Abstract
We recently proposed a role for the two-pore-domain K+ (K2P) channel Trek-1 in the regulation of cytokine release from mouse alveolar epithelial cells (AECs) by demonstrating decreased interleukin-6 (IL-6) secretion from Trek-1-deficient cells, but the underlying mechanisms remained unknown. This study was designed to investigate the mechanisms by which Trek-1 decreases IL-6 secretion. We hypothesized that Trek-1 regulates tumor necrosis factor-α (TNF-α)-induced IL-6 release via NF-κB-, p38-, and PKC-dependent pathways. We found that Trek-1 deficiency decreased IL-6 secretion from mouse and human AECs at both transcriptional and translational levels. While NF-κB/p65 phosphorylation was unchanged, p38 phosphorylation was decreased in Trek-1-deficient cells, and pharmacological inhibition of p38 decreased IL-6 secretion in control but not Trek-1-deficient cells. Similarly, pharmacological inhibition of PKC also decreased IL-6 release, and we found decreased phosphorylation of the isoforms PKC/PKDμ (Ser744/748), PKCθ, PKCδ, PKCα/βII, and PKCζ/λ, but not PKC/PKDμ (Ser916) in Trek-1-deficient AECs. Phosphorylation of PKCθ, a Ca2+-independent isoform, was intact in control cells but impaired in Trek-1-deficient cells. Furthermore, TNF-α did not elevate the intracellular Ca2+ concentration in control or Trek-1-deficient cells, and removal of extracellular Ca2+ did not impair IL-6 release. In summary, we report the expression of Trek-1 in human AECs and propose that Trek-1 deficiency may alter both IL-6 translation and transcription in AECs without affecting Ca2+ signaling. The results of this study identify Trek-1 as a new potential target for the development of novel treatment strategies against acute lung injury.
Keywords: Trek-1, interleukin-6 tumor necrosis factor-α, lung, cytokines, calcium, epithelium, acute lung injury, acute respiratory distress syndrome
acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) remain devastating illnesses in both adult (47) and pediatric populations (62). Treatment and complications of ALI/ARDS pose a significant burden on our health care budget and, therefore, the development of new therapeutic strategies represents a major research interest. The main treatment strategies for ALI/ARDS consist of mechanical ventilation and oxygen supplementation (16). While potentially life-saving, both therapies accentuate further lung injury and promote the release of proinflammatory cytokines into alveoli (1, 17).
In particular, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are found abundantly in bronchoalveolar lavage (BAL) fluid from patients with ALI (17) and are associated with increased mortality in this population (27, 36). In addition to inflammatory cells, alveolar epithelial cells (AECs) are another source for cytokine secretion (11, 50). From studies in macrophages, neutrophils, and mast cells, we learned that changes in the cell membrane potential commonly precede secretory events (21, 30), but the mechanisms regulating inflammatory cytokine secretion from AECs remain poorly understood. As in many biological systems, opening of Ca2+ channels is thought to cause membrane depolarization and mediator secretion (35, 57), whereas opening of K+ channels is generally associated with membrane repolarization and stabilization of the resting membrane potential (29, 40). A relatively new family of K+ channels, two-pore-domain K+ (K2P) channels, are strong candidates as important regulators of the cell membrane potential (4). In general, K2P channels conduct “background” or “leak” currents that lead to membrane repolarization and reestablishment of the resting membrane potential (28). However, little is known about the regulation and potential function of K2P channels in the lung. We recently reported the expression of the K2P channels Trek-1, Trek-2, and TRAAK in AECs and proposed a role for Trek-1 in TNF-α-induced cytokine and chemokine secretion (49). In this study, we focus on investigating the signaling mechanisms that lead to decreased IL-6 secretion from Trek-1-deficient AECs. We demonstrate, for the first time, that the decrease in TNF-α-induced IL-6 release from AECs is due to alterations in IL-6 gene transcription and protein translation in Trek-1-deficient cells, and that TNF-α-induced IL-6 secretion from AECs can occur independently of the intra- and extracellular Ca2+ concentration.
MATERIALS AND METHODS
Cell culture.
MLE-12 mouse and human A549 AECs were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in DMEM (GIBCO, Carlsbad, CA) supplemented with 10% FBS (GIBCO), 1% penicillin/streptomycin (GIBCO), 20 mM HEPES (Sigma Aldrich, St. Louis, MO), and 2 mM l-glutamine (GIBCO). Once cells were 80–90% confluent they were treated with TNF-α (5 ng/ml; R&D Systems) at 37°C. Cell viability was determined to be >90% under all conditions by Trypan blue staining.
Development of a stable Trek-1 short-hairpin RNA-transfected A549 cell line.
Similar to our previously described Trek-1-deficient MLE-12 cell line (49), we developed a stable Trek-1-deficient A549 cell line using a commercially available pRFP-C-RS vector (Trek-1 specific probe no. FI348008; control scrambled peptide no. TR30015; Origene) following the manufacturer's instructions. Details of the vector containing the control plasmid and Trek-1 short-hairpin RNA (shRNA) can be obtained from the company website (origene.com/rna/vector_information.mspx). Briefly, cells were cultured to 50% confluence before transfection using a TurboFectin 8.0-based transfection system (TF81001; Origene). Stable clones containing Trek-1 shRNA were selected and propagated in puromycin-containing culture medium. Knockdown of Trek-1 protein was confirmed by Western blotting and immunostaining using confocal microscopy.
Confocal microscopy.
We seeded 8 × 104 cells on sterile acid-treated glass cover slips (Fisher Scientific, Fair Lawn, NJ) and cultured the cells to 80–90% confluence. Cells were then fixed with 4% paraformaldehyde containing 0.2% Triton X-100 for 5 min at 4°C and blocked with 2% BSA containing 0.2% Triton X-100 for 30 min. The cover slips were incubated with antibodies against Trek-1 (1:500; Alamone Labs) overnight at 4°C. The following day, the samples were incubated with an Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (1:500; Molecular Probes) for 30 min at room temperature. Nuclear staining was obtained using Fluoro Gel II mounting medium containing DAPI (Electron Microscopy Sciences, Hatfield, PA). As a negative control, a species-specific IgG antibody was substituted for the primary antibody.
Images were acquired using the Zeiss 710 confocal imaging system available in the Department of Physiology at the University of Tennessee Health Science Center. Emitted fluorescence was collected using a ×63 magnification objective lens (NA 1.4 oil), and the images were recorded using Zen 2009 Light Edition software (Zeiss).
Western blot analysis.
Antibodies were purchased from the following companies and used in the dilutions recommended by the manufacturers: Trek-1 (1:500; Santa Cruz), TNF-α receptor-1 (1:1,000, TNFR1; Abcam), total NF-κB/p65 (1:1,000; Cell Signaling), phospho-NF-κB/p65 (1:1,000; Cell Signaling), total p38 (1:1,000; Cell Signaling), phospho-p38 (1:1,000; Cell Signaling), and a isoform-specific phospho-PKC antibody sampler kit (Cell Signaling) containing antibodies against the phosphorylated PKC isoforms μ (Ser744/748), μ (Ser916), θ, δ, α/βII, and ζ/λ. GAPDH (1:2,000; Cell Signaling) was used as a loading control. Initially, 8 × 104 cells were seeded into 12-well tissue culture plates (MidWest Science, St. Louis, MO). Once cells reached 80–90% confluence, they were lysed on ice in RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, and 0.1% SDS) with a protease inhibitor cocktail (Roche, Burlington, NC). Lysates were centrifuged at 4°C and 17,000 g for 15 min, and total protein concentrations were measured using the Bradford assay (Bio-Rad, Hercules, CA). A total of 45–60 μg protein of each sample was separated by SDS-PAGE on 4–12% NuPage Bis-Tris gradient gels (Invitrogen) and transferred onto nitrocellulose membranes at 35 mV for 2 h. All membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (Bio-Rad) containing 0.1% Tween 20 for 1 h at 37°C. The membranes were then incubated overnight with the indicated primary antibodies at 4°C. The next day, membranes were incubated for 1 h with the following secondary antibodies: for Trek-1 we used an anti-goat horseradish peroxidase (HRP)-conjugated IgG antibody (1:5,000; Santa Cruz); for TNFR1, total NF-κB/p65, phospho-NF-κB/p65, total p38, phospho-p38, phospho-PKC; for the antibodies contained in the anti-phospho-PKC isoform sampler kit and for GAPDH we used an anti-rabbit HRP-conjugated IgG (1:5,000; Cell Signaling). Bands were visualized by enhanced chemoluminescence with ECL SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL). Band densitometry measurements to determine relative quantities of protein were performed using ImageJ 1.42 software for Windows.
IL-6 ELISA measurements.
Initially, 1 × 105 MLE-12 cells or 8 × 104 A549 cells were seeded in 12-well culture plates and grown to 80–90% confluence. Cells were then incubated in the presence or absence of TNF-α (5 ng/ml) at room air for 6 or 24 h at 37°C. In experiments using the p38 kinase inhibitor SB-202190 (5 μM; Sigma), the PKC inhibitor calphostin C (0.2 μM in the presence of a 8-W light source; Sigma), the myristolated PKCθ pseudosubstrate inhibitor (Myr-LHQRRGAIKQAKVHHVKC-NH2, 20 μM; Calbiochem), the translation inhibitor cycloheximide (0.2 μg/ml; Sigma), and the Ca2+ reuptake inhibitor thapsigargin (0.5 μM; Sigma), cells were incubated with the inhibitor for 30 min before stimulation with TNF-α. When IL-6 measurements were performed in the absence of extracellular Ca2+, cells were incubated in DMEM without Ca2+ (catalog no. 21068–028; GIBCO) supplemented with 10% FBS (GIBCO), 1% penicillin/streptomycin (GIBCO), 20 mM HEPES (Sigma Aldrich), and 2 mM l-glutamine (GIBCO) during TNF-α stimulation. Cell viability was assessed after 6 and 24 h using Trypan blue staining and was consistently >90%. Furthermore, total intracellular protein concentrations were measured in each experiment using the Bradford assay and remained consistent under all experimental conditions, suggesting that no unspecific leakage of intracellular proteins occurred. Supernatants were collected at 6 and 24 h, and IL-6 concentrations from MLE-12 and A549 cells were determined using BD Bioscience OptEIA species-specific IL-6 ELISA kits.
Gene expression by real-time PCR.
Total RNA was isolated from 2 × 106 MLE-12 cells using a High Pure RNA Isolation Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions. Single-stranded DNA was synthesized from 1 μg total RNA, and Reverse Transcription PCR was performed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer's instructions. Real-Time PCR was performed using a TaqMan Gene Expression assay (Invitrogen). Primer sets for human IL-6 were purchased from Cell Signaling. In preliminary experiments we confirmed that hypoxanthine-guanine phosphoribosyltranserase (HGPRT) levels were unchanged between control and Trek-1-deficient A549 cells, and, therefore, IL-6 mRNA levels were normalized to HGPRT expression. All experiments were repeated a minimum of three times, and each sample was run in triplicates.
Fura 2-AM Ca2+ measurements.
For continuous fura 2-AM fluorescence measurements, 3 × 105 MLE-12 cells or 2 × 105 A549 cells were seeded into no. 0 thickness uncoated glass-bottom microwell dishes of 20 mm diameter (MatTek, Ashland, MA) and grown to 70–80% confluence. Cells were then serum starved for 12 h and washed with HBSS buffer before incubation in HEPES-buffered solution (in mM: 134 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, with pH adjusted to 7.4 with NaOH) containing the ratiometric fluorescent Ca2+ indicator fura 2-AM (5 μM; Sigma) and 2% pluronic F-127 (Sigma) for 45 min at room temperature, followed by a 20-min wash. Fura 2-AM-loaded cells were alternately excited at 340 or 380 nm using a PC-driven Hyperswitch (Ionoptix). Background-corrected ratios were collected every 1 s at 510 nm using a Dage MTI iCCD camera and Ionwizard software (Ionoptix) (61). Changes in the intracellular Ca2+ concentration were analyzed by calculating the fura 2-AM ratio at 340 nm emission to 380 nm emission.
For experiments where fura 2 fluorescence was recorded after 6 h of TNF-α stimulation, 2 × 104 cells/well were seeded into 96-well plates and grown to 80–90% confluence in phenol red-free culture medium (GIBCO). Cells were serum-starved for 12 h before fura 2-AM loading (5 μM) with 2% pluronic F-127 for 45 min at room temperature in Krebs buffer. Cells were treated with TNF-α (5 ng/ml for 6 h before fluorescence determination) and/or ATP (10 μM, added immediately before fluorescence determination; Sigma), and fluorescence data were collected over a 70-s reading window using an automated FlexStation 3 spectrophotometer with SoftMax Pro software (Molecular Devices) to calculate 340/380 nm fluorescence ratios.
Statistical analysis.
All values were expressed as means ± SE. Student's t-test was used to compare means of two different groups. Real-time PCR data were analyzed using the ΔΔCt method, and a change in gene expression greater than twofold was considered significant. Additionally, in cytokine and phosphorylation studies, ANOVA was used to compare means of different groups. All statistical analyses were performed using SigmaStat 3.5 software, and P < 0.05 was considered significant.
RESULTS
Validation of a stable human Trek-1 shRNA-transfected A549 cell line.
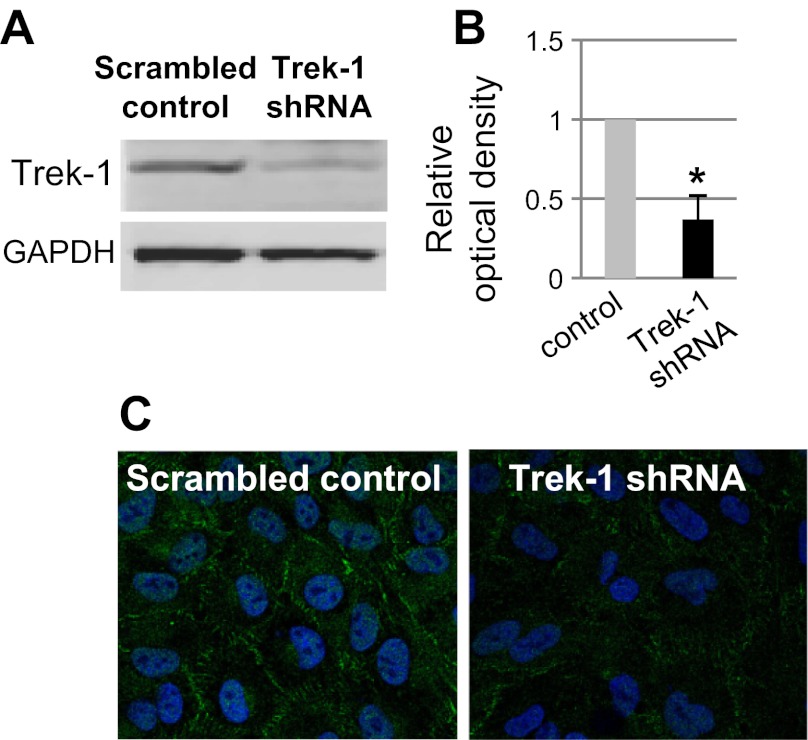
We previously reported decreased IL-6 release from Trek-1-deficient mouse AECs (MLE-12) after 24 h of TNF-α stimulation (49). In this study, we further investigated the time course of IL-6 secretion from both mouse and human AECs after TNF-α stimulation. For this purpose, we developed a stable Trek-1 shRNA-transfected A549 cell line. Figure 1A shows a representative Western blot experiment depicting decreased Trek-1 protein expression in Trek-1-deficient A549 cells, and Fig. 1B shows densitometry analysis of three representative experiments demonstrating a decrease in Trek-1 protein expression of 63% in Trek-1 shRNA-transfected cells. A representative experiment showing decreased Trek-1 immunostaining (green) in Trek-1-deficient cells using confocal microscopy is shown in Fig. 1C.
Fig. 1.
Decreased Trek-1 protein expression in A549 cells after short-hairpin RNA (shRNA) transfection. A: a representative Western blot experiment. B: densitometry analysis of 3 Western blot experiments after normalization of band intensities to GAPDH. C: decreased Trek-1 immunofluorescence by confocal microscopy (Trek-1 in green; n = 3) can be seen. Nuclei are stained with DRAQ5 (blue; magnification ×60). *P < 0.05 compared with control.
IL-6 release was decreased in Trek-1-deficient AECs.
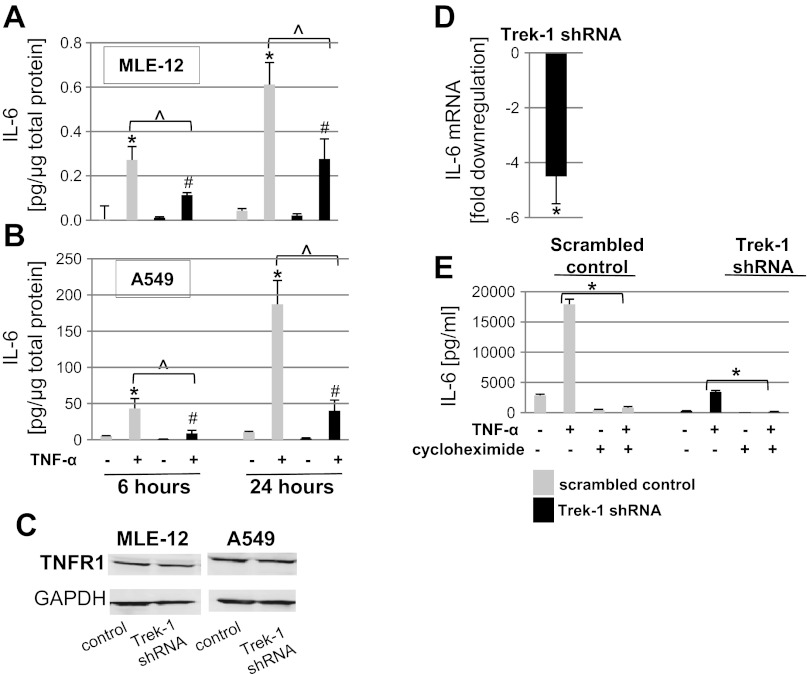
We studied baseline and TNF-α-induced IL-6 release from both mouse MLE-12 (Fig. 2A) and human A549 (Fig. 2B) cells. To determine the time course of IL-6 release, we focused on an early time point (6 h) and a later time point (24 h). A TNF-α dose of 5 ng/ml was chosen as a stimulus based on the results obtained in our previous study (49). Stimulation of control cells with TNF-α increased IL-6 release from both MLE-12 and A549 cells in a time-dependent manner. Of note, the amount of IL-6 released from A549 cells was consistently higher than from MLE-12 cells at both time points. Similar to control cells, TNF-α induced IL-6 release from Trek-1-deficient MLE-12 and A549 cells. However, TNF-α-induced IL-6 secretion from both Trek-1-deficient cell lines was decreased at the 6- and 24-h time points compared with their respective controls transfected with a scrambled peptide. These results point toward a role for Trek-1 in the regulation of TNF-α-induced IL-6 release from AECs.
Fig. 2.
Decreased interleukin-6 (IL-6) secretion from Trek-1-deficient MLE-12 (A) and A549 (B) cells after 6 and 24 h of tumor necrosis factor-α (TNF-α) treatment (P < 0.05, n ≥ 3; *compared with untreated cells transfected with a scrambled control peptide; #compared with untreated Trek-1 shRNA-transfected cells; ^TNF-α stimulated control cells compared with TNF-α-stimulated Trek-1-deficient cells). TNF-α receptor-1 (TNFR1) expression was unchanged in Trek-1-deficient cells (C; n = 3). Baseline IL-6 mRNA levels were decreased in Trek-1-deficient A549 cells compared with controls (D; 4.5-fold decrease, P < 0.05, n = 3; we considered a greater than 2-fold change in gene expression as significant). An inhibitor of protein translation, cycloheximide, decreased TNF-α-induced IL-6 release by >95% (*P < 0.05) in both control and Trek-1-deficient cells after 6 h of treatment (E).
To investigate whether the observed decrease in IL-6 release from Trek-1-deficient cells was due to downregulation of TNF-α receptors on these cells, we performed Western blot experiments using an antibody against TNFR1 and found no difference in TNFR1 protein expression between control and Trek-1-deficient cells in both MLE-12 and A459 cells (Fig. 2C). Densitometry analysis of three Western blot experiments confirmed equal expression of TNFR1 protein in both cell types regardless of Trek-1 deficiency (data not shown).
IL-6 release from Trek-1-deficient AECs is regulated at transcriptional and translational levels.
To investigate whether the decrease in IL-6 release from Trek-1-deficient cells was caused by alterations in IL-6 gene expression, we measured baseline IL-6 mRNA levels in control and Trek-1-deficient A549 cells using real-time PCR (Fig. 2D). We found that baseline IL-6 mRNA levels were decreased 4.5-fold in Trek-1-deficient cells compared with controls transfected with a scrambled peptide.
In addition, to evaluate whether the decrease in IL-6 release from Trek-1-deficient cells was due to alterations in IL-6 protein translation, we added an inhibitor of protein translation, cycloheximide, to our cell cultures 1 h before stimulation with TNF-α for 6 h (Fig. 2E). Cycloheximide inhibited IL-6 release in both control and Trek-1-deficient cells by >95%, suggesting that the majority of the IL-6 protein measured in the supernatants from AECs was newly synthesized and not preformed or stored within the cells.
TNF-α-induced NF-κB/p65 expression and phosphorylation was unchanged in Trek-1-deficient AECs.
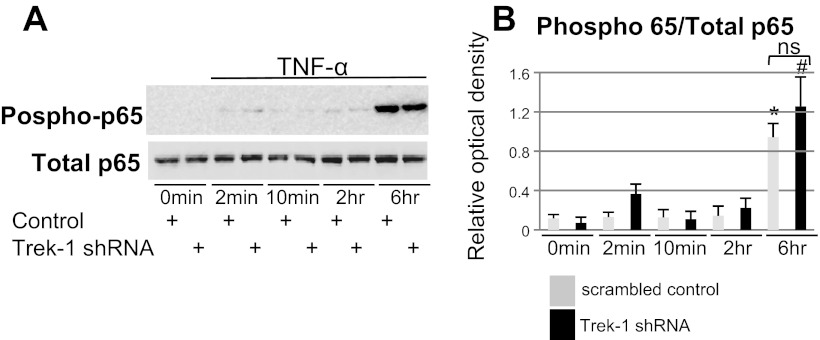
To investigate whether the decrease in IL-6 release from Trek-1-deficient AECs was due to alterations in NF-κB/p65-dependent signaling pathways, we studied TNF-α-induced p65 protein expression and phosphorylation in MLE-12 cells using Western blot (Fig. 3A). Total p65 levels were unchanged between cells transfected with a scrambled control peptide and Trek-1 shRNA-transfected cells both at baseline and during a 6-h time course of TNF-α (5 ng/ml) stimulation. Phosphorylation of p65 increased equally after 6 h of TNF-α treatment in control and Trek-1-deficient cells compared with their respective controls at time point 0 min. Densitometry analysis of three Western blot experiments and normalization to total p65 protein expression confirmed an equal increase in p65 phosphorylation after 6 h of TNF-α stimulation (Fig. 3B) in control and Trek-1-deficient cells. These results suggest that altered NF-κB/p65 signaling is unlikely to be responsible for the decrease in IL-6 secretion from Trek-1-deficient AECs.
Fig. 3.
Expression and phosphorylation of NF-κB/p65 in MLE-12 cells using Western blot. Total p65 protein expression was unchanged between control and Trek-1-deficient cells. After 6 h of TNF-α stimulation (5 ng/ml), p65 phosphorylation increased equally in control and Trek-1-deficient cells (A). Densitometry analysis of 3 experiments is shown in B. Treatment of cells with TNF-α resulted in increased p65 phosphorylation in both control (*) and Trek-1-deficient cells (#) compared with their respective controls at 0 min (P < 0.05). Phosphorylation of p65 was normalized to total p65 protein and was unchanged between control and Trek-1-deficient cells after 6 h of TNF-α stimulation (ns, not significant, P > 0.05).
TNF-α-induced p38 phosphorylation was decreased in Trek-1-deficient AECs.
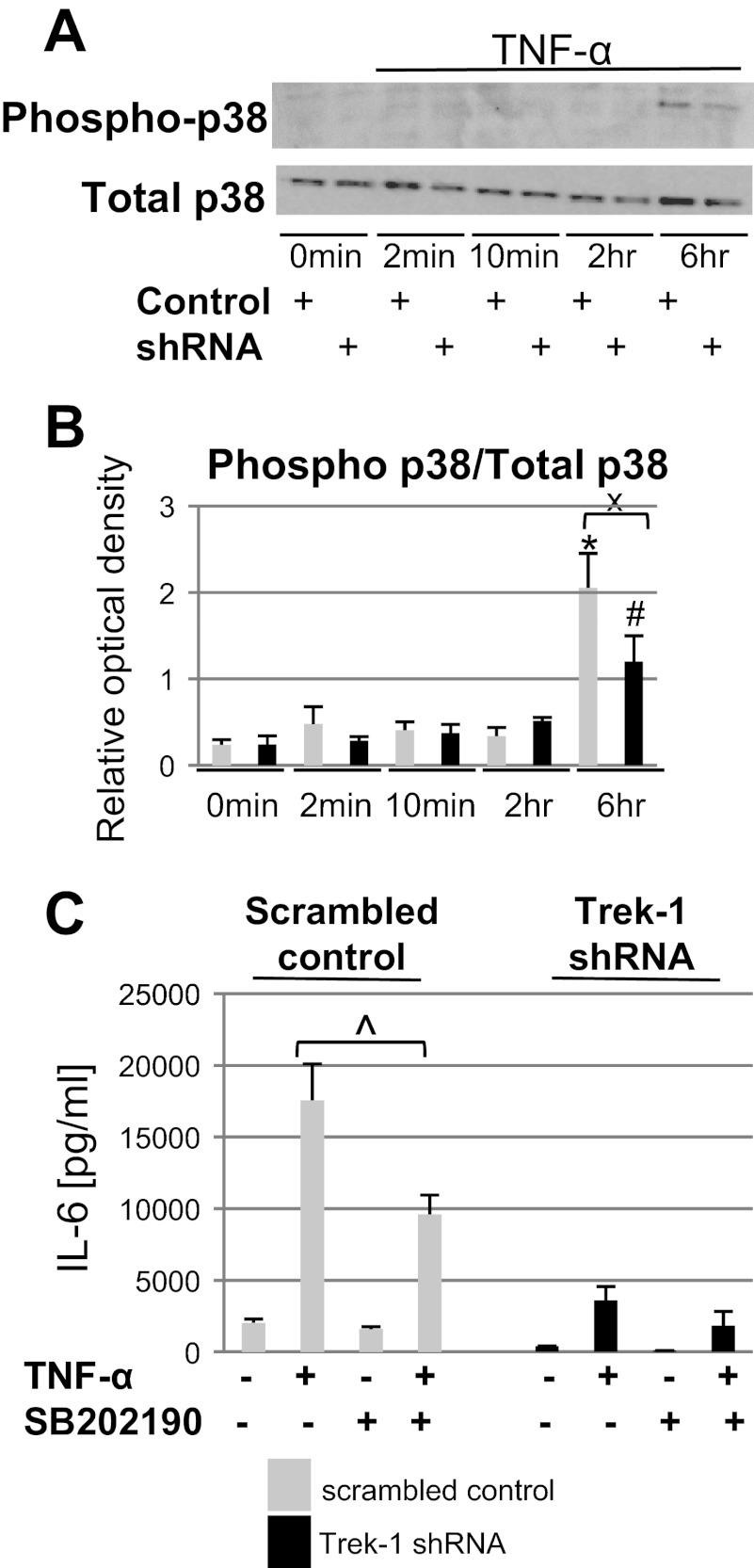
To determine whether alterations in p38 kinase signaling were responsible for the decrease in IL-6 release from Trek-1-deficient cells, we measured TNF-α-induced p38 expression and phosphorylation in MLE-12 cells over a 6-h time period. Using Western blot experiments, we found no changes in total p38 expression between control and Trek-1-deficient cells, but p38 phosphorylation was decreased in Trek-1-deficient cells after 6 h of TNF-α stimulation compared with control cells (Fig. 4A). Densitometry analysis of three experiments and normalization of phosphorylated p38 to total p38 protein band intensities confirmed an increase in p38 phosphorylation after 6 h of TNF-α treatment in both control and Trek-1-deficient cells compared with their respective controls at 0 min. However, p38 phosphorylation at 6 h occurred to a lesser degree in Trek-1-deficient cells than in controls (Fig. 4B).
Fig. 4.
Expression and phosphorylation of p38 kinase in MLE-12 cells using Western blot. Total p38 protein expression was unchanged between control and Trek-1-deficient cells at baseline and after 6 h of TNF-α (5 ng/ml) stimulation, but p38 phosphorylation was decreased in Trek-1-deficient cells at 6 h compared with cells transfected with a scrambled control (A). Densitometry analysis of 3 experiments depicting phosphorylated p38 levels normalized to total p38 protein confirms a decrease in p38 phosphorylation levels in Trek-1-deficient cells at 6 h (B, n = 3, P < 0.05; *compared with control cells at 0 min, #compared with Trek-1-deficient cells at 0 min, xcontrol cells at 6 h compared with Trek-1-deficient cells at 6 h). Treatment of cells with the p38 inhibitor SB-202190 before TNF-α stimulation deceased IL-6 release in control but not in Trek-1-deficient cells (C; n = 5, ^P < 0.05).
To evaluate whether impaired p38 phosphorylation in Trek-1-deficient cells could lead to a decrease in IL-6 release, we treated A549 cells with the p38 inhibitor SB-202190 for 30 min before stimulation with TNF-α (5 ng/ml; Fig. 4C). Because p38 phosphorylation occurred after 6 h of TNF-α treatment, we chose this time point to study the effects of SB-202190 on TNF-α-induced IL-6 release. Treatment of cells with SB-202190 before stimulation with TNF-α decreased IL-6 secretion from control cells by 46% but did not affect IL-6 release from Trek-1-deficient cells. Addition of SB-202190 to unstimulated control or Trek-1-deficient cells had no effect on baseline IL-6 secretion.
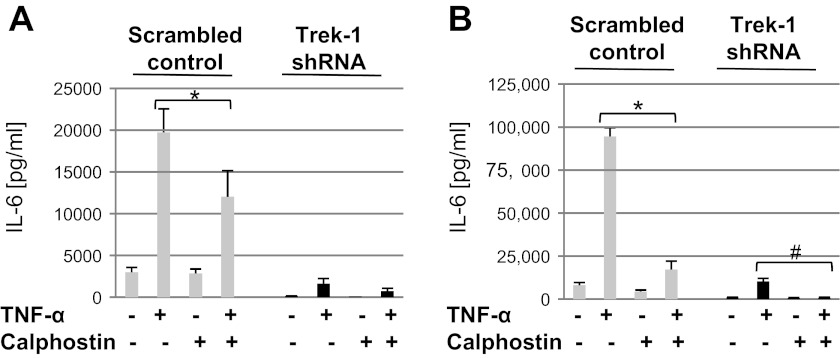
PKC signaling was altered in Trek-1-deficient AECs.
PKC is one of the key regulatory kinases in TNF-α-activated signaling pathways (10, 18, 24). To determine whether alterations in PKC expression could be responsible for the decrease in IL-6 release from Trek-1-deficient AECs, we studied the effect of the PKC inhibitor calphostin C on IL-6 secretion from control and Trek-1-deficient A549 cells after 6 and 24 h of TNF-α stimulation (5 ng/ml). After 6 h of TNF-α stimulation, calphostin C decreased IL-6 release from control cells by 40% but showed no further inhibitory effect in Trek-1-deficient cells (Fig. 5A). After 24 h of TNF-α stimulation, inhibition of PKC by calphostin C decreased TNF-α-induced IL-6 release from control cells by 82% and from Trek-1-deficient cells by 90% compared with TNF-α alone (Fig. 5B). The addition of calphostin C to unstimulated cells had no effect on IL-6 release. Collectively, these data suggest that the regulation of IL-6 release by PKC may be more important at earlier times of TNF-α stimulation (6 h) than after 24 h.
Fig. 5.
After 6 h of TNF-α treatment, calphostin C (0.2 μM) inhibited TNF-α-induced IL-6 release from control but not Trek-1-deficient cells (A), whereas after 24 h of TNF-α treatment, calphostin C inhibited IL-6 release from both control and Trek-1-deficient cells (B; #P < 0.05, n = 3–5). *P < 0.05 compared with control.
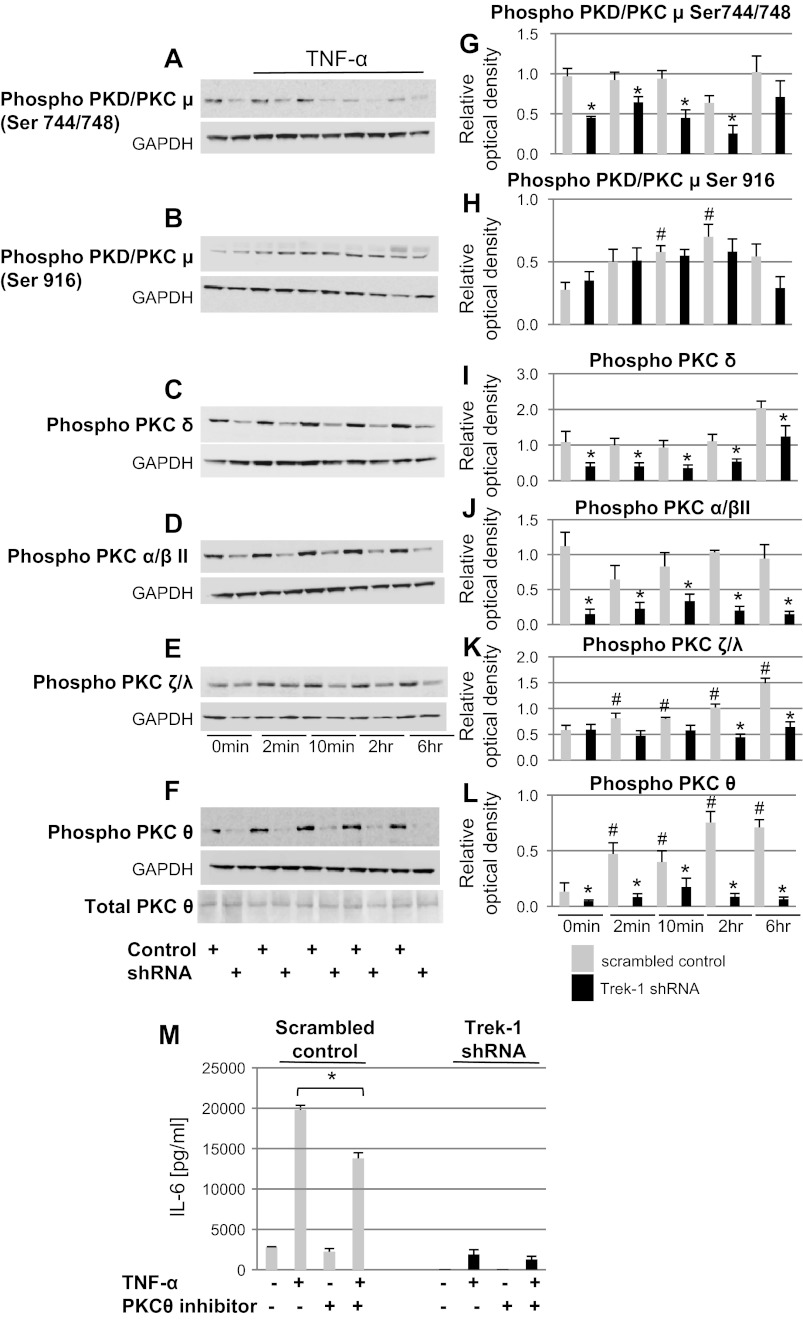
Phosphorylation of PKC isoforms was altered in Trek-1-deficient AECs.
To determine whether phosphorylation of specific-PKC isoforms was altered in Trek-1-deficient cells, we used Western blot experiments to study the phosphorylation of PKC/PKDμ (Ser744/748), PKC/PKDμ (Ser916), PKCδ, PKCα/βII, PKCζ/γ, and PKCθ. A representative 6-h time course for each isoform in control and Trek-1-deficient MLE-12 cells is shown in Fig. 6, A–F. Densitometry analyses of five experiments for each isoform are shown in Fig. 6, G–L. We chose to normalize band intensities of phosphorylated PKC isoforms to GAPDH, since antibodies against all nonphosphorylated PKC isoforms analyzed in this study are not commercially available. In addition, in preliminary experiments we found significant alterations in nonphosphorylated PKCα and PKCβ expression between control and Trek-1-deficient cells (data not shown).
Fig. 6.
Trek-1 deficiency alters phosphorylation of PKC isoforms at baseline and after TNF-α stimulation in MLE-12 cells. A–F: representative Western blot experiments for the phosphorylated isoforms PKC/PKDμ (Ser744/748), PKC/PKDμ (Ser916), PKCδ, PKCα/βII, PKCζ/γ, and PKCθ. F also includes a blot showing unchanged total PKCθ expression in control and Trek-1-deficient cells. G–L: densitometry analyses of 5 experiments/isoform. Band densities were normalized to GAPDH (P < 0.05, *compared with scrambled control at each time point, #compared with scrambled control at 0 min). M: a decrease in IL-6 release from control but not Trek-1-deficient A549 cells in the presence of a myristolated PKCθ pseudosubstrate inhibitor (*P < 0.05, n = 3) can be seen. Baseline IL-6 secretion from control or Trek-1-deficient cells was not affected by this PKCθ inhibitor.
Phosphorylation of PKC/PKDμ (Ser744/748) was decreased at baseline and after TNF-α stimulation in Trek-1-deficient cells (Fig. 6G), whereas phosphorylation of PKC/PKDμ (Ser916) was unchanged between Trek-1-deficient cells and controls at each time point (Fig. 6H). Furthermore, TNF-α stimulation did not induce phosphorylation of PKC/PKDμ (Ser744/748) in control cells (Fig. 6G), whereas phosphorylation of PKC/PKDμ (Ser916) was increased in control but not in Trek-1-deficient cells after TNF-α stimulation compared with their respective controls at time 0 min (Fig. 6H).
Phosphorylation of PKCδ was decreased in Trek-1-deficient cells at baseline and after TNF-α stimulation compared with controls, and TNF-α exposure did not induce PKCδ phosphorylation in either control or Trek-1-deficient cells compared with their respective controls at time 0 min (Fig. 6I).
Similarly, phosphorylation of PKCα/βII was decreased in Trek-1-deficient cells at baseline and after TNF-α stimulation compared with controls, and TNF-α treatment did not induce PKCα/βII phosphorylation in either control or Trek-1-deficient cells compared with their respective controls at time 0 min (Fig. 6J).
Phosphorylation of PKCζ/λ was decreased in Trek-1-deficient cells after 2 and 6 h of TNF-α stimulation but not at earlier time points, and TNF-α treatment induced PKCζ/λ phosphorylation in control but not Trek-1-deficient cells compared with their respective controls at time 0 min (Fig. 6K).
Phosphorylation of PKCθ was decreased in Trek-1-deficient cells at baseline and after TNF-α stimulation compared with controls, and TNF-α treatment increased PKCθ phosphorylation in control cells but not in Trek-1-deficient cells compared with their respective controls at time 0 min (Fig. 6L). Despite changes in PKCθ phosphorylation, total PKCθ expression was unchanged in control and Trek-1-deficient cells (Fig. 6F).
Inhibition of PKCθ decreased IL-6 release from control but not Trek-1-deficient cells.
To further investigate the role of PKCθ in IL-6 release, we measured IL-6 concentrations in supernatants from A549 cells after 6 h of TNF-α stimulation in the presence of a myristolated PKCθ pseudosubstrate inhibitor (Fig. 6M). In the presence of the PKCθ inhibitor, TNF-α-induced IL-6 release was decreased in control cells but not in Trek-1-deficient cells. Baseline IL-6 secretion from control or Trek-1-deficient cells was not affected by the PKCθ inhibitor.
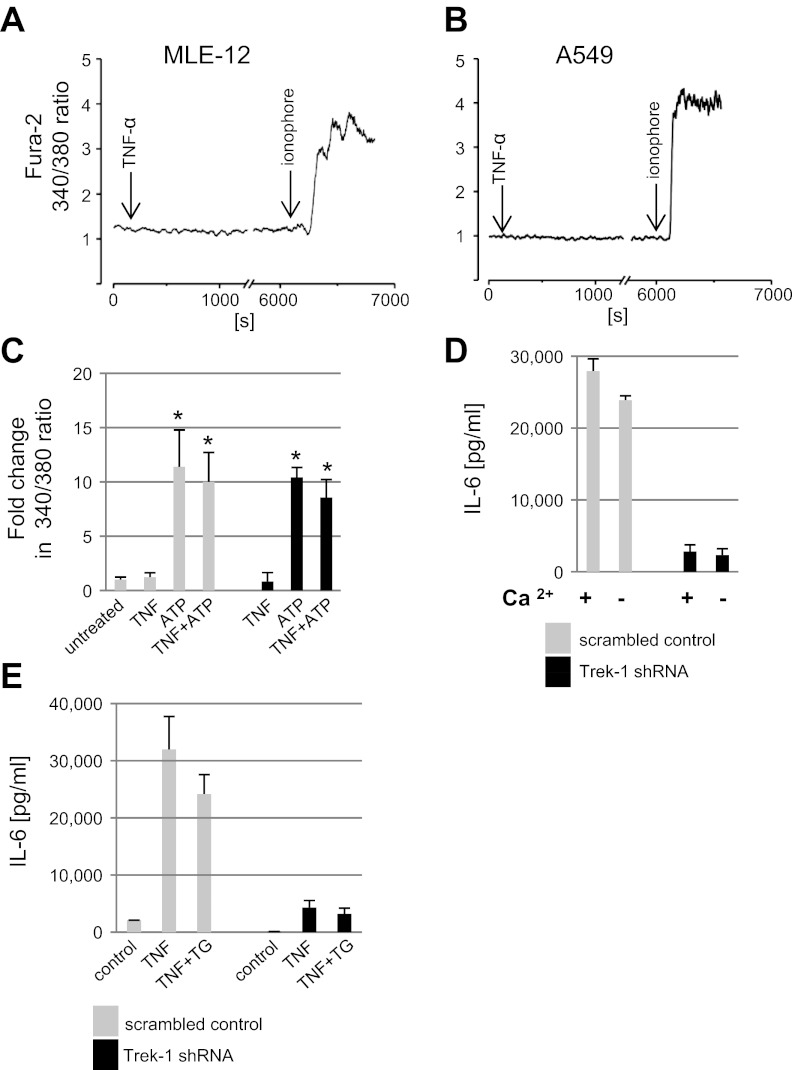
TNF-α stimulation did not induce a change in the intracellular Ca2+ concentration.
In general, mediator secretion from inflammatory cells is thought to be a Ca2+-dependent process (44, 45). Therefore, we investigated whether the decreased IL-6 secretion from Trek-1-deficient A549 cells could be the result of acute or delayed alterations in intracellular Ca2+ release mechanisms. Addition of TNF-α (5 ng/ml) to fura 2-AM-loaded MLE-12 (Fig. 7A) or A549 (Fig. 7B) cells had no effect on the intracellular Ca2+ concentration over a 2-h time period. As a control for fura 2-AM loading and cell viability, ionomycin (10 μM) was added to both cell types at the end of the experiment and resulted in an immediate elevation in the intracellular Ca2+ concentration as indicated by an increase in the fura 2-AM 340/380 nm ratio. These results suggest that the decreased IL-6 release from Trek-1-deficient cells was unlikely caused by an acute alteration in the intracellular Ca2+ concentration after TNF-α stimulation.
Fig. 7.
TNF-α did not induce changes in the intracellular Ca2+ concentration. The intracellular Ca2+ concentration of MLE-12 (A, n = 5) and A549 cells was analyzed (B, n = 12) by calculating the fura 2 340/380 nm ratio. TNF-α and ionophore were added at the times indicated by the arrows. Both cell types showed a response to ionophore indicating adequate fura 2 loading. C: data show that ATP-induced intracellular Ca2+ release in A549 cells was not altered by Trek-1 deficiency. Data are depicted as fold change in fura 2 340/380 nm ratio compared with untreated cells (*P < 0.05). The intracellular Ca2+ concentration did not change after addition of TNF-α (5 ng/ml for 6 h) but increased with ATP (10 μM) treatment (P < 0.05, n = 7), and the combination of ATP and TNF-α showed no further increase. The intracellular Ca2+ response to TNF-α (n = 3), ATP (n = 7), and the combination of these two stimuli (n = 3) was similar in Trek-1-deficient and control cells. TNF-α-induced IL-6 secretion from A549 cells was independent of the extra- and intracellular Ca2+ concentration (D and E). Cells were stimulated with TNF-α (5 ng/ml for 6 h) in a 20 mg/dl Ca2+-containing and a low Ca2+-containing culture medium supplemented with 10% FBS (final Ca2+ concentration 1 mg/dl) before IL-6 quantification in the supernatants. Lowering of the extracellular Ca2+ concentration did not affect IL-6 release from either control or Trek-1-deficient cells (D). Similarly, the presence of thapsigargin (TG, 0.5 μM) did not affect TNF-α-induced IL-6 release from either control or Trek-1-deficient cells (E).
To determine whether Trek-1-deficient cells showed delayed alterations in the intracellular Ca2+ concentration after TNF-α stimulation (5 ng/ml), we treated control and Trek-1-deficient A549 cells with TNF-α for 6 h before measuring the intracellular Ca2+ concentration (Fig. 7C). Treatment of both control and Trek-1-deficient A549 cells with TNF-α alone did not evoke an increase in the intracellular Ca2+ concentration above baseline. However, treatment of cells with ATP (10 μM), a stimulus known to release Ca2+ from intracellular stores (48), elevated the intracellular Ca2+ concentration in both control and Trek-1-deficient cells compared with unstimulated, scrambled peptide-transfected control cells. The combination of TNF-α and ATP had no additional effect over ATP alone. Collectively, these data suggest that TNF-α treatment did not cause an increase in the intracellular Ca2+ concentration of A549 cells within the first 2 h or after 6 h of stimulation. Furthermore, the release of Ca2+ from intracellular stores appeared intact in Trek-1-deficient cells, as our results with ATP indicate. Therefore, the decrease in TNF-α-induced IL-6 secretion from these cells seemed unlikely to be related to either acute or delayed alterations in the intracellular Ca2+ concentration of AECs.
IL-6 secretion from AECs occurred independently of the extracellular and intracellular Ca2+ concentration.
To determine whether the decrease in IL-6 secretion from Trek-1-deficient cells was related to alterations in the intracellular Ca2+ concentration of AECs, we measured TNF-α-induced IL-6 release from A549 cells in a Ca2+-rich (20 mg/dl) and a low-Ca2+-containing solution (Ca2+-free DMEM with 10% FBS, final Ca2+ concentration 1 mg/dl). Lowering of the extracellular Ca2+ concentration did not affect TNF-α-induced IL-6 secretion from either control or Trek-1-deficient AECs (Fig. 7D). Similarly, inhibition of Ca2+ reuptake mechanisms by thapsigargin did not impede TNF-α-induced IL-6 secretion from either control or Trek-1-deficient AECs (Fig. 7E). Collectively, these data suggest that IL-6 secretion can occur independently of the extracellular and intracellular Ca2+ concentration in both control and Trek-1-deficient AECs and that an alteration in Ca2+ signaling mechanisms was unlikely to account for the decrease in IL-6 release from Trek-1-deficient AECs.
DISCUSSION
The mortality rates of patients with ALI and ARDS remain high (47, 62). Current treatment regimens include mechanical ventilation and oxygen supplementation, although both therapies are known to further propagate lung injury, a finding supported by data recently published by our group (33, 34). Both therapeutic regimens result in an increase in inflammatory cytokine and chemokine levels, in particular IL-6 and TNF-α, in the BAL fluid of both animals (39) and patients (17, 36) with ALI/ARDS. However, the specific pathways leading to these findings remain unclear. In our search for new therapeutic approaches for ALI/ARDS, we are particularly interested in elucidating the cellular mechanisms that regulate mediator secretion from AECs. In this study, we showed for the first time expression of the K2P channel Trek-1 in human AECs, and we investigated potential alterations in TNF-α-induced signaling mechanisms that could result in a decrease in IL-6 secretion from Trek-1-deficient cells. Our previous data had shown a decrease in IL-6 secretion from Trek-1-deficient mouse AECs (49), but the underlying mechanisms remained unexplored. It is important to note that a unique characteristic of Trek-1 and other K2P channels consists in the fact that these channels are constitutively open, allowing for a continuous potassium efflux or so-called “leak current.” In contrast to almost all other channel types, which in a resting cell are constitutively closed, Trek-1 currents and associated downstream signaling may rely more on the presence or absence of such channel proteins than on channel activation. The creation of a mouse and human Trek-1-deficient cell line provides, therefore, a useful tool to study the role of Trek-1 in cell signaling events and cellular effector functions. While possible effects of TNF-α on Trek-1 certainly need to be addressed by future studies, the focus of this manuscript concerns the consequences of Trek-1 deficiency on TNF-α-induced IL-6 secretion.
TNF-α is known to exert its proinflammatory effects via several signaling pathways, including activation of the transcription factor NF-κB/p65 and phosphorylation of p38 kinase and PKC (24, 43, 56). We found no differences in the expression or phosphorylation of NF-κB/p65 between control and Trek-1-deficient AECs, although TNF-α is known to employ NF-κB/p65-dependent pathways to promote inflammation in the lung, and activation of NF-κB/p65-dependent pathways by a variety of stimuli is required for IL-8 secretion and IL-6 gene expression in lung epithelial cells (46, 56). Our data confirm that NF-κB/p65 phosphorylation occurs in AECs upon TNF-α stimulation but suggest that altered expression or phosphorylation of this transcription factor was unlikely the cause for the impaired IL-6 secretion from Trek-1-deficient AECs. It is important to note that NF-κB activity is not solely related to p65 phosphorylation and that nuclear translocation and DNA binding provide other levels of NF-κB regulation. However, because we did not find any significant differences in NF-κB/p65 phosphorylation at any of the time points studied, we did not further pursue NF-κB activation in this study. Interestingly, in an infectious model of alveolar inflammation, inhibition of PKC resulted in decreased NF-κB/p65 phosphorylation (14), whereas our data show that NF κB/p65 phosphorylation was not altered in Trek-1-deficient AECs despite significant alterations in the phosphorylation pattern of several PKC isoforms. It is also important to recognize that Trek-1 deficiency may affect the function of other transcription factors that could lead to a decrease in IL-6 secretion from AECs. In fact, activating transcription factor-3 (ATF-3) has been reported to regulate IL-6 release from A549 cells (53). In this study we focused on NF-κB/p65 since this particular transcription factor has been associated with increased levels of IL-6 and TNF-α in the BAL fluid of rats in an in vivo model of ALI (60). Further experiments will be necessary to expand our knowledge on potential alterations in the activation of other transcription factors in Trek-1-deficient AECs that may affect cytokine secretion, including ATF-3, C/EBPβ, and specificity protein factor-1 (2, 22). Importantly, IL-6 mRNA was downregulated at baseline in Trek-1-deficient cells, suggesting alterations in IL-6 synthesis in Trek-1-deficient cells. Whether such changes persist after cytokine stimulation has never been documented and needs to be addressed in future studies. Interestingly, although unstimulated cells secrete only low levels of IL-6 at baseline, the amounts of IL-6 secreted from untreated cells were equal between control and Trek-1-deficient cells. Only stimulation with TNF-α uncovered differences in IL-6 secretion as shown in Fig. 2. It, therefore, appears that Trek-1 may be involved in regulating IL-6 secretion under both baseline and TNF-α-stimulated conditions. Furthermore, baseline secretion of IL-6 from Trek-1-deficient cells is unlikely related to the observed alterations in p38 or PKC signaling, since inhibitors of these pathways had no effect on baseline IL-6 release. Of importance, an inhibitor of translation, cycloheximide, almost completely abolished IL-6 secretion form both control and Trek-1-deficient cells, suggesting that the majority of IL-6 released from either cell type was newly synthesized rather than preformed.
In contrast to NF-κB/p65, phosphorylation of p38 kinase was decreased in Trek-1-deficient cells only after 6 h of TNF-α exposure, suggesting that alterations in p38 phosphorylation are unlikely to be regulating IL-6 release occurring before 6 h of TNF-α stimulation. Interestingly, we recently found a similar time course of p38 phosphorylation in an in vivo mouse model of ALI (personal communication from Dr. Scott Sinclair). Whether decreased p38 phosphorylation could be responsible for the decrease in IL-6 secretion from Trek-1-deficient cells at later time points needs to be tested in future experiments.
p38 kinase is recognized as one of the key molecules in TNF-α-activated signaling cascades in airway epithelial cells (8) and is thought to participate in the development of lung inflammation, in part, via regulation of IL-8 gene expression (5, 51). The important role of p38 kinase in promoting lung inflammation is underscored by the fact that, in addition to NF-κB inhibition, decreased activation of p38 kinase is described as one of the mechanisms by which corticosteroids decrease lung inflammation (25). Interestingly, p38 kinase-independent IL-6 and IL-8 gene expression has also been reported in lung epithelial cells (23), implying that the transcription of the IL-6 and IL-8 genes can occur independently of p38 kinase activation but that the secretion of IL-6 and IL-8 protein does require p38 kinase phosphorylation.
In addition to NF-κB/p65 and p38 kinase, activation of PKC is one of the cornerstones of TNF-α signaling in the lung (6, 42), and TNF-α-induced IL-6 secretion in airway epithelial cells is thought to be PKC-dependent (19, 54). To date, three families of PKC isoforms, including 12 different isoenzymes, have been described in the literature (9). Interestingly, PKCδ knockout mice showed decreased levels of IL-6 in their BAL fluid after exposure to asbestos (52), and inhibition of PKCε resulted in decreased IL-6 release from bronchial epithelial cells (59). In contrast, PKCζ-deficient airway epithelial cells produced increased levels of IL-6 (59). Other PKC isoforms have been reported to regulate IL-6 release in tissues other than the lung (26, 55). Our data suggest that decreased PKC activity may be associated with impaired IL-6 secretion from AECs, as indicated by our data showing inhibition of IL-6 release by the PKC inhibitor calphostin C (Fig. 5). To further investigate the specific isoforms responsible for the decrease in IL-6 secretion from Trek-1-deficient AECs, we studied the phosphorylation patterns of several PKC isoforms (Fig. 6). Interestingly, in eosinophils and mast cells, the activation of PKCβ is thought to be crucial for mediator release (3, 41). However, our results show that in AECs phosphorylation of PKCθ may be of particular importance for IL-6 release, since TNF-α-induced phosphorylation of this isoform appeared intact in control cells but was impaired in Trek-1-deficient AECs, and pharmacological inhibition of PKCθ decreases IL-6 release in stimulated control cells but not in stimulated Trek-1-deficient cells. Interestingly, PKCθ is a member of the so-called “novel” PKC isoforms (7) and is a Ca2+-independent isoform lacking the Ca2+-binding C2 domain (15). In turn, PKCθ has also been reported to regulate the intracellular Ca2+ concentration of T cells (20). To determine whether the decrease in IL-6 secretion from Trek-1-deficient AECs was caused by altered Ca2+ signaling mechanisms, we measured changes in the intracellular Ca2+ concentration upon TNF-α stimulation in control and Trek-1-deficient cells. To our surprise, treatment of AECs with TNF-α did not induce an acute or delayed increase in the intracellular Ca2+ concentration, and inhibition of Ca2+ reuptake mechanisms by thapsigargin did not affect IL-6 secretion. Furthermore, the response to ATP, a known stimulus of intracellular Ca2+ release (37), was unchanged in Trek-1-deficient cells compared with controls. These data suggest that the mechanisms regulating intracellular Ca2+ release were intact in Trek-1-deficient cells and that alterations in these mechanisms were unlikely the cause for the decrease in IL-6 release from Trek-1-deficient AECs. In addition, removal of extracellular Ca2+ from the culture medium did not affect IL-6 release from either control or Trek-1-deficient cells. This is an important finding, since cytokine release is generally thought to be a Ca2+-dependent process (58). However, most of the reported studies have been performed in secretory or inflammatory cells (31, 32), and very little is known about the mechanisms of cytokine secretion from AECs. Influx of extracellular Ca2+ via transient receptor potential channel ankryn 1 channels has been reported to be required for IL-8 secretion from AECs (38), and Ca2+ influx induced by staphylococcal α-toxin appeared necessary for IL-6 release from AECs via activation of Ca2+-dependent PKC isoforms. Furthermore, nucleotide-induced IL-6 release from airway epithelial cells has been reported to be Ca2+-dependent (12). Collectively, these data suggest that the requirement for Ca2+ to induce IL-6 secretion from lung epithelial cells may be stimulus-, and potentially cell type-specific, with significant differences between airway and AECs. Of note, it is important to consider that FBS itself contains Ca2+, but a solution containing 10% FBS would only contain about 1 mg/dl of Ca2+ (assuming a normal serum Ca2+ concentration of 10 mg/dl), whereas our standard culture medium contained 20 mg/dl of Ca2+ (GIBCO).
In conclusion, this is the first report demonstrating Trek-1 expression in human AECs and proposing a potential role for this K2P channel in the development of alveolar inflammation by regulating IL-6 secretion. It is important to recognize that the decrease in IL-6 release from Trek-1-deficient cells may not be limited to stimulation of cells with TNF-α. Stimulation of Trek-1-deficient cells with other proinflammatory cytokines may result in a similar decrease in IL-6 release, which would make it intriguing to speculate that Trek-1 could be “master regulator” of IL-6 release under both basal and stimulated conditions. Therefore, to clarify whether Trek-1 was directly inhibiting TNF-α-mediated secretion of IL-6, in future experiments we may investigate whether bypassing this pathway could overcome the suppressive effects of Trek-1 deficiency.
The fact that IL-6 release may occur independently of the extra- and intracellular Ca2+ concentration is particularly important in the search for the underlying mechanisms that lead to decreased IL-6 secretion from Trek-1-deficient AECs. A theoretical model where accumulation of positive K+ charges inside Trek-1-deficient cells decreases the driving force for Ca2+ influx (13) resulting in decreased cytokine secretion appears unlikely in the light of our results. Off-target effects of Trek-1 shRNA treatment are unlikely to contribute to our findings, since gene expression of a closely related K2P channel, namely Trek-2, and of an unrelated, Ca2+-permeable non-K2P channel, transient receptor potential channels of the vanilloid receptor subtype 4, remained unchanged by real-time PCR in both MLE-12 and A549 cells (data not shown). Although further studies are necessary to investigate how Trek-1 deficiency ultimately leads to impaired IL-6 secretion form AECs, Trek-1 may represent a novel therapeutic target to reduce alveolar inflammation.
GRANTS
This study was supported by a grant from the Le Bonheur Children's Medical Center Research Foundation of the University of Tennessee Health Science Center and National Heart, Lung, and Blood Institute HL-094366 (C. M. Waters) and HL-67061, HL-094378, and HL-110247 (J. H. Jagger).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., M.C.G., G.J.T., J.H.J., and C.M.W. conception and design of research; A.S., B.T., M.C.G., K.G.L., and D.N. performed experiments; A.S., B.T., and D.N. analyzed data; A.S., K.G.L., J.H.J., and C.M.W. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., G.J.T., J.H.J., and C.M.W. edited and revised manuscript; A.S., B.T., M.C.G., K.G.L., G.J.T., D.N., J.H.J., and C.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Drs. S. E. Sinclair and P. Makena for help with Western blot experiments and Dr. E. Fitzpatrick for supplying IL-6 ELISA plates.
REFERENCES
- 1. Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 13: 73–78, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Andreoni KA, Wang X, Huang SM, Huang ES. Human cytomegalovirus hyperimmune globulin not only neutralizes HCMV infectivity, but also inhibits HCMV-induced intracellular NF-kappaB, Sp1, and PI3-K signaling pathways. J Med Virol 67: 33–40, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bates ME, Bertics PJ, Calhoun WJ, Busse WW. Increased protein kinase C activity in low density eosinophils. J Immunol 150: 4486–4493, 1993 [PubMed] [Google Scholar]
- 4. Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci 29: 566–575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bezzerri V, Borgatti M, Finotti A, Tamanini A, Gambari R, Cabrini G. Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. J Immunol 187: 6069–6081, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Boehringer N, Hagens G, Songeon F, Isler P, Nicod LP. Differential regulation of tumor necrosing factor-alpha (TNF-alpha) and interleukin-10 (IL-10) secretion by protein kinase and phosphatase inhibitors in human alveolar macrophages. Eur Cytokine Net 10: 211–218, 1999 [PubMed] [Google Scholar]
- 7. Culp DJ, Zhang Z, Evans RL. Role of calcium and PKC in salivary mucous cell exocrine secretion. J Dental Res 90: 1469–1476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dauletbaev N, Eklove D, Mawji N, Iskandar M, Di Marco S, Gallouzi IE, Lands LC. Down-regulation of cytokine-induced interleukin-8 requires inhibition of p38 mitogen-activated protein kinase (MAPK) via MAPK phosphatase 1-dependent and -independent mechanisms. J Biol Chem 286: 15998–16007, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 279: L429–L438, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Deng B, Xie S, Wang J, Xia Z, Nie R. Inhibition of protein kinase C beta(2) prevents tumor necrosis factor-alpha-induced apoptosis and oxidative stress in endothelial cells: the role of NADPH oxidase subunits. J Vasc Res 49: 144–159, 2012 [DOI] [PubMed] [Google Scholar]
- 11. dos Santos CC, Han B, Andrade CF, Bai X, Uhlig S, Hubmayr R, Tsang M, Lodyga M, Keshavjee S, Slutsky AS, Liu M. DNA microarray analysis of gene expression in alveolar epithelial cells in response to TNFalpha, LPS, and cyclic stretch. Physiol Genomics 19: 331–342, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Douillet CD, Robinson WP, 3rd, Milano PM, Boucher RC, Rich PB. Nucleotides induce IL-6 release from human airway epithelia via P2Y2 and p38 MAPK-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291: L734–L746, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol 32: 490–497, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farah CA, Sossin WS. The role of C2 domains in PKC signaling. Adv Exp Med Biol 740: 663–683, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care 7: 233–241, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonzalez-Lopez A, Garcia-Prieto E, Batalla-Solis E, Amado-Rodriguez L, Avello N, Blanch L, Albaiceta GM. Lung strain and biological response in mechanically ventilated patients. Intensive Care Med 38: 240–247, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation (Abstract). J Neuroinflam 9: 82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graness A, Chwieralski CE, Reinhold D, Thim L, Hoffmann W. Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (IL-6) and IL-8 secretion. J Biol Chem 277: 18440–18446, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol 46: 213–224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janiszewski J, Huizinga JD, Blennerhassett MG. Mast cell ionic channels: significance for stimulus-secretion coupling. Can J Physiol Pharmacol 70: 1–7, 1992 [DOI] [PubMed] [Google Scholar]
- 22. John AE, Zhu YM, Brightling CE, Pang L, Knox AJ. Human airway smooth muscle cells from asthmatic individuals have CXCL8 hypersecretion due to increased NF-kappa B p65, C/EBP beta, and RNA polymerase II binding to the CXCL8 promoter. J Immunol 183: 4682–4692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawaguchi M, Onuchic LF, Huang SK. Activation of extracellular signal-regulated kinase (ERK)1/2, but not p38 and c-Jun N-terminal kinase, is involved in signaling of a novel cytokine, ML-1. J Biol Chem 277: 15229–15232, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Kim JK, Lee SM, Suk K, Lee WH. A novel pathway responsible for lipopolysaccharide-induced translational regulation of TNF-alpha and IL-6 expression involves protein kinase C and fascin. J Immunol 187: 6327–6334, 2011 [DOI] [PubMed] [Google Scholar]
- 25. King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-kappaB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J Biol Chem 284: 26803–26815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kondo M, Ugi S, Morino K, Fuke T, Obata T, Yoshizaki T, Nishio Y, Maeda S, Kashiwagi A, Maegawa H. Postprandial activation of protein kinase Cmicro regulates the expression of adipocytokines via the transcription factor AP-2beta. Int J Mol Med 28: 95–100, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Lee YL, Chen W, Chen LY, Chen CH, Lin YC, Liang SJ, Shih CM. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. J Crit Care 25: e177–e176, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol 279: F793–F801, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Liao Y, Kristiansen AM, Oksvold CP, Tuvnes FA, Gu N, Runden-Pran E, Ruth P, Sausbier M, Storm JF. Neuronal Ca2+-activated K+ channels limit brain infarction and promote survival. PLoS One 5: e15601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu D, Zhang J, Wu J, Zhang C, Xu T. Altered calcium-induced exocytosis in neutrophils from allergic patients. Int Arch Allergy Immunol 134: 281–287, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol 111: 923–933, 2003 [PubMed] [Google Scholar]
- 32. Ma HT, Beaven MA. Regulators of Ca(2+) signaling in mast cells: potential targets for treatment of mast cell-related diseases? Adv Exp Med Biol 716: 62–90, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Makena PS, Gorantla VK, Ghosh MC, Bezawada L, Balazs L, Luellen CL, Parthasarathi K, Waters CM, Sinclair SE. Lung injury caused by high tidal volume mechanical ventilation and hyperoxia is dependent on oxidant-mediated c-Jun NH2-terminal kinase (JNK) activation. J Appl Physiol 111: 1467–1476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makena PS, Luellen CL, Balazs L, Ghosh MC, Parthasarathi K, Waters CM, Sinclair SE. Preexposure to hyperoxia causes increased lung injury and epithelial apoptosis in mice ventilated with high tidal volumes. Am J Physiol Lung Cell Mol Physiol 299: L711–L719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, Lieben L, Torrekens S, Moermans K, Vanden Bosch A, Bouillon R, Nilius B, Carmeliet G. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab 8: 257–265, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107: 1062–1073, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Mikulski Z, Hartmann P, Jositsch G, Zaslona Z, Lips KS, Pfeil U, Kurzen H, Lohmeyer J, Clauss WG, Grau V, Fronius M, Kummer W. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration (Abstract). Respir Res 11: 133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mukhopadhyay I, Gomes P, Aranake S, Shetty M, Karnik P, Damle M, Kuruganti S, Thorat S, Khairatkar-Joshi N. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Receptor Signal Trans Res 31: 350–358, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Nagato AC, Bezerra FS, Lanzetti M, Lopes AA, Silva MA, Porto LC, Valenca SS. Time course of inflammation, oxidative stress and tissue damage induced by hyperoxia in mouse lungs. Int J Exp Pathol 93: 269–278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nerbonne JM. Repolarizing cardiac potassium channels: multiple sites and mechanisms for CaMKII-mediated regulation. Heart Rhythm 8: 938–941, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noll T, Dieckmann D, Gibbs BF, Nitschke M, Albrecht C, Vollrath I, Tamaoki T, Wolff HH, Amon U. Heterogeneity of signal transduction mechanisms in human basophils and human skin mast cells. II. Effects of 7-O-methyl-UCN-01, NPC 15437 and bryostatin 1 and 2, four protein kinase C-modulatory agents, on mediator release. Biol Signals 6: 1–10, 1997 [PubMed] [Google Scholar]
- 42. Oenema TA, Kolahian S, Nanninga JE, Rieks D, Hiemstra PS, Zuyderduyn S, Halayko AJ, Meurs H, Gosens R. Pro-inflammatory mechanisms of muscarinic receptor stimulation in airway smooth muscle (Abstract). Respir Res 11: 130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal 22: 977–983, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ribeiro CM. The role of intracellular calcium signals in inflammatory responses of polarised cystic fibrosis human airway epithelia. Drugs in R&D 7: 17–31, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem 280: 17798–17806, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Rose F, Dahlem G, Guthmann B, Grimminger F, Maus U, Hanze J, Duemmer N, Grandel U, Seeger W, Ghofrani HA. Mediator generation and signaling events in alveolar epithelial cells attacked by S. aureus α-toxin. Am J Physiol Lung Cell Mol Physiol 282: L207–L214, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Salter MW, Hicks JL. ATP causes release of intracellular Ca2+ via the phospholipase C beta/IP3 pathway in astrocytes from the dorsal spinal cord. J Neurosci 15: 2961–2971, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwingshackl A, Teng B, Ghosh M, West AN, Makena P, Gorantla V, Sinclair SE, Waters CM. Regulation and function of the two-pore-domain (K2P) potassium channel Trek-1 in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 302: L93–L102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 293: L105–L113, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Shi JX, Su X, Xu J, Zhang WY, Shi Y. MK2 posttranscriptionally regulates TNF-alpha-induced expression of ICAM-1 and IL-8 via tristetraprolin in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 302: L793–L799, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Shukla A, Lounsbury KM, Barrett TF, Gell J, Rincon M, Butnor KJ, Taatjes DJ, Davis GS, Vacek P, Nakayama KI, Nakayama K, Steele C, Mossman BT. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-delta knockout mice. Am J Pathol 170: 140–151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Signorelli S, Jennings P, Leonard MO, Pfaller W. Differential effects of hypoxic stress in alveolar epithelial cells and microvascular endothelial cells. Cell Physiol Biochem 25: 135–144, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Sun Y, Wu F, Sun F, Huang P. Adenosine promotes IL-6 release in airway epithelia. J Immunol 180: 4173–4181, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Tokuda H, Hosoi T, Hayasaka K, Okamura K, Yoshimi N, Kozawa O. Overexpression of protein kinase C-delta plays a crucial role in interleukin-6-producing pheochromocytoma presenting with acute inflammatory syndrome: a case report. Hormone Metab Res 41: 333–338, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Wang JY, Chen L, Zheng Z, Wang Q, Guo J, Xu L. Cinobufocini inhibits NF-kappaB and COX-2 activation induced by TNF-alpha in lung adenocarcinoma cells. Oncology Rep 27: 1619–1624, 2012 [DOI] [PubMed] [Google Scholar]
- 57. Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 326: 443–452, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990 [DOI] [PubMed] [Google Scholar]
- 59. Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ. Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase Cepsilon in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 293: L1163–L1170, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Zhang LN, Ai YH, Gong H, Dai XG, Peng L, Liu ZY, Zhao SP. The experiment study of treatment of infectious acute lung injury by intravenous administration of adenovirus borne inhibitor of nuclear factor-KappaB gene in rat. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 23: 559–562, 2011 [PubMed] [Google Scholar]
- 61. Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol 295: C1376–C1384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 124: 87–95, 2009 [DOI] [PubMed] [Google Scholar]