Abstract
This study tested the hypothesis that oxidative mitochondrial-targeted DNA (mtDNA) damage triggered ventilator-induced lung injury (VILI). Control mice and mice infused with a fusion protein targeting the DNA repair enzyme, 8-oxoguanine-DNA glycosylase 1 (OGG1) to mitochondria were mechanically ventilated with a range of peak inflation pressures (PIP) for specified durations. In minimal VILI (1 h at 40 cmH2O PIP), lung total extravascular albumin space increased 2.8-fold even though neither lung wet/dry (W/D) weight ratios nor bronchoalveolar lavage (BAL) macrophage inflammatory protein (MIP)-2 or IL-6 failed to differ from nonventilated or low PIP controls. This increase in albumin space was attenuated by OGG1. Moderately severe VILI (2 h at 40 cmH2O PIP) produced a 25-fold increase in total extravascular albumin space, a 60% increase in W/D weight ratio and marked increases in BAL MIP-2 and IL-6, accompanied by oxidative mitochondrial DNA damage, as well as decreases in the total tissue glutathione (GSH) and GSH/GSSH ratio compared with nonventilated lungs. All of these injury indices were attenuated in OGG1-treated mice. At the highest level of VILI (2 h at 50 cmH2O PIP), OGG1 failed to protect against massive lung edema and BAL cytokines or against depletion of the tissue GSH pool. Interestingly, whereas untreated mice died before completing the 2-h protocol, OGG1-treated mice lived for the duration of observation. Thus mitochondrially targeted OGG1 prevented VILI over a range of ventilation times and pressures and enhanced survival in the most severely injured group. These findings support the concept that oxidative mtDNA damage caused by high PIP triggers induction of acute lung inflammation and injury.
Keywords: albumin spaces, macrophage inflammatory protein-2, IL-6, glutathione, pulmonary edema, vascular permeability
the mortality rate for acute lung injury (ALI) and its most severe form, the acute respiratory distress syndrome (ARDS), remains high at 30–40% (27). A reduction in tidal volume from 12 ml/kg to 6 ml/kg during mechanical ventilation support has been one of the few treatments that has resulted in a significant reduction in this mortality, indicating a significant augmentation of the underlying lung pathology by ventilator-induced lung injury (VILI) (4, 27). Reactive oxygen (ROS) and nitrogen (RNS) species are major contributors to the lung injury in both VILI and ALI/ARDS (4, 33, 38, 46). In patients with ARDS, plasma lipid peroxidation products are increased and antioxidants are decreased, and oxidized glutathione (GSH) is increased in alveolar lining fluid (28). The inflammatory ARDS process favors ROS and RNS production by phagocytic and nonphagocytic cells, as well as increases in inflammatory cytokines, proteases, and other injurious mediators (4, 38, 46). Although many pathways have been implicated, the primary essential target for propagation of inflammatory lung injury has not been identified. As a result, previous clinical studies using antioxidants or pharmacological blockade of individual inflammatory cytokines have not dramatically improved survival in ALI/ARDS (27, 47).
Although mitochondria continuously produce ROS during oxidative phosphorylation, superoxide leaking from the electron transport chain is scavenged by mitochondrial and cytoplasmic superoxide dismutase (SOD) and GSH peroxidases at the expense of GSH (44). However, high levels of cyclical stretch of endothelium and lung epithelium lead to excessive ROS production from mitochondria and NADPH oxidases, which can deplete antioxidant defenses (1, 5). Although cyclical stress of lung epithelial cells produces DNA damage that is partly due to NADPH oxidase activity (45), whether the mitochondrial genome is damaged in VILI and, if so, whether mitochondrial-targeted DNA (mtDNA) damage contributes to lung inflammation and injury have not previously been explored.
Multiple lines of provocative evidence suggest that mtDNA damage may link the oxidant stress associated with high peak inspiratory pressure (PIP) to induction of ALI. For example, in lung endothelial as well as other cell types, overexpression of constructs encoding DNA repair enzymes linked to the mitochondrial-targeting sequence (MTS) from Mn-SOD reduces oxidant-induced mtDNA damage, apoptosis, and cell death (10, 11, 15, 41). Recently, fusion protein constructs consisting of the TAT sequence (to facilitate cellular entry), an MTS, and a DNA glycosylase, either the mammalian 8-oxoguanine DNA glycosylase (OGG1) or the bacterial glycosylase, Endonuclease III, have been shown to protect against hydrogen peroxide-induced injury in isolated perfused lungs. However, the effect of mitochondrial-targeted repair enzymes on mechanical lung injury in intact animals has not previously been determined (6). Accordingly, the present study capitalized on the availability of mitochondrial-targeted DNA repair enzymes to test the hypothesis that oxidative mtDNA damage is a trigger for lung inflammation and injury in the setting VILI.
MATERIALS AND METHODS
All experimental protocols were approved by the institutional Animal Care and Use Committee of the University of South Alabama College of Medicine. Anesthetized mice were euthanized by exsanguination at the time of flushing blood out from the lung.
Fusion protein constructs.
Codon-optimized constructs were placed in plasmids for expression in Escherichia coli of fusion proteins containing OGG1 coupled to a TAT sequence to facilitate cellular uptake, the MTS from MnSOD, a hemaglutin (HA) tag for immunological localization, and a histidine tail as previously described (20). Liquid cultures of bacterial cells transfected with plasmids containing the constructs were grown to an OD60 = 0.6 and induced with isopropylthiogalactoside for 3 h. Bacteria were pelleted by centrifugation and resuspended in buffer A [20 mM Tris·HCl pH 8.0, 500 mM NaCl, 1× protein inhibitor cocktail EDTA-free (Calbiochem), 100 mM PMSF, and 5 mM imidazole]. Bacteria were lysed by sonication with a Branson Sonifier 250. After sonication, bacterial lysates were spun in a Beckman Ultracentrifuge for 20 min at 105 g. After centrifugation, cleared lysates were incubated with Ni-NTA-agarose. The Ni-NTA-agarose was placed in a column and washed with several volumes of wash buffer (buffer A containing 30 mM imidazole). The bound protein was eluted from the column with elution buffer (buffer A containing 500 mM imidazole), and purity of the eluted protein was assessed using SDS-PAGE. All reagents for fusion protein production were obtained from Sigma unless otherwise indicated. Previous studies have established that the OGG1 fusion construct localizes almost exclusively in the mitochondrial subcellular fraction with little or no detectable accumulation in nucleus or cytosol (6).
Treatment with mt-targeted OGG1.
Approximately 24 h before ventilation, C57BL/6 male mice (Charles River Laboratory), weighing 20.2–41.9 g (25.7 ± 4.0 g), were anesthetized with an intraperitoneal injection of Ketamine (90 mg/kg) and pentobarbital sodium (25 mg/kg). The left jugular vein was exposed, and the mice were infused intravenously with fusion protein constructs containing OGG1 (60 μg) diluted in PBS to 30 μl. Untreated mice were injected with PBS only. After bleeding stopped, the incision was sutured and the mice were allowed to recover.
Experimental protocols.
Ventilation protocols were designed to produce three levels of severity of lung injury ranging from minimal to very severe. Mice were anesthetized with an intraperitoneal injection of Ketamine (90 mg/kg) and pentobarbital sodium (25 mg/kg). The trachea was cannulated, and the mice were ventilated with 100% oxygen using a Harvard rodent ventilator (model no. 683; Harvard). Mice received either no ventilation (No Vent., n = 5), ventilation for 1 h with either 10 cmH2O PIP (PIP10 × 1 h Vent., n = 5), 40 cmH2O PIP ventilation only (PIP40 × 1 h Vent., n = 5), 40 cmH2O PIP ventilation with OGG1 (PIP40 × 1 h + OGG1, n = 5), 2-h ventilation with 40 cmH2O PIP only (PIP40 × 2 h Vent., n = 5), 2-h ventilation with 40 cmH2O PIP after OGG1 (PIP40 × 2 h + OGG1, n = 6), 2-h ventilation with 50 cmH2O PIP only (PIP50 × 2 h Vent., n = 5), or 2-h ventilation with 50 cmH2O PIP after OGG1 (PIP50 × 2 h + OGG1, n = 5). The approximate tidal volumes used were 0.3 ml (12 ml/kg) for the 10 cmH2O PIP group; 0.8 ml (32 ml/kg) for the 40 cmH2O PIP groups, and 0.95 (36 ml/kg) for the 50 cmH2O PIP groups (42, 49). After the ventilation period, mice were injected with 50 IU heparin into the peritoneal space, blood was collected by cardiac puncture of the left ventricle, and blood gases were determined using a Radiometer ABL5 blood gas machine. Ventilation rates were decreased during high PIP ventilation compared with low PIP ventilation groups, but this reduction was not sufficient to prevent some degree of hyperventilation and hypocapnia in the high PIP ventilation groups. A suture was placed around the pulmonary artery and aorta, and a cannula (0.86-mm internal diameter, 1.27-mm outside diameter) was placed in the pulmonary artery. The hilum of right lung was tied off, and the left ventricle was clipped. The left lung was flushed of blood with 2 ml of 10% PBS, and bronchoalveolar lavage (BAL) was performed twice with 0.3 ml of saline on the left lung. After BAL, the left lung was harvested, minced, and sonicated using a Missonex XL 2000 sonicator in 3-s bursts with 0.5 ml 10% PBS. After centrifugation to obtain the supernatant, the pellet was dried to a constant weight for tissue dry weight. Collected blood was centrifuged, and serum was separated.
Western immunoblot analysis of subcellular fusion protein localization.
Subcellular fractions were prepared from lung homogenates as described previously (6). Lung tissue (1 g) was homogenized in a glass homogenizer with Teflon pestle eight times using 6 ml of homogenization buffer (0.25 M sucrose, 20 mM Hepes-NaOH, pH 7.4, and 1 mM EDTA). Protease inhibitor cocktail (Sigma-Aldrich) was added to all isolation buffers. The homogenate was filtered through 70-μm mesh (BD Biosciences) and centrifuged on a cushion (5 ml) containing 0.35 M sucrose, 20 mM Hepes-NaOH, pH 7.4, and 1 mM EDTA at 700 g for 10 min at 4°C. The fraction around and above the interphase was collected as crude mitochondria and reserved for mitochondrial isolation. The nuclear pellet was suspended in 3 ml of nuclear isolation buffer (0.25 M sucrose, 20 mM Hepes-NaOH, pH 7.4, 25 mM KCl, and 5 mM MgCl2) and purified in 3 ml cushion containing 0.8 M sucrose, 20 mM Hepes-NaOH, pH 7.4, 25 mM KCl, and 5 mM MgCl2 at 3,000 g for 15 min at 4°C. The nuclear pellet so obtained was washed with nuclear isolation buffer and centrifuged at 1,000 g for 10 min. The pellet containing purified nuclei was suspended in 300 μl of RIPA buffer (Cell Signaling Technology), incubated for 30 min on ice, and centrifuged at 18,000 g for 15 min. The supernatant was designated as the “nuclear fraction.” The crude mitochondrial fraction, collected as described above, was centrifuged at 18,000 g for 20 min to pellet mitochondria, which were suspended in 2 ml of mitochondrial isolation buffer (0.2 M mannitol, 50 mM sucrose, 20 mM Hepes-NaOH, pH 7.4, and 1 mM EDTA) and centrifuged under the same conditions. This supernatant was designated as the cytosolic fraction, whereas the pellet containing mitochondria was suspended in 300 μl of RIPA buffer (Cell Signaling Technology), incubated for 30 min on ice and centrifuged at 18,000 g for 15 min. This latter supernatant was designated as the mitochondrial fraction. Cytosolic, nuclear, and mitochondrial fractions were subjected to Western immunoblot analysis for specific markers and for HA-tagged fusion protein constructs.
Western blot analyses were performed as described earlier using antibodies against the HA tag (Sigma-Aldrich) to determine subcellular distribution of the fusion proteins (6). The mitochondrial fraction was characterized using an antibody (Sigma-Aldrich) against the cytoplasmic loop of the voltage-dependent anion channel (porin-1), the pore-forming unit in the outer mitochondrial membrane, which serves as an adenine nucleotide translocator. The nuclear fraction was characterized using an antibody against Lamin B1 (Santa Cruz Biotechnology), a component of the nuclear envelope. An antibody against β-actin was used as a loading control for total lysate and cytosolic fractions.
Measurement of albumin plasma equivalents.
Albumin quantities in BALF fluid (BALF), supernatant of homogenized left lung tissue, and serum were measured by using an ELISA kit (Bethyl Laboratories) for mouse albumin. The left lung was minced and sonicated in 500 μl PBS using a Missonex XL 2000 sonicator. Supernatant samples were removed, and the left lung tissue was desiccated at 80°C for 5 days to obtain a stable dry weight. Initial dilutions for ELISA were as follows: 1 × 106 for serum, 4 × 103 for BAL, and 6 × 103 for tissue supernatant. Serum samples were further diluted by 1:3, and then 200 μl of diluted serum, BAL, and tissue supernatant samples were each spotted and followed by three successive 1:2 dilutions. Four wells each of PBS blank and positive albumin controls in the sample concentration range were also included. Then 100 μl of antialbumin detection anybody was added to each well, and the plate was incubated for 1 h at room temperature on an orbital shaker. Contents were discarded, and the plate was washed four times. Next, 100 μl of horseradish peroxidase solution was added, and the plate was incubated for 30 min at room temperature on an orbital shaker. This was followed by 100 μl of colorimetric substrate incubated for 30 min followed by 100 μl of the stop solution supplied in the ELISA kit. The resultant yellow color was read at 450 nm with a Dynex MXR plate reader. Sample albumin concentrations were calculated from each serial dilution and checked for consistency. Mouse albumin standard was mixed with PBS to obtain an initial concentration of 0.9 μg/ml. Two standard curves were determined for each plate using seven 1:3 dilutions with final volumes of 200 μl per well and expressed using a four-parameter curve fit. Residual albumin in the tissue supernatant was assumed to represent primarily interstitial albumin, and alveolar compartment albumin was assumed to be largely removed by the BAL procedure. Total albumin masses (QA) were calculated for tissue (QA,tiss) and alveolar spaces (QA,alv), and total extravascular albumin (QA,total = QA,tiss + QA,alv) in the left lung were normalized to tissue dry weight (DLW) and plasma albumin concentration (CA,pl) and expressed as plasma equivalents (PE) in microliters/micrograms, where PE = QA/CA,pl/DLW.
Use of this normalization technique corrects for differences in plasma albumin concentration and lung weights between experiments. The sensitivity of this kit was 20 ng/ml. Lung vascular injury was evaluated using increases in the total, BAL, and tissue endogenous albumin plasma equivalents in each group.
Lung wet/dry weight ratios.
The right lower lobe was weighed (W) and desiccated at 80°C for 1 wk to obtain a stable dry weight (D) for calculation of the wet/dry weight ratio (W/D ratio).
Measurement of MIP-2 and IL-6.
Macrophage inflammatory protein (MIP)-2 and IL-6 in BALF were measured using mouse ELISA kits (R&D Systems). The sensitivity of these kits were 1 pg/ml for MIP-2 and 2.5 pg/ml for IL-6.
Analysis of mtDNA content and oxidative damage.
Immediately after perfusion, lungs were snap-frozen in liquid nitrogen and saved for determination of oxidative mtDNA damage. Total DNA was isolated from lung samples and powdered with a mortar and pestle using previously described methods (36, 41). Purified DNA samples were digested with PpuMI and AhdI restriction enzymes (New England Biolaboratories) and used for further analyses.
To measure oxidative damage to the mitochondrial genome, a quantitative Southern blot analysis was performed as described previously with minor modifications (38). In brief, digested DNA samples were precipitated, dissolved in TE buffer, and precisely quantified on the Hoefer DyNA Quant 200 Fluorometer (Hoefer) using Hoechst 33258 dye. To reveal oxidative base modifications, DNA was treated with formamidopyrimidine glycosylase (Fpg; New England Biolaboratories), a bacterial DNA repair enzyme that cleaves DNA at sites of oxidized purines, thereby creating single-strand breaks. Subsequently, Fpg-treated and -untreated samples were incubated with 0.1 N NaOH for 15 min at 37°C, mixed with loading dye, and resolved in 0.6% agarose alkaline gel. After electrophoresis, DNA was vacuum transferred to a nylon membrane (Roche Diagnostics) and hybridized with a PCR-generated probe to the corresponding region of mtDNA. The mtDNA probe, labeled with a DIG-labeling kit (Roche Diagnostics), was generated using rat mtDNA sequence as a template and the following primers: 5′-CCCTACTTACTGGCTTCAATCTAC-3′ for the sense strand and 5′-CATACCATACCTATATATCCGAAGG-3′ for the anti-sense strand. The 1,016-bp product was hybridized with a 13.6-kb fragment of rat mtDNA obtained after PpuMI and AhdI digestion. Hybridization bands were detected with Amersham Hyperfilm ECL (GE Healthcare) and a Gel Logic 1500 Imaging System (Kodak). Changes in the equilibrium lesion density of Fpg-detectable base oxidation lesions within each experimental group were calculated as negative ln of the quotient of hybridization intensities in Fpg-treated and non-Fpg bands, normalized to 10 kb (3), and are independent of the total amount of mtDNA.
Statistical analysis.
All values are expressed as means ± SE. One-way ANOVA with repeated measures followed by a Student-Newman-Keuls posttest was used. Significant differences were determined where P < 0.05.
RESULTS
Subcellular localization of the fusion protein.
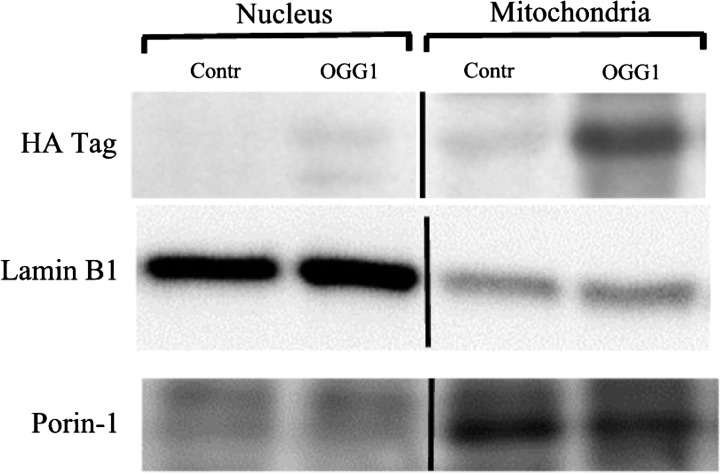
Figure 1 displays a representative Western immunoblot of the HA-tagged fusion protein in the nuclear and mitochondrial fractions of lung homogenates in control mice and mice 1 h after injection with the OGG1 fusion protein construct. Also indicated are the nuclear protein, laminin B1, and the mitochondrial protein, porin-1, in the respective subcellular fractions. The HA tag was not detected in the nuclear fraction but was present in the mitochondrial fraction. Some slight nonspecific antibody binding also occurred in the control mice but was far less prominent than in the fusion-protein-injected mice.
Fig. 1.
Western immunoblot probed for the hemaglutin (HA) tag of the fusion protein in nuclear and mitochondrial subcellular fractions of lungs from control mice (Contr) and mice injected with the 8-oxoguanine-DNA glycosylase 1 (OGG1) fusion protein construct. Immunoblots labeled for the nuclear protein, lamin B1, and the mitochondria protein, porin-1, are also shown.
Arterial blood gases.
Terminal blood gases obtained from left ventricular puncture at the end of the experiments are shown in Table 1. Although respiratory rate was reduced at the higher PIP levels, hypocapnia was present in the high PIP ventilation groups. PaCO2 values were significantly lower in the OGG1-treated groups ventilated at 40 and 50 cmH2O PIP compared with nonventilated mice although significantly more alkalosis was not present. PaO2 values were significantly greater in the 40 cmH2O PIP groups ventilated for 1 h compared with the nonventilated group. Although there were no significant differences between OGG1-treated and -untreated groups within each time and PIP protocol using one-way ANOVA, the PaCO2 values of OGG1-treated groups ventilated at 40 cmH2O PIP for 1 and 2 h were significantly lower than the untreated groups when compared by unpaired t-test, suggestive of a borderline difference.
Table 1.
Ventilation times, PIPs, and ventilation rates of all groups with terminal arterial blood gases and pH for all groups surviving the 2-h ventilation protocol
| Treatment | PIP, cmH2O | Vent. Time, min | PaO, mmHg | PaCO2, mmHg | pH | Vent. Rate, breaths/min |
|---|---|---|---|---|---|---|
| NV | 10 | 11.2 ± 1.2 | 211 ± 11 | 36.0 ± 1.2 | 7.28 ± 0.01 | 110 |
| Low PIP | 10 | 60 | 293 ± 7 | 34.5 ± 2.4 | 7.33 ± 0.01 | 110 ± 2 |
| PIP40 | 40 | 60 | 466 ± 14* | 27.6 ± 1.1 | 7.35 ± 0.01 | 28.3 ± 0.5 |
| PIP40 OGG1 | 40 | 60 | 499 ± 8* | 21.6 ± 0.3* | 7.43 ± 0.02* | 29.6 ± 0.4 |
| PIP40 | 40 | 120 | 344 ± 4 | 26.3 ± 0.5 | 7.36 ± 0.01 | 28.3 ± 0.3 |
| PIP40 OGG1 | 40 | 120 | 448 ± 21 | 20.4 ± 0.6† | 7.35 ± 0.01 | 29.7 ± 2.4 |
| PIP50 | 50 | 96.6 ± 0.7 | NM | NM | NM | 25.6 ± 0.2 |
| PIP50 OGG1 | 50 | 120 | 331 ± 11 | 18.8 ± 0.6† | 7.29 ± 0 | 25.8 ± 0.2 |
Applicable values are means ± SE.
P < 0.05 vs. no ventilation (NV) group;
P < 0.05 vs. NV and low-peak-inspiratory-pressure (PIP) group. Vent, ventilation; OGG1, 8-oxoguanine-DNA glycosylase 1; NM, not measured.
Extravascular albumin spaces and edema.
The ventilation times and peak inspiratory pressure permeability effects of ventilation were evaluated using the plasma-equivalent extravascular spaces for endogenous albumin partitioned into interstitial and alveolar space components. Normalizing albumin spaces for tissue weight and plasma volume allows inferences about the permeability of alveolar and extra alveolar vessel lung vascular segments, as well as relative spaces available to albumin in the interstitial and alveolar compartments. This proved to be a sensitive method in that significantly increased albumin spaces were detected at the lowest level of injurious ventilation even without a significant increase in lung wet/dry weight ratio as described below.
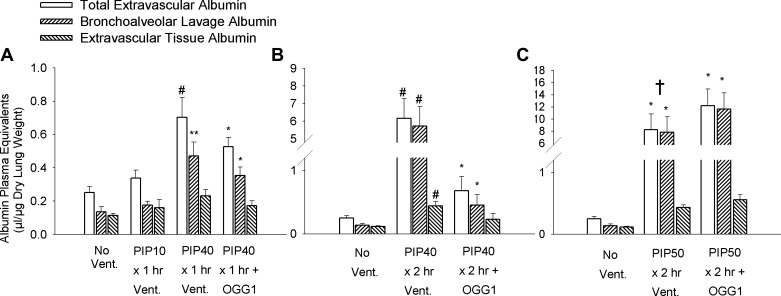
Figure 2 is a composite showing the total, alveolar, and tissue albumin equivalent spaces after three different ventilation protocols. Figure 2A shows the least injurious protocols consisting of 1 h of ventilation with either 10 cmH2O PIP or 40 cmH2O PIP. Total extravascular albumin spaces increased significantly in the 1 h 40 cmH2O PIP group by 2.8-fold compared with unventilated lungs and 2.2-fold compared with the 10 cmH2O PIP ventilation group. Extravascular albumin spaces in groups treated with the mtDNA repair enzymes increased significantly by some twofold compared with unventilated mice. However, the increases were significantly less for the fusion-protein-treated groups compared with that of the untreated 40 cmH2O PIP ventilation group. Partitioning of the total albumin into tissue and alveolar components indicated significant increases in BAL albumin spaces in all groups ventilated with 40 cmH2O PIP compared with either no ventilation or ventilation 1 h with 10 cmH2O PIP. Although the mean values for separate BAL and tissue albumin spaces in the untreated group were greater than those in groups treated with the fusion protein constructs, the differences were not statistically significant.
Fig. 2.
Mouse lung extravascular plasma equivalent albumin spaces showing bronchoalveolar lavage albumin space, extravascular tissue albumin space, and the total extravascular albumin space after ventilation for different times and with different peak inspiratory pressures (PIP). A: mice received either no ventilation (No Vent.), or 1 h of ventilation with 10 cmH2O PIP (PIP10), or 40 cmH2O PIP only (PIP40), or 40 cmH2O PIP with OGG1 (PIP40 OGG1). B: mice received either no ventilation, or 2 h of ventilation with 40 cmH2O PIP only (PIP40), or 40 cmH2O PIP with OGG1 (PIP40 OGG1). C: mice received either no ventilation, or 2 h of ventilation with 50 cmH2O PIP only (PIP50), or 50 cmH2O PIP with OGG1 (PIP50 OGG1). *P < 0.05 vs. No Vent. group. **P < 0.05 vs. No Vent. group and PIP10 groups. #P < 0.05 vs. all other groups. †All animals in the untreated PIP50 group died before 2 h.
Figure 2B indicates the albumin spaces after ventilation with 2 h of 40 cmH2O PIP compared with unventilated mouse lungs. In untreated mice ventilated at 40 cmH2O PIP, total, BAL, and tissue extravascular albumin spaces increased significantly by 25-fold, 42-fold, and 3.7-fold, respectively, compared with nonventilated lungs. However, total, BAL, and tissue extravascular albumin spaces increased by only ∼2.5-fold in the OGG1 fusion protein DNA repair enzyme-treated groups compared with nonventilated lungs, and these values were all significantly less than those in the untreated 40 cmH2O PIP-ventilated group.
Extravascular albumin spaces for the most severely injured groups ventilated for 2 h at 50 cmH2O PIP are shown in Fig. 2C. Massive lung edema was produced by this ventilation protocol, and total, BAL, and tissue extravascular albumin spaces in all high PIP-ventilated groups increased significantly by 28- to 48-fold, 47- to 86-fold, and 3.7- to 4.8-fold, respectively, compared with nonventilated controls. There were no statistical differences between untreated and fusion-protein-treated groups ventilated with 50 cmH2O PIP. However, all of the mice in the untreated 50 cmH2O PIP groups died 20–30 min before completing the entire 2-h ventilation protocol, whereas none of the mice in groups treated with the OGG1 repair enzyme constructs died during the experiments.
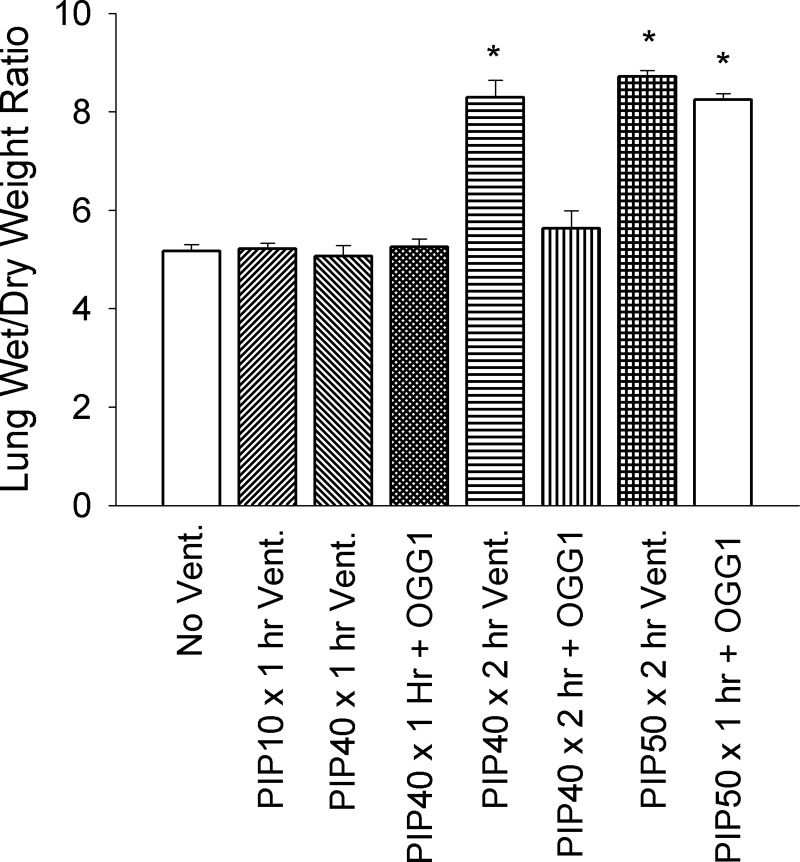
Figure 3 summarizes the lung wet/dry weight ratios in all groups. There were no significant differences in wet/dry weight ratios between the groups with no ventilation, 1-h ventilation with 10 cmH2O PIP or 40 cmH2O PIP without treatment, or groups ventilated for either 1 or 2 h at 40 cmH2O PIP treated with OGG1. However, 2 h of ventilation with 40 cmH2O PIP-untreated group resulted in a significant 60% increase in lung wet/dry weight ratios in untreated mice as did ventilation for 2 h at 50 cmH2O PIP in untreated or DNA repair enzyme-treated mice. There were no significant differences between the groups ventilated with 50 cmH2O PIP and the group ventilated for 2 h at 40 cmH2O PIP.
Fig. 3.
Lung wet/dry weight ratios for all experimental groups. *P < 0.05 vs. No Vent., PIP10 × 1 h Vent. only, PIP40 × 1 h Vent. only PIP40 × 1 h + OGG1, and PIP40 × 2 h + OGG1 groups. All animals in the untreated PIP50 group died before 2 h.
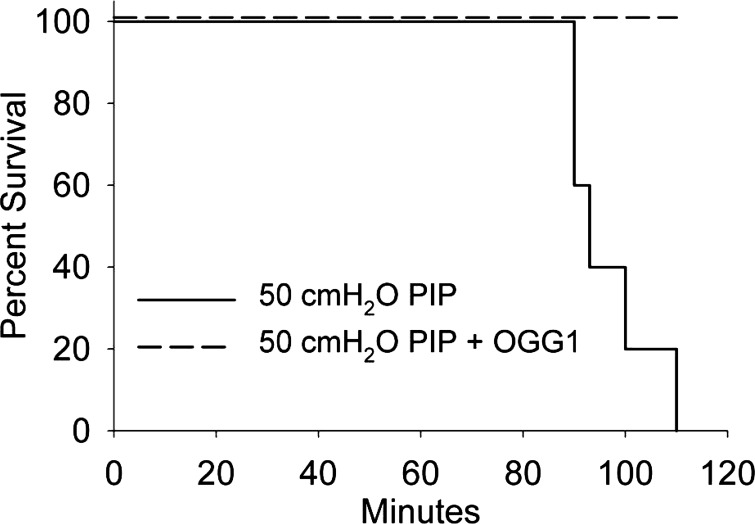
Kaplan-Meier survival plot.
Figure 4 shows the Kaplan-Meier survival plot for the groups ventilated at 50 cmH2O PIP for 2 h with and without pretreatment with the OGG1 fusion protein. All of the OGG1-treated animals survived 2 h of ventilation, whereas all of the untreated animals died. The average survival time for the untreated group was 96.6 ± 0.7 min.
Fig. 4.
Kaplan-Meier survival plot for the group ventilated at 50 cmH2O PIP for 2 h with (dashed line) and without (solid line) pretreatment with the OGG1 fusion protein.
Cytokine production during high PIP ventilation.
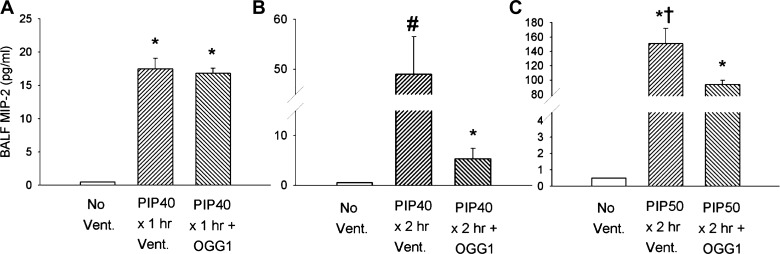
Cytokine production as measured by cytokine concentrations in the BALF also exhibited increases that were dependent on ventilation time and peak airway pressures. BALF cytokine responses mirrored the protective effect of mtDNA repair enzymes on edema and albumin accumulation in that the lowest level of injury produced the least effect on cytokine concentrations. Figure 5 summarizes the effect of the three levels of lung injury on BAL MIP-2 concentrations. All groups ventilated for 1 h at 40 cmH2O PIP had increased BAL MIP-2 compared with nonventilated values, which were close to the threshold for detection as shown in Fig. 5A. Figure 5B indicates that MIP-2 values increased significantly approximately threefold in all mice ventilated for 2 h at 40 cmH2O PIP compared with mice ventilated for 1 h at the same PIP. BALF MIP-2 was significantly higher in untreated animals than for the DNA repair enzyme-treated group. Figure 5C indicates the large increases in BALF MIP-2 values after 2-h ventilation with 50 cmH2O PIP, which was approximately ninefold that in mice ventilated for 1 h at 40 cmH2O PIP. Although there were no significant differences between MIP-2 values in treated and untreated mice; however, as noted above, all untreated mice died before completing the 2-h ventilation protocol, whereas all mtDNA repair enzyme-treated mice survived the entire 2 h.
Fig. 5.
Macrophage inflammatory protein-2 (MIP-2) concentrations in bronchoalveolar lavage fluid (BALF) from lungs of mice after different ventilatory protocols. A: groups received either no ventilation, ventilation for 1 h without treatment at 10 cmH2O PIP (PIP10), or ventilation for 1 h with 40 cmH2O PIP (PIP40) with and without pretreatment with OGG1. B: groups received either no ventilation or ventilation for 2 h with 40 cmH2O PIP (PIP40) with and without pretreatment with OGG1. C: groups received either no ventilation or ventilation for 2 h with 50 cmH2O PIP (PIP50) with and without pretreatment with OGG1. *P < 0.05 vs. No Vent. group. #P < 0.05 vs. all other groups. †All animals in the untreated PIP50 group died before 2 h.
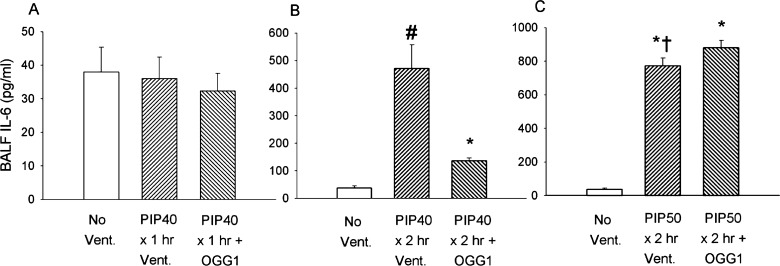
Figure 6 indicates a similar effect of the three levels of lung injury on BALF IL-6 concentrations. Shown in Fig. 6A, IL-6 did not increase significantly in any of the groups after 1-h ventilation with 40 cmH2O PIP compared with nonventilated controls. Figure 6B indicates that IL-6 values markedly increased 12-fold with the more severe lung injury after 2 h of 40 cmH2O PIP ventilation compared with unventilated controls. BALF IL-6 was also significantly higher in untreated mice compared with the group treated with the mtDNA repair enzymes. As shown in Fig. 6C, IL-6 was markedly increased in treated and untreated mice after ventilation for 2 h with 50 cmH2O PIP. Increases in these groups were up to 23 times that in nonventilated controls, but there were no significant differences in BALF IL-6 values between untreated and mtDNA repair enzyme-treated groups. As previously described, all untreated mice died before completing the 2-h ventilation protocol, whereas all mtDNA repair enzyme-treated mice survived the entire 2 h.
Fig. 6.
Interleukin-6 (IL-6) concentrations in BALF from lungs of mice after different ventilatory protocols. A: groups received either no ventilation, ventilation for 1 h without treatment at 10 cmH2O PIP (PIP10), or ventilation for 1 h with 40 cmH2O PIP (PIP40) with and without pretreatment with OGG1. B: groups received either no ventilation or ventilation for 2 h with 40 cmH2O PIP (PIP40) with and without pretreatment with OGG1. C: groups received either no ventilation or ventilation for 2 h with 50 cmH2O PIP (PIP50) with and without pretreatment with OGG1. *P < 0.05 vs. No Vent. group. #P < 0.05 vs. all other groups. †All animals in the untreated PIP50 group died before 2 h.
Tissue GSH concentrations.
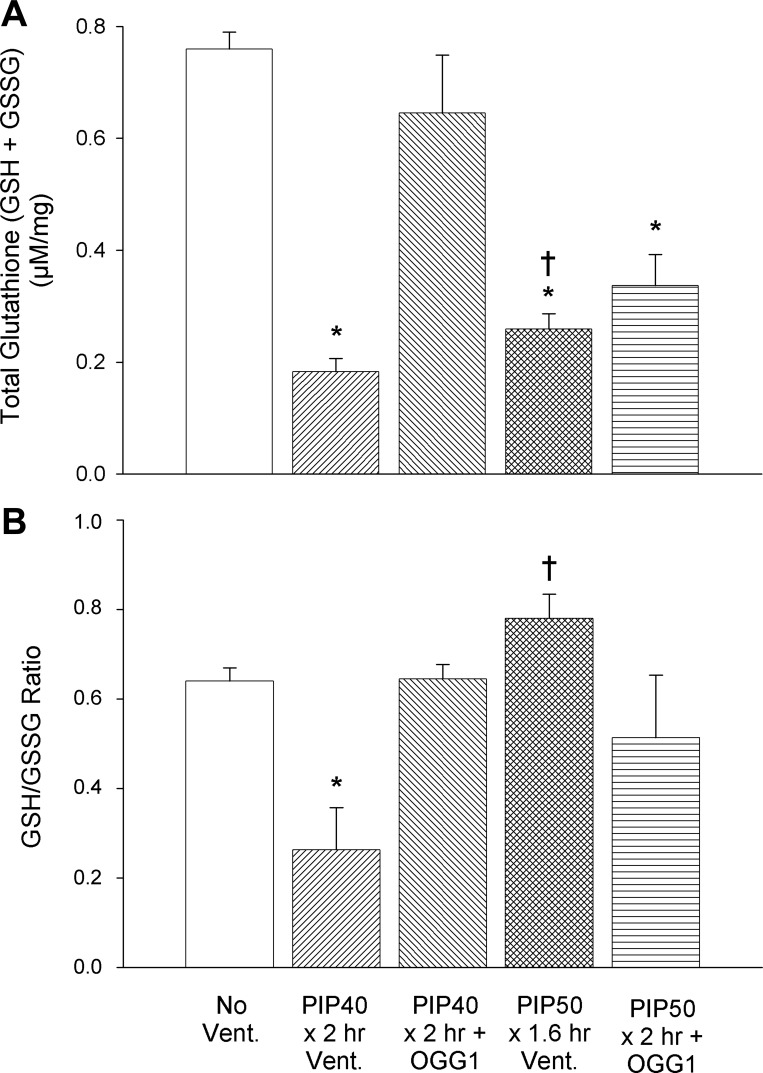
To assess the redox status of lung tissue, the total GSH pool (GSH + GSSH) consisting of the reduced (GSH) and oxidized (GSSG) components of the GSH oxidant scavenger system were measured. As indicated by Fig. 7A, the total lung GSH pool was significantly reduced in untreated mice ventilated at 40 cmH2O or 50 cmH2O PIP for 2 h compared with nonventilated controls. However, treatment with the OGG1 construct prevented the decrease in total GSH after 2-h ventilation with 40 cmH2O but not after ventilation with 50 cmH2O. There was no significant difference between the OGG1-treated group ventilated for 2 h at 40 cmH2O and the nonventilated control group. Figure 7B indicates that the GSH/GSSG ratio was also significantly reduced in the group ventilated for 2 h at 40 cmH2O PIP compared with nonventilated controls, but the 2-h 40 cmH2O PIP OGG1-treated group was not significantly different from the control group. Unexpectedly, the GSH/GSSG ratios did not significantly decrease in the treated and untreated groups ventilated with 50 cmH2O PIP, as these values were not significantly different from the nonventilated controls. Thus treatment with the mtDNA repair enzyme significantly preserved the GSH antioxidant system when ventilated for 2 h with 40 cmH2O PIP but did not protect the lung during the more injurious ventilation for 2 h at 50 cmH2O PIP.
Fig. 7.
Lung tissue glutathione (GSH) concentrations in nonventilated controls and groups ventilated for 2 h with 40 cmH2O PIP and 2 h with 50 cmH2O PIP with and without OGG1 pretreatment. Shown are the total glutathione pool (A) and GSH/GSSG ratios (B). *P < 0.05 vs. nonventilated controls; †all untreated PIP50 group members died before 2 h.
Mitochondrial DNA damage.
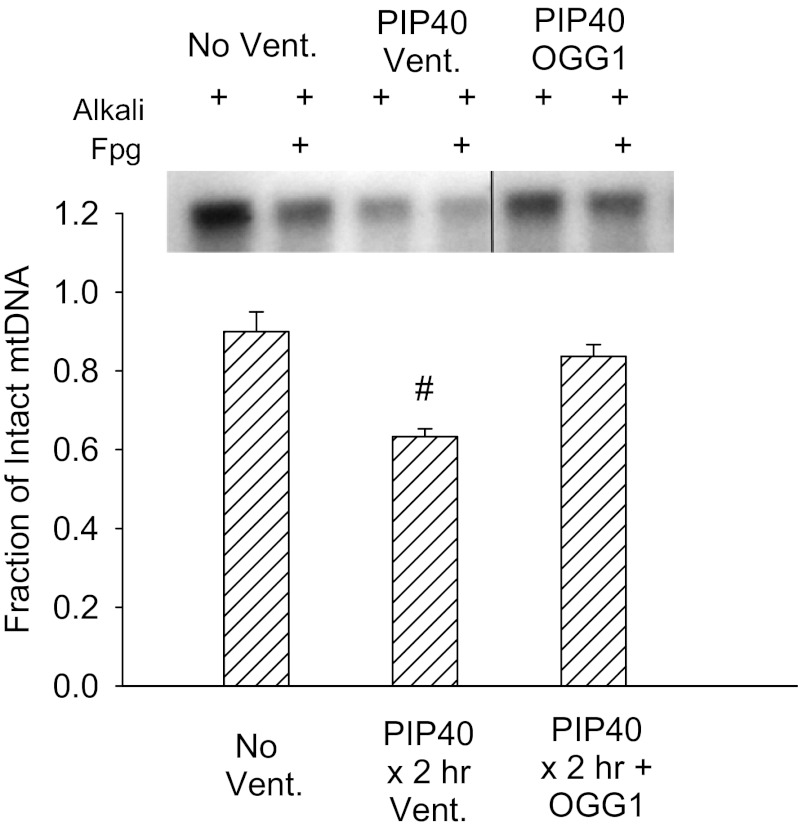
Figure 8 summarizes the Southern blot analysis of Fpg-detectable oxidative base damage. Only the nonventilated group and groups ventilated for 2 h at 40 cmH2O PIP were analyzed because these groups demonstrated the greatest differential effect of the targeted mtDNA repair enzymes on injury. The fraction of intact mtDNA after Fpg treatment in lungs ventilated at 40 cmH2O PIP was significantly reduced in untreated lungs compared with nonventilated controls and mice treated with mitochondrial-targeted OGG1, indicating significant oxidative base damage by high PIP ventilation that was prevented or reversed by OGG1 pretreatment. In addition, the pixel intensity of samples treated with alkali only, which is indicative of oxidative damage to the deoxyribose backbone of mtDNA, was decreased by high PIP to 47% of that in nonventilated controls but was restored to 94% of baseline by OGG1 treatment.
Fig. 8.
Southern blot analysis of mitochondrially targeted DNA (mtDNA) in lung tissue in groups ventilated for 2 h with 40 cmH2O PIP. Samples were treated with alkali to reveal mtDNA strand breaks and alkali + formamidopyrimidine glycosylase (Fpg) to indicate base damage. Line on blot denotes that the OGG1 portion of the blot was moved over in the same blot picture. Bar graph summarizes ratios of blot intensities of alkali+EFG/alkali bands in lungs of nonventilated controls (No Vent.), and mice either untreated (PIP40 × 2 h Vent.) or OGG1 treated (PIP40 × 2 h + OGG1) ventilated for 2 h at 40 cmH2O PIP. #P < 0.05 vs. all other groups.
DISCUSSION
We show here for the first time that VILI in mice is accompanied by oxidative base damage to the mitochondrial genome and, more interestingly, that protection against mtDNA oxidative damage with an mtDNA repair enzyme suppresses the increased pulmonary vascular permeability and inflammation induced by mechanical lung injury in intact mice. Collectively, these observations point to the novel concept that mtDNA damage in response to elevated PIP may trigger VILI. Before discussing evidence pertaining to this conclusion, however, we first address certain attributes of the murine model of VILI.
Yoshikawa et al. (53) demonstrated a direct relationship of lung vascular permeability and edema to both the ventilation time and extent of PIP elevation during mechanical ventilation in mice. In our study, we titrated three levels of lung injury ranging in severity from mild to fatal by increasing the ventilation duration and PIP. In addition, some degree of hyperventilation and hypocapnia were also present in all groups ventilated with high PIP levels. Hypocapnic alkalosis in itself has been shown to promote increased vascular permeability and edema and reduce alveolar edema clearance (8), but its contribution to VILI appears to be modest (22). The extent that hypocapnia may have contributed the lung damage at the highest PIP levels in the present study is not known, but the borderline lower PaCO2 values in OGG1-treated groups ventilated at 40 cmH2O PIP would be expected to increase the lung injury rather than afford protection as observed after treatment with DNA repair enzymes. Thus it is unlikely that hypocapnia significantly influenced the effects of OGG1 treatment on lung injury. Although the exact cause is uncertain, regional hyperventilation of some lung units may have produced the unexpectedly high GSH/GSSG ratio in groups ventilated with 50 cmH2O PIP even though the GSH pool was depleted overall due to extensive lung damage. An alternative explanation could be that some type of ventilation/perfusion mismatch increased regional hypoxia in the presence of global hyperventilation because hypoxia was found to actually increase the GSH/GSSG ratio (48).
Another important methodological feature of this study was our use of a novel and sensitive method for evaluating vascular injury based on partitioning of the endogenous serum albumin spaces. In normally hydrated, passively drained postmortem lung tissue, ∼20% of the lung weight is residual blood volume and extravascular lung water is approximately evenly divided between cellular and interstitial spaces (43). The extravascular albumin space expressed as plasma-equivalent volumes comprises ∼30% of extravascular fluid volume due to exclusion of albumin by interstitial matrix molecules from portions of the interstitial fluid (34, 43). Most previous albumin-distribution studies have employed radiolabeled albumin as a tracer molecule, and an increase in extravascular radiolabel is used as an indicator of increased permeability of the pulmonary capillary barrier (14). Increases in extravascular albumin accumulation are considered to be more sensitive indicators of early capillary damage than edema quantified using the lung wet/dry weight ratio (33). Among many examples, Matalon and Egan (26) showed an increase in extravascular albumin equivalents of 76–210% in lungs of rabbits exposed to 65 h of 100% O2 even though the wet/dry weight ratio did not change significantly. This is comparable to the 180% extravascular albumin increase we observed in the 1-h 40 cmH2O PIP without a detectable change in wet/dry weight ratios. Similarly, in rat lungs damaged by high airway pressures, Dreyfuss et al. (14) observed a 430% increase in lung extravascular albumin plasma equivalents despite the fact that wet/dry weight ratios increased by only 14%.
However, few studies have used unlabeled endogenous albumin for evaluating lung extravascular albumin spaces, nor is it common to separate extravascular albumin into interstitial and alveolar fluid compartments, as done in the present report. The ELISA method described here appears to be as sensitive as radiolabeled albumin for detecting vascular injury without the added risk and expense that accompanies the use of radioisotopes. In support of this contention, we found that, in the group ventilated for 1 h at 40 cmH2O PIP untreated, total extravascular albumin plasma equivalents increased by 180% compared with nonventilated lungs with no difference in wet/dry weight ratios. Extravascular albumin also increased 150% in the group ventilated for 2 h at 40 cmH2O PIP after treatment with OGG1, also with no significant increase in wet/dry weight ratio. In more severe injury, after ventilation for 2 h at 40 cmH2O PIP, lung extravascular albumin increased by 2,100%, whereas wet/dry weight ratio increased by only 61%. Partitioning of albumin spaces into interstitial and alveolar compartments also was informative; the interstitial space displayed limited maximal increase in endogenous albumin of only approximately three- to fourfold compared with an almost unlimited space available in the alveolar compartment. The significant increases in interstitial space also occurred before significant alveolar flooding, which confirms the relationship of interstitial edema to alveolar flooding previously described (43). Viewed collectively, it appears that ELISA measurement of the extravasation of endogenous albumin into lung interstitial and alveolar compartments presents an attractive alternative method to the conventional use of radiolabeled albumin to assess the severity of lung injury evoked by high PIP.
One of the key findings reported herein is that VILI is accompanied by an increased equilibrium level of oxidative base damage in the mitochondrial genome. In some ways, this is not surprising. Involvement of ROS and RNS in VILI is well documented (38). Rapid production of ROS in response to cyclical stretch has been demonstrated for both endothelial cells and lung epithelial cells, with endothelial ROS generated by mitochondria within 15 min (1, 5). The mitochondrial genome also is considerably more sensitive than nuclear DNA to oxidative damage; thus the VILI-related DNA damage reported in previous studies most likely reflects damage to the mitochondrial genome. That mtDNA damage seen in the present study is related to an oxidant stress is unambiguously supported by the detection of damage by the enzyme Fpg, which selectively recognizes an end-product of oxidant stress, 8-oxoguanine, in a biologically relevant target, DNA.
As noted above, one of the interesting findings reported herein is that an mtDNA repair enzyme, OGG1, had a significant protective effect at every level of high PIP-induced lung injury. Disposition of the fusion protein construct in lung tissue as well as its biological activity were similar to that reported previously for its use in perfused rat lungs (6). In particular, we found in both the isolated rat lung and intact mouse models that the fusion protein rapidly accumulated in lung tissue and specifically in the mitochondrial but not in nuclear fractions. Furthermore, the mitochondrially targeted OGG1 suppressed oxidative mtDNA damage in perfused lungs evoked by glucose oxidase-glucose-generated hydrogen peroxide (6) and, in the present study, by elevated PIP. Thus the protective actions of the fusion protein discussed below are most likely attributed to interactions with oxidatively damaged mtDNA.
Inhibition of oxidative mtDNA damage afforded by mitochondrially targeted OGG1 was associated with attenuation of the 2.8-fold increase in total extravascular albumin space in lungs ventilated for 1 h at 40 cmH2O PIP even though there were no significant differences in BAL cytokines or lung wet/dry weight ratios. The most dramatic protection associated with fusion protein treatment was observed with moderately severe injury (2 h at 40 cmH2O PIP) that produced a 25-fold increase in lung total extravascular albumin space and a 60% increase in lung wet/dry weight ratio. Mitochondrially targeted OGG1 prevented about 90% of the increase in vascular albumin leak, as well as the increases in wet/dry weight ratios and inflammatory cytokines and the decreases in the tissue GSH pool and GSH/GSSG ratio. Some protective effect of OGG1 occurred even at the highest level of injury (2 h at 50 cmH2O PIP), where massive lung edema, albumin leak, and cytokine production occurred in all groups. In this instance, although the mtDNA repair enzymes did not prevent lung injury, there was a striking effect on survival because none of the untreated, 50 cmH2O PIP-ventilation mice survived the protocol, whereas all of the OGG1-treated mice survived for the entire 2-h ventilation period.
The lavage concentrations of MIP-2 and IL-6 were used to evaluate the effect of OGG1 on the magnitude of the innate immune inflammatory response. IL-6 is produced by lymphocytes and macrophages in response to trauma and has both inflammatory and anti-inflammatory effects. IL-6 highly correlates with the severity of lung injury in both mice and humans (18, 47, 52), but IL-6-blocking antibodies actually augmented VILI-induced lung permeability in mice because neutrophil IL-6 reduced neutrophil adhesion and transmigration (50). MIP-2α (CXCL2), the mouse analog of IL-8 in humans, is secreted by activated alveolar macrophages and is elevated by injurious ventilation (37). Belperio et al. (2) also demonstrated protection against VILI in CXCR2 knockout mice. The mtDNA repair enzyme protected against both the vascular permeability increase and propagation of the inflammatory response, as indicated by significant reductions in both MIP-2 and IL-6 in BALF in OGG1-treated mice ventilated for 1 h and 2 h at 40 cmH2O PIP.
Also in support of the present findings, Jafari et al. (19) observed a reciprocal relationship of levels of the cytokines IL-8 and IL-6 with the intracellular GSH concentration after cyclical stretch of alveolar type II epithelial cells. When N-acetylcysteine was used to scavenge free radicals and preserve the intracellular GSH pool, the excess IL-8 and IL-6 production as well as NF-κB and activated protein-1 binding were suppressed. This inverse relationship of IL-6 to the GSH pool was also observed in lungs of intact mice in the present study. In other studies, Kuipers et al. (21) linked these innate immune cytokine responses to activation of the NALP3 inflammasome because the increases in inflammatory cytokines and chemokines, BAL protein, and neutrophil accumulation were suppressed in lungs of NALP3 knockout mice after high-volume ventilation. It is tempting to speculate that mitochondrially targeted OGG1 in the present study reduced the release of mtDNA fragments that serve as damage-associated molecular pattern compounds to bind Toll-like receptors and activate the NALP3 inflammasome.
In light of the above findings that mitochondrially targeted OGG1 protected against high-PIP-induced mtDNA damage as well as a constellation of functional and biochemical effects of high PIP, the question arises as to how mtDNA damage is mechanistically linked to VILI at the cellular level. The mitochondrial genome is 10 to 100 times more susceptible to oxidative damage than nuclear DNA (51), and augmentation of mtDNA repair by transfection of genes encoding repair enzymes targeted to mitochondria has previously been shown to maintain mitochondrial function and prevent apoptosis during oxidative stress in multiple cell types including lung endothelial cells (10, 11, 16, 23). More recently, the same fusion protein construct used herein was found to protect isolated lungs from acute endothelial barrier dysfunction evoked by hydrogen peroxide (6). In cultured cell studies, mtDNA repair enzymes were found to enhance the rate of mtDNA repair after oxidative injury and reduce the equilibrium level of damage, thus pointing to the conclusion that oxidative mtDNA damage triggered cell death and dysfunction pathways (11).
Traditional concepts hold that mtDNA damage, through disruption of mtDNA transcription, leads to deficiencies in mitochondrially encoded subunits of oxidative phosphorylation and attendant increases in ROS production, culminating in a vicious, feed-forward cycle responsible for cell death and dysfunction (9, 44). In an extension of this idea, the present data are largely consistent with the idea that the mtDNA repair protein is effective because it speeds the rate of mtDNA repair after oxidative damage, thereby preventing the rise in equilibrium damage to a level that deranges mitochondrial ROS homeostasis. In support of this concept, mitochondrially targeted OGG1 prevented depletion of total GSH pool and the GSH/GSSG ratio in mice ventilated for 2 h with 40 cm H2O PIP, thus suggesting that preservation of mtDNA integrity afforded protection against a degree of persistent oxidant stress capable of overwhelming endogenous ROS scavenging.
In some ways, however, the beneficial actions of mtDNA OGG1 is not so easily ascribed to its mtDNA repair activity per se. One general line of evidence against this prospect is related to the time course of lung injury and its protection by mitochondrially targeted OGG1. For example, hydrogen peroxide-induced increases in isolated lung Kf occurred rapidly and were, not only inhibited by pretreatment, but also reversed by the mtDNA repair proteins even when they were administered after injury was established. The protective effects of the mtDNA repair enzymes on VILI noted in the present study also are prominent because of the rapidity by which high PIP is translated into endothelial barrier dysfunction; VILI, manifest as increases in lung vascular permeability, is evident within 10 to 30 min of high-PIP ventilation (12, 13, 35, 53). Both the protective effects of the fusion protein noted previously in isolated, hydrogen peroxide-injured lungs and the rapid onset of VILI-related vascular permeability are difficult to explain based on the feed-forward model because the time required for accumulation of oxidative mtDNA damage and its translation into defective electron transport and attendant ROS leakage seems too long to explain such rapid biological effects (7).
There are, however, other plausible mechanisms whereby mtDNA damage could be linked to the induction of VILI. For example, Reyes et al. (39) reported that mitochondrial nucleoids, the mtDNA multiprotein complex, are linked to the cytoskeleton via transmitochondrial membrane spanning nonmuscle myosin and actin filaments. Interestingly, previous studies indicate that inhibition of nonmuscle myosin light chain kinase attenuates increases in lung vascular permeability resulting from a variety of insults including VILI (29, 32, 40). Thus it is easy to envision how damage to mtDNA and resulting destabilization of nucleoid interactions with the cytoskeleton could trigger cytoskeletal derangements required for stretch-mediated endothelial barrier disruption. In our animal model, mitochondrially targeted OGG1 may be protective by virtue of its ability to stabilize nucleoid cytoskeleton. Kamp and colleagues (31) reported that both wild-type and an enzymatically inactive mutant OGG1 protected against asbestos-induced cytotoxicity in A549 cells and suggested that these beneficial effects might be related to the ability of OGG1 to bind the proapoptotic aconitiase (31).
OGG1 also appears to independently activate pathways that could modulate vascular permeability and inflammation. In this context, OGG1 has recently been reported to act as a guanine exchange factor which, after binding the common oxidative base damage product, 8-oxoguanine, activates RAS family GTPases, thereby leading to phosphorylation of the mitogen-activated kinases, MEK 1/2 and ERK 1/2 (3). Thus mitochondrially targeted OGG1 could bind free 8-oxoguanine and thereby suppress the RAS-mediated evolution of VILI. Although ERK 1/2 phosphorylation increases dramatically during high pressure ventilation, inhibition or genetic deletion of ERK 1/2 did not attenuate VILI (24). However, all isoforms of phosphatidylinositol 3-kinase have Ras interaction sites, and pharmacological inhibition or genetic deletion of phosphatidylinositol 3-kinase markedly attenuates the increases in lung permeability and edema during high-volume ventilation (17, 25, 30).
In summary, an mtDNA repair enzyme, OGG1, reduced oxidative mtDNA damage, preserved the redox status of the lung, and attenuated the permeability and inflammatory cytokine responses to high-PIP ventilation. Under conditions of severe elevations in PIP, the fusion protein also suppressed VILI-related mortality. It is tempting to speculate that such mtDNA repair protein fusion could have clinical application during mechanical ventilation in patients with ARDS to protect against VILI and inflammation. The generalized systemic effects may also be protective against multiple organ failure, which may have accounted for the increased survival in the repair enzyme-treated 50 cmH2O PIP groups.
GRANTS
This work was supported in part by grants from the National Institutes of Health (HL092992, HL058234, HL073244, and Project #3 in P01 HL66299).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.H., M.M., J.M.C., O.M.G., M.V.R., B.J.P., and G.L.W. performed experiments; M.H., M.M., J.M.C., M.V.R., B.J.P., M.N.G., and J.C.P. analyzed data; M.H., B.J.P., M.N.G., and J.C.P. interpreted results of experiments; M.H., M.M., M.N.G., and J.C.P. prepared figures; M.H. drafted manuscript; M.H., M.N.G., and J.C.P. edited and revised manuscript; M.H., B.J.P., G.L.W., M.N.G., and J.C.P. approved final version of manuscript; G.L.W., M.N.G., and J.C.P. conception and design of research.
REFERENCES
- 1. Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L486–L496, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 110: 1703, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boldogh I, Hajas G, Guilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, Sur S, Hazra TK, Mitra S. Activation of Ras Signaling by 8-oxoguanine DNA glycosylase Bound to Its Excision Product 8-oxoguanine. J Biol Chem 287: 20769–20773, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L834–L841, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN. Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am J Physiol Lung Cell Mol Physiol 301: L892–L898, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clayton DA. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol 7: 453–478, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Crit Care 14: 220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol 283: L205–L210, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J Biol Chem 275: 37518–37523, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 132: 880–884, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Dreyfuss D, Saumon G. Ventilator induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Dreyfuss D, Soler P, Saumon G. Spontaneous resolution of pulmonary edema caused by short periods of cyclic overinflation. J Appl Physiol 72: 2081–2089, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Druzhyna NM, Hollensworth SB, Kelley MR, Wilson GL, LeDoux SP. Targeting human 8-oxoguanine glycosylase to mitochondria of oligodendrocytes protects against menadione-induced oxidative stress. Glia 42: 370–378, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Druzhyna NM, Musiyenko SI, Wilson GL, LeDoux SP. Cytokines induce nitric oxide-mediated mtDNA damage and apoptosis in oligodendrocytes. Protective role of targeting 8-oxoguanine glycosylase to mitochondria. J Biol Chem 280: 21673–21679, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Fanelli V, Puntorieri V, Assenzio B, Martin E, Elia V, Bosco M, Delsedime L, Del Sorbo L, Ferrari A, Italiano S, Ghigo A, Slutsky A, Hirsch E, Ranieri V. Pulmonary-derived phosphoinositide 3-kinase gamma (PI3K+¦) contributes to ventilator-induced lung injury and edema. Inten Care Med 36: 1935–1945, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Haitsma JJ, Uhlig S, Verbrugge SJ, Goggel R, Poelma DL, Lachmann B. Injurious ventilation strategies cause systemic release of IL-6 and MIP-2 in rats in vivo. Clin Physiol Funct Imag 23: 349–353, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology 9: 43–53, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Koczor CA, Snyder JW, Shokolenko IN, Dobson AW, Wilson GL, LeDoux SP. Targeting repair proteins to the mitochondria of mammalian cells through stable transfection, transient transfection, viral transduction, and TAT-mediated protein transduction. Methods Mol Biol 554: 233–249, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Kuipers MT, Aslami H, Janczy JR, van der Sluijs KF, Vlaar AP, Wolthuis EK, Choi G, Roelofs JJ, Flavell RA, Sutterwala FS, Bresser P, Leemans JC, van der PT, Schultz MJ, Wieland CW. Ventilator-induced lung injury is mediated by the NLRP3 inflammasome. Anesthesiology 116: 1104–1115, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Laffey JG, Engelberts D, Kavanagh BP. Injurious effects of hypocapnic alkalosis in the isolated lung. Am J Respir Crit Care Med 162: t-405, 2000 [DOI] [PubMed] [Google Scholar]
- 23. LeDoux SP, Wilson GL. Base excision repair of mitochondrial DNA damage in mammalian cells. Prog Nucleic Acid Res Mol Biol 68: 273–284, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Li LF, Liao SK, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: a prospective, controlled animal experiment. Crit Care 11: R25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lionetti V, Lisi A, Patrucco E, De Giuli P, Milazzo MG, Ceci S, Wymann M, Lena A, Gremigni V, Fanelli V, Hirsch E, Ranieri VM. Lack of phosphoinositide 3-kinase-gamma attenuates ventilator-induced lung injury. Crit Care Med 34: 134–141, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Matalon S, Egan EA. Interstitial fluid volumes and albumin spaces in pulmonary oxygen toxicity. J Appl Physiol 57: 1767–1772, 1984 [DOI] [PubMed] [Google Scholar]
- 27. Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome—Four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33: 319–327, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merenyi G, Lind J. Free radical formation in the peroxynitrous acid (ONOOH)/peroxynitrite (ONOO-) system. Chem Res Toxicol 11: 243–246, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, Lussier YA, Watterson DM, Dudek SM, Garcia JG. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol 44: 40–52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyahara T, Hamanaka K, Weber DS, Drake DA, Anghelescu M, Parker JC. Phosphoinositide 3-kinase, Src, and Akt modulate acute ventilation-induced vascular permeability increases in mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L11–L21, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, de Souza-Pinto NC, Bohr VA, Budinger GR, Schumacker PT, Weitzman SA, Kamp DW. Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic Biol Med 47: 750–759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parker JC. Inhibitors of myosin light chain kinase, phosphodiesterase and calmodulin attenuate ventilator induced lung injury. J Appl Physiol 89: 2241–2248, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Parker JC. Acute lung injury and pulmonary vascular permeability: use of transgenic models. In: Comprehensive Physiology. Walden, MA: Wiley, 2011, pp. 835–882 [DOI] [PubMed] [Google Scholar]
- 34. Parker JC, Falgout HJ, Parker RE, Granger DN, Taylor AE. The effect of fluid volume loading on exclusion of interstitial albumin and lymph flow in the dog lung. Circ Res 45: 440–450, 1979 [DOI] [PubMed] [Google Scholar]
- 35. Parker JC, Townsley MI, Rippe B, Taylor AE, Thigpen J. Increased microvascular permeability in dog lungs due to high peak airway pressures. J Appl Physiol 57: 1809–1816, 1984 [DOI] [PubMed] [Google Scholar]
- 36. Pastukh V, Ruchko M, Gorodnya O, Wilson GL, Gillespie MN. Sequence-specific oxidative base modifications in hypoxia-inducible genes. Free Radic Biol Med 43: 1616–1626, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol 93: 517–525, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal 9: 2003–2012, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Reyes A, He J, Mao CC, Bailey LJ, Di RM, Sembongi H, Kazak L, Dzionek K, Holmes JB, Cluett TJ, Harbour ME, Fearnley IM, Crouch RJ, Conti MA, Adelstein RS, Walker JE, Holt IJ. Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Res 39: 5098–5108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossi JL, Velentza AV, Steinhorn DM, Watterson DM, Wainwright MS. MLCK210 gene knockout or kinase inhibition preserves lung function following endotoxin-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 292: L1327–L1334, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells. Free Radic Biol Med 50: 1107–1113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tankersley CG, Rabold R, Mitzner W. Differential lung mechanics are genetically determined in inbred murine strains. J Appl Physiol 86: 1764–1769, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Taylor AE, Parker JC. The interstitial spaces and lymph flow. In: Handbook of Physiology: The Respiratory System. Circulation and Nonrespiratory Function., edited by Fishman AP, Fisher AB. Bethesda, MD: Am. Physiol. Soc., 1985, p. 167–320 [Google Scholar]
- 44. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Upadhyay D, Correa-Meyer E, Sznajder JI, Kamp DW. FGF-10 prevents mechanical stretch-induced alveolar epithelial cell DNA damage via MAPK activation. Am J Physiol Lung Cell Mol Physiol 284: L350–L359, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27: 337–349, 2006 [DOI] [PubMed] [Google Scholar]
- 48. White CW, Jackson JH, McMurtry IF, Repine JE. Hypoxia increases glutathione redox cycle and protects rat lungs against oxidants. J Appl Physiol 65: 2607–2616, 1988 [DOI] [PubMed] [Google Scholar]
- 49. Wilson MR, Choudhury S, Goddard ME, O'Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol 95: 1385–1393, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, Frank JA. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol 182: 8056–8062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yakes FΓ, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94: 514–519, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoshikawa S, King JA, Lausch RN, Penton AM, Eyal FG, Parker JC. Acute ventilator-induced vascular permeability and cytokine responses in isolated and in situ mouse lungs. J Appl Physiol 97: 2190–2199, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Yoshikawa S, King JA, Reynolds SD, Stripp BR, Parker JC. Time and pressure dependence of transvascular Clara cell protein, albumin, and IgG transport during ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 286: L604–L612, 2004 [DOI] [PubMed] [Google Scholar]