Abstract
Simulating ventilator-induced lung injury (VILI) in the laboratory requires stretching of lung alveolar tissue. Whereas precision-cut lung slices (PCLSs) are widely used for studying paracrine signaling pathways in the lungs, their use in stretch studies is very limited because of the technical challenge of fixing them to a stretchable substrate, stretching them uniformly, or holding them in a stretch device without causing rupture. We describe a novel method for attaching PCLSs to silicone membranes by stitching them together in a star-shaped pattern. Using a device that was previously designed in our laboratory for stretching primary alveolar epithelial cell monolayers, we demonstrate that in the central region of the PCLSs stretch is uniform, equibiaxial, and, after a short preconditioning period, also reproducible. The stitched and stretched PCLSs showed equal or better viability outcomes after 60 min of cyclic stretch at different magnitudes of physiological stretch compared with primary pulmonary alveolar epithelial cell monolayers. Preparing and stitching the PCLSs before stretch is relatively easy to perform, yields repeatable outcomes, and can be used with tissue from any species. Together with the ensuring uniform and equibiaxial stretch, the proposed methods provide an optimal model for VILI studies with PCLSs.
Keywords: lung injury, mechanical deformation, PCLS, pulmonary mechanics, tissue slices
ventilator-induced lung injury (VILI) is associated with overinflation of the lungs and overstretching of the alveoli walls (16), leading to increased permeability of the alveolar epithelial barrier, which can severely compromise gas exchange by forming or worsening of edema in the alveolar air spaces (14, 19). Studies that simulate the mechanical stretch of the alveolar wall during VILI utilize different models ranging from pulmonary alveolar epithelial type II cell lines (3, 29), primary alveolar epithelial type I and II cells (1, 2, 15), excised intact lungs (5, 6), and living animals (28, 32). Although VILI studies with stretched monolayers of cells or ventilated animals can be complicated, stretching precision-cut lung slices (PCLSs) presents additional technical challenges because of the need to fix the slices to a stretchable substrate or hold them in a way that allows deformation without rupture.
There is a growing interest in using PCLSs as an in vitro model that bridges between pulmonary cells and the intact lung (27), and unstretched PCLSs have been in wide use (4, 8, 26). However, only a limited number of studies stretched PCLSs, clamping them onto a thin and flexible polydimethylsiloxane (PDMS) membrane and inflating them (12), or mounting them on a gel under a silicone mesh and depressing the mesh-PCLS complex into the gel using a circular indenter (20), both resulting in a nonuniform deformation over the surface of the PCLS. Other methods for attaching precision-cut slices to stretchable substrates have been previously used in studies with other tissues. For example, a mixture of poly-d-lysine and laminin in water was used to adhere organotypic brain hippocampal slice cultures to flexible silicone membranes (23). From our own experience as well as from others, attempts to fix or glue PCLSs to silicone membranes using various adhesion materials are unsuccessful (12), probably because the majority of the PCLS cut surface is composed of air spaces, which offer no purchase for adhesion.
Unstretched PCLSs have already been defined as an excellent model for studying airway and pulmonary vascular smooth muscle responses (10, 25). However, there are also several clear advantages to studying VILI in PCLSs over other experimental platforms. First, unlike cell monolayers, PCLSs contain the natural composition and arrangement of the alveolar epithelial, endothelial, mesenchymal, and parenchymal cells, providing a bridge between idealized cell monoculture exposed to a uniform stretch stimulus and intact animals with a heterogeneous lung environment where systemic responses may mask the measured signals. Second, the PCLSs contain the natural extracellular matrix of the alveolar epithelial cells and thus do not require coating of the stretchable membrane with matrix proteins to enhance cell adhesion (31). Third, stretch responses can be compared across species (12). Additionally, the PCLSs are relatively easy to prepare and can stay viable in culture for at least 6 days, maintaining the epithelial cell phenotype. In this paper, we present a novel method for stretching PCLSs in a uniform and equibiaxial manner by stitching them to silicone membranes using silk suture and using a stretch device that has been previously designed in our laboratory (30).
MATERIALS AND METHODS
Preparation of rat PCLSs.
PCLSs were prepared from rat lungs (N = 12 rats) as previously described by others (9, 12), with slight modifications. Briefly, after injection of pentobarbital to male Sprague-Dawley rats (at 65 mg/kg ip), the trachea was cannulated and the animals were mechanically ventilated. Rats were exsanguinated by cutting the inferior vena cava, and the lungs were subsequently perfused via the pulmonary artery to remove blood. The animal protocols used in this study were reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
After the lungs were excised, they were rinsed twice via the cannula with physiological saline containing 3 mM NaPO4, 6 mM KCl, 2 mM CaCl2, 1.5 mM MgSO4, 1% Pen-Strep, and 12 mM HEPES, and thereafter the liquid was drained. The lungs were then filled through the cannula with a 2% wt/vol low-melting-point agarose solution (5 ml per 100 g body wt, SeaPlaque, Rockland, ME) and kept on ice for 20 min to facilitate cutting thin sections. Cylindrical pieces were cut from the lung lobes with a 15-mm diameter corer while care was taken to exclude major airways and blood vessels. Subsequently, 250-μm-thick PCLSs were sliced in ice-cold PBS by use of a Leica VT 1000S tissue slicer. Typical yield from a pair of rat lungs was five cores and 6–10 slices per core. The PCLSs were then incubated floating in serum-free minimal essential medium (MEM) supplemented with 0.5 μl/ml gentamicin and 1 μl/ml amphotericin B and kept in a CO2 incubator at 37°C. To remove residual agarose and cellular debris, the medium was changed every 30 min for first 2 h, every 1 h for the next 2 h, and every 24 h thereafter.
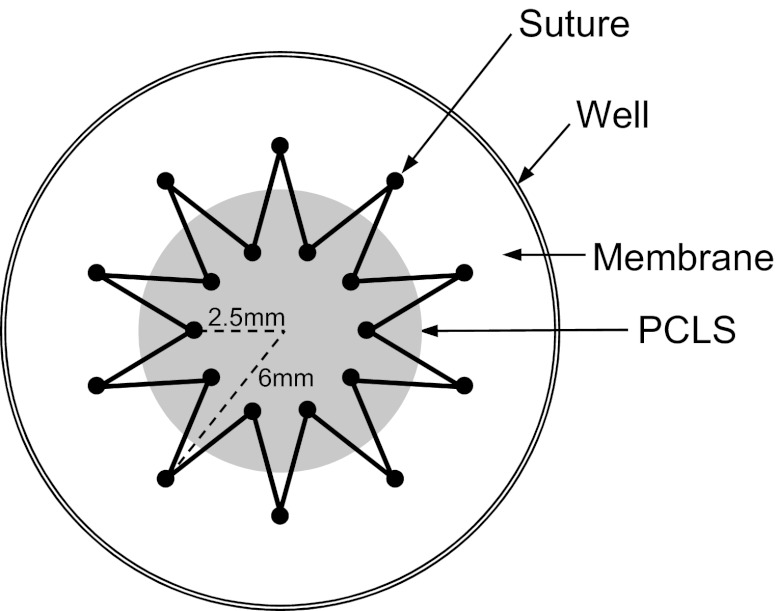
Immediately prior to stretch experiments, the PCLSs were stitched to flexible Silastic membranes (Specialty Manufacturing, Saginaw, MI) mounted in custom-designed wells (30), by using a 5-0 nonabsorbable silk suture (Surgik LC, Broken Arrow, OK), in a star-shaped pattern (Fig. 1), with an inner diameter of 5 mm and an outer diameter of 12 mm (a total number of 20 points at which the suture crosses the membrane and the PCLS). All the measurements were obtained in the 5-mm diameter central region of the PCLS.
Fig. 1.
Top-view drawing of the star-shaped pattern stitched precision-cut lung slices (PCLS) onto the Silastic membrane in the custom-designed well. Solid black circles indicate the points where the suture crosses the membrane and the PCLS.
Phenotype characterization.
To characterize the stability of the alveolar type I and type II cell phenotype in the PCLSs over time, we immunostained 30 PCLSs from 7 different rats with specific markers on different culture days. We used antibodies for RTI40 (a generous gift from Dr. L. Dobbs, University of California San Francisco, San Francisco, CA) and aquaporin-5 (AQP5, Santa Cruz Biotechnology, Santa Cruz, CA) as type I cell markers, at a 1:500 dilution. The type II cells were labeled with pro-surfactant protein C (pro-SPC, Millipore, Billerica, MA) antibody at a 1:200 dilution. Briefly, the PCLSs were fixed in 4% paraformaldehyde for 20 min and treated with 1 mg/ml sodium borohydride in phosphate-buffered saline (PBS) to reduce endogenous fluorescence. Nonspecific binding was blocked with normal goat serum followed by incubation with primary antibodies for type I cells for 1 h at room temperature. The PCLSs were subsequently treated with Triton X-100 to permeabilize the cell membrane and allow the pro-SPC primary antibody to enter the type II cells. The PCLSs were incubated with the pro-SPC antibody for 1 h at room temperature. After thorough washings, fluorescently tagged secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were added for 2 h at room temperature. At least three regions per PCLS were randomly chosen for imaging on a confocal microscope (Leica TCS SP5), and a vertical stack of ten 1-μm images from the midthickness region of the PCLS was captured. The phenotypic markers on different days of culture were assessed qualitatively by observation of the maximal projection from each stack of tissue images.
Measurement of change in alveolar surface area in PCLSs during quasi-static stretch.
To determine the relationship between the Silastic membrane deformation and PCLS deformation, we manually stretched seven stitched PCLSs from five different rats by turning a circular indenter that had been previously designed in our laboratory and that deformed the membrane uniformly and equibiaxially (30). The PCLSs were stretched to 38 or 60% change in membrane surface area (ΔMSA) and back to the resting position for three cycles (and also up to 20 cycles at 38% ΔMSA), and light microscope images were taken after each turn of the well on the indenter. Before capturing an image, we carefully focused the microscope light on specific alveoli in the PCLS with cross sections parallel to the membrane plane that were predetermined for tracking in the beginning of each experiment. Using ImageJ (version 1.45s), we measured the 2D alveolar cross-sectional area (ASA) of two to eight alveoli in each PCLS image and calculated the percent of change in cross-sectional area (ΔASA) for each alveolus, as
| (1) |
where ASAD is the area of an alveolus during membrane stretch and ASAU is the initial area of that same alveolus prior to stretch. Values of %ΔASA were then plotted against the successive clockwise (stretching) and counterclockwise (releasing) indenter turns and also against %ΔMSA values, which were previously calibrated to the indenter displacement (30).
Radial and circumferential strains during quasi-static stretch.
To assess the deformation field, six stitched PCLSs from four different rats were manually stretched by using the indenter described above to 38% ΔMSA and back to resting position for three cycles. Images were taken with a Nikon D70 camera at the resting point and at 38% ΔMSA for each cycle. Multiple distinguishing points (16–24 per PCLS) were tracked on each PCLS (Adobe Photoshop, CS4) and expressed as radial and azimuthal coordinates (r and θ; Fig. 2A). The radial displacement of each point, ur, was calculated as the difference between r at 38% ΔMSA and the initial unstretched r value (r0) and averaged across the three cycles. The radial strain (εrr) for each PCLS was defined as the slope of the ur-r relationship (Eq. 2; Ref. 17). After verifying that the circumferential displacement, uθ, does not change significantly with the angle θ (the slope ∂uθ/∂θ ranges from 0.001 to 0.01, i.e., almost zero), the circumferential strain (εθθ) was calculated by using Eq. 3 (17),
| (2) |
| (3) |
Fig. 2.
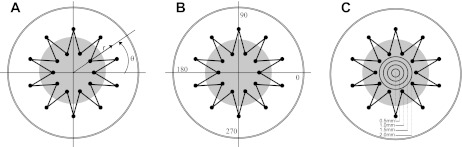
A: a cylindrical coordinate system with an origin in the center of the well was used to measure the radius r and angle θ for each distinguishing point. All the distinguishing points from multiple slices were classified according to their angle (B), i.e., the specific quadrants of the coordinate system that the points lie in, and their radii (C), i.e., the specific concentric ring that the points lie in.
The distinguishing points from all the PCLSs were divided into four quadrants (Fig. 2B) and four concentric circular rings with different radii (Fig. 2C) according to their θ and r coordinates, respectively. Then the radial and circumferential strains were separately plotted against the four different quadrants and four different rings.
Viability after cyclic stretch.
Cell viability was evaluated with and without exposing 22 day 2–3 PCLSs from six different rats to cyclic stretch. The PCLSs were stretched with a stretching device that was previously designed in our laboratory, at a frequency of 0.25 Hz and across a range of relevant physiological magnitudes including 12, 25, and 37% ΔMSA, roughly corresponding to 64, 86, and 100% total lung capacity, respectively, as previously described (30).
After stitching, the PCLSs were incubated in serum-free Dulbecco's minimum essential medium (DMEM) containing 20 mM HEPES for 1 h at 37°C. Subsequently, 0.6 μM calcein AM and 0.28 μM ethidium homodimer-1 (LIVE/DEAD, Molecular Probes, Grand Island, NY) were added to the wells to stain for live and dead cells in the PCLSs, respectively. PCLSs were cyclically stretched for 1 h or remained unstretched. Then at least three regions per PCLS were randomly chosen for imaging on a confocal microscope (Leica TCS SP5), and a vertical stack of ten 1-μm images from the midthickness region of the PCLS was captured.
An image processing algorithm was then implemented in MATLAB (R2010b, version 7.11, The MathWorks, Natick, MA) to semiquantify the percent of dead cells in each PCLS image. For each image, we computed separately the total number of green (calcein AM) and red (ethidium homodimer-1) pixels above background threshold values. Then, assuming the area of live and dead stained cells is approximately the same, we calculated the percent of area of dead cells in each image. To obtain only the dead cells attributable to stretch, we subtracted the average percent of area of dead cells in unstretched preparations from the same study day from the values obtained from images of stretched PCLSs.
Quantification of p65 translocation in type I alveolar epithelial cells in the PCLS.
Quantification of the translocation of the p65 subunit of NF-κB in type I alveolar epithelial cells was based on an image-processing algorithm implemented in MATLAB (R2010b, version 7.11, The MathWorks, Natick, MA) and applied on immunofluorescent images of PCLSs that were triple labeled for p65, AQP5, and DAPI. Images from the AQP5 channel were used to narrow the region of interest to type I cells only. Within this region of interest, images from the DAPI channel were used as masks to generate from each p65 image two images: cytoplasmic p65 image and nucleic p65 image. The sum of pixel values in each image was then computed and divided by the number of nonzero pixels in the image.
Statistical analysis.
All the experiments were performed at least four times, each time with PCLSs from a different animal. Multiple PCLSs were studied from each animal for each test, and in each PCLS multiple alveoli were examined for characterizing the stretching behavior of the slices, as explicitly described in results. All the data presented are expressed as means ± SE, except for the percent of dead cells in Table 1, which are presented as means ± SD.
Table 1.
Comparison between the percent of dead cells in precision-cut lung slices stretched for 60 min at different magnitudes and zero, i.e., unstretched cells, and type I-like alveolar epithelial cell monolayers
| Precision-Cut Lung Slices | Type I-Like Alveolar Epithelial Cell Monolayers | |
|---|---|---|
| 12% ΔMSA | −5.0 ± 3.1*† | 0.7 ± 0.5 |
| 25% ΔMSA | 3.3 ± 7.3 | 3.9 ± 3.4 |
| 37% ΔMSA | 0.9 ± 8.3† | 8.9 ± 3.9 |
Data are presented as means ± SD and calculated as described in materials and methods. Data for cell monolayers were reproduced from Tschumperlin and Margulies, 1998 (21). ΔMSA, change in membrane surface area.
Significantly lower than zero, i.e., unstretched cells (P ≤ 0.01).
Significantly lower than in type I-like alveolar epithelial cells (P ≤ 0.0001).
All the statistical tests were implemented in JMP (version 9.0.0). Statistical significance of differences between two groups was determined by a two-tailed, paired or unpaired (as appropriate) t-test. One sample t-test was used to compare a group of data to a constant value. One-way ANOVA for repeated measures was performed, followed by a Tukey's post hoc test, to determine statistical significance between three or more datasets. Analyses were deemed statistically significant at P ≤ 0.05.
RESULTS
Alveolar epithelial type I and II cells in PCLSs.
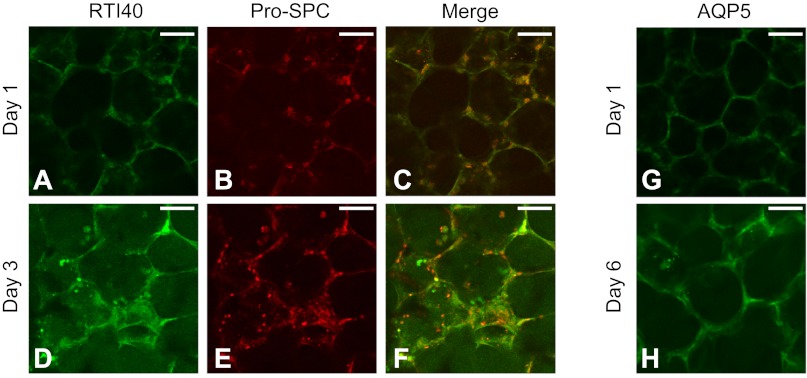
Alveolar epithelial type I and II cells in the PCLSs were immunostained with specific phenotypic markers, i.e., RTI40 and AQP5 for type I cells and pro-SPC for type II cells (Fig. 3). Both types of cells were present in the PCLSs 1 and 3 days after slicing. The type I cells appeared flat and spanned the entire walls of the alveoli, whereas the type II cells appeared cuboidal and were found usually at the junctions between adjacent alveoli. Type I cells in PCLSs were also stained after 6 days in culture using AQP5, and the outcome was very similar to the RTI40 stain.
Fig. 3.
Alveolar epithelial type I and II cells in the PCLSs were immunostained using RTI40 (A and D) and AQP5 (G and H) for type I cells and pro-surfactant protein C (pro-SPC; B and E) for type II cells, after 1 day in culture (A–C and G), 3 days in culture (D–F), and 6 days in culture (H). AQP5 for day 3 PCLSs was very similar to days 1 and 6; thus not shown. Bar = 50 μm.
Relationship between ΔMSA and ΔASA.
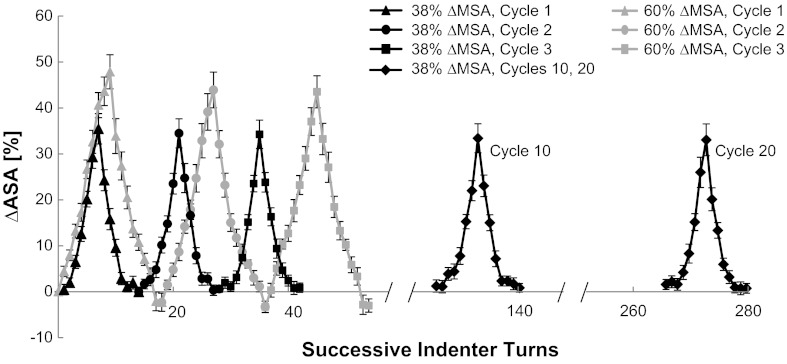
To determine the transient and steady-state relationship between ΔMSA and ΔASA, the stitched PCLSs were stretched with a manual indenter for 20 cycles to a maximal magnitude of 38% ΔMSA and for three cycles to a maximal magnitude of 60% ΔMSA, and images were captured at 8–10 prespecified indentation positions. The percent ΔASA was estimated in every image during the stretch and release cycles and plotted in temporal succession (Fig. 4). For this study, we analyzed data from 31 alveoli in seven PCLSs from five different rats.
Fig. 4.
Change in alveolar surface area (%ΔASA) as a function of successive indenter turns. Data are means ± SE.
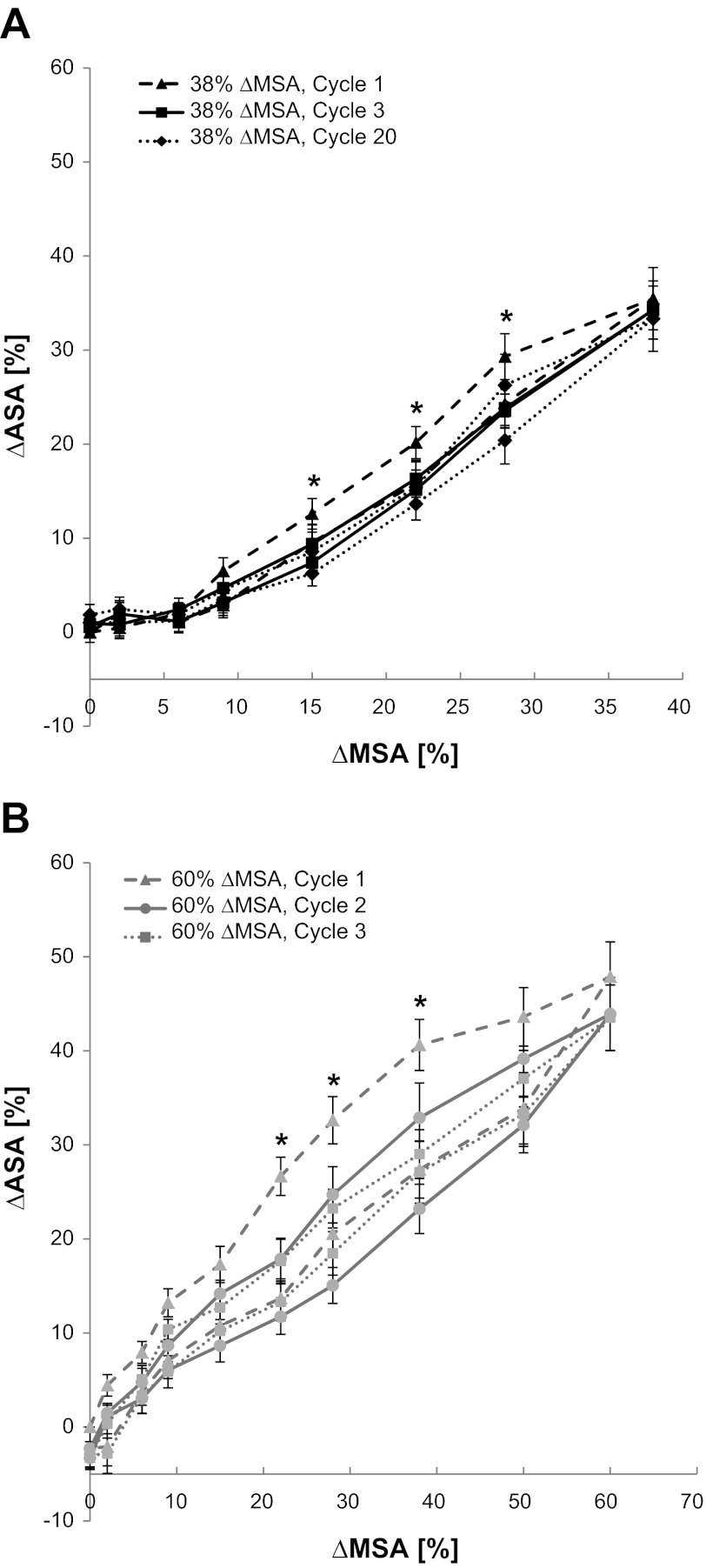
To determine the relationship between the ΔMSA and ΔASA and quantify preconditioning and hysteresis, we examined cycles 1–3, 10, and 20 when stretching to a maximal ΔMSA of 38% (Fig. 5A), and cycles 1–3 when stretching to a maximal ΔMSA of 60% (Fig. 5B). Stretch to 38% ΔMSA yielded maximal %ΔASA values of 35.47 ± 3.31, 34.48 ± 3.16, and 34.26 ± 3.09%, in cycles 1 through 3, respectively. The maximal %ΔASA at cycle 10 was 33.41 ± 3.16% and at cycle 20 it was 33.33 ± 3.47%. These values were not statistically different from 38%, the imposed change in membrane surface area (ΔMSA). At 60% ΔMSA, the maximal values for %ΔASA for cycles 1 through 3 were 47.87 ± 3.72, 43.91 ± 3.88, and 43.52 ± 3.48%, respectively. Although there was no effect of preconditioning at peak stretch, all these values indicate significantly lower PCLS stretch than the Silastic membrane, possibly because of tears of the PCLS near the suture contact points. This partial release of the PCLS from the membrane may also have caused the negative %ΔASA values at the zero %ΔMSA after cycle 1 for these very large stretch magnitudes. Altogether, this suggests that the PCLS stretch should not exceed 38% ΔASA.
Fig. 5.
Percent change in ASA as a function of the change in the Silastic membrane surface area (%ΔMSA) at cycles 1, 3, and 20 when stretching to a maximal ΔMSA of 38% (A), and cycles 1, 2, and 3 when stretching to a maximal ΔMSA of 60% (B). At several specific %ΔMSA, the estimated %ΔASA in cycle 1 was significantly different than in the other cycles. *For the 38% ΔMSA study, at 15% ΔMSA: F(4,72) = 4.0612, P = 0.0058; at 22% ΔMSA: F(4,72) = 5.3925, P = 0.0010; at 28% ΔMSA: F(4,72) = 6.5550, P = 0.0002. For the 60% ΔMSA study, at 22% ΔMSA: F(2,42) = 12.1827, P = 0.0002; at 28% ΔMSA: F(2,42) = 5.3590, P = 0.0107; at 38% ΔMSA: F(2,42) = 9.0500, P = 0.009. Data are means ± SE.
To evaluate the effect of preconditioning in detail, we compared the values of estimated %ΔASA after each successive indenter turn throughout the cycles of stretch, using a one-way ANOVA with repeated measures. At both 38 and 60% ΔMSA, we found several midcycle membrane stretch magnitudes (%ΔMSA) in which the estimated %ΔASA in the first cycle was significantly different than in the other cycles (P ≤ 0.05, see * in Fig. 5, A and B). However, in both experiments, the second cycle was no longer significantly different than the subsequent cycles. Additionally, to evaluate hysteresis, we compared the estimated %ΔASA on the ascending stretch curve to the estimated %ΔASA on the descending stretch curve of each cycle using a paired t-test. In both experiments there were several points in the first and second cycles at which the estimated %ΔASA on the ascending stretch curve was significantly larger than on the descending stretch curve, yet these differences were not present in the third cycle. Overall, these data suggest that starting from the third cycle the PCLS stretch profile reached a steady state at which it deformed steadily in time and presented a nonhysteresis strain behavior.
We further tested the repeatability of the PCLS stretch in eight alveoli in five PCLSs from two different rats that were stretched to 38% ΔMSA for 20 cycles, then remained unstretched for 90 min, and then were stretched again for 20 cycles. The percent ΔASA was estimated during the stretch and release cycles 1, 2, 3, 10, and 20 before and after rest as described above. The average maximal %ΔASA in these cycles was 28.91 ± 0.56 and 27.37 ± 0.63, before and after rest, respectively. Comparing the maximal %ΔASA in these cycles before and after rest by using a paired t-test yielded insignificant differences between the two groups, suggesting the stretching of the PCLS is repeatable at least for a ∼2-h period of time.
Uniform, equibiaxial stretch of the PCLSs.
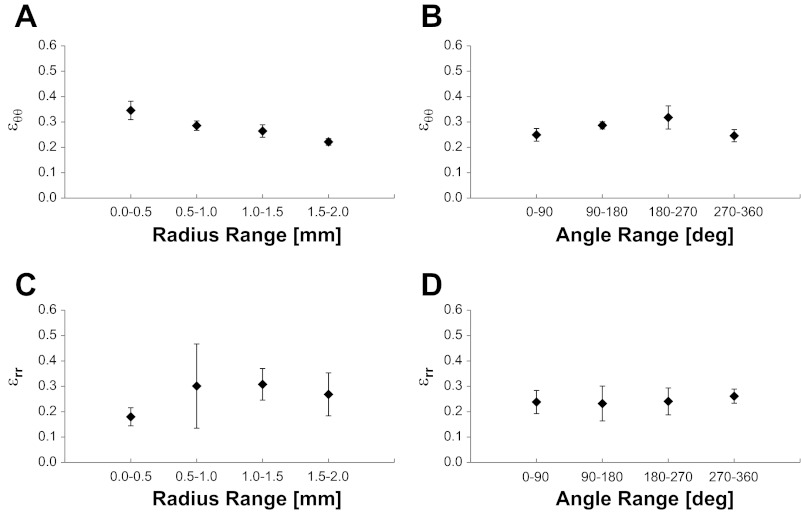
The radial and circumferential strains were calculated at 115 distinguishing points in six PCLSs from four different rats by Eqs. 2 and 3 (Fig. 6). A one-way ANOVA with repeated measures revealed no significant differences in the strains with quadrant [εrr: F(3,16) = 0.0167, P = 0.9969; εθθ: F(3,16) = 0.8940, P = 0.4672] or radius [εrr: F(3,18) = 0.1940, P = 0.8987; εθθ: F(3,18) = 2.9324, P = 0.0742], and we concluded that the PCLS strain is spatially uniform. In addition, in a paired t-test we found that there was no significant difference between the radial and circumferential strains, suggesting that the PCLS stretch is also equibiaxial.
Fig. 6.
Circumferential (εθθ; A and B) and radial (εrr; C and D) strains at multiple distinguishing points on PCLSs that were stretched at 38% ΔMSA for 3 cycles. Data are means ± SE and presented as a function of the specific range of radial (A and C) and quadrant (B and D) location for each distinguishing point.
Viability studies after exposure of the PCLSs to cyclic stretch.
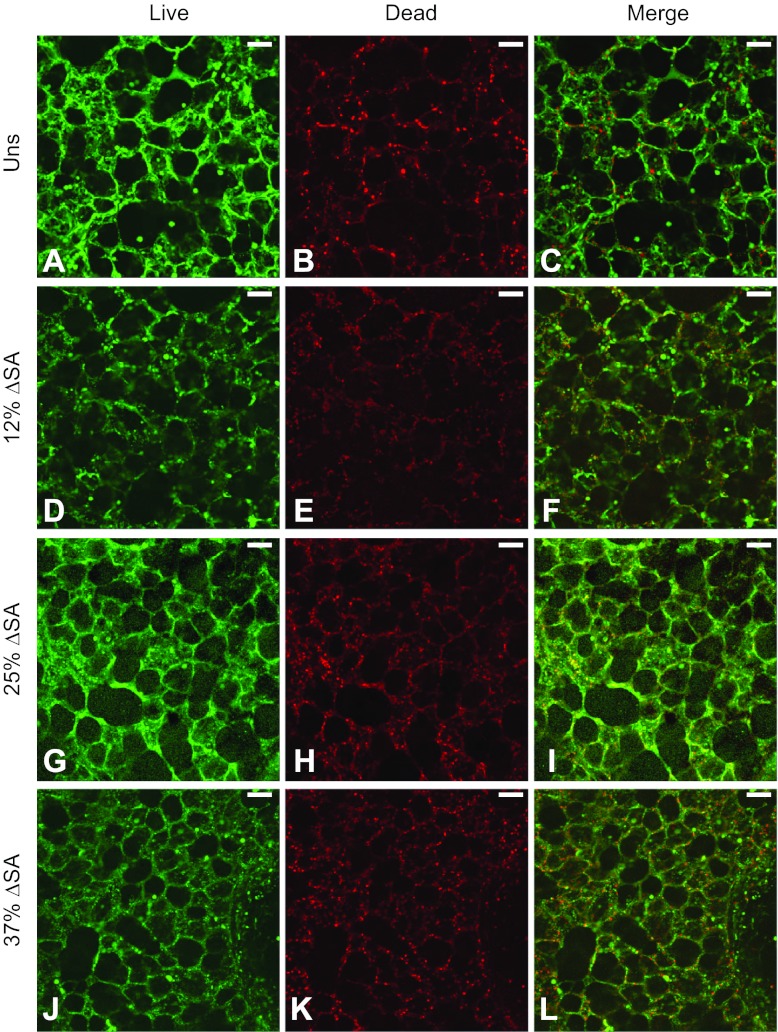
To evaluate cell viability, we cyclically stretched rat PCLSs for 60 min at a frequency of 0.25 Hz at magnitudes of 12, 25, and 37% ΔMSA. Viability was assessed in 211 images taken at different randomly chosen locations in 22 PCLSs from six different rats by a semiquantitative image processing algorithm, as described in materials and methods. The percent of dead cells was not significantly different from zero for PCLSs stretched at 25 or 37% (Table 1, Fig. 7), but we found significantly fewer dead cells in PCLSs that were stretched at 12% ΔMSA than in unstretched PCLSs. We then compared the percent of dead cells in the stretched PCLSs to our previously published data in type I-like alveolar epithelial cells (30), which line more than 95% of the alveolar wall surface area (11). We found that at both 12 and 37% ΔMSA, the percent of dead cells was significantly lower in the PCLSs than in the type I-like cell monolayers, and at 25% ΔMSA the difference was not statistically significant (Table 1). Altogether, these data suggest that the rat PCLSs stay viable during physiological stretch, showing even better viability than cell monolayers at 12 and 37% ΔMSA. These outcomes might originate from different regulation of cell death in the PCLSs because of better adhesion of the cells to the natural extracellular matrix in the PCLSs.
Fig. 7.
Representative live/dead stain images for day 2–3 rat PCLSs that remained unstretched (A–C) or stretched at 12% ΔMSA (D–F), 25% ΔMSA (G–I), and 37% ΔMSA (J–L). Bar = 50 μm.
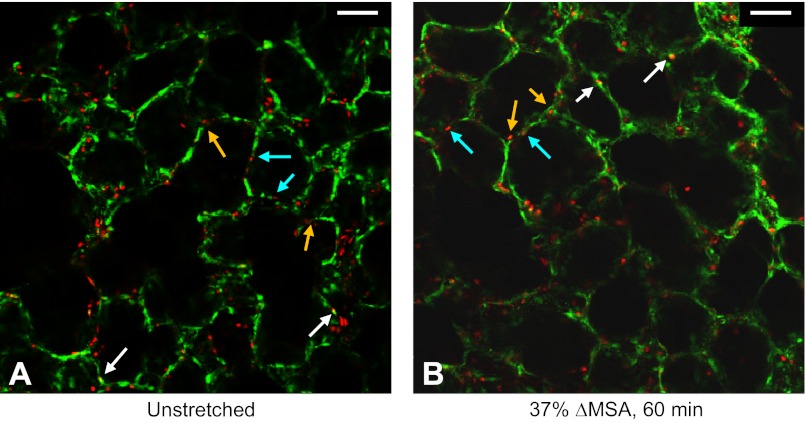
We qualitatively examined cell death in distinctive locations in the alveoli, which may be indicative of cell type-specific association with cell death, in 48 alveoli in five PCLSs from two different rats that were stretched at 37% ΔMSA for 60 min. By observing the stained live vs. dead cells in each alveolus, we categorized each cell based on its location and counted the dead cells in each category per alveolus. Cells were classified as being located on the alveolar wall, far from junctions between adjacent alveoli, which is the typical location of type I cells, or as being located in junctions between adjacent alveoli, as typically seen for type II cells (for example, see light blue and orange arrows pointing to these two distinctive locations, respectively, in Fig. 8). When it was impossible to determine the cell-specific location, no score was added to any of the groups. The number of dead cells in the two groups did not significantly differ, i.e., the average number of dead cells per alveolus was 1.89 ± 0.18 for cells far from alveolar junctions, and 2.26 ± 0.20 for cells at alveolar junctions, suggesting similar sensitivity of different epithelial cell populations to injury.
Fig. 8.
Representative live/dead stain images for day 2–3 rat PCLSs that remained unstretched (A) or stretched for 60 min at 37% ΔMSA (B). In these preparations, we added the dead cell probe ethidium homodimer-1 immediately before stretch, then stretched the PCLSs, and 1 h after stretch we added the live cell probe calcein AM to the wells. White arrows point to double stained cells. Light blue and orange arrows point to distinctive locations of alveolar epithelial cells, which may be indicative of type I and type II cells, respectively. Bar = 50 μm.
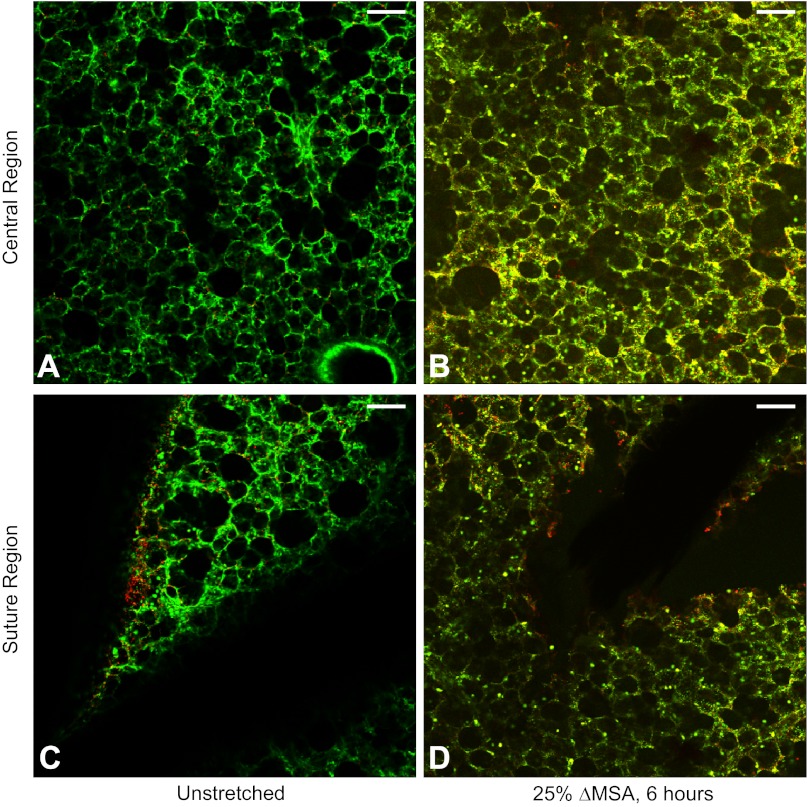
Next, we tested the feasibility of using the stitched PCLS model in chronic stretch experiments. Using 11 additional PCLSs from two different rats, we show qualitatively that cells in the PCLSs remained generally viable even after 6 h of stretch at 25% ΔMSA (Fig. 9B). Notably, the amount of dead cells in PCLSs that remained unstretched yet stitched for 6 h was negligible (Fig. 9A). In addition, stitching four slices from two different rats onto the membrane and keeping them stitched for 24 h resulted in just a slight increase in cell death (data not shown), compared with PCLSs that were stretched for 1–6 h (Figs. 7, A–C, and 9A).
Fig. 9.
Representative live/dead stain images for day 2–3 rat PCLSs that remained stitched and unstretched for 6 h (A and C) or stretched for 6 h at 25% ΔMSA (B and D). A and B: images captured in the 5-mm-diameter central region of the PCLS. C and D: images captured in the peripheral suture region of the PCLS. The silk suture appears dark in C and D. Bar = 50 μm.
To test the amount of cell injury due to stitching of the PCLSs, we performed viability studies on eight PCLSs from three different rats that were stretched at 25% ΔMSA for 6 h or remained unstretched, by imaging the contact regions of the suture with the slice (Fig. 9, C and D). In regions very close to the suture, we observed more dead cells than in the central region of the PCLS (Fig. 9, C and D vs. 9, A and B); however, this was very local and did not affect cells that were even slightly further away from the suture. Because the amount of cell death near the suture was very mild, we do not expect it to have a significant effect on the general cell viability in the PCLSs or on other responses to stretch.
p65 translocation in type I alveolar epithelial cells.
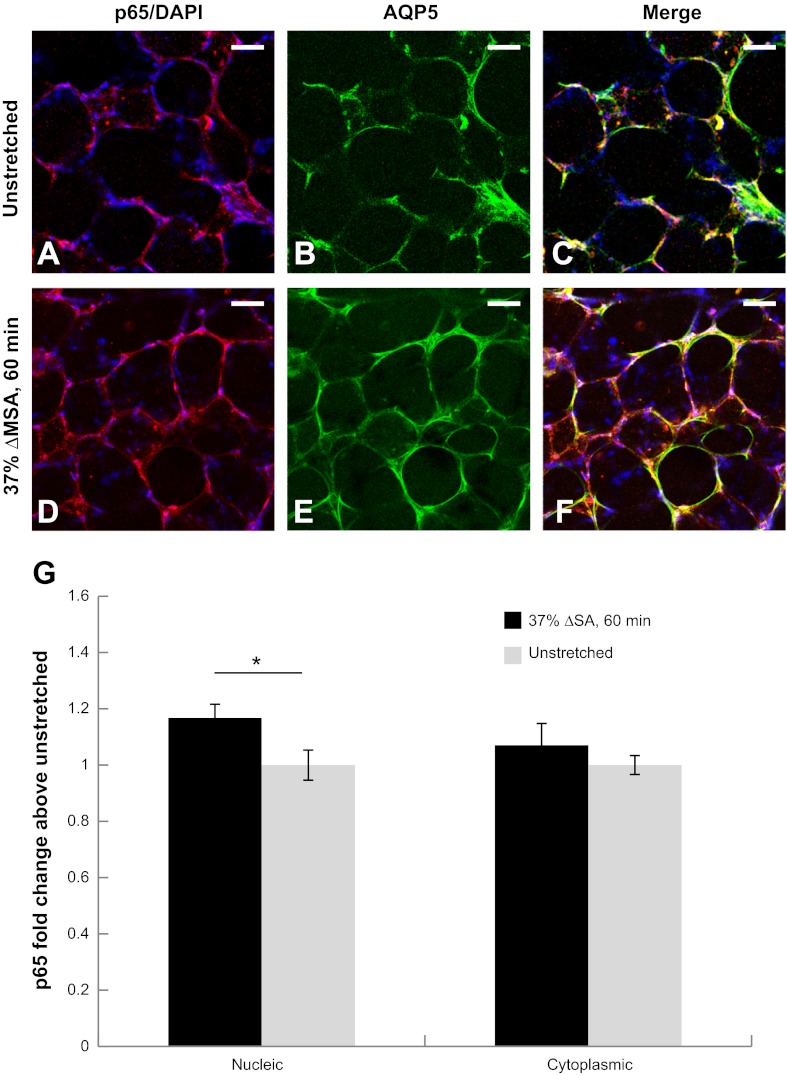
We quantified stretch-induced translocation of the p65 subunit of NF-κB from the cell cytoplasm to the nucleus in AQP5-labeled cells, i.e., type I alveolar epithelial cells, in the PCLSs. In PCLSs that were stretched at 37% ΔMSA for 60 min, nuclear p65 was significantly higher than the cytoplasmic p65, whereas in unstretched PCLSs we did not find a significant difference between the two cell domains (Fig. 10).
Fig. 10.
Translocation of the p65 subunit of NF-κB due to stretch. Representative images of p65 (red) and DAPI (blue; A and D); and the type I alveolar epithelial cell marker AQP5 (green) staining (B and E) in unstretched PCLSs (A–C) and PCLSs stretched for 60 min at 37% ΔMSA (D–F). G: quantification of nuclear and cytoplasmic p65 staining in unstretched and stretched PCLSs as described in materials and methods. Data are expressed as means ± SE of fold change above unstretched cells (*P < 0.05 vs. unstretched). Bar = 50 μm.
DISCUSSION
In the present study, PCLSs were prepared from rat lungs and were kept floating in culture medium 2–3 days prior to stretch experiments. During this time, the PCLSs stayed viable (Fig. 7, A–C) and maintained the type I and type II alveolar epithelial cell phenotypes (Fig. 3). Thereafter, the PCLSs were fastened to a flexible Silastic membrane with silk suture in a star-shaped pattern (Fig. 1) and were stretched quasi-statically with a manual indenter for characterizing the stretch profile. We found that the PCLSs stretch uniformly and equibiaxially (Fig. 6) and remain viable after 60 min of cyclic stretch at magnitudes of 12, 25, and 37% ΔMSA (Fig. 7, Table 1) and after 6 h at 25% ΔMSA (Fig. 9, A and B). In addition, testing cell viability near the suture contact points revealed only mild and local cell death (Fig. 9, C and D) that is not expected to generate significant toxicity to affect the general cell responses in the PCLS.
Although we have previously showed that cultured type II alveolar epithelial cells are more prone to cell death under stretch conditions compared with cultured type I-like cells (30), when we cultured the type II cells on type I-like cell extracellular matrix, stretch-induced cell death was significantly reduced compared with type II cell cultures seeded on fibronectin and was not different than in type I-like cell cultures (24). Here we show that in the PCLS, where the extracellular matrix is native, cell death is not associated with specific epithelial cell location (Fig. 8), suggesting that both type I and type II alveolar epithelial cells are similarly sensitive to stretch-induced cell death. Comparison with the level of cell death after stretch in type I-like cultures revealed equal or better viability in the PCLSs (Table 1). This can result from better adhesion of the cells to the native extracellular matrix, paracrine regulation of cell viability and survival by the different cell types in the PCLS, or by the natural geometrical orientation of the cells in the PCLS. However, the amount of cell death in stretched type I-like cell cultures is still very low (Table 1), and the better viability found here does not challenge the validity of that model.
In addition, to ensure that the ethidium homodimer-1 (dead cell probe)-stained cells are indeed dead and not stained because of a transient stretch-induced increase in the cell membrane permeability to the probe, we tested the presence of double-stained cells in 14 PCLSs from two different rats. In these preparations, we added the ethidium homodimer-1 immediately before stretch, then stretched the PCLSs at 37% ΔMSA for 60 min, and 1 h after stretch we added the calcein AM (live cell probe) to the wells. We added the probes at the exact same time course to unstretched PCLSs for comparison. Images from three different fields were examined and no difference was found between stretched and unstretched PCLSs, with only two to three cells per 500 μm × 500 μm field found to be double stained (white arrows in Fig. 8, A and B). This suggests that the ethidium homodimer-1 stain is reliable under stretch conditions, as has also been shown in alveolar epithelial cell monolayers (30).
Up to 38% ΔMSA the PCLS stretch was reproducible after the first cycle and indistinguishable from the membrane stretch (Figs. 4 and 5). However, at higher stretch magnitudes, the maximal estimated ΔASA was significantly lower than the applied ΔMSA (60%), likely because of local tears of the tissue at the contact points with the suture, which loosen the tissue from the membrane. In addition, stretch was reproducible even after 90 min of rest between stretch experiments at 38% ΔMSA. Finally, the radial and circumferential strain magnitudes in the quasi-statically stretched PCLSs (Fig. 6) were very similar to previously estimated strains in cell monolayers, which were calculated by a similar technique (30). These strain magnitudes, ranging from 23 to 32%, were correlated to breathing or ventilating at 80–90% total lung capacity, which is at the higher end of breathing efforts, found usually only at deep sighs. We propose that, taken together, the stretched PCLS model can be used to simulate the physiological range of alveolar inflation.
As an example of application, and as an effort to better understand the signaling pathways that are activated by stretch in specific types of cells in the lung, we quantified stretch-induced translocation of the p65 subunit of NF-κB from the cell cytoplasm to the nucleus in type I alveolar epithelial cells in the PCLSs by labeling them with AQP5 and applying image analysis methods on the labeled cells exclusively. The data showed higher nuclear p65 in PCLSs that were stretched at 37% ΔMSA for 60 min compared with the cytoplasmic p65, whereas no difference was found in unstretched PCLSs (Fig. 10). These results agree with data from type I-like alveolar epithelial cell monolayers that were stretched at the same magnitude and duration (21). In a recent study, we have linked NF-κB activation by stretch to increases in the epithelial barrier permeability in type I-like cell monolayers (13). Future studies with the PCLS-stretch model may explore NF-κB activation in other types of lung cells, e.g., type II alveolar epithelial cells, endothelial cells, etc. In addition, the model can be used to study pharmacological treatments to prevent stretch-induced increases in permeability that characterize VILI, by targeting different signaling pathways in a specific type of cells and testing their effects on other cell populations in the lung. Although measuring permeability directly in the PCLSs is difficult without using a flexible porous membrane as a substrate, it is possible to quantify changes that indirectly correlate with changes in permeability, such as the distribution and content of tight junctional proteins, e.g., occludin.
The focus of this study was to develop a platform for VILI studies with lung alveolar tissue by stretching PCLSs equibiaxially and uniformly. A number of other studies have stretched PCLSs of similar and greater thickness. For example, Lavoie et al. (20) stretched human PCLSs nonuniformly by depressing a silicone mesh-PCLS complex into a polyacrylamide gel to simulate breathing conditions and looked at airway constriction with and without exposure to acetylcholine. In the present study, to eliminate tissue stress by active contracting elements (i.e., airway smooth muscle cells), we intentionally avoided having large airways in the PCLSs that we chose to study. Dassow et al. (12) clamped PCLSs onto a PDMS membrane and inflated this complex to generate stretch, which resulted in a nonuniform and anisotropic stretch in two directions. Although these methods bypass stitching-related issues (see limitations below), they do not produce a uniform deformation of the PCLS, which prevents comparison of measurements performed grossly in different regions of a single PCLS. The goal in the study of Lavoie et al. was to study individual airways where one may assume that the specific airway deformation is uniform in a very local scale. However, to study whole-tissue response or perform measurements in multiple fields in the PCLS, this assumption does not hold, limiting the use of this method to small regions.
There are several interesting mechanical aspects to consider in the stretched PCLS model. First, it should be noted that because the stiffness of the basement membrane in the PCLS, i.e., 2–30 mPa (33), is in the same order of magnitude as the stiffness of the Silastic membrane, i.e., 2.7 ± 0.09 MPa (measured in 12 samples, at a pulling rate of 12 in./s by use of a custom-designed materials testing machine, Ref. 7), the mechanical response of the PCLS to stretch depends on the mechanical properties of the tissue and not on the Silastic membrane mechanical properties. In addition, we compared the average diameter of stitched, unstretched PCLSs to the diameters of alveoli reported by others for rat lungs (22). Mercer et al. (22) fixed rat lungs approximately at functional residual volume and found an average alveolar diameter of 72.7 or 91.8 μm (mouth-opening or caliper-opening diameters, respectively) and they also found that the general shape of most alveoli was spherical. We measured the alveolar cross-sectional area of eight alveoli using ImageJ and (assuming spherical shape) the average diameter of stitched unstretched alveoli was calculated to be 74.8 ± 3.9 μm, in very good agreement with Mercer et al. These measurements suggest that the stitched PCLSs are not prestretched beyond the stretch that occurs at functional residual volume.
Third, similar to a thin shell (radius ≫ thickness), an alveolus in the intact lung experiences simultaneous stress in two dimensions (two circumferential strains with negligible thinning of the alveolar wall). Similarly, in the PCLS preparations, the two-dimensional planar stretch imposes primarily circumferential expansion of alveoli (not to be confused with the cylindrical coordinate system used to describe radial and circumferential strains in our wells). Using thicker sections would permit viewing of intact alveoli by appropriate staining and imaging methods, but unless the thick slice is also expanded perpendicularly to the membrane plane (likely loosening the slice from the Silastic membrane), it would be easier and more insightful to study three-dimensional subpleural alveoli in an isolated intact lung (18).
The stitch-stretch-PCLS model allows immunostaining and imaging as demonstrated here, but also lysing for Western blotting and immunoprecipitation, extracting mRNA, and performing other routine biochemical assays. In principle, the described model can also be used for multiwell systems; however, the manual stitching, which is currently the rate-limiting step, should then be addressed. In addition, there are several potential limitations to stretching the PCLSs using the silk suture. First, suturing may activate local cell signaling near the contact points with the suture. Second, we documented a 25% failure rate for 60 min of cyclic stretch at 37% ΔMSA, when PCLSs tore at the suture entry points, increasing the number of tissue samples required for each stretch study. However, this failure rate is similar to gluing or clamping the PCLSs, but these other methods do not produce a uniform, equibiaxial stretch profile.
The method described in this paper for preparing, stitching, and stretching PCLSs is relatively easy, produces repeatable results, and allows us to evaluate in situ paracrine signaling pathways involved in the mechanotransduction in the lungs. The method can be applied to lungs in different species, including humans, and can assist in the translation of studies between species. Most importantly, because stretching the PCLSs with the stitching method produces a uniform and equibiaxial stretch in the central region of the PCLS, we believe that it is an optimal way of utilizing PCLSs for acute and chronic stretch studies.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01-HL-057204.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author (s).
AUTHOR CONTRIBUTIONS
N. D. and S. S. M. conception and design of research; N. D. and J. H. performed experiments; N. D. and J. H. analyzed data; N. D. and S. S. M. interpreted results of experiments; N. D. and J. H. prepared figures; N. D. and J. H. drafted manuscript; N. D. and S. S. M. edited and revised manuscript; S. S. M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gladys Gray Lawrence (University of Pennsylvania) for technical assistance with imaging the PCLSs on the confocal microscope, Dr. Brittany Coats (The University of Utah) for performing the experiments for calculating the Silastic membrane stiffness, and Dr. Min Jae Song for processing the AQP-p65 images and analyzing the data.
REFERENCES
- 1. Budinger GR, Urich D, DeBiase PJ, Chiarella SE, Burgess ZO, Baker CM, Soberanes S, Mutlu GM, Jones JC. Stretch-induced activation of AMP kinase in the lung requires dystroglycan. Am J Respir Cell Mol Biol 39: 666–672, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cavanaugh KJ, Cohen TS, Margulies SS. Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am J Physiol Cell Physiol 290: C1179–C1188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charles PE, Tissieres P, Barbar SD, Croisier D, Dufour J, Dunn-Siegrist I, Chavanet P, Pugin J. Mild-stretch mechanical ventilation upregulates toll-like receptor 2 and sensitizes the lung to bacterial lipopeptide. Crit Care 15: R181, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chew AD, Hirota JA, Ellis R, Wattie J, Inman MD, Janssen LJ. Effects of allergen on airway narrowing dynamics as assessed by lung-slice technique. Eur Respir J 31: 532–538, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Chiang CH, Chuang CH, Liu SL, Chian CF, Zhang H, Ryu JH. N-acetylcysteine attenuates ventilator-induced lung injury in an isolated and perfused rat lung model. Injury 43: 1257–1263, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Chiang CH, Chuang CH, Liu SL, Lee TS, Kou YR, Zhang H. Apocynin attenuates ventilator-induced lung injury in an isolated and perfused rat lung model. Intensive Care Med 37: 1360–1367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coats B, Margulies SS. Material properties of human infant skull and suture at high rates. J Neurotrauma 23: 1222–1232, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, Hornby PJ, Panettieri RA., Jr TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol 297: L530–L537, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Cooper PR, Poll CT, Barnes PJ, Sturton RG. Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol 43: 220–226, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Cooper PR, Zhang J, Damera G, Hoshi T, Zopf DA, Panettieri RA., Jr C-027 inhibits IgE-mediated passive sensitization bronchoconstriction and acts as a histamine and serotonin antagonist in human airways. Allergy Asthma Proc 32: 359–365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126: 332–337, 1982 [DOI] [PubMed] [Google Scholar]
- 12. Dassow C, Wiechert L, Martin C, Schumann S, Muller-Newen G, Pack O, Guttmann J, Wall WA, Uhlig S. Biaxial distension of precision-cut lung slices. J Appl Physiol 108: 713–721, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Davidovich N, Dipaolo BC, Lawrence GG, Chhour P, Yehya N, Margulies SS. Cyclic-stretch induced oxidative stress increases alveolar epithelial permeability. The Biomedical Engineering Society Annual Fall Meeting Hartford, CT, USA Landover, MD: Biomedical Engineering Society, 2011. http://bmes.org/files/bmes_2011_program_book__211pgs_(1).pdf [Google Scholar]
- 14. de Prost N, Ricard JD, Saumon G, Dreyfuss D. Ventilator-induced lung injury: historical perspectives and clinical implications. Ann Intensive Care 1: 28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiPaolo BC, Lenormand G, Fredberg JJ, Margulies SS. Stretch magnitude and frequency-dependent actin cytoskeleton remodeling in alveolar epithelia. Am J Physiol Cell Physiol 299: C345–C353, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Fung YC. A First Course in Continuum Mechanics: For Physical and Biological Engineers and Scientists. Englewood Cliffs, NJ: Prentice Hall, 1994 [Google Scholar]
- 18. Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol 291: L596–L601, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Kim KJ, Crandall ED. Effects of lung inflation on alveolar epithelial solute and water transport properties. J Appl Physiol 52: 1498–1505, 1982 [DOI] [PubMed] [Google Scholar]
- 20. Lavoie TL, Krishnan R, Siegel HR, Maston ED, Fredberg JJ, Solway J, Dowell ML. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am J Respir Crit Care Med 186: 225–232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine GK, Deutschman CS, Helfaer MA, Margulies SS. Sepsis-induced lung injury in rats increases alveolar epithelial vulnerability to stretch. Crit Care Med 34: 1746–1751, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Mercer RR, Laco JM, Crapo JD. Three-dimensional reconstruction of alveoli in the rat lung for pressure-volume relationships. J Appl Physiol 62: 1480–1487, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Morrison B, 3rd, Cater HL, Benham CD, Sundstrom LE. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J Neurosci Methods 150: 192–201, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Oswari J, Matthay MA, Margulies SS. Keratinocyte growth factor reduces alveolar epithelial susceptibility to in vitro mechanical deformation. Am J Physiol Lung Cell Mol Physiol 281: L1068–L1077, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Perez-Zoghbi JF, Sanderson MJ. Endothelin-induced contraction of bronchiole and pulmonary arteriole smooth muscle cells is regulated by intracellular Ca2+ oscillations and Ca2+ sensitization. Am J Physiol Lung Cell Mol Physiol 293: L1000–L1011, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 125: 535–553, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther 24: 452–465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Syrkina O, Jafari B, Hales CA, Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology 13: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Trepat X, Puig F, Gavara N, Fredberg JJ, Farre R, Navajas D. Effect of stretch on structural integrity and micromechanics of human alveolar epithelial cell monolayers exposed to thrombin. Am J Physiol Lung Cell Mol Physiol 290: L1104–L1110, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Tschumperlin DJ, Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 275: L1173–L1183, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Tschumperlin DJ, Oswari J, Margulies SS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162: 357–362, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Vaneker M, Halbertsma FJ, van Egmond J, Netea MG, Dijkman HB, Snijdelaar DG, Joosten LA, van der Hoeven JG, Scheffer GJ. Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology 107: 419–426, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Welling WL, Zupka MT, Welling DJ. Mechanical properties of basement membrane. Physiology 10: 30–35, 1995 [Google Scholar]