Abstract
Although it is known that polycyclic aromatic hydrocarbons (PAHs) can be found in smoked meats, little is known about their prevalence in Native American smoked fish. In this work, the effect of traditional Native American fish smoking methods on dietary exposure to PAHs and possible risks to human health has been assessed. Smoking methods considered smoking structure (tipi or shed) and wood type (apple or alder). Neither smoking structure nor wood type accounted for differences in smoked salmon content of 33 PAHs. Carcinogenic and non-carcinogenic PAH loads in traditionally smoked salmon were 40–430 times higher than those measured in commercial products. Dietary exposure to PAHs could result in excess lifetime cancer risks between 1E-5 and 1E-4 at a daily consumption rate of 5 g d−1 and could approach 1E-2 at 300 g d−1. Hazard indexes approached 0.005 at 5 g d−1, or approximately 0.3 at 300 g d−1. Levels of PAHs present in smoked salmon prepared using traditional Native American methods may pose elevated cancer risks if consumed at high consumption rates over many years.
Keywords: Food safety, risk assessment, relative potency factor, smoked fish, Native American
INTRODUCTION
Traditional Native American food cooking and consumption serve an important role in many tribal nations. This is especially true for salmon preparation among tribes in the Pacific Northwest. The Confederated Tribes of the Umatilla Indian Reservation (CTUIR), situated in the Columbia River Basin, have historically relied on smoked salmon for sustenance and trading. Their smoking methods involve exposing salmon fillets directly to smoke from smoldering wood for several hours to 2–3 days. It is known that this process leads to deposition of combustion by-products on smoked foods. The traditional smoking of salmon is still practiced today and is a significant component of the CTUIR’s cultural and spiritual identity 1. However, smoking processes can introduce potentially harmful combustion by-products into the smoked fillets.
Polycyclic aromatic hydrocarbons (PAHs) are a group of combustion by-products that occur as mixtures in the particulate and gas phase of combustion smoke. Some PAHs are known to be mutagens and carcinogens in mammals 2–4. They are volatile to semi-volatile in character, composed predominately of carbon and hydrogen and consist of two or more fused aromatic rings with varying degrees of functional group substitution 5–6. Their physico-chemical properties allow them to sorb to meats during smoking processes with the degree of deposition being highly variable and related to smoking temperature 7, wood type 8–9, smoking technology 10, smoking duration 11, and the properties of the food being smoked 12. Additionally, PAH abundance profiles generated during combustion are closely related to combustion fuel type as demonstrated by PAH profile differences between coal, gasoline, diesel and wood fuels 8–9.
A few studies have investigated the impact of industrial smoking technologies on PAH food deposition 7, 10, 12–18. These studies identified smoking process factors critical to the generation of PAHs and proposed strategies to minimize their formation in order to achieve levels in compliance with regulatory standards. However, the generation and deposition of PAHs on foods from traditional subsistence smoking methods has received less attention 11–12, 19–20. The CTUIR’s Department of Science and Engineering requested information on PAH loads and associated risks for use by the Tribe’s Environmental Health Program.
In order to assist the Tribe’s Environmental Health Program, this study sought to: 1) characterize the effect of different traditional CTUIR smoking methods on the profile and concentration of parent and substituted PAHs in commonly smoked salmon, 2) compare traditionally smoked salmon PAH levels to those found in commercially available smoked salmon and 3) estimate potential risks associated with consumption of traditionally smoked salmon. Salmon catchment, preparation, smoking and analysis were carried out in collaboration between CTUIR members and researchers and Oregon State University researchers. This is the first known study to evaluate the impact of PAH exposure resulting from traditional Native American fish smoking methods on human health.
MATERIAL AND METHODS
Study design
Although smoking is done in both open and enclosed structures, this work describes smoking that takes place in enclosed spaces. Traditional smoking structures used by CTUIR include smoke sheds and tipis, which are large enough to smoke entire tanned hides, many salmon, and/or large quantities of game (Figure 1a and d). They are typically 2.5 × 2.5 × 2.5 m or larger and contain a smoke source in the bottom (hearth stoked with chunks of various types of wood) (Figure 1c and f). Raw foods destined for smoking are arranged on metal mesh racks within the shed or on hanging lines for the tipi (Figure 1b and e). In both cases, the goal is to generate as much smoke as possible in order to preserve the raw meat material. For this study, smoking events considered two factors, smoking structure (tipi or shed) and the type of wood used to smoke the salmon (apple or alder). The first round of smoking used apple wood in both structures, followed by a second round using alder. All salmon samples were prepared by traditional CTUIR smoking methods as if to be eaten.
Figure 1.

CTUIR shed and tipi smoking structures (A, D), salmon placement (B, E), and wood orientation (C, F) used for traditional salmon smoking.
Salmon catchment, filleting and smoking
Twenty spring Chinook salmon were purchased early on the morning of May 15, 2011 from a commercial CTUIR fisherman near Celilo, Oregon on the Columbia River. Whole salmon were immediately placed in ice filled coolers and transported to the smoking location on the Confederated Tribes of the Umatilla Indian Reservation four hours away. Salmon preparation and filleting took place in an enclosed building on an impermeable surface washed with a 10% bleach solution. The weight of each salmon was recorded and ranged from 4.5 –7.7 kg, with a mean weight of 6.1 kg (data not shown). Ten salmon to be used for the first round of smoking (with apple wood) were filleted during the afternoon of the day of the catch, stored in covered stainless steel trays and refrigerated at 4.5 °C until smoking. The ten remaining whole salmon were filleted just prior to the second smoking event (alder wood), which occurred one day later. Salmon were stored on ice at all times until being filleted. No brine or liquid smoke was used on any of the samples.
Just prior to smoking, ten fillets were placed skin down on a rack above/around the smoke shed smoke source (see Figure 1a–c), ten fillets were hung on lines strung above/across the middle of the tipi smoke source (Figure 1d-f) and ten ~ 100 g portions of non-smoked salmon were immediately placed into pre-cleaned amber jars and frozen at < -10° C. Salmon smoking generally proceeded as follows: the smoker arranged fish fillets in the smoking structures, started the fire, and periodically entered to add wood and check on the condition of the fillets until the desired dryness or hardness was reached. The first set of smoking events was conducted using ten salmon fillets and apple wood obtained from a local apple orchard. Apple wood smoking required approximately 22 hours in the shed and 24 hours in the tipi. The process required replenishment of the wood approximately every 2.5 – 3 hours throughout the entire process. The second set of smoking events was conducted using the remaining ten salmon and alder wood taken from creek banks along Iskuulpa Creek located within the Reservation boundaries. This smoking event proceeded as described for the first smoking event yielding a total of 20 non-smoked salmon samples for the study. Fillets were smoked for approximately 32 hours in the smoke shed and for approximately 33 hours in the tipi. The alder wood had been recently harvested and was therefore wetter, requiring more time to smoke and dry the fillets.
Post-smoking storage and transport
Smoked samples from both the tipi and the smoke shed were stored and transported in an identical manner, as required by OSU’s standard operating procedures which were drafted for this study. After the smoking process was completed, ~ 100 g full-thickness slices with skin were collected from each of the ten smoked salmon fillets and transferred to individual organics cleaned amber glass jars appropriately labeled for the wood and structure type. One sample was taken from each of the ten fillets yielding 40 smoked salmon samples. Jars were filled one-half to three-quarters full, immediately stored at <-10 °C for 20 days, transported on ice to the Food Safety and Environmental Stewardship laboratory at Oregon State University, and stored immediately at −20°C until extraction.
Laboratory methods
Chemical analysis
A total of 33 PAHs were quantified in this study. Standards composed of 16 EPA priority pollutant PAHs [naphthalene (NAP), acenaphthylene (ACY), acenaphthene (ACE), fluorine (FLO), phenanthrene (PHE), anthracene (ANT), pyrene (PYR), fluoranthene (FLA), chrysene (CHR), benz[a]anthracene (BAA), benzo[b]fluoranthene (BBF), benzo[k]fluoranthene (BKF), benzo[a]pyrene (BAP), indeno[1,2,3-cd]pyrene (IPY), benzo[g,h,i]perylene (BPY), dibenz[a,h]anthracene (DBA)], a custom mixture of 11 PAHs [1-methylnaphthalene (1-NAP), 2-methylnaphthalene (2-NAP), 1,2-dimethylnaphthalene (1,2-NAP), 1,6-dimethylnaphthalene (1,6-NAP), 1-methylphenanthrene (1-PHE), 2-methylanthracene (2-ANT), 1-methylpyrene (1-PYR), 6-methylchrysene (6-CHR), dibenzo[a,l]pyrene (DBP), dibenzothiophene (DBT), retene (RET)] and 6 single PAHs (2-methylphenanthrene (2-PHE), 3,6-dimethylphenanthrene (3,6-PHE), 9-methylanthracene (9-ANT), 2,3-dimethylanthracene (2,3-ANT), 9,10-dimethylanthracene (9,10-ANT), benzo[e]pyrene (BEP)] were purchased from AccuStandard (New Haven, CT) and guaranteed to be greater than 97% pure. Isotopically labeled acenaphthylene-D8, phenanthrene-D10, fluoranthene-D10, benzo[a]pyrene-D12, perylene-D12, indeno[1,2,3-cd]pyrene-D12 and benzo[g,h,i]perylene-D12 were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA) while naphthalene-D8 and chrysene-D12 were supplied by C/D/N Isotope Inc. (Quebec, Canada). Optima grade ethyl acetate, acetone, hexane and pesticide grade isooctane were purchased from Fisher Scientific (Pittsburgh, PA). High purity water was supplied by a Barnstead EASYpure UV compact ultrapure water system (Dubuque, IA). Commercially available Sampli-Q QuEChERS AOAC extraction salts (6 g of magnesium sulfate, 1.5 g sodium acetate/package) and 2 mL AOAC fatty sample dispersive solid-phase extraction tubes (50 mg PSA, 50 mg C18EC and 150 mg magnesium sulfate) were obtained from Agilent Technologies (Santa Clara, CA). Three commercially available smoked salmon were purchased from a local grocery store and analyzed in replicates of five for comparison to CTUIR smoked salmon.
PAH extraction, clean-up and quantification were conducted following methods previously described by Forsberg et al. 21 with some modifications. Briefly, smoked salmon fillet homogenates (1 g) were spiked with 100 μL of a 5 μg mL−1 surrogate PAH solution composed of naphthalene-D8, acenaphthylene-D8, phenanthrene-D10, fluoranthene-D10, chrysene-D12, benzo[a]pyrene-D12, benzo[g,h,i]perylene-D12, and extracted into a solution of high purity acetone, ethyl acetate, and isooctane (2:2:1; v/v/v). Extracts (1 mL) were then cleaned by dispersive solid-phase extraction, spiked with a solution of perylene-D12 and indeno[1,2,3-cd]pyrene-D12 internal standards, and analyzed using an Agilent 5975B GC-MS (Santa Clara, CA) with electron impact ionization (70 eV) and a DB-5MS column (30 m length, 0.25 μm film thickness, 0.25 mm I.D., Agilent J&W). See Supplemental Material, Table 1 for PAH instrument specific parameters. Sample extracts were stored in the dark at -20 °C for no greater than 10 days prior to analysis. Calibration curves of PAH relative response ratios were generated from seven calibration standards ranging from 1–1000 pg μL−1. Standards contained surrogate PAHs at 250 pg μL−1 and recovery internal standards at 500 pg μL−1 and 490 pg μL−1 for perylene-D12 and indeno[1,2,3-cd]pyrene-D12 respectively. Surrogate PAHs were used to quantify native PAHs. Recovery internal standards were used to quantify surrogate PAHs and provide estimates of losses incurred during laboratory analysis.
Quality assurance/control
Each analytical batch contained a minimum of 15% quality control samples. QC samples included matrix over-spikes, extraction duplicates, instrument blanks, continuing calibration verification standard analysis, and method blanks. Matrix over-spikes and extraction duplicates were performed along with every batch of ten fish samples. Matrix over spikes were within ±10% of expected values for all analytes except dibenzo[a,l]pyrene whose average recovery was ±60%. The average relative percent difference of analyte levels between extraction duplicates was < 15% except for pyrene (23%) and benzo[a]pyrene (19%). Instrument blanks were consistently below detection indicating GC-MS system cleanliness. Continuing calibration verification standards were all within ±15% of expected values except for dibenzo[a,l]pyrene which was within ±30%. Method blanks identified average background levels of naphthalene, 1-methyl naphthalene, 2-methyl naphthalene, fluoranthene, pyrene and benzo[g,h,i]perylene at 250, 17, 48, 13, 51, 4.6 μg kg−1 respectively. Contamination was sourced to dispersive solid-phase extraction materials. All reported values were subsequently background subtracted and evaluated by method reporting limits presented by Forsberg et al. 22 (see Supporting Information, Table 1).
Exposure, cancer and non-cancer risk calculations
Estimates of human dietary PAH exposure doses (mg kg−1 BW d−1) occurring over a lifetime were determined using equation 1, where C is the concentration (μg kg−1) of PAHs measured in smoked salmon, CF is a conversion factor (0.001 mg μg−1), IR is the CTUIR ingestion rate of smoked fish and BW represents body weight which was set to 70 kg.
| (Eq. 1) |
Among CTUIR members, fish consumption rates can be binned into categories of low (<100 g d−1), moderate (100–454 g d−1), and high or heritage (454–1000 g d−1) where 5–50% of total fish consumed are smoked. As a result, average daily doses were calculated for smoked fish consumption rates of 5 g d−1 (5% of 100 g d−1) and 300 g d−1 (50% of 600 g d−1) 23.
Carcinogenic risk estimates resulting from dietary exposure to PAHs were determined for each traditional smoking method across the range of average daily doses. All carcinogenic risk calculations were conducted using the potency factor adjusted level of benzo[a]pyrene equivalents (ΣPAH6),where ΣPAH6 represents the summed level of benzo[a]pyrene and relative potency factor adjusted fluoranthene, benzo[b]fluoranthene, benz[a]anthracene, chrysene and benzo[k]fluoranthene 24 (see Supporting Information, Table 2). Life-time excess cancer risks were calculated as the product of the dietary carcinogen exposure dose (mg kg−1 BW d−1) and benzo[a]pyrene’s slope factor value of 7.3 (mg kg−1 d−1)−1 (Eq. 2).
| (Eq. 2) |
Risk associated with dietary exposure to non-carcinogenic PAHs was evaluated using a hazard quotient approach for the previously described range of average daily doses. Hazard quotients represent a ratio of the exposure dose for each PAH divided by an oral chronic reference dose (RfD), where RfDs provide “an estimate of a daily oral exposure to the human population that is likely to be without an appreciable risk of deleterious effects during a lifetime” (Eq. 3) 25. For the purposes of this risk assessment, alkylated naphthalenes, phenanthrenes, anthracenes and pyrenes were summed with their parent PAHs and evaluated by non-alkylated parent PAH RfDs as described by the US FDA 26 (see Supporting Information, Table 2). Summation of individual hazard quotients (ΣPAH16 HQs) results in a hazard index (Eq. 4).
| (Eq. 3) |
| (Eq. 4) |
Statistical evaluation
Smoked salmon samples were chemically analyzed for thirty-three PAHs. Data below method reporting limits were not inputted. A two-way ANOVA accounting for the smoking method (tipi or shed) and wood used for smoking (apple or alder) was performed for each PAH. The model included the fixed main effects (STRUCTURE, WOOD) and the interaction (STRUCTURE × WOOD). The least significant difference for all pair-wise comparisons of the STRUCTURE × WOOD interactions was performed if the ANOVA F-test was statistically significant. An effect was deemed statistically significant for p≤0.05. Statistical analyses were performed using Matlab R2011a (version 7.12.0.635).
RESULTS
In total, 75 salmon samples prepared using traditional CTUIR and commercial smoking methods were chemically analyzed. Of the 33 PAHs analyzed, 10 were consistently below method reporting limits in all CTUIR smoked salmon (see Supporting Information, Table 3). These included dibenzothiophene, di-alkylated phenanthrenes and anthracenes, 6-methylchrysene and PAHs with molecular weights greater than 252 g mol−1 (i.e. indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene, benzo[ghi]perylene and dibenzo[a,l]pyrene). All PAHs were below detection in non-smoked CTUIR fresh salmon (controls) except for trace levels of fluorene (2.7 μg kg−1 over 20 replicate analyses). The lack of PAHs in non-smoked salmon indicates that the PAHs measured in smoked salmon fillets were wholly attributable to CTUIR’s smoking processes. The three commercially available smoked salmon products had ≤4 PAHs above reporting limits, where fluorene was the only PAH found in all commercial foods analyzed. Other PAHs quantified in commercially smoked salmon included phenanthrene, acenaphthylene, and acenaphthene; all at concentrations <28 μg kg−1 with most occurring at <12 μg kg−1 (see Supporting Information, Figure 1). Known carcinogenic PAHs were not found in any of the commercially produced smoked salmon.
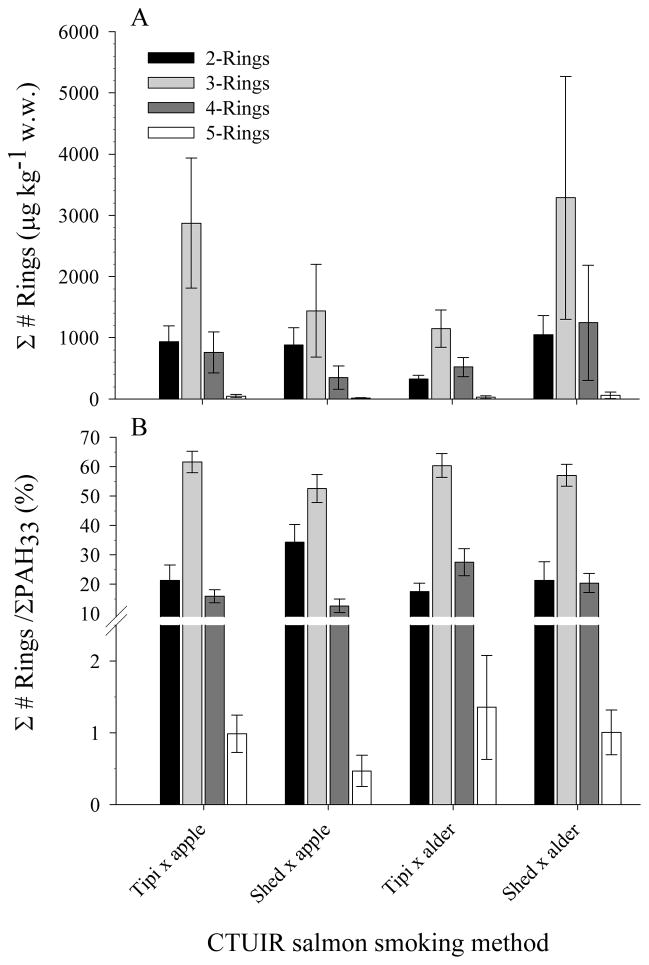
Average levels and chemical profiles for individual and structurally grouped PAHs measured in CTUIR smoked salmon are presented in Figures 2 and 3 respectively. Individual PAH levels ranged from < 2 – 3800 μg kg−1. Phenanthrene was the most abundant PAH found in all CTUIR traditionally smoked salmon followed by acenaphthylene, naphthalene, fluoranthene, pyrene, fluorene and anthracene. The summation of these 7 analytes accounted for 75–80% of the total mass of PAHs measured across all smoked salmon. Other PAHs that provided minor contributions to total PAH mass included mono- and di- alkylated naphthalenes (ave ΣPAH4 ~ 11% of total; 2-methyl NAP > 1-methyl NAP > 1,6-dimethyl NAP > 1,2-dimethyl NAP) and mono-alkylated phenanthrenes (ave ΣPAH2 ~ 5% of total; 2-methyl PHEN>1-methyl PHEN), where mono-alkylated naphthalene levels were on average 4 times greater than di-alkyl substituted naphthalenes (Figure 2a–d). Together, 2-ring, 2+3-ring, and 2+3+4-ring PAHs accounted for roughly 25%, 80% and > 98% of the total PAH mass measured across all CTUIR smoked salmon samples respectively (Figure 3a, b).
Figure 2.
Levels and profiles (mean ± SD) of non-carcinogenic (A-D) and carcinogenic (E-H) PAHs measured in salmon smoked by traditional Native American methods: tipi using apple wood (A, E), tipi using alder wood (B, F), shed using apple wood (C, G), and shed using alder wood (D, H). Individual effects (structure, wood) were dependent on each other and were not interpreted as significant (two-way ANOVA, interaction p-value < 0.001). ‘X’ indicates that an analyte was below method reporting limits.
Figure 3.
PAH abundance (mean ± SD) grouped by number of PAH rings expressed as (A) concentration and (B) proportion of total for salmon smoked by traditional Native American methods.
Several PAHs with ≥5 rings were consistently measured in CTUIR smoked salmon. In order of roughly decreasing amount, these included chrysene > benz[a]anthracene > benzo[b]fluoranthene > benzo[e]pyrene > benzo[a]pyrene ≈ benzo[k]fluoranthene ≈ benzo[b]fluoranthene. Regardless of smoking method, the summed level of these 7 compounds were generally ≤50 μg kg−1 and their contribution to total PAH mass was less than 2% (Figures 2e–h, 3). These levels are amongst the highest reported for modern day smoked foods 17, 27. Though individual PAH levels differed significantly within and between smoking methods, a statistically significant interaction of smoking method and wood type was observed for 21 of the 23 PAHs above detection limits. Therefore, individual effects (structure, wood) were dependent on each other and were not interpreted as significant (two-way ANOVA, interaction p-value < 0.001).
The average summed amounts of PAHs measured for each CTUIR salmon preparation method and three commercially available smoked salmon foods are presented in Figure 4. All salmon smoked by CTUIR, regardless of smoking method, had greater amounts of PAH residues than non-smoked and commercially available smoked salmon. PAH levels were generally 140–430 times greater in CTUIR smoked salmon than corresponding non-smoked salmon and between 40–430 times greater than those measured in commercially available smoked salmon samples. Marked differences in the PAHs identified were also evident as previously described.
Figure 4.
Summed levels of PAHs (mean ± SD) measured in salmon smoked by four traditional Native American methods, non-smoked wild caught salmon and three different commercially available smoked salmon (CTUIR smoked salmon, n = 10/smoking method; non-smoked salmon, n = 20; commercial smoked salmon, n = 5 replicate analyses/sample).
Levels of PAHs measured in CTUIR smoked salmon grouped by mode of toxicity are presented in Table 1. Of the 40 CTUIR smoked salmon samples analyzed, four had benzo[a]pyrene levels that were below reporting limits (< 2 μg kg−1), seven contained 2–5 μg kg−1, thirteen contained 5–10 μg kg−1,fifteen were 10–30 μg kg−1and one was > 35 μg kg−1. Average levels of benzo[a]pyrene and ΣPAH6 were highest in salmon smoked in the shed using alder wood (17 ± 15; 110 ± 89 μg kg−1) and tipi using apple wood (13 ± 7; 71 ± 35 μg kg−1) followed by salmon smoked in the tipi using alder wood (8 ± 6; 49 ± 24 μg kg−1) and shed using apple wood (6 ± 2; 26 ± 17 μg kg−1). Across all smoking methods, fluoranthene was the largest contributor to the RPF-adjusted concentration of carcinogenic PAHs followed by benzo[b]fluoranthene, benz[a]anthracene and chrysene. Similarly, average summed levels of non-carcinogenic PAHs (ΣPAH16) were highest in salmon smoked in the shed using alder wood (4700 ± 2800 μg kg−1) and tipi using apple wood (3900 ± 1400 μg kg−1) and lower in those smoked in the shed using apple wood (2300 ± 1000 μg kg−1) and tipi with alder wood (1700 ± 410 μg kg−1). Phenanthrene, acenaphthylene, fluoranthene, pyrene, fluorene, anthracene, and naphthalene levels were the major contributors to ΣPAH16 in all CTUIR smoked salmon.
Table 1.
Carcinogenic and non-carcinogenic PAH loads, average daily doses and risks (mean ± SD) for traditionally smoked salmon at two ingestion rates.
| Native American Salmon Smoking Methodsa
|
|||||
|---|---|---|---|---|---|
| Toxicity category | Parameter | Tipi × apple | Shed × apple | Tipi × alder | Shed × alder |
| Carcinogenic | PAH Load (ug kg−1 w.w.)b | ||||
| ΣPAH6 | 7.1 ± 3.5 × 101 | 2.6 ± 1.7 × 101 | 4.9 ± 2.4 × 101 | 1.1 ± 0.9 × 102 | |
| Average Daily Dose (mg kg−1 BW d−1) | |||||
| 5 g d−1 ΣPAH6 | 5.1 ± 2.5 × 10−6 | 1.9 ± 1.2 × 10−6 | 3.5 ± 1.7 × 10−6 | 7.8 ± 6.4 × 10−6 | |
| 300 g d−1 ΣPAH6 | 3.1 ± 1.5 × 10−4 | 1.1 ± 0.7 × 10−4 | 2.1 ± 1.0 × 10−4 | 4.7 ± 3.8 × 10−4 | |
| Life-time Excess Cancer Risk | |||||
| 5 g d−1 ΣPAH6 | 3.7 ± 1.8E-5 | 1.4 ± 0.9E-5 | 2.6 ± 1.2E-5 | 5.7 ± 4.7E-5 | |
| 300 g d−1 ΣPAH6 | 2.2 ± 1.1E-3 | 8.2 ± 5.2E-4 | 1.5 ± 0.8E-3 | 3.4 ± 2.8E-3 | |
| Non-carcinogenic | PAH Load (ug kg−1 w.w.)c | ||||
| ΣPAH16 | 3.9 ±1.4 × 103 | 2.3 ± 1.0 × 103 | 1.7 ± 0.4 × 103 | 4.7 ± 2.8 × 103 | |
| Average Daily Dose (mg kg−1 BW d−1) | |||||
| 5 g d−1 ΣPAH16 | 2.8 ± 1.0 × 10−4 | 1.6 ± 0.7 × 10−4 | 1.2 ± 0.3 × 10−4 | 3.4 ± 2.0 × 10−4 | |
| 300 g d−1 ΣPAH16 | 1.7 ± 0.6 × 10−2 | 9.8 ± 4.2 × 10−3 | 7.4 ± 1.8 × 10−3 | 2.0 ± 1.2 × 10−2 | |
| Hazard Indexd | |||||
| 5 g d−1 ΣPAH16 HQs | 0.0058 ±0.0018 | 0.0044 ±0.0015 | 0.0024 ±0.0005 | 0.0071 ±0.0034 | |
| 300 g d−1 ΣPAH16 HQs | 0.35 ±0.11 | 0.26 ±0.09 | 0.14 ±0.03 | 0.43 ±0.21 | |
All estimates were calculated from n = 10 salmon fillets/method.
ΣPAH6 represents the summed level of benzo[a]pyrene and RPF-adjusted carcinogenic PAHs.
ΣPAH16 represents the summed level of non-carcinogenic PAHs.
ΣPAH16 HQ represents the summed level of RfD-adjusted non-carcinogenic PAHs.
Dietary PAH average daily doses (mg kg−1 d−1) and their corresponding risk estimates are presented in Table 1 and are reflective of a wide range of smoked salmon ingestion rates. After converting PAH concentrations to benzo[a]pyrene equivalents and then to daily doses of benzo[a]pyrene equivalents, risks were estimated using the benzo[a]pyrene cancer slope factor . Estimated life-time excess cancer risks resulting from exposure to carcinogenic PAHs (ΣPAH6) ranged from 1.4 ± 0.9 E-5 to 5.7 ± 4.7 E-5 (at a consumption rate of 5 g d−1) and from 8.2 ± 5.2 E-4 to 3.4 ± 2.8 E-3 (at a consumption rate of 300 gd−1) across all smoking methods. Exposure to non-carcinogenic PAH mixtures resulted in hazard indexes (ΣPAH16 HQs) ranging from 0.0024 ± 0.0005 to 0.0071 ± 0.0034 (at 5g d−1) and from 0.14 ± 0.03 to 0.43 ± 0.21 (at 300 g d−1) across all smoking methods. All smoking methods resulted in hazard indexes less than unity. Salmon smoked in the shed with alder wood consistently produced the largest observed values for carcinogenic and non-carcinogenic PAHs.
DISCUSSION
A number of different PAHs associated with biomass combustion were identified in traditionally smoked CTUIR salmon fillets. These included PAHs composed of 2–6 benzene rings, predominantly those with ≤4 rings, which displayed a low degree of alkylation. The PAHs identified were similar to those reported in traditional Nigerian smoked fish 20, fish prepared using traditional German smoking kilns 15, and other smoked meat studies 12, 18. Levels of PAHs in CTUIR smoked salmon, however, were of the highest reported and were paralleled only by those measured in traditionally smoked Nigerian fishes and fish prepared by ‘home-smoking’ methods 13, 19–20.
It is known that several factors can influence the concentration of PAHs in smoked meats. For instance, Duedhal-Olesen et al. 7 reported a 200% increase in the average sum of 25 PAHs in smoked salmon associated with hot ( 65–80 °C ) versus cold (15–30 °C) smoking and a 120–180% increase when herring and mackerel fillets received direct versus indirect combustion smoke exposure. Similar trends have been found for the influence of different combustion woods and smoking duration on smoked food PAH content where soft resinous woods and longer smoking durations resulted in higher PAH content foods 7–8, 11. However, regardless of method used, all CTUIR fish were smoked under ‘hot’ conditions (90–120 °C) with direct exposure to combustion smoke generated from two types of hard wood. Although we expected to find substantial differences in smoked salmon PAH content related to one of the smoking factors (wood type or smoking structure), no statistically significant differences were found. The remarkable similarity in PAH profiles observed for CTUIR smoked salmon and the lack of a treatment effect demonstrate that all CTUIR smoking methods produce a smoked food with a similar level and profile of PAH deposition (Figures 2–3).
Although no differences in PAH loads related to smoking structure or wood type were observed, substantial differences were noted between CTUIR’s smoked salmon and commercially produced smoked salmon. Across all store purchased smoked salmon samples PAH loads were < 60 μg kg−1 and two-thirds of the samples contained ~ 15 μg kg−1. These levels were 40–430 times lower than those measured in CTUIR smoked salmon and comparable to CTUIR’s non-smoked wild caught salmon (Figure 4). Furthermore, none of the commercially smoked salmon had detectable amounts of carcinogenic PAHs. The observed differences probably reflect the highly automated, controlled and standardized smoking systems used in modern smoke houses. These methods often use computer controlled external smoke generators, standardized temperature programs and relatively short smoking durations 13, 15, 20. Conversely, the PAH content in CTUIRs smoked fish likely depends on factors related directly to the smoking event such as smoking intensity, duration and wood moisture content.
To estimate human health impacts resulting from exposure to CTUIR’s traditionally smoked salmon, risks were estimated using standard risk equations, a body weight of 70 kg and ingestion rates of 5 and 300 g d−1. Estimates of excess life-time cancer risk at 5 g d−1 were between 1E-5 and 1E-4, and at 300 g d−1 they were close to or above 1E-3. Inclusion of RPF-adjusted PAHs into risk models led to cancer risk estimates up to 6 times greater than those based on benzo[a]pyrene alone. Similar results have been reported for dietary exposure to commonly consumed Nigerian smoked fish prepared by traditional smoking methods 20. These levels will require careful deliberation when crafting health advice since they are above the “point of departure” (1E-6) for risk assessment.
Though estimated average daily doses to non-carcinogenic PAHs were routinely 25–80 times greater than those of carcinogenic PAHs (Table 1), non-carcinogenic PAHs produced hazard indexes less than or approaching one; a level described by the EPA as generally having no appreciable risk for the development of non-cancer health effects. Taken together, risks associated with carcinogenesis pose the largest threat to human health. This coincides with other smoked food risk assessments and is the basis for establishment of regulatory limits for carcinogenic PAHs in smoked meats by the European Food Safety Authority, specifically for food contaminated with benzo[a]pyrene 20, 27.
It is important to emphasize that the aim of the present study was to estimate potential risk associated with consumption of traditional smoked salmon and not to quantify actual risk for CTUIR members. When the Tribe’s environmental health program interpret the results, the assumptions used and uncertainties that exist will be considered. For instance, the effect of PAHs and other chemicals not included in this assessment which have tested positive for carcinogenic effects, such as benzo[j]fluoranthene and benzo[c]fluorine, is not well understood 27. Additionally, the use of relative potency factors for determining cancer risk resulting from exposure to chemical mixtures has many assumptions that could affect risk estimates; assumptions described as problematic by the European Food Safety Authority 27. It is also not clear how smoked salmon moisture content affects exposure PAH concentrations; the conversion to caloric intake may need to be considered. Lastly, it was outside the scope of this study to assess the impact of other co-risk factors, such as environmental PAH exposures, underlying health and nutrition, and individual/ethnic differences in metabolism on calculated risk values 28–29.
We routinely measured 16 non-carcinogenic and 6 carcinogenic PAHs in salmon smoked by traditional Native American methods. No differences in PAH content related to smoking structure or wood type were found. PAH profiles agree well with other reports, but levels were of the highest reported and were significantly greater than those measured in commercially prepared store purchased smoked salmon. Levels of PAHs present in smoked salmon prepared using traditional Native American methods may pose elevated cancer risks if consumed at high consumption rates over many years. The CTUIR will use the reported findings to assist in the development of culturally-appropriate risk management strategies.
Supplementary Material
Supporting Information, Table 1. Retention times, monitored quantitation and confirmation ions, and method reporting limits (MRLs) for 33 PAHs and 9 isotopically labeled PAHs by GC-MS
Supporting Information, Table 2. PAH reference toxicity values used to generate risk estimates for exposure to CTUIR traditionally smoked salmon
Supporting Information, Table 2. PAH reference toxicity values used to generate risk estimates for exposure to CTUIR traditionally smoked salmon
Supporting Information, Table 3. PAH ranges (μg kg−1w.w.) and number of replicates (n) above reporting limits measured in salmon smoked by traditional Native American methods.
Supporting Information, Figure 1. PAH profiles measured in commercially available smoked salmon (n = 5).
Acknowledgments
Thanks are owed to the CTUIR (Chris Harris, Michelle Burke, James Bronson, Deborah Harris) and OSU (Glenn Wilson, Ricky Scott, Kristin Pierre, Jorge Padilla, Kevin Hobbie, Lane Tidwell, and Oleksii Motorykin) participants for contributions to the completion of the study.
Abbreviations
- CTUIR
Confederated Tribes of the Umatilla Indian Reservation
- PAH
polycyclic aromatic hydrocarbon
- OSU
Oregon State University
- AOAC
Association of Official Analytical Chemists
- EC
European Commission
- GC-MS
gas chromatography-mass spectrometry
- PSA
primary/secondary amine
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged and Safe
- SIM
selective ion monitoring
- EPA
Environmental Protection Agency
- QC
Quality Control
- ATSDR
Agency for Toxic Substance and Disease Registry
- FDA
Food and Drug Administration
- EFSA
European Food Safety Authority
- RPF
Relative Potency Factors
Footnotes
Supporting Information Description: Three additional tables and one figure have been provided as supplemental information. They include SI Table 1, Retention times, monitored quantitation and confirmation ions, and method reporting limits for 33 PAHs and 9 isotopically labeled PAHs by GC-MS; SI Table 2, PAH toxicity reference values used to generate risk estimates for exposure to CTUIR traditionally smoked salmon; SI Table 3, PAH ranges and number of replicates above reporting limits measured in salmon smoked by traditional Native American methods; and SI Figure 1, PAH profiles measured in commercially available smoked salmon. This information is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Harris SG, Harper BL. A Native American exposure scenario. Risk Anal. 1997;17:789–795. doi: 10.1111/j.1539-6924.1997.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 2.Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon- DNA adducts and mechanism of action. Environ Mol Mutagen. 2005;45:106–114. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- 3.Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol Appl Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 5.Layshock J, Simonich SM, Anderson KA. Effect of dibenzopyrene measurement on assessing air quality in Beijing air and possible implications for human health. J Environ Monit. 2010;12:2290–2298. doi: 10.1039/c0em00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem. 2002;33:489–515. [Google Scholar]
- 7.Duedahl-Olesen L, Christensen JH, Hojgard A, Granby K, Timm-Heinrich M. Influence of smoking parameters on the concentration of polycyclic aromatic hydrocarbons (PAHs) in Danish smoked fish. Food additives & contaminants. 2010;27:1294–1305. doi: 10.1080/19440049.2010.487074. [DOI] [PubMed] [Google Scholar]
- 8.Stumpe-Viksna I, Bartkevics V, Kukare A, Morozovs A. Polycyclic aromatic hydrocarbons in meat smoked with different types of wood. Food Chem. 2008;110:794–797. [Google Scholar]
- 9.Khalili NR, Scheff PA, Holsen TM. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos Environ. 1995;29:533–542. [Google Scholar]
- 10.Kuhn K, Nowak B, Behnke A, Seidel A, Lampen A. Effect-based and chemical analysis of polycyclic aromatic hydrocarbons in smoked meat: a practical food-monitoring approach. Food additives & contaminants. 2009;26:1104–1112. doi: 10.1080/02652030902855414. [DOI] [PubMed] [Google Scholar]
- 11.Djinovic J, Popovic A, Jira W. Polycyclic aromatic hydrocarbons (PAHs) in traditional and industrial smoked beef and pork ham from Serbia. Euro Food Res Tech. 2008;227:1191–1198. [Google Scholar]
- 12.Santos C, Gomes A, Roseiro LC. Polycyclic aromatic hydrocarbons incidence in Portuguese traditional smoked meat products. Food Chem Toxicol. 2011;49:2343–2347. doi: 10.1016/j.fct.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Larsson BK. Polycyclic aromatic hydrocarbons in smoked fish. Z Lebensm Forsch A. 1982;174:101–107. [Google Scholar]
- 14.Djinovic J, Popovic A, Jira W. Polycyclic aromatic hydrocarbons (PAHs) in different types of smoked meat products from Serbia. Meat Sci. 2008;80:449–456. doi: 10.1016/j.meatsci.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Karl H, Leinemann M. Determination of polycyclic aromatic hydrocarbons in smoked fishery products from different smoking kilns. Z Lebensm Forsch A. 1996;202:458–464. doi: 10.1007/BF01197265. [DOI] [PubMed] [Google Scholar]
- 16.Moret S, Conte L, Dean D. Assessment of polycyclic aromatic hydrocarbon content of smoked fish by means of a fast HPLC/HPLC method. J Agric Food Chem. 1999;47:1367–1371. doi: 10.1021/jf9808877. [DOI] [PubMed] [Google Scholar]
- 17.Wretling S, Eriksson A, Eskhult GA, Larsson B. Polycyclic aromatic hydrocarbons (PAHs) in Swedish smoked meat and fish. J Food Compos Anal. 2010;23:264–272. [Google Scholar]
- 18.Lorenzo JM, Purriños L, Bermudez R, Cobas N, Figueiredo M, García Fontán MC. Polycyclic aromatic hydrocarbons (PAHs) in two Spanish traditional smoked sausage varieties: “Chorizo gallego” and “Chorizo de cebolla”. Meat Sci. 2011;89:105–109. doi: 10.1016/j.meatsci.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Afolabi OA, Adesulu EA, Oke OL. Polynuclear aromatic hydrocarbons in some Nigerian preserved freshwater fish species. J Agric Food Chem. 1983;31:1083–1090. doi: 10.1021/jf00119a040. [DOI] [PubMed] [Google Scholar]
- 20.Akpambang VOE, Purcaro G, Lajide L, Amoo IA, Conte LS, Moret S. Determination of polycyclic aromatic hydrocarbons (PAHs) in commonly consumed Nigerian smoked/grilled fish and meat. Food Addit Contam. 2009;26:1096–1103. doi: 10.1080/02652030902855406. [DOI] [PubMed] [Google Scholar]
- 21.Forsberg ND, Wilson GR, Anderson KA. Determination of parent and substituted polycyclic aromatic hydrocarbons in high-fat salmon using a modified QuEChERS extraction, dispersive SPE and GC–MS. J Agric Food Chem. 2011a;59:8108–8116. doi: 10.1021/jf201745a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsberg ND, Wilson GR, Anderson KA. Addition to determination of parent and substituted polycyclic aromatic hydrocarbons in high-fat salmon using a modified QuEChERS extraction, dispersive SPE and GC–MS. J Agric Food Chem. 2011b;59:10773–10773. doi: 10.1021/jf201745a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper BL, Harding AK, Waterhous T, Harris SG. Traditional Tribal subsistence exposure scenario and risk assessment guidance manual. Corvallis: Oregon State University; 2007. [accessed 27 January 2012]. URL ( http://www.box.com/shared/70r3579u5gh7ysdugfv7.) [Google Scholar]
- 24.USEPA (US Environmental Protection Agency) External review draft. Washington, D.C: U.S. EPA, Integrated Risk Information System; 2010. Development of a relative potency factor (RPF) approach for polycyclic aromatic hydrocarbon (PAH) mixtures. [Google Scholar]
- 25.ATSDR. Oregon Department of Human Services Superfund Health Investigation and Education Program. U.S. Department of Health and Human Services; Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2006. Public Health Assessment: Portland Harbor, Multnomah County, Oregon, EPA Facility ID: OR0001297969. [Google Scholar]
- 26.USFDA (US Food and Drug Administration) Protocol for interpretation and use of sensory testing and analytical chemistry results for re-opening oil-impacted areas closed to seafood harvesting due to the Deepwater Horizon Oil Spill. U.S. Food and Drug Administration; 2010. [accessed 21 October 2010]. Available: URL ( http://www.fda.gov/food/ucm217601.htm.) [Google Scholar]
- 27.EFSA. Polycyclic aromatic hydrocarbons in food: scientific opinion of the panel on contaminants in the food chain. The EFSA J. 2008;724:1–114. [Google Scholar]
- 28.Donatuto J, Harper BL. Issues in evaluating fish consumption rates for Native American tribes. Risk Anal. 2008;28:1497–1506. doi: 10.1111/j.1539-6924.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- 29.Harris SG, Harper BL. A Native American exposure scenario. Risk Anal. 1997;17:789–795. doi: 10.1111/j.1539-6924.1997.tb01284.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information, Table 1. Retention times, monitored quantitation and confirmation ions, and method reporting limits (MRLs) for 33 PAHs and 9 isotopically labeled PAHs by GC-MS
Supporting Information, Table 2. PAH reference toxicity values used to generate risk estimates for exposure to CTUIR traditionally smoked salmon
Supporting Information, Table 2. PAH reference toxicity values used to generate risk estimates for exposure to CTUIR traditionally smoked salmon
Supporting Information, Table 3. PAH ranges (μg kg−1w.w.) and number of replicates (n) above reporting limits measured in salmon smoked by traditional Native American methods.
Supporting Information, Figure 1. PAH profiles measured in commercially available smoked salmon (n = 5).