Abstract
Background
Life altering anxiety disorders, such as post-traumatic stress disorder (PTSD), can co-occur at high rates with substance use disorders. Alcoholism, compared to other substance use disorders, is particularly common. Rodent studies of acute stress effects on alcohol consumption show stress can alter ethanol (EtOH) consumption. This study examined voluntary EtOH consumption in male Long-Evans rats that had undergone a stress-enhanced fear learning (SEFL) procedure.
Methods
Adult Long-Evans rats were exposed to a stress that consisted of 15 inescapable foot-shocks (1 mA, 1 sec) known to cause a long-lasting non-associative enhancement of subsequent fear learning. Control animals received no shock. One day later, animals were placed in a novel and very different context and received a single foot-shock. On day 3, animals were returned to the single shock context and freezing was used as a measure of learned fear. The intermittent access two-bottle choice (2BC) regimen, consisted of 1 bottle of water and 1 bottle of experimental solution, either 19% EtOH or 28.4% sucrose-0.08% quinine, for a 24 hr period, 3 days a week, and all other times 2 water bottles. This regimen lasted until stable levels of experimental solution drinking were reached, at which point the experimental solution was removed for 40 days and then returned to measure resumption of consumption.
Results
Rats that received stress prior to ethanol consumed significantly more EtOH than control rats before and after reinstatement. Rats that received stress after drinking was established did not consume significantly more EtOH when the drug was returned. Stress had no significant effect on sucrose-quinine drinking, our calorie and taste control for EtOH.
Conclusions
A single traumatic event sufficient to produce long-lasting enhancement of fear-learning increases voluntary EtOH consumption, but does not alter previously acquired EtOH drinking habits or alter consumption of a calorically equivalent sweet-bitter tasting solution.
Keywords: Post-traumatic stress disorder, two-bottle choice, ethanol, sucrose, quinine
INTRODUCTION
Fear is an adaptive response that initiates defensive behavior to protect animals and humans from danger when it is in proportion to the threat. However, anxiety disorders, such as post-traumatic stress disorder (PTSD), can arise when fear is inappropriately regulated. PTSD occurs when symptoms caused by a traumatic experience, such as anxiety, avoidance of potential reminders of the trauma, re-experiencing of the trauma and increased vigilance, persist longer than 1 month (American Psychiatric Association., 2000). Substance use disorders, particularly alcoholism, have high comorbidity rates with PTSD (Back et al., 2005; Dedert et al., 2009; Farrugia et al., 2011; Freeman and Kimbrell, 2004; Kulka, 1990; McDevitt-Murphy et al., 2010; Ray et al., 2009; Savarese et al., 2001). PTSD treatment typically involves exposure-based psychotherapy and pharmacotherapy. Such treatments appear to decrease general anxiety, however, PTSD is often resistant to exposure therapy (Kessler et al., 1995).
Most animal models of stress and fear focus on Pavlovian fear-learning to study the neurobiology of stress (Ehrlich et al., 2009; Martijena et al., 2002; Pape and Pare, 2010) as well as effects of stress on alcohol consumption (Breese et al., 2005; Higley et al., 1991; Sommer et al., 2008). However, animal models of PTSD have been developed in order to determine the brain mechanisms underlying this disorder. Researchers have utilized predator scent (Corley et al., 2011), prolonged restraint (Rodriguez Manzanares et al., 2005), and electric shock (Rau and Fanselow, 2009) to mimic traumatic events experienced by humans which can lead to PTSD. Such stress inducing techniques produce non-associative sensitization to subsequent associative fear-conditioning (Rau et al., 2005; Rodriguez Manzanares et al., 2005) analogous to symptoms of PTSD such as exaggerated startle response and enhanced fear to similar events (American Psychiatric Association., 2000). Non-associative sensitization of fear has been shown to produce PTSD-like symptoms in the SEFL rats (Rau et al., 2005).
The animal model we used to study non-associative sensitization to fear learning and alcohol consumption is a well-characterized rat model of stress-enhanced fear learning (SEFL) that exhibits behavioral components of associative and non-associative fear (Rau et al., 2005; Rau and Fanselow, 2009). Like in PTSD, the non-associative sensitization which enhances future fear learning in SEFL rats is resistant to extinction training and pharmacological inactivation of contextual memory formation (McFall et al., 1992; Rau et al., 2005). The experience of stress causes persistent changes in expression of genes in the SEFL model (Ponomarev et al., 2010) that have been specifically implicated in PTSD in humans (Ressler et al., 2011). The SEFL procedure produces long-lasting exaggerated acquisition of novel fears, and similar to other stressors might affect how alcohol drinking habits are learned. These observations lead to our hypothesis that SEFL rats, which have increased fear, will also have increased alcohol consumption.
In order to study voluntary EtOH consumption in SEFL rats an intermittent access two-bottle choice (2BC) drinking procedure that leads rats to gradually escalate EtOH consumption to high levels (5–6 g/kg/24h) without resorting to sucrose fadeout procedures or forced EtOH administration (Simms et al., 2008; Wise, 1975) was used. SEFL conditioning in EtOH naïve rats resulted in increased voluntary EtOH consumption compared to controls rats. We also show this effect is specific to EtOH as there was no significant difference in calorically equivalent non-alcoholic Sucrose-Quinine solution consumed. Together these experiments show that a highly stressful experience sufficient to produce long-lasting enhancement of fear-learning also increases voluntary EtOH consumption.
METHODS
Subjects
Experimentally naive Long-Evans male rats, approximately 90 days old (Harlan; Indianapolis, IN) were used. Rats were singly housed in stainless steel wire mesh cages upon arrival at the University of California Los Angeles (UCLA) Psychology Department vivarium or in Plexiglas cages upon arrival at the UCLA Center for Health Sciences (CHS) vivarium. Food and water were available ad libitum, in a 12-hr light-dark cycle (lights on at 6:00 a.m.) maintained in colony rooms. SEFL experimental procedures took place in the Psychology vivarium during the light cycle, beginning around 8:00 a.m. A week before experiments, animals were handled daily for approximately 20 s each to adapt to the experimenters. All 2BC drinking presentations and measurements were done in the CHS vivarium and started within 15 minutes of the beginning of the dark cycle. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (7th Edition, National Academy Press, Washington, D.C., 1996) and were approved by the Chancellor's Animal Research Committee of the UCLA Office for the Protection of Research Subjects.
SEFL conditioning apparatus
Procedures took place in two distinct training/testing environments, Context A, the stress environment and Context B, the novel environment. Each context contained distinguishable background noise, lighting, and odor in fear conditioning boxes that differed in interior size/shape, texture and grid floor pattern designed to minimize generalization between the contexts. Context A chambers (28×21×22 cm; Lafayette Instruments, Lafayette, IN) were aluminum sided with an opaque Plexiglas back and clear Plexiglas front door piece. Floor grids (18 stainless steel rods, 4 mm diameter and 1.5 cm apart) were connected to a shock generator and scrambler (Lafayette Instrument Co.; Lafayette, IN). Overhead fluorescent lighting was used. A ventilation fan provided 70 dB of background noise. Fear conditioning boxes were cleaned and dried between each session using a 10% sodium hydroxide solution. Stainless steel pans were placed beneath each grid floor in the chambers; these contained 4–5 sprays of atomized Simple Green as the context odor. Metal scaffolding attached to a cart was used to transport the animals in their home cages to Context A and back.
The interior of the Context B chambers, initially the same as described above, were modified by inserting white Plexiglas along the rear wall and two white Plexiglas side-walls at 60° angles that formed an A-frame. As in Context A, the front door piece consisted of a clear Plexiglas panel. The grid floor, connected to a shock generator and scrambler (Lafayette Instrument Co.; Lafayette, IN), consisted of 17 stainless steel rods (4 mm diameter) spaced in offset rows 1 cm vertically and 2.6 cm horizontally. Lighting consisted of one red 30 w incandescent bulb. A white noise generator produced background noise (70 dB, A-scale). Fear conditioning chambers were cleaned, using a 1% acetic acid solution, and dried between each session. Stainless steel pans were placed beneath each grid floor in the chambers; these contained 4–5 sprays of an atomized 11% coconut extract mixture as the context odor. Animals were transported to and from their home cages in a covered, black rubberized box subdivided into four areas by black Plexiglas panels.
SEFL procedure
Rats were randomly assigned to one of two groups that received 15 shocks (SEFL) or no shock (control) during pre-exposure in Context A. Shock pre-exposure on day 1 consisted of 15 shocks (1 mA, 1 s) spaced with a variable intershock interval of 240–480 s. Both non-shock controls and shocked animals were placed in chambers for 90 min. On all subsequent days SEFL and control animals were conditioned and tested under the same conditions. On day two, animals were given one shock in Context B. The shock (1 mA, 1 s) was given 180 s after placement in the chamber, and animals were removed from the chambers 300 s later. On day 3, animals were given an 8 min context test in Context B. Freezing, defined as the absence of all movement except that necessary for respiration, is a consistent response to learned fear stimuli (Fanselow, 1980). We used an automated activity monitoring system to provide an index of freezing prior to the day 2 Context B single shock and during the day 3 Context B context test.
Intermittent access two-bottle choice (2BC) drinking paradigm
The intermittent access to 2BC drinking paradigm was adapted from Wise (1973). All fluids were presented in 50-ml graduated plastic conical tubes with stainless-steel low-leak drinking spouts accessible to rats through the top of their home cage. On the Monday following the end of a 7 day post-transfer housing acclimatization period, rats was given access to 1 bottle of drinking water and 1 bottle of the experimental solution for a 24 hr period on Mondays, Wednesdays, and Fridays. Bottles were weighed at the beginning and end of each 24 hr drinking period; measurements were taken to the nearest 1/100 g. The weight of each rat was also measured at the end of the 24 hr drinking period (Tuesdays, Thursdays, and Saturdays) and rat weight was used to calculate the grams of solution consumed per kilogram of body weight per 24 hr drinking session (g/kg/24 hrs). Preference for experimental solutions was calculated as the ratio of experimental solution over the total fluid consumed in a 24 hr drinking session. Experimental drinking solutions were prepared in drinking water provided by UCLA veterinary staff. Upon completion of each drinking session the experimental solution was replaced with a second water bottle until the next presentation. The rats had unlimited access to 2 bottles of water over the weekend after the 24 hr measurements were taken on Saturday. Bottle placement was alternated each drinking session to control for side preferences. EtOH presentations were made until after rats had reached a steady high level of drinking (25 or 31 presentations). Then rats were withdrawn from the experimental solution for 40 days followed by reintroduction of drinking solution for 7–22 2BC presentations.
Early-SEFL & 19% EtOH
SEFL/control conditioning of EtOH naïve animals was done 10 days prior to 2BC EtOH drinking. The 19% EtOH drinking solution was made from 95% (v / v) ethanol (Sigma Aldrich). EtOH was presented without changes in concentration or addition of sweeteners. Animals received 31 2BC EtOH presentations. The EtOH was withdrawn for 40 days. Upon re-instatement, 7 2BC EtOH presentations were made.
Late-SEFL & 19% EtOH
Prior to SEFL/control conditioning, animals received 25 2BC 19% EtOH presentations. EtOH was withdrawn for 40 days. At the beginning of the withdrawal period animals were transferred to the Psychology vivarium. Animals were separated into SEFL and control groups based on the amount of intake on the final day of 2BC before the removal of EtOH for 40 days. Animals were paired by roughly equivalent levels of intake, such that the two highest drinkers were paired, then the next two highest drinkers were paired, and this was repeated until each rat had an equal pair. The initial separation into either SEFL or control group the highest group was randomly assigned, and each subsequent pair was systematically alternated between group so that neither group always received the slightly higher or lower drinker of the pair. SEFL/control conditioning began on day 28 of withdrawal, and upon completion of the three day conditioning process animals were transferred back to the CHS vivarium to reacclimatize to the 2BC surroundings during the remainder of their withdrawal period. Upon re-instatement, 22 2BC EtOH presentations were made.
Early-SEFL & Sucrose-Quinine
After SEFL/control conditioning, Sucrose-Quinine naïve animals were allowed to acclimate to the new 2BC environment for 7 days. The 28.4% sucrose-0.08% quinine-HCl (Sucrose-Quinine) was made up as a w/v ratio from pure sucrose and quinine-HCl (both Sigma Aldrich). Sucrose-Quinine was presented without changes in concentration, additional sweeteners, or EtOH. Animals received 31 2BC Sucrose-Quinine presentations and were then withdrawn for 40 days. Upon re-instatement, 19 2BC Sucrose-Quinine presentations were made.
Data and Statistical Analysis
A freezing index was used as a measure of learned fear and defined as a lack of movement except that necessary for respiration (Fanselow, 1980; Rau et al., 2005; Rau and Fanselow, 2009). Behavior was recorded using near infrared video equipment and computer software (VideoFreeze, Med-Associates Inc.) that determined pixel changes from frame to frame. Freezing was calculated using the automated software and defined as activity below a minimum threshold for longer than one second (correlation of r>0.9 between automated system and highly trained human observers) as previously described (Jacobs et al., 2010). Average freezing scores were calculated for baseline freezing in the novel context for 3 minutes prior to the single shock; post-shock freezing in the novel context was assessed for 5 minutes. SEFL Context test in the novel context measured freezing for 8 minutes. Drinking data and rat weight were recorded by hand and compiled daily electronically. Student's paired and un-paired t-tests were used to test for statistical differences in freezing within groups between contexts, and between groups within contexts. 2-way repeated measures (RM) ANOVA with Bonferroni pairwise comparisons was used to test for statistical difference between groups for EtOH and Sucrose-Quinine consumption.
RESULTS
Early-SEFL & 2BC EtOH
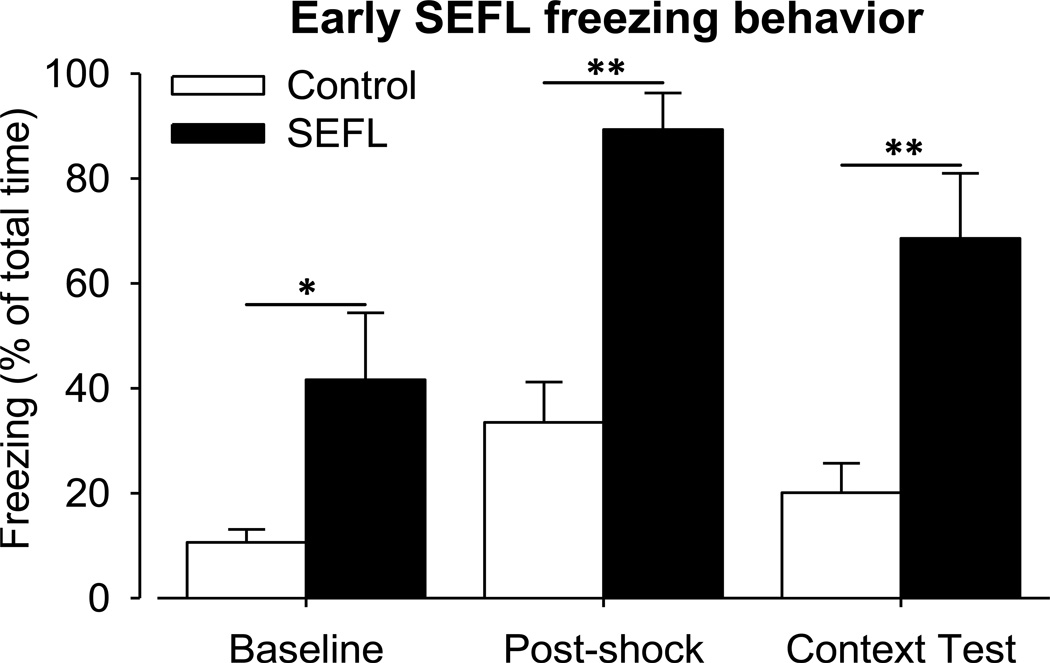
SEFL conditioning resulted in significantly increased freezing (Fig. 1) of SEFL rats (n = 10) compared to control rats (n = 12) within each sub-context measurement of animal freezing activity: Baseline (un-paired t-test; p = 0.041), Post-shock and Context Test (un-paired t-test; p < 0.001). Control rat freezing was significantly different between Baseline and Post-shock freezing (paired t-test; p < 0.011), however, there was no significant difference between Baseline and Context Test or Post-shock and Context Test freezing (paired t-test; p = 0.604). This is important because it shows Post-shock increase in fear to be transient. SEFL rat freezing was significantly different between Baseline and Post-shock (paired t-test; p < 0.001) and Baseline and Context Test (paired t-test; p < 0.001) contexts. The difference was not significant between Post-shock and Context Test freezing (paired t-test; p = 0.31), and this is important because it shows the permanency of the enhanced-learned fear.
Figure 1. Early SEFL: freezing behavior.
Ten days before beginning 2BC 19% EtOH drinking SEFL treatment was done. Behavioral differences in fear response between control (n = 12) that only received a single shock and SEFL (n = 10) rats that received 15 shocks in context A as well as a single shock in the novel context. The % freezing time is a reliable measure of fear. The Context Test measures the amount of fear learning that occurred to the novel context subsequent to the single shock. SEFL conditioning resulted in significantly increased freezing SEFL compared to control rats within each sub-context measurement of animal freezing activity as tested with un-paired t-test: Baseline (*, p = 0.041), Post-shock and Context Test (**, p < 0.001).
The EtOH consumption during the initial EtOH presentation days was similar for both groups (Fig. 2A). However, the SEFL group began to consume more EtOH than the control counterparts. Two-way RM ANOVA comparison showed that across the 31 pre-withdrawal presentations there was a between group effect of SEFL conditioning such that there was significantly increased voluntary EtOH consumption in SEFL rats compared to control rats (F(1, 20) = 5.364, p = 0.031). There was no statistically significant interaction between SEFL treatment and presentation day (F(30, 599) = 1.358, p = 0.098).
Figure 2. Early SEFL: 2BC 19% EtOH.
Ten days before the beginning of 2BC EtOH drinking, SEFL treatment was done to produce SEFL (n = 10) and control (n = 12) rats. A) There was a significant group effect of increased consumption in SEFL compared to control rats across the initial 31 presentations (2-way RM ANOVA; †, F(1,20) = 5.364, p = 0.031). EtOH treatment was removed for 40 days. Upon reintroduction rats rapidly returned to high levels of EtOH consumption with a significant separation in the amount of EtOH consumed between the two groups (2-way RM ANOVA; ‡, F(1,20) = 7.708; p = 0.012). B) 2-way RM ANOVA showed significant group differences only prior to 40 d removal of EtOH (†, F(1,20) = 5.660; p = 0.027), and a trend of increased preference post-withdrawal (F(1,20) = 3.301; p = 0.097).
After the 40 d period of EtOH removal 2BC EtOH drinking was reintroduced. The first EtOH consumption for the SEFL group was significantly decreased compared to the final day of drinking before the 40d removal (paired t-test; p = 0.041). A 2-way RM ANOVA confirmed that after reintroduction, EtOH consumption of SEFL rats was also significantly higher compared to the control rat consumption for all post-withdrawal presentations (F(1, 20) = 7.708; p = 0.012). Together with the pre-removal drinking data, these data suggest that SEFL conditioning before 2BC EtOH drinking leads to significantly increased voluntary EtOH consumption. After reintroduction there remained no significant interaction between SEFL treatment and presentation day (F(6, 120) = 0.260, p = 0.954).
EtOH preference in the SEFL group before EtOH removal was significantly increased compared to control rat group according to 2-way RM ANOVA (Fig. 2B; F(1, 20) = 5.660, p = 0.027). Preference for EtOH continued to increase for both groups relative to pre-removal preferences, however, the average SEFL group preference was still greater than that of the control group. 2-way RM ANOVA test showed that there was no longer a significant group difference in preference following reintroduction of EtOH (F(1, 20) = 3.301; p = 0.097). This shift of increased post-withdrawal preference in control rats seems to be due to decreased water consumption (data not shown).
Late-SEFL & 2BC EtOH
Naïve rats, placed on 2BC 19% EtOH drinking, had no significant between groups difference in pre-removal EtOH consumption (Fig. 3A; 2-way RM ANOVA, F(1, 30) = 0.0067, p = 0.935). There was also no interaction between groups (note: SEFL treatment had not happened yet) and presentation days (F(24, 719) = 0.493, p = 0.981). This is expected since there was no experimental difference between the groups of rats at this point in time, as they had not yet had the SEFL conditioning. After the 25th presentation, 2BC EtOH presentations were halted for 40 days.
Figure 3. Late SEFL: 2BC 19% EtOH.
A cohort of stress naive rats (n = 30) began 2BC 19% EtOH drinking for 25 initial sessions. 2BC EtOH presentations were stopped for 40 days. On day 28 of withdrawal rats were separated into SEFL (n = 15) and control (n = 15) groups using a counter-balanced method based upon their 25th EtOH presentation drinking levels. SEFL treatment was done, and 2BC EtOH drinking was reinstated, for 22 presentations, at the end of 40 days. A) There was no significant group effect of different 19% EtOH consumption in SEFL compared to control rats across the initial 25 presentations (2-way RM ANOVA; F(1,30) = 0.0067; p = 0.935). Upon re-introduction, rats rapidly returned to high levels of EtOH consumption, maintaining a lack of significant difference in EtOH consumed between the two groups (2-way RM ANOVA; F(1,30) = 0.615; p = 0.439). B) Although 37 of the 38 SEFL group (n = 15) treatments before and after withdrawal had a higher average EtOH preference than the control group (n = 15), 2-way RM ANOVA showed no significant group differences prior to 40 d withdrawal (F(1,30) = 0.289; p = 0.595) or after withdrawal F(1,30) < 0.001; p > 0.05).
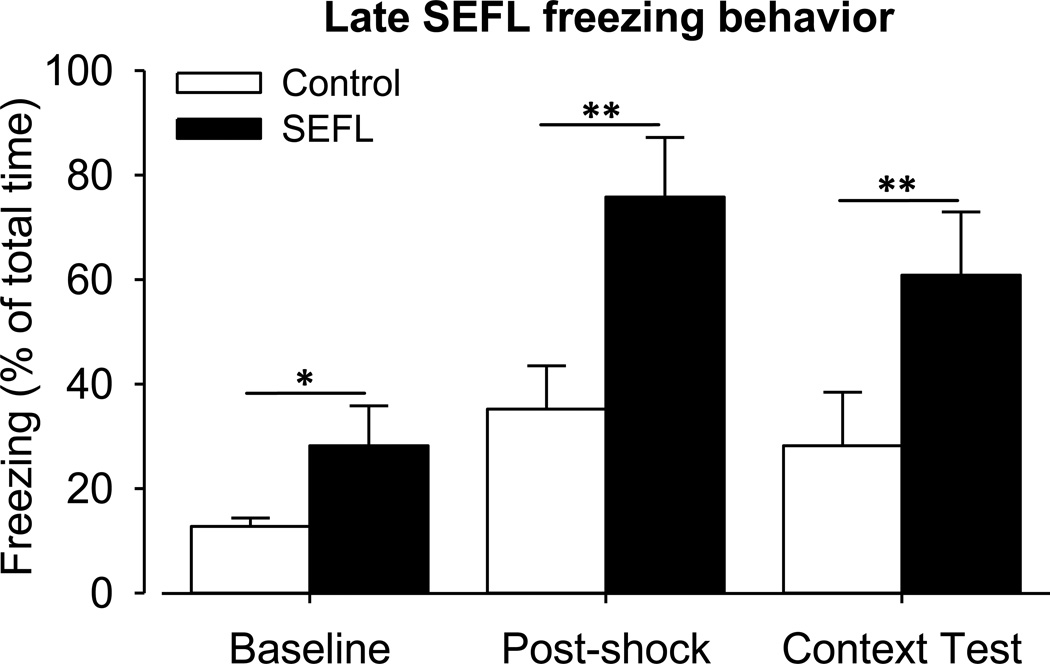
On the 28th day of withdrawal SEFL conditioning was performed for SEFL (n =15) and control animals (n = 15) resulting in significantly increased freezing (Fig. 4) of SEFL rats for each sub-context measurement of animal activity: Baseline (un-paired t-test; p = 0.001), Post-shock and the Context Test measurement (un-paired t-test; p < 0.001). Within control rats there were significant sub-context differences between Baseline and Post-shock (paired t-test; p < 0.001) and Post-shock and Context Test freezing (paired t-test; p = 0.031), however, Baseline and Context-test freezing was not significantly different (paired t-test; p = 0.062). Within SEFL rats Baseline and Post-shock and Baseline and Context-test freezing were significantly different (paired t-test; both p < 0.001), but Post-shock and Context-test were not (paired t-test; p = 0.620). As validation of SEFL conditioning across different cohorts it is important that these data follow the same trend as the freezing data from experiment 1.
Figure 4. Late SEFL: freezing behavior.
On the 28th day of withdrawal SEFL treatment was performed for SEFL (n =15) and control animals (n = 15). Behavioral differences in fear response between control and SEFL rats resulted in significantly increased freezing of SEFL rats for each sub-context measurement of animal activity: Baseline (un-paired t-test; *, p = 0.001), Post-shock and the Context Test measurement (un-paired t-test; **, p < 0.001).
After completion of SEFL treatment the 2BC EtOH drinking was reintroduced for 22 presentations. There was no difference in EtOH consumption between groups (Fig. 3A; 2-way RM ANOVA; F(1, 30) = 0.615, p = 0.439). There was also no interaction between SEFL treatment groups and presentation days (F(21, 629) = 0.654, p = 0.878). These data show that SEFL conditioning does not have an effect upon previously acquired drinking behaviors.
Control and SEFL rats started at similar preference levels and increased through the 25th presentation (Fig. 3B), and there were no between group differences across the entire pre-withdrawal drinking period when tested with 2-way RM ANOVA (F(1, 30) = 0.289, p = 0.595). During the 22 post-withdrawal presentations the control group increased their preference, however, this did not reach significance (2-way RM ANOVA; F(1, 30) < 0.001, p > 0.05).
Early-SEFL & 2BC Sucrose-Quinine
SEFL conditioning, done before 2BC drinking with Sucrose-Quinine, resulted in significantly increased freezing (Fig. 5) of SEFL (n = 7) compared to control rats (n = 7) with a group effect of significantly increased freezing of SEFL rats for each sub-context measurement: Baseline (un-paired t-test; p < 0.034), and both Post-shock and the Context Test measurement (un-paired t-test; p < 0.010). The freezing of the rats for experiment 3 was the same as that seen for experiments 1 and 2.
Figure 5. Early SEFL: freezing behavior.
Approximately 10 days before beginning 2BC Sucrose-Quinine drinking SEFL treatment was done. Behavioral differences in fear response between control (n = 7 that only received a single shock and SEFL (n = 7) rats that received 15 shocks in context A as well as a single shock in the novel context. The % freezing time is a reliable measure of fear. The Context Test measures the amount of fear learning that occurred to the novel context subsequent to the single shock. SEFL treatment resulted in significantly increased freezing compared to control rats within each sub-context measurement of animal freezing activity: Baseline (un-paired t-test; *, p < 0.05), Post-shock and Context Test (un-paired t-test; **, p < 0.01).
After SEFL treatment animals were given intermittent access to 2BC Sucrose-Quinine drinking. Sucrose-Quinine solution was used as an equivalent caloric substitute for 19% EtOH as well as mimicking some of the taste components of EtOH without the pharmacological properties (Di Lorenzo et al., 1986; Kiefer and Mahadevan, 1993). In the control group, Sucrose-Quinine consumption continued to increase until the 31st presentation (Fig. 6A), while the SEFL group Sucrose-Quinine consumption reached a plateau by the 17th presentation. These group differences, however, were not statistically significant; as we found no effect of SEFL treatment on Sucrose-Quinine consumption across pre-removal presentations using the 2-way RM ANOVA test (F(1, 12) = 0.331, p = 0.576).
Figure 6. Early SEFL: 2BC 28.4% sucrose-0.08% quinine.
Ten days before the beginning of 2BC 28.4% sucrose-0.08% quinine drinking was introduced, SEFL treatment was done to produce SEFL (n = 7) and control (n = 7) rats. A) There was no significant group effect of 28.4% sucrose-0.08% quinine consumption in SEFL compared to control rats across the initial 31 presentations (2-way RM ANOVA; F(1,12) = 0.331; p =0.576). The 28.4% sucrose-0.08% quinine was removed for 40 days. Upon re-introduction, control rats rapidly returned to pre-removal levels of 28.4% sucrose-0.08% quinine consumption while consumption by SEFL rats remained low. However, there was no significant difference in consumption after the 40 d removal (2-way RM ANOVA; F(1,12) = 1.609; p = 0.229). B) There was no significant difference in 28.4% sucrose-0.08% quinine preference between control and SEFL rats before or after removal (2-way RM ANOVA; F(1,12) = 0.0034; p = 0.955 and F(1,12) = 1.200; p = 0.295, respectively). While not significant, the average SEFL rat preference for 28.4% sucrose-0.08% quinine preference was lower than the control rat average preference for every presentation after the 40 d removal.
Sucrose-Quinine was removed for 40 days. Upon reintroduction, the average Sucrose-Quinine consumption of the SEFL and control groups were slightly, but not significantly, decreased (Fig. 6A). There were no significant group differences in Sucrose-Quinine consumption across the post-withdrawal presentations (2-way RM ANOVA; F(1, 12) = 1.609, p = 0.229). These data suggest that it is not the caloric or taste value of 19% EtOH which accounts for the significantly increased EtOH consumption of the SEFL group compared to the control group that was shown with the voluntary EtOH drinking in the first experiment. It is interesting to note that the trend of decreased Sucrose-Quinine consumption is opposite to the significantly increased EtOH consumption from Experiment 1 (Fig. 2A).
Prior to removal, Sucrose-Quinine preference of SEFL and control groups did not significantly differ (Fig. 6B, 2-way RM ANOVA; F(1, 12) = 0.0034, p = 0.955). There was also no post-removal group differences in Sucrose-Quinine preference (2-way RM ANOVA; F(1, 12) = 1.200, p = 0.295).
DISCUSSION
SEFL alters future EtOH drinking
We demonstrate that a single episode of un-signaled, inescapable foot-shock stress produces increased voluntary EtOH consumption in alcohol naïve Long-Evans rats. The increase in EtOH consumption is persistent even after 40 days of withdrawal and consumption is higher for previously stressed rats relative to unstressed controls upon reintroduction of EtOH (Fig. 2A). To our knowledge, only one previous study exposed EtOH naïve rats to foot-shock stress prior to voluntary EtOH drinking (Anisman and Waller, 1974). In contrast to our findings, Anisman and Waller (1974) observed that rats that received foot-shock stress increased their consumption transiently and did not maintain high level of EtOH consumption without daily foot-shock stress. However, differences in experimental parameters between the Anisman and Waller study and the present study may account for the differences in findings. In our study the SEFL treatment context and 2BC EtOH drinking context were absolutely different. This demonstrates that a single stressful event can increase voluntary EtOH drinking even in an environment completely dissociable from the stress environment. Analogous dissociation of drinking and stress environments may occur in populations with co-morbid PTSD and alcohol use disorder. Thus, highly differentiable stress and drinking environments are important procedural manipulations to incorporate into animal models of PTSD and voluntary EtOH consumption. Furthermore, the persistent increases in consumption and preference for EtOH, that we show to be produced by a single traumatic event, are in agreement with human data that shows persistent alcoholism years after experiencing a life-altering trauma (Dedert et al., 2009; Farrugia et al., 2011; McDevitt-Murphy et al., 2010; Ray et al., 2009; Savarese et al., 2001).
The large volume of data showing high rates of co-morbid AUDs and PTSD (Farrugia et al., 2011; Kehle et al., 2012; Kulka, 1990; McDevitt-Murphy et al., 2010; Simpson et al., 2012), in general show a correlation between the strength of PTSD symptoms and increased alcohol consumption. There are significant gender effects, such that, women with primary onset AUD and men with primary onset PTSD, have higher rates of depression compared to their counterparts (Back et al., 2005). Also evidence exists which shows earlier age of onset for drinking alcohol, higher rates of comorbid substance use disorders, poorer clinical prognosis and decreased effectiveness of cognitive therapy for patients comorbid for AUD and PTSD, when patients first experienced trauma beginning in childhood as compared to trauma experiences beginning as an adult (Farrugia et al., 2011). Importantly, PTSD severity leads to greater alcohol craving, whereas the reverse is not true; alcohol craving does not lead to increased PTSD symptom severity (Simpson et al., 2012). This clinical evidence is in agreement with the theory of self-medication, whereby patients will use drugs, such as alcohol, in order to treat their symptoms of PTSD (Roberts et al., 2000; Stewart, 1996). The Simpson et al., (2012) findings are analogous to the increased EtOH consumption of SEFL rats we have shown here (Fig. 2A). Further, SEFL-induced freezing in animals that already acquired EtOH drinking habits (Fig. 4) was not different from freezing behavior in EtOH-naïve SEFL rats (Fig. 1).
SEFL does not alter previously formed EtOH drinking habits
We have also demonstrated that rats previously exposed to voluntary EtOH drinking do not change their EtOH consumption after the stress of SEFL treatment. This finding is similar to many other studies where rats are exposed to foot-shock stress after baseline EtOH drinking phase (Choca et al., 1977; Darnaudery et al., 2007; Fidler and LoLordo, 1996); however, some studies have shown increases (Fullgrabe et al., 2007; Siegmund et al., 2005; Vengeliene et al., 2003) or decreases (Brunell and Spear, 2005) in EtOH consumption during foot-shock treatment days. Another group showed that withdrawal from forced EtOH administration facilitated contextual foot-shock fear-learning and increased subsequent 2BC EtOH drinking, and both increased EtOH drinking and increased fear were reduced by extinction training (Bertotto et al., 2010). It is well-established that chronic intermittent EtOH consumption results in numerous neuroadaptations in brain circuitry (Breese et al., 2005; Koob, 2003; Kumar et al., 2009), and our data suggests that voluntary EtOH consumption and withdrawal alters brain circuitry in a way that prevents SEFL conditioning from affecting established EtOH drinking habits (Fig. 3A).
While our experiments addressed issues of order of onset, very few clinical studies have addressed the order of onset of AUD and PTSD. A recent study evaluated personality traits and PTSD symptomatology in a group of National Guard soldiers deployed to Operation Iraqi Freedom to help differentiate between pre- and post-deployment onset AUD (Kehle et al., 2012). By dividing soldiers into four groups: 1) never had AUD, 2) had AUD prior to Iraq deployment, but no AUD symptoms after return, 3) persistent AUD before and after Iraq deployment, and 4) AUD onset after Iraq deployment, the authors showed that the only difference between groups 3 and 4 was that soldiers with new onset AUD reported more severe symptoms of post-deployment PTSD (Kehle et al., 2012). These results are consistent with literature demonstrating that PTSD symptoms are associated with heightened risk of increased drinking behavior and often precede substance use disorders (Davidson et al., 1990; McFarlane, 1998).
Our data appears to contradict studies which demonstrated selective stress-induced increases in voluntary EtOH consumption of rats previously made alcohol dependent using forced chronic intermittent EtOH (CIE) treatment (Bertotto et al., 2010; Sommer et al., 2008). It is worth noting that we tested EtOH consumption more than a week after SEFL conditioning whereas other studies measured EtOH consumption during stress days. It is also important to consider that while our voluntary EtOH drinking study may represent a model of habitual EtOH consumption it may not necessarily represent a model of alcohol dependence. The voluntary EtOH drinking paradigm we used results in blood EtOH levels of <150 mg/dl (Simms et al., 2008), and we did not observe significant withdrawal symptoms in these rats on the water days, however see (Li et al., 2011). Forced CIE administration by gavage, or vapor inhalation (Roberts et al., 2000; Sommer et al., 2008) consistently results in blood EtOH levels of >150 mg/dl and these high levels are necessary both to produce observable withdrawal symptoms after individual dosing, and for the long-lasting dependence on ethanol that follows CIE treatment and withdrawal (Liang et al., 2007; Roberts et al., 2000; Sommer et al., 2008). Therefore, established alcohol dependence may be a necessary condition for the detection of stress-induced alteration of voluntary alcohol consumption.
SEFL effect is specific to EtOH consumption
SEFL-induced increases in EtOH consumption may have been due to its caloric and taste properties rather than its pharmacological properties. Therefore, our last experiment was designed to determine the effects of SEFL on consumption of a caloric- and taste-equivalent control for 19% EtOH. The taste of EtOH is best mimicked by a mixture of bitter and sweet flavors, the closest cocktail is sucrose and quinine-HCl (Di Lorenzo et al., 1986; Kiefer and Mahadevan, 1993). SEFL conditioning did not significantly affect consumption of the Sucrose-Quinine solution (Fig. 6A). Our data are in agreement with a previous report showing that stress does not lead to increased preference for sucrose (Calvo-Torrent et al., 1999; Kant and Bauman, 1993). Thus, the increased EtOH consumption we observed in early-SEFL rats is likely due to the pharmacological effects of EtOH. Intriguingly, there was a trend for decreased Sucrose-Quinine average consumption of SEFL rats, especially after the 20th 2BC presentation and continuing after reintroduction from the 40 day withdrawal period. This trend of reduced Sucrose-Quinine consumption in SEFL rats is consistent with previous reports of reduced sucrose preference and increased finickiness towards quinine and may be an indication of anhedonia (Dess et al., 1988; Muscat and Willner, 1992).
CONCLUSIONS
In summary, this work shows that SEFL induced in EtOH-naïve rats by unpredictable and inescapable foot-shocks results in increased voluntary EtOH consumption, but does not affect previously acquired EtOH drinking behavior. Increased voluntary EtOH consumption is specific to EtOH since SEFL conditioning did not significantly affect Sucrose-Quinine consumption. These behavioral findings further validate the SEFL model as a rodent model of human PTSD. Future studies will seek to identify specific biochemical and functional brain changes produced by SEFL conditioning in order to develop treatments for this psychiatric disorder.
ACKNOWLEDGEMENTS
We are grateful to Douglas Mill, Ryan Tsuchida, Chriselle Ebreo, and Yatendra Mulpuri for help with treatment of experimental animals. We are grateful to Nidhi Shetty and the UCLA Department of Biostatistics for the statistical consulting work contributed to this manuscript. We also thank Dr. Selena Bartlett for helpful suggestions on experimental design. This study was made possible by NIH grants MH62122 (MSF), MH088184 (MSF) and AA016100 (IS).
References
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Anisman H, Waller TG. Effects of inescapable shock and shock-produced conflict on self selection of alcohol in rats. Pharmacol Biochem Behav. 1974;2:27–33. doi: 10.1016/0091-3057(74)90131-2. [DOI] [PubMed] [Google Scholar]
- Back SE, Jackson JL, Sonne S, Brady KT. Alcohol dependence and posttraumatic stress disorder: differences in clinical presentation and response to cognitive-behavioral therapy by order of onset. J Subst Abuse Treat. 2005;29:29–37. doi: 10.1016/j.jsat.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Bussolino DF, Molina VA, Martijena ID. Increased voluntary ethanol consumption and c-Fos expression in selected brain areas induced by fear memory retrieval in ethanol withdrawn rats. Eur Neuropsychopharmacol. 2010;20:568–581. doi: 10.1016/j.euroneuro.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a "kindling"/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Calvo-Torrent A, Brain PF, Martinez M. Effect of predatory stress on sucrose intake and behavior on the plus-maze in male mice. Physiol Behav. 1999;67:189–196. doi: 10.1016/s0031-9384(99)00051-7. [DOI] [PubMed] [Google Scholar]
- Choca JP, Wilson AS, Garcia TJ. Effects of shock and darkness on alcohol consumption by rats. J Stud Alcohol. 1977;38:2184–2187. doi: 10.15288/jsa.1977.38.2184. [DOI] [PubMed] [Google Scholar]
- Corley MJ, Caruso M, Takahashi LK. Stress-induced enhancement of fear conditioning and sensitization facilitates extinction-resistant and habituation-resistant fear behaviors in a novel animal model of posttraumatic stress disorder. Physiol Behav. 2011;105:408–416. doi: 10.1016/j.physbeh.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Louvart H, Defrance L, Leonhardt M, Morley-Fletcher S, Gruber SH, Galietta G, Mathe AA, Maccari S. Impact of an intense stress on ethanol consumption in female rats characterized by their pre-stress preference: modulation by prenatal stress. Brain Res. 2007;1131:181–186. doi: 10.1016/j.brainres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Kudler HS, Saunders WB, Smith RD. Symptom and comorbidity patterns in World War II and Vietnam veterans with posttraumatic stress disorder. Compr Psychiatry. 1990;31:162–170. doi: 10.1016/0010-440x(90)90020-s. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Green KT, Calhoun PS, Yoash-Gantz R, Taber KH, Mumford MM, Tupler LA, Morey RA, Marx CE, Weiner RD, Beckham JC. Association of trauma exposure with psychiatric morbidity in military veterans who have served since September 11, 2001. J Psychiatr Res. 2009;43:830–836. doi: 10.1016/j.jpsychires.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dess NK, Chapman CD, Minor TR. Inescapable shock increases finickiness about drinking quinine-adulterated water in rats. Learn Motiv. 1988;19:408–424. [Google Scholar]
- Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol. 1986;3:55–61. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Farrugia PL, Mills KL, Barrett E, Back SE, Teesson M, Baker A, Sannibale C, Hopwood S, Rosenfeld J, Merz S, Brady KT. Childhood trauma among individuals with co-morbid substance use and post traumatic stress disorder. Ment Health Subst Use. 2011;4:314–326. doi: 10.1080/17523281.2011.598462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Lolordo VM. Failure to find postshock increases in ethanol preference. Alcohol Clin Exp Res. 1996;20:110–121. doi: 10.1111/j.1530-0277.1996.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Freeman T, Kimbrell T. Relationship of alcohol craving to symptoms of posttraumatic stress disorder in combat veterans. J Nerv Ment Dis. 2004;192:389–390. doi: 10.1097/01.nmd.0000126735.46296.a4. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs NS, Cushman JD, Fanselow MS. The accurate measurement of fear memory in Pavlovian conditioning: Resolving the baseline issue. J Neurosci Methods. 2010;190:235–239. doi: 10.1016/j.jneumeth.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant GJ, Bauman RA. Effects of chronic stress and time of day on preference for sucrose. Physiol Behav. 1993;54:499–502. doi: 10.1016/0031-9384(93)90242-8. [DOI] [PubMed] [Google Scholar]
- Kehle SM, Ferrier-Auerbach AG, Meis LA, Arbisi PA, Erbes CR, Polusny MA. Predictors of postdeployment alcohol use disorders in National Guard soldiers deployed to Operation Iraqi Freedom. Psychol Addict Behav. 2012;26:42–50. doi: 10.1037/a0024663. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Mahadevan RS. The Taste of Alcohol for Rats as Revealed by Aversion Generalization Tests. Chem Senses. 1993;18:509–522. [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS. Trauma and the Vietnam war generation: Report of the findings fro mteh national Vietnam veterans readjustment study. New York: Brunner/Mazel; 1990. [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martijena ID, Rodriguez Manzanares PA, Lacerra C, Molina VA. Gabaergic modulation of the stress response in frontal cortex and amygdala. Synapse. 2002;45:86–94. doi: 10.1002/syn.10085. [DOI] [PubMed] [Google Scholar]
- McDevitt-Murphy ME, Williams JL, Bracken KL, Fields JA, Monahan CJ, Murphy JG. PTSD symptoms, hazardous drinking, and health functioning among U.S. OEF and OIF veterans presenting to primary care. J Trauma Stress. 2010;23:108–111. doi: 10.1002/jts.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall ME, Mackay PW, Donovan DM. Combat-related posttraumatic stress disorder and severity of substance abuse in Vietnam veterans. J Stud Alcohol. 1992;53:357–363. doi: 10.15288/jsa.1992.53.357. [DOI] [PubMed] [Google Scholar]
- McFarlane AC. Epidemiological evidence about the relationship between PTSD and alcohol abuse: the nature of the association. Addict Behav. 1998;23:813–825. doi: 10.1016/s0306-4603(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev. 1992;16:507–517. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Rau V, Eger EI, Harris RA, Fanselow MS. Amygdala transcriptome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology. 2010;35:1402–1411. doi: 10.1038/npp.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Ray LA, Capone C, Sheets E, Young D, Chelminski I, Zimmerman M. Posttraumatic stress disorder with and without alcohol use disorders: diagnostic and clinical correlates in a psychiatric sample. Psychiatry Res. 2009;170:278–281. doi: 10.1016/j.psychres.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese VW, Suvak MK, King LA, King DW. Relationships among alcohol use, hyperarousal, and marital abuse and violence in Vietnam veterans. J Trauma Stress. 2001;14:717–732. doi: 10.1023/A:1013038021175. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Stappenbeck CA, Varra AA, Moore SA, Kaysen D. Symptoms of Posttraumatic Stress Predict Craving Among Alcohol Treatment Seekers: Results of a Daily Monitoring Study. Psychol Addict Behav. 2012 Feb 27; doi: 10.1037/a0027169. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stewart SH. Alcohol abuse in individuals exposed to trauma: a critical review. Psychol Bull. 1996;120:83–112. doi: 10.1037/0033-2909.120.1.83. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wise RA. Maximization of ethanol intake in the rat. Adv Exp Med Biol. 1975;59:279–294. doi: 10.1007/978-1-4757-0632-1_19. [DOI] [PubMed] [Google Scholar]