Abstract
Over the last two decades, significant research attention has been given to the acute effect of a single bout of exercise on postprandial lipaemia. A large body of evidence supports the notion that an acute bout of aerobic exercise can reduce postprandial triacylglycerol (TAG) concentrations. However, this effect is short-lived emphasising the important role of regular physical activity for lowering TAG concentrations through an active lifestyle. In 1995, the concept of accumulating physical activity was introduced in expert recommendations with the advice that activity can be performed in several short bouts throughout the day with a minimum duration of 10 minutes per activity bout. Although the concept of accumulation has been widely publicised, there is still limited scientific evidence to support it but several studies have investigated the effects of accumulated activity on health-related outcomes to support the recommendations in physical activity guidelines. One area, which is the focus of this review, is the effect of accumulating exercise on postprandial lipaemia. We propose that accumulating exercise will provide additional physical activity options for lowering postprandial TAG concentrations relevant to individuals with limited time or exercise capacity to engage in more structured forms of exercise, or longer bouts of physical activity. The benefits of accumulated physical activity might translate to a reduced risk of cardiovascular disease in the long-term.
Keywords: Physcal activity, Postprandial period, Lipid metabolism, Cardiovascular disease
INTRODUCTION
In 1995 the Centers for Disease Control and Prevention and the American College of Sports Medicine published physical activity guidelines which stated that adults should accumulate 30 minutes of moderate intensity activity on most, preferably all, days of the week [1]. The concept of accumulating physical activity was novel at this time, but the guidelines included the caveat that the minimum duration of any one bout should be 10 minutes. Shortly after, the US National Institutes of Health [2] and the US Surgeon General's report [3] made similar recommendations and the concept of accumulation has been endorsed by the Department of Health in the United Kingdom [4]. Within all these guidelines a minimum exercise duration of 10 minutes has been recommended for individual activity bouts.
The latest US Physical Activity Guidelines define accumulation as, "the concept of meeting a specific physical activity dose or goal by performing activity in short bouts, then adding together the time spent during each of these bouts" [5]. The rationale behind the concept of accumulation for health is based on the assumption that the activities reported in many epidemiological studies, such as walking, stair climbing and gardening, are intermittent in nature [6-10]. A feature of many of these activities is that they can be accumulated throughout the day rather than being performed in one continuous bout. The nature of accumulating physical activity is distinct from intermittent exercise training where athletes use repeated bouts of high intensity work intervals to achieve improvements in exercise performance. Nonetheless, the definition of accumulation includes no statement on the intensity of physical activity or the time spent in each bout. Thus, there is considerable variation in the physical activity bouts used in studies examining the accumulation of exercise.
Evidence that accumulating activities translates into health benefits and the inclusion of accumulation as a recommendation into physical activity guidelines was not strongly supported initially [1,11,12]. Nevertheless, since the original guidelines were issued better evidence has emerged to support this concept. For example, a previous large prospective study indicates that accumulating short bouts of physical activity confers equal benefit in reducing coronary heart disease risk as performing one longer bout, provided that the total amount of energy expended is similar [13]. However, caution is required when interpreting the results of this study. The activity levels in this report are based solely on observation, therefore the precise activity patterns required to confer protection from cardiovascular disease are unknown. Another study investigated whether lifestyle physical activity improves cardiorespiratory fitness and blood pressure to a similar extent as structured exercise in sedentary men and women [14]. In the lifestyle physical activity group, participants were asked to accumulate at least 30 minutes of moderate-intensity physical activity (i.e., adapted to each individual's lifestyle) on most days of the week for 6 months and they were asked to maintain this activity for a further 18 months at the end of the initial six months. The structured exercise group received a 'traditional' exercise prescription, performing exercise 20 to 60 minutes at a time at an intensity of 50% to 85% of maximum oxygen uptake for 3 to 5 days a week. After 24 months, both groups showed a significant reduction in resting systolic and diastolic blood pressure, and a significant improvement in physical activity and cardiorespiratory fitness. Murphy et al. [15] studied 21 middle-aged sedentary individuals over a 6-week period. In a randomised, cross-over design, participants performed brisk walking 5 days a week either in three 10-minute sessions throughout the day or one 30-minute session. The psychological parameters of tension/anxiety decreased in both groups. There were also favourable effects on blood lipids, body composition and cardiorespiratory fitness in both exercise programmes.
In addition, several intervention studies have investigated the issue of accumulating exercise for health [16]. The majority of these studies have reported that regular accumulated exercise confers a positive benefit on fitness, blood lipids, blood pressure, blood glucose, insulin and body composition. The findings from these studies are supportive of the concept that accumulating activity is beneficial to health. For specific details regarding these studies readers are referred to a relevant review article [16]. The purpose of the present review is to examine the effects of accumulating exercise on postprandial triacylglycerol (TAG) concentrations and to provide practical guidance for those wishing to engage in exercise that may lower TAG concentrations. The issue of accumulating exercise for health is important because if exercise sessions can be partitioned throughout the day there are possible applications for sedentary or older populations who might find it easier to perform shorter bouts of exercise rather than one long bout due to their low physical fitness levels.
A search was made using PubMed from December 1975 through February 2012 and 180 studies were located. Key words used in combination included "postprandial triacylglycerol," and "postprandial triglyceride," and "exercise". The search incorporated any article that was published in English and was cross-checked, and supplemented using the authors' personal libraries. Criteria for inclusion in this review were: 1) the dependent variable was postprandial TAG concentration collected from humans, 2) studies were designed to compare the effects of exercise performed in one continuous bout with two or more accumulated bouts and a control resting condition, and 3) studies were designed to compare the effects of exercise performed in two or more accumulated bouts and a control resting condition. Criteria for exclusion of a study were: 1) short intermittent bouts of repeated exercise performed with short recovery (i.e., 1 to 10 minutes) and 2) exercise training studies. Nine studies met inclusion criteria from the 180 studies retrieved. One reference was added to this review since this study [17] was not available in PubMed at the time of publication. A summary of the 10 included studies is provided in Table 1.
Table 1.
Summary of short-term studies examining the effects of accumulated exercise on postprandial lipaemia
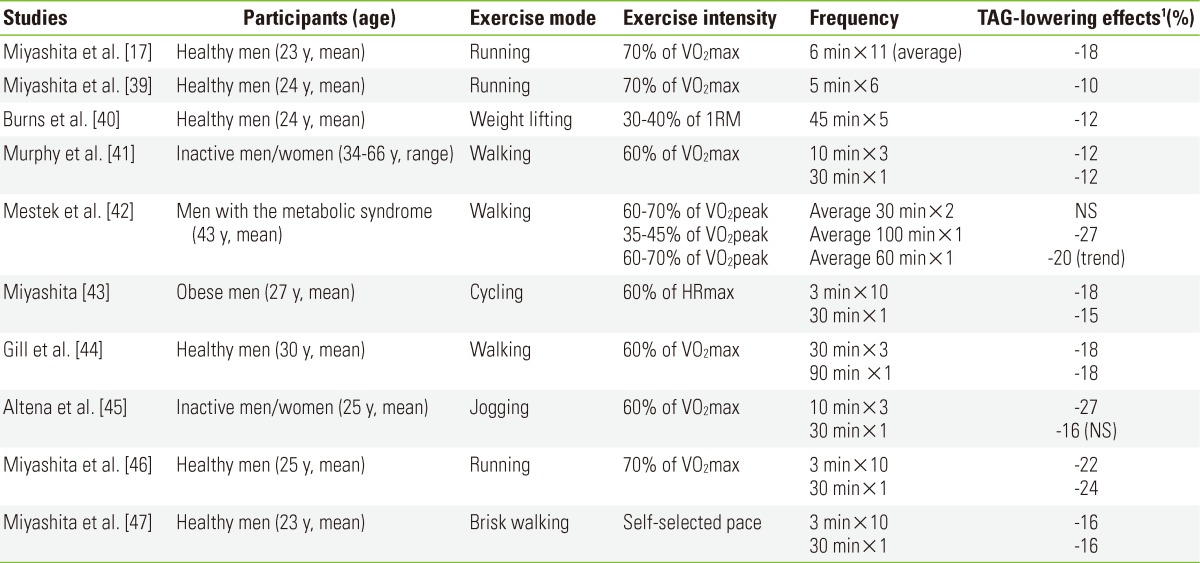
TAG, triacylglycerol; VO2, oxygen uptake; RM, repetition maximum; HR, heart rate; NS, not significant.
1Values are compared with the control trial.
EVIDENCE THAT THE POSTPRANDIAL STATE IS IMPORTANT TO ASSESS
In 1979, Zilversmit [18] proposed that atherosclerosis was a postprandial phenomenon. Good evidence now supports the notion that the impaired clearance of TAG-rich lipoproteins, namely chylomicrons and very low density lipoproteins, and increased hepatic secretion of very low density lipoproteins are a strong risk factor for coronary heart disease [19, 20]. Measurement of fasting TAG concentration does not necessarily provide the best index of TAG metabolic capacity, as the major catabolic pathway of TAG-rich lipoproteins occurs during the postprandial state [21]. Moreover, people spend most of their lifetime, up to three-quarters of each day, in the postprandial state. Thus, repeated daily episodes of increased concentrations of postprandial TAG, often termed postprandial lipaemia, and prolonged residence in the circulation of TAG-rich lipoproteins are considered a risk factor for cardiovascular disease [22,23]. This notion is supported by the findings of case-control studies, which reveal that postprandial plasma TAG concentrations are higher in coronary heart disease patients than in healthy control participants [24,25]. Large prospective cohort studies confirm that elevated non-fasting TAG concentrations are an independent risk factor for cardiovascular disease in men and women [26,27]. In the Copenhagen City Heart Study the age-adjusted hazard ratio for myocardial infarction was 16.8 in women and 4.6 in men whose non-fasting TAG concentrations were ≥5.0 mmol/L compared with those whose values were <1.0 mmol/L. Those ratios were attenuated (to 5.4 and 2.4, respectively) but remained significant after multivariate adjustment for confounding factors [26]. These findings are consistent with those of the US Women's Health Study [27]. Thus, it is important to consider lifestyle modifications which may be effective in reducing repeated daily episodes of exaggerated postprandial lipaemia.
THE IDEA FOR RESEARCH ON ACCUMULATED EXERCISE AND POSTPRANDIAL LIPAEMIA
The contribution of a single bout of aerobic exercise in attenuating postprandial TAG concentrations has been known for some time (for a review of relevant studies see [28]). Although recent studies with adolescents conducted by Tolfrey and colleagues failed to identify a dose-dependent effect of the exercise-induced reduction in lipaemia [29,30], it was established early on that energy expenditure was the most important determinant of the exercise-induced reduction in lipaemia in adults [31]. A study by Tsetsonis and Hardman [32] extended this finding and paved the way for the concept that accumulated exercise might influence postprandial lipaemia. They observed similar reductions in postprandial TAG concentrations after participants walked on a treadmill for one long session (180 minutes) at a low intensity (32% of maximum oxygen uptake) and one shorter session (90 minutes) at a moderate intensity (63% of maximum oxygen uptake) with an equivalent energy expenditure [32]. The findings from this study suggested that the total energy expenditure of exercise, and not exercise intensity, was the key to lowering postprandial TAG concentrations. This finding was pivotal as it suggested that other factors in the exercise prescription, such as intensity and duration, were of minor importance compared with energy expenditure for reducing postprandial lipaemia. Moreover, the logical progression of the finding is that the pattern of energy expenditure is also probably unimportant compared with total energy expenditure. Substrate contributions during exercise, which are determined by exercise intensity, do also not appear to be an important consideration, as suppression of exercise-induced lipolysis by acipimox does not affect the exercise lowering of postprandial TAG [33]. Collectively, these findings suggest that if exercise sessions can be partitioned throughout the day there are possible applications for sedentary populations who might find it easier (due to their low physical fitness levels) to perform shorter bouts of exercise rather than one long bout. However, it is important to recognise that performing multiple bouts of low-moderate intensity exercise to accumulate sufficient energy expenditure may also be challenging for unfit adults to achieve. Thus, investigation of a minimum threshold of energy expenditure from exercise for lowering postprandial lipaemia could have public health implications. Clarity on this threshold could provide practical guidance to the general public which could assist with exercise adherence. In one study self-report data revealed that overweight women who accumulated short bouts of exercise, with a minimum duration of 10 minutes per session, had better exercise adherence than women who did longer bouts of exercise over 20 weeks of intervention [34].
EMPIRICAL STUDIES ON ACCUMULATED EXERCISE AND POSTPRANDIAL LIPAEMIA
The studies which have examined whether accumulating exercise can reduce postprandial lipaemia are summarised in Table 1. Reductions in postprandial lipaemia with exercise are transient and rapidly lost with detraining [35,36], so all of the studies reported have examined the acute effect of accumulated exercise on TAG concentrations. The reduction in TAG with exercise may be mediated through an increase in skeletal muscle lipoprotein lipase activity, which enhances the uptake of TAG into previously exercised muscle [37], and/or a reduced rate of secretion of hepatic very-low-density lipoprotein (VLDL)-TAG [38]. It is important to note that exercise needs to be performed frequently for continued benefit. The majority of these studies have reported that accumulated exercise confers a positive benefit by reduced circulating concentrations of postprandial TAG. For example, in our previous study, we examined the effect of accumulating a large volume (4.18 MJ=1000 kcal) of exercise in short (6 minutes) bouts performed throughout the day on postprandial TAG concentrations in young healthy men [17]. These bouts of running lowered postprandial TAG concentrations by 18% the next day compared with a control resting condition. We subsequently found that accumulating short 5-minute bouts of running for a total of 30 minutes on the same day as test meals are consumed also lowers postprandial TAG concentrations in healthy young men [39]. The total energy expended during exercise was 1.76 MJ (420 kcal) and postprandial TAG concentrations were reduced by 10% compared with a resting control condition.
Only one study has examined the effect of accumulating resistance exercise on postprandial lipaemia [40]. We investigated how performing multiple bouts of light resistance exercise over the course of a day might affect postprandial lipaemia in 24 young, healthy adult males [40]. On day 1 of the exercise trials participants completed five, 45-minute bouts of resistance exercise. Each bout comprised 20 sets of 15 repetitions of 5 different resistance exercises. Across the five, 45-minute bouts a total of 100 sets and 1500 repetitions were completed. Exercises were performed at 30% to 40% of 1-repetition maximum. On the control trial participants rested on day 1. On day 2 of each trial participants consumed a high fat test meal. The total area under the postprandial TAG concentration versus time curve was 12% lower in the exercise than in the control trial. There was no direct evidence for the mechanism behind the reduction in TAG after the resistance exercise. There was a tendency toward increased non-esterified fatty acid (NEFA) concentrations in the exercise trial. Increased delivery of NEFA to the liver in the postprandial period could increase hepatic VLDL-TAG output. However, the significant increase in ketone body (i.e., 3-hydroxybutyrate) concentration in the exercise trial in this study suggests that NEFAs were being oxidised rather than esterified in the liver, possibly in response to the energy deficit created by exercise. There is some evidence, therefore, for a decreased VLDL output after resistance exercise. Other researchers have suggested increased skeletal muscle LPL activity is the main mechanism behind improvements in postprandial lipaemia after resistance exercise [48], but again direct evidence is lacking. In terms of the practical application of our findings with resistance exercise, these suggest that workers whose jobs involve heavy manual labour throughout the day (e.g., builders and construction workers) may gain some benefit in terms of lowered postprandial lipaemia.
Whilst almost all of the existing studies support an effect of accumulated exercise on postprandial TAG concentrations caution is needed in translating these data to public health recommendations. Almost all of these studies have involved healthy young populations. Data for inactive, overweight/obese individuals at risk of cardiovascular disease and older populations are needed because excursions in TAG concentrations after meals are likely to be exaggerated in these individuals compared with their healthy peers [49-51]. Moreover, many of the studies presented in Table 1 involved the accumulation of a relatively high volume of exercise (≥2 MJ =>478 kcal), which may be unattainable for some individuals on a regular basis. Only three studies have attempted to examine obese, older or at risk patients with mixed results [41-43]. Thus, further evaluation of the minimum amount of accumulated physical activity for reducing postprandial lipaemia is needed in these populations if laboratory efficacy studies are to be translated into lifestyle recommendations.
POSTPRANDIAL LIPAEMIA: STUDIES COMPARING ACCUMULATED EXERCISE WITH CONTINUOUS EXERCISE
To our knowledge, four studies have compared the effects on postprandial lipaemia of exercise performed in short bouts ≥10 minutes with one continuous bout, matched for total energy expenditure [41,42,44,45]. Gill et al. [44] were the first to demonstrate that in healthy men there was a comparable reduction in postprandial plasma TAG concentrations after three, 30 minute bouts of running versus one continuous 90 minute run of equal intensity (60% of maximum oxygen uptake). The total postprandial plasma TAG response to a standardised fat meal measured over a 6-hour observation period was reduced by 18% compared with the control trial in both the continuous and short bouts of exercise trials. Murphy et al. [41] extended the findings of Gill and colleagues by examining accumulated exercise of a duration more consistent with the minimum recommended volume of physical activity. In this study participants performed 30 minutes of walking either in one continuous bout before breakfast or in three short (10 minute) bouts; one before breakfast, one before lunch and one before an early evening meal. The exercise intensity was the same on both trials (60% of maximum oxygen uptake). Both patterns of exercise reduced postprandial plasma TAG concentrations to a similar extent with the greatest reductions in TAG concentrations observed in the late afternoon/early evening. Since plasma NEFA concentrations did not differ significantly between control and exercise trials, the authors concluded that increased lipoprotein lipase activity was the most likely explanation for the reductions in postprandial TAG concentrations during the exercise trials.
Similarly, Altena et al. [45] have shown that accumulating short bouts of exercise (3×10 minutes, 20 minutes recovery between bouts) reduce incremental plasma TAG concentrations in healthy young males and females during an 8 hour postprandial observation period. However, this study did not observe any reduction in incremental plasma TAG concentrations after continuous exercise (30 minutes) despite a similar energy expenditure (1 MJ=245 kcal) in the accumulated and continuous exercise trials. This study concluded that energy expenditure may not be the only determinant of postprandial TAG responses, and the authors speculated that excess post-exercise oxygen consumption may be one factor responsible for reducing postprandial TAG concentrations. We attempted to evaluate this proposal in one study. In order to address this issue we measured recovery oxygen uptake for 5 minutes after each bout of running in an accumulated exercise trial (10×3 minutes, 30 minutes recovery between bouts), and these measurements were also made at equivalent time points in a continuous exercise (1×30 minutes), and control trial (data were collected in the study described in [46]). We found a significantly higher total oxygen uptake in the accumulated exercise trial (38.2±1.7 L) than in the continuous exercise (20.1±0.9 L) and control (16.8±0.8 L) trials (Unpublished data). However, there was a comparable reduction in postprandial TAG concentrations between accumulated and continuous exercise trials the next day [46]. Our data indicate that excess post-exercise oxygen consumption was not the factor responsible for the reduction in postprandial lipaemia. Excess post-exercise oxygen consumption is short lived after short bouts of exercise and the extra energy expended from this is probably insufficient to influence postprandial TAG concentrations. It is worth noting that there was no control for menstrual cycle phase in the females in the study by Altena et al. [45] and this was a confounding factor since it is known that postprandial plasma TAG responses are lower in the luteal than the follicular phase of the menstrual cycle [52]. Finally, Mestek et al. [42] examined TAG responses to a standardised high-fat test meal after walking with a total estimated energy expenditure of 2.1 MJ (500 kcal). The participants were sedentary men with the metabolic syndrome who completed four trials: 1) control, 2) continuous low intensity walking (35% to 45% of peak oxygen uptake), 3) continuous moderate intensity walking (60-70% of peak oxygen uptake), and 4) accumulated moderate intensity walking. In contrast to the three previous studies [41,44,45], the key finding was that single bouts of low- and moderate-intensity walking, but not accumulated walking (2×1.05 MJ/session) lowered postprandial TAG concentrations. The reason that Mestek et al. [42] did not observe a reduction in postprandial lipaemia after accumulated exercise is unclear but they speculated that exercise may need to be distributed over more than two sessions. This suggestion is feasible because increases in muscle blood flow after multiple bouts of exercise may enhance exposure of TAG to skeletal muscle lipoprotein lipase, the enzyme responsible for hydrolysis of circulating TAG [53]. However, there is little direct data to support this hypothesis and further study is required to address this issue.
The majority of evidence from these four studies [41,42,44,45] suggests that accumulated exercise is as effective as continuous exercise for reducing postprandial TAG concentrations and that reductions can be achieved with a total energy expenditure in line with the physical activity guidelines [5,54,55]. However, none of these studies employed exercise bouts lasting less than 10 minutes in duration. Thus, based on these studies it is not clear if accumulating 30 minutes of exercise in very short bouts lasting less than 10 minutes in length, lowers postprandial TAG concentrations. This is an important question to answer for individuals whose occupational activity patterns involve short bouts of activity repeated throughout the day (e.g., postman, gardeners and cleaners) and for individuals with a limited exercise capacity who cannot exercise in long continuous bouts. Moreover, from a physiological standpoint, if energy expenditure is the prime determinant of postprandial TAG reductions, it should follow that individuals should be able to accumulate exercise in almost any pattern and still gain benefit as long as the total daily energy expenditure of exercise is sufficient.
Three studies have examined this issue and compared the effect on postprandial lipaemia of exercise performed in very short bouts (<10 minutes) versus one continuous bout [43, 46,47]. In one of our previous studies, we compared the effects of continuous versus accumulated running with a total energy expenditure of 2.0 MJ (476 kcal) in young healthy males [46]. The participants completed three, two-day trials: 1) control, 2) 30 minutes of continuous running, 3) 10, 3-minute bouts of running with 30 minutes rest between each. The exercise intensity was ≥70% of maximum oxygen uptake. The total area under the postprandial plasma TAG concentration versus time curve was 22% and 24% lower on the accumulated and continuous running trials, respectively, compared with the control trial. We extended this finding [47] by examining short bouts of walking as opposed to running [46]. Accumulating ten, 3 minute walks (1.1 MJ=265 kcal in total) at a self-selected pace, over the course of one day, reduced postprandial plasma TAG concentrations on the next day to a similar extent as that observed after 30 minutes of continuous walking. These findings are supported by a study by Miyashita [43] showing similar reductions in postprandial lipaemia after low volume (total estimated energy expenditure 0.9 MJ (200 kcal) cycling performed in either one continuous 30 minute bout or in 10, 3-minute bouts. Importantly the participants in this study were obese young men exercising at approximately 60% of maximum heart rate.
The evidence from these three studies is consistent and collectively demonstrates that accumulating exercise in short (<10 minutes) bouts is sufficient and as effective as continuous exercise for lowering postprandial lipaemia in young healthy and obese men [43,46,47]. To date, no one has established the shortest exercise duration that can be accumulated to lower postprandial TAG concentrations. It is also worth noting that it is not known to what extent these observations are clinically relevant as there is no defined clinical cut-off point for postprandial lipaemia. Nonetheless, elevated non-fasting TAG concentrations are an independent risk factor for cardiovascular disease and all-cause mortality in men and women [26,27]. An increase in non-fasting TAG from 1.00-1.99 mmol/L to 2.00-2.99 mmol/L in the Copenhagen City Heart Study increased the age-adjusted hazard ratio for myocardial infarction to 2.2 in females and 1.6 in males. A Norwegian study found that non-fasting TAG levels of >3.5 mmol/L versus <1.5 mmol/L were associated with a 5-fold increase in risk of death from coronary heart disease and a 2-fold risk of all-cause death in women [56]. Thus, a small change in postprandial TAG concentrations brought about by physical activity could have important public health implications from the point of view of reducing cardiovascular disease risk and mortality. Indeed the 16% reduction in postprandial TAG concentrations we observed with only 30 minutes of accumulated walking (1.1 MJ) would be sufficient to reduce an individual's non-fasting TAG from 3.5 to <3.0 mmol/L [47]. Moreover, a recent prospective cohort study has demonstrated that as little as 90 minutes a week (15 minutes a day) of physical activity is sufficient to reduce mortality and extend life expectancy among Taiwanese men and women [57]. Thus, it seems feasible that the small volume of physical activity needed to change postprandial TAG concentrations is likely to be clinically important despite a lack of long-term observations relating to physical activity, non-fasting TAG and clinical endpoints.
IMPLICATIONS FOR PROMOTING PHYSICAL ACTIVITY/EXERCISE
It is difficult to determine if individuals in real-life would ever choose to accumulate leisure-time physical activity in such disparate patterns (i.e., performing lots of short bouts of activity throughout the day) as used in our experiments [43,46,47]. Nevertheless, we and other groups demonstrate that for reductions in postprandial TAG to occur the total energy expenditure of physical activity is more important than the pattern of physical activity. Collectively, such data have implications for individuals whose occupational activity is intermittent. Moreover, older adults, with limited capacity to perform exercise for long periods because of low fitness or pre-existing disease, could potentially benefit from these findings because they may be able to perform bouts of physical activity similar in duration to those described here. Future studies need to investigate these factors. Furthermore, the finding that a reduction in postprandial lipaemia may be achieved by accumulating exercise in multiple short bouts may encourage more individuals to incorporate a small amount of activity into their lives. Such factors are important for public health policy because estimates in many countries suggest that most individuals do not complete sufficient amounts of physical activity to meet the guidelines set out by expert panels [5,54,55].
SUMMARY AND CONCLUSIONS
This review highlights recent data suggesting that accumulating activity is an additional option for lowering postprandial TAG concentrations. Although more research is needed to support the efficacy studies described in this review, it is hoped that the information provided here will help shape future guidelines on physical activity and health. In particular, the available data could have implications for older adults with an impaired capacity to achieve daily exercise guidelines and future research should address this question. Furthermore, there is no data from free-living situations demonstrating that accumulating physical activity can attenuate daytime TAG concentrations. Given that most individuals should be able to achieve bouts of activity similar in duration and intensity to some of those described here it is important to examine individuals undergoing their daily routines.
Footnotes
The authors have no conflicts of interest with the material presented in this paper.
References
- 1.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 2.US National Institutes of Health. Physical activity and cardiovascular health: National Institutes of Health consensus development conference statement. Bethesda: National Institutes of Health; 1995. p. 8. [Google Scholar]
- 3.US Department of Health and Human Services. Physical activity and health: a report of the surgeon general. Washington, DC: US Department of Health and Human Services; 1996. p. 44. [Google Scholar]
- 4.UK Department of Health. At least five a week: evidence on the impact of physical activity. London: UK Department of Health; 2004. p. 27. [Google Scholar]
- 5.US Department of Health and Human Services, Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008. p. C-3. [Google Scholar]
- 6.Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet. 1953;265(6795):1053–1057. doi: 10.1016/s0140-6736(53)90665-5. [DOI] [PubMed] [Google Scholar]
- 7.Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet. 1953;265(6796):1111–1120. doi: 10.1016/s0140-6736(53)91495-0. [DOI] [PubMed] [Google Scholar]
- 8.Morris JN, Crawford MD. Coronary heart disease and physical activity of work; evidence of a national necropsy survey. Br Med J. 1958;2(5111):1485–1496. doi: 10.1136/bmj.2.5111.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 10.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 11.DeBusk RF, Stenestrand U, Sheehan M, Haskell WL. Training effects of long versus short bouts of exercise in healthy subjects. Am J Cardiol. 1990;65(15):1010–1013. doi: 10.1016/0002-9149(90)91005-q. [DOI] [PubMed] [Google Scholar]
- 12.Ebisu T. Splitting the distance of endurance running: on cardiovascular endurance and blood lipids. Jpn J Phys Educ. 1985;30(1):37–43. [Google Scholar]
- 13.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and coronary heart disease risk in men: does the duration of exercise episodes predict risk? Circulation. 2000;102(9):981–986. doi: 10.1161/01.cir.102.9.981. [DOI] [PubMed] [Google Scholar]
- 14.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, 3rd, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281(4):327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- 15.Murphy M, Nevill A, Neville C, Biddle S, Hardman A. Accumulating brisk walking for fitness, cardiovascular risk, and psychological health. Med Sci Sports Exerc. 2002;34(9):1468–1474. doi: 10.1097/00005768-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MH, Blair SN, Murtagh EM. Accumulated versus continuous exercise for health benefit: a review of empirical studies. Sports Med. 2009;39(1):29–43. doi: 10.2165/00007256-200939010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Miyashita M, Burns SF, Stensel DJ. Accumulating short bouts of running exercise throughout the day reduces postprandial plasma triacylglycerol concentrations and resting blood pressure in healthy young men. J Phys Act Health. 2006;3(1):112–123. [Google Scholar]
- 18.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60(3):473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 19.Karpe F, Hamsten A. Postprandial lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol. 1995;6(3):123–129. doi: 10.1097/00041433-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Harchaoui KE, Visser ME, Kastelein JJ, Stroes ES, Dallinga-Thie GM. Triglycerides and cardiovascular risk. Curr Cardiol Rev. 2009;5(3):216–222. doi: 10.2174/157340309788970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weintraub MS, Grosskopf I, Rassin T, Miller H, Charach G, Rotmensch HH, et al. Clearance of chylomicron remnants in normolipidaemic patients with coronary artery disease: case control study over three years. BMJ. 1996;312(7036):935–939. doi: 10.1136/bmj.312.7036.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohn JS. Postprandial lipemia: emerging evidence for atherogenicity of remnant lipoproteins. Can J Cardiol. 1998;14(Suppl B):18B–27B. [PubMed] [Google Scholar]
- 23.Karpe F. Lipid-related factors. In: Stanner S, editor. Cardiovascular disease: diet, nutrition and emerging risk factors. 1st ed. Oxford: Blackwell Publishing; 1995. p. 52. [Google Scholar]
- 24.Patsch JR, Miesenböck G, Hopferwieser T, Mühlberger V, Knapp E, Dunn JK, et al. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12(11):1336–1345. doi: 10.1161/01.atv.12.11.1336. [DOI] [PubMed] [Google Scholar]
- 25.Groot PH, van Stiphout WA, Krauss XH, Jansen H, van Tol A, van Ramshorst E, et al. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991;11(3):653–662. doi: 10.1161/01.atv.11.3.653. [DOI] [PubMed] [Google Scholar]
- 26.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 27.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 28.Peddie MC, Rehrer NJ, Perry TL. Physical activity and postprandial lipidemia: are energy expenditure and lipoprotein lipase activity the real modulators of the positive effect? Prog Lipid Res. 2012;51(1):11–22. doi: 10.1016/j.plipres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Tolfrey K, Bentley C, Goad M, Varley J, Willis S, Barrett L. Effect of energy expenditure on postprandial triacylglycerol in adolescent boys. Eur J Appl Physiol. 2012;112(1):23–31. doi: 10.1007/s00421-011-1936-x. [DOI] [PubMed] [Google Scholar]
- 30.Tolfrey K, Doggett A, Boyd C, Pinner S, Sharples A, Barrett L. Postprandial triacylglycerol in adolescent boys: a case for moderate exercise. Med Sci Sports Exerc. 2008;40(6):1049–1056. doi: 10.1249/MSS.0b013e31816770fe. [DOI] [PubMed] [Google Scholar]
- 31.Tsetsonis NV, Hardman AE. Effects of low and moderate intensity treadmill walking on postprandial lipaemia in healthy young adults. Eur J Appl Physiol Occup Physiol. 1996;73(5):419–426. doi: 10.1007/BF00334418. [DOI] [PubMed] [Google Scholar]
- 32.Tsetsonis NV, Hardman AE. Reduction in postprandial lipemia after walking: influence of exercise intensity. Med Sci Sports Exerc. 1996;28(10):1235–1242. doi: 10.1097/00005768-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Malkova D, Hardman AE, Bowness RJ, Macdonald IA. The reduction in postprandial lipemia after exercise is independent of the relative contributions of fat and carbohydrate to energy metabolism during exercise. Metabolism. 1999;48(2):245–251. doi: 10.1016/s0026-0495(99)90042-2. [DOI] [PubMed] [Google Scholar]
- 34.Jakicic JM, Wing RR, Butler BA, Robertson RJ. Prescribing exercise in multiple short bouts versus one continuous bout: effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. Int J Obes Relat Metab Disord. 1995;19(12):893–901. [PubMed] [Google Scholar]
- 35.Hardman AE, Lawrence JE, Herd SL. Postprandial lipemia in endurance-trained people during a short interruption to training. J Appl Physiol. 1998;84(6):1895–1901. doi: 10.1152/jappl.1998.84.6.1895. [DOI] [PubMed] [Google Scholar]
- 36.Herd SL, Hardman AE, Boobis LH, Cairns CJ. The effect of 13 weeks of running training followed by 9 d of detraining on postprandial lipaemia. Br J Nutr. 1998;80(1):57–66. doi: 10.1017/s0007114598001779. [DOI] [PubMed] [Google Scholar]
- 37.Seip RL, Semenkovich CF. Skeletal muscle lipoprotein lipase: molecular regulation and physiological effects in relation to exercise. Exerc Sport Sci Rev. 1998;26:191–218. [PubMed] [Google Scholar]
- 38.Gill JM, Mees GP, Frayn KN, Hardman AE. Moderate exercise, postprandial lipaemia and triacylglycerol clearance. Eur J Clin Invest. 2001;31(3):201–207. doi: 10.1046/j.1365-2362.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 39.Miyashita M, Burns SF, Stensel DJ. Acute effects of accumulating exercise on postprandial lipemia and C-reactive protein concentrations in young men. Int J Sport Nutr Exerc Metab. 2009;19(6):569–582. doi: 10.1123/ijsnem.19.6.569. [DOI] [PubMed] [Google Scholar]
- 40.Burns SF, Miyashita M, Ueda C, Stensel DJ. Multiple bouts of resistance exercise and postprandial triacylglycerol and serum C-reactive-protein concentrations. Int J Sport Nutr Exerc Metab. 2007;17(6):556–573. doi: 10.1123/ijsnem.17.6.556. [DOI] [PubMed] [Google Scholar]
- 41.Murphy MH, Nevill AM, Hardman AE. Different patterns of brisk walking are equally effective in decreasing postprandial lipaemia. Int J Obes Relat Metab Disord. 2000;24(10):1303–1309. doi: 10.1038/sj.ijo.0801399. [DOI] [PubMed] [Google Scholar]
- 42.Mestek ML, Plaisance EP, Ratcliff LA, Taylor JK, Wee SO, Grandjean PW. Aerobic exercise and postprandial lipemia in men with the metabolic syndrome. Med Sci Sports Exerc. 2008;40(12):2105–2111. doi: 10.1249/MSS.0b013e3181822ebd. [DOI] [PubMed] [Google Scholar]
- 43.Miyashita M. Effects of continuous versus accumulated activity patterns on postprandial triacylglycerol concentrations in obese men. Int J Obes (Lond) 2008;32(8):1271–1278. doi: 10.1038/ijo.2008.73. [DOI] [PubMed] [Google Scholar]
- 44.Gill JM, Murphy MH, Hardman AE. Postprandial lipemia: effects of intermittent versus continuous exercise. Med Sci Sports Exerc. 1998;30(10):1515–1520. doi: 10.1097/00005768-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Altena TS, Michaelson JL, Ball SD, Thomas TR. Single sessions of intermittent and continuous exercise and postprandial lipemia. Med Sci Sports Exerc. 2004;36(8):1364–1371. doi: 10.1249/01.mss.0000135793.43808.6c. [DOI] [PubMed] [Google Scholar]
- 46.Miyashita M, Burns SF, Stensel DJ. Exercise and postprandial lipemia: effect of continuous compared with intermittent activity patterns. Am J Clin Nutr. 2006;83(1):24–29. doi: 10.1093/ajcn/83.1.24. [DOI] [PubMed] [Google Scholar]
- 47.Miyashita M, Burns SF, Stensel DJ. Accumulating short bouts of brisk walking reduces postprandial plasma triacylglycerol concentrations and resting blood pressure in healthy young men. Am J Clin Nutr. 2008;88(5):1225–1231. doi: 10.3945/ajcn.2008.26493. [DOI] [PubMed] [Google Scholar]
- 48.Petitt DS, Arngrímsson DS, Cureton KJ. Effect of resistance exercise on postprandial lipemia. J Appl Physiol. 2003;94(2):694–700. doi: 10.1152/japplphysiol.00377.2002. [DOI] [PubMed] [Google Scholar]
- 49.Castro Cabezas M, Halkes CJ, Meijssen S, van Oostrom AJ, Erkelens DW. Diurnal triglyceride profiles: a novel approach to study triglyceride changes. Atherosclerosis. 2001;155(1):219–228. doi: 10.1016/s0021-9150(00)00554-2. [DOI] [PubMed] [Google Scholar]
- 50.Fisher RM, Coppack SW, Gibbons GF, Frayn KN. Post-prandial VLDL subfraction metabolism in normal and obese subjects. Int J Obes Relat Metab Disord. 1993;17(5):263–269. [PubMed] [Google Scholar]
- 51.Halkes CJ, Castro Cabezas M, van Wijk JP, Erkelens DW. Gender differences in diurnal triglyceridemia in lean and overweight subjects. Int J Obes Relat Metab Disord. 2001;25(12):1767–1774. doi: 10.1038/sj.ijo.0801831. [DOI] [PubMed] [Google Scholar]
- 52.Gill JM, Malkova D, Hardman AE. Reproducibility of an oral fat tolerance test is influenced by phase of menstrual cycle. Horm Metab Res. 2005;37(5):336–341. doi: 10.1055/s-2005-861481. [DOI] [PubMed] [Google Scholar]
- 53.Miyashita M, Stensel D. Aerobic exercise and postprandial lipemia: issues on volume and frequency of exercise. Med Sci Sports Exerc. 2009;41(4):965. doi: 10.1249/MSS.0b013e318199bd75. [DOI] [PubMed] [Google Scholar]
- 54.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 55.O'Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, et al. The ABC of Physical Activity for Health: a consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci. 2010;28(6):573–591. doi: 10.1080/02640411003671212. [DOI] [PubMed] [Google Scholar]
- 56.Stensvold I, Tverdal A, Urdal P, Graff-Iversen S. Non-fasting serum triglyceride concentration and mortality from coronary heart disease and any cause in middle aged Norwegian women. BMJ. 1993;307(6915):1318–1322. doi: 10.1136/bmj.307.6915.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]


