Abstract
Numerous studies have detailed the extensive conservation of developmental signaling pathways between the model system, Drosophila melanogaster, and mammalian models, but researchers have also profited from the unique and highly tractable genetic tools available in this system to address critical questions in physiology. In this review, we have described contributions that Drosophila researchers have made to mathematical dynamics of pattern formation, cardiac pathologies, the way in which pain circuits are integrated to elicit responses from sensation, as well as the ways in which gene expression can modulate diverse behaviors and shed light on human cognitive disorders. The broad and diverse array of contributions from Drosophila underscore its translational relevance to modeling human disease.
Keywords: dorsal vessel, mechanosensation, mushroom bodies, transient receptor potential channels, transgenics
the extensive tractability of the Drosophila model system has facilitated the discovery of numerous genes involved in multiple physiological pathways that share tremendous conservation with mammalian systems. Although a fly is not a human, there is no question that the basic pathways in cell, tissue, and organ development and function are absolutely conserved, and thus, information gleaned from understanding fly physiology has had an enormous impact on our understanding of human physiology. In fact, without the “lowly” fruit fly, our understanding would be greatly lacking for such critical processes as programmed cell death, cardiac development, ion channel function, and embryonic development, to name only a very few research areas. What are the advantages to this invertebrate model system that have enabled researchers to elucidate components of so many different pathways?
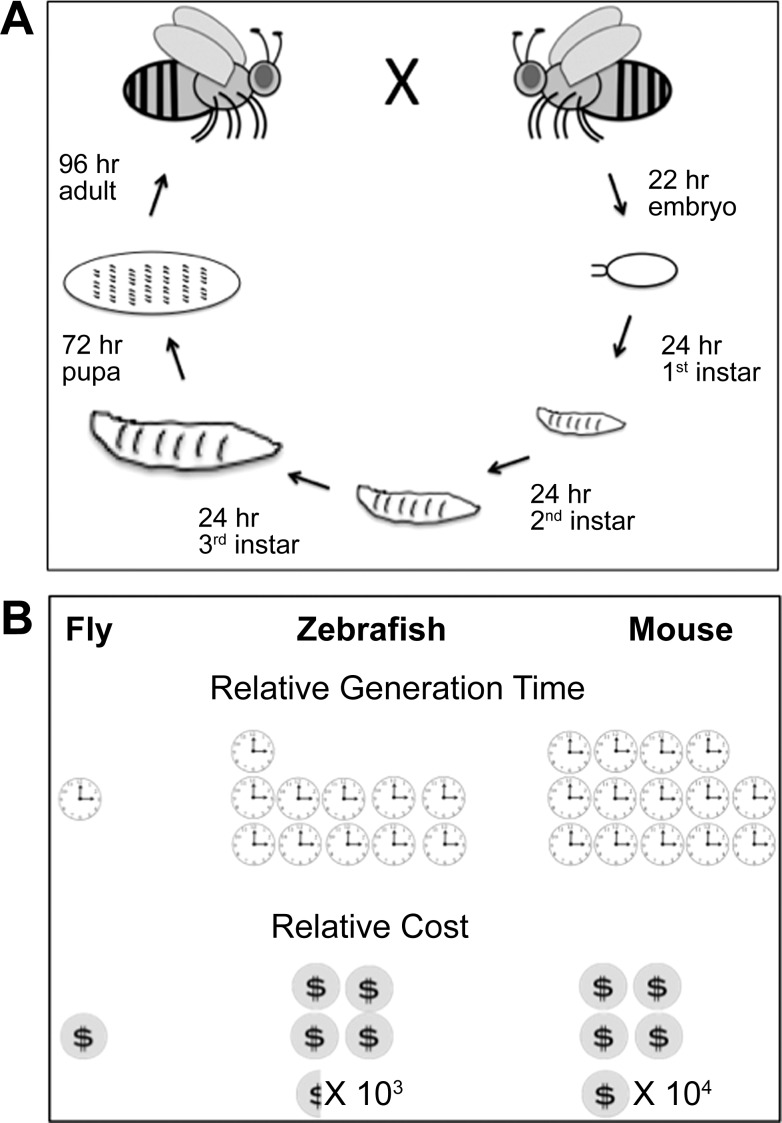
Certainly, their rapid generation time (∼10 days) is key (Fig. 1A). The adult fly, upon emergence from the pupal case, is sexually immature, and sexually mature behaviors, pheromonal complement, and gonadal development occur within 48–72 h. Senescence can commence within 2 wk, since a simple habituation paradigm is compromised by that time (88). Compare this to months for rodents and years for humans.
Fig. 1.
Life cycle and comparison with two vertebrate models. A: life cycle of Drosophila melanogaster. B: comparative length of time per generation and relative cost to maintain the same number of animals between Drosophila, zebrafish, and mouse.
Another major plus, especially during current times of significantly reduced funding, is their relative lack of expense to maintain. On average, flies cost 20 cents/mo per 100 animals; zebrafish are 900 times more expensive, and mice over 10,000 times the cost (66) (Fig. 1B).
But by far and away its arguably greatest advantage is the incredible genetic tractability of Drosophila. Several crucial characteristics facilitated initial genetic studies in this animal. There are only four chromosomes in the haploid Drosophila genome, and numerous easily scored outward characteristics have been identified that are also highly mutable. While the contributions to classical genetics from early studies on Drosophila have been overwhelming, the range and complexity of genetic and molecular tools now available have enabled Drosophila researchers to perform unbiased genetic screens for an overwhelming number of different physiological processes. Not only can mutations in critical genes be uncovered, they can be maintained in populations through the use of balancer chromosomes (chromosomes with multiple small inverted regions that prevent correct alignment of the chromatids and thus crossing over). Balancer chromosomes are homozygous lethals, and carry easily scored phenotypic markers so that the chromosome carrying the mutation can be identified.
Another advantage is that signaling pathways and interacting factors of lethal mammalian genes may be elucidated in Drosophila by targeting their expression to a nonvital tissue, such as the eye. The Drosophila researcher is fortunate enough to employ several unique tools in the scientific toolbox (Supplemental Table S1). These include an array of aneuploid strains (lines carrying chromosomes with deficiencies or duplicated regions), classical mutations, and transgenic lines, including Gal4/UAS and later systems generated along the same concept. The Gal4/UAS system is bipartite: a line encoding the yeast transcription activator protein Gal4 is crossed with a UAS line carrying the Upstream Activation Sequence, which is an enhancer to which Gal4 specifically binds to activate gene transcription, and the progeny then expresses the gene downstream of the UAS at a time and in the tissue(s) directed by the promoter subcloned upstream of Gal4 (Fig. 2) (12). The majority of Gal4 lines are generated by constructs derived from specific promoters or by fragments that randomly “trap” spatially and temporally specific enhancers upstream of Gal4 driven by a minimal promoter. Not only can a gene be expressed outside of its normal milieu, its expression may be reduced via the use of RNAi transgenes, or monitored through the use of reporter transgenes, and exogenous genes may be expressed to establish they are functional orthologs of their Drosophila counterpart (see Supplemental Table S1). While only some of the vast array of tools are described in Supplemental Table S1, more can be found on FlyBase (www.flybase.org), a free website with links to multiple stock centers (Bloomington, IN, USA; Kyoto, Japan; and Vienna, Austria), other resources (Drosophila Genomics Resource Center, Berkeley Drosophila Genome Project), and, for all annotated genes (the vast majority), links to sequence, predicted protein, expression profiles, interaction pathways, available lines, and references.
Fig. 2.
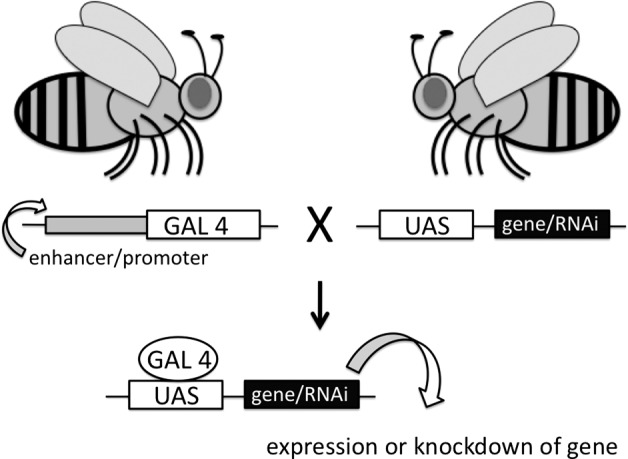
The Gal4-UAS bipartite transgenic system. A line encoding the yeast transcription activator protein Gal4 is crossed with a UAS line carrying the Upstream Activation Sequence; Gal4 specifically binds to this sequence to activate gene transcription. The temporal and spatial expression is directed by promoter or enhancer sequences upstream of Gal4, and the progeny will correspondingly express the gene downstream of the UAS sequences.
The beauty of the unbiased genetic screens possible in this system is that genes isolated based on seemingly unimportant phenotypes were later demonstrated to be key players in the etiology of human disease. For example, Notch, first identified in Drosophila in 1915 as a wing mutation, has since been shown to be the cause of human T-lymphoblastic neoplasms. Notch is also critical for neurogenesis, neuronal differentiation, bone development, and cardiogenesis, in addition to other crucial developmental pathways (reviewed in Ref. 6).
In this review, we offer up only a limited number of major contributions made by Drosophilists to understanding the dynamics of pattern formation, cardiac function, thermal and pain sensation, and the basis of specific behaviors, including sleep and learning. We have included a table listing recent reviews for several research areas (Supplemental Table S2), but only these few are presented in greater depth in this review. We are limited not by the contributions, but by page constraints, with the hope that the reader will come to appreciate how much clinically relevant information about fundamental physiological processes has been derived from the small and unassuming fruit fly.
Organogenesis, Gradients, and the Mathematical Dynamics of Pattern Formation
Female Drosophila lay their eggs on or near a food source for the hatching larvae to feast upon, and then fly away. While humans, in general, nurture their offspring until mature, their embryos also require factors to initiate the developmental program. In flies, such factors are maternally deposited in the nurse cells of the oocyte (hence their designation as maternal effect genes); their role is to activate the first wave of zygotically expressed genes (gap genes). However, these genes are known to be critical for organogenesis, in general, and the detailed genetic characterization and expression profiles for some of these genes have enabled researchers to use mathematical modeling to establish how dynamic pattern gradients are formed, which is critical to our understanding of organogenesis and the physiological pathways relevant to individual organ systems.
The first zygotic genes expressed in the developing embryo subdivide it into broad regions: terminal, “head,” and central (reviewed in Ref. 101); mutations in these genes create “gaps” by deletion of specific segments. The earliest expression patterns of the gap genes are independently determined by the maternal effect genes, but later expression is dependent on the other gap genes, establishing multiple gradients to define the individual domains (reviewed in Ref. 60). The gap genes encode transcription factors, and while they are key players in early development, they are also necessary for organ development. However, much remains to be elucidated to understand the molecular mechanisms guiding their function. It has been proposed that the ancestral role of the gap genes may be in neurogenesis and head patterning (60), but they have also proven invaluable in increasing our understanding of the evolution of gene networks.
A mutation in a homeotic gene causes a given morphological structure—like an antenna—to develop into a copy of a structure that belongs elsewhere on the body, like a leg. These are the genes responsible not only for transforming segments into body parts, but also for organogenesis, in general. The homeotic genes in Drosophila are divided into two clusters: the Antennapedia cluster (ANTP-C), and the bithorax complex (BX-C). The ANTP-C genes specify how the thorax and abdomen of the fly are constructed (74). The proteins encoded by the homeotic genes are transcription factors that activate diverse downstream targets termed realizator genes, which, when first described, were proposed to mediate cell adhesion, polarity, and shape (40). The first description of the network of realizator genes controlled by a homeotic gene (in this case, Abdominal-B) identified targets such as GAP and GEF cytoskeleton regulators, cell adhesion molecules (e.g., E-cadherin) and Crumbs, which is critical for determining apical-basal polarity in the cell. While the individual organ structures will not necessarily be conserved between Drosophila and humans, similar networks of homeotic gene-controlled cascades likely determine the changes in groups of cells that result in formation of specific organs.
Characterization of one of the critical maternal effect genes, bicoid (bcd), has been fundamental to elucidating how expression gradients are established. Embryos derived from bcd females lack anterior structures; normally, Bicoid protein is expressed in a gradient with the highest concentration in the anterior of the embryo. The protein is not detected in oocytes, evidence that the maternally deposited mRNA is translationally regulated (24, 36). Several elegant studies have demonstrated that maternally derived bcd mRNA is anchored to the anterior pole of the oocyte by virtue of specific sequences in its 3′ untranslated region via the actions of the products of several other genes (8).
Using fluorescently tagged protein, Gregor et al. (44) have shown that the gradient of Bicoid protein is established within 90 min. The gradient is remarkably precise from embryo to embryo, since, in larger embryos, the gradient is appropriately scaled up. These studies of bcd were the first to demonstrate that positional information derived from a concentration gradient could establish polarity within an embryo and has made a seminal contribution to our understanding of pattern development.
Bcd also acts as a downregulator of maternally expressed caudal protein by binding to its mRNA via the homeodomain in Bcd. caudal (cad) is required for posterior development of the embryo and forms a posterior-to-anterior gradient; the action of Bcd inhibits cad translation in the anterior half of the embryo (102, 103).
How does Bcd stay concentrated in the anterior portion of the embryo, instead of diffusing throughout; that is, how is the gradient maintained, since the integrity of the gradient is obviously crucial for the next developmental stages? While spatial distribution of bcd mRNA is clearly important, protein movement is also necessary to establish the gradient (75), as is degradation of Bcd protein via targeted ubiquitylation (77). Although numerous models have been proposed to explain how the gradient is established, none completely explain all the experimental observations, a demonstration of the incredible complexity of this critical biological question (45, 98). Just as gap genes have been used as a tool to assess the mathematical dynamics of pattern formation, bcd has served as a model for mathematical modeling of a morphogenetic gradient (60).
While bcd is essential for anterior-posterior patterning of the embryo, Dorsal (Dl) is key for dorsoventral patterning. As for bcd, expression patterns of the multiple genes regulated by Dl have been extensively characterized. Computational modeling of the Dl gradient suggests its pattern is dynamic, with increasing amplitude but constant shape—very different from that observed for the bcd gradient, which is expressed at the same developmental time (65).
Since the mRNA and protein expression patterns for the gap-gene network in Drosophila have been so extensively detailed, mathematical models have also been constructed to define the temporal embryological topology of these genes. While some limitations obviously exist (i.e., assuming only one enhancer per gene), such models can reasonably predict at least some of the interactions between genes that are critical for anterior-posterior patterning (9). These kinds of computational approaches to predicting interacting genes within a network are almost impossible to attempt in other species.
Drosophila as a Model for Human Cardiac Physiology
The heart, or dorsal vessel in Drosophila, functions to pump hemolymph from posterior to anterior, and consists of cardiomyocytes surrounded by noncontractile pericardial cells. One feature that makes this an ideal system for the study of both cardiac development and physiology is that the dorsal vessel can be visualized using only a light microscope during both larval and adult stages, which makes it possible for researchers to observe physiological parameters in the intact animal. This, combined with the many genetic tools available in Drosophila, has made it possible to identify and characterize many of the key factors in cardiac development. One of the best known examples of a cardiogenic factor identified by a candidate gene approach in Drosophila is tinman (tin), which was named as a result of the absence of a dorsal vessel in tin mutants (11). As has been the case for many of the cardiogenic factors identified in Drosophila, impairments in NKX2–5, the human homolog for tin, results in congenital heart disease (107). This speaks to the translational value of the Drosophila dorsal vessel, since so many of the genes involved in cardiac development are conserved between vertebrates and invertebrates, and the resulting defects in cardiac physiology as a consequence of misexpression of these genes are also similar (reviewed in Refs. 10 and 93) (Supplemental Table S3).
One of the most notable differences between Drosophila and mammals is that the dorsal vessel is part of an open circulatory system. This difference actually works to the advantage of the Drosophila investigator, because an open circulatory system allows for some transport of hemolymph even without a fully functioning dorsal vessel, making it possible to identify roles for cardiogenic factors that would otherwise result in lethality. Additionally, Drosophila has less genetic redundancy than vertebrate systems, which can assist in the identification of novel cardiogenic factors. Together, these features have facilitated the identification of novel genes involved in cardiac development, elucidated complex interactions between multiple cardiogenic factors, and resulted in the creation of models with translational value to mammalian cardiac physiology.
Following the initial development of the larval heart, some additional modifications take place during metamorphosis to create the adult dorsal vessel. One of the most important differences between the larval and adult dorsal vessels is that the adult dorsal vessel receives direct neuronal input, which increases complexity and allows for adaptation to different conditions (26). Importantly, many of the ion transporters required for contractility of the heart in vertebrates are also present and function in the dorsal vessel (76).
Several methods have been developed for high throughput and translational cardiac analyses. One of the first studies to look at the age-related decline of cardiac function in Drosophila used a light microscrope and video recording to measure heart rate and end systolic and end diastolic diameter in response to stress at different ages (95). By inducing heart failure via external electrical pacing, it was found that as flies age, maximal heart rate decreases, similar to what is observed in humans (32, 95). Green fluorescent protein can be used in conjunction with the Gal4/UAS system to allow for visualization and identification of specific cell types within the dorsal vessel (e.g., 95). For continuous analysis, partially dissected preparations in artificial hemolymph have been used to measure tension generated by the cardiac muscle using carbon fibers (91). Analysis of video traces from these types of preparations, as well as from intact preparations, can offer several useful parameters, such as heart rate, systolic and diastolic intervals, systolic and diastolic diameters, percent fractional shortening, contraction wave velocity, and cardiac arrhythmicity (31). An additional technique that involves enclosing a single fly in a small chamber to prevent large movements and detecting the internal space of the heart tube using optical coherence tomography has been used to establish Drosophila as a useful model to study dilated cardiomyopathy (121). For the study of electrical activity, recordings of intracellular action potentials can be performed even as the heart contracts (71). One caveat for these types of studies is that care must be taken to use a medium that mimics physiological conditions specific for hemolymph (111). A video demonstrating the preparations and uses for several of the above mentioned techniques can be found at http://www.jove.com/index/details.stp?id=1596 (20).
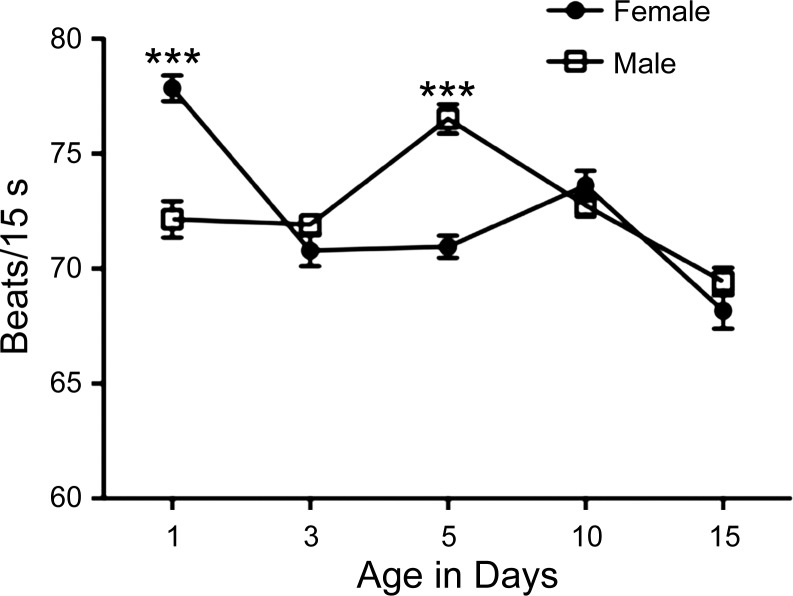
The Drosophila system offers several advantages that make it attractive to modeling cardiac diseases beyond the ease with which one can manipulate cardiac gene expression in vivo. These above techniques, along with the advantage of a short lifespan, have made Drosophila ideally suited to study cardiac aging. Several similarities exist between the aging dorsal vessel and the mammalian heart. Figure 3 shows that for both male and female Drosophila, there is an overall trend toward decreasing heart rate with increasing age, similar to what is observed in mammals (32). Our analysis of heart rate in the aging fly also shows that the changes with age are sexually dimorphic. These changes with increasing age have been correlated with alterations in the contraction pattern, suggesting that the likelihood of arrhythmias increase with increasing age (91). Age-related cardiac decline has been linked to decreasing levels of the Drosophila ortholog of human KCNQ1, which encodes a potassium channel α-subunit, and of dSur (an ATP-sensitive potassium channel) (91). In humans, KCNQ1 alterations have been linked to an increased risk for arrhythmia (see Ref. 61 for review). In flies, KCNQ has been shown to be responsible for the slower repolarizing current, and its overexpression in older flies reduces the number of arrhythmias (91). Similarly, it appears that in both flies and mammals, dSur protects against pacing stress and hypoxic stress (2, 46). Lastly, for flies, as well as for mammals, exercise training reduces age-related cardiac decline (97, 113).
Fig. 3.
Heart rate decreases with increasing age in a sexually dimorphic manner. The heart rates of age-matched male and female wild-type (Canton S) flies were compared over time. n = 40 individuals for each age (1, 3, 5, 10 or 15 days old). Individual heart rates were the average of 5 intervals of 15 s each with 15 s between each interval. Statistically significant differences indicated by asterisks over lines covering the bars. ***P < 0.001, Students t-test.
Insulin-IGF receptor (InR) signaling has a known role in controlling life span (see Ref. 115 for review), making the aging dorsal vessel an ideal model for studying organ-specific aging in response to manipulation of insulin signaling. Mutant lines with defects in the insulin-like receptor (InR) or its substrate chico have an increased life span (19, 114). Direct manipulation of insulin signaling in the dorsal vessel by overexpressing the phosphatase dPTEN (a negative regulator of insulin/IGF signaling) or the forkhead transcription factor dFOXO (a negatively regulated target of insulin/IGF signaling), prevents the decline in cardiac performance that is typically seen with increasing age, indicating that decreased insulin signaling not only increases life span, but also specifically prevents age-related organ decline (119).
Beyond its value as a model for cardiac aging, the dorsal vessel in Drosophila can be used to evaluate general cardiac dysfunction. Drosophila has homologs for all six cardiac disease genes examined in a survey of 287 human disease genes (34). In addition, many of the proteins needed to carry out cardiac function in vertebrates, such as ion channels and contractile proteins, are conserved (91). Mutations in several of the ion channels and transporters have been found to alter heart rate. Specifically, mutations in SERCA, the sarcoendoplasmic reticulum Ca2+-ATPase, which has been linked to cardiac malfunction in mammals, significantly reduces beat frequency and alters electrical activity and rhythmicity (106). Mutations in the Ca2+ channel encoded by cacophony results in an abnormally fast heartbeat that does not change in response to temperature (100). ORK1, an outward rectifying potassium channel, was shown to regulate the pacemaker frequency in Drosophila (71). This was the first evidence that a two-pore domain potassium channel could regulate automatic cardiac activity. Inactivation of ORK1 increases heart rate, and its overexpression abolishes the heartbeat (71). Recently, a Drosophila model for atrial fibrillation was established using tachypacing to induce cardiomyocyte remodeling, which causes atrial fibrillation in humans (124), enabling researchers to harness the power of Drosophila genetics to search for novel targets for cardiomyocyte remodeling (124).
Hypoxia has been another physiological problem for which Drosophila is ideally suited to aid in research. Hypoxia is a major concern for human health because it is the leading cause of necrotic cell death in myocardial infarction, as well as having a fundamental role in embryogenesis, obstructive sleep apnea, and sickle cell anemia. Adult Drosophila are tolerant to low oxygen environments and can withstand anoxia for ∼3 to 4 h without experiencing cell damage (48). Genes important in hypoxia were discovered by exposing Drosophila to either severe intermittent (present in humans during obstructive sleep apnea, central hypoventilation syndrome, and intermittent vascular occlusion in sickle cell anemia) or constant (present in humans during pulmonary diseases such as asthma and congenital heart disease with right to left shunt) hypoxic conditions, and performing microarray analysis to identify up-regulated or downregulated transcripts (3). Overexpression of Hsp70 and Hsp23 during constant hypoxia, and Mdr49 and l(2)08717 during intermittent hypoxia, increased survival (3). Other correlates have been discovered: for example, trehalose, a glucose dimer, plays a role in protecting both fly and mammalian cells from anoxic stress. Overexpression of trehalose-6-phosphate synthase 1 (tps1), which synthesizes trehalose, increases tolerance to anoxia in flies, and disruption of the tps1 gene results in lethality (15). Increased resistance to low oxygen stress has been observed in mammalian human embryonic kidney cells transfected with Drosophila tps1 (16). Hypoxia-inducible factor, the metabolic AMP-activated protein kinase, and nitric oxide signaling have all been shown to be involved in the response to hypoxia in the mammalian heart (62, 67, 83); not surprisingly, all are present and functional in flies (72, 94, 120). Feala and colleagues are compiling data from the annotated Drosophila genome (1), the KEGG database of enzymes and pathways (92), and Drosophila biochemistry data from the literature to build a database of all metabolic pathways in Drosophila myocytes (28) to create a genome-wide model that includes all genes, enzymes, metabolites, and regulatory proteins, as well as protein-protein interaction pathways, that are involved in the hypoxia defenses (29).
Transient Receptor Potential Channels, Pain, and Thermal Sensation
Every organism must sense and respond to deleterious changes in their environment to survive; these include changes in temperature, gustatory responses, vision, olfaction, and pain. Not surprisingly, the neuronal mechanisms in place to sense environmental disruptions are highly conserved across species. While channels necessary for sensing these alterations have been extensively characterized in the nematode, C. elegans, and several have also been cloned from humans, what Drosophila has been able to offer is an understanding of how the circuit is integrated, from perception to sensation to response.
It is critical for organismal survival to maintain an appropriate internal body temperature, as well as to seek out a suitable external thermal environment; thus, all organisms tend to actively avoid deleterious thermal conditions. There is a temperature optimum for the normal regulation of cellular physiology, such as membrane fluidity and biochemical reactions, which have evolved to function best within a limited temperature range. Unlike mammals, which maintain a fairly constant internal temperature, Drosophila (and other ectotherms) are dependent on the external environment to regulate their internal temperature, and rely substantially on behavioral mechanisms to maintain an appropriate body temperature (110). It is not unreasonable to expect that the mechanisms for sensation of different thermal conditions are ancient, since this is a fundamental physiological process necessary for survival and fitness.
While adult Drosophila can subsist between the temperatures of ∼16°C and 30°C, they flourish at the mean of 23–25°C. Since flies are small, they readily equilibrate to the surrounding temperature. Therefore, if flies are to maintain an appropriate body temperature for normal physiological processes, they must have evolved a rapid thermal sensing capability. And since they have two very different life stages that must address the same problem, elucidating the mechanisms for larval and adult thermal regulation should yield information not only about thermal sensors in general, but about the evolution of thermal regulation.
However, before one can understand the molecular mechanisms underlying thermosensation, it is necessary to identify the temperature-sensing neurons and the organs in which they reside, and this has proven to be comparatively facile using the Drosophila model system. One of the first studies to identify thermoresponsive cells and organs made use of transgenic Drosophila expressing cameleon 2.1, a calcium-sensitive protein with two fluorophores whose fluorescence ratio changes with a conformational change in the protein (e.g., when there are changes in the intracellular calcium concentration). Cameleon 2.1 was expressed in all neurons in Drosophila larvae, which were then exposed to differing cold and warm temperatures. These experiments identified neurons in the terminal organ in the larval head that acted as cold sensors, and specific body wall neurons that acted as heat sensors; this was confirmed using electrophysiological techniques (78). Additional heat sensors were found to reside in central neurons (see below).
The navigational decisions made by Drosophila larvae upon encountering even small changes in temperature (0.005°C/s gradient, 81) have been elegantly teased out. Drosophila larvae crawling on a substrate frequently stop and “sweep” their heads from side to side and then choose to orient along a specific thermal gradient. This occurs via negative and positive thermosensors (similar to mammalian thermosensation) (reviewed in Ref. 42), and downstream neural circuits integrate the input from one set of sensory neurons to initiate turning or aligning behavior (81). These experiments were some of the first to elucidate the stochastic mechanisms underlying a goal-directed behavior requiring executive decisions.
“Heat” signals are transduced, at least in part, by members of the TRP family, which share homology with the mammalian TRP family genes. TRP channels are ancient, with extensive functional conservation amongst the subfamily members (86). The first TRP channel identified in Drosophila, painless, is found in neurons within the larval body wall, and is activated by high heat (>42°C, well outside the fly's optimal temperature, and thus considered noxious) (116); it is also activated in response to mechanical insult. Consistent with this phenotype, painless is expressed in the multidentritic neurons in the periphery (116). painless is the Drosophila ortholog of the mammalian wasabi receptor, TRPA1, and was isolated by screening an insertional mutation library for animals that did not produce the stereotypical kink-and-coil reaction in response to the touch of a hot (∼40°C) probe. It shares homology with a member of the TRPN subfamily, no mechanoreceptor potential-C (NOMP-C), which is also required for mechanosensation. The isolation of painless is yet another testimony to the utility of the Drosophila genetic screen: 1,500 lines were screened in short order and yielded 49 candidates with decreased sensitivity to noxious heat, of which painless was the first one characterized.
There are three other members of the TRPA subfamily: dTRPA1, dTRPA2, and dTRPA3. dTRPA1 is also activated by higher temperatures and is found within specific cell clusters in the larval central nervous system, as well as in cells found within the corpus cardiacum of the ring gland, the fly's endocrine organ, and in cells dispersed throughout the gastrointestinal tract. However, it is only the anterior cell neurons in the brain that are required for thermal sensation (49). This channel modulates selection of an environmental temperature of ∼18°C when the animal is exposed to only slightly warmer (∼24°C) conditions (69). Recently, the G protein-coupled receptor necessary for thermosensory signaling by dTRPA1 was shown to be a rhodopsin-ninaE, acting in a pathway independent of any activation by light (108). These studies determined that Gq, phospholipase C, and TRPA1 are downstream elements in the pathway initiating with RH1, the opsin encoded by ninaE; more importantly, and again demonstrating conservation across species, the human ortholog (melanopsin) can rescue the thermal sensory defects in ninaE mutant larvae (108).
How do TRPA1 channels discriminate between thermal and sensory cues? Such specificity may be established by selective expression of different isoforms: TRPA1(A) is insensitive to warmth and is expressed in gustatory neurons; the chemically sensitive TRPA1(B) isoform is instead expressed in these cells (64). This is specified by a unique region within the B isoform that significantly reduces its thermosensitivity (64).
dTRPA2 was isolated from an insertional mutagenesis screen of more than 27,000 lines. Known as pyrexia (a medical term meaning fever), it is expressed in both multidendritic and nondendritic cells lining the larval epidermis; in adults, it is expressed in sensory neurons underneath the eye bristles, and in neurons innervating the back of the thorax, maxillary palps, proboscis, and antennae. In all cases, it is expressed throughout the neurite, suggesting it functions in body-wide responses. Channels encoded by pyrexia are involved in the sensation of noxious heat, and animals carrying a mutation in this gene become paralyzed after a short exposure to noxious (40°C) heat (73).
dTRPA3, also known as water witch, functions as a hygroreceptor to detect humidity (79). A second TRP channel, nanchung (a TRPV channel like inactive, below), detects dry air; interestingly, the compartmentalization of sensation for detecting moist vs. dry is similar to the compartmentalization of receptors that detect cold vs. heat.
Several “cold” larval receptors in Drosophila have also been described; these include the TRPC channels TRP and TRPL, which detect relatively small differences in temperature (105). Larvae carrying a genetic disruption in each of the 13 TRP channels were tested in a simple choice assay (between 14°C and 17.5°C); this screen identified the two TRPV channels (70). The TRP channel Iav was shown to function in thermal discrimination of cooler temperatures in the chordotonal organs by genetically targeted disruption, resulting in increased turning angles of the larva along the substrate (70). Adult cold sensors have been identified in a simple adult behavioral screen of insertional mutations (fly strains in which a small transposable element was inserted within or adjacent to a gene, disrupting expression of that gene) (39). This screen uncovered brivido (brv)—Italian for shiver—a member of the TRP polycistin subgroup of the TRP ion channel family. A second TRPP mutation, brv2, was identified by homology; flies lacking brv2 function also display impaired avoidance of cold (as low as 11°C) temperatures. A third TRPP-homologous gene, brv3, was similarly shown to act as a cold thermosensor. The three brv genes are coexpressed in the same three neurons within the arista (part of the fly antenna) and serve as cold sensors; the other three aristal neurons are heat sensors. Tracking the projections from the aristal neurons to the brain (using a targeted green fluorescent protein, and a few genetic tricks to specify projections from only the cold or the heat thermosensors) demonstrated that they converged onto adjacent but distinct glomeruli (39). These elegant experiments demonstrated that distinct cell populations coordinate behavioral responses to specific temperatures.
The TRP family members discussed above were all initially identified years before their human homologues (Supplemental Table S4). In some cases, mutations were characterized long before their functions in pain or thermal sensation were recognized (i.e., trp, trp-like [trpl] and inactive [iav]). Additional TRP family members were isolated by homology, or by simple, high-throughput behavioral screens, or by a combination of screening only those lines carrying mutations in genes known to be homologous to the TRP channel family. In fact, the human TRP homolog was identified via homology with the Drosophila trp gene (118).
While the TRP channels are key in thermal sensation, they are not, however, the whole story. Another forward genetic screen, using the same 27,000 insertion lines mentioned above, identified elements of the histamine signaling pathway, including histidine decarboxylase and two histamine receptors, as demonstrating a low tolerance for high temperatures, and increased tolerance for cold (57). Independent confirmation was achieved using known mutant alleles corresponding to these genes. In addition, treatment with histamine receptor antagonists was shown to affect temperature preference. Histamine is found in a limited number (∼20) of neurons, but arborizations from these cells are heavily distributed throughout the adult brain; the receptors are also widely expressed in neural tissue (57). These studies demonstrated a novel role for histamine beyond its functions in phototransduction, mechanosensation, and sleep, and they suggest a possible involvement in seasonal acclimation that may occur with changes in daylight and temperature.
Similar to human neuropeptide Y, the Drosophila homolog, neuropeptide F (NPF), has been shown to play a role in nociception by modulating TRP channel-mediated responses to noxious heat stimuli (123). A G protein-coupled NPF receptor is coexpressed with painless in peripheral thoracic neurons; additional experiments have shown that expression of the rat capsaicin receptor TRPV1 in neurons expressing painless resulted in larval aversion, which was rescued by coexpression of NPRF1 (123). These studies have very elegantly demonstrated a conservation of mechanism across species.
Thus, through the use of extensive genetic screening, multiple genes in different pathways have been identified as being critical for thermal sensation and pain. In addition, the subpopulations of cells, in different tissues and organs, necessary for perceiving these signals, as well as some of the molecules required for their transduction, have been characterized in an effort to integrate these pathways. The beauty of the model is that a simple assay—alignment along a small thermal gradient, or reduced aversion to a noxious stimulus—which takes only a few minutes to complete, has enabled researchers to begin to piece together the step-wise process of an evolutionarily conserved goal-directed behavior. And of course, acknowledging the conservation across species, the first mammalian TRP family members were isolated by using the Drosophila gene as a stepping-stone.
Using Drosophila to Elucidate Biobehavioral Mechanisms
Aggression.
Aggression is a complex behavior seen in almost all social species, including Drosophila. It has many purposes, but often in the animal kingdom it plays a role in territory defense, survival or reproduction, and is also observed in psychiatric disorders in humans. The first account of aggressive behavior in Drosophila came from Jacobs, who described males fighting for food sources (59). Drosophila has since been used to identify several genes involved in aggressive behavior, which may have implications for aggression in humans. Many of the studies that have been done to date have focused on selectively breeding for males that display increased aggressiveness, are more territorial, and have greater success when fighting other males, and identifying upregulated and downregulated genes relative to wild-type in these selected populations (22, 27). Several of the genes found to be associated with altered aggressiveness in males have been previously identified to have roles in a wide range of biological processes, including, sex determination, neurotransmission, learning and memory, pheromone detection, and nervous system development (see Ref. 104 for review). Importantly, many of these genes also have human homologs (29).
In addition, several studies have assessed the role of biogenic amines in aggression. Baier et al. (5) showed that octopamine, dopamine, and a region of the adult fly brain known as the mushroom bodies (analogous to the mammalian hippocampus) all show reduced levels of aggression (5).
Sleep.
Although the exact purpose of sleep is unknown, it is conserved in all animal species that have been examined thus far (18). Drosophila, like all other animals studied, demonstrate a cycle of ∼24 h even when separated from external cues, such as temperature and light, and there are distinct periods of sleep and rest, demonstrated by differing levels in activity. One of the best known contributions from Drosophila to the study of sleep was the discovery of the clock genes (reviewed in Ref. 96). The first clock gene was discovered by identifying flies with abnormal circadian patterns of eclosion, and it was named period (68); years later, it was found that period has homologs in many other species, including some mammals (96).
Sleep in a fly is defined as any period of immobility lasting for more than 5 min, which shares most of the features of sleep in humans (53, 58). In flies, it is possible to measure sleep depth by looking at parameters such as sleep continuity (58). Electrophysiological recordings can also be performed, but these are generally invasive (89). Many studies have focused on identifying those genes involved in sleep in the hope that, by understanding the biological processes modulated by these genes, the reason for sleep will be revealed.
Another well-known example is the forward genetic screen that identified Shaker, which selected for short-cycle sleeping mutants and identified minisleep, a Shaker allele. Shaker encodes the α-subunit of a voltage-dependent potassium channel, which is involved in membrane repolarization and transmitter release (17). Knocking out Kcna2, the mouse Shaker ortholog, also results in reduced sleep (23).
Reverse genetic screens have also been used to further investigate the role of genes and pathways already known to be involved in sleep. For example, previous studies had shown that the cAMP/PKA/CREB pathway is important for learning and memory, and that CREB expression displays a circadian pattern (7). Increasing cAMP or CREB activity in Drosophila decreases sleep time and rebound after sleep deprivation (54); later studies in mice showed similar results (43).
Drosophila as a model to study learning and memory.
Investigation of the neurological processes underlying learning and memory may provide progress toward elucidating the etiologies of human cognitive disorders. In addition to the genetic screens that have repeatedly proven to be beneficial, there are also numerous behavioral assays developed for Drosophila that have facilitated a greater understanding of the molecular mechanisms underlying learning and memory. The first technique to be used was based on a fly's ability to learn to avoid an odor associated with an electric shock (99). Since its initial development, the odorant learning method has been modified and generates a remembered association that can last up to a week (117). Odorant learning methods have been used to identify several genes involved in learning and memory that are generally expressed in the mushroom bodies and central complex, two structural regions within the Drosophila brain that mediate higher-order behaviors (51, 52). Another popular learning paradigm is conditioned courtship suppression, which is based on the fact that mated females will block male copulation attempts. Rejected males will eventually learn to suppress their courtship behavior toward other females (109). This paradigm is less widely used for large-scale screens than the odor association method, since courtship studies are more tedious and limited to males. A parallel technique exists that is based on the courting of newly eclosed males by older males. Since the courtship attempts in this case will not end in copulation, the older male eventually learns that his attempts are futile and reduces his courtship attempts (38). This technique was used to establish a role for dopamine in habituation learning (87). An additional technique that is simple, useful for both sexes, and sufficiently high throughput for use in a large-scale genetic screen, is the use of a heat box. A heat box is a small chamber into which a single fly is placed, and permitted to roam freely, but when it crosses the midline of the box, the temperature will be increased in the chamber, training the fly to prefer the cooler side of the chamber (122).
Dunce (dnc) was the first of the learning mutants to be discovered using the odorant learning paradigm. dnc mutants displayed normal phototaxis, an ability to sense odors and normal deterrence of electric shock (25), yet they were unable to learn to associate a particular odor with an electric shock (25). dnc was later found to encode cyclic AMP (cAMP) phosphodiesterase II (13). An additional learning mutant, rutabaga (rut), was identified using the same behavioral paradigm as for dnc and encodes a Ca2+/calmodulin-sensitive adenylyl cyclase (80). A third learning mutant, amnesiac, encodes the pituitary adenylyl cyclase-activating peptide (30, 99a). The results from dnc and rut provide strong evidence that regulation of Gs signaling and cAMP levels are essential for normal learning and memory in Drosophila.
In addition, Drosophila models exist for many of the human disease-associated cognitive deficits. Neurofibromatosis is a dominant genetic disorder in humans characterized by benign neuronal tumors and defects in movement and learning due to mutations in Nf1 (see Ref. 63 for review). A Drosophila strain lacking Nf1 was generated to help identify molecular pathways involved in this disease (47). The authors found that Nf1 may be involved in cAMP signaling by regulating activation of rut (47). Drosophila has also been used to model tauopathies. Overexpression of TAU in Drosophila results in deficits in learning and memory (85). The most notable symptom of trisomy 21 (Down's syndrome) is mental retardation, believed to be due to overexpression of DSCR1, a gene that encodes calcipresin, an inhibitor of the serine/threonine protein phosphatase calcineurin (37). Overexpression of the Drosophila ortholog, nebula, results in severe immediate memory and consolidated memory defects with decreases in protein kinase A activity and phosphorylated CREB, additional evidence for the involvement of CAMP signaling in learning and memory (14). Fragile X syndrome, characterized by mental retardation, is caused by disruption of the FMR1 gene (see Ref. 55 for review). Disruption of FMR1 in Drosophila results in abnormal mushroom body development and defects in memory in the courtship conditioning paradigm (84). Glutamate receptor antagonists and lithium can restore learning and memory deficits in this model, which may be helpful for human patients (84).
Stress.
On a daily basis, animals of all species must deal with changing physiological and environmental conditions. Starvation is a commonly encountered stress for a variety of species, and prenatal malnutrition in humans has been linked to diseases, such as schizophrenia and other forms of psychosis (reviewed in Ref. 82). Starvation stress is modeled in flies by providing access to water but no food. One study showed that starvation resistance is heritable by placing flies on a decomposing lemon, which has insufficient nutritional value, and selecting for increased resistance to starvation (50). Although there are a few ways in which animals may increase their starvation resistance, it has been shown that it is most commonly accomplished in Drosophila by increasing energy stores (reviewed in Ref. 56). In addition, animals selected for starvation resistance had increased resistance to other stressors but no increase in longevity (50).
Another commonly encountered stress is oxidative stress, which can trigger numerous diseases, such as cancer, stroke, Parkinson's disease, cardiovascular disease, and many other diseases of aging (reviewed in Ref. 33). In Drosophila, oxidative stress is easily accomplished by feeding the animals paraquat or by genetic manipulations to the electron transport chain, because mitochondria are the primary source of reactive oxygen species. One study used paraquat to show that increasing oxidative stress acts to increase tau-induced neurodegeneration in Drosophila (21). Sun and colleagues found that overexpression of mitochondrial Mn-superoxide dismutase increases longevity (112). To reduce the rate of reactive oxygen species by the mitochondria, researchers overexpressed human uncoupling protein 2 in adult Drosophila neurons and observed decreased production of reactive oxygen species, decreased oxidative damage increased resistance to paraquat and increased longevity (35).
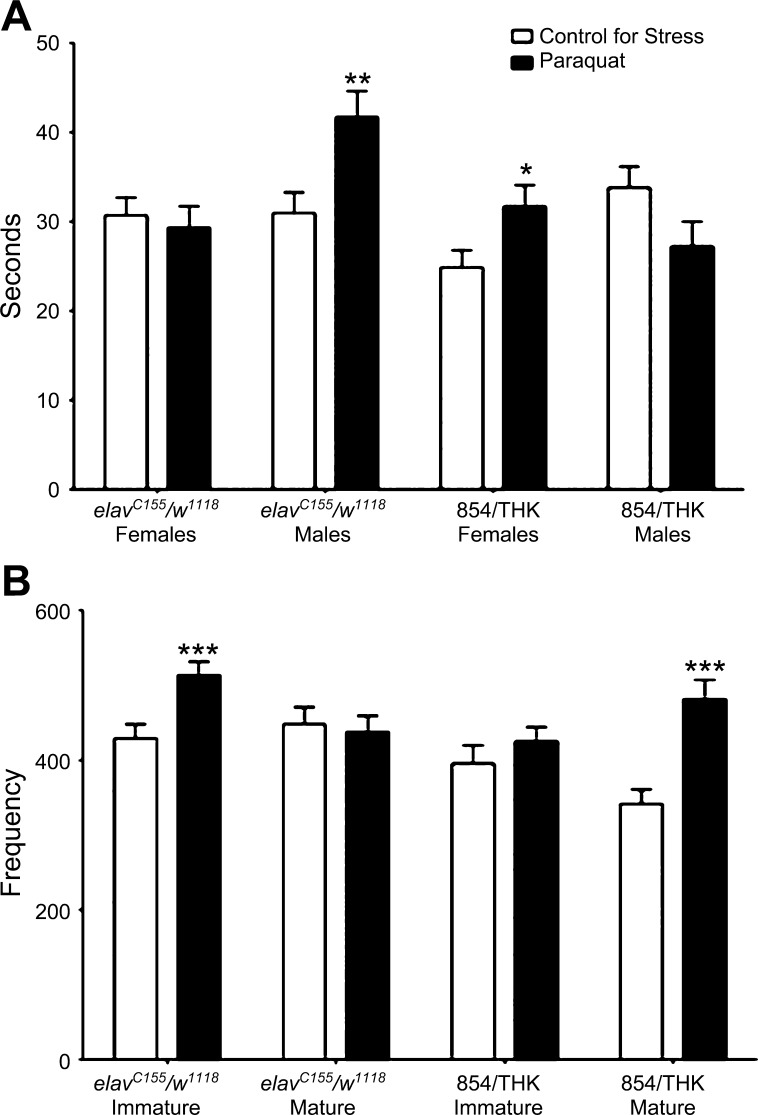
Recent studies in our laboratory have focused on sexual and temporal dimorphisms in the stress response, which have shown that the behavioral response to stress differs depending on whether the individual is a sexually immature or mature male or female. For example, the total distance moved in an open field study with Canton-S (wild-type) animals exposed to starvation or oxidative (paraquat) stress depends on the age [1 day (sexually immature) or 5 days (sexually mature)] and sex (male or female) of the animals being assayed (Fig. 4). Further analysis using a series of Gal4 lines to target expression of a tyrosine hydroxylase (rate-limiting enzyme for dopamine synthesis) RNAi (THK) to different subsets of dopamine neurons demonstrated that differential recruitment of dopamine neurons into the stress response circuitry underlies the sexually and temporally dimorphic behavioral responses observed in sexually immature and mature males and females. For example, sexually mature animals from the 854/THK line, which targets expression of THK to the mushroom body neurons, display a significant 3-way interaction (genotype × sex × stress) for freezing duration (a predatory response behavior) in response to paraquat stress compared with control (elavC155/w1118) (P < 0.001). elavC155/w1118 males increased the duration of freezing (P < 0.01), while females of the genotype showed no significant effect, but 854/THK females increased their freezing duration (P < 0.05), and 854/THK males show no significant effect (Fig. 4A). 854/THK females assayed for freezing frequency in response to paraquat exposure displayed a significant 3-way interaction (genotype × age × stress) compared with elavC155/w1118 controls (P < 0.001). Sexually immature elavC155/w1118 animals increased their freezing frequency (P < 0.001), while sexually mature animals of the same genotype showed no significant effect, but the opposite was true for 854/THK flies: 854/THK sexually immature animals showed no significant effect, while sexually mature animals increased their freezing duration (P < 0.001) (Fig. 4B). These data provide an excellent example of how the refined genetics of Drosophila can be used in combination with high-throughput behavioral paradigms to shed light on extremely complex questions.
Fig. 4.
Recruitment of dopamine neurons into the stress response circuitry is dependent on sex and level of sexual maturity. A: sexually mature male and females were assayed for time spent freezing following a 24-h exposure to paraquat (oxidative) stress. Three-way ANOVA (genotype × sex × stress) P < 0.001, two-way ANOVA (sex × stress) P < 0.05 for elavC155/w1118 and P < 0.01 for 854/THK, ANOVA (stress) **P < 0.01, *P < 0.05. B: sexually immature and mature females were assayed for frequency of freezing bouts following a 24-h exposure to paraquat stress. Three-way ANOVA (genotype × age × stress) P < 0.001, two-way ANOVA, P < 0.05 for both, ANOVA (stress) ***P < 0.001. elavC155/w1118, control; 854/THK, targeted knockdown in dopamine synthesis in a subset of dopamine neurons within the mushroom bodies. n = 45.
Summary
The contributions of the Drosophila model to our understanding of numerous diverse physiological pathways cannot be overemphasized. These studies also serve as a shining example of the pivotal importance of unfettered basic research, since so much of what we now realize to be of critical significance in the etiology of human disease can trace its lineage to genetic mutations identified in Drosophila.
Glossary
Central complex: an associated group of structures within the insect brain critical for locomotion, coordination between the two brain hemispheres, and some aspects of cognitive behavior.
Chordotonal organs: the stretch receptor organs in arthropods that help detect vibrations caused by sound, or the position of body appendages.
Eclosion: When the adult fly emerges from the pupal case after metamorphosis.
Forward screen: a (generally unbiased) genetic screen to identify genes based on a known phenotype. A reverse screen begins with the mutation and identifies the associated phenotype(s).
Homeotic gene: genes critical in development, expressed in specific patterns, and which, when mutated, result in the transformation of one body region or appendage for another.
Mushroom bodies: structures within the insect brain composed of Kenyon cells and dense neuropil, considered the analogue of the mammalian hippocampus.
Pupariation: formation of the pupal case by the larva during metamorphosis.
GRANTS
This work was supported in part by R01MH083771 from the National Institutes of Health to W. S. Neckameyer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.S.N. and K.J.A. prepared figures; W.S.N. and K.J.A. drafted manuscript; W.S.N. edited and revised manuscript; W.S.N. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1. Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Sidén-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Akasaka T, Klinedinst S, Ocorr K, Bustamante EL, Kim SK, Bodmer R. The ATP-sensitive potassium (KATP) channel-encoded dSUR gene is required for Drosophila heart function and is regulated by tinman. Proc Natl Acad Sci USA 103: 11999–12004, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azad P, Zhou D, Russo E, Haddad GG. Distinct mechanisms underlying tolerance to intermittent and constant hypoxia in Drosophila melanogaster. PLoS One 4: e5371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azpiazu N, Frasch M. Tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev 7: 1325–1340, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol 205: 1233–1240, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bellen H, Tong C, Tsuda H. 100 years of research and its impact on vertebrate neuroscience: a history lesson for the future. Nature Rev Neurosci 11: 514–522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belvin MP, Zhou H, Yin JC. The Drosophila dCREB2 gene affects the circadian clock. Neuron 22: 777–787, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berleth T, Burri M, Thoma G, Bopp D, Richstien S, Frigerio G, Noll M, Nusslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J 7: 1749–1756, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bieler J, Pozzorini C, Naef F. Whole-embryo modeling of early segmentation in Drosophila identifies robust and fragile expression domains. Biophysical J 101: 287–296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bodmer R. Heart development in Drosophila and its relationship to vertebrates. Trends Cardiovasc Med 5: 21–28, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Bodmer R, Jan LY, Jan YN. A new homeobox-containing gene, msh-2, is transiently expressed early during mesoderm formation of Drosophila. Development 110: 661–669, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature 289: 79–81, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Chang KT, Shi YJ, Min KT. The Drosophila homolog of Down's syndrome critical region 1 gene regulates learning: implications for mental retardation. Proc Natl Acad Sci USA 100: 15794–15799, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Q, Ma E, Behar KL, Xu T, Haddad GG. Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster. J Biol Chem 277: 3274–3279, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Chen Q, Behar KL, Fan C, Haddad GG. Expression of Drosophila trehalose-phosphate synthase in HEK-293 cells increases hypoxia tolerance. J Biol Chem 278: 29113–29118, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature 434: 1087–1092, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Cirelli C, Tononi G. Is sleep essential? PloS Biol 6: e216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292: 104–106, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Cooper AS, Rymond KE, Ward MA, Bocook EL, Cooper RL. Monitoring heart function in larval Drosophila melanogaster for physiological studies. J Vis Exp 33: e1596, doi:10.3791/1596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dias-Santagata D, Fulga TA, Duttaroy A, Feany MB. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J Clin Invest 117: 236–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet 38: 1023–2031, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Douglas CL, Vyazovskiy V, Southard T, Chiu SY, Messing A, Tononi G, Cirelli C. Sleep in Kcna2 knockout mice. BMC Biol 9: 5–42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Driever W, Nusslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature 337: 138–143, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA 73: 1684–1688, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dulcis D, Levine RB. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J Comp Neurol 465: 560–578, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Edwards AC, Rollmann SM, Morgan TJ, Mackay TF. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet 2: e154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feala JD, Coquin L, McCulloch AD, Paternostro G. Flexibility in energy metabolism supports hypoxia tolerance in Drosophila flight muscle: metabolomic and computational systems analysis. Mol Syst Biol 3: 99, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feala JD, Coquin L, Paternostro G, McCulloch AD. Integrating metabolomics and phenomics with systems models of cardiac hypoxia. Prog Biophys Mol Biol 96: 209–225, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Feany MB, Quinn WG. A neuropeptid gene defined by the Drosophila memory mutant amnesiac. Science 268: 869–873, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Fink M, Callol-Massot C, Chu A, Ruiz-Lozano P, Izpisua Belmonte JC, Giles W, Bodmer R, Ocorr K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 46: 101–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol 78: 890–900, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Floyd RA, Towner RA, He T, Hensley K, Maples KR. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med 51: 931–941, 42. 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fortini ME, Skupski MP, Boguski MS, Hariharan IK. A survey of human disease gene counterparts in the Drosophila genome. J Cell Biol 150: F23–F30, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fridell YW, Sánchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab 1: 145–152, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Frohnhofer H, Lehmann R, Nusslein-Volhard C. Manipulating the anteroposterior pattern of the Drosophila embryo. J Embryol Exp Morph 97 (Suppl): 169–179, 1986 [PubMed] [Google Scholar]
- 37. Fuentes JJ, Genescà L, Kingsbury TJ, Cunningham KW, Pérez-Riba M, Estivill X, de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet 9: 1681–1690, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Gailey DA, Jackson FR, Siegel RW. Male courtship in Drosophila: the conditioned response to immature males and its genetic control. Genetics 102: 771–782, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gallio M, Ofstad T, Macpherson L, Wang J, Zuker C. The coding of temperature in the Drosophila brain. Cell 144: 614–624, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcıa-Bellido A. Genetic control of wing disc development in Drosophila. Ciba Found Symp 0: 161–182, 1975 [DOI] [PubMed] [Google Scholar]
- 41. Garrity P. Modulation of TRPA1 thermosensitivity enables sensory discrimination in Drosophila. Nature 481: 76–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garrity P, Goodman M, Samuel A, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev 24: 2365–2382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Graves LA, Hellman K, Veasey S, Blendy JA, Pack AI, Abel T. Genetic evidence for a role of CREB in sustained cortical arousal. J Neurophysiol 90: 1152–1159, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Gregor T, Wieschaus E, McGregor A, Bialek W, Tank D. Stability and nuclear dynamics of the bicoid morphogen gradient. Cell 130: 141–152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grimm O, Coppey M, Wieschaus E. Modelling the bicoid gradient. Development 137: 2253–2264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res 84: 973–979, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Guo HF, Tong J, Hannan F, Luo L, Zhong Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature 403: 895–898, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Haddad GG, Wyman RJ, Mohsenin A, Sun Y, Krishnan SN. Behavioral and electrophysiologic responses of Drosophila melanogaster to prolonged periods of anoxia. J Insect Physiol 43: 203–210, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Hamada F, Rosenzweig M, Kang K, Pulver S, Ghezzi A, Jegia T, Garrity P. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454: 217–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harshman LG, Hoffmann AA, Clark AG. Selection for starvation resistance in Drosophila melanogaster: physiological correlates, enzyme activities and multiple stress responses. J Evol Biol 12: 370–379, 1999 [Google Scholar]
- 51. Heisenberg M. Mutants of brain structure and function: what is the significance of the mushroom bodies for behavior? Basic Life Sci 16: 373–390, 1980 [DOI] [PubMed] [Google Scholar]
- 52. Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet 2: 1–30, 1985 [DOI] [PubMed] [Google Scholar]
- 53. Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron 25: 129–138, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Hendricks JC, Williams JA, Panckeri K, Kirk D, Tello M, Yin JC, Sehgal A. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci 4: 1108–1115, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Heulens I, Kooy F. Fragile X syndrome: from gene discovery to therapy. Front Biosci 16: 1211–1232, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Hoffmann AA, Harshman LG. Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity (Edinb) 83: 637–643, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Hong S, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci 26: 7245–7256, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huber R, Hill SL, Hollada C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep 27: 628–639, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Jacobs ME. Influence of light on mating of Drosophila melanogaster. Ecology 41: 182–188, 1960 [Google Scholar]
- 60. Jaeger J. The gap gene network. Cell Mol Life Sci 68: 243–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40: 16–23, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Jouhilahti EM, Peltonen S, Heape AM, Peltonen J. The pathoetiology of neurofibromatosis 1. Am J Pathol 178: 1932–1939, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kang K, Panzano V, Chang E, Ni L, Dainis A, Jenkins A, Regna K, Muskavitch M, Garrity P. Modulation of TRPA1 thermosensitivity enables sensory discrimination in Drosophila. Nature 481: 76–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kanodia J, Rikhy R, Kim Y, Lund V, DeLotto R, Lippincott-Schwartz J, Shvartsman S. Dynamics of the dorsal morphogen gradient. Proc Natl Acad Sci USA 106: 21707–21712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kasai Y, Cagan R. Drosophila as a tool for personalized medicine: a primer. Personalized Med 7: 621–632, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol 46: 2116–2124, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA 68: 2112–2116, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kwon Y, Shim H, Wang X, Montell C. Control of thermotactic behavior via the coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci 11: 871–873, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Kwon Y, Shen W, Shim H, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci 30: 10465–10471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lalevée N, Monier B, Sénatore S, Perrin L, Sémériva M. Control of cardiac rhythm by ORK1, a Drosophila two-pore domain potassium channel. Curr Biol 16: 1502–1508, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Lavista-Llanos S, Centanin L, Irisarri M, Russo DM, Gleadle JM, Bocca SN, Muzzopappa M, Ratcliffe PJ, Wappner P. Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein similar. Mol Cell Biol 22: 6842–6853, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong A, Bae E, Kaang B, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 37: 305–310, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Lewis R, Wakimoto B, Denell R, Kaufman T. Genetic analysis of the Antennapedia gene complex (ANT-C) and adjacent chromosomal regions of Drosophila melanogaster. II. Polytene chromosome segments 84A-84B12 Genetics 95: 383–397, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Little S, Tkacik G, Kneeland T, Wieschaus E, Gregor T. The formation of the bicoid morphogen gradient requires protein movement from anteriorly localized mRNA. PLOS Biol 9: e1000596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron 26: 35–43, 2000 [DOI] [PubMed] [Google Scholar]
- 77. Liu J, Ma J. Fates-shifted is an F-box protein that targets Bicoid for degradation and regulates developmental fate determination in Drosophila embryos. Nat Cell Biol 13: 22–29, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu L, Yermolaieva O, Johnson W, Abboud F, Welsh M. Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci 6: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh M. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 450: 294–298, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophia learning mutant. Cell 37: 205–215, 1984 [DOI] [PubMed] [Google Scholar]
- 81. Luo L, Gershow M, Rosenzweig M, Kang K, Fang-Yen C, Garrity P, Samuel A. Navigational decision-making in Drosophila thermotaxis. J Neurosci 30: 4261–4272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology (Berl) 214: 89–106, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10: 1247–1255, 2000 [DOI] [PubMed] [Google Scholar]
- 84. McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, McDonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45: 753–764, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Mershin A, Pavlopoulos E, Fitch O, Braden BC, Nanopoulos DV, Skoulakis EM. Learning and memory deficits upon TAU accumulation in Drosophila mushroom body neurons. Learn Mem 11: 277–287, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Montell C. Drosophila TRP channels. Pflugers Arch 451:19–28, 2005 [DOI] [PubMed] [Google Scholar]
- 87. Neckameyer WS. Dopamine and mushroom bodies in Drosophila: experience-dependent and -independent aspects of sexual behavior. Learn Mem 5: 157–165, 1998 [PMC free article] [PubMed] [Google Scholar]
- 88. Neckameyer W, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiol Aging 21: 145–152, 2000 [DOI] [PubMed] [Google Scholar]
- 89. Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol 12: 1934–1940, 2002 [DOI] [PubMed] [Google Scholar]
- 90. Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801, 1980 [DOI] [PubMed] [Google Scholar]
- 91. Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW, Bodmer R. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci USA 104: 3943–3948, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 27: 29–34, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Olson EN, Srivastava D. Molecular pathways controlling heart development. Science 272: 671–676, 1996 [DOI] [PubMed] [Google Scholar]
- 94. Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochm J 367: 179–186, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Paternostro G, Vignola C, Bartsch DU, Omens JH, McCulloch AD, Reed JC. Age-associated cardiac dysfunction in Drosophila melanogaster. Circ Res 88: 1053–1058, 2001 [DOI] [PubMed] [Google Scholar]
- 96. Peschel N, Helfrich-Förster C. Setting the clock-by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett 585: 1435–1442, 2011 [DOI] [PubMed] [Google Scholar]
- 97. Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One 4: e5886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Porcher A, Dostatni N. The bicoid morphogen system. Current Biol 20: R249–R254, 2010 [DOI] [PubMed] [Google Scholar]
- 99. Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA 71: 708–712, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99a. Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature 277: 212–214, 1979 [DOI] [PubMed] [Google Scholar]
- 100. Ray VM, Dowse HB. Mutations in and deletions of the Ca2+ channel-encoding gene cacophony, which affect courtship song in Drosophila, have novel effects on heartbeating. J Neurogenet 19: 39–56, 2005 [DOI] [PubMed] [Google Scholar]
- 101. Rivera-Pomar R, Jackle H. From gradients to stripes in Drosophila embryogenesis: filling in the gaps. Trends Genet 14: 478–483, 1996 [DOI] [PubMed] [Google Scholar]
- 102. Rivera-Pomar R, Lu X, Perrimon N, Taubert H, Jackle H. Activation of posterior gap gene expression in the Drosophila blastoderm. Nature 376: 253–256, 1995 [DOI] [PubMed] [Google Scholar]
- 103. Rivera-Pomar R, Niessing D, Schmidt-Ott U, Gehring W, Jackle H. RNA binding and translational suppression by bicoid. Nature 379: 746–749, 1996 [DOI] [PubMed] [Google Scholar]
- 104. Robin C, Daborn PJ, Hoffmann AA. Fighting fly genes. Trends Genet 23: 51–54, 2007 [DOI] [PubMed] [Google Scholar]
- 105. Rosensweig M, Kang K, Garrity P. Distinct TRP channels are required for warm and cold avoidance in Drosophila melanogaster. Proc Natl Acad Sci USA 105: 14668–14673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sanyal S, Jennings T, Dowse H, Ramaswami M. Conditional mutations in SERCA, the Sarco-endoplasmic reticulum Ca2+-ATPase, alter heart rate and rhythmicity in Drosophila. J Comp Physiol B 176: 253–263, 2006 [DOI] [PubMed] [Google Scholar]
- 107. Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak Maron BJ JP, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2–5. Science 281: 108–111, 1998 [DOI] [PubMed] [Google Scholar]
- 108. Shen W, Kwon Y, Adegbola A, Loo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science 331: 1333–1336, 2011 [DOI] [PubMed] [Google Scholar]
- 109. Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci USA 76: 3430–3434, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stevenson R. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am Naturalist 126: 362–386, 1985 [Google Scholar]
- 111. Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A 175: 179–191, 1994 [DOI] [PubMed] [Google Scholar]
- 112. Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics 161: 661–672, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tabet JY, Meurin P, Driss AB, Weber H, Renaud N, Grosdemouge A, Beauvais F, Cohen-Solal A. Benefits of exercise training in chronic heart failure. Arch Cardiovasc Dis 102: 721–730, 2009 [DOI] [PubMed] [Google Scholar]
- 114. Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292: 107–110, 2001 [DOI] [PubMed] [Google Scholar]
- 115. Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003 [DOI] [PubMed] [Google Scholar]
- 116. Tracey W, Wilson R, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell 113: 261–273, 2003 [DOI] [PubMed] [Google Scholar]
- 117. Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell 79: 35–47, 1994 [DOI] [PubMed] [Google Scholar]
- 118. Wes P, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA 92: 9652–9656, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet 36: 1275–1281, 2004 [DOI] [PubMed] [Google Scholar]
- 120. Wingrove JA, O'Farrell PH. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 98: 105–114, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wolf MJ, Amrein H, Izatt JA, Choma MA, Reedy MC, Rockman HA. Drosophila as a model for the identification of genes causing adult human heart disease. Proc Natl Acad Sci USA 103: 1394–1399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wustmann G, Rein K, Wolf R, Heisenberg M. A new paradigm for operant conditioning of Drosophila melanogaster. J Comp Physiol A 179: 429–436, 1996 [DOI] [PubMed] [Google Scholar]
- 123. Xu J, Li M, Shen P. A G-protein coupled neuropeptide Y-like receptor suppresses behavioral and sensory response to multiple stressful stimuli in Drosophila. J Neurosci 30: 2504–2512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang D, Ke L, Mackovicova K, Van Der Want JJ, Sibon OC, Tanquay RM, Morrow G, Henning RH, Kampinga HH, Brundel BJ. Effects of different small HSPB members on contractile dysfunction and structural changes in a Drosophila melanogaster model for atrial fibrillation. J Mol Cell Cardiol 51: 381–389, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.