Abstract
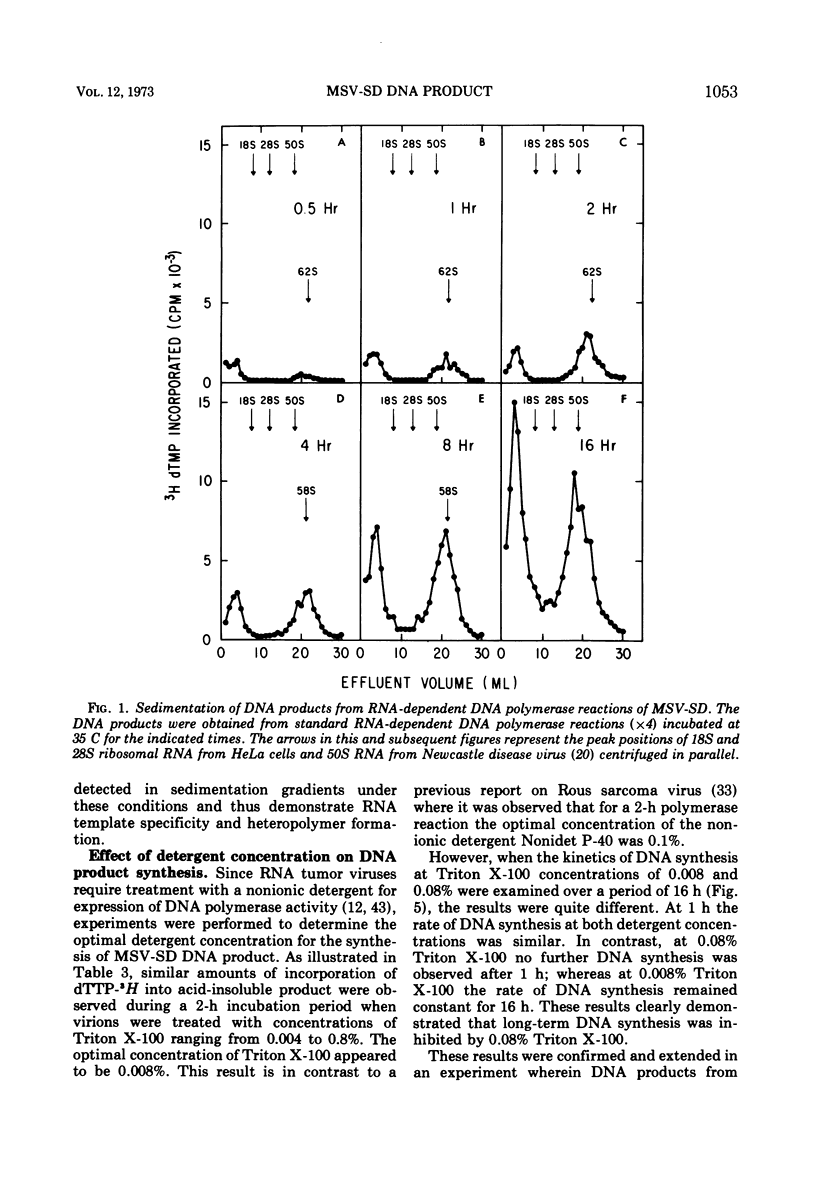
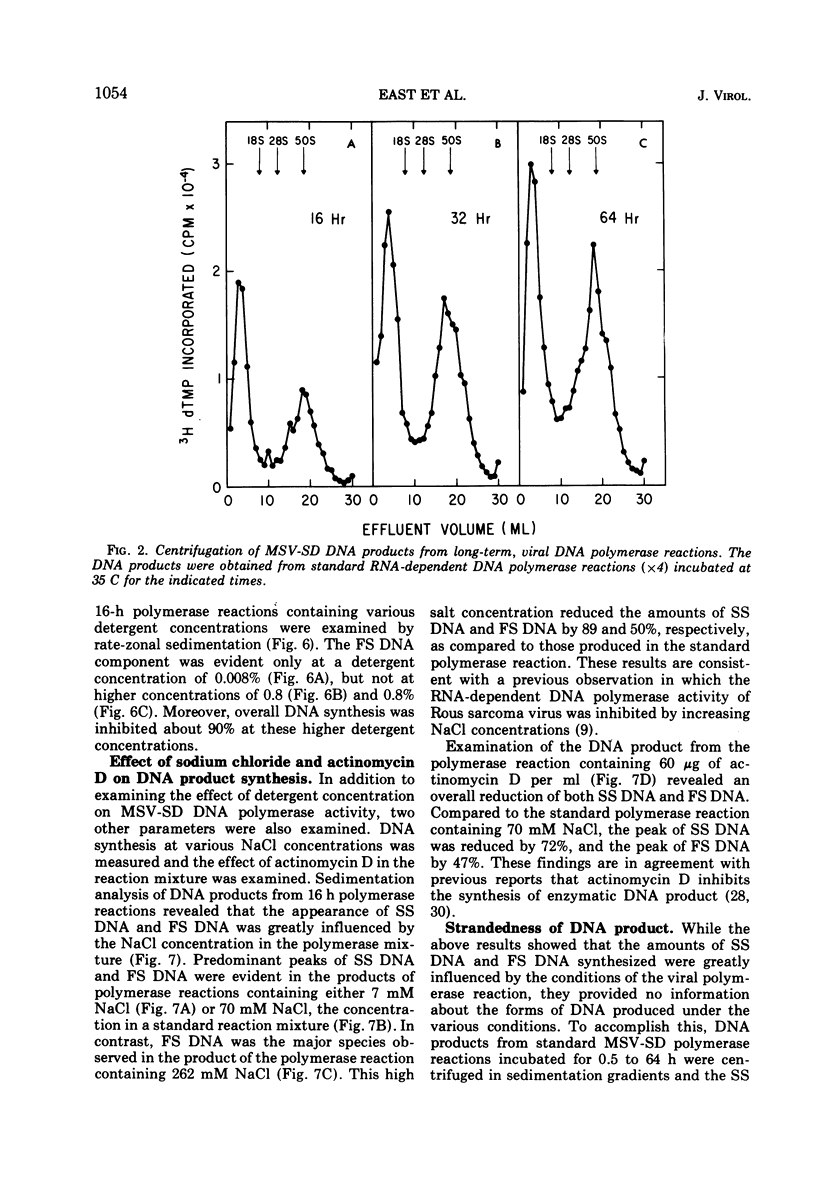
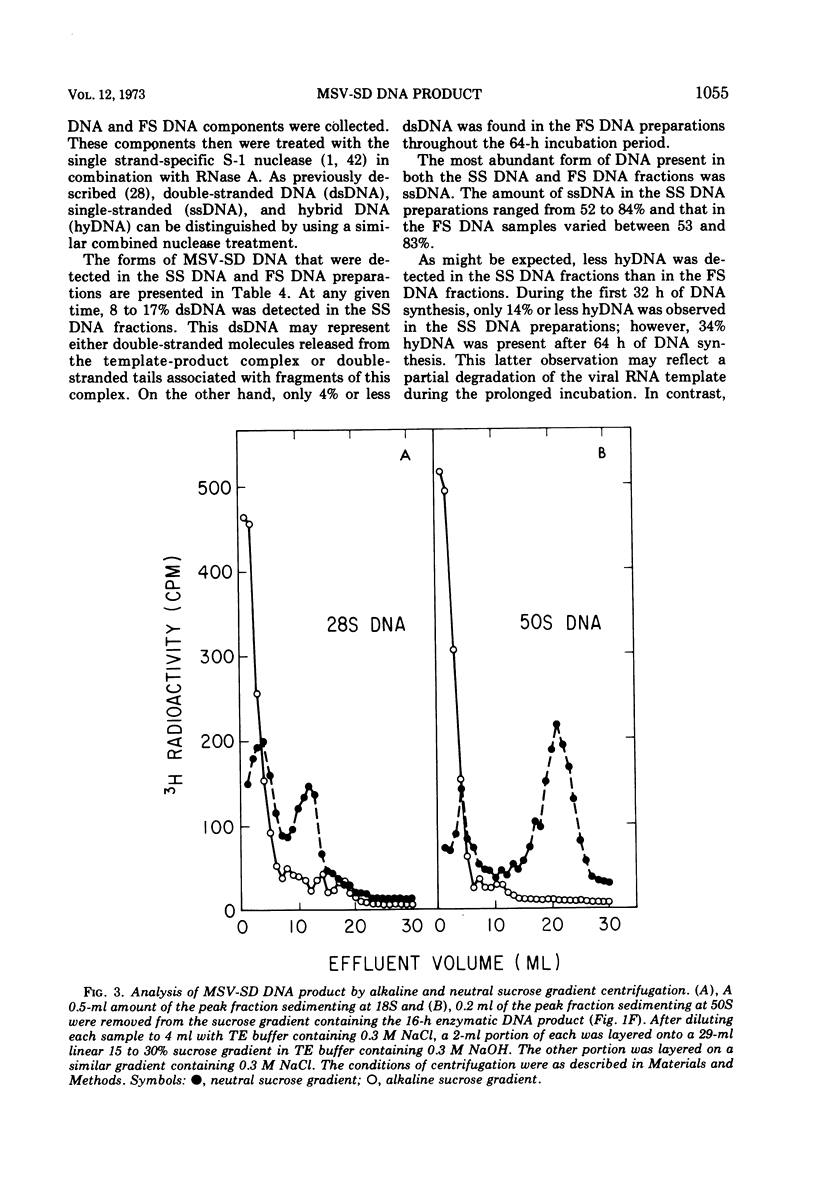
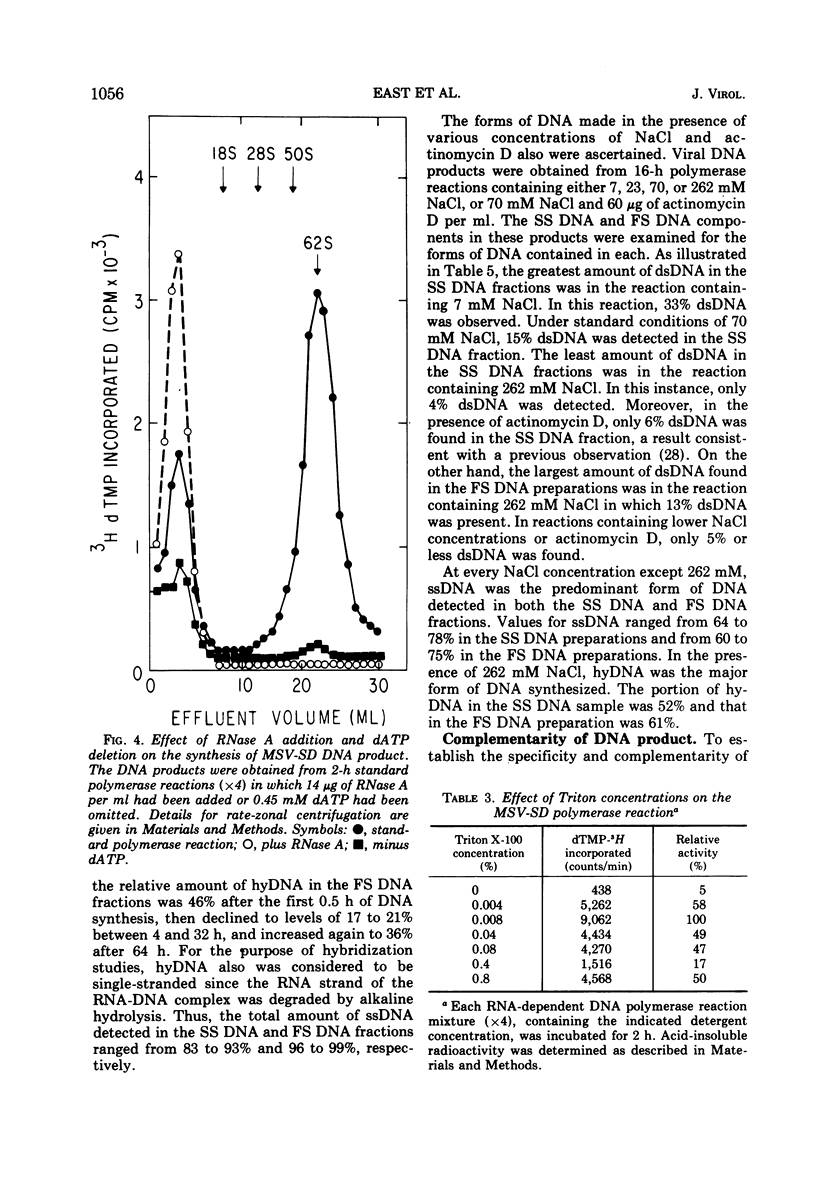
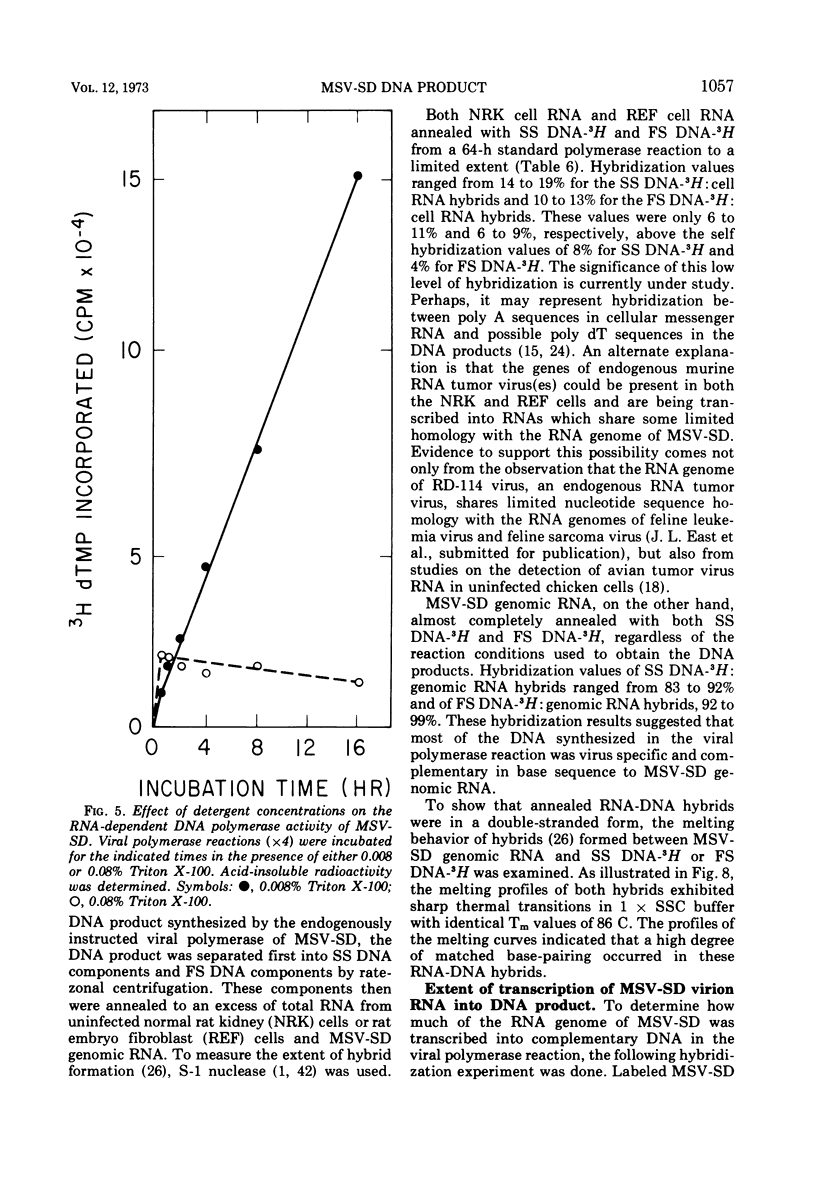
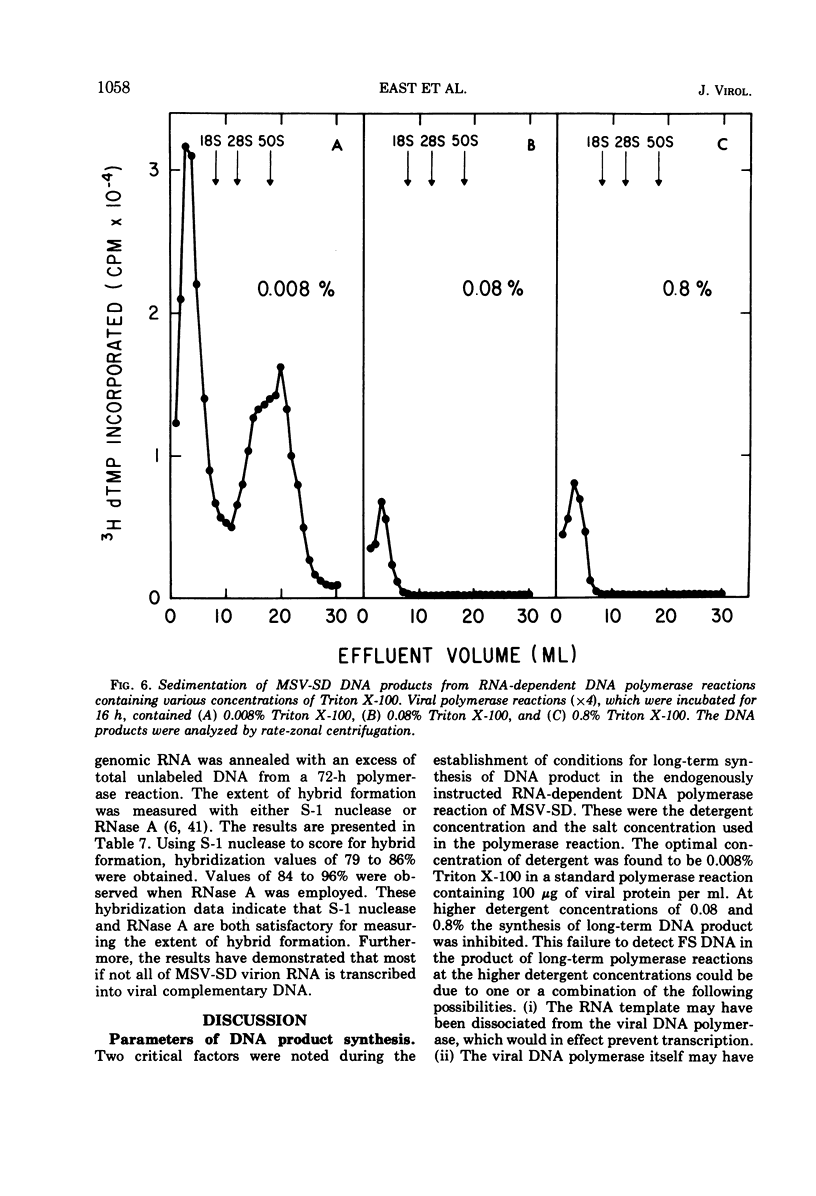
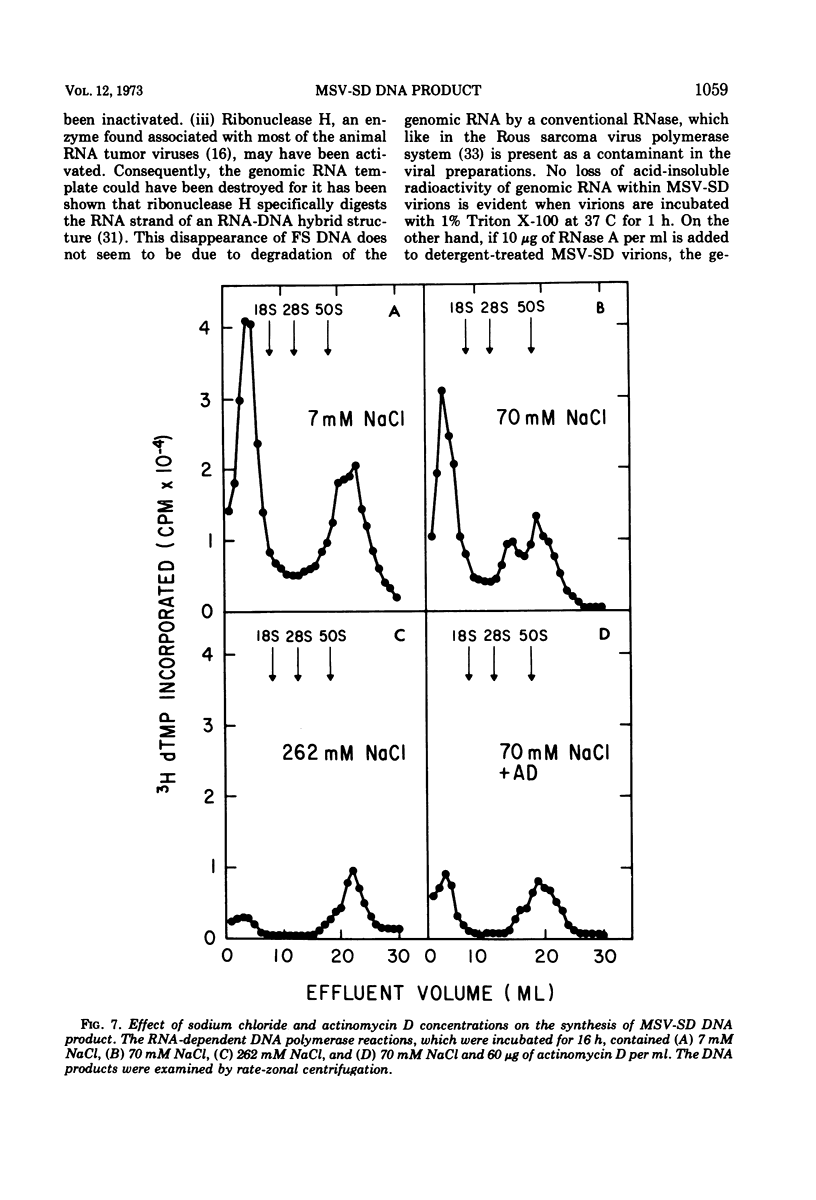
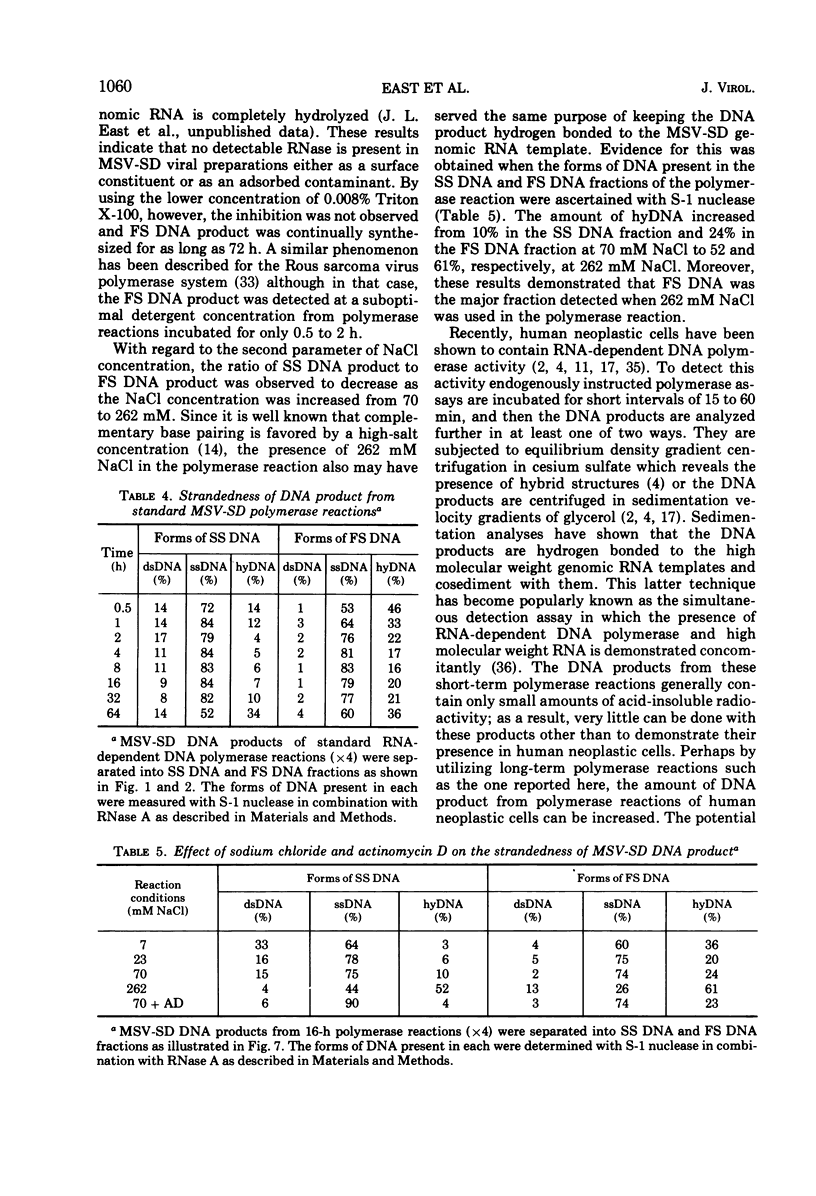
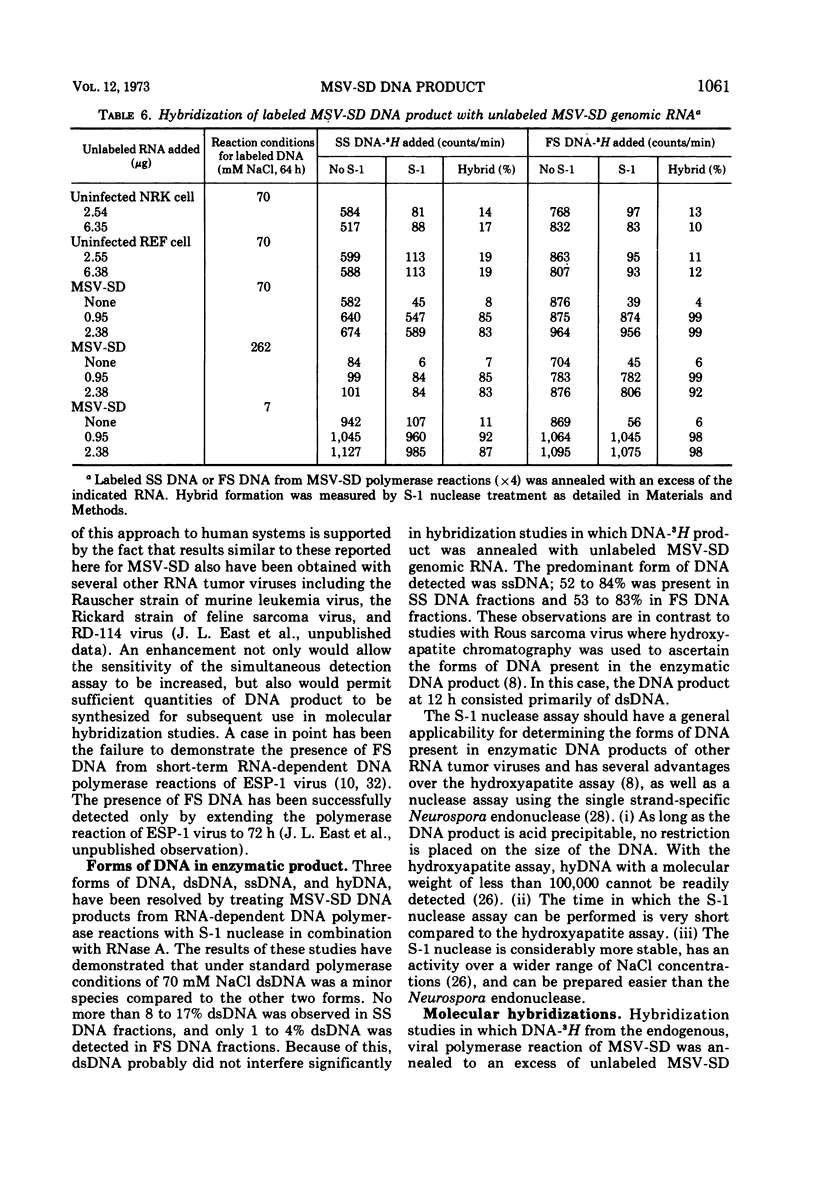
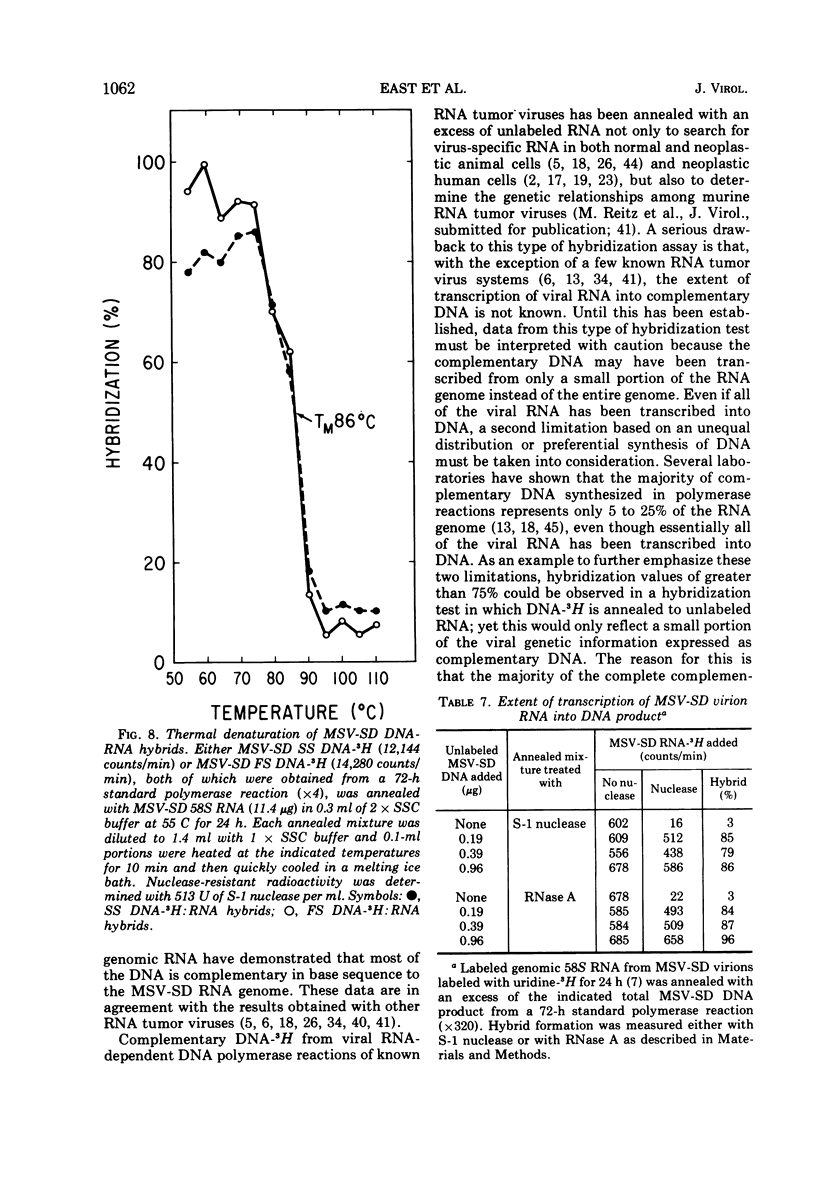
The DNA product of the endogenously instructed RNA-dependent DNA polymerase reaction of murine sarcoma virus continued to be synthesized for as long as 64 h in the presence of 0.008% Triton X-100. Higher detergent concentrations and actinomycin D inhibited DNA product synthesis. The DNA product from long-term polymerase reactions consisted of small DNA fragments as shown by sedimentation in alkaline sucrose gradients. The enzymatic DNA product was separated into a slow sedimenting fraction and a fast sedimenting fraction by rate-zonal centrifugation. Fast sedimenting DNA was the predominant fraction made in viral polymerase reactions containing 262 mM NaCl. By using a combination of S-1 nuclease and pancreatic RNase A, the amount of single-stranded DNA, double-stranded DNA, and DNA-RNA hybrid present in the slow-sedimenting and fast-sedimenting fractions was determined. Under standard polymerase conditions of 70 mM NaCl, single-stranded DNA was the major form of DNA found in both fractions. In contrast, the prevalent form of DNA made in the presence of 262 mM NaCl was DNA-RNA hybrid. Hybridization studies in which either S-1 nuclease or pancreatic RNase A was used to measure hybrid formation demonstrated not only that the DNA product was complementary in base sequence to the RNA genome, but also that at least 79 to 84% of the RNA genome was transcribed into complementary DNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Axel R., Gulati S. C., Spiegelman S. Particles containing RNA-instructed DNA polymerase and virus-related RNA in human breast cancers. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3133–3137. doi: 10.1073/pnas.69.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baxt W., Hehlmann R., Spiegelman S. Human leukaemic cells contain reverse transcriptase associated with a high molecular weight virus-related RNA. Nat New Biol. 1972 Nov 15;240(98):72–75. doi: 10.1038/newbio240072a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Canaani E. Complementarity between Rous sarcoma virus (RSV) RNA and the in vitro-synthesized DNA of the virus-associated DNA polymerase. Virology. 1970 Nov;42(3):783–788. doi: 10.1016/0042-6822(70)90325-9. [DOI] [PubMed] [Google Scholar]
- East J. L., Allen P. T., Knesek J. E., Chan J. C., Bowen J. M., Dmochowski L. Structural rearrangement and subunit composition of RNA from released Soehner-Dmochowski murine sarcoma virions. J Virol. 1973 May;11(5):709–720. doi: 10.1128/jvi.11.5.709-720.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanshier L., Garapin A. C., McDonnell J., Faras A., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase associated with avian tumor viruses: secondary structure of the deoxyribonucleic acid product. J Virol. 1971 Jan;7(1):77–86. doi: 10.1128/jvi.7.1.77-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Sarin P. S., Allen P. T., Newton W. A., Priori E. S., Bowen J. M., Dmochowski L. Reverse transcriptase in type C virus particles of human origin. Nat New Biol. 1971 Aug 4;232(31):140–142. doi: 10.1038/newbio232140a0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Yang S. S., Ting R. C. RNA dependent DNA polymerase of human acute leukaemic cells. Nature. 1970 Dec 5;228(5275):927–929. doi: 10.1038/228927a0. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., McDonnell J. P., Levinson W., Quintrell N., Fanshier L., Bishop J. M. Deoxyribonucleic acid polymerase associated with Rous sarcoma virus and avian myeloblastosis virus: properties of the enzyme and its product. J Virol. 1970 Nov;6(5):589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Aaronson S. A., Martin M. A. Heterogeneity of murine leukemia virus in vitro DNA; detection of viral DNA in mammalian cells. Science. 1971 Jun 25;172(3990):1353–1355. doi: 10.1126/science.172.3990.1353. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. Ribonuclease H: a ubiquitous activity in virions of ribonucleic acid tumor viruses. J Virol. 1972 Dec;10(6):1136–1142. doi: 10.1128/jvi.10.6.1136-1142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati S. C., Axel R., Spiegelman S. Detection of RNA-instructed DNA polymerase and high molecular weight RNA in malignant tissue. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2020–2024. doi: 10.1073/pnas.69.8.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlmann R., Kufe D., Spiegelman S. Viral-related RNA in Hodgkins' disease and other human lymphomas. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1727–1731. doi: 10.1073/pnas.69.7.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. I. Isolation and preliminary characterization of RNA from virus particles. J Mol Biol. 1966 Jun;18(1):195–203. doi: 10.1016/s0022-2836(66)80085-2. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Kufe D., Hehlmann R., Spiegelman S. Human sarcomas contain RNA related to the RNA of a mouse leukemia virus. Science. 1972 Jan 14;175(4018):182–185. doi: 10.1126/science.175.4018.182. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell J. P., Garapin A. C., Levinson W. E., Quintrell N., Fanshier L., Bishop J. M. DNA polymerases of Rous sarcoma virus: delineation of two reactions with actinomycin. Nature. 1970 Oct 31;228(5270):433–435. doi: 10.1038/228433a0. [DOI] [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Priori E. S., Dmochowski L., Myers B., Wilbur J. R. Constant production of type C virus particles in a continuous tissue culture derived from pleural effusion cells of a lymphoma patient. Nat New Biol. 1971 Jul 14;232(28):61–62. doi: 10.1038/newbio232061a0. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Fanshier L., Evans B., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase(s) of Rous sarcoma virus: effects of virion-associated endonuclease on the enzymatic product. J Virol. 1971 Jul;8(1):17–27. doi: 10.1128/jvi.8.1.17-27.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokutanda M., Rokutanda H., Green M., Fujinaga K., Ray R. K., Gurgo C. Formation of viral RNA-DNA hybrid molecules by the DNA polymerase of sarcoma-leukaemia viruses. Nature. 1970 Sep 5;227(5262):1026–1028. doi: 10.1038/2271026a0. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Shishido K., Ando T. Estimation of the double-helical content in various single-stranded nucleic acids by treatment with a single strand-specific nuclease. Biochim Biophys Acta. 1972 Dec 22;287(3):477–484. doi: 10.1016/0005-2787(72)90292-4. [DOI] [PubMed] [Google Scholar]
- Soehner R. L., Dmochowski L. Induction of bone tumours in rats and hamsters with murine sarcoma virus and their cell-free transmission. Nature. 1969 Oct 11;224(5215):191–192. doi: 10.1038/224191a0. [DOI] [PubMed] [Google Scholar]
- Soehner R. L., Fujinaga S., Dmochowski L. Neoplastic bone lesions induced in rats and hamsters by Moloney and Harvey murine sarcoma viruses. Bibl Haematol. 1970;(36):593–599. doi: 10.1159/000391757. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Murine sarcoma and leukemia viruses: genetic differences determined by RNA-DNA hybridization. Virology. 1971 Nov;46(2):480–484. doi: 10.1016/0042-6822(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Levinson W. E., Bishop J. M. Extent of transcription by the RNA-dependent DNA polymerase of Rous sarcoma virus. Nat New Biol. 1971 Sep 1;233(35):19–21. doi: 10.1038/newbio233019a0. [DOI] [PubMed] [Google Scholar]



