanisms (14, 30). In both cases, cell surface adhesions, cytoskeleton and membrane tension cooperate to transmit forces which eventually affect the conformation of “mechanosensors” and trigger the mechanoresponse(10, 14). Interestingly, numerous GEFs and GAPs are known to associate with cytoskeletal and cell adhesion components, suggesting that mechanical forces can directly affect the activity or the localization of RhoA regulators.
Figure 3. Mechanical forces in cell biology.
Diagram summarizing the different types of force that cells can experience. These can be externally applied (A) or generated by the cell itself and its own cytoskeleton (B). The effect on RhoA activity is indicated for each example. Force is a vector with magnitude and direction that causes an object with mass to change its velocity (SI unit Newton). Stress: force per unit of area (SI unit Pascal).
GEFs
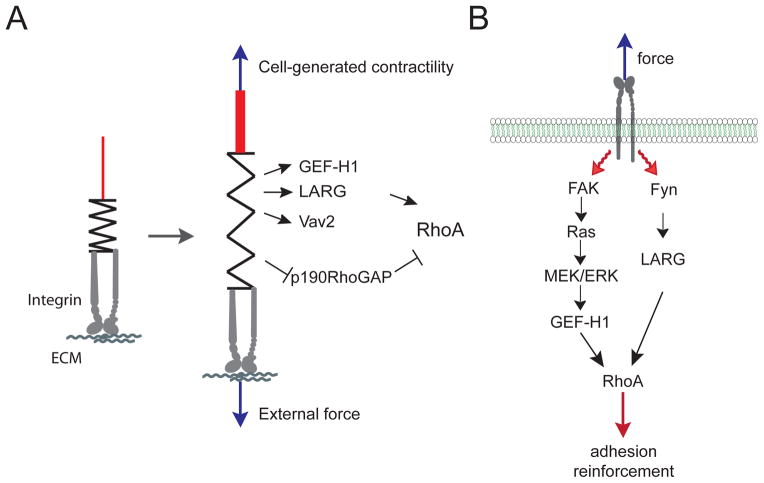
Some GEFs specific for RhoA have been found to associate with the cytoskeleton and adhesions (Table 1). Integrin-based adhesions constitute a major site of mechanotransduction (31) and experience very diverse types of forces. For example, they are subjected to tensional forces when the ECM is stretched or when cells are grown on rigid substrates and generate more myosin-dependent contractility (Figure 3). Therefore, it’s not surprising that the GEFs associated with Cell-ECM adhesions are involved in the mechanoresponse (Figure 4A). Among them, vav2 was reported to be phosphorylated and activated in response to cyclic stretch in mesangial cells (32). Vav2 phosphorylation required EGFR transactivation. Depletion of vav2, as well as EGFR inhibition, prevented stretch-induced RhoA activation (32). Applying tensional forces on fibronectin-coated beads bound to fibroblasts, our group recently showed that force on integrins activates RhoA through two GEFs, GEF-H1 and LARG(33) (Figure 4B). We observed that mechanical forces induce the recruitment of GEF-H1 and LARG to the adhesions. We found that Fyn regulates LARG activity, whereas GEF-H1 is activated by a FAK/Ras/ERK signaling pathway. Consistent with these findings, Waterman and colleagues observed that myosin-dependent contractility promotes GEF-H1 recruitment to Cell-ECM adhesions (34). Interestingly, another group found that GEF-H1 is more active when epithelial cells are grown on rigid substrates (35). This suggests that both externally applied forces and cell-generated forces activate the same GEFs, reinforcing the idea that these two distinct mechanical signals trigger the same signaling pathways. However, on rigid substrates, Heck and colleagues observed that microtubule stability, and not the Ras/ERK pathway, regulates GEF-H1 activity (35). This apparent discrepancy could be due to the difference of cell types that were used in these studies. Indeed, working with fibroblasts another group observed that RhoA activation in response to stretch was not affected by taxol-induced microtubule stabilization (36), whereas in endothelial cells RhoA activation in response to stretch requires GEF-H1 and is prevented by taxol (37).
Table 1.
RhoA GEFs and GAPs which associate with the cytoskeleton or adhesions.
| GEFs | localization | references |
|---|---|---|
| p115 (ArhGEF1) | Cell-ECM adhesion | (33, 108) |
|
| ||
| GEF-H1 (ArhGEF2) | Cell-ECM adhesion | (33, 34) |
| Cell-Cell adhesion | (41, 42) | |
| microtubule | (145) | |
|
| ||
| LARG (ArhGEF12) | Cell-ECM adhesion | (33, 108) |
|
| ||
| Vav | Cell-ECM adhesion | (146) |
|
| ||
| p190RhoGEF | Cell-ECM adhesion | (109) |
| microtubule | (109) | |
|
| ||
| p114RhoGEF (ArhGEF18) | Cell-Cell adhesion | (43) |
|
| ||
| Trio | Intermediate filaments | (147) |
|
| ||
| PDZRhoGEF (ArhGEF11) | Cell-ECM adhesion | (148) |
|
| ||
| GAPs | ||
|
| ||
| p190RhoGAP | Cell-ECM adhesion | (149) |
|
| ||
| DLC1 | Cell-ECM adhesion | (44) |
| Cell-Cell adhesion | (150) | |
|
| ||
| Myo-IXA | Cell-Cell adhesion | (54) |
(151)
Figure 4. RhoA GEFs and GAPs regulated by force.
A. Schematic diagram showing the GEFs and GAPs whose activities are regulated by external force or cell-generated tension on Cell-ECM adhesion.
B. Diagram showing the signaling pathways that regulate GEF-H1 and LARG activity in response to force on integrin (33).
Cell-cell adhesions are also subjected to tensional forces which are generated by neighboring cells or by the cell’s own contractile machinery (Figure 3). It is now clear that tugging forces play an important role in intercellular junction maturation and growth (see below) (38–40). Interestingly, some RhoA GEFs have been found to localize at intercellular adhesions. GEF-H1 associates with cingulin at tight junctions (41, 42), however this interaction was reported to inhibit GEF-H1 and RhoA. More recently, p114RhoGEF was shown to localize at tight junctions and to activate RhoA locally, leading to junction assembly (43). Since mechanical tension induces junction maturation (38), it would be interesting to determine if p114RhoGEF activity is regulated by tugging forces.
GAPs
Mirroring the GEFs, some RhoA GAPs localize at adhesions (table 1) and play a role during the mechanoresponse. DLC1 (44–47) and p190RhoGAP (48–51) associate with Cell-ECM adhesion components. Shear stress regulates p190RhoGAP activity in a biphasic pattern in endothelial cells (52). It was found that short term application of shear stress (<5 min) activates p190RhoGAP through Src Family Kinase-mediated phosphorylation, but longer application of shear stress (>30 min) induces p190RhoGAP dephosphorylation and inactivation. This biphasic regulation of p190RhoGAP leads first to RhoA inactivation followed by activation, similar to what has been observed during adhesion to matrix. Interestingly, p190RhoGAP is necessary for stress fiber alignment in response to shear stress (52). In addition, p190RhoGAP was shown to be necessary for the regulation of two transcription factors, GATAII and TFII-I, in response to increased matrix rigidity in a model of capillary tube formation (53). This suggests that cell-generated contractility may affect p190RhoGAP activity, although this remains to be determined. More recently, Myo-IXA, a single headed myosin with a GAP domain, has been shown to associate with actin at cell-cell junctions, locally restraining RhoA activity to allow proper junction formation (54). It would be interesting to analyze if application of tensional force on intercellular junctions affects Myo-IXA activity or localization.
Other Rho proteins
There is extensive crosstalk between RhoA and Rac1 that contributes to processes such as cell migration (55). In the context of mechanotransduction several pathways have been identified, particularly where high RhoA activity and mechanical tension may depress Rac1 activity. During polarized migration it is important to confine protrusion to the front of the migrating cell and to suppress inappropriate protrusions at other sites on a cell’s periphery. Recent studies have implicated membrane tension generated during cell migration in the suppression of Rac1 activity at sites away from the leading edge and in the maintenance of cell polarity during migration (56). Several potential pathways are suggested by previous work. In migrating leukocytes, high RhoA/ROCK activity was shown to restrict membrane protrusion to the leading edge, in part via the LIM kinase pathway inhibiting cofilin and thereby stabilizing actin filaments at the cell periphery away from the cell front (57). In other work, inhibiting the Rac GAP FilGAP was found to increase membrane protrusions around the periphery of cells (58) suggesting that this Rac GAP confines protrusion to the cell front. Interestingly, FilGAP activity was activated by ROCK-mediated phosphorylation, providing a mechanism by which high RhoA activity can inhibit Rac1 activity. In a subsequent study using a reconstituted actin gel with several purified proteins, it was shown that FilGAP dissociates from filamin A in response to mechanical tension (59). When released it was suggested to relocate to the plasma membrane where it can act to inhibit Rac1 activity. The related Rac GAP, ArhGAP22, is activated in cells by endogenous mechanical force to depress Rac1 activity (60). Blocking myosin activity, either directly with blebbistatin or indirectly by inhibiting ROCK activity, decreased ArhGAP22 activity (60). The Rac GEF βPIX binds to Myosin II and actomyosin contractility induces βPIX dissociation from cell substrate adhesions (34). This contributes to Rac1 inhibition during adhesion maturation. It seems likely that all of these mechanisms may synergize to confine Rac1 activity and membrane protrusion to the leading edge of migrating cells and away from regions of high mechanical tension and RhoA/ROCK activity. As a consequence of the competitive binding to RhoGDI, increasing the binding affinity of one Rho protein leads to the release and degradation and/or activation of other Rho proteins (61). Interestingly, actomyosin contractility induces GDI dissociation from cell-matrix adhesions(34). However, the mechanism of GDI recruitment to adhesions is not known.
Experimentally manipulating force
Before considering some of ways that force can be applied to cells, it is useful to consider some of the forces that cells can exert and experience. The force exerted by a single myosin motor is between 1 and 8 pN (62–64). (1 Newton = 105 dynes. Dynes were used for many of the more classical measurements of force, but today Newtons are the unit of force generally used.) The maximum tension developed by striated muscle has been calculated to be ~ 3 × 106 dynes/cm2 (= ~300 nN/μm2), which translates to ~3 × 10−5 dynes per thin filament (i.e. ~300 pN per thin filament) (65). For cells in culture, various forms of traction force microscopy have been used to measure the tension that they generate on their adhesions and the substratum. Here we will consider just a few of the values that have been obtained. In some of the first experiments investigating the force generated on the substratum by cultured cells, Harris and colleagues calculated an approximate value of 10 nN per μm of cell length (6). Lee and coworkers concluded that the maximum force generated by fish keratocytes was ~20 nN (66). Using a cantilever device, Galbraith and Sheetz obtained a force of 0.2 to 4 nN/μm2 for migrating fibroblasts (67). Geiger’s lab examined the tension developed by focal adhesions and found that the stress was proportional to the size of the focal adhesion with a value of about 5.5 nN/μm2 (68, 69). In general, the area of a focal adhesion relates to the diameter of the stress fiber attached to it. Consequently, because the number of force-generating myosin molecules will relate to the diameter of a stress fiber, intuitively one might expect there to be a constant ratio between the size of a focal adhesion and the force that is being transmitted through it to the substratum. However, an unexpected discovery was made by Beningo et al. who found that in migrating cells, more force was transmitted to the substratum by small nascent adhesions at the leading edge of cells than in larger more mature focal adhesions behind the leading edge (70). A possible resolution to the apparent discrepancy between these two sets of results comes from the work of Chen’s lab, who have studied traction generated by cells plated on deformable micro-posts (micro-needles) (71). Like the Geiger lab they found that for most adhesions there was a correlation between the size of a focal adhesion and the stress exerted at the adhesion. Indeed, they found a similar value of ~4–5 nN/μm2. However, in their work they also found a subset of smaller adhesions less that 1 μm2 that generated high levels of stress that did not correlate with the size of the adhesion (71). These latter adhesions most likely relate to the adhesions studied by Beningo et al. at the leading edge of migrating cells (70).
Manipulations of the ECM and flexible substrata can be used to mimic the tensional forces cells experience in the body. Simply plating cells on more rigid rather than on more compliant substrata increases the tension generated by cells on their underlying matrix due to increased RhoA activity (see discussion below) (72). Various devices have been developed that allow investigators to stretch cells by stretching the substratum to which the cells are adhering. The development of culture dishes with a flexible base that can be stretched by applying a vacuum facilitated subjecting cells to periods of cyclic stretch (73, 74). The period of the stretch as well as the degree of stretch imposed on cells can be readily varied and a large literature now exists describing many signaling pathways that become activated in response to cyclic stretch. Tension has also been applied to individual cells using glass rods or needles (8, 75). With these it is often more difficult to know the precise force that is being applied to cells, although the amount of force required to bend a needle by a certain angle can be determined experimentally.
Stretching or deforming a cell via a flexible substratum or by a glass rod or needle simultaneously affects many properties, including cell shape, the cytoskeleton, as well as a cell’s adhesion to the matrix and/or its neighbors. In order to examine the effects of tension on specific adhesion molecules different approaches have been developed taking advantage of optical (laser) tweezers or magnetic tweezers to exert forces on beads that are attached to cells via specific ligands or antibodies. Wang and colleagues used ferromagnetic beads that were attached to cells via integrin ligands (76). The beads were magnetized in one direction and then a second magnetic field was applied at 90° inducing the beads to twist and exert a shear force. This allowed them to show that there was a stiffening response as force was applied and that this depended on the cytoskeleton (76). Sheetz’s group used optical tweezers to manipulate beads similarly coated with integrin ligands or antibodies (77). They used the optical tweezers to restrain individual beads against the force exerted by the cell. It was observed that cells sensed the restraining force and strengthened the cytoskeletal linkages to oppose this. One advantage of optical tweezers is that beads can be individually manipulated with great precision, allowing them to be placed at different points on a cell’s surface and to be moved in different directions. Optical tweezers can generate forces up to about 500 pN, but in the higher range of forces heat generated by the laser can be detrimental and limit the use of this approach. Whereas an advantage is the ease of examining single cell responses, optical tweezers are not suitable for bulk biochemical analyses of signaling pathways.
Ingber and his group used an electromagnetic microneedle to apply force on magnetic beads coated with adhesion molecule ligands or antibodies (78, 79). With this system it is easy to apply predetermined pulses of force on beads by turning the current on for defined periods. The time between the magnet being on can also be varied so that the behavior of cells responding to the cessation of force can also be examined. The magnitude of the force generated by magnetic tweezers can be easily varied by altering the magnetic field and bead size, resulting in forces ranging from 1 pN to 100 nN (78). This wide range of force that can be generated is a potential advantage of the technique. However, the application of force is unidirectional and the position of the beads relative to the cell surface is essentially random, reflecting where the beads have dropped. However, a significant advantage of using magnetic beads to generate force on cells is that tension can be applied to all the cells in a dish provided that sufficient beads are added and a permanent magnet is used (80). This facilitates biochemical analysis of signaling pathways induced by sustained force (33, 81). The forces generated on cells using magnetic beads and permanent magnets have been discussed in detail elsewhere (80, 82). As an example, studying the application of collagen-coated 3 μm magnetic beads to fibroblasts growing in a 60 mm dish and using a permanent ceramic magnet 2 cm above the dish, Zhao et al. calculated that they exerted 480 pN per cell or 0.65 pN/μm2 (81).
A large body of work has examined the effects of flow and shear force particularly on endothelial cells. Because of their location lining blood vessels, these are exposed and respond to blood flow throughout their existence. Hemodynamic forces vary over a wide range within the vasculature, but most work has focused on the arterial system because the high flow within arteries is critical not only to their normal physiology but also is a major factor in the pathological development of atherosclerosis. Endothelial cells experience force perpendicular to the endothelium as a result of blood pressure and force parallel to the blood vessel wall as a result of flow. The frictional force of blood flow generates shear stress that acts at the surface of endothelial cells (83). This has pronounced effects on endothelial behavior (13, 15, 83–85). Straight regions of arteries result in laminar flow but this becomes disturbed when a vessel curves, bifurcates or branches. The mean wall shear stress of large arteries has been determined to be between 20 and 40 dynes/cm2 (83, 86), but much higher values (exceeding 100 dynes/cm2) have been recorded transiently at the peak of pulsatile flow resulting from the heart beat (87). Turbulent flow results in shear stress experienced by the endothelium that has been calculated to vary from negative values through zero to levels of between 40 to 50 dynes/cm2 (87). Several devices have been developed to allow the effects of flow and shear stress to be examined on cells in culture. These include the cone plate viscometer, in which flow is generated by the rotation of a cone above cells growing in a culture dish (88, 89). The shear stress and whether flow is laminar or turbulent are determined by the angle of the cone, the viscosity of the medium and speed of rotation. Parallel plate flow chambers are frequently used to study the effects of flow on cells. In these, fluid is pumped between two glass sheets, on one of which the cells of interest have been cultured (90, 91). Cells can also be grown in capillary tubes through which fluid is similarly pumped at levels determined by the investigator to mimic the desired shear forces (92, 93). With both parallel plate chambers and capillary tubes, turbulent flow can be generated by reversing the direction of flow or by stopping and starting flow. Shear stress values can be generated that cover the full range experienced by arterial endothelial cells in vivo (83, 86).
Not only can exogenously applied force be experimentally modulated, but the endogenous forces generated by actomyosin contractility within cells can also be controlled by the investigator. This can be achieved by directly affecting myosin activity or by modifying upstream signaling pathways. Myosin ATPase activity can be inhibited by the drug blebbistatin, which has become a valuable tool for cell biologists interested in decreasing endogenous tension (94). The major limitation using this drug is that it is photo-sensitive and therefore cannot easily be used with live cell imaging. Alternatively, the expression of myosin II isoforms (usually myosin IIA or IIB) can be knocked down using siRNA techniques. Given the key role of RhoA and ROCK in regulating myosin activity in cells, contractility is often manipulated by inhibiting the RhoA/ROCK signaling pathway. Direct inhibition of RhoA is achieved using treatment with the Botulinum exotransferase C3 which ADP-ribosylates RhoA. Several ROCK inhibitors have been developed, but the most frequently used experimentally is Y27632 (95). The disadvantage of perturbing the RhoA/ROCK pathway is that contractility is only one of many downstream signaling events that is affected, often making interpretation of results difficult. Stimulating contractility can be induced in several ways. Expression of constitutively active RhoA drives activation of the ROCK pathway and elevates myosin activity, but again there will be many other effects. The level of MLC phosphorylation can also be enhanced by inhibiting phosphatase activity pharmacologically, for example with calyculin A. This potently stimulates contractility (96), but here too there will be many side effects. The phosphorylation state of the regulatory MLC can also be mimicked by expression of mutant MLCs in which one or both of the critical phosphorylatable residues (threonine18 and serine19) are mutated to aspartic acid. These generate constitutively active forms and have been used in several studies (see for example (97)). The difficulty with these mutants is that the dynamic nature of regulation by phosphorylation is blocked because the myosin molecules are locked into a single activated state.
Rigid substrata, stress fibers and focal adhesions
On substrata of different compliance, cells exhibit strikingly different behaviors. Compared with when they are cultured on more rigid surfaces, on more compliant substrates fibroblasts are less able to develop stress fibers and focal adhesions but migrate more rapidly (98). Culturing cells on substrates of different compliance can also have profound effects on gene expression (99, 100). The behavior of cells on relatively soft substrata relates to the general observation that in tissue culture many cells develop stress fibers and focal adhesions, although the same cells within their host tissues rarely develop these structures (101). What is it about tissue culture and rigid substrata that promote the formation of these structures that often dominate a cell’s cytoskeletal appearance? In tissue culture, frequently one factor is the presence of agents in serum such as LPA and S1P that activate RhoA (18). These derive from platelet secretions during blood clot formation. In wound healing they probably contribute to the contraction of cells surrounding a wound site. Notably, tissue culture has often been likened to a wound response. However, even in the absence of serum and these factors, many fibroblasts develop stress fibers and focal adhesions when plated on rigid substrata coated with matrix proteins. Conversely, even in the presence of serum, cells adhering to soft substrata are unable to assemble these structures (98). The rigidity of the substratum is a second factor contributing to the development of focal adhesions and stress fibers. On rigid substrata cells such as fibroblasts generate strong tractional forces to the matrix components adsorbed to the surface of the culture dish or cover glass. The resulting isometric tension was suggested many years ago as a factor in the development of these structures (29). Subsequent work has shown that culturing cells on rigid surfaces elevates RhoA activity (102, 103). The importance of tension in the development of these structures is supported by a large body of evidence, including numerous experiments showing that inhibiting the RhoA/ROCK pathway or myosin activity blocks the development of stress fibers and focal adhesions, and leads to the disassembly of these structures if they have already formed (18, 20, 104–106). The development of stress fibers and focal adhesions on rigid substrata is the quintessential example of endogenously generated tension affecting the organization of the cytoskeleton and cell behavior. Synergy between endogenously generated tension and tension applied exogenously promoting the assembly of these structures was elegantly demonstrated by Riveline and coworkers who showed that applying tension on cells adhering to rigid substrata promoted the growth of focal adhesions (75).
In addition to endogenous tension contributing to the assembly of focal adhesions and stress fibers, a major contribution to the activation of RhoA derives from integrin engagement with the ECM. This is a complex biphasic response, in which integrin-mediated adhesion initially depresses and then elevates RhoA activity (49, 107). The RhoA GEFs, p115/Lsc, LARG and p190RhoGEF were all shown to be activated upon adhesion to fibronectin (108, 109). Both the engagement of integrins and the mechanical tension exerted on these adhesion molecules leads to the activation of RhoA (33, 108, 109).
ECM Compliance and gene expression
It has been known for a long time that the differentiated phenotype of many cells is often lost when they are grown on rigid plastic substrates as opposed to being cultured on more appropriate ECM proteins. This is particularly true when the growth and differentiation characteristics of epithelial cells are compared between cultures growing on plastic or on ECM components that recapitulate many of the characteristics of basement membrane (110). Many studies revealed that the expression of differentiated genes depends not only on the presence of appropriate growth factors but also on an appropriate ECM. For example, the morphology and gene expression exhibited by breast epithelial cells were profoundly influenced not only by the composition of the matrix but also its physical state. Thus it was shown early on that culturing breast epithelial cells on floating collagen gels, which are compliant, compared with collagen gels anchored to rigid culture dishes affected the expression of specific genes (111).
With hindsight, many of the effects of matrix rigidity or cell shape on the differentiated phenotype can be understood in the context of RhoA/ROCK signaling. Numerous studies have led to the conclusion that the level of RhoA activity affects differentiation and gene expression (112, 113). For example, Sordella and coworkers studying the phenotype of the p190-B RhoGAP null mouse discovered that mice lacking this major negative regulator of RhoA activity, not only had elevated RhoA activity, but were defective in adipogenesis and had enhanced myogenesis. They concluded that there was a Rho-dependent switch that regulated stem cells to differentiate in a myoblast direction under conditions of high RhoA activity but to differentiate into adipocytes under low RhoA activity (114). This work was extended by others. For example, McBeath and colleagues using human mesenchymal stem cells (MSCs) in culture demonstrated that their commitment into osteoblasts or adipocytes was determined by their cell shape and that MSCs that flattened and spread became osteoblasts whereas the same cells prevented from spreading became adipocytes (115). These investigators found that inhibiting the RhoA pathway drove the MSCs toward the adipocyte pathway, but activating RhoA induced the osteoblast lineage. They went on to show that this latter pathway was mediated by the RhoA effector, ROCK, and that expression of activated ROCK was sufficient to drive osteogenesis. Interestingly, this occurred even when the cells were kept in a rounded state, whereas expressing activated RhoA was not sufficient to overcome the inhibitory effect of cell rounding on osteogenesis. These results suggested that the link between RhoA and ROCK could be uncoupled by cell rounding. Pursuing this further, Chen’s group showed that indeed in rounded cells there was high RhoA activity but low ROCK activity and that the level of myosin light chain phosphorylation was similarly low. Additionally, they found that inhibiting endogenous cell tension either by disrupting the cytoskeleton with cytochalasin or by blocking myosin with blebbistatin also inhibited ROCK activity, although in the case of cytochalasin treatment this decrease in ROCK activity occurred in the presence of high RhoA activity (106). Their results suggested a positive feedback mechanism by which mechanical tension is needed to maintain high ROCK activity. In terms of the uncoupling between ROCK and RhoA activities, tyrosine phosphorylation of ROCK2 was shown to inhibit its activation by RhoA (116). This tyrosine phosphorylation was found to occur in response to adhesion and likely allows RhoA signaling to activate mDia but not ROCK, such that actin polymerization and cell spreading are promoted but contraction is inhibited. The high activity of RhoA that has been detected at the leading edge of migrating cells (117) has been difficult to explain in terms of models where RhoA drives contractility but can be easily accommodated in models where there is a regulatory bifurcation downstream from RhoA such that ROCK is inhibited while mDia is activated. However, in the case of cell rounding leading to ROCK inhibition, we suspect that this may involve other pathways because cell rounding is usually associated with decreased levels of tyrosine phosphorylation for many proteins (118).
In a detailed study in which MSCs were cultured on matrices that closely related to the compliance of their endogenous tissue environments, it was shown that soft substrata resembling the stiffness of brain induced a neurogenic pattern of gene expression, whereas on stiffer substrata mimicking muscle the same cells were myogenic, and on the stiffest matrices resembling collagenous bone the cells were osteogenic (100). Significantly, it was found that blocking myosin II activity inhibited the effects of matrix compliance on the resulting phenotype providing further support for the importance of myosin and tension in the sensing of matrix rigidity. Exploring how transcription may be regulated by matrix rigidity, cytoskeletal tension and RhoA activity, Piccolo’s group examined the transcriptional profiles of several cell types on substrates of differing compliance and identified the YAP/TAZ transcriptional regulators as key factors in controlling the enhanced expression of specific genes on more rigid substrates (119). Specifically, the distribution of these factors in the nucleus or in the cytoplasm was found to be determined by rigid versus soft matrices, respectively. Inhibiting RhoA, ROCK or myosin II activity was found to keep YAP and TAZ in the cytoplasm, whereas active RhoA drove them into the nucleus and induced the expression of genes associated with rigid matrices. It will be interesting in the future to learn how this is accomplished, but together with many of the studies mentioned above this work establishes a pathway by which a cell responds to the rigidity or compliance of its environment and alters its pattern of gene expression accordingly.
Tension at Cell-Cell junctions
The role of RhoA activity and mechanical tension in cell-cell junctions is complex. Numerous studies with agents that increase endothelial permeability, such as thrombin, have implicated both increased RhoA activity and myosin-based contractility with opening of endothelial junctions and increased permeability (120, 121). However, other work has indicated a role for RhoA in junction assembly (43, 122–125). Not only is RhoA activity required for junction assembly, but several studies have shown that myosin-induced tension downstream from RhoA can promote junction assembly (38, 126–128). These results appear at first sight to be contradictory. We suspect that under conditions where tension is associated with junctional disruption other factors must contribute to weakening the junctions. Support for this idea comes, for example, from studies of HIV-induced encephalitis in which there is increased monocyte passage across the blood/brain barrier and disruption of endothelial tight junctions. The weakening of tight junctions was related to ROCK-mediated phosphorylation of two tight junction proteins, occludin and claudin-5 (129). It seems likely that many agents that increase permeability and open cell-cell junctions simultaneously increase tension while weakening the adhesive strength of the junctional CAMs. On the other hand in situations where RhoA and increased contractility enhance junction assembly, we assume that the signals must segregate such that the junctional CAMs maintain their adhesive strength or increase it so that increased tension does not break the adhesions and open gaps between the cells. With respect to the strengthening of junctions in response to mechanical force, it was discovered that α-catenin changes its conformation in response to tension on epithelial junctions to expose a cryptic site that can bind vinculin (128). In parallel work, it was shown that vinculin is recruited to adherens junctions in response to mechanical tension and that tension on E-cadherin leads to a stiffening response that is dependent on vinculin (40). Together these studies support a model in which RhoA-mediated tension on cell-cell junctions can have opposite effects depending on the adhesive strength of the junctional CAMs and their associated protein complexes.
Cadherin engagement has been found to either decrease (130, 131) or increase RhoA activity(132–134). Differences in these results may reflect in part the different signaling pathways initiated downstream from different cadherins. However, some of the differences may be due to the presence or absence of force on the cadherins. Working with endothelial cells and VE-cadherin, Nelson and colleagues observed that sustained adhesion via VE-cadherin resulted in a peak of RhoA activity 6 hours following VE-cadherin engagement and they provided evidence that this was dependent on tension being transmitted to the sites of cell-cell adhesion. In contrast, the depression in RhoA activity upon E-cadherin engagement was rapid (130). Consistent with the idea that mechanical force on the cadherin may switch the signaling pathway from depressing RhoA activity to elevating it, we have found that while simple engagement of E-cadherin leads to decreased RhoA activity, applying force to the cadherins elevates RhoA activity (Marjoram and Guilluy, unpublished results). It will be interesting to identify the GEFs that become activated in response to tension on E-cadherin.
In many situations mechanical force on cells is associated with increased proliferation. Investigating the role of cell-cell adhesions versus cell-matrix adhesions in mechanical signaling to induce cell proliferation, Chen’s group compared the response of endothelial cells and vascular smooth muscle cells to mechanical force (135). Subjecting both cell types to stretching stimulated proliferation but endothelial cells required cell-cell adhesion and engagement of VE-cadherin for proliferation to occur, whereas smooth muscle cells responded to stretch by proliferation in the absence of cell-cell contact. Interestingly, the authors found that stretching endothelial cells activated Rac1 and this was required for proliferation. However, upon stretching smooth muscle cells RhoA activation was needed for proliferation (135). This result is in contrast to the absence of RhoA activation found by Schwartz’ group when smooth muscle cells were stretched, but differing conditions probably account for the apparent discrepancy (136).
Cancer
During development, the rigidity/compliance of different regions of embryos is thought to have a major impact on the differentiation and organization of various tissues and organs. This view is supported by the large body of work studying cells grown in culture that indicates the importance of the physical characteristics as well as the composition of the microenvironment. There are also disease situations where the rigidity of tissues alters and affects cell behavior. Examples include many solid tumors, the hardening of arterial walls that occurs with age, atherosclerosis, and fibrotic diseases where there is increased deposition of ECM. Solid tumors are often detected by physical palpation, an indication that they are less compliant than the surrounding tissues. The increased rigidity of tumors not surprisingly has been associated with increased RhoA activity and other altered signaling pathways (103). Many epithelial cell types adopt a more normal morphology and phenotype when grown in relatively soft 3D matrices and this is lost when the same cells are cultured on rigid two dimensional surfaces (137). Working with breast epithelial cells in culture, it was found that changing the compliance of the ECM alone promoted a more malignant cancer phenotype (103). Cells grown on a stiff matrix exhibited larger colony size, increased ERK activity, elevated RhoA activity, more focal adhesions, and greater tractional force applied to the ECM compared to cells grown on a soft ECM. Blocking ROCK activity caused the cells on the stiff ECM to behave more like cells grown on a compliant ECM. Elevated RhoA-dependent signaling disrupted the normal epithelial morphology of the breast epithelial cells, which in soft matrices grow as spheroids with a cell polarity mimicking that found in the normal gland. On more rigid substrata the cells lost their polarized organization and the cell aggregates failed to develop lumens. These changes are reminiscent of the changes associated with malignancy (103, 138). Elevated rigidity has been shown to have protumorigenic effects in other cell types as well. For example, expression of activated forms of ROCK2 in skin resulted in increased stiffening associated with increased collagen deposition (139). This was associated with nuclear accumulation of β-catenin, transcriptional activation and hyperproliferation. Interestingly, when human skin squamous cell carcinomas were examined, the majority were found to have elevated ROCK expression and activity (139).
Mechanical tension in tumors is associated not only with increased cell proliferation but also with enhanced invasion (140). Tumor cells migrate along aligned collagen fibrils and this is promoted by increased mechanical tension within the tumor (141). When tumor cells move in tissues either they migrate as cell collectives where a group of cells migrate together while maintaining their cell-cell contacts or they migrate as individual cells (142). In the latter situation they have been found to migrate in two distinct ways, which have been described as mesenchymal versus amoeboid or rounded (143, 144). These two types of migration appear to be interchangeable and the mesenchymal form can be driven to become the amoeboid type by inhibiting proteases involved in degrading the ECM or by elevating RhoA and ROCK activity. Conversely, the mesenchymal mode of migration is promoted by high Rac1 but low RhoA activity (60, 142).
Future directions
The discovery that mechanical forces exerted exogenously on cells or generated endogenously within them leads to Rho protein activation and signaling has many implications. This pathway is important in development, preferentially driving stem cell differentiation along one lineage versus another. With the increasing interest in potential stem cell therapies, recognition of the impact of the physical properties of the environment is important. However, knowing that these effects of the environment are driven by the RhoA/ROCK pathway should permit these environmental influences to be overridden by manipulating this signaling pathway so as to direct the differentiation of stem cells along predetermined lines. Elucidating the signaling pathways from mechanical force to Rho protein activation may also impact the approach to various pathologies such as fibrosis and cancer. However, with tumors there is a red flag in that tumor cells can switch their mode of migration from a mesenchymal type to an amoeboid type according to the relative activities of Rac1 and RhoA. Consequently, the tempting idea that decreasing tumor cell RhoA activity may be beneficial, leading to decreased cell proliferation and favoring a more normal phenotype, may have unexpected consequences converting invasive tumor cells from one migratory phenotype to another. Nevertheless, when combined with other therapies, such as inhibiting Rac1-driven migration, targeting RhoA activity in tumors may be advantageous. The identification of upstream signaling components such as GEFs promises to provide novel targets for therapeutic development, not only for certain cancers but also for other disease where mechanosensitive signaling may be involved.
Acknowledgments
E.L. thanks the Integrative Vascular Biology Training Program (TL32HL069768) for funding support. C.G. thanks the European Commission Seventh Framework Programme (FP7/2007–2013) for a Marie Curie Outgoing International Fellowship (254747). K.B. thanks the Kenan Foundation for support and support from grants from the National Institutes of Health (GM029860 and HL080166).
This field is expanding rapidly and we apologize to the many authors whose work we failed to acknowledge due to space limitations.
References
- 1.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 2.Curtis AS, Seehar GM. The control of cell division by tension or diffusion. Nature. 1978;274:52–53. doi: 10.1038/274052a0. [DOI] [PubMed] [Google Scholar]
- 3.Vandenburgh H, Kaufman S. In vitro model for stretch-induced hypertrophy of skeletal muscle. Science. 1979;203:265–268. doi: 10.1126/science.569901. [DOI] [PubMed] [Google Scholar]
- 4.Bray D. Mechanical tension produced by nerve cells in tissue culture. J Cell Sci. 1979;37:391–410. doi: 10.1242/jcs.37.1.391. [DOI] [PubMed] [Google Scholar]
- 5.Bray D. Axonal growth in response to experimentally applied mechanical tension. Dev Biol. 1984;102:379–389. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]
- 6.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 7.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 8.Kolega J. Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. J Cell Biol. 1986;102:1400–1411. doi: 10.1083/jcb.102.4.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18:472–481. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 11.Mammoto A, Mammoto T, Ingber DE. Rho signaling and mechanical control of vascular development. Current opinion in hematology. 2008;15:228–234. doi: 10.1097/MOH.0b013e3282fa7445. [DOI] [PubMed] [Google Scholar]
- 12.Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker’s guide to mechanobiology. Dev Cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circulation research. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012 doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberts BJA, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 5. Garland Science; 2007. [Google Scholar]
- 18.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 19.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 20.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. Journal of Cell Biology. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annual review of cell and developmental biology. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 22.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 23.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) Journal of Biological Chemistry. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 26.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng JH, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-Associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 27.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. [see comments] Nature Cell Biology. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 29.Burridge K. Are stress fibres contractile? Nature. 1981;294:691–692. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- 30.Chen CS. Mechanotransduction - a field pulling together? Journal of cell science. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 31.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 32.Peng F, Zhang B, Ingram AJ, Gao B, Zhang Y, Krepinsky JC. Mechanical stretch-induced RhoA activation is mediated by the RhoGEF Vav2 in mesangial cells. Cellular signalling. 2010;22:34–40. doi: 10.1016/j.cellsig.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Guilluy C, Swaminathan V, Garcia-Mata R, Timothy O’Brien E, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:724–729. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo JC, Han X, Hsiao CT, Yates JR, III, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heck JN, Ponik SM, Garcia-Mendoza MG, Pelhke CA, Inman Dr, Eliceiri KW, Keely PJ. Microtubules Regulate GEF-H1 in Response to Extracellular Matrix Stiffness. Mol Biol Cell. 2012 doi: 10.1091/mbc.E11-10-0876. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. Journal of cell science. 2009;122:3644–3651. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. American journal of physiology Lung cellular and molecular physiology. 2010;298:L837–848. doi: 10.1152/ajplung.00263.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brevier J, Montero D, Svitkina T, Riveline D. The asymmetric self-assembly mechanism of adherens junctions: a cellular push-pull unit. Physical biology. 2008;5:016005. doi: 10.1088/1478-3975/5/1/016005. [DOI] [PubMed] [Google Scholar]
- 40.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Benais-Pont G, Punn A, Flores-Maldonado C, Eckert J, Raposo G, Fleming TP, Cereijido M, Balda MS, Matter K. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol. 2003;160:729–740. doi: 10.1083/jcb.200211047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–166. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li G, Du X, Vass WC, Papageorge AG, Lowy DR, Qian X. Full activity of the deleted in liver cancer 1 (DLC1) tumor suppressor depends on an LD-like motif that binds talin and focal adhesion kinase (FAK) Proc Natl Acad Sci U S A. 2011;108:17129–17134. doi: 10.1073/pnas.1112122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao YC, Shih YP, Lo SH. Mutations in the focal adhesion targeting region of deleted in liver cancer-1 attenuate their expression and function. Cancer Res. 2008;68:7718–7722. doi: 10.1158/0008-5472.CAN-08-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark K, Howe JD, Pullar CE, Green JA, Artym VV, Yamada KM, Critchley DR. Tensin 2 modulates cell contractility in 3D collagen gels through the RhoGAP DLC1. Journal of cellular biochemistry. 2010;109:808–817. doi: 10.1002/jcb.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawai K, Iwamae Y, Yamaga M, Kiyota M, Ishii H, Hirata H, Homma Y, Yagisawa H. Focal adhesion-localization of START-GAP1/DLC1 is essential for cell motility and morphology. Genes to cells : devoted to molecular & cellular mechanisms. 2009;14:227–241. doi: 10.1111/j.1365-2443.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- 48.Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 50.Noren NK, Arthur WT, Burridge K. Cadherin engagement inhibits RhoA via p190RhoGAP. J Biol Chem. 2003;278:13615–13618. doi: 10.1074/jbc.C200657200. [DOI] [PubMed] [Google Scholar]
- 51.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang B, Radel C, Hughes D, Kelemen S, Rizzo V. p190 RhoGTPase-activating protein links the beta1 integrin/caveolin-1 mechanosignaling complex to RhoA and actin remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:376–383. doi: 10.1161/ATVBAHA.110.217794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omelchenko T, Hall A. Myosin-IXA Regulates Collective Epithelial Cell Migration by Targeting RhoGAP Activity to Cell-Cell Junctions. Curr Biol. 2012 doi: 10.1016/j.cub.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD. Membrane Tension Maintains Cell Polarity by Confining Signals to the Leading Edge during Neutrophil Migration. Cell. 2012;148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Worthylake RA, Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem. 2003;278:13578–13584. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- 58.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho and ROCK-regulated GAP for Rac binds filamin A to control actin remodeling. Nature Cell Biology. 2006 doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 59.Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478:260–263. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 61.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishijima A, Doi T, Sakurada K, Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991;352:301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- 63.Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DC. Movement and force produced by a single myosin head. Nature. 1995;378:209–212. doi: 10.1038/378209a0. [DOI] [PubMed] [Google Scholar]
- 64.Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 65.Tonomura Y, Oosawa F. Molecular mechanism of contraction. Annu Rev Biophys Bioeng. 1972;1:159–190. doi: 10.1146/annurev.bb.01.060172.001111. [DOI] [PubMed] [Google Scholar]
- 66.Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galbraith CG, Sheetz MP. A micromachined device provides a new bend on fibroblast traction forces. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9114–9118. doi: 10.1073/pnas.94.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. [see comments] Nature Cell Biology. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 69.Schwarz US, Balaban NQ, Riveline D, Bershadsky A, Geiger B, Safran SA. Calculation of forces at focal adhesions from elastic substrate data: the effect of localized force and the need for regularization. Biophys J. 2002;83:1380–1394. doi: 10.1016/S0006-3495(02)73909-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. Journal of Cell Biology. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci. 1985;75:35–42. doi: 10.1242/jcs.75.1.35. [DOI] [PubMed] [Google Scholar]
- 74.Sumpio BE, Banes AJ, Levin LG, Johnson G., Jr Mechanical stress stimulates aortic endothelial cells to proliferate. J Vasc Surg. 1987;6:252–256. [PubMed] [Google Scholar]
- 75.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. Journal of Cell Biology. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. [see comments] Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 77.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin- cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 78.Matthews BD, Overby DR, Alenghat FJ, Karavitis J, Numaguchi Y, Allen PG, Ingber DE. Mechanical properties of individual focal adhesions probed with a magnetic microneedle. Biochem Biophys Res Commun. 2004;313:758–764. doi: 10.1016/j.bbrc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 80.Glogauer M, Ferrier J. A new method for application of force to cells via ferric oxide beads. Pflugers Arch. 1998;435:320–327. doi: 10.1007/s004240050518. [DOI] [PubMed] [Google Scholar]
- 81.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 82.Tim O’Brien E, Cribb J, Marshburn D, Taylor RM, 2nd, Superfine R. Chapter 16: Magnetic manipulation for force measurements in cell biology. Methods Cell Biol. 2008;89:433–450. doi: 10.1016/S0091-679X(08)00616-X. [DOI] [PubMed] [Google Scholar]
- 83.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malek AM, Izumo S. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertens. 1994;12:989–999. [PubMed] [Google Scholar]
- 87.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 88.Bussolari SR, Dewey CF, Jr, Gimbrone MA., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum. 1982;53:1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- 89.Schnittler HJ, Franke RP, Akbay U, Mrowietz C, Drenckhahn D. Improved in vitro rheological system for studying the effect of fluid shear stress on cultured cells. Am J Physiol. 1993;265:C289–298. doi: 10.1152/ajpcell.1993.265.1.C289. [DOI] [PubMed] [Google Scholar]
- 90.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 91.Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 92.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 93.Ziegelstein RC, Cheng L, Capogrossi MC. Flow-dependent cytosolic acidification of vascular endothelial cells. Science. 1992;258:656–659. doi: 10.1126/science.1329207. [DOI] [PubMed] [Google Scholar]
- 94.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 95.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho- associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 96.Peterson LJ, Rajfur Z, Maddox AS, Freel CD, Chen Y, Edlund M, Otey C, Burridge K. Simultaneous stretching and contraction of stress fibers in vivo. Molecular Biology of the Cell. 2004 doi: 10.1091/mbc.E03-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 100.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 101.Wong AJ, Pollard TD, Herman IM. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983;219:867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]
- 102.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. Journal of Cell Biology. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 104.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313:3616–3623. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO Journal. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 109.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 111.Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nie M, Aijaz S, Leefa Chong San IV, Balda MS, Matter K. The Y-box factor ZONAB/DbpA associates with GEF-H1/Lfc and mediates Rho-stimulated transcription. EMBO reports. 2009;10:1125–1131. doi: 10.1038/embor.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 114.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 115.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 116.Lee HH, Chang ZF. Regulation of RhoA-dependent ROCKII activation by Shp2. J Cell Biol. 2008;181:999–1012. doi: 10.1083/jcb.200710187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 118.Burridge K, Turner CE, Romer LH. Tyrosine Phosphorylation of Paxillin and Pp125(Fak) Accompanies Cell-Adhesion to Extracellular-Matrix - A Role in Cytoskeletal Assembly. Journal of Cell Biology. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 120.Moy AB, Shasby SS, Scott BD, Shasby DM. The effect of histamine and cyclic adenosine monophosphate on myosin light chain phosphorylation in human umbilical vein endothelial cells. Journal of Clinical Investigation. 1993;92:1198–1206. doi: 10.1172/JCI116690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability [Review] [164 refs] Journal of Applied Physiology. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 122.Zhong CL, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of ras-transformed epithelial cells. Molecular Biology of the Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. Journal of Cell Biology. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. Journal of Cell Biology. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res. 2006;312:1637–1650. doi: 10.1016/j.yexcr.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 128.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 129.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. Journal of Biological Chemistry. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 131.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Molecular Biology of the Cell. 2002;13:1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calautti E, Grossi M, Mammucari C, Aoyama Y, Pirro M, Ono Y, Li J, Dotto GP. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. Journal of Cell Biology. 2002;156:137–148. doi: 10.1083/jcb.200105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin- dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell. 2004;15:2943–2953. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007;101:e44–52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 136.Katsumi A, Milanini J, Kiosses WB, Del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol. 2002;158:153–164. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, Barker N, Clevers H, Sansom OJ, Anderson KI, Weaver VM, Olson MF. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26:273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 141.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC medicine. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2009;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 145.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273:34954–34960. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 146.Hobert O, Schilling JW, Beckerle MC, Ullrich A, Jallal B. SH3 domain-dependent interaction of the proto-oncogene product Vav with the focal contact protein zyxin. Oncogene. 1996;12:1577–1581. [PubMed] [Google Scholar]
- 147.Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–892. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- 148.Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, Parsons JT. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci. 2008;121:895–905. doi: 10.1242/jcs.020941. [DOI] [PubMed] [Google Scholar]
- 149.Sharma SV. Rapid recruitment of p120RasGAP and its associated protein, p190RhoGAP, to the cytoskeleton during integrin mediated cell-substrate interaction. Oncogene. 1998;17:271–281. doi: 10.1038/sj.onc.1201921. [DOI] [PubMed] [Google Scholar]
- 150.Tripathi V, Popescu NC, Zimonjic DB. DLC1 Interaction with alpha-Catenin Stabilizes Adherens Junctions and Enhances DLC1 Antioncogenic Activity. Mol Cell Biol. 2012;32:2145–2159. doi: 10.1128/MCB.06580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Haudenschild DR, Chen J, Pang N, Lotz MK, D’Lima DD. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum. 2010;62:191–200. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]