Abstract
The receptor for advanced glycation end products (RAGE) is a multiligand pattern recognition receptor implicated in multiple disease states. Although RAGE is expressed on systemic vascular endothelium, the expression and function of RAGE on lung endothelium has not been studied. Utilizing in vitro (human) and in vivo (mouse) models, we established the presence of RAGE on lung endothelium. Because RAGE ligands can induce the expression of RAGE and stored red blood cells express the RAGE ligand Nε-carboxymethyl lysine, we investigated whether red blood cell (RBC) transfusion would augment RAGE expression on endothelium utilizing a syngeneic model of RBC transfusion. RBC transfusion not only increased lung endothelial RAGE expression but enhanced lung inflammation and endothelial activation, since lung high mobility group box 1 and vascular cell adhesion molecule 1 expression was elevated following transfusion. These effects were mediated by RAGE, since endothelial activation was absent in RBC-transfused RAGE knockout mice. Thus, RAGE is inducibly expressed on lung endothelium, and one functional consequence of RBC transfusion is increased RAGE expression and endothelial activation.
Keywords: receptor for advanced glycation end products, red blood cell transfusion, endothelial cell, lung inflammation, red blood cells
the receptor for advanced glycation end products (RAGE) is widely expressed on systemic endothelium and functions as a pattern recognition receptor for multiple ligands, including advanced glycation end products (AGEs), high mobility group box 1 (HMGB1), calgranulins (s100A12 and s100B), amyloid β-proteins, Mac-1, phosphotidylserine, and lipopolysaccharide (10, 24, 26, 35). AGEs, a heterogenous group of adducts formed during pathological states, hyperglycemia, and periods of increased oxidative stress, lead to increased generation of reactive oxygen species (ROS) and inflammatory cytokines following ligation of RAGE expressed on vascular endothelium (25, 31, 32). We have previously shown that stored red blood cells (RBCs) that express the RAGE ligand Nε-carboxymethyl lysine (Nε-CML) trigger lung endothelial ROS generation that was attenuated by soluble receptor for advanced glycation end products (sRAGE), the extracellular ligand-binding domain of the receptor that acts as a decoy by binding RAGE ligands (19). This finding supports the hypothesis that RAGE ligands on the surface of stored RBCs promote activation of lung endothelium, thus contributing to the pathogenesis of lung injury in susceptible transfusion recipients.
Because RAGE is abundantly expressed on alveolar epithelium, particularly type I epithelial cells, and is utilized as a specific marker of alveolar epithelial injury, the existence of RAGE on pulmonary endothelium has come into question (9, 28, 30). Indeed, prior studies failed to detect RAGE in lung endothelium (9, 28). However, the complexities of RAGE biology render it a difficult molecule to study, since RAGE is known to exist in several different isoforms, subject to cleavage by proteases, rapidly internalized and recycled, and minimally expressed under basal conditions (14, 15, 22, 23, 38). Because RAGE is a critical player in systemic endothelial dysfunction in vascular disease and diabetes, it is surprising that the presence of RAGE on lung endothelium is questioned and that RAGE is accepted as a specific marker of epithelial injury (3, 4, 28, 30). As a consequence, little is known about the expression and function of RAGE on lung endothelium in health and disease. We thus sought to reexamine the expression of RAGE using multiple approaches under basal conditions and following perturbation of human lung endothelial cells and mouse lung. Because it has been suggested that ligation of RAGE leads to upregulation of the receptor itself, we hypothesized that transfusion of RBCs expressing the AGE Nε-CML would augment RAGE expression in the lung microvasculature (29). In this report, we demonstrate that lung endothelial RAGE is expressed at very low levels at baseline but is induced by RBC transfusion. Furthermore, transfusion of RBCs augments expression of the endothelial cell adhesion molecule vascular cell adhesion molecule 1 (VCAM-1) in the lung through a RAGE-dependent mechanism.
MATERIALS AND METHODS
Preparation of RBCs.
All studies involving human subjects were approved by the University of Pennsylvania Institutional Review Board. Leukoreduced RBC units were obtained from the blood bank at the Hospital of the University of Pennsylvania and used within 15 days of the expiry date. For in vitro experiments utilizing fresh RBCs, whole blood was obtained from a healthy volunteer in EDTA-containing tubes (BD Biosciences, Franklin Lakes, NJ). The whole blood was leukoreduced with leukoreduction filters (Purecell Neo; Pall, Port Washington NY), and the eluent was centrifuged at 800 g for 10 min. RBCs were washed three times in sterile PBS immediately before use.
Generation of a RAGE-expressing cell line.
Human embryonic kidney (HEK 293) cells were transfected with human advanced glycation end products receptor (accession no. NM_001136.4; InvivoGen, San Diego, CA) using Lipofectamine LTX (Invitrogen, Carlsbad, CA) selected with Blastocidin.
Stimulation of endothelial cells with RBCs.
Primary human lung microvascular endothelial cells (HMVEC-L) were obtained from Lonza. Immortalized lung microvascular endothelial cells (IMVEC-L) have been previously described and were a kind gift from Dr. Aron Fisher at the Institute for Environmental Medicine (17). HMVEC-L or IMVEC-L were seeded in six-well plates and grown to confluence. The endothelial cells (ECs) were washed with PBS and then incubated with 1 × 108 stored RBCs in endothelial basal media (Lonza, Walkersville, MD) for 4 h at 37°C. Following the incubation the ECs were washed with PBS, and cell lysates were prepared using buffer containing 50 mmol/l Tris·HCl (pH 8), 150 mmol/l NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitor cocktail (Mini Complete; Roche Applied Sciences) and stored at −80°C.
Endothelial RAGE expression.
The proximity ligation assay (PLA) was performed on lungs from naïve C57Bl/6J, RAGE knockout (KO), or platelet endothelial cell adhesion molecule (PECAM) KO mice that were inflated with 3 ml of optimum cutting temperature (OCT)/PBS, OCT embedded, frozen, and sectioned (4–6 μm thick) using a commercially available kit (Duolink; Olink Bioscience). Mouse monoclonal α-PECAM-1 (provided by Dr. Peter Newman, final concentration, 10 μg/ml) and rabbit polyclonal (IgG) α-RAGE antibody (final concentration, 5 μg/ml; Abcam) were used for the PLA assay. Images were acquired using an epifluorescence microscope (Olympus).
Detection of supernatant sRAGE by ELISA.
HMVEC-L in monolayer were incubated with 1 × 108 RBCs as described above. Supernatants were aspirated, centrifuged at 7,000 g for 5 min to pellet out RBCs, and frozen at −80°C. Supernatant sRAGE concentrations were assayed using a commercially available kit (R&D Systems, Minneapolis, MN).
Detection of RAGE transcripts.
Cells (2 × 106) were stimulated with 1 × 108 RBC in serum-free M199 medium. Following stimulation, the cells were washed four times with PBS, and RNA was extracted using the RNeasy Plus kit (Qiagen, Valencia, CA). RNA (1 μg) was reverse transcribed using Platinum Pfx DNA polymerase (Invitrogen), and the presence of RAGE transcripts was analyzed by PCR using primers against full-length RAGE (forward: 5′-CAGCCGGAACAGCAGTTGGA-3′, reverse: 5′-AGGGAGCTGATGGATGGGAT-3′). The PCR cycling conditions were 94°C for 5 min, 94°C for 15 s, 56°C for 30 s, and 68°C for 1.5 min for 40 cycles. The cDNA was diluted one hundred-fold for amplification of β-actin transcripts (forward: 5′-AACTGGGACGACATGGAGAA-3′, reverse: 5′-TCGTAGATGGGCACAGTGTG-3′). The PCR products were analyzed on a 0.8% agarose gel. The 1.2- and 1.4-kb bands were excised, and DNA was extracted (Qiaex gel extraction kit; Qiagen) and sequenced on both strands by cycle sequencing using dideoxy termination technology, on an ABI sequencer (University of Pennsylvania DNA sequencing facility). The sequences were aligned with published human RAGE sequences (NG_029868).
Immunoblotting.
Immunoblotting was performed on cell lysates as previously described using the following antibodies: rabbit polyclonal α-RAGE antibody (Abcam, Cambridge, MA, or Genetex, Irvine, CA), mouse monoclonal α-RAGE antibody (Abcam), goat α-RAGE antibody (Santa Cruz Biotechnology, Santa Cruz, CA), mouse α-PECAM antibody (a kind gift from Dr. Peter J. Newman), mouse α- or β-actin (Jackson ImmunoResearch Laboratories, West Grove, PA), and horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibody (Jackson ImmunoResearch Laboratories) (19). For select experiments, RAGE antibody (Abcam) was incubated with RAGE peptide [CWRKRQPR(R/L)EERKAPESQED; Abcam] or scramble peptide (sc-8629-P; Santa Cruz) according to the manufacturer's directions before immunoblotting.
Flow cytometry.
RBCs were obtained from leukoreduced RBC units as described above and washed immediately before use. EC were incubated with 1 × 109 RBCs for 4 h at 37.5°C. The cells were washed with fluorescence-activated cell sorter (FACS) buffer (2% FBS plus PBS), and red cells were lysed with ammonium chloride lysing buffer (BD Biosciences). Cells were labeled on ice with monoclonal mouse (IgG2a) α-RAGE antibody (10 μg/ml; Abcam). The appropriate isotype controls were used (10 μg/ml; Southern Biotech). ECs were washed two times with FACS buffer and labeled with phycoerythrin-conjugated secondary antibody (Sigma Aldrich). FACS analysis was performed using a flow cytometer (FACS Calibur; Becton-Dickinson, Franklin Lakes, NJ). Data analysis was performed using Flowjo software (version 7.2.5; Treestar, Ashland, OR).
Experimental animals.
C57Bl/6J animals were purchased from The Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Wilmington, MA). RAGE KO mice were provided by Dr. Ann Marie Schmidt. The generation of homozygous RAGE KO mice has been previously described (13). PECAM KO mice were a kind gift from Dr. Horace DeLisser. All experimental procedures were performed in 8- to 12-wk-old male mice. Animal studies were approved by and conducted in accordance with the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Isolation of murine RBCs.
Whole blood was drawn from C57Bl/6J mice via intracardiac puncture and collected in sterile syringes. Citrate-phosphate-dextrose (Sigma-Aldrich, St. Louis, MO) solution was added to the whole blood (1:9). The whole blood was leukoreduced with leukoreduction filters (Purecell Neo; Pall). The filters were washed with sterile PBS, and the eluent was centrifuged at 800 g for 10 min. RBCs were combined with storage solution (150 mmol/l sodium chloride, 45 mmol/l dextrose, 29 mmol/l mannitol, and 2 mmol/l adenine, 3:1 ratio of RBCs to storage solution) and transferred to 100-ml plasticizer storage bags (Charter Medical, Winston-Salem, NC). The bags were heat sealed and stored at 4°C for 12–14 days before transfusion.
Detection of Nε-CML on murine RBCs.
Nε-CML on intact murine RBCs was detected using competitive ELISA as previously described (19).
Transfusion of RBCs.
Murine packed red blood cells (PRBCs) were warmed to room temperature, washed until the supernatant was clear with sterile PBS, and concentrated to a hematocrit of 60–70% immediately before transfusion. C57/Bl6 mice or RAGE KO mice (where indicated) were transfused with 10 μl/g 1-day stored RBCs, 12- to 14-day stored RBCs, or 10 μl/g sterile PBS via tail vein. For select studies, animals were transfused with 14-day stored RBCs with sRAGE (2 μg/g sRAGE; Sino Biological, Daxing, China). Animals were killed with 80 mg/kg intraperitoneal ketamine and 10 mg/kg intraperitoneal xylazine at 4, 6, or 24 h following transfusion.
Immunohistochemistry.
Lungs were fixed in 10% formalin, expanded by vacuum for 14 h, paraffin embedded, and sectioned. Deparaffinization and rehydration were performed by heating unstained slides at 56°C for 1 h followed by graded washes in xylene, ethanol, and water. Antigen retrieval was performed by heating slides in Tris-EDTA buffer (10 mM Tris base, 1 mM EDTA, 0.05% Tween, pH 9). Sections were incubated in blocking buffer (5% goat serum or 2% BSA in PBS) and then labeled with primary antibody for 1 h at room temperature [rat anti-mouse VCAM-1, 20 μg/ml (R&D systems and MinnInvivoGen)]. Endogenous peroxidases were blocked in 0.6% hydrogen peroxide in methanol. Sections were incubated in biotinylated secondary antibody for 30 min (7.5 μg/ml; Vector Laboratories, Burlingame, CA). VECTASTAIN ABC Reagent and DAB substrate kit (Vector Laboratories) were added followed with methyl green. Images were acquired using a light microscope (Olympus, Center Valley, PA). VCAM-1 staining was quantified in RBC-transfused mice by counting VCAM-1-positive vessels/high-powered field. Quantification was performed by two blinded reviewers.
Immunofluorescence.
Animals were transfused as described above. Immediately following euthanasia lungs were inflated with OCT/PBS (1:1), frozen in OCT at −80°C, and sectioned. Unfixed tissue sections were labeled with rabbit anti-RAGE and rat anti-PECAM antibodies (10 μg/ml) for 1 h at room temperature. Dylight 594-conjugated mouse anti-rabbit and Dylight 488-conjugated goat anti-rat (7.5 μg/ml; Jackson ImmunoResearch Laboratories) secondary antibodies were used. Images were acquired using an epifluorescence microscope (Olympus).
Vascular perfusion.
C57Bl/6J mice were transfused with PBS or RBCs. Four hours following transfusion, mice were anesthetized, the right ventricle was cannulated, and lungs were perfused two times with 10 ml ice-cold sterile PBS; the lungs were subsequently perfused with 5 ml ice-cold rabbit polyclonal (IgG) α-RAGE antibody (final concentration, 10 μg/ml; Abcam) and rat monoclonal (IgG2a) α-PECAM-1 (390, final concentration 10 μg/ml) or rat IgG and rabbit IgG (final concentration 10 μg/ml; Millipore) followed by 10 ml sterile PBS. The whole lung was inflated with 3 ml of OCT/PBS, OCT embedded, frozen, and sectioned. Immunofluorescence staining was performed by incubating with the appropriate secondary antibodies for 1 h following a blocking step. Images were acquired using an inverted epifluorescence microscope (Olympus). Fluorescence intensity was quantified using Image J software.
Immunoblotting of whole lungs.
Whole lungs were homogenized in cell lysis buffer and incubated at 4°C for 1 h. Homogenates were sonicated for 5 min and centrifuged for 10 min at 16,000 g. Supernatant protein concentrations were assayed (Pierce, Rockford, IL), and immunoblotting was performed using rabbit α-RAGE antibody, mouse α-HMGB1 antibody, and HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) (19).
Immunoelectron microscopy.
Mice were transfused with 10 μl/g stored RBCs or PBS. Four hours following transfusion, mice were killed and lungs were perfused two times with 5 ml ice-cold PBS via the right ventricle and then perfused with 5 ml 2% paraformaldehyde. Lungs were inflated with 2 ml 2% paraformaldehyde and fixed at 4°C for 1 h. Lungs were then transferred to PBS and stored at 4°C until processing. Pieces (1 mm3) of the tissue were infused in polyvinylpyrrolidone cryoprotectant overnight at 4°C (25% polyvinylpyrrolidone, 2.3 M sucrose, and 0.055 M Na2CO3, pH 7.4). Tissue was frozen on ultracryotome stubs under liquid nitrogen and stored in liquid nitrogen until use. Ultrathin sections (70–100 nm) were cut using a Leica UC7 ultramicrotome with a FC7 cryo-attachment, lifted on a small drop of 2.3 M sucrose, and mounted on Formvar-coated copper grids. Sections were washed three times with PBS and then three times with PBS containing 0.5% bovine serum albumin and 0.15% glycine (PBG buffer) followed by a 30-min incubation with 5% normal goat serum in PBG. Sections are labeled with anti-RAGE primary antibodies at room temperature for 1 h. Sections were washed four times with PBG and then labeled with secondary antibodies conjugated to colloidal gold (goat anti-rabbit 5 nm; Amersham, Piscataway, NJ) for 1 h at room temperature. Sections were washed three times in PBG and three times in PBS and then fixed in 2.5% glutaraldehyde in PBS for 5 min, washed two times in PBS, and washed six times in double-distilled H2O (ddH2O). Sections were poststained in 2% neutral uranyl acetate for 7 min, washed three times in ddH2O, stained 2 min in 4% uranyl acetate, and then embedded in 1.25% methyl cellulose. Labeling was observed on a JEOL JEM 1011 electron microscope (Peabody, MA) at 80 kV fitted with a side-mount AMT 2k digital camera (Advanced Microscopy Techniques, Danvers, MA).
RESULTS
Human lung and human lung ECs express three RAGE isoforms.
We first determined the ability of three different antibodies to detect RAGE in human lung tissue and HEK 293 cells transfected with human RAGE (293-RAGE). Mouse monoclonal antibody raised against full-length RAGE detected a 45-kDa isoform in the whole lung homogenate and 45- and 51-kDa isoforms in 293-RAGE cells (Fig. 1). Antibody to the NH2-terminus (H-16) confirmed these results (Fig. 1B). With longer exposure, both the 45- and 51-kDa isoforms were detected in human lung in addition to a 64-kDa RAGE isoform (Fig. 1B). Lastly, we examined RAGE expression using a polyclonal antibody raised to the COOH-terminus. This antibody also detected the previously visualized isoforms (Fig. 1C). Thus, similar to findings reported in murine lung tissue, human lung expresses at least three major isoforms of RAGE, with the 45-kDa isoform being the predominant form expressed (12). Human lung also expresses a 64-kDa RAGE isoform at low levels.
Fig. 1.
Receptor for advanced glycation end products (RAGE) expression in whole lung and lung microvascular endothelial cells. A: monoclonal antibody detects a 45-kDa RAGE isoform in human lung. B: polyclonal antibody raised to the NH2-terminus confirms the presence of the 45-kDa isoform and detects additional 51- and 64-kDa isoforms. C: polyclonal antibody raised to the COOH-terminus detects all three RAGE isoforms in human lung. D: monoclonal antibody only detects RAGE in immortalized lung endothelial cells after a long exposure (20 min). IMVEC-L, immortalized lung microvascular endothelial cells. F: polyclonal antibody raised to the COOH-terminus detects RAGE in immortalized lung endothelial cells.
After establishing the validity of the antibodies, we next examined RAGE expression in both immortalized and primary human lung microvascular ECs (IMVEC-L and HMVEC-L, respectively). With short exposure, monoclonal antibody raised against full-length RAGE detected the 45-kDa RAGE isoform in lung homogenate and the 51-kDa RAGE isoform in 293-RAGE cells but did not detect either of these bands in IMVEC-L (Fig. 1D). However, with longer exposure, this antibody detected both the 51-kDa isoform and a 64-kDa band in IMVEC-L (Fig. 1D). The 64-kDa isoform was more readily detectable with the polyclonal antibody raised to the COOH-terminus of RAGE (Fig. 1E). Thus, lung ECs in culture express two major RAGE isoforms, albeit in low levels under basal conditions.
RAGE is inducible by endotoxin, RAGE ligands, and RBCs in lung ECs.
Previous reports demonstrated the presence of NF-κB-like binding sites in the RAGE promoter and increased lung RAGE expression in mice treated with lipopolysaccharide (LPS) (12, 18). We therefore examined the effects of endotoxin on the expression of lung endothelial RAGE. Stimulation of IMVEC-L with endotoxin increased expression of the 64-kDa RAGE isoform (Fig. 2A). Because we have previously shown the RAGE ligand Nε-CML on the surface of banked human RBCs and RAGE ligands are known to upregulate the receptor itself, we asked whether the RAGE ligand AGE-BSA or intact human RBCs obtained from RBC units would induce RAGE in primary lung ECs (1, 19). As shown in Fig. 2A, AGE-BSA increased expression of the 64-kDa RAGE isoform. We next asked whether human RBCs obtained from a healthy volunteer or leukoreduced RBC units would increase RAGE expression. Figure 2B demonstrates that, although fresh RBCs did not induce RAGE, RBCs from two of the three RBC units tested increased the 64-kDa isoform. To ensure that alterations in oxygen tension were not contributing to the observed variation in RAGE upregulation by stored RBC units, we measured Po2 in the RBC-treated cell culture supernatants. RBCs did not elicit hypoxia in cultured EC. In addition, we observed minimal variability in Po2 across five different RBC-treated EC samples (data not shown). Thus, it appears that lung ECs express a 64-kDa RAGE isoform that is inducible by LPS and by banked RBCs. Because the polyclonal antibody detected several bands in the IMVEC-L and HMVEC-L lysates, we confirmed specificity of the detected 64- and 51-kDa isoforms using a synthetic peptide corresponding to an 18-amino acid sequence in the COOH-terminus of RAGE (Fig. 2C).
Fig. 2.
RAGE expression following stimulation with lipopolysaccharide (LPS), RAGE ligand, or red blood cell (RBC) concentrates. A: RAGE expression is increased in human lung microvascular endothelial cells (HMVEC-L) 4 h following stimulation with LPS (10 ng/ml), advanced glycation end products (AGE) BSA (10 μg/ml), or RBC concentrates (1 × 108 RBCs). EC, endothelial cell. B: RBCs obtained from 2 of 3 stored leuokoreduced RBC units increased RAGE expression, whereas fresh RBC did not. Three independent experiments were performed; data are representative of one experiment. C: the 64- and 51-kDa bands are absent in the presence of RAGE peptide but not Scramble (Sc) peptide. Short (s) and long (l) exposure shown. RBCs from stored leukoreduced RBC units induce RAGE expression to a variable-degree HMVEC-L, monoclonal antibody (m), polyclonal antibody (p) (D).
We next examined the ability of RBCs from different RBC units to induce RAGE expression in lung ECs. Over half of the stored RBCs tested increased RAGE expression in HMVEC-L, although there was considerable variability in RAGE induction by RBCs (Fig. 2D). Both the rabbit polyclonal and mouse monoclonal antibodies were used to detect RAGE in HMVEC-L (Fig. 2D).
Lung EC surface RAGE expression is increased following stimulation with RBC.
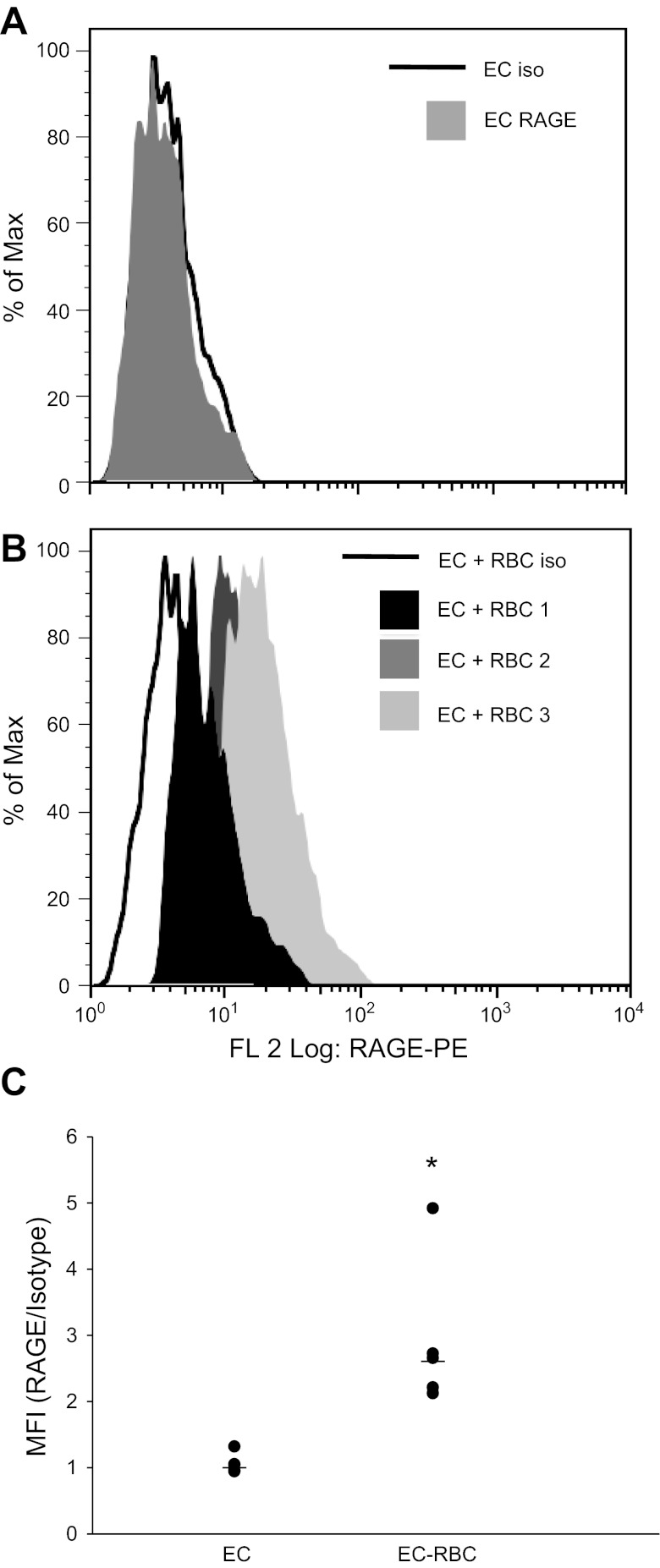
Previous studies have demonstrated increased expression of RAGE following stimulation with proinflammatory cytokines, suggesting that preformed RAGE is stored intracellularly and mobilized to the cell surface during inflammatory states (11). We therefore asked whether surface expression of RAGE would be increased following stimulation with RBCs from leukoreduced stored RBC units. Although the RAGE expression in the total population did not change with RBC stimulation (data not shown), we observed a significant increase in the mean fluorescence intensity of RAGE when we gated on the RAGE-expressing cells (Fig. 3, A–C). These data suggest that RAGE is expressed (but at low levels) on a subpopulation of EC and that, after stimulation with RBCs, RAGE expression increases on the surface of this subset of cells.
Fig. 3.
Surface expression of RAGE is increased following RBC stimulation. A: basal RAGE expression on IMVEC-L. B: RAGE-expressing cells demonstrate increased surface expression following stimulation with RBCs; 3 of 5 different RBC units tested are shown. C: mean fluorescence intensity {MFI [RAGE/isotype (Iso)]} for EC and EC + RBC; n = 5 RBC U/experiment. Each experiment was repeated two times. Data are representative of one experiment.
Soluble RAGE is detectable in supernatants of lung ECs and is increased in the presence of RBC.
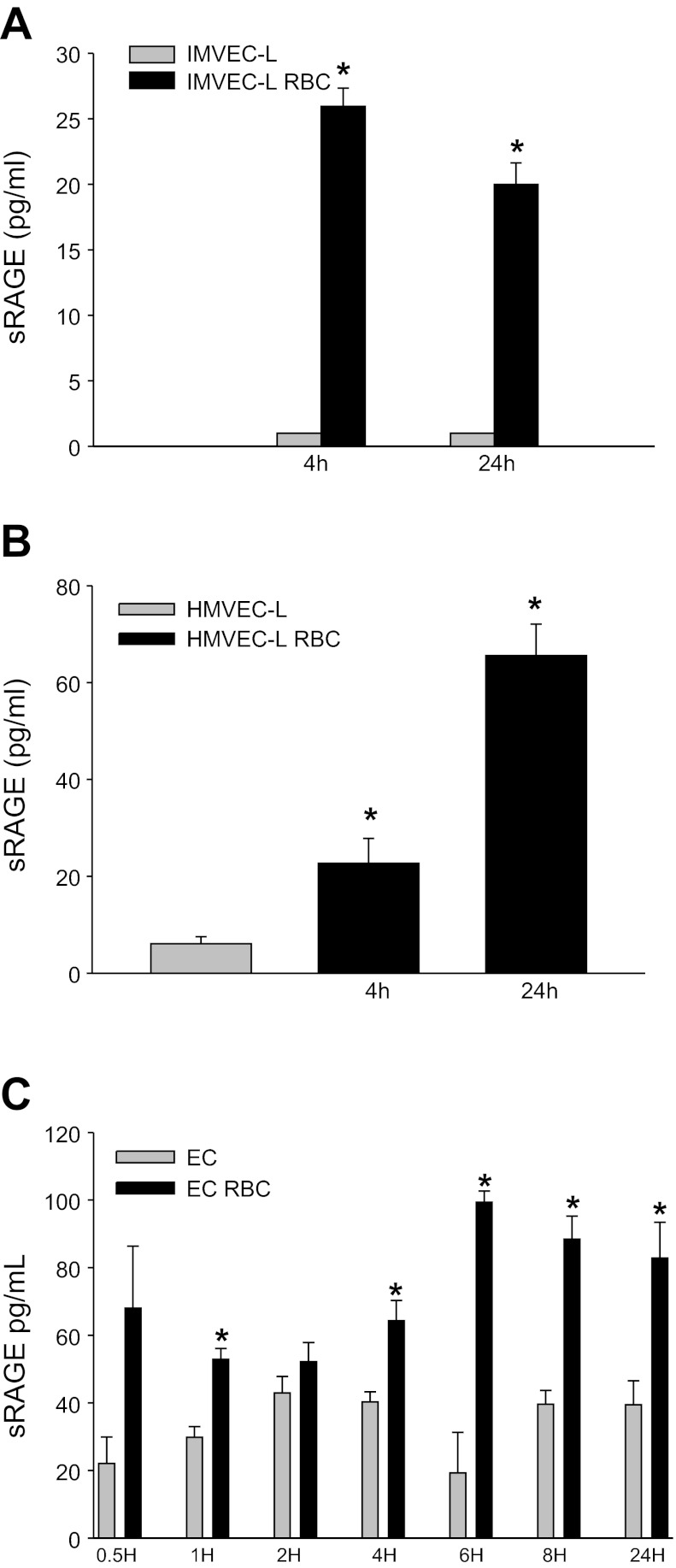
Total RAGE expression may be reflected by not only surface RAGE but also cleaved sRAGE given that metalloproteases, including ADAM 10 and MMPs 3, 13, and 9, are known to cleave RAGE (23, 34, 38). Thus, we also determined sRAGE concentrations in the supernatant (SN) of cultured lung EC under basal conditions and following stimulation with stored RBCs. sRAGE was minimally detectable under basal conditions, and RBC concentrates increased sRAGE in the supernatant (P < 0.001 at 4 h and P = 0.003 at 24 h, IMVEC-L; P = 0.036 at 4 h and P < 0.001 at 24 h, HMVEC-L; Fig. 4, A and B). Notably, sRAGE is undetectable in the presence of serum-containing media, since the addition of serum to SN-containing sRAGE abrogated the ability to detect RAGE (data not shown). We next examined the kinetics of sRAGE generation by naïve EC and ECs in the presence of stored RBCs. Following stimulation with stored RBCs, sRAGE expression increased at early and late time points (Fig. 4C).
Fig. 4.
Soluble RAGE levels in conditioned media from lung EC. A: soluble receptor for advanced glycation end products (sRAGE) is increased in SN from IMVEC-L following stimulation with RBCs (P < 0.001 at 4 h and P = 0.003 at 24 h, HMVEC-L P = 0.036 at 4 h and P < 0.001 at 24 h). B and C: sRAGE is increased in the SN from IMVEC-L following stimulation with RBCs (P = 0.007, 0.286, 0.023, 0.004, 0.014, and 0.027) for 1, 2, 4, 6, 8, and 24 h, respectively. *P < 0.05.
HMVECs express RAGE transcripts.
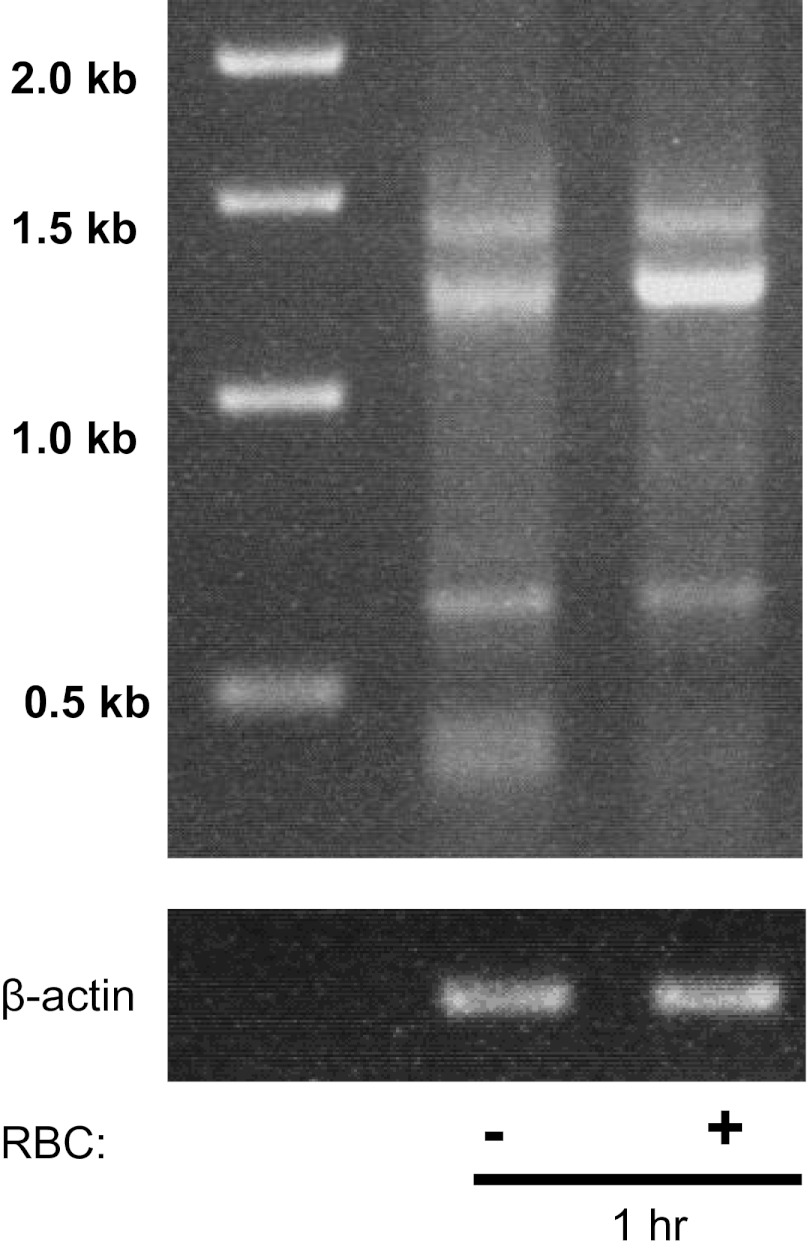
Over 19 different splice variants have been detected in human lung and predominantly three major splice variants and RAGE isoforms have been reported in microvascular ECs (14, 37). To determine whether lung ECs express RAGE transcripts, we performed PCR on naïve and RBC-treated IMVEC-L using primers against full-length RAGE. Consistent with other reports demonstrating the presence of multiple RAGE transcripts in microvascular ECs, we observed the presence of three different RAGE transcripts in naïve EC of 1.43, 1.25, and ∼0.65 kb. RBC stimulation led to an increase in the 1.25-kb transcript at 1 h (Fig. 5). The 1.43 and 1.25 transcripts were fully sequenced and were confirmed to be previously reported RAGE transcripts corresponding with NH2-truncated and full-length isoforms (14, 37).
Fig. 5.
RAGE transcripts in naïve IMVEC-L and following RBC stimulation. Several RAGE transcripts were detected in IMVEC-L. Stimulation with RBCs from leukoreduced (LR) RBC units (1 × 108) increased expression of the 1.25-kb isoform at 1 h.
RAGE is expressed at low levels on mouse lung endothelium in situ.
As demonstrated in Fig. 6A, the polyclonal rabbit anti-RAGE antibody detects RAGE in lung tissue of WT but not RAGE−/− mice. Figure 6, B and C, demonstrates that RAGE and PECAM antibodies used for immunofluorescence are specific for their respective antigens. Consistent with previous reports, bronchi and large vessels did not express RAGE, whereas type I alveolar epithelial cells demonstrated high levels of RAGE positivity (Fig. 6D) (7, 9).
Fig. 6.
RAGE expression on murine lung tissue. A: immunoblotting of lung tissue from C57Bl/6J and RAGE knockout (KO) mice. Immunofluorescence of RAGE (B), platelet endothelial cell adhesion molecule (PECAM) KO (C), and wild-type (WT) lungs (B and C) reveals that antibodies are specific. D: localization of RAGE on major lung structures (green, PECAM; red, RAGE). Only small amounts of EC RAGE are visualized at baseline (arrowheads). E: proximity ligation assay (PLA) demonstrates RAGE and PECAM colocalization in murine lung tissue. PLA on lung sections from RAGE KO (a), PECAM KO (b), or WT (c) mice. Lung sections from WT mice display a positive signal.
Because of the close anatomic proximity of alveolar epithelium and endothelium, localization to one cell type is challenging, especially since, under basal conditions, the alveolar endothelium likely displays low expression of RAGE. Using the in situ PLA, which incorporates PCR technology to amplify signal and detect proteins within 40 nm of each other, we determined whether RAGE and the endothelial marker PECAM interact within lung endothelium. Control lung sections from RAGE−/− and PECAM−/− mice did not demonstrate any positive signal (Fig. 6, Ea and Eb). However, RAGE was found to localize with PECAM in alveolar endothelium of wild-type (WT) mice (Fig. 6Ec). Thus, our findings confirm low-level RAGE expression on murine lung endothelium under basal conditions.
Lung endothelial RAGE expression is increased following RBC transfusion.
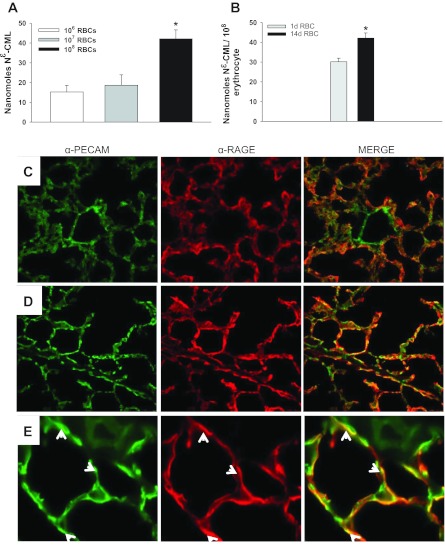
Because Nε-CML is detectable on stored murine RBCs (Fig. 7, A and B), we asked whether RBC transfusion can augment lung endothelial RAGE expression in vivo (1, 19, 27). Although PBS-transfused mice displayed minimal endothelial RAGE staining, endothelial RAGE expression was increased 24 h following RBC transfusion (Fig. 7, C-E). Similar results were obtained when lung endothelial RAGE expression was assessed 4 h following transfusion (data not shown).
Fig. 7.
The RAGE ligand Nε-carboxymethyl lysine (Nε-CML) is detectable on murine RBCs, and endothelial RAGE expression is increased following RBC transfusion. A: there is a dose-dependent increase in detectable Nε-CML on intact murine RBCs (P = 0.004). B: Nε-CML on 1- and 14-day-old murine RBCs (P = 0.020). PECAM and RAGE expression following 24 h of PBS transfusion (C) or RBC transfusion (D), magnification ×20. E: ×100 magnification of lung from RBC-transfused animal; white arrows denote RAGE and PECAM-labeled endothelial cells. *P < 0.05.
To quantify endothelial RAGE expression under basal conditions and following RBC transfusion, we used a vascular perfusion technique. Mice were transfused with either PBS or RBC, and then the lung was selectively perfused with anti-RAGE and anti-PECAM antibodies or the appropriate IgG control antibodies 4 h following transfusion. The lungs were then harvested. Because the tissues were not permeabilized before fixation and imaging, only the vasculature was exposed for labeling, providing a technique to assess endothelial-specific RAGE expression both in the naïve and perturbed mouse lung. RBC transfusion led to increased endothelial RAGE expression compared with PBS treatment, where endothelial RAGE expression was minimal (P < 0.001, Fig. 8). These results were confirmed with immunoelectron microscopy showing that RAGE labeling was limited to the basement membrane of type I cells in naïve mice but was evident in the capillary lumen following RBC transfusion (Fig. 9).
Fig. 8.
Endothelial RAGE and PECAM expression in naïve and RBC-transfused mice. A: RBC-transfused mice were perfused with IgG control antibodies. B: PBS-treated mice perfused with α-PECAM and α-RAGE antibodies. C: RBC-transfused mice perfused with α-PECAM and α-RAGE antibodies. D: quantification of endothelial RAGE expression from 3 random fields using Image J software. Endothelial RAGE expression is increased 4 h following RBC transfusion, P < 0.001. Representative images from 1 experiment; 3 independent experiments were conducted. Original magnification ×40. *P < 0.05.
Fig. 9.
Immunoelectronmicroscopy of RAGE in murine lung tissue. RAGE labeling following PBS transfusion (A) or RBC transfusion (B). White arrows denote positive labeling on alveolar epithelium, and black arrows denote endothelial labeling.
RBC transfusion augments lung VCAM-1 expression and HMGB1 release in a RAGE-dependent manner.
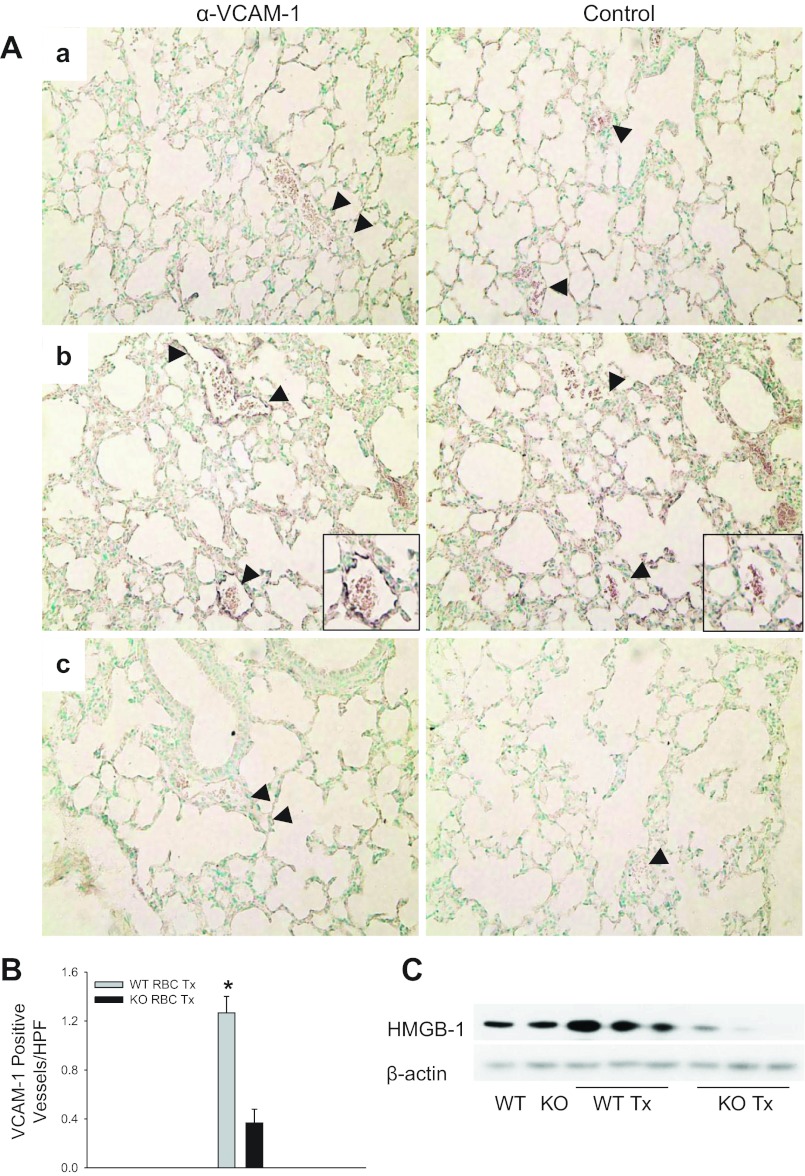
We next asked whether RBC transfusion would increase VCAM-1 expression in the lung, an adhesion molecule upregulated during endothelial activation (13, 16, 25). Lung endothelium of WT mice transfused with stored RBCs displayed increased VCAM-1 expression following transfusion compared with lungs of RAGE KO mice (1.27 vs. 0.367 positive vessels/high-powered field, P < 0.001 for WT vs. RAGE KO-transfused mice; Fig. 10, A and B). Transfusion also increased expression of the danger signal HMGB1, a known RAGE ligand, in the lungs of WT mice and downregulated HMGB1 expression in RAGE KO mice, suggesting cross-regulation of RAGE and RAGE ligands (Fig. 10C). Thus, transfusion of stored RBCs augments vascular activation and heightens the inflammatory response through a RAGE-dependent mechanism.
Fig. 10.
RBC transfusion increases lung vascular cell adhesion molecule 1 (VCAM-1) expression and high mobility group box 1 (HMGB1) release. A: VCAM-1 is increased in WT mice 6 h following transfusion. a, PBS-transfused (Tx) WT mouse; b, RBC transfusion increased VCAM-1 expression in WT mouse; c, RBC-transfused RAGE KO mouse. Representative images; n = 6 mice/group. Magnification X40. B: RBC-transfused WT mice displayed increased VCAM-1 staining in the lungs compared with RAGE KO mice or untreated controls (1.27 vs. 0.367 positive vessels/high-powered field, P < 0.001 for WT vs. RAGE KO-transfused mice). Two independent experiments were conducted (n = 3 mice/group for each experiment). C: HMGB1 is increased in WT mice following transfusion. Immunoblot of whole lungs from untransfused or RBC-transfused WT and RAGE KO mice reveals a RAGE-dependent increase in whole lung HMGB1 expression. *P < 0.05. Black arrowheads point to vessels within the lung. Insets are magnifications of the vessels.
sRAGE attenuates RAGE induction by transfused RBCs.
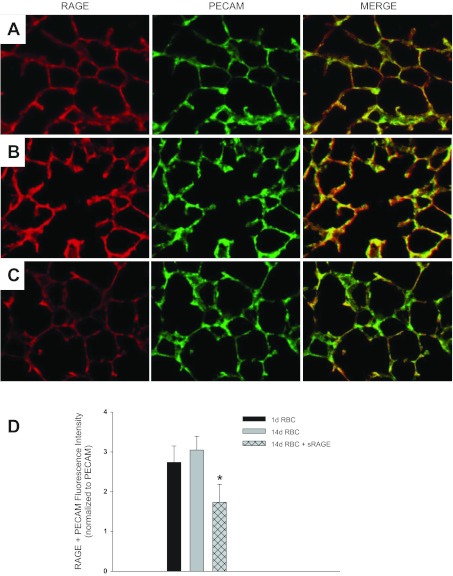
To determine whether RAGE ligands on RBCs mediated lung endothelial RAGE upregulation following transfusion, we asked whether sRAGE would decrease RAGE upregulation by stored 14-day RBCs. Lung endothelial RAGE expression was attenuated in animals transfused sRAGE + 14-day RBCs compared with animals receiving 14-day RBCs alone (Fig. 11, A–D). These findings suggest that RAGE ligands on transfused RBCs augment lung endothelial RAGE upregulation following RBC transfusion.
Fig. 11.
sRAGE attenuates lung endothelial RAGE upregulation by transfused RBCs. PECAM and RAGE expression 24 h following 1-day RBC transfusion (A), 14-day RBC transfusion (B), or 14-day RBC + sRAGE transfusion (C). Quantification of PECAM and RAGE colocalization from 5 random fields/animal, 5 mice in each group, P = 0.05 (D). Images are representative from 1 experiment; 2 independent experiments were conducted. Original magnification X20.
DISCUSSION
Several major findings of significant consequence are presented in this study. Utilizing multiple approaches, we demonstrate that murine lung endothelium and human lung ECs express RAGE at low levels. In vitro we establish the presence of three major RAGE isoforms in human lung tissue and microvascular ECs. Endothelial RAGE is readily inducible in response to stored RBCs. This effect appears to be occurring in a subpopulation of RAGE-expressing cells where increased surface expression of RAGE is detectable following stimulation with RBCs. In vivo findings paralleled our in vitro studies, since RAGE expression on lung endothelium is minimal under basal conditions but highly inducible by transfusion of stored 14-day RBCs. Furthermore, transfusion of RBCs directly mediated lung endothelial activation through increased VCAM-1 and RAGE expression that was attenuated in the absence of RAGE signaling.
Interestingly, baseline RAGE expression is low throughout the body with exception of the lung where it is expressed constitutively at high levels on alveolar epithelium (2, 28). Consequently, examination of RAGE in the pathobiology of lung disease has focused on derangements of epithelial function, and RAGE biology within the lung endothelium has been largely ignored. To our knowledge, this report represents the first detailed examination of lung endothelial RAGE. Although initial studies demonstrated RAGE expression in lung endothelium from bovine tissue, subsequent studies have failed to detect RAGE on pulmonary endothelium, localizing RAGE to the basolateral membrane of type I alveolar epithelial cells. Consequently, RAGE has become rather well accepted in the pulmonary literature as a specific marker of epithelial damage (2, 5, 7, 9, 28, 30). However, several different groups have detected RAGE on the pulmonary endothelium. Durr et al. have detected RAGE expression in rat lung endothelium and Morbini and colleagues have detected endothelial RAGE expression in human lung sections that was increased during inflammatory states (8, 20). Consistent with these findings, we and others have observed increased endothelial dysfunction in response to RAGE ligands that was attenuated with disruption of the RAGE axis (19, 33). Given these observations, we sought to establish the presence of RAGE on lung endothelium and in human lung ECs using several approaches.
To determine the expression of RAGE on intact lung endothelium, we examined murine lung tissues. Basal endothelial RAGE expression in naïve mice was low, although PLA detected the presence of RAGE in ECs in lung tissue. Because RAGE is known to be inducible by its ligands, we asked whether transfusion of Nε-CML-expressing murine RBCs would increase endothelial RAGE expression (19). Enhanced endothelial RAGE expression following transfusion was confirmed utilizing immunofluorescence and immunoelectron microscopy. Consistent with previous reports localizing RAGE to the basolateral membrane of type I cells, our studies also detected RAGE primarily in the same location under basal conditions (9, 28). However, we detected increased RAGE on endothelium following transfusion. Thus, it appears that RAGE is expressed on lung endothelium at very low levels under basal conditions but readily upregulated. Corroborating these findings are the observations of Frommhold and colleagues demonstrating low basal levels of endothelial RAGE in cremaster muscle venules that is upregulated rapidly following cytokine stimulation (11). Collectively, these findings suggest that RAGE, a multiligand damage-associated molecular pattern receptor, is readily inducible and mobilized to the EC surface during inflammatory states.
VCAM-1 is primarily expressed on activated ECs; however, it is not expressed under quiescent conditions and is thus an ideal marker of endothelial activation (6). Transfusion of stored RBCs increased VCAM-1 expression in WT mice compared with PBS-transfused mice or RAGE KO mice. Immunohistochemistry showed increased VCAM-1 immunostaining on larger vessels but not alveolar endothelium. Our data showed increased RAGE expression in endothelium adjacent to alveoli following stimulation compared with larger vessels although focal RAGE immunostaining on large vessels was detectable. One explanation for these findings is that the kinetics of RAGE upregulation following inflammatory stimuli differ by vascular bed, and our studies did not capture the time point at which large vessel RAGE is maximally upregulated. Alternatively, these findings may suggest an alternative mechanism for RAGE-mediated VCAM-1 upregulation by transfusion of stored RBCs that is independent of direct receptor ligation.
Enhanced endothelial VCAM-1 expression was not the only RAGE-mediated functional consequence, since RBC transfusion increased HMGB1 expression in WT mice compared with KO mice. Previous studies have reported the release of HMGB1 by ECs following RAGE ligation; however, whether or not HMGB1 is released as a result of cellular activation or necrosis of endothelium has not been described (13, 36). Our findings are consistent with previous reports demonstrating elevated HMGB1 levels in patients receiving PRBC transfusions (21). Posttransfusion levels of HMGB1 were higher than what has been reported in transfusates, suggesting that HMGB1 is secreted or released as part of the response to RBC transfusion (21). Because we did not observe this effect in RAGE KO mice, we propose that HMGB1 is released following direct RAGE ligation by transfused RBCs. Collectively, these data demonstrate that transfused RBCs augment lung inflammation through a RAGE-dependent mechanism. Further studies to elucidate both the cell type and mechanism of HMGB1 release will be critical in understanding its role in lung inflammation following transfusion.
Lastly, we examined whether RBC-mediated RAGE upregulation could be attenuated with sRAGE. Our data demonstrate that administration of sRAGE at the time of RBC transfusion can decrease lung endothelial RAGE upregulation. These findings support our data demonstrating enhanced lung endothelial RAGE expression following transfusion is dependent on RBC RAGE ligands. Furthermore, these observations suggest that RAGE-blocking agents may be of therapeutic benefit in treating the vascular complications of transfusion.
In summary, we provide evidence for the existence of RAGE on lung microvasculature and demonstrate that transfusion of stored RBC increases RAGE expression and augments vascular activation through a RAGE-dependent mechanism. Although RAGE ligand-expressing RBCs do not incite lung injury themselves, the findings of enhanced vascular activation as demonstrated by increased RAGE expression and increased VCAM-1 expression may predispose susceptible hosts to subsequent injury, since RAGE is a pattern recognition receptor known to bind endogenous danger signals. Indeed, the discovery of inducible RAGE on lung endothelium represents a critical first step in developing a deeper understanding of this pattern recognition receptor in the pathogenesis of lung disorders. Given that RAGE is known to modulate inflammatory responses, maintain endothelial barrier function, directly mediate inflammatory cell recruitment, and participate in tumor metastasis, the potential for mechanistic studies to elucidate the role of lung endothelial RAGE in a broad spectrum of disorders from tumor biology and pulmonary hypertension to ischemia-reperfusion injury and acute lung injury is vast. Our data suggest that future therapies targeting the RAGE axis in the lung may be useful in ameliorating the burden of these lung diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-098362, the National Blood Foundation, and the American Lung Association (N. S. Mangalmurti).
DISCLOSURES
All authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Author contributions: N.S.M. conception and design of research; N.S.M., J.L.F., L.-C.W., D.B.S., and G.M. performed experiments; N.S.M. analyzed data; N.S.M. and S.M.A. interpreted results of experiments; N.S.M., L.-C.W., and G.M. prepared figures; N.S.M. drafted manuscript; N.S.M., D.L.S., A.M.S., J.S.L., and S.M.A. edited and revised manuscript; N.S.M., J.L.F., L.-C.W., D.B.S., G.M., D.L.S., A.M.S., J.S.L., and S.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Shirley Arrington (Blood Bank, Hospital of the University of Pennsylvania) for providing the RBC units.
REFERENCES
- 1. Bopp C, Bierhaus A, Hofer S, Bouchon A, Nawroth PP, Martin E, Weigand MA. Bench-to-bedside review: the inflammation-perpetuating pattern-recognition receptor RAGE as a therapeutic target in sepsis (Abstract). Crit Care 12: 201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 143: 1699–1712, 1993 [PMC free article] [PubMed] [Google Scholar]
- 3. Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, Matthay MA. Elevated levels of RAGE, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest 135: 269–275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, Arroliga AC. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant 26: 675–680, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA. Plasma receptor for advanced glycation end-products and clinical outcomes in acute lung injury. Thorax 63: 1083–1089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol 171: 223–229, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res 323: 475–488, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol 22: 985–992, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 44: 1147–1157, 1998 [PubMed] [Google Scholar]
- 10. Friggeri A, Banerjee S, Biswas S, de Freitas A, Liu G, Bierhaus A, Abraham E. Participation of the receptor for advanced glycation end products in efferocytosis. J Immunol 186: 6191–6198, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frommhold D, Kamphues A, Hepper I, Pruenster M, Lukic IK, Socher I, Zablotskaya V, Buschmann K, Lange-Sperandio B, Schymeinsky J, Ryschich E, Poeschl J, Kupatt C, Nawroth PP, Moser M, Walzog B, Bierhaus A, Sperandio M. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood 116: 841–849, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gefter JV, Shaufl AL, Fink MP, Delude RL. Comparison of distinct protein isoforms of the receptor for advanced glycation end-products expressed in murine tissues and cell lines. Cell Tissue Res 337: 79–89, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest 118: 183–194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J 22: 1572–1580, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J 23: 1766–1774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 274: 31740–31749, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Krump-Konvalinkova V, Bittinger F, Unger RE, Peters K, Lehr HA, Kirkpatrick CJ. Generation of human pulmonary microvascular endothelial cell lines. Lab Invest 81: 1717–1727, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem 272: 16498–16506, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Mangalmurti NS, Chatterjee S, Cheng G, Andersen E, Mohammed A, Siegel DL, Schmidt AM, Albelda SM, Lee JS. Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products. Transfusion 50: 2353–2361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol 19: 1437–1445, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock 32: 17–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perrone L, Peluso G, Melone MA. RAGE recycles at the plasma membrane in S100B secretory vesicles and promotes Schwann cells morphological changes. J Cell Physiol 217: 60–71, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 22: 3716–3727, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt AM, Hofmann M, Taguchi A, Yan SD, Stern DM. RAGE: a multiligand receptor contributing to the cellular response in diabetic vasculopathy and inflammation. Semin Thromb Hemost 26: 485–493, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest 96: 1395–1403, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 1498: 99–111, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108: 949–955, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, Hata Y. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 9: 165–174, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation (Abstract). J Transl Med 7: 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest 97: 238–243, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280: E685–E694, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Wolfson RK, Chiang ET, Garcia JG. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res 81: 189–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamakawa N, Uchida T, Matthay MA, Makita K. Proteolytic release of the receptor for advanced glycation end products from in vitro and in situ alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L516–L525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamamoto Y, Harashima A, Saito H, Tsuneyama K, Munesue S, Motoyoshi S, Han D, Watanabe T, Asano M, Takasawa S, Okamoto H, Shimura S, Karasawa T, Yonekura H, Yamamoto H. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J Immunol 186: 3248–3257, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 59: 249–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 370: 1097–1109, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L, Bukulin M, Kojro E, Roth A, Metz VV, Fahrenholz F, Nawroth PP, Bierhaus A, Postina R. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem 283: 35507–35516, 2008 [DOI] [PubMed] [Google Scholar]