Key Points
During inflammation, serotonin released by platelets activates vessel wall promoting leukocyte adhesion and recruitment.
Absence of platelet serotonin improves survival after lipopolysaccharide-induced endotoxic shock.
Abstract
The majority of peripheral serotonin is stored in platelets, which secrete it on activation. Serotonin releases Weibel-Palade bodies (WPBs) and we asked whether absence of platelet serotonin affects neutrophil recruitment in inflammatory responses. Tryptophan hydroxylase (Tph)1–deficient mice, lacking non-neuronal serotonin, showed mild leukocytosis compared with wild-type (WT), primarily driven by an elevated neutrophil count. Despite this, 50% fewer leukocytes rolled on unstimulated mesenteric venous endothelium of Tph1−/− mice. The velocity of rolling leukocytes was higher in Tph1−/− mice, indicating fewer selectin-mediated interactions with endothelium. Stimulation of endothelium with histamine, a secretagogue of WPBs, or injection of serotonin normalized the rolling in Tph1−/− mice. Diminished rolling in Tph1−/− mice resulted in reduced firm adhesion of leukocytes after lipopolysaccharide treatment. Blocking platelet serotonin uptake with fluoxetine in WT mice reduced serum serotonin by > 80% and similarly reduced leukocyte rolling and adhesion. Four hours after inflammatory stimulation, neutrophil extravasation into lung, peritoneum, and skin wounds was reduced in Tph1−/− mice, whereas in vitro neutrophil chemotaxis was independent of serotonin. Survival of lipopolysaccharide-induced endotoxic shock was improved in Tph1−/− mice. In conclusion, platelet serotonin promotes the recruitment of neutrophils in acute inflammation, supporting an important role for platelet serotonin in innate immunity.
Introduction
Platelets store serotonin in their dense granules at millimolar concentration and secrete it when they become activated.1,2 This requires a complex mechanism of uptake, storage, and targeted release that is similar to that in neurons, with the exception that platelets are not stationary but circulate in high numbers throughout the vasculature. Platelets do not synthesize serotonin but incorporate and store serotonin that is synthesized in duodenal enterochromaffin cells and secreted into blood. Several different effects of non-neuronal serotonin have been unraveled in the past, including prohemostatic (on platelets and vascular smooth muscle cells),3,4 mitogenic (on hepatocytes and pulmonary smooth muscle cells),5,6 and immunomodulatory (on lymphocytes, monocytes, and smooth muscle cells)7–9 functions. In vitro studies have shown that serotonin also activates the release of Weibel-Palade bodies (WPBs) from endothelial cells, which would promote leukocyte rolling via the WPB constituent P-selectin.10,11 However, it is not clear whether serotonin influences neutrophil-endothelial interactions, a central step in early innate immune responses.
We chose 2 approaches to study serotonin effects on leukocyte rolling and recruitment: genetic deficiency of non-neuronal serotonin, as has been established in Tph1−/− mice,12 and pharmacologic depletion of platelet serotonin. Tryptophan hydroxylase (TPH) 1 is the rate-limiting enzyme for the synthesis of serotonin in non-neuronal cells and has been identified in enterochromaffin cells (primary source), pulmonary endothelial cells, mast cells, and with limited indirect evidence, monocytes/macrophages.12–15 It is distinct from the neuronal isoform TPH2.12 Tph1−/− mice are known to have defects in hemostasis, liver regeneration, pulmonary arterial pressure regulation, and erythropoiesis, but acute inflammatory responses mediated by neutrophil recruitment have not been addressed.3,6,13,16,17
Here we present evidence that platelet serotonin modulates steady state leukocyte rolling mediated by P-selectin. The reduced neutrophil interaction with the vessel wall in the absence of platelet serotonin is translated into defective neutrophil recruitment to sites of acute inflammation.
Methods
Mice
C57BL/6J mice were purchased from The Jackson Laboratory. Tph1−/− mice were on C57BL/6J background.12 Experimental procedures were approved by the Animal Care and Use Committee of the Immune Disease Institute and the Regierungspraesidium of Baden-Wuerttemberg, Germany.
Blood cell count
Blood was collected from the retro-orbital venous sinus into EDTA-containing tubes (5mM) using heparin-coated glass capillaries (Kimble Chase) under anesthesia with 4% isoflurane. The numbers of red blood cells, white blood cells, and platelets were read on a Sysmex XE 2100 analyzer (Sysmex) and the differential count of neutrophils, monocytes, and lymphocytes was performed on a Hemavet 950 FS analyzer (Drew Scientific). Fluorescent beads at a defined concentration (Sphero Rainbow, Spherotech) were used for quantification of cell counts in lavages in flow cytometry.
Flow cytometry
Whole blood was incubated with red blood cell lysis buffer (Lonza) for 10 minutes on ice and centrifuged for 3 minutes at 600g. Supernatant was removed and pelleted cells were resuspended in phosphate-buffered saline (PBS). Samples were incubated with anti–mouse Ly-6G (Gr-1) and anti–mouse CD11b (eBiocience) and neutrophils were gated according to forward/side scatter characteristics and Gr-1/CD11b-positivity. Anti–mouse CD62L (L-selectin) and anti–mouse CD162 (PSGL-1, BD Pharmingen) were used to determine the cell surface expression of these adhesion molecules on a FACSCalibur flow cytometer (Becton Dickinson) using CellQuest Version 3.3 (BD Bioscience) and FlowJo Version 6.4.7 (TreeStar) software.
Quantification of serotonin, TNFα, and soluble L-selectin
To estimate the biologically available serotonin content in whole blood, serum was prepared by strong single-agonist activation (thrombin) of platelets and coagulation. Blood was collected from the retro-orbital sinus with noncoated glass capillaries and incubated with 0.1 U/mL thrombin for 30 minutes at room temperature (Sigma-Aldrich). Clot and blood cells were pelleted by centrifugation at 1000g for 5 minutes and serum was purified by centrifugation at 16 000g for 5 minutes. Serotonin concentration was measured with the Serotonin Fast Track ELISA (Labordiagnostika Nord) according to the manufacturer's instructions. TNFα-concentration in serum was measured with a mouse TNFα ELISA (eBioscience). Plasma was separated from EDTA-anticoagulated whole blood by centrifugation (1000g for 10 minutes, followed by 16 000g for 5 minutes). Concentrations of soluble murine L-selectin were measured with a sandwich ELISA (R&D Systems) according to the manufacturer's instructions.
Platelet serotonin depletion by serotonin uptake inhibition
To estimate the efficacy of fluoxetine for inhibiting platelet serotonin uptake in vitro, platelet-rich plasma (PRP) was obtained by 2 × 5 minutes of centrifugation of heparinized blood at 100g. PRP was incubated with fluoxetine (Sigma-Aldrich) at the indicated concentration for 10 minutes and then with [14C]-serotonin (57 mC/mmol/mL) for 30 minutes at 37°C. Serotonin uptake was terminated by adding 1 volume of 4% ice-cold paraformaldehyde solution. The samples were then centrifuged at 2500g for 5 minutes, and the supernatants were used for scintillation counting. Total [14C]-serotonin was determined in samples lyzed with 0.5% triton X-100. For in vivo platelet serotonin depletion, 10 mg/kg fluoxetine was administered in the drinking water for 21 days.
Intravital microscopy of mesenteric veins
Six-week-old male mice were pretreated with an intraperitoneal injection of 20 mg/kg Escherichia coli serotype 055:B5 lipopolysaccharide (LPS), 50 mg/kg serotonin, or vehicle. After 4 hours, mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine intraperitoneally. Platelets and white blood cells were fluorescently labeled by retro-orbital injection of 50 μL rhodamine 6G (1 mg/mL, Sigma-Aldrich). After median laparotomy a loop of ileum was exteriorized in a temperature-controlled, humidified plastic chamber and a small mesenteric vein with a diameter of approximately 200 μm was visualized with an Axiovert 200M inverted microscope and an AxioCam MRm camera using AxioVision Rel. 4.6 software (Zeiss). Shear rate was determined with an Optical Doppler Velocimeter (Microcirculation Research Institute). Blood cell interactions with the endothelium were recorded for 1 minute in 3 veins/mouse and averaged. Leukocyte adhesion was defined as no visible movement for 30 seconds. Where indicated, leukocyte rolling 10 minutes after superperfusion of mesenterium with 20 μL of 5mM histamine (Sigma-Aldrich) was compared with baseline rolling. All analyses were carried out off-line by an independent investigator blinded to genotype and treatment.
Transwell migration assay of isolated neutrophils
Neutrophils were isolated from murine bone marrow by negative immuno-selection using magnetic beads (Miltenyi Biotec) as previously described (neutrophil purity was at least > 90%).18 One-hundred thousand neutrophils in 100 μL RPMI 1640 with 10mM HEPES and 0.5% BSA (Sigma-Aldrich) were placed in the upper well of a 12-transwell plate (Corning) and attracted with serotonin, vehicle or leukotriene B4 (LTB4; Sigma-Aldrich) in 600 μL of the same buffer in the lower well for 1 hour at 37°C and 5% CO2. Neutrophil transmigration through the 5-μm diameter membrane pores was assessed by flow cytometry.
Platelet-neutrophil rosetting
As previously described,19 blood was collected into either 3.2% citrate (platelets) or 15mM EDTA + 1% BSA (neutrophils) containing tubes. Platelets were washed in modified Tyrode buffer and activated with thrombin (0.2 U/mL, Sigma-Aldrich) for 10 minutes at 37°C after which hirudin (1 U/mL, Sigma-Aldrich) was added to inhibit further thrombin activity. Neutrophils were isolated from bone marrow as described and incubated in a ratio of 10 platelets per 1 neutrophil for 45 minutes at 37°C. After fixation in 2% paraformaldehyde platelets were stained with a rat anti-CD41 antibody (BD Pharmingen) and neutrophil nuclei with Hoechst 33342 (Invitrogen). One-hundred neutrophils were analyzed by fluorescence microscopy in a blinded manner. A neutrophil was considered positive for rosetting when 2 or more platelets were bound to it.
Aseptic wound induction
Wounds were punched into a double-layer of dorsal skin using a standard dermatology biopsy punch (4-mm diameter, Kai Medical) as previously described.20,21 Wounds were photographed every 2 days next to a phantom punch for normalization. In a separate set of mice, wounds were excised 4 hours after wound induction with a 6-mm punch. After washing, samples were homogenized in PBS at 4°C using a polytron homogenizer. Homogenate was incubated with an equal volume of hexadecyltrimethylammonium bromide for 2 minutes. Myeloperoxidase (MPO) activity was determined after centrifugation by adding tetramethylbenzidine substrate (absorbance was read at 630 nm). Wound samples from a third set of mice were histologically stained with hematoxylin-eosin.
Sterile peritonitis
Eight- to 12-week-old male mice were treated with 1 mL of 4% thioglycollate intraperitoneally for chemical irritation or with the yeast cell wall particle zymosan A for biologic inflammation by toll-like receptor (TLR) 2 and complement receptor stimulation (0.6 mg/kg intraperitoneally, both Sigma-Aldrich) or vehicle.21,22 After 4 hours cells were recovered by peritoneal lavage with 8 mL of RPMI medium and counted by flow cytometry.
LPS lung inflammation
Acute lung inflammation was induced by intratracheal administration of LPS (300 μg/kg; E coli serotype 026:B6, Sigma-Aldrich, in a volume of 80 μL) to ketamine/xylazine-anesthetized mice. Bronchoalveolar lavage fluid was collected after 24 hours by washing the lungs 3 times with 1 mL of PBS containing 0.1mM EDTA23 and analyzed by flow cytometry.
LPS endotoxic shock survival
Eight- to 10-week-old male mice were challenged by an intraperitoneal injection of E coli serotype 055:B5 or E coli 0111:B4 LPS (both Sigma-Aldrich) at a dose (20 mg/kg) that was determined as the lethal dose (LD) 60 to 80 in preliminary experiments (not shown). Mice were provided with fluid gel and food on the cage floor and monitored every 2 hours. The end point was a composite of severely labored breathing, somnolence, paralysis, bleeding, sustained severe tremor, lack of righting reflex, or death.
Electrocardiogram
Electrocardiogram (ECG) was recorded noninvasively in awake mice with an ECGenie system and analyzed using e-Mouse Version 2.1 software (Mouse Specifics).
Statistical analysis
Data are presented as mean ± SEM and were analyzed by unpaired, 2-tailed student t test or ANOVA with Bonferroni correction. P values < .05 were regarded as statistically significant.
Results
Serotonin deficiency in Tph1−/− mice is associated with mild leukocytosis
To test the hypothesis that non-neuronal serotonin may modulate innate immune functions by affecting leukocyte extravasation, we first performed a complete blood cell count in age and sex-matched WT and Tph1−/− mice. The absence of non-neuronal serotonin in Tph1−/− mice was associated with an increased white blood cell count compared with WT (9.1 ± 0.4 versus 6.1 ± 0.5 × 103/μL, P < .0001; Figure 1). Although there was no difference in platelet count, the red blood cell count was reduced in Tph1−/− mice compared with WT (8.9 ± 0.3 versus 9.4 ± 0.7 × 103/μL, P < .01). In differential counting, circulating neutrophils were 40% more numerous in Tph1−/− blood compared with WT (1.45 ± 0.1 versus 1.06 ± 0.1 × 103/μL, P < .005). A 35% increase was seen for monocytes (0.27 ± 0.02 in Tph1−/− versus 0.20 ± 0.02 × 103/μL, P < .02), but not for lymphocytes, which circulated in similar numbers in Tph1−/− and WT mice (5.71 ± 0.4 in Tph1−/− versus 5.98 ± 0.4 × 103/μL, ns). Granulopoiesis in bone marrow was similar in WT and Tph1−/− mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that the observed difference in peripheral blood were not because of enhanced neutrophil production. These results suggest that genetic ablation of non-neuronal serotonin results in mild leukocytosis primarily driven by an increase in the number of circulating neutrophils.
Figure 1.
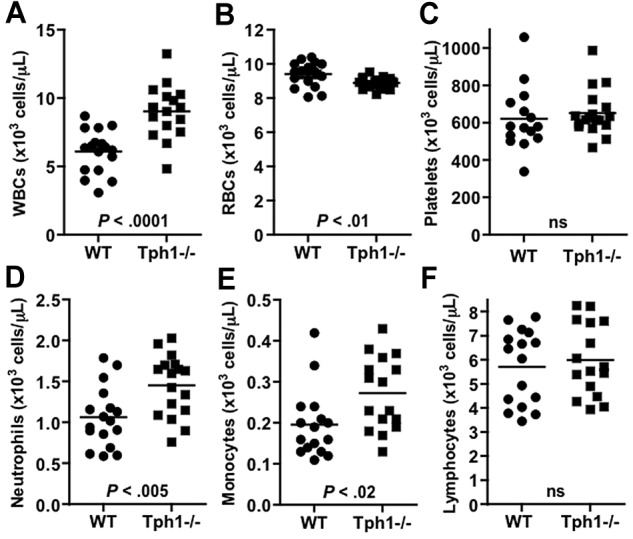
Serotonin deficiency in Tph1−/− mice is associated with mild leukocytosis. Whole blood counts of white blood cell (WBCs, A), red blood cells (RBCs, B), platelets (C), neutrophils (D), monocytes (E), and lymphocytes (F). N = 16-18. ns indicates not significant.
L-selectin shedding is slightly enhanced in Tph1−/− neutrophils
We next measured the surface expression of 2 important neutrophil ligands, P-selectin glycoprotein ligand (PSGL)–1 and L-selectin, because changes in surface ligand density could affect neutrophil-endothelial interactions or reflect cell-aging processes. In circulating neutrophils, the expression of L-selectin was reduced by 20% in Tph1−/− mice compared with WT (Figure 2B). PSGL-1 expression on the other hand was unaffected (Figure 2A). Plasma of Tph1−/− mice contained more soluble L-selectin than plasma from WT mice (Figure 2C). These results indicate that Tph1−/− mice show increased shedding of L-selectin from circulating neutrophils possibly reflecting longer time spent in circulation.19
Figure 2.
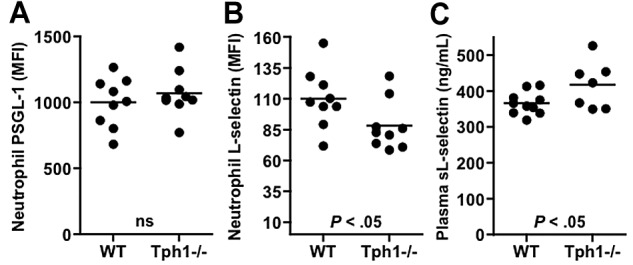
L-selectin shedding from neutrophils in Tph1−/− mice. (A) Surface expression of P-selectin glycoprotein ligand (PSGL)–1 (A) and L-selectin (B) in flow cytometry. (C) Plasma soluble L-selectin was determined by ELISA. N = 7-10.
Leukocyte rolling on unstimulated endothelium is faster in the absence of platelet serotonin
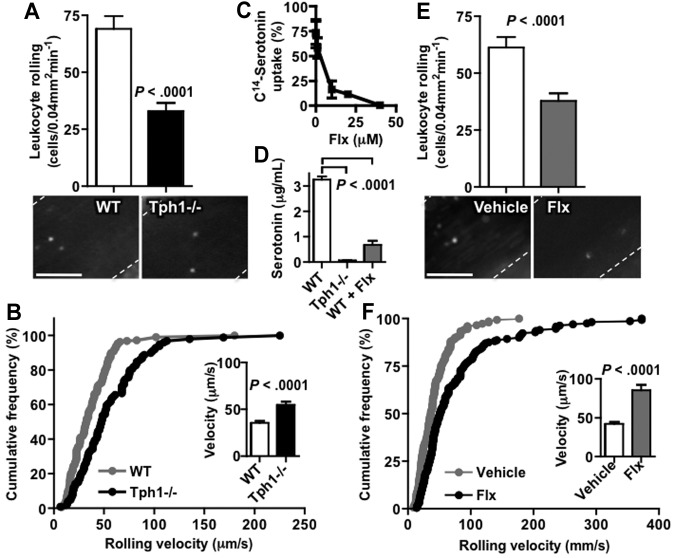
Intravital microscopy of unstimulated mesenteric veins was used to examine whether a difference in leukocyte-endothelial interactions may explain the mild leukocytosis seen in Tph1−/− mice. The number of rolling leukocytes was reduced by 50% in Tph1−/− mice compared with WT (32 ± 4 versus 69 ± 6 cells/0.04 mm2min−1, P < .0001; Figure 3A). The leukocytes that did roll were significantly faster in Tph1−/− than in WT mice (55 ± 3 versus 35 ± 2 μm/s, P < .0001; Figure 3B), indicating fewer selectin-mediated interactions.
Figure 3.
Leukocyte rolling on unstimulated endothelium is decreased and faster in the absence of platelet serotonin. (A) Rhodamine-stained leukocyte rolling in resting mesenteric veins of WT and Tph1−/− mice after laparotomy without further stimulation (n = 8). (B) Resting leukocyte-rolling velocity in these mice. (C) In vitro uptake of labeled serotonin after incubation of isolated platelets with fluoxetine (n = 3). (D) Serum serotonin in Tph1−/− and WT mice after a 3-week treatment with fluoxetine or vehicle in the drinking water (n = 11). Leukocyte rolling (E) and velocity (F) in WT mice after treatment with fluoxetine for 3 weeks (n = 9). Only negligible leukocyte adhesion was observed in these experiments. Bar represents 50 μm.
Because sources of peripheral serotonin other than platelets could promote endothelial activation, a pharmacologic model of platelet serotonin depletion was applied. Platelets, monocytes/macrophages, mast cells, and endothelial cells have been shown to express the serotonin transporter SERT (which is inhibited by selective serotonin reuptake inhibitors, SSRI), but only platelets are not capable of synthesizing their own serotonin, because unlike these other cell types, platelets do not express TPH1.2,13,14,24 The SSRI fluoxetine inhibited the uptake of 14C-serotonin into isolated platelets with an IC80 of 10 μM (Figure 3C). A 3-week treatment course with fluoxetine in the drinking water reduced serum serotonin, that is available platelet serotonin, in WT mice by more than 80% (from 3.2 ± 0.1 to 0.6 ± 0.2 μg/mL, P < .0001; Figure 3D). Fluoxetine treatment reduced the number of rolling leukocytes by 38% (P < .0001) and their rolling was faster than in control WT mice (Figure 3E-F). Only negligible leukocyte adhesion was observed in these experiments (≤ 0.1 cells/0.04 mm2 in all groups). The groups were well matched with respect to body weight (19 ± 0.4 and 20 ± 0.4 g in WT and Tph1−/− mice, respectively; P > .05), vessel diameter (233 ± 11 and 237 ± 9 μm; P > .05), and Newtonian wall shear rate (131 ± 7 and 126 ± 9 s−1; P > .05). Systemic administration of serotonin normalized the number of rolling leukocytes in Tph1−/− mice but did not significantly increase the number of rolling leukocytes in WT mice (supplemental Figure 2A). Transplantation of WT bone marrow into Tph1−/− mice neither increased serum serotonin levels nor leukocyte rolling significantly 6 weeks after transplantation, suggesting that serotonin synthesized by nonhematopoietic, probably enterochromaffin cells enhanced leukocyte rolling (supplemental Figure 2C-D). In summary, absence of non-neuronal serotonin in Tph1−/− mice or platelet serotonin depletion with fluoxetine were associated with a 40% to 50% decrease in the number of rolling leukocytes and a concomitant increase in rolling velocity, suggesting that platelet serotonin regulates endothelial baseline activation.
Local administration of histamine increases leukocyte rolling irrespective of non-neuronal serotonin
To evaluate whether leukocyte rolling in Tph1−/− mice could be normalized by a physiologic secretagogue of WPBs, we stimulated endothelium by local superperfusion of the mesentery with histamine.25 Ten minutes after this stimulation the number of rolling leukocytes increased considerably and was similar in WT (197 ± 25 cells/0.04 mm2min−1) and Tph1−/− mice (204 ± 31 cells/0.04 mm2min−1; P > .05, n = 8). This indicates that the secretory response of endothelium to a stimulus was not defective and that leukocytes in Tph1−/− mice could roll normally when given adequately activated endothelium.
Leukocyte adhesion on LPS-stimulated endothelium is decreased in the absence of platelet serotonin
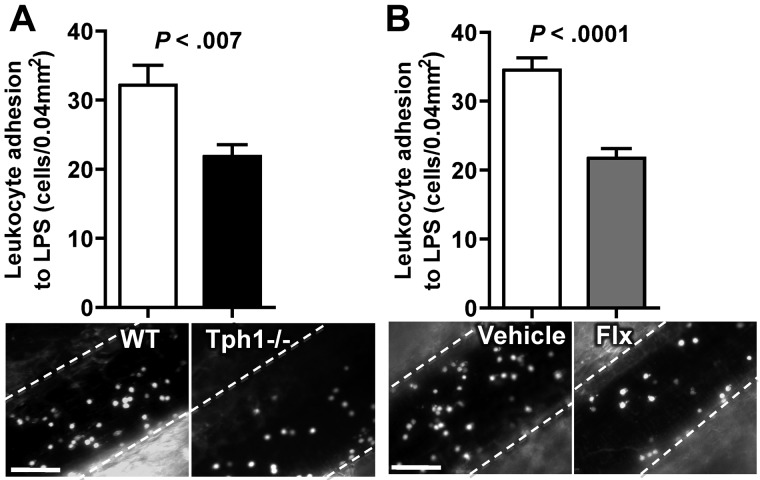
Leukocyte-endothelial interactions without or with only weak stimulation consist of rolling and almost no firm adhesion.26 To examine whether low levels of rolling in the absence of serotonin also reduces firm adhesion of leukocytes, endothelium was activated by intraperitoneal injection of the TLR4 agonist LPS. Four hours after this challenge, rolling was slow and a large number of leukocytes firmly adhered to the endothelium. We observed an approximately 30% decrease in leukocyte adhesion in Tph1−/− mice or WT mice pretreated for 3 weeks with fluoxetine compared with untreated WT mice (Figure 4B-D). Systemic administration of serotonin normalized the LPS-induced leukocyte adhesion in Tph1−/− mice (supplemental Figure 2B). These data indicate that the absence of non-neuronal and in particular platelet serotonin is associated with a decrease in LPS-induced leukocyte adhesion on venous endothelium.
Figure 4.
LPS-induced leukocyte adhesion is decreased in Tph1−/− mice. (A) Leukocyte adhesion 4 hours after intraperitoneal injection of 20 mg/kg LPS in WT and Tph1−/− mice. (B) Leukocyte adhesion in chronically fluoxetine-treated mice 4 hours after intraperitoneal injection of LPS. N = 10. Bar represents 50 μm.
Fewer neutrophils extravasate in acute peritonitis, lung inflammation, and in aseptic skin wounds in the absence of non-neuronal serotonin
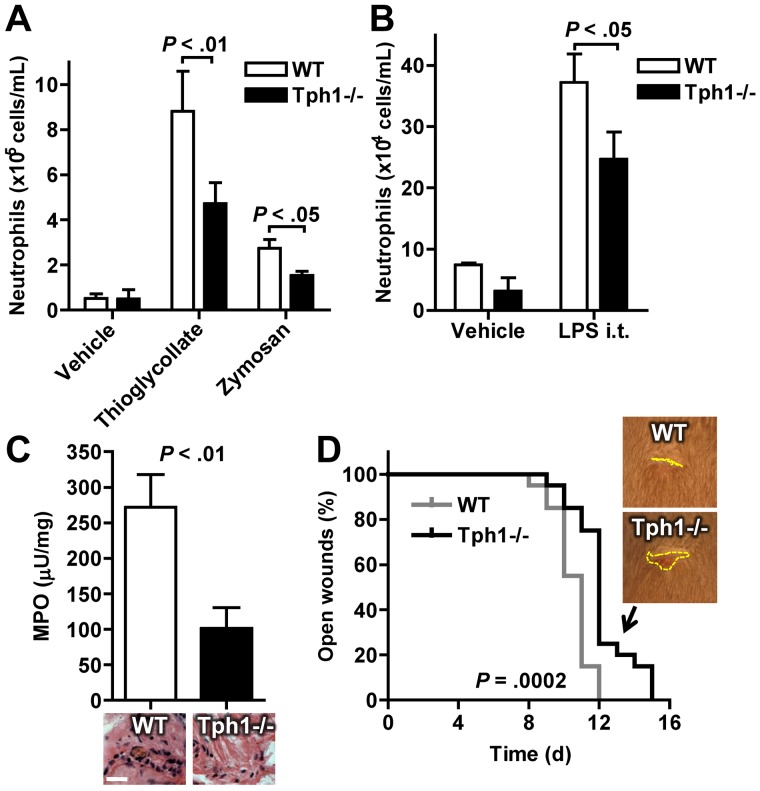
Early leukocyte recruitment to sites of inflammation (within 24 hours) mainly involves neutrophils.27 The extravasation of neutrophils into different acutely inflamed organs was therefore investigated in WT and Tph1−/− mice. Four hours after induction of acute peritonitis, the number of neutrophils that could be retrieved from the abdominal cavity by lavage was reduced by approximately 50% in Tph1−/− mice compared with WT (Figure 5A). Acute lung inflammation was induced by inhalation of LPS and extravasated cells were harvested by bronchoalveolar lavage. Again, significantly fewer neutrophils were detected in Tph1−/− lavage compared with WT (Figure 5B). These data show that neutrophil extravasation in acute inflammation is reduced in the absence of non-neuronal serotonin.
Figure 5.
Fewer neutrophils extravasate in acute peritonitis, lung inflammation, and skin wounds in the absence of non-neuronal serotonin. (A) Gr-1 and CD11b-positive neutrophils in abdominal lavages of WT and Tph1−/− mice 4 hours after intraperitoneal injection of thioglycollate (n = 10) or zymosan (n = 4). (B) Neutrophil recovery in bronchoalveolar lavage 24 hours after inhalation with LPS (n = 6). (C) Tissue MPO concentration and hematoxylin-eosin staining in 24-hour-old skin wounds (n = 10). (D) Time to closure of skin wounds (n = 10).
To examine how non-neuronal serotonin influences wound healing, aseptic skin wounds were punched into the dorsal skin of Tph1−/− and WT mice. Neutrophil extravasation after 4 hours was reduced in Tph1−/− mice as assessed by histologic staining and chemical detection of the neutrophil enzyme MPO (Figure 5C). This decrease in neutrophil extravasation was associated with a significantly prolonged time to wound closure in Tph1−/− mice (Figure 5D); thus it appears that the reduction in neutrophil extravasation in Tph1−/− mice is pathophysiologically relevant.
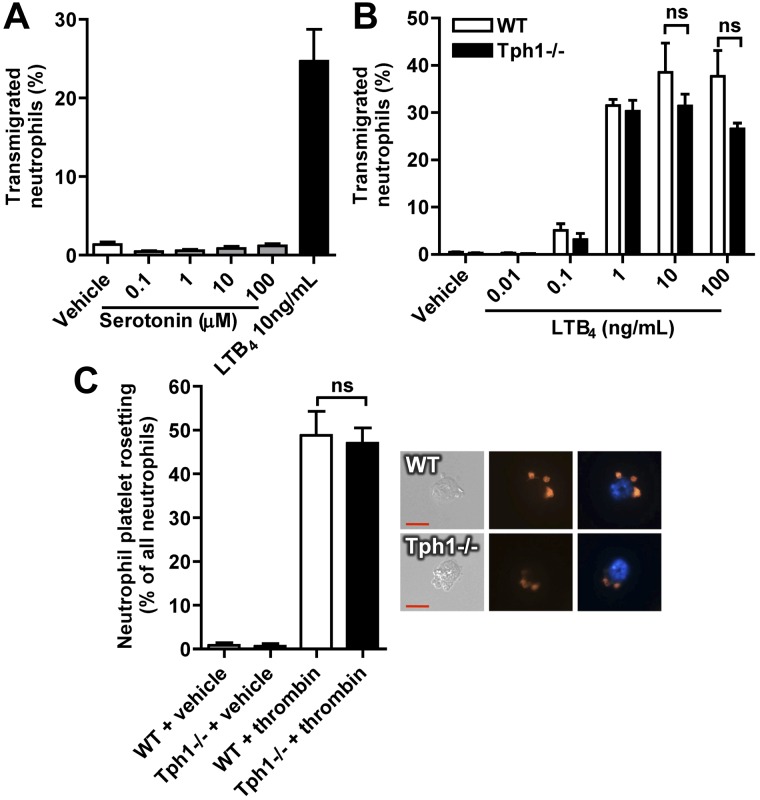
Neutrophil migration during in vitro chemotaxis and platelet-neutrophil rosetting are not affected by serotonin
Serotonin did not act as a chemoattractant in an in vitro transmigration assay using transwell chambers (Figure 6A). There was also no significant difference between neutrophils from WT and Tph1−/− mice in their chemotactic response to the strong chemoattractant LTB4 (only a nonsignificant trend toward reduced migration in neutrophils from Tph1−/− mice at very high LTB4 concentrations, Figure 6B). A defect in platelet/neutrophil binding could perhaps also result in reduced inflammatory recruitment in Tph1 −/− mice. We therefore evaluated platelet and neutrophil rosetting after platelet activation with thrombin. We found it was similar in isolated cells from WT and Tph1−/− mice (Figure 6C). Thus, serotonin does not appear to be a chemoattractant for neutrophils and its absence does not prevent P-selectin–mediated adhesion of platelets to leukocytes.
Figure 6.
In vitro neutrophil migration and platelet-neutrophil rosetting are not affected by serotonin. (A) Migration of isolated murine neutrophils toward serotonin or LTB4. (B) LTB4-induced migration of neutrophils that were isolated from WT and Tph1−/− mice. (C) The capacity to form mixed aggregates was analyzed in isolated neutrophils and a 10-fold excess of thrombin-activated platelets after a 45-minute incubation period. Rosetting was defined as 2 or more platelets binding to 1 neutrophil. N = 4.
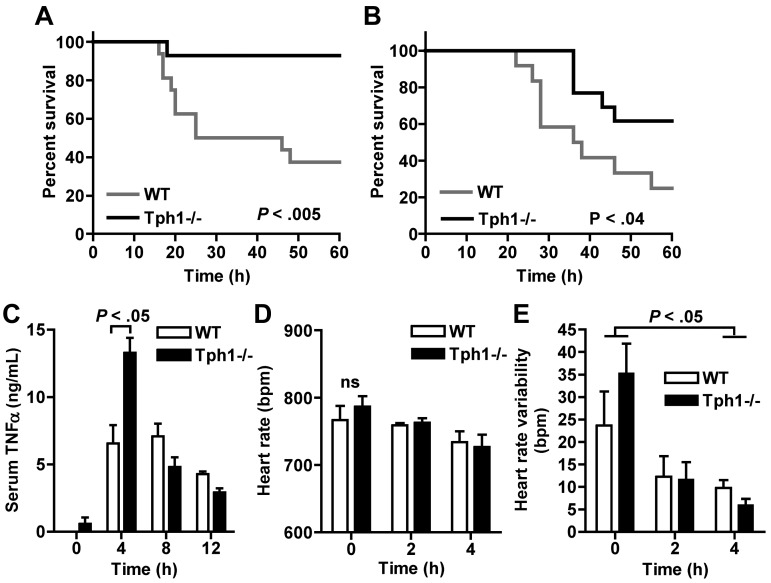
Survival of LPS endotoxic shock is improved in Tph1−/− mice
An extreme manifestation of neutrophil-driven acute inflammation is the systemic response in septic shock, which is associated with high mortality (up to 60%).28 We hypothesized that the absence of peripheral serotonin in Tph1−/− mice may provide protection from septic shock because neutrophil recruitment was reduced in these mice. After injection of a dose of E coli serotype 055:B5 LPS that was lethal for approximately 60% of WT animals, survival in male Tph1−/− mice was significantly better (with 10% mortality after 60 hours; P = .0048; Figure 7A). Challenge with LPS from another E coli serotype (0111:B4) with a dose that was lethal for approximately 80% of WT mice was again associated with improved survival in the absence of non-neuronal serotonin (with 40% mortality in Tph1−/− mice; Figure 7B). This survival benefit of Tph1−/− mice was not associated with a suppression of the proinflammatory cytokine TNFα, but rather an increase in plasma after 4 hours (Figure 7C). Although LPS slightly reduced heart rate and significantly suppressed heart rate variability, ECG analysis revealed no significant differences between Tph1−/− and WT mice (Figure 7D-E). These results suggest that non-neuronal serotonin contributes to septic shock mortality independently of TNFα release or influences on heart rhythm.
Figure 7.
Survival of LPS endotoxic shock is improved in Tph1−/− mice. (A) Kaplan-Meier survival analysis after intraperitoneal injection of E coli serotype 055:B5 LPS into male mice (n = 14). (B) Survival after intraperitoneally injection of E coli 0111:B4 LPS (n = 12). (C) Serum TNFα levels at the indicated time points after E coli 055:B5 LPS injection (n = 3). Heart rate (D) and heart rate variability (E) were measured noninvasively in awake mice at the depicted time points after E coli 055:B5 LPS challenge (n = 6).
Discussion
Based on the previous observation that serotonin induces WPB secretion from endothelial cells in vitro,10,11 we hypothesized that peripheral serotonin may be involved in the recruitment of leukocytes during acute inflammatory processes. We report now that the targeted migration of neutrophils into inflamed organs is indeed promoted by non-neuronal, probably platelet-derived, serotonin.
In vitro studies previously addressed possible effects of serotonin on neutrophils, but the results were confusing, including diametrically opposing findings.29–34 To date, the existence or nonexistence of serotonergic components in neutrophils has not been established. Some groups propose direct effects of serotonin on neutrophils in oxidative burst whereas others attribute serotonin-induced effects to the release of messengers from endothelial cells or to direct extracellular effects of serotonin.29,30 Serotonin did not induce significant superoxide production in an investigation of human neutrophils,31 but decreased the production of reactive oxygen species in other studies.32 Recently, it was reported that serotonin inhibited the oxidative burst in total leukocyte preparations from human blood, but not in isolated neutrophils.33 In vitro migration of polymorphonuclear cells was not influenced by serotonin, which is in accordance with our observations.34
In vivo studies examining vascular permeability have shown that perivascular mast cells secrete serotonin to induce an early, leukocyte-independent phase of edema formation.35 The recruitment of leukocytes did not depend on mast cell-derived serotonin in these studies. However, early leukocyte adhesion (within 60 minutes, ie, predominantly neutrophil adhesion) after injection of LPS depends on the activation of serotonin receptors as shown by pharmacologic blockade in another study.36 Although this study did not examine which cell types were involved, it supports our conclusion that non-neuronal serotonin is required for neutrophil recruitment.
The absence of non-neuronal serotonin in Tph1−/− mice was associated with mild leukocytosis, which was predominantly driven by an increased neutrophil count that occurred in the absence of increased granulopoiesis. In accordance with previous results, RBC count was slightly reduced and platelet count was unaltered in Tph1−/− mice.3,17 Although monocytes were also increased in Tph1−/− mice, there was no significant difference in lymphocyte count. This phenotype suggested a role of peripheral serotonin in neutrophil homeostasis that is reminiscent of the phenotype of P-selectin–deficient mice, where neutrophil clearance is delayed resulting in an increased neutrophil count.19,26 Supporting a prolongation of the time that neutrophils spent in circulation, L-selectin shedding was increased in Tph1−/− mice.19 Direct activation of the endothelium with the WPB secretagogue histamine induced the same response in WT and Tph1−/− mice with similar numbers of rolling leukocytes; also, injection of serotonin could normalize leukocyte rolling. This indicates that the machinery involved in WPB release was intact, mediating early leukocyte rolling via P-selectin, and that leukocytes from Tph1−/− mice could roll normally.25 In contrast, leukocyte adhesion after systemic inflammatory stimulation with LPS was reduced in Tph1−/− mice, suggesting that serotonin amplifies inflammatory leukocyte-endothelial interactions.
We used several models of acute inflammation to demonstrate that the defect in leukocyte-endothelial interactions translated into impairment of neutrophil extravasation in Tph1−/− mice. Closure of aseptic wounds, a neutrophil-driven process,37 was prolonged in Tph1−/− mice. Whether SSRI-treated patients suffer from prolonged wound healing or show defective neutrophil recruitment is an interesting question that has not been investigated. The decrease in neutrophil extravasation during peritonitis and acute lung injury in Tph1−/− mice was in the same magnitude as was reported in P-selectin−/− mice but not as striking as in P-/E–selectin double knock-out mice.38,39 The phenotype we observed in Tph1−/− mice is also similar to that of VWF-deficient mice, which cannot present P-selectin on stimulated endothelium because storage of P-selectin is defective.40 These mice also show reduced numbers of rolling leukocytes, rolling with increased velocity, and reduced neutrophil extravasation. Thus, it is probable that the defective neutrophil recruitment in Tph1−/− mice results from a decrease in basal WPB secretion. Serotonin directly modulates functions of several other immune cells, but unlike in the case of dendritic cells, mast cells, and eosinophils, serotonin did not act as a chemoattractant to induce in vitro migration of neutrophils.7,41–44 In vitro platelet-neutrophil rosetting was also independent of the platelet serotonin content, indicating that serotonin does not influence platelet P-selectin expression and platelet-neutrophil interactions. It is more probable that serotonin enhances neutrophil extravasation by regulating endothelial selectin expression, because serotonin induces WPB exocytosis in cultured endothelial cells10,11 and injection of serotonin enhanced leukocyte rolling in the Tph1−/− mice.
A very similar defect in leukocyte-endothelial interactions as observed in Tph1−/− mice could be reproduced by pharmacologic depletion of the serotonin storage pool in circulating platelets with the standard SSRI fluoxetine. Depressed patients who take an effective daily dose of SSRI drop their whole blood serotonin content by 90% after 3 weeks.45 The experimental conditions were established by in vitro (isolated platelets) and in vivo (intraperitoneal injection into mice, not shown) treatment with fluoxetine, which efficiently inhibited the uptake of labeled serotonin into platelets. Fluoxetine administration in the drinking water reduced the pool of available serotonin in WT mice, that is, the amount of serotonin that can be released after maximal stimulation into blood serum, by > 80%. Unlike other cellular sources of peripheral serotonin, platelets do not express TPH1 and therefore cannot synthesize serotonin on their own.3 Mast cells are a local source of serotonin, mediating edema formation and modulating vascular tone.46 However, it is very unlikely that SSRI treatment also depletes mast cell serotonin, because mast cells synthesize serotonin in their cytoplasm with TPH1, which means that interrupting serotonin uptake with SSRI would not reduce intracellular serotonin contents.14 The same reasoning applies to endothelial cells and monocytes/macrophages.13,24 A limitation of our study remains the lack of direct evidence that serotonin derived from other peripheral cells is not involved in the recruitment of neutrophils. Still, a very recent report supports the importance of platelets for the targeted serotonin release in inflammatory reactions, showing that joint edema formation in arthritis depends on serotonin release from platelets.47
A striking inflammatory response is septic shock. We tested the hypothesis that non-neuronal serotonin enhances this neutrophil-driven disorder, which was suggested previously by pharmacologic studies with 5-HT2 receptor antagonists and fluoxetine.36,48 Indeed, Tph1−/− mice were protected from lethal LPS-induced septic shock. In the early response to LPS challenge, heart rate and blood pressure slightly decrease, TNFα levels increase, and heart rate variability drops significantly in mice.49 Because Tph1−/− mice showed a similar response in these parameters as WT mice, they appear to be independent of non-neuronal serotonin (serotonin-enhanced sympathetic tachycardia is primarily mediated by neuronal serotonin receptors).50 Serotonin is a known platelet coactivator and enhances the procoagulant properties of activated platelets, which may also affect intravascular coagulopathy in sepsis.3,4 Together, our data indicate that Tph1−/− mice are protected from septic shock because of reduced neutrophil recruitment (and possibly reduced platelet activation), but not because of a difference in cytokine secretion or cardiovascular changes.
If future studies show that platelet serotonin also drives neutrophil recruitment in humans, this mechanism could have important clinical implications. Possible anti-inflammatory therapeutic strategies targeting platelet serotonin include reducing non-neuronal serotonin synthesis (by TPH1 inhibition), depleting platelet serotonin stores (by SERT inhibition), or preventing excessive platelet serotonin release (by general or specific antiplatelet therapy). We conclude that platelet serotonin constitutively enhances leukocyte rolling and surveillance of the vessel wall. Platelets also deliver serotonin to sites of inflammation to enhance the recruitment of neutrophils in innate immune reactions. The release of serotonin is yet another way by which platelets enhance inflammatory responses. Excessive neutrophil recruitment may thus also become a target of antiserotonergic treatment strategies.
Supplementary Material
Acknowledgments
The authors thank Lesley Cowan for assistance in preparing the paper, Freya Roming for assistance in carrying out the fluoxetine experiments, and Daniela Stallmann for blinded analysis of leukocyte-endothelial interactions and immunofluorescent staining.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grants R01 HL095091 (D.D.W.) and R01 HL041002 (D.D.W.). D.D. was supported by a fellowship of the Deutsche Forschungsgemeinschaft (DU 1190/3-1).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.D. designed and performed experiments, analyzed data, and wrote the paper; D.D., G.L.S., M.D., N.H., A.B., M.M., and S.M.C. performed intravital microscopy; G.L.S. and M.D. performed platelet-leukocyte rosetting and FACS analysis of L-selectin and PSGL-1; G.L.S. determined soluble L-Selectin levels; C.C. performed neutrophil migration assays; D.D., S.M.C., and N.H. performed histology and stainings; M.M. prepared and analyzed bone marrow smears, S.C. and M.I. performed bone marrow transplantation and lung inflammation experiments; M.B. provided the Tph1−/− mice, valuable advice and critical reading of the paper; C.B. provided helpful advice and critical reading of the paper; and D.D.W designed the experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denisa D. Wagner, Immune Disease Institute, 3 Blackfan Cir, Third Fl, Boston, MA 02115; e-mail: denisa.wagner@childrens.harvard.edu; or Daniel Duerschmied, Heart Center Freiburg University, Hugstetter Str 55, 79106 Freiburg, Germany; e-mail: daniel.duerschmied@uniklinik-freiburg.de.
References
- 1.McNicol A, Israels SJ. Platelet dense granules: structure, function and implications for haemostasis. Thromb Res. 1999;95(1):1–18. doi: 10.1016/s0049-3848(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 2.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walther DJ, Peter JU, Winter S, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115(7):851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 4.Dale GL, Friese P, Batar P, et al. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415(6868):175–179. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- 5.Eddahibi S, Fabre V, Boni C, et al. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells: relationship with the mitogenic action of serotonin. Circ Res. 1999;84(3):329–336. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- 6.Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312(5770):104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 7.Durk T, Panther E, Muller T, et al. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol. 2005;17(5):599–606. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- 8.Iken K, Chheng S, Fargin A, Goulet AC, Kouassi E. Serotonin up-regulates mitogen-stimulated B lymphocyte proliferation through 5-HT1A receptors. Cell Immunol. 1995;163(1):1–9. doi: 10.1006/cimm.1995.1092. [DOI] [PubMed] [Google Scholar]
- 9.Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther. 2008;327(2):316–323. doi: 10.1124/jpet.108.143461. [DOI] [PubMed] [Google Scholar]
- 10.Palmer DS, Aye MT, Ganz PR, Halpenny M, Hashemi S. Adenosine nucleotides and serotonin stimulate von Willebrand factor release from cultured human endothelial cells. Thromb Haemost. 1994;72(1):132–139. [PubMed] [Google Scholar]
- 11.Schluter T, Bohnensack R. Serotonin-induced secretion of von Willebrand factor from human umbilical vein endothelial cells via the cyclic AMP-signaling systems independent of increased cytoplasmic calcium concentration. Biochem Pharmacol. 1999;57(10):1191–1197. doi: 10.1016/s0006-2952(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 12.Walther DJ, Peter J-U, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 13.Eddahibi S, Guignabert C, Barlier-Mur AM, et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation. 2006;113(15):1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- 14.Kushnir-Sukhov NM, Brown JM, Wu YL, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119(2):498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Finocchiaro LM, Arzt ES, Fernandez-Castelo S, Criscuolo M, Finkielman S, Nahmod VE. Serotonin and melatonin synthesis in peripheral blood mononuclear cells: stimulation by interferon-gamma as part of an immunomodulatory pathway. J Interferon Res. 1988;8(6):705–716. doi: 10.1089/jir.1988.8.705. [DOI] [PubMed] [Google Scholar]
- 16.Dempsie Y, Morecroft I, Welsh DJ, et al. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation. 2008;117(22):2928–2937. doi: 10.1161/CIRCULATIONAHA.108.767558. [DOI] [PubMed] [Google Scholar]
- 17.Amireault P, Hatia S, Bayard E, et al. Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc Natl Acad Sci U S A. 2011;108(32):13141–13146. doi: 10.1073/pnas.1103964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergmeier W, Goerge T, Wang HW, et al. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117(6):1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RC, Mayadas TN, Frenette PS, et al. Blood cell dynamics in P-selectin-deficient mice. Blood. 1995;86(3):1106–1114. [PubMed] [Google Scholar]
- 20.Subramaniam M, Saffaripour S, Van De Water L, et al. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150(5):1701–1709. [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan AK, Kisucka J, Brill A, Walsh MT, Scheiflinger F, Wagner DD. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. 2008;205(9):2065–2074. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullaly SC, Kubes P. Mast cell-expressed complement receptor, not TLR2, is the main detector of zymosan in peritonitis. Eur J Immunol. 2007;37(1):224–234. doi: 10.1002/eji.200636405. [DOI] [PubMed] [Google Scholar]
- 23.Idzko M, Hammad H, van Nimwegen M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13(8):913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 24.Mossner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun. 1998;12(4):249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 25.Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 26.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 27.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111(11):5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 29.Schuff-Werner P, Splettstosser W, Schmidt F, Huether G. Serotonin acts as a radical scavenger and is oxidized to a dimer during the respiratory burst of human mononuclear and polymorphonuclear phagocytes. Eur J Clin Invest. 1995;25(7):477–484. doi: 10.1111/j.1365-2362.1995.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 30.Doukas J, Hechtman HB, Shepro D. Vasoactive amines and eicosanoids interactively regulate both polymorphonuclear leukocyte diapedesis and albumin permeability in vitro. Microvasc Res. 1989;37(2):125–137. doi: 10.1016/0026-2862(89)90032-0. [DOI] [PubMed] [Google Scholar]
- 31.Simpson PJ, Schelm JA, Jakubowski JA, Smallwood JK. The role of serotonin (5HT2) receptor blockade in myocardial reperfusion injury: effects of LY53857 in a canine model of myocardial infarction. J Pharmacol Exp Ther. 1991;258(3):979–985. [PubMed] [Google Scholar]
- 32.Jancinova V, Drabikova K, Nosal R, et al. Inhibition of FMLP-stimulated neutrophil chemiluminescence by blood platelets increased in the presence of the serotonin-liberating drug chloroquine. Thromb Res. 2003;109(5-6):293–298. doi: 10.1016/s0049-3848(03)00239-1. [DOI] [PubMed] [Google Scholar]
- 33.Pracharova L, Okenkova K, Lojek A, Ciz M. Serotonin and its 5-HT(2) receptor agonist DOI hydrochloride inhibit the oxidative burst in total leukocytes but not in isolated neutrophils. Life Sci. 2010;86(13-14):518–523. doi: 10.1016/j.lfs.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Bondesson L, Nordlind K, Liden S, Sundstrom E. Inhibiting effects of serotonin and serotonin antagonists on the migration of mononuclear leucocytes. Immunopharmacol Immunotoxicol. 1993;15(2-3):243–250. doi: 10.3109/08923979309025997. [DOI] [PubMed] [Google Scholar]
- 35.Kubes P, Gaboury JP. Rapid mast cell activation causes leukocyte-dependent and -independent permeability alterations. Am J Physiol. 1996;271(6 Pt 2):H2438–2446. doi: 10.1152/ajpheart.1996.271.6.H2438. [DOI] [PubMed] [Google Scholar]
- 36.Walther A, Petri E, Peter C, Czabanka M, Martin E. Selective serotonin-receptor antagonism and microcirculatory alterations during experimental endotoxemia. J Surg Res. 2007;143(2):216–223. doi: 10.1016/j.jss.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Kim MH, Liu W, Borjesson DL, et al. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008;128(7):1812–1820. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamochi M, Kamochi F, Kim YB, et al. P-selectin and ICAM-1 mediate endotoxin-induced neutrophil recruitment and injury to the lung and liver. Am J Physiol. 1999;277(2 Pt 1):L310–319. doi: 10.1152/ajplung.1999.277.2.L310. [DOI] [PubMed] [Google Scholar]
- 39.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84(4):563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 40.Denis C, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98(7):4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K. mRNA expression of serotonin receptors in cells of the immune tissues of the rat. Brain Behav Immun. 2000;14(3):219–224. doi: 10.1006/brbi.1999.0579. [DOI] [PubMed] [Google Scholar]
- 42.Muller T, Durk T, Blumenthal B, et al. 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One. 2009;4(7):e6453. doi: 10.1371/journal.pone.0006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boehme SA, Lio FM, Sikora L, et al. Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J Immunol. 2004;173(6):3599–3603. doi: 10.4049/jimmunol.173.6.3599. [DOI] [PubMed] [Google Scholar]
- 44.Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, et al. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol. 2006;177(9):6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 45.Hergovich N, Aigner M, Eichler HG, Entlicher J, Drucker C, Jilma B. Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin Pharmacol Ther. 2000;68(4):435–442. doi: 10.1067/mcp.2000.110456. [DOI] [PubMed] [Google Scholar]
- 46.Kanerva K, Lappalainen J, Makitie LT, Virolainen S, Kovanen PT, Andersson LC. Expression of antizyme inhibitor 2 in mast cells and role of polyamines as selective regulators of serotonin secretion. PLoS One. 2009;4(8):e6858. doi: 10.1371/journal.pone.0006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cloutier N, Pare A, Farndale RW, et al. Platelets can enhance vascular permeability. Blood. 2012;120(6):1334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 48.Roumestan C, Michel A, Bichon F, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fairchild KD, Saucerman JJ, Raynor LL, et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. Am J Physiol Regul Integr Comp Physiol. 2009;297(4):R1019–1027. doi: 10.1152/ajpregu.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramage AG, Villalon CM. 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. 2008;29(9):472–481. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.