Abstract
Prevalence of hypertension (HT) increases in women after menopause, and there is evidence that HT is not as well controlled in postmenopausal women as men. The reasons for this are not clear but may be related to the lack of adequate blockade of the systems contributing to HT in women. This study aimed to determine the roles of three of the systems known to contribute to HT in animal studies: angiotensin II (ANG II; enalapril inhibitor), eicosanoids [1-aminobenzotriazole (1-ABT) inhibitor], and endothelin (ETA receptor antagonist), on blood pressure (BP) in three groups of female spontaneously hypertensive rats (SHR), aged 18 mos (postmenopausal rat, PMR). After baseline telemetry BP, three drug periods were performed for 5 days each: single blockade (ABT or enalapril), double blockade (ABT+enalapril or enalapril+ABT), and triple blockade (all 3 drugs). Controls received no treatment until the third period when they received ETA receptor antagonist alone. Single drug blockade reduced BP in PMR to similar levels. Double blockade reduced mean arterial pressure more in ABT+enalapril rats than in the other group (enalapril+ABT). Triple drug blockade reduced BP to similar levels in both groups, but the BP remained ∼110 mmHg. The data suggest that these three systems, ANG II, eicosanoids, and endothelin, contribute together and independently to BP control in old female SHR. However, other systems also contribute to the HT since the BP was not normalized, supporting the notion that HT in postmenopausal women may require complex multidrug therapy to be better controlled and that may require the development of additional drugs.
Keywords: enalapril, 20-HETE, endothelin, angiotensin II, women
the incidence of hypertension increases in women after menopause (6), and there is evidence that hypertension is not as well controlled in postmenopausal women as men (5). The mechanisms responsible for postmenopausal hypertension in women are not clear.
In the past several years we have characterized the aging female spontaneously hypertensive rat (SHR) as a model of postmenopausal hypertension. The female SHR has lower blood pressure (BP) than males until they stop estrous cycling, between 10–12 mo of age (4). By the time they reach 16 mo of age, the BP in these aging intact females is equivalent to or higher than in males. BP in the males does not change after ∼9 mo of age (4). In aging male SHR (16–18 mo), the hypertension can be abolished with angiotensin AT1 receptor antagonist losartan (15), suggesting that ANG II is a major cause of hypertension in aging male SHR.
The mechanisms responsible for the hypertension in aging female SHR are more complex. When the renin-angiotensin system (RAS) with the AT1 receptor antagonist losartan (13), was blocked, BP in young females (aged 3–4 mo) was normalized with losartan but in old females (aged 16–18 mo), BP was reduced with losartan but was not normalized (5, 14, 15).
Similarly we determined the role played by endothelin (ET) ETA receptor in mediating the hypertension in postcycling female SHR (aged 18 mo) and found that ETA receptor antagonism had no effect on BP in young female SHR or in age-matched (to the old females) old males but modestly reduced the BP in old females (13). Again the BP was not normalized in old females with ETA receptor antagonists.
From the data of Roman's study (8) that the arachidonic acid products, including 20-HETE, play a role in vasoconstriction of the renal vasculature, we determined the effect chronic blockade on the BP in young and old female SHR (aged 16–18 mo). Both a nonspecific inhibitor of arachidonic acid metabolism, 1-aminobenzotriazole (1-ABT), and a specific inhibitor of ω-hydroxylase (thus 20-HETE synthesis) (HET-0016) both reduced BP in old females without affecting BP in young females (12). However, as in the previous studies, blockade of the arachidonic acid pathways did not normalize the BP in old females.
These studies led us to believe that the hypertension in postcycling SHR (PMR) was multifactorial in origin but caused us to question whether we could normalize their BP with a combination of blockade of some of the systems known to contribute to hypertension Therefore, we tested the hypothesis that blockade of two or three of these pathways together is necessary to normalize BP in old female SHR.
METHODS
Rats.
Female SHRs, 8 mo of age, were obtained from Taconic Farms (Germantown, NY) and were aged in the Laboratory Animal Facility of the University of Mississippi Medical Center, until they were 18 mo of age. The animals were maintained on standard rat chow (Teklad, Harlan SD, Indianapolis, IN) and tap water in an environment with 12 h-12 h light-dark cycles. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center, and studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals, National Institutes of Health.
Rationale.
ANG II contributes to 20-HETE synthesis (8). ANG II can also cause synthesis of endothelin (1). Thus we wanted to know what contribution ANG II had to eicosanoid synthesis-mediated hypertension in old females (enalapril first, then 1-ABT). Also, we wanted to know what contribution the eicosanoid synthesis had to the ANG II-mediated hypertension in the old females (1-ABT first then enalapril). Finally, we wanted to know if we blocked both of these systems, did endothelin contribute independently to the BP in aging female SHR. Hence, we designed the experiment as described below.
Experimental design.
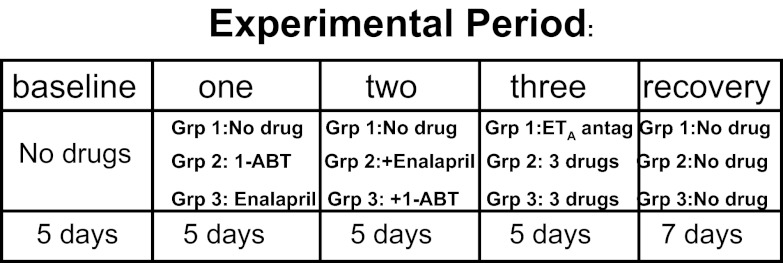
As shown in Fig. 1, SHR female rats, aged 18 mo, were divided into three groups (n = 5–6 per group). Telemetry transmitters were implanted as described below. After 2 wk recovery from surgery, BP was measured during a 5-day baseline period. Group 1 served as a control and received no drugs for the first two experimental periods and then received the endothelin ETA receptor antagonist Abt 627 (5 mg·kg−1·day−1 in drinking water) in the third experimental period (13). Rats in group 2 received the nonselective arachidonic acid metabolism inhibitor 1-ABT (100 mg·kg−1·day−1 ip) (12), during the first experimental period; 1-ABT plus enalapril (250 mg·kg−1·day−1 po in drinking water) (7) during the second experimental period; and 1-ABT plus enalapril plus ETA antagonist during the third experimental period. Group 3 rats received enalapril in the first experimental period; enalpril plus 1-ABT in the second experimental period; and enalapril plus 1-ABT plus ETA receptor antagonist in the third period. In the fourth experimental period, all drugs were stopped for recovery of BP. All rats drank similar amounts of water per day (30–35 ml/day) and excreted similar amounts of urine per day (29.6 ± 3.5 ml/day), and body weights were similar throughout the study.
Fig. 1.
Experimental design. Postcycling female spontaneously hypertensive rats (PMR), aged 18 mo, were implanted with radiotelemeters and 2 wk later, mean arterial blood pressure (MAP) was measured for 5 days in the baseline period. Thereafter, rats in group (Grp) 2 received 1-aminobenzotriazole (1-ABT; 100 mg·kg−1·days−1 ip) for 5 days (single therapy; experimental period 1), then 1-ABT + enalapril (250 mg·kg−1·days−1 po) for 5 days (double therapy; experimental period 2), and then 1-ABT + enalapril + ETA receptor antagonist (Abt 627; 5 mg·kg−1·days−1 po) for an additional 5 days (triple therapy; experimental period 3). Rats in Grp 3 received enalapril for 5 days (single therapy; experimental period 1), then enalapril + 1-ABT for 5 days (double therapy; experimental period 2), and then enalapril + 1-ABT + ETA antagonist for an additional 5 days (triple therapy; experimental period 3). Grp 1 rats were controls for 1-ABT and enalapril and received no drug in experimental periods 1 and 2. In experimental period 3, Grp 1 rats received ETA antagonist alone. In the fourth experimental period rats in Grps 1–3 received no drug for 7 days (recovery).
Blood pressure measurement.
With gas anesthesia using isoflurane (Malinkrodt Veterinary, Hazelwood, CA) and with aseptic technique, rats were implanted with radiotelemetry transmitters (TA11PA-C40; Data Sciences International, St. Paul, MN) into the abdominal aorta below the renal arteries, as we previously described (12–15). The transmitter was secured to the abdominal muscle. Rats were placed into individual cages above a receiver (RLA-3000) and allowed 2 wk of recovery. Telemetry BP measurements were obtained during a 10-s sampling period (500 Hz), recorded, and averaged every 5 min for 24 h per day.
Statistics.
Data are presented as means ± SE. Time series BP studies were analyzed by repeated measures two-way ANOVA followed by Student-Newman-Keuls post hoc comparisons. The percentage reductions in mean arterial pressure (MAP) (Fig. 3) in each rat in each group for days 4 and 5 of each experimental protocol were averaged and compared by ANOVA. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed with SigmaPlot v11 (Systat Software, San Jose, CA).
RESULTS
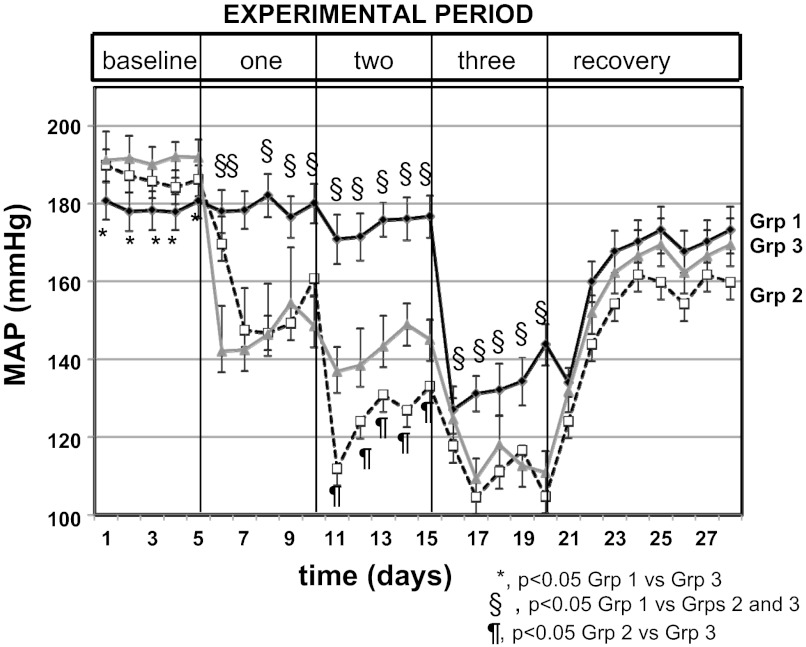
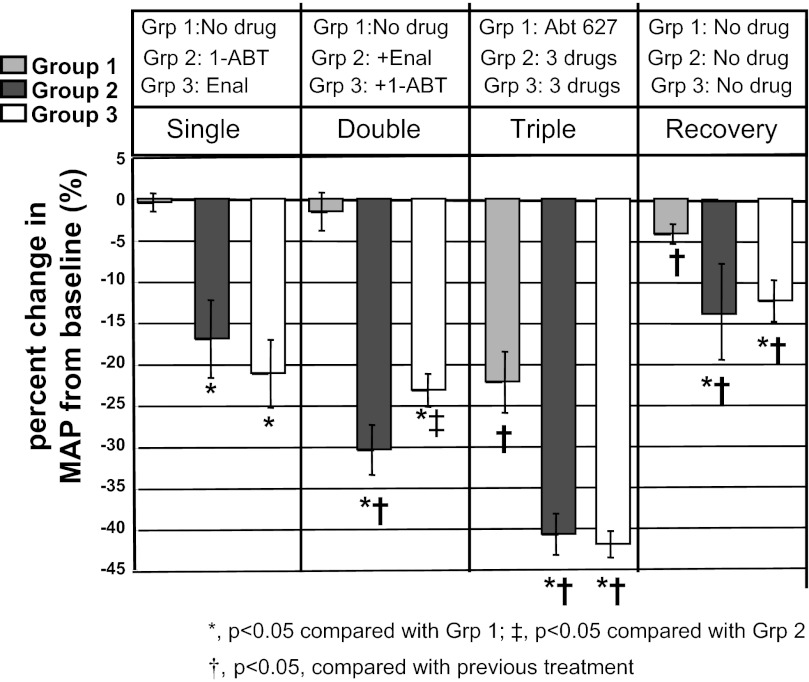
As shown in Fig. 2, baseline BPs were slightly higher in group 3 (191 ± 4 mmHg) than in group 2 but similar to group 1 [group 2: 179 ± 5 (P < 0.05 vs. group 3; P = NS vs. group 2; group 1: 186 ± 4 mmHg, P = NS vs. group 1 or 3)]. In the first experimental period, administration of 1-ABT alone significantly decreased MAP in group 2 rats, and enalapril alone reduced MAP in group 3 rats. There was no difference in percentage reduction of MAP between group 2 or group 3 rats in experimental period 1 (P = NS) (Fig. 3), and the MAPs remained above 150 mmHg for both groups (Fig. 2). MAP did not change in group 1 rats receiving only water.
Fig. 2.
MAP in old female SHR in baseline, first, second and third experimental periods (drug treatments), and fourth experimental period (recovery). Data are the average daily MAP for each rat, averaged together for each group, and presented as means ± SE for each day. *P < 0.05, grp 1 compared with Grp 3; §P < 0.05, for Grp 1 compared with Grps 2 and 3; ¶P < 0.05, Grp 2 compared with Grp 3. There are no symbols for differences compared with the previous experimental period. See Figs. 3 and 4 for differences with previous experimental periods.
Fig. 3.
Percent change in MAP with drugs in first, second and third experimental periods and during recovery (fourth experimental period) in old female SHR. 1-ABT: 1-aminobenzotriazole, epoxygenase and ω-hydroxylase inhibitor; Enal: enalapril, angiotensin II-converting enzyme (ACE) inhibitor; Abt627: endothelin ETA receptor antagonist. Data are the average change in MAP in percent averaged for each rat in the last 2 days of each experimental period, averaged for each group, and presented as means ± SE. *P < 0.05 compared with Grp 1; ‡P < 0.05 compared with Grp 2; †P < 0.05 compared with previous treatment.
In the second experimental period, 1-ABT plus enalapril in group 2 reduced MAP compared with 1-ABT alone in the first period, but enalapril plus 1-ABT in group 3 failed to further reduce MAP compared with the first experimental period (Figs. 2 and 3). MAP in both groups 2 and 3 was still above 130 mmHg with double therapy. Rats in group 1 had no change in MAP.
In the third experimental period, rats in group 1 received ETA receptor antagonist alone, which caused a significant reduction in their MAP (Fig. 2). Rats in groups 2 and 3 that received triple therapy (1-ABT+enalapril+ETA antagonism or enalapril+1-ABT+ETA antagonism) exhibited reductions in MAP to ∼110 mmHg (Fig. 2). The percentage changes in MAP with triple therapy were similar in both groups 2 and 3 (Fig. 3).
During the recovery period (experimental period 4, no drugs in any rats), MAPs were similar between all three groups but were still reduced in all rats compared with their relative baseline levels.
DISCUSSION
The findings of the present study are that 1) single therapy with either an arachidonic acid metabolite synthesis inhibitor, 1-ABT, or an ANG II synthesis inhibitor, enalapril, reduces MAP to similar levels that were still above 150 mmHg; 2) double therapy with both enalapril and 1-ABT had differential effects on MAP depending on which drug was given first, but MAP was still above 130 mmHg; 3) triple therapy with enalapril, 1-ABT, and the ETA receptor antagonist reduced MAP to ∼110 mmHg in both groups. The data show that the three systems studied in this work, ANG II, endothelin and eicosanoids, contribute to the hypertension in aging female SHR in an independent manner, but that despite the triple blockade, the MAP was not normalized (to 100 mmHg).
In previous studies we had determined the individual mechanisms by which blood pressure increased in postcycling female SHR compared with young females and/or males. For example, we found that there was a sex difference in the response to losartan in old SHR. Male SHR, aged 16 mos, had MAP that was 183 ± 5 mmHg compared with 193 ± 8 mmHg in females (14). Treatment of both groups with losartan reduced the MAP to 90 ± 8 mmHg in males but to only 123 ± 11 in females. Thus MAP decreased by >50% in old males but by less than 37% in old females. In other studies in young female SHR, losartan reduced MAP to ∼90 mmHg as well (15). Taken together, these data lead to the conclusion that other mechanisms contributed to BP in aging female SHR but not males.
Our conclusions were also supported by earlier studies in which we evaluated the role of endothelin and, specifically, ETA receptor in hypertension in aging male and female SHR. In those studies we found that blockade of ETA receptor had no effect on MAP in males (unpublished data, Yanes and Reckelhoff) or young females but significantly reduced MAP in old females, but only by ∼20% (13).
Finally, in recent years we evaluated the contribution of 20-HETE to the hypertension in old female SHR. We found that blockade of 20-HETE synthesis by the nonspecific synthesis inhibitor 1-ABT, which we used in the present study, reduced MAP by ∼20 mmHg in old female SHR but not young females (12). We also found that intravenous infusion of the specific inhibitor of 20-HETE synthesis (HET-0016) reduced MAP by 30 mmHg in old females thus did not normalize it (12). We have not determined as of yet whether there is a sex difference in the depressor response to 1-ABT since we have not studied males.
In the present study, it was surprising to us that enalapril, which blocks synthesis of ANG II, had a similar effect on MAP as did 1-ABT. While 1-ABT inhibits synthesis of 20-HETE via it's effects of ω-hydroxylase, it also inhibits synthesis of vasodilator EETs via it's effects on epoxygenase activity (8). These data indicate that 20-HETE makes the same relative contribution to hypertension in old female SHR as does ANG II. In addition, there was a difference in the depressor response in rats with double therapy in which either 1-ABT was given first or enalapril was given first. In rats given 1-ABT first, enalapril caused a greater drop in MAP than in rats given enalapril first and 1-ABT given second. While we expected that by the end of 5 days, the BP would have been similar in both groups given the same drugs, the data suggest that part of the arachidonic acid contribution to the hypertension in old female SHR is mediated via ANG II (3), and thus if ANG II synthesis is already blocked, the further contribution of arachidonic acid metabolites is minimal. This is consistent with the hypothesis that ANG II causes arachidonic acid release from membrane fractions in cells (8). Thus, if ANG II synthesis is inhibited, there is a reduction in the substrate for eicosanoid synthesis and thus further eicosanoid synthesis inhibition would have little effect on MAP. Similarly, Cheng and colleagues showed that 20-HETE caused increased expression of angiotensin converting enzyme (ACE) expression (2). A reduction in 20-HETE with 1-ABT then would also have attenuated ANG II synthesis by the resulting reduction in ACE.
In the present studies we did not use the more specific 20-HETE synthesis inhibitor, HET-0016, due to the difficulty in use (solubility, can't use minipump, etc.) and the length and complexity of the current experiment, and we had already determined the ability of 1-ABT to reduce MAP in aging female SHR that was not different from the HET-0016 (12). Another caveat is any potential role played by EETs in the hypertension of aging female SHR. However, since EETs are vasodilators, inhibition of their synthesis with 1-ABT would have had no effect on MAP if EET synthesis is already reduced in old female SHR. If EETs are elevated, a reduction with 1-ABT would have offset any reduction in vasoconstriction due to 20-HETE. In any case, if EETs are elevated in old female SHR, their impact on the hypertension is likely to be less than the contribution of 20-HETE since 1-ABT significantly reduced the MAP.
As mentioned, we had previously shown that endothelin, mediated via ETA receptor, played a role in mediating the hypertension in aging female SHR. We had found that endothelin levels were significantly higher in renal cortex of old females than young female SHR, and these data were supported by the downregulation of ETA receptor expression (13). Perhaps the reduced level of ETA receptor expression in old female SHR is one reason why the ETA receptor blockade failed to lower the MAP to normotensive levels. ANG II has been shown to upregulate preproendothelin in the kidney (1). Thus ETA receptor blockade on top of ANG II synthesis blockade further reduced the MAP in old females, thus suggesting that there is a contribution of endothelin to the hypertension in old female SHR that is independent of ANG II. The role played by ETB receptors in the hypertension in old female SHRs remains to be determined. However, based on the fact that ETA receptor blockade reduced MAP suggests that ETB may mediate anti-hypertensive effects in old female SHR.
Finally, although MAP was reduced with triple drug therapy in old female SHR, the MAP remained about 110 mmHg, and thus still not normotensive (“normotensive” defined as MAP = 100 mmHg). In previous studies we found that losartan reduced MAP to 90 ± 8 mmHg in old male SHR (14) and to similar levels in young female SHR (15). Thus it is possible that there may be systems other than the eicosanoids, RAS, and endothelin that contribute to hypertension in old females, such as the sympathetic nervous system. Whether there is an additional component to the hypertension in old females due to the sympathetic nervous system that is independent of the systems we have tested in this study will need to be determined in future studies.
Perspectives and Significance
In aging women, heart disease is the condition most commonly contributing to both morbidity and mortality (9). Hypertension is a major risk factor for heart disease. While normotensive levels of blood pressure are only achieved in ∼35% of individuals afflicted with hypertension, studies show that this number is even lower in women (10, 11). The fact that hypertension is less well controlled in women despite the facts that they see their physicians more regularly than men and are typically more compliant with the medications, suggests that either women are not being treated as aggressively as men for their hypertension, or as our studies showed, hypertension is more complicated in aging women than age-matched men.
There are no gender-specific guidelines for drug treatment of hypertension, so postmenopausal women are treated the same as men (9). Clinical trials have shown the efficacy of thiazide diuretics, β-adrenergic blockers, angiotensin-converting enzyme inhibitors (ACEI), and angiotensin AT1 receptor blockers (ARB), and calcium channel blockers as treatment for hypertension. In individuals with high cardiovascular risk as would be found in many postmenopausal women, the American Heart Association recommends treatment with a β-blocker and/or an ARB or ACEI in combination with a thiazide diuretic (9). The National Health and Nutrition Examination Survey (NHANES) IV (1999–2004) reported that 50.8% of women and 55.9% of men had controlled BP (5). Comparison between NHANES III (ending in 1999) with NHANES IV showed that hypertension was less well controlled in women than men, although the drug treatment between men and women was similar (5). Our present data suggest that new clinical studies need to be done that are powered to delineate responses to antihypertensive drugs between men and women, especially in individuals who have “resistant” hypertension.
GRANTS
The authors thank the National Heart, Lung and Blood Institute for funding: RO1 HL-66072, RO1 HL-69194, PO1 HL-51971 (to J. F. Reckelhoff), and the American Heart Association Scientist Development Grant no. 0830239N (to L. L. Yanes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L., L.L.Y., and D.D.D. performed experiments; R.L., L.L.Y., D.D.D., and J.F.R. analyzed data; R.L., L.L.Y., D.D.D., and J.F.R. interpreted results of experiments; R.L., L.L.Y., D.D.D., and J.F.R. prepared figures; R.L., L.L.Y., D.D.D., and J.F.R. approved final version of manuscript; J.F.R. conception and design of research; J.F.R. drafted manuscript; J.F.R. edited and revised manuscript.
REFERENCES
- 1. Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mRNA during chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol 280: R1388–R1392, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Cheng J, Garcia V, Ding Y, Wu CC, Thakar K, Falck JR, Ramu E, Schwartzman ML. Induction of Angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeicosatetraenoic acid-mediated endothelial dysfunction. Arterioscler Thromb Vasc Biol 32: 1917–1924, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Croft KD, McGiff JC, Sanches-Mendoza A, Carrol MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol 279: F544–F551, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Fortepiani LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension 41: 640–645, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Kim JK, Alley D, Seeman T, Karlamangla A, Crimmins E. Recent Changes in cardiovascular risk factors among women and men. J Womens Health (Larchmt) 15: 734–746, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Reckelhoff JF, Fortepiani LA. Novel Mechanisms Responsible for Postmenopausal Hypertension. Hypertension 43: 918–923, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Roman RJ. P-450 metabolites of arachdonic acid in control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, Kaplan NM, O'Connor CM, O'Gara PT, Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease. A scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 115: 761–2788, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Solimene MC. Coronary heart disease in women: A challenge for the 21st century. Clinics 65: 99–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and his treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension 36: 780–789, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Yanes LL, Lima R, Moulana M, Romero DG, Yuan K, Ryan MJ, Baker R, Zhang H, Fan F, Davis DD, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of-HETE. Am J Physiol Regul Integr Comp Physiol 300: R1543–R1548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol 288: R229–R233, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 291: R383–R390, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: role of the Renin-Angiotensin system. Hypertension 56: 359–363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]