Abstract
Twelve weeks of resistance training (3 days/wk) combined with daily consumption of the cyclooxygenase-inhibiting drugs acetaminophen (4.0 g/day; n = 11, 64 ± 1 yr) or ibuprofen (1.2 g/day; n = 13, 64 ± 1 yr) unexpectedly promoted muscle mass and strength gains 25–50% above placebo (n = 12, 67 ± 2 yr). To investigate the mechanism of this adaptation, muscle biopsies obtained before and ∼72 h after the last training bout were analyzed for mRNA levels of prostaglandin (PG)/cyclooxygenase pathway enzymes and receptors [arachidonic acid synthesis: cytosolic phospholipase A2 (cPLA2) and secreted phospholipase A2 (sPLA2); PGF2α synthesis: PGF2α synthase and PGE2 to PGF2α reductase; PGE2 synthesis: PGE2 synthase-1, -2, and -3; PGF2α receptor and PGE2 receptor-4], cytokines and myokines involved in skeletal muscle adaptation (TNF-α, IL-1β, IL-6, IL-8, IL-10), and regulators of muscle growth [myogenin, myogenic regulatory factor-4 (MRF4), myostatin] and atrophy [Forkhead box O3A (FOXO3A), atrogin-1, muscle RING finger protein 1 (MuRF-1), inhibitory κB kinase β (IKKβ)]. Training increased (P < 0.05) cPLA2, PGF2α synthase, PGE2 to PGF2α reductase, PGE2 receptor-4, TNF-α, IL-1β, IL-8, and IKKβ. However, the PGF2α receptor was upregulated (P < 0.05) only in the drug groups, and the placebo group upregulation (P < 0.05) of IL-6, IL-10, and MuRF-1 was eliminated in both drug groups. These results highlight prostaglandin and myokine involvement in the adaptive response to exercise in older individuals and suggest two mechanisms underlying the enhanced muscle mass gains in the drug groups: 1) The drug-induced PGF2α receptor upregulation helped offset the drug suppression of PGF2α-stimulated protein synthesis after each exercise bout and enhanced skeletal muscle sensitivity to this stimulation. 2) The drug-induced suppression of intramuscular PGE2 production increased net muscle protein balance after each exercise bout through a reduction in PGE2-induced IL-6 and MuRF-1, both promoters of muscle loss.
Keywords: acetaminophen, ibuprofen, sarcopenia
we previously reported that daily consumption of over-the-counter doses of acetaminophen (4.0 g/day) or ibuprofen (1.2 g/day) promoted muscle mass and strength gains 25–50% above a placebo consuming group in 60- to 78-yr-old men and women completing strength training 3 days per wk for 12 wk (10, 59). These results were surprising given that consumption of the same drugs and doses blocked the normal muscle protein synthesis response to a single bout of resistance exercise (61) through a cyclooxygenase (COX)-derived PGF2α-mediated mechanism (60, 61). Additional studies (7, 69), along with analysis of muscle biopsies taken before and after the 12 wk of training (59) have concluded COX-1 is the specific isoform likely involved with the prostaglandin production after exercise in healthy humans and is sensitive to both acetaminophen and ibuprofen in human skeletal muscle. Furthermore, the initial attempt at understanding the mechanism underlying the unexpected drug effects on muscle growth showed there were no drug-specific effects on muscle COX enzyme levels after training (59).
The impetus for our human studies in this area was the early studies of Rodemann and Goldberg (46), Palmer and colleagues (39, 40), and Vandenburg and colleagues (63) in isolated animal muscle and muscle cell culture. They showed an increase in arachidonic acid flux through the COX enzyme, either through stretch or direct arachidonic acid supplementation, promoted the muscle production of PGF2α and PGE2, which in turn stimulated muscle protein synthesis or degradation, respectively. Interestingly, in the aforementioned training study only muscle exposed to both exercise and either of the two drugs over the 12 wk experienced this supplemental growth (59), suggesting a muscle stretch and/or loading-related mechanism. Although we speculated that the COX inhibitors may have had a stronger effect on reducing PGE2-related protein degradation (59), the actual mechanism behind the drug effects on muscle growth is still unknown. Understanding how these drugs work is important not only for the millions of individuals worldwide that consume these drugs regularly along with healthcare professionals that may prescribe chronic consumption of these drugs, but it also provides insight into the regulation of skeletal muscle adaptations to exercise.
The goal of the current investigation was to take a comprehensive, yet targeted, approach to understand the COX-inhibiting drug effects on muscle growth (59). Including our previous measurements of the COX enzymes (59), we examined 27 components (enzymes, receptors, cytokines, myokines, transcription factors, and growth factors) involved in the regulation of the PG/COX pathway and muscle mass. In the muscle biopsy samples taken before and after training we examined the COX pathway enzymes that are upstream and downstream of COX and responsible for enhancing general prostaglandin production (i.e., PGH2, the product of COX and precursor to all prostaglandins) and specific enzymes and receptors that regulate the production and initiate the effects of PGF2α and PGE2, respectively (Fig. 1). Specifically, we examined phospholipase A2 (PLA) enzymes [cyctosolic (cPLA2) and secreted (sPLA2)] that convert membrane phospholipids to the COX-substrate arachidonic acid (8, 20, 23, 50); the enzymes that convert PGH2 to PGF2α (PGF2α synthase) (29, 30, 36, 68) and PGE2 to PGF2α (PGE2 to PGF2α reductase) (34, 51, 68); the enzymes that convert PGH2 to PGE2 (PGE2 synthase-1, -2, and -3) (37, 41, 47, 70); the PGF2α receptor (1, 6, 35, 38); and PGE2 receptor-4 (6, 55) (Table 1). We also examined several cytokines and myokines that have been shown to regulate skeletal muscle metabolism and adaptation, specifically, tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-8, and IL-10 (42, 43). Additionally, we measured several known regulators of muscle growth [myogenin, myogenic regulatory factor-4 (MRF4), myostatin] (16, 25, 48) and atrophy [Forkhead box O3A (FOXO3A), atrogin-1, muscle RING finger protein 1 (MuRF-1), and inhibitory κB kinase β (IKKβ)] (16, 17, 31, 48). The findings from these analyses resulted in two proposed mechanisms as to how COX-inhibiting drugs promote muscle mass gains during resistance training in older individuals.
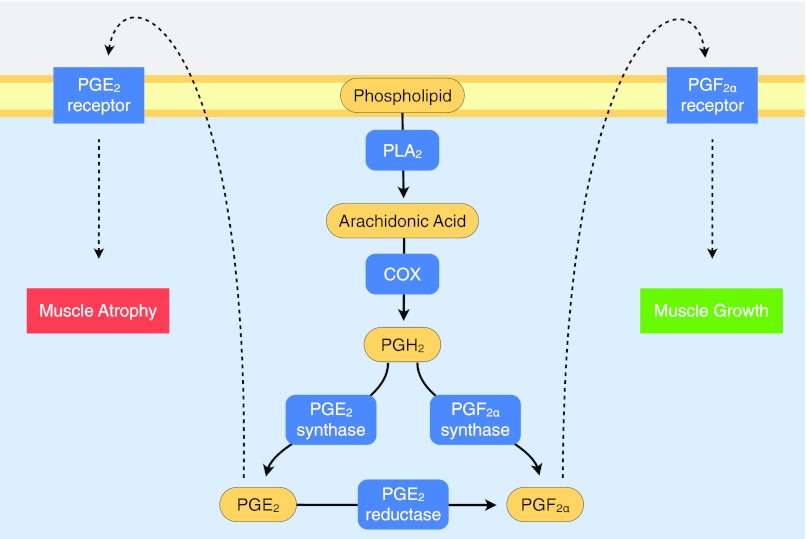
Fig. 1.
Schematic of the prostaglandin (PG) producing cyclooxygenase (COX) pathway and specific receptors that influence growth and atrophy in skeletal muscle. See Table 1 for additional nomenclature, isoform, variant, and gene information. All of the enzymes and receptors in blue were measured in the current study.
Table 1.
Nomenclature, gene information, and mRNA primer characteristics
| Gene Name | Accession No. | Sequence (5′ → 3′) | Amplicon Size, bp | mRNA Region, bp | Annealing Temp, °C | |
|---|---|---|---|---|---|---|
| Prostaglandin/COX pathway and receptors | ||||||
| Cytosolic phospholipase A2 (cPLA2) | PLA2G4A | NM_024420.2 | CCTTACCAGCACATTATAGTGGAGCACC | 110 | 221–330 | 60 |
| GGAGTATCAAGCATGTCACCAAAGGC | ||||||
| Secreted phospholipase A2 (sPLA2) | PLA2G10 | NM_003561.1 | CTGTGCGGACCGGCAGAGAACAAAT | 136 | 789–924 | 59 |
| GCGAGTCCGGCTCACATAGGAACT | ||||||
| PGF2α synthase1 | AKR1C3 | NM_003739.5b | ACTCAAGTACAAGCCTGTCTGCAACC | 150 | 612–761 | 60 |
| TTCGGGTCCACCCATCGTTTGTC | ||||||
| PGE2 to PGF2α reductase2 | CBR1 | NM_001757.2 | ATAAAACCCCAAGGGAGAGTGGTGAACG | 120 | 514–633 | 60 |
| CAGCTCCTCCTCAGTGATGGTCTCA | ||||||
| PGE2 synthase-13 | PTGES | NM_4878.4 | CGGAAGAAGGCCTTTGCCAACC | 125 | 171–295 | 59 |
| GGGTAGATGGTCTCCATGTCGTTCC | ||||||
| PGE2 synthase-24 | PTGES2 | NM_025072.6c | AGAGCAGGCACCGCCTCCAGG | 146 | 1359–1504 | 61 |
| ACGCAGCACGCCATACACCGC | ||||||
| PGE2 synthase-35 | PTGES3 | NM_006601.5 | AGGCCCGCCCACCAGTTCGC | 82 | 254–335 | 61 |
| AGTCCCTTCGATCGTACCACTTTGCAG | ||||||
| PGF2α receptor6 | PTGFR | NM_000959.3d | CTGTATTTGTTGGAGCCCATTTCTGGTTAC | 111 | 1011–1121 | 60 |
| CATGTTGCCATTCGGAGAGCAAAA | ||||||
| PGE2 receptor-47 | PTGER4a | NM_000958.2 | GCTCGTGGTGCGAGTATTCGTCAACC | 122 | 1453–1574 | 60 |
| TCCAGGGGTCTAGGATGGGGTTCA | ||||||
| Cytokines and myokines | ||||||
| TNF-α | TNF | NM_000594.2 | CCCAGGCAGTCAGATCATCTTCTCGAA | 149 | 390–538 | 58 |
| CTGGTTATCTCTCAGCTCCACGCCATT | ||||||
| IL-1β | IL1B | NM_000576.2 | GGATATGGAGCAACAAGTGGTG | 113 | 661–773 | 61 |
| CGCAGGACAGGTACAGATTCT | ||||||
| IL-6 | IL6 | NM_000600.3 | CTATGAACTCCTTCTCCACAAGCGCCTT | 127 | 61–187 | 59 |
| GGGGCGGCTACATCTTTGGAATCTT | ||||||
| IL-8 | IL8 | NM_000584.3 | GCTCTGTGTGAAGGTGCAGTTTTGCCAA | 135 | 153–287 | 60 |
| GGCGCAGTGTGGTCCACTCTCAAT | ||||||
| IL-10 | IL10 | NM_000572.2 | GGCGCTGTCATCGATTTCTTCC | 101 | 430–530 | 60 |
| GGCTTTGTAGATGCCTTTCTCTTG | ||||||
| Muscle growth and atrophy regulators | ||||||
| Myogenin | MYOG | NM_002479.4 | CAGTGCACTGGAGTTCAGCGCCAA | 139 | 599–737 | 60 |
| TTCATCTGGGAAGGCCACAGACACAT | ||||||
| MRF4 | MYF6 | NM_002469.2 | CCCCTTCAGCTACAGACCCAAACAAGAA | 100 | 542–641 | 60 |
| CCCCCTGGAATGATCGGAAACAC | ||||||
| Myostatin | MSTN | NM_005259.2 | GACCAGGAGAAGATGGGCTGAATCCGTT | 96 | 861–956 | 60 |
| GCTCATCACAGTCAAGACCAAAATCCCTT | ||||||
| FOXO3A | FOXO3 | NM_201559.2e | GAACGTGGGGAACTTCACTGGTGCTA | 98 | 2278–2375 | 59 |
| GGTCTGCTTTGCCCACTTCCCCTT | ||||||
| Atrogin-1 | FBXO32 | NM_058229.3f | TATTGCACCCTGGGGGAAGCTTTCAA | 92 | 481–572 | 59 |
| TCCAACAGCCGGACCACGTAGTTAAA | ||||||
| MuRF-1 | TRIM63 | NM_032588.3 | CTCAGTGTCCATGTCTGGAGGCCGTT | 147 | 328–474 | 58 |
| GGCCGACTGGAGCACTCCTGTTTGTA | ||||||
| IKKβ | IKBKB | NM_001556.2g | ATGTCATCCGATGGCACAATCAGG | 127 | 260–386 | 60 |
| TGGGTCAGCCTTCTCATGATCTGG | ||||||
Other common aliases: 1PGH2 9,11-endoperoxide reductase; 2PGE2 9-ketoreductase; 3microsomal prostaglandin E synthase-1 (mPGES-1); 4microsomal prostaglandin E synthase-2 (mPGES-2); 5cytosolic prostaglandin E synthase (cPGES); 6FP; 7EP4. Top sequence reflects the Forward primer and the bottom sequence reflects the Reverse primer.
PTGER4 was the only PGE2 receptor examined, as our recent transcriptome analysis of a relatively large cohort of young and old adults showed infrequent and low level expression of PTGER1, PTGER2, and PTGER3; while PTGER4 was abundantly expressed in all of the individuals studied (45). bPrimers detect both variant 1, isoform 1 (NM_003739.5) and variant 2, isoform 2 (NM_001253908.1). cPrimers detect variant 1, isoform 1 (NM_025072.6). dPrimers detect both variant 1, isoform a (NM_000959.3) and variant 2, isoform b (NM_001039585.1). ePrimers detect both variant 1 (NM_001455.3) and variant 2 (NM_201559.2). fPrimers detect all variants: variant 1, isoform 1 (NM_058229.3); variant 2, isoform 2 (NM_148177.2) and variant 3, isoform 3 (NM_001242463.1). gPrimers detect all variants: variant 1, isoform 1 (NM_001556.2); variant 2, isoform 2 (NM_001190720.2) and variant 7, isoform 5 NM_001242778.1). See text for definitions of abbreviations.
MATERIALS AND METHODS
Overall Study Design
This study was a randomized, placebo-controlled, double-blind 12-wk investigation. During the 12 wk subjects completed a progressive resistance training program of the lower extremities three times per week and consumed a placebo (n = 12, 67 ± 2 yr), acetaminophen (4.0 g/day; n = 11, 64 ± 1 yr), or ibuprofen (1.2 g/day; n = 13, 64 ± 1 yr). The study was conducted at the Human Performance Laboratory at Ball State University and Ball Memorial Hospital and approved by the Institutional Review Boards of both institutions. All study procedures, risks, and benefits were explained to the subjects before giving written consent to participate. A detailed presentation of the subject characteristics, screening, and exclusion criteria; resistance training program; COX-inhibitor consumption, compliance, and side-effect monitoring; and the muscle size (via MRI) and strength measurements and related findings have been reported previously (59).
Targeted muscle mRNA measurements of potential COX inhibitor-related regulators of muscle mass were examined in previously obtained muscle biopsies taken before and after training (59). In addition, we completed microdialysis-based measurements of muscle proteolysis (21, 22, 24, 56) to address the hypothesis that resistance exercise training would reduce the previously reported age-related elevation (58) and provide supplementary information on the potential impact of the COX-inhibiting drugs on basal resting proteolysis.
Muscle Biopsy
Subjects underwent a muscle biopsy (3) of the m. vastus lateralis before and at the end of the resistance training program in the basal state (i.e., no testing or training was completed for 3 days before each biopsy). Biopsies were obtained in the early morning (∼7 AM) after at least 30 min of supine rest and after an overnight fast of ∼12 h. The evening meals before the biopsy were supplied in liquid form (Ensure Plus, 57% carbohydrate, 15% protein, and 28% fat) and provided 50% of the estimated daily caloric need (1.5 times the predicted resting metabolic rate) to standardize the composition, amount, and timing (i.e., duration of the fast) of the final meal consumed before the biopsy. At the end of training, drug consumption (59) continued until the biopsies were obtained. After the biopsy, excess blood, visible fat, and connective tissue were removed, and a portion of the muscle to be used for mRNA analysis was immediately stored in 0.5 ml RNAlater (Ambion, Austin, TX) at 4°C for 24 h and then placed at −20°C until analysis.
Muscle mRNA Measurements
Quantitative PCR (qPCR) was completed to determine the mRNA levels of the components listed in Table 1. Decisions regarding the specific components of the prostaglandin/COX pathway were based on relevant literature and our recent skeletal muscle transcriptome analysis on a relatively large cohort of young and old men and women (45). mRNA analyses were completed on 33 individuals (minus one subject from each group).
Total RNA extraction and RNA quality check.
Total RNA was extracted in TRI Reagent (Molecular Research Center, Cincinnati, OH). The quality and integrity (RIN of 8.12 ± 0.03) of extracted RNA (113.28 ± 4.51 ng/μl) were evaluated using a RNA 6000 Nano LabChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) as we have previously described (7, 59, 69).
qPCR.
Oligo (dT) primed first-strand cDNA was synthesized (150 ng of total RNA) using SuperScript II RT (Invitrogen, Carlsbad, CA). Quantification of mRNA levels (in duplicate) was performed in a 72-well Rotor-Gene 3000 Centrifugal Real-Time Cycler (Corbett Research, Mortlake, NSW, Australia). Housekeeping gene GAPDH was used as a reference gene, as we have previously described (28, 44, 69). All primers used in this study were mRNA specific (on different exons and/or crossing over an intron) and designed for qPCR (Vector NTI Advance 9 software, Invitrogen) using SYBR Green chemistry. Details about primer characteristics are presented in Table 1. A melting curve analysis was generated for all qPCR runs to validate that only one product was present. A serial dilution curve (cDNA made from 500 ng of total RNA of human skeletal muscle; Ambion) was generated for each qPCR run to evaluate reaction efficiencies. The amplification calculated by the Rotor-Gene software was specific and highly efficient (efficiency = 1.07 ± 0.02; R2 = 0.99 ± 0.00; slope = 3.19 ± 0.04). Gene expression before and after the 12 wk of resistance exercise training was compared using the 2−ΔΔCT (fold change) relative quantification method (7, 32, 33, 44).
Muscle Microdialysis Measurements
Resting skeletal muscle proteolysis was examined with microdialysis sampling of interstitial 3-methyhistidine (3MH) concentration (21, 22, 24, 56, 58) before and after training the same morning as the muscle biopsies but from the opposite leg. These secondary measurements focused on the placebo group, but due to the double-blind nature of the protocol, all subjects were examined. As we have previously described in detail (24, 58), a preperfused CMA 60 microdialysis catheter was placed in the m. vastus lateralis, followed by an unperfused 2.5-h rest period, and then perfused with a calibrated microinfusion pump for 1.5 h at 2.0 μl/min with sterile Ringer supplemented with Dextran-70. The initial 30-min dialysate sample was discarded, followed by four 15-min samples, with the first two used for 3MH determination. Dialysate weights were similar to the expected weight across drug groups and pre- to posttraining (overall average: 30.0 ± 0.1 mg), suggesting no net fluid transport across the microdialysis membrane (19). Dialysate 3MH concentration was determined in duplicate by HPLC as previously described (24, 56, 58) and corrected for probe recovery based on our previously obtained nonexercising recovery data for 3MH in the m. vastus lateralis of older subjects (58), and the two 15-min determinations were averaged to represent the pre- or posttraining value for that subject. Subjects with subcutaneous fat thickness over the thigh region that prevented microdialysis sampling of only the muscle (determined with MRI) were excluded; therefore, 26 total individuals were studied [placebo: 9 (8 males, 1 female), acetaminophen: 10 (9 male, 1 female), ibuprofen: 7 (7 male)].
Statistical Analysis
Data were analyzed with two-way (group and time) analysis of variance ANOVA with repeated measures, and post hoc comparisons were made with Tukey's test. Significance was accepted at P < 0.05. Data are presented as means ± SE.
RESULTS
Prostaglandin/COX Pathway and Receptors
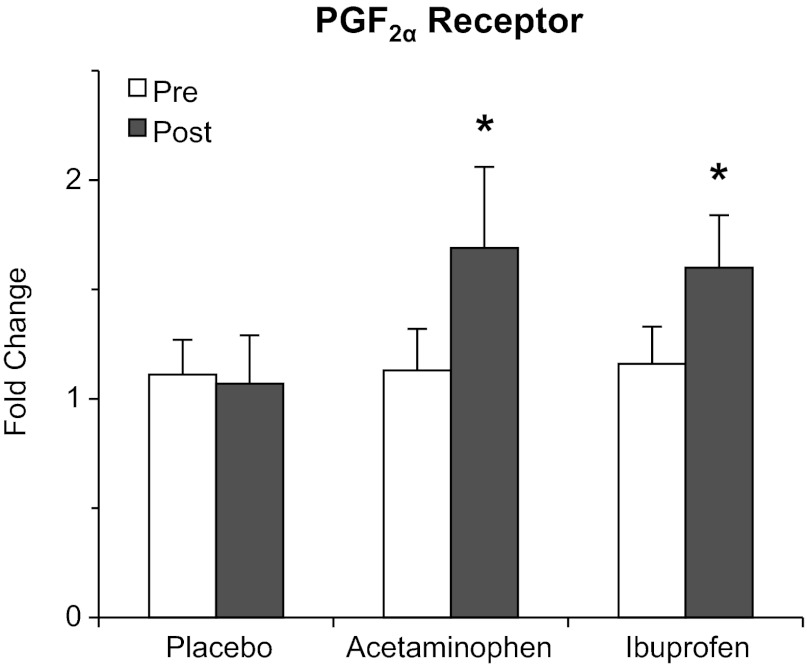
The prostaglandin-producing COX pathway components and the receptors for PGF2α and PGE2 are presented in Table 2 and Fig. 2. Several components of the COX pathway were increased (P < 0.05) due to training (cPLA2: 120%, PGF2α synthase: 222%, PGE2 to PGF2α reductase: 99%). sPLA2 and PGE2 synthase-1, -2, and -3 remained unchanged (P > 0.05) from pre- to posttraining in all three groups. The PGF2α receptor was upregulated (P < 0.05) only in the drug groups (∼70%) over the 12 wk. PGE2 receptor-4 was upregulated (P < 0.05) by 60% due to training.
Table 2.
Fold changes from pre- to posttraining in the placebo and two drug groups
| Placebo | Acetaminophen | Ibuprofen | |
|---|---|---|---|
| Prostaglandin/COX pathway and receptors | |||
| cPLA2 | |||
| Pre | 1.23 ± 0.25 | 1.12 ± 0.18 | 1.20 ± 0.24 |
| Post∗ | 2.63 ± 0.83 | 2.78 ± 0.75 | 1.31 ± 0.18 |
| sPLA2 | |||
| Pre | 1.19 ± 0.23 | 1.05 ± 0.11 | 1.11 ± 0.16 |
| Post | 1.39 ± 0.26 | 1.63 ± 0.41 | 0.91 ± 0.11 |
| PGF2α synthase | |||
| Pre | 1.12 ± 0.17 | 1.15 ± 0.18 | 1.27 ± 0.26 |
| Post∗ | 1.67 ± 0.22 | 5.81 ± 3.20 | 1.67 ± 0.62 |
| PGE2 to PGF2α reductase | |||
| Pre | 1.13 ± 0.17 | 1.02 ± 0.06 | 1.05 ± 0.11 |
| Post∗ | 1.41 ± 0.18 | 3.61 ± 1.64 | 1.45 ± 0.14 |
| PGE2 synthase-1 | |||
| Pre | 1.12 ± 0.17 | 1.24 ± 0.26 | 1.21 ± 0.23 |
| Post | 2.09 ± 0.87 | 1.10 ± 0.17 | 1.21 ± 0.14 |
| PGE2 synthase-2 | |||
| Pre | 1.04 ± 0.08 | 1.07 ± 0.12 | 1.06 ± 0.10 |
| Post | 0.93 ± 0.08 | 1.32 ± 0.22 | 0.89 ± 0.09 |
| PGE2 synthase-3 | |||
| Pre | 1.07 ± 0.13 | 1.03 ± 0.09 | 1.18 ± 0.27 |
| Post | 0.83 ± 0.09 | 1.16 ± 0.23 | 0.85 ± 0.07 |
| PGE2 receptor-4 | |||
| Pre | 1.24 ± 0.25 | 1.17 ± 0.21 | 1.18 ± 0.18 |
| Post∗ | 1.68 ± 0.39 | 2.24 ± 0.80 | 1.46 ± 0.22 |
| Cytokines and myokines | |||
| TNF-α | |||
| Pre | 1.39 ± 0.32 | 1.13 ± 0.19 | 1.47 ± 0.39 |
| Post∗ | 1.99 ± 0.40 | 2.13 ± 0.48 | 1.35 ± 0.18 |
| IL-1β | |||
| Pre | 1.38 ± 0.29 | 1.24 ± 0.29 | 1.38 ± 0.32 |
| Post∗ | 1.95 ± 0.44 | 2.41 ± 0.47 | 1.42 ± 0.32 |
| IL-8 | |||
| Pre | 1.06 ± 0.10 | 1.17 ± 0.20 | 1.18 ± 0.20 |
| Post∗ | 2.33 ± 0.33 | 2.63 ± 0.65 | 1.74 ± 0.34 |
| Muscle growth and atrophy regulators | |||
| Myogenin | |||
| Pre | 1.09 ± 0.14 | 1.07 ± 0.15 | 1.08 ± 0.12 |
| Post | 1.04 ± 0.12 | 0.90 ± 0.13 | 0.97 ± 0.08 |
| MRF4 | |||
| Pre | 1.11 ± 0.15 | 1.13 ± 0.19 | 1.07 ± 0.12 |
| Post | 1.51 ± 0.24 | 1.57 ± 0.56 | 0.99 ± 0.10 |
| Myostatin | |||
| Pre | 1.37 ± 0.30 | 1.13 ± 0.18 | 1.23 ± 0.20 |
| Post | 1.40 ± 0.27 | 1.02 ± 0.25 | 1.12 ± 0.13 |
| FOXO3A | |||
| Pre | 1.10 ± 0.15 | 1.10 ± 0.14 | 1.08 ± 0.12 |
| Post | 1.12 ± 0.12 | 1.28 ± 0.26 | 0.86 ± 0.09 |
| Atrogin-1 | |||
| Pre | 1.05 ± 0.10 | 1.06 ± 0.12 | 1.04 ± 0.09 |
| Post | 1.11 ± 0.10 | 0.94 ± 0.09 | 0.91 ± 0.07 |
| IKKβ | |||
| Pre | 1.06 ± 0.11 | 1.03 ± 0.09 | 1.06 ± 0.11 |
| Post∗ | 1.54 ± 0.21 | 1.28 ± 0.16 | 1.05 ± 0.10 |
Fig. 2.
Muscle mRNA levels of the PGF2α receptor, the only component upregulated in both drug groups and not the placebo group from the beginning (pre) to the end (post) of the 12-wk resistance exercise training and drug intervention. *Significant increase in both drug groups from pretraining, P < 0.05.
Cytokines and Myokines
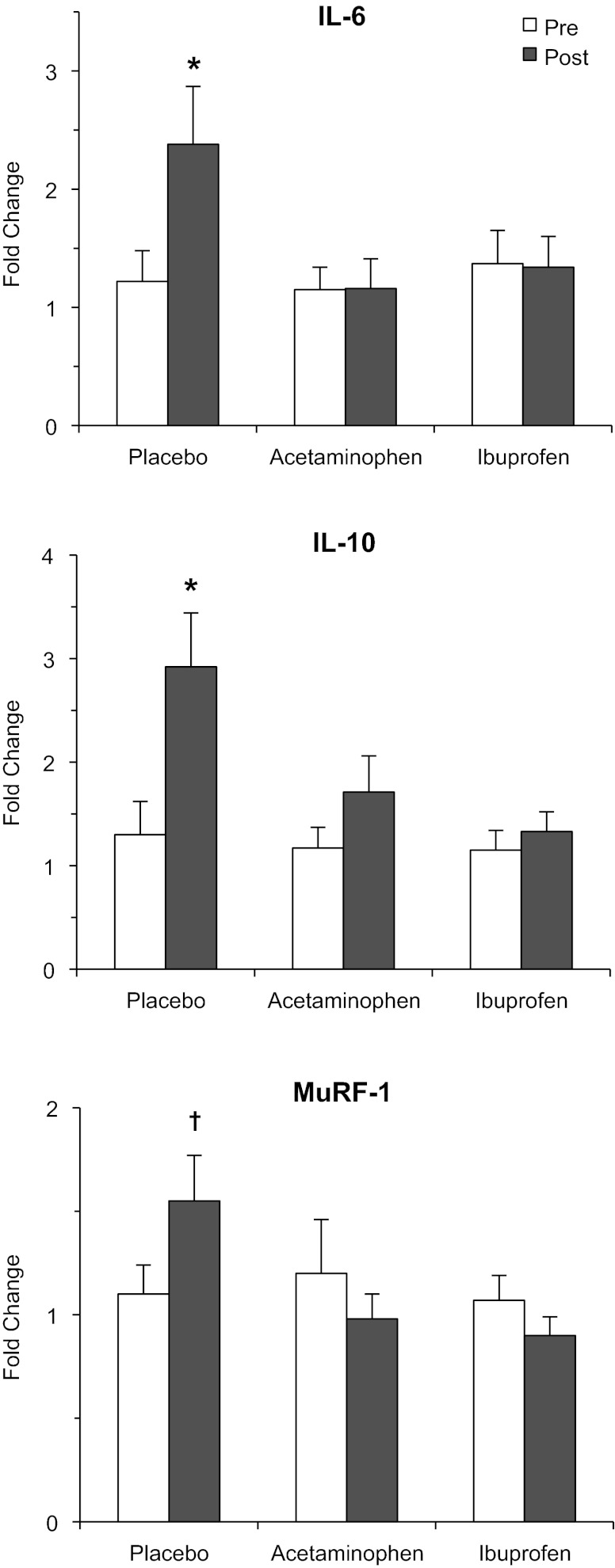
TNF-α (98%), IL-1β (132%), and IL-8 (114%) were increased (P < 0.05) due to training (Table 2). IL-6 (139%) and IL-10 (211%) were both upregulated (P < 0.05) only in the placebo group (Fig. 3).
Fig. 3.
Muscle mRNA levels of the components upregulated only in the placebo group and not the drug groups from the beginning (pre) to the end (post) of the 12-wk resistance exercise training and drug intervention. MuRF-1, muscle RING finger protein 1. *P < 0.05 from pretraining in the placebo group. †P < 0.05 from posttraining in the acetaminophen and ibuprofen groups.
Muscle Growth and Atrophy Regulators
The growth regulators myogenin, MRF4, and myostatin, as well as the atrophy regulators FOXO3A and atrogin-1, remained unchanged (P > 0.05) from pre- to posttraining in all three groups (Table 2). MuRF-1 was differentially upregulated in the placebo group compared with the acetaminophen and ibuprofen groups (Fig. 3). IKKβ was increased (P < 0.05) due to training (Table 2), which appeared to be driven primarily by the placebo group.
Muscle 3MH
Basal muscle myofibrillar proteolysis, as estimated from muscle interstitial levels of 3MH, was not influenced (P > 0.05) by resistance training or drug consumption (placebo: pre, 4.64 ± 0.34; post, 4.73 ± 0.48 nmol/ml; acetaminophen: pre; 4.03 ± 0.30; post, 4.29 ± 0.36 nmol/ml; ibuprofen: pre, 4.97 ± 0.76; post, 4.74 ± 0.91 nmol/ml; all subjects: pre, 4.60 ± 0.32; post, 4.61 ± 0.39 nmol/ml).
DISCUSSION
The main goal of this investigation was to provide mechanistic insight as to how the over-the-counter COX-inhibiting drugs acetaminophen and ibuprofen altered skeletal muscle metabolism and promoted supplemental muscle growth by 25–50% during resistance training in older individuals (59). The findings from the current analyses resulted in two proposed mechanisms: 1) The drug-induced PGF2α receptor upregulation helped offset the drug suppression of PGF2α-stimulated protein synthesis after each exercise bout and enhanced skeletal muscle sensitivity to this stimulation. 2) The drug-induced suppression of intramuscular PGE2 production increased net muscle protein balance after each exercise bout through a reduction in PGE2-induced IL-6 and MuRF-1, both promoters of muscle loss. In addition, most of the prostaglandin/COX pathway enzymes and receptors had not previously been examined in human skeletal muscle or in response to exercise. Many of these components were elevated over the 12 wk and further suggest the prostaglandin/COX pathway is part of the adaptive response of skeletal muscle to resistance exercise training.
Our original hypothesis was that daily consumption of the COX-inhibiting drugs would chronically blunt the COX-mediated production of PGF2α and subsequent stimulation of muscle protein synthesis after resistance exercise (60, 61), ultimately blunting the amount of exercise training-induced hypertrophy. Recent studies of the PGF2α receptor (1, 6, 38) in skeletal muscle (35), using agonists and competitive inhibitors, have confirmed that PGF2α binding to its receptor stimulates muscle cell growth and have delineated the intracellular signaling pathways triggered by this receptor. Additional PGF2α receptors in the muscle of the drug groups, coupled with a general training increase in COX (59) and the PGF2α-producing enzymes (PGF2α synthase and PGE2 to PGF2α reductase), would make the muscle less susceptible to the same daily COX-inhibiting drug doses and more sensitive to any PGF2α that was produced following exercise. The overall contribution of these responses to the supplementary muscle growth in the drug groups is unclear but would be better understood if the time course for the upregulation of the receptor and enzymes was known. It would also be interesting to know the related consequences of longer-term exercise training and drug consumption.
The supplemental muscle growth in the drug groups also appears to be driven by lower levels of IL-6 and MuRF-1 compared with the placebo group (Fig. 3). IL-6 has been shown in numerous population-based studies of older individuals to be associated with a reduction in muscle mass and functional independence (2, 11, 13, 49). In addition, acute IL-6 infusion in humans to raise plasma concentration to postexercise levels reduces muscle protein turnover by 50%, resulting in an increased net muscle protein degradation (62). In animals, chronic infusion of IL-6 to simulate postexercise levels and low-level inflammation retards growth (5) and promotes muscle atrophy (18). Interestingly, PGE2 stimulates IL-6 transcription in nonskeletal muscle cells (12, 14, 15, 52, 65–67) through a nuclear factor (NF)-κB-mediated mechanism; thus a reduction in intramuscular PGE2 production due to the COX-inhibiting drugs (60) is a plausible explanation for the lower IL-6 transcription in the drug groups. In support of this concept, Vella et al. (64) have shown an increase in NF-κB DNA binding to the IL-6 promoter region in the hours following an acute bout of resistance exercise. The IL-10 results (Fig. 3) also support the IL-6 findings, as IL-6 infusion increases IL-10 in humans (53) and IL-10 knockout animals have an exaggerated skeletal muscle IL-6 response to an inflammatory insult (26), suggesting IL-10 is a regulator of IL-6.
MuRF-1, an E3 ubiquitin ligase that mediates skeletal muscle proteolysis, has also been shown to be regulated via NF-κB (9, 48). Thus it is intriguing to speculate that the drug effects on IL-6 and MuRF-1 are linked through a common PGE2 receptor-NF-κB-mediated mechanism. This notion is supported by the IKKβ findings (Table 2) showing the main effect for an increase with training of this NF-κB regulator (17, 31, 64) was primarily driven by the increase in the placebo group. It should be noted that the IL-6 and MuRF-1 data are generated from biopsies taken in the basal state (3 days after the final exercise bout). IL-6, and to a lessor degree MuRF-1, are substantially more elevated during the first 24 h following an acute exercise bout (33, 44, 54) than those reported here for the placebo group. Thus the proposed effects we describe here were likely influenced to a greater degree during the repeated acute responses to each exercise bout. It is also possible older individuals have an exaggerated response following exercise (57) that the drugs help dampen.
Many of the prostaglandin/COX pathway components measured in this study have not been previously examined in skeletal muscle or with exercise training and the training-induced effects are noteworthy. The upregulation of cPLA2 (Table 2) along with COX-1 upregulation (59) suggest an overall enhanced ability to produce more arachidonic acid and PGH2, the precursor to all prostaglandins. The increase in PGF2α synthase and PGE2 to PGF2α reductase suggests an increased capacity of skeletal muscle to produce PGF2α directly and through the conversion of PGE2 (Fig. 1). Although the enzymes that convert PGH2 to PGE2 were unchanged, the PGE2 receptor-4 was upregulated with exercise training (Table 2). Thus the potential of PGF2α and PGE2 effects are enhanced, albeit through different adaptive approaches (enzyme vs. receptor level increases). Overall, it appears the prostaglandin/COX pathway in skeletal muscle is part of the adaptive response to resistance exercise training in older adults.
Several of the training-induced effects (i.e., main effect for training) appeared to have a nonstatistically significant influence by one of the drugs. cPLA2 and two of the classic inflammatory cytokines (TNF-α and IL-1β) were suppressed by ibuprofen and uninfluenced by acetaminophen (Table 2). While these observations do not lend insight into the drug effects on the muscle adaptations examined in the current study, they do support the classic view that ibuprofen, but not acetaminophen, has anti-inflammatory effects in peripheral tissues. These findings may have implications for interactions of acetaminophen and ibuprofen with other metabolic and inflammatory processes in skeletal muscle. In addition, the two PGF2α-producing enzymes (PGF2α synthase and PGE2 to PGF2α reductase) showed a more variable response in the acetaminophen group due to a very large response in the same two individuals. This is intriguing since these two enzymes have different structures and catalytic designs (29, 30, 34, 36, 51, 68). Although speculative, this response may be related to the influence of glutathione on these enzymes (34, 68), considering glutathione is also involved in other pathways directing the removal of acetaminophen (4, 27).
The myofibrillar proteolysis measurements were added to the current study with the primary goal of determining whether resistance exercise training could reduce the previously reported age-related elevation (58). While 12 wk of resistance training was unable to influence basal myofibrillar proteolysis, some age-specific findings were apparent. Our previous investigation (58) examined young (27 ± 2 yr) compared with older individuals over the age of 70 yr (mean 75 ± 4, range: 71–83 yr), and when the age of the current study cohort is considered, those above 70 yr had a 33% higher basal proteolysis than those between 60 and 70 yr (irrespective of training: 4.39 vs. 5.83 nmol/ml). Considering the previously reported magnitude of age-related elevation (44%, 4.28 ± 0.27 vs. 6.16 ± 0.56 nmol/ml), it may be that accelerated muscle proteolysis becomes more apparent in the eighth decade of life. The proteolysis data from the drug groups suggest there was no impact of the COX-inhibiting drugs on basal resting proteolysis.
Perspectives and Significance
We propose two mechanisms through which chronic consumption of acetaminophen and ibuprofen influence skeletal muscle metabolism and promote supplemental muscle growth during resistance training in older individuals. In addition, we define for the first time the adaptation of the prostaglandin/COX pathway enzymes and receptors, in relation to PGF2α and PGE2, the two prostaglandins that stimulate muscle protein turnover. These findings highlight the involvement of prostaglandins and myokines in the adaptive response to resistance exercise in older individuals and underscore the need for more acute and chronic studies in this area. Considering the potency of the drug effects on muscle mass and function, these mechanistic findings also have important implications for the understanding of sarcopenia, the age-related loss of skeletal muscle mass and function, and the further development of strategies to combat sarcopenia.
GRANTS
This investigation was funded by NIH Grant R01 AG-020532.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.A.T., R.A.S., and B.J. conception and design of research; T.A.T., R.A.S., B.J., and C.C.C. performed experiments; T.A.T., R.A.S., and B.J. analyzed data; T.A.T., R.A.S., B.J., C.C.C., and S.W.T. interpreted results of experiments; T.A.T. prepared figures; T.A.T. drafted manuscript; T.A.T., R.A.S., B.J., C.C.C., and S.W.T. edited and revised manuscript; T.A.T., R.A.S., B.J., C.C.C., and S.W.T. approved final version of manuscript.
REFERENCES
- 1. Abramovitz M, Boie Y, Nguyen T, Rushmore TH, Bayne MA, Metters KM, Slipetz DM, Grygorczyk R. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem 269: 2632–2636, 1994 [PubMed] [Google Scholar]
- 2. Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab 284: E481–E487, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 7–110, 1962. 13862378 [Google Scholar]
- 4. Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 31: 55–138, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bodell PW, Kodesh E, Haddad F, Zaldivar FP, Cooper DM, Adams GR. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol 106: 443–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Ann Rev Pharm Tox 41: 661–690, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Burd NA, Dickinson JM, Lemoine JK, Carroll CC, Sullivan BE, Haus JM, Jemiolo B, Trappe SW, Hughes GM, Sanders CE, Jr, Trappe TA. Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am J Physiol Endocrinol Metab 298: E354–E361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther 23: 49–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Carroll CC, Dickinson JM, Lemoine JK, Haus JM, Weinheimer EM, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of acetaminophen and ibuprofen on in vivo patellar tendon adaptations to knee extensor resistance exercise in older adults. J Appl Physiol 111: 508–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Gonzalo-Calvo D, de Luxan-Delgado B, Rodriguez-Gonzalez S, Garcia-Macia M, Suarez FM, Solano JJ, Rodriguez-Colunga MJ, Coto-Montes A. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: A translational approach. Cytokine 58: 193–198, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol 14: 4443–4454, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47: 639–646, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Fiebich BL, Hull M, Lieb K, Gyufko K, Berger M, Bauer J. Prostaglandin E2 induces interleukin-6 synthesis in human astrocytoma cells. J Neurochem 68: 704–709, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Fiebich BL, Schleicher S, Spleiss O, Czygan M, Hull M. Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: possible involvement of EP4-like receptors, p38 mitogen-activated protein kinase and protein kinase C. J Neurochem 79: 950–958, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol 346: 267–278, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE 2006: re13, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hamrin K, Rosdahl H, Ungerstedt U, Henriksson J. Microdialysis in human skeletal muscle: effects of adding a colloid to the perfusate. J Appl Physiol 92: 385–393, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Hanasaki K, Ono T, Saiga A, Morioka Y, Ikeda M, Kawamoto K, Higashino K, Nakano K, Yamada K, Ishizaki J, Arita H. Purified group X secretory phospholipase A2 induced prominent release of arachidonic acid from human myeloid leukemia cells. J Biol Chem 274: 34203–34211, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Hansen M, Langberg H, Holm L, Miller BF, Petersen SG, Doessing S, Skovgaard D, Trappe T, Kjaer M. Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scand J Med Sci Sports 21: 62–72, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Hansen M, Trappe T, Crameri RM, Qvortrup K, Kjaer M, Langberg H. Myofibrillar proteolysis in response to voluntary or electrically stimulated muscle contractions in humans. Scand J Med Sci Sports 19: 75–82, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Haq S, Kilter H, Michael A, Tao J, O'Leary E, Sun XM, Walters B, Bhattacharya K, Chen X, Cui L, Andreucci M, Rosenzweig A, Guerrero JL, Patten R, Liao R, Molkentin J, Picard M, Bonventre JV, Force T. Deletion of cytosolic phospholipase A2 promotes striated muscle growth. Nat Med 9: 944–951, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Haus JM, Miller BF, Carroll CC, Weinheimer EM, Trappe TA. The effect of strenuous aerobic exercise on skeletal muscle myofibrillar proteolysis in humans. Scand J Med Sci Sports 17: 260–266, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Huey KA, McCusker RH, Kelley KW. Exaggerated expression of skeletal muscle-derived interleukin-6, but not TNFalpha, in mice lacking interleukin-10. J Neuroimmunol 199: 56–62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31: 1499–1506, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Komoto J, Yamada T, Watanabe K, Takusagawa F. Crystal structure of human prostaglandin F synthase (AKR1C3). Biochemistry 43: 2188–2198, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Komoto J, Yamada T, Watanabe K, Woodward DF, Takusagawa F. Prostaglandin F2α formation from prostaglandin H2 by prostaglandin F synthase (PGFS): crystal structure of PGFS containing bimatoprost. Biochemistry 45: 1987–1996, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol 103: 388–395, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103: 1744–1751, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Malatkova P, Maser E, Wsol V. Human carbonyl reductases. Curr Drug Metab 11: 639–658, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Markworth JF, Cameron-Smith D. Prostaglandin F2α; stimulates PI3K/ERK/mTOR signaling and skeletal myotube hypertrophy. Am J Physiol Cell Physiol 300: C671–C682, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Mindnich RD, Penning TM. Aldo-keto reductase (AKR) superfamily: genomics and annotation. Hum Genomics 3: 362–370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murakami M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prost Other Lipid Mediat 68–69: 383–399, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Ocklind A, Lake S, Krook K, Hallin I, Nister M, Westermark B. Localization of the prostaglandin F2α receptor in rat tissues. Prostaglandins Leukot Essent Fatty Acids 57: 527–532, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Palmer RM. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids 39: 95–104, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Palmer RM, Reeds PJ, Atkinson T, Smith RH. The influence of changes in tension on protein synthesis and prostaglandin release in isolated rabbit muscles. Biochem J 214: 1011–1014, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119: 229–240, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298: R1485–R1495, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Pedersen BK. Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol 107: 1006–1014, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62: 1407–1412, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112: 1625–1636, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2α influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem 257: 1632–1638, 1982 [PubMed] [Google Scholar]
- 47. Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharm Rev 59: 207–224, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23: 160–170, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 64: 1183–1189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761: 1246–1259, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Schieber A, Frank RW, Ghisla S. Purification and properties of prostaglandin 9-ketoreductase from pig and human kidney. Identity with human carbonyl reductase. Eur J Biochem 206: 491–502, 1992 [DOI] [PubMed] [Google Scholar]
- 52. St-Jacques B, Ma W. Role of prostaglandin E2 in the synthesis of the pro-inflammatory cytokine interleukin-6 in primary sensory neurons: an in vivo and in vitro study. J Neurochem 118: 841–854, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285: E433–E437, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab 283: E1272–E1278, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 282: 11613–11617, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol 105: 902–906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thalacker-Mercer AE, Dell'Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trappe T, Williams R, Carrithers J, Raue U, Esmarck B, Kjaer M, Hickner R. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. J Physiol 554: 803–813, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ. Skeletal muscle PGF2α and PGE2 in response to eccentric resistance exercise: influence of ibuprofen and acetaminophen. J Clin Endocrinol Metab 86: 5067–5070, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 282: E551–E556, 2002 [DOI] [PubMed] [Google Scholar]
- 62. van Hall G, Steensberg A, Fischer C, Keller C, Moller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Vandenburgh HH, Hatfaludy S, Sohar I, Shansky J. Stretch-induced prostaglandins and protein turnover in cultured skeletal muscle. Am J Physiol Cell Physiol 259: C232–C240, 1990 [DOI] [PubMed] [Google Scholar]
- 64. Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, Russell AP, Cameron-Smith D. Resistance exercise increases NF-kappaB activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 302: R667–R673, 2012 [DOI] [PubMed] [Google Scholar]
- 65. Wang P, Zhu F, Konstantopoulos K. Interleukin-6 synthesis in human chondrocytes is regulated via the antagonistic actions of prostaglandin (PG)E2 and 15-deoxy-Delta(12,14)-PGJ2. PLos One 6: e27630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66. Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-κB activation. Am J Physiol Cell Physiol 298: C1445–C1456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang P, Zhu F, Lee NH, Konstantopoulos K. Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-kappaB activation. J Biol Chem 285: 24793–24804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Watanabe K. Prostaglandin F synthase. Prost Other Lipid Mediat 68–69: 401–407, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Weinheimer EM, Jemiolo B, Carroll CC, Harber MP, Haus JM, Burd NA, Lemoine JK, Trappe SW, Trappe TA. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol 292: R2241–R2248, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Yamada T, Komoto J, Watanabe K, Ohmiya Y, Takusagawa F. Crystal structure and possible catalytic mechanism of microsomal prostaglandin E synthase type 2 (mPGES-2). J Mol Biol 348: 1163–1176, 2005 [DOI] [PubMed] [Google Scholar]