Abstract
ADAM15 is a disintegrin and metalloprotease recently implicated in cancer and chronic immune disorders. We have recently characterized ADAM15 as a mediator of endothelial barrier dysfunction. Whether this molecule contributes to acute inflammation has not been evaluated. The purpose of this study was to investigate the role of ADAM15 in mediating pulmonary microvascular leakage during acute inflammatory injury. Immunofluorescent staining and Western blotting revealed that the endothelium was the main source of ADAM15 in lung tissue. In a mouse model of acute lung injury induced by lipopolysaccharide (LPS), upregulation of ADAM15 was observed in association with pulmonary edema and neutrophil infiltration. The LPS-induced inflammatory injury, as demonstrated by bronchoalveolar lavage neutrophil count, lung wet-to-dry weight ratio, and myeloperoxidase activity, was significantly attenuated in Adam15−/− mice. Studies with primary cell culture demonstrated abundant ADAM15 expression in endothelial cells (ECs) of mouse lung but not in neutrophils. Deficiency of ADAM15 in ECs had no obvious effect on basal permeability but significantly attenuated hyperpermeability response to LPS as evidenced by albumin flux assay and measurements of transendothelial electrical resistance, respectively. ADAM15 deficiency also reduced neutrophil chemotactic transmigration across endothelial barriers in the presence or absence of formyl-methionyl-leucyl-phenylalanine (fMLP). Rescue expression of ADAM15 in Adam15−/− ECs restored neutrophil transendothelial migration. These data indicate that ADAM15 upregulation contributes to inflammatory lung injury by promoting endothelial hyperpermeability and neutrophil transmigration.
Keywords: vascular permeability, metalloproteinase, endothelial dysfunction, inflammation
the adams (a disintegrin and metalloproteinase) are multifunctional transmembrane glycoproteins that are involved in a variety of biological processes (5, 17). In general, ADAMs are metalloproteinases that are capable of cleaving cell surface protein ectodomains and inducing signaling events through receptor shedding, and/or outside-in signaling (8, 26). ADAM15 is a less studied ADAM isoform that is found in human vein endothelium (13) and is associated with certain types of cancer and chronic inflammatory or immunological disorders (5, 14, 21). In particular, ADAM15 supports cancer metastasis by promoting tumor cell migration and angiogenesis (21, 24). Increased ADAM15 expression is also detected in atherosclerotic lesions, rheumatoid synovium, angiogenic retinas, and the intestines of patients with inflammatory bowl disease (1, 3–5). This suggests that increased ADAM15 expression is involved in a variety of inflammatory conditions.
Our recent studies of the effects of thrombin on human umbilical vein endothelial cells (HUVECs) has identified ADAM15 as an important mediator of endothelial barrier dysfunction. In general, endothelial barriers are formed by a layer of closely apposed endothelial cells that are anchored to the extracellular matrix at the basement membrane. The cell-cell and cell-matrix adhesion structures act coordinately with the cytoskeleton to maintain the integrity of the barrier with a low basal permeability (22, 31). However, these structures can be degraded or shed by proteases like ADAMs (8, 26) or may undergo conformational changes in response to pathological signaling (12, 18, 31). In HUVEC monolayers, ADAM15 is necessary for both endothelial hyperpermeability and increased neutrophil transmigration in response to thrombin exposure (28). Moreover, these ADAM15-dependent effects are mediated largely by endothelial intracellular signaling through Src kinase and extracellular regulated kinase (ERK)-1/2, and not through receptor ectodomain shedding. These interesting findings prompted us to further investigate the role of ADAM15 in pathological states involving endothelial barrier dysfunction.
Acute lung injury (ALI) is a condition that involves pulmonary microvascular leakage in response to systemic or local lung inflammation. Unresolved ALI can progress into respiratory distress syndrome, a major cause of mortality and morbidity in patients with infectious disease or sepsis. Despite theories advanced regarding its pathogenesis, there has been no major breakthrough in treatment or prevention of ALI. This is largely owing to the complexity of the disease and our incomplete understanding of its endpoint cellular mechanisms (6, 15). Microvascular barrier dysfunction is a common endpoint of inflammatory response characterized by endothelial hyperpermeability, plasma leakage, and leukocyte infiltration in the lungs, leading to respiratory distress and multiple organ failure (7, 16). On the basis of the observation that ADAM15 is involved in several inflammatory conditions, coupled with our results showing thrombin induced barrier dysfunction in umbilical vein endothelium is ADAM15 dependent, we infer that ADAM15 plays a role in endothelial barrier dysfunction during inflammation in other tissues including the lungs.
In the present study we examined the role of ADAM15 in the lungs and in pulmonary endothelium, under experimental inflammatory conditions [exposure to bacterial lipopolysaccharide (LPS)]. LPS exposure is known to induce edema formation and neutrophil infiltration in the lungs. We find that LPS exposure increases ADAM15 expression in the murine lungs. LPS-induced pulmonary edema and neutrophil infiltration are greatly attenuated in gene knockout Adam15−/− mice, and similarly ADAM15 deficiency decreases LPS-induced endothelial hyperpermeability and neutrophil transendothelial migration in vitro in primary cultured mouse lung endothelial cell (MLEC) monolayers. Together the data support a causal role for ADAM15 in endothelial barrier dysfunction during LPS-induced inflammation in the lungs.
MATERIALS AND METHODS
Animals.
Adam15−/− mice were kindly provided by Dr. Carl P. Blobel at the Hospital for Special Surgery, New York, NY. Adam15−/− mice were bred with C57/BL6 mice (Charles River Laboratories) to obtain Adam15+/− mice, which were used to generate Adam15−/− mice and Adam15+/+ control littermates. All animal procedures were conducted in compliance with the NIH guidelines for animal research and approved by the Institutional Animal Care and Use Committee.
In vivo mouse experiments.
Ten-week-old mice were injected intraperitoneally with Pseudomonas aeruginosa LPS (Sigma) at a dose of 10 mg/kg body wt or with an equivalent volume of PBS. After 24 h, lungs were excised and the tissue wet weight was measured immediately, followed by drying at 80°C for 36 h to obtain dry weight measurements. For lung histopathology, 6-μm sagittal cryosections through central and peripheral areas of each lobe were stained with hematoxylin and eosin and observed under a microscope. Neutrophils were examined by immunohistological staining with a conventional ABC assay using a rat anti-mouse antibody raised against the neutrophil marker, Ly-6G (Invitrogen). Inflammatory injury was assessed in a blind manner, grading four histological findings, each on a scale of 0–4: 1) alveolar congestion, 2) hemorrhage, 3) infiltration of neutrophils into the extravascular space, and 4) thickness of the alveolar wall as previously described (23). A composite injury score was calculated for each mouse on a scale of 0 to 16, by averaging five randomly selected fields under ×20.
BAL neutrophil count and MPO assay.
To quantitatively assess neutrophil infiltration, neutrophils in the bronchoalveolar lavage (BAL) fluid (BALF) were counted and neutrophil myeloperoxidase (MPO) in the lung measured. Briefly, the lung was lavaged through a tracheal cannula with 0.7 ml saline, four times. For each mouse, ∼2.5 ml of BALF was retrieved. Total cell number in BALF was determined with a hemocytometer and neutrophil differentiation determined by examining 200 cells on Wright-Giemsa-stained cytocentrifuge slide. Neutrophil number was calculated by multiplying the total cell count by the neutrophil fraction. Lung MPO activity was assessed on the basis of H2O2-dependent oxidation of o-dianisidine. Briefly, MPO in the lung tissue was extracted with 0.5% hexadecyltrimethylammonium bromide (Sigma). The enzymatic reaction was initiated by adding a 10-ml sample to 190 ml potassium phosphate buffer containing 0.167 mg/ml o-dianisidine (Sigma) and 0.005% H2O2 in each well of a 96-well plate. Kinetic absorbance at 460 was read at 5 min. MPO activity was calculated according to the definition that one unit of MPO activity is the amount required to increase OD460 by 0.001/min at 25°C and normalized to protein concentration determined by BCA assay (Bio-Rad).
Albumin transendothelial flux and TER.
As indicators of barrier properties, albumin permeability across endothelial monolayers and transendothelial electrical resistance (TER) were measured as previously described (27). For albumin permeability, endothelial cells (ECs) were grown to confluence on a 0.3-μm pore Transwell membrane, and followed by FITC-labeled albumin (15 mM) added to the top chamber. After 2 h, concentrations of albumin in the top and bottom chambers were measured with a fluorescence microplate reader (BioTek). The permeability coefficient of albumin (Pa) was determined as Pa = [A]/t × 1/A × V/[L], where brackets denote albumin concentration in the bottom chamber [A] or the top chamber [L], t is time (s), A is the area of the membrane (cm2), and V is the volume of the bottom chamber. At the same time, parallel wells were subjected to an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche) and calcein-AM (Invitrogen) staining to confirm cell viability and confluence. For TER, 105 cells were seeded onto (8 × 1 cm2 chamber) electrical cell impedance sensing (ECIS) electrode arrays (Applied Biophysics) and grown overnight. With a 1-V, 4,000-Hz alternating current signal supplied through a 1-MΩ resistor to a constant-current source, in-phase voltage and out-of-phase voltage were recorded with ECMS 1.0 software (CET). Endothelial barrier function was expressed as TER normalized to the baseline prior to LPS addition, and the time when LPS added was set = 0.
Neutrophil isolation and transmigration assay.
Murine polymorphonuclear leukocytes were purified from bone marrow (mPMN) by using a discontinuous Percoll (Sigma) gradient as previously described (20). Cell purity (>95% of granulocytes) was assessed by flow cytometry with an anti-Ly-6G antibody. ECs were grown to confluence on 96-well Transwell membranes (3 μm pore size) (Millipore, CA), and 5×104 mPMNs were added to the top well, with or without 10 nM formyl-methionyl-leucyl-phenylalanine (fMLP) (Sigma, MO) in the bottom well. After 2 h, 5×104 polystyrene beads (Polysciences) were added to the bottom well and transmigrated neutrophils were quantified via flow cytometry by gating granulocytes and were normalized to the number of beads. Parallel wells were subjected to MTT assay and calcein-AM staining to confirm cell viability and confluence.
Western blotting.
Cells were lysed in RIPA buffer (Upstate) plus phosphatase (Sigma) and protease inhibitors (Roche). Protein concentration of cell lysates was determined by BCA assay (Bio-Rad). Cell lysates corresponding to 20 μg protein were fractioned by SDS-PAGE under reducing conditions and electrophoretically transferred to polyvinylidene difluoride membranes (Amersham Biosciences). Membranes were blocked in 3% BSA in TBS with 0.1% Tween 20, and membranes were incubated overnight at 4°C with primary antibody, followed by 2 h at room temperature with horseradish peroxidase-conjugated second antibody. Membranes were developed using Pico Supersignal chemiluminescent substrate (Pierce). Stripping buffer (Thermo) was applied to the same membrane for reprobing when necessary. Films were scanned and intensity of bands quantified with NIH software Image J.
Immunofluorescence.
Lung tissue slides were fixed with ice-cold acetone and incubated at room temperature for 1 h with a primary antibody, followed by another 1 h with FITC- or Cy3-labeled secondary antibodies (Jackson ImmunoResearch). Cell nuclei were counterstained with Hoechst 33342 (Invitrogen). Imaging was performed with a Zeiss Axio Observer 200M inverted microscope equipped with Zeiss AxioVision software.
Flow cytometry.
ECs were detached with enzyme-free cell dissociation solution (Chemicon). To examine the purity of mouse primary cells, mPMN or MLEC were stained with phycoerythrin-conjugated anti-Ly-6G antibody (Invitrogen) or FITC-labeled anti-mouse CD31 antibody (Invitrogen) on ice for 30 min prior to flow cytometric analysis. To examine the surface expression of ADAM15, transfected MLEC were stained with anti-ADAM15 antibody (R&D) for 30 min on ice followed by incubation with Cy3-conjugated secondary antibody (Jackson ImmunoResearch) for another 30 min. In all experiments, an identical amount of isotype IgG was applied as a control for nonspecific staining.
Statistical analysis.
Nonparametric lung-injury scores were presented as mean ± interquartile range and analyzed with a Mann-Whitney test. Other data were presented as means ± SE. An unpaired Student's t-test was used to compare differences between two groups and ANOVA followed by Newman-Keuls posttest for comparison of multiple groups. Statistical significance was defined as P ≤ 0.05.
RESULTS
The endothelium is a dominant source of ADAM15 in lung.
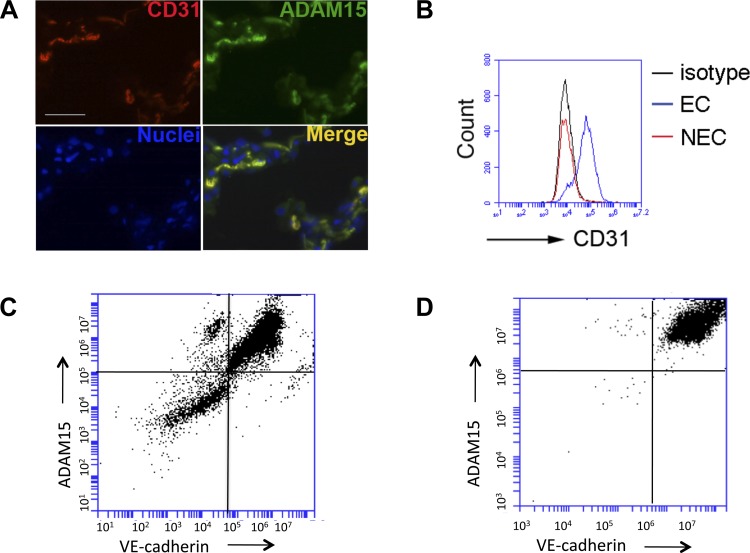
To determine whether ADAM15 is expressed in lung endothelium, immunohistological staining was performed with antibodies raised against ADAM15 or the endothelial specific marker CD31. In tissue sections, expression of ADAM15 was strongly colocalized with CD31 (Fig. 1A), suggesting that endothelium is the predominant site/source of ADAM15 in lung tissue (13). This was further confirmed by flow cytometry analysis of endothelial vs. nonendothelial cells segregated by immunomagnetic assay (Fig. 1, B–D). CD31 staining suggested the high purity of the isolated endothelial cells (Fig. 1B). Costaining with another EC-specific marker, VE-cadherin, further demonstrated a higher level of ADAM15 expression in VE-cadherin-positive EC than in VE-cadherin-negative nonendothelial cells isolated from the same lung tissue (Fig. 1, C and D).
Fig. 1.
The endothelium is a predominant source of ADAM15 in lung. A: ADAM15 colocalizes with CD31 in lung tissue. Immunofluorescence microscopy demonstrates colocalization of CD31 and ADAM15. Lung sections were labeled with CD31 and ADAM15 antibody, followed by rhodamine and FITC-conjugated second antibody, respectively. Nuclei are counterstained with Hoechst 33342 (blue). Images are captured with Zeiss Axio Observer 200M inverted microscope equipped with Zeiss AxioVision software (scale bar = 25 μm). B: FACS analysis: primary lung endothelial cells (EC) or non-EC (NEC) isolated from wild-type (WT) mice were labeled with an anti-CD31 antibody or IgG isotype control. C: FACS analysis of ADAM15 demonstrated coexpression with VE-cadherin positive endothelial cells vs. NEC. D: after CD31 selection, EC cells were cultured to confluence and assayed for VE-cadherin and ADAM15 expression as shown.
ADAM15 is upregulated in the lung tissue of mice receiving LPS.
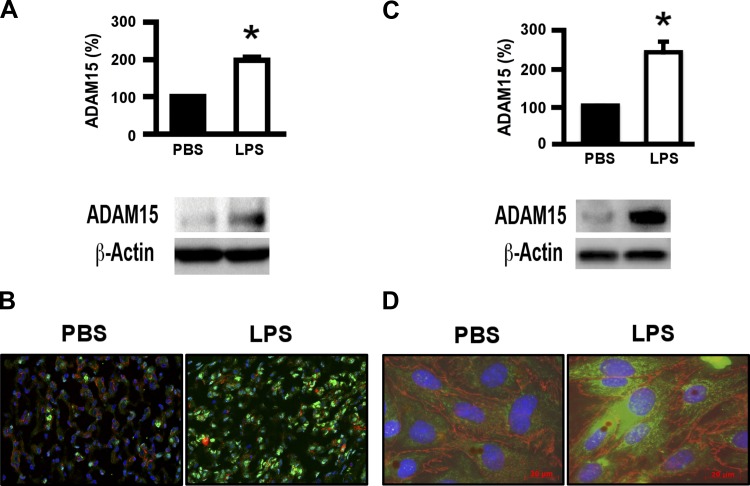
On the basis of our previous observations of ADAM15-mediated hyperpermeability in HUVEC cells, and that ADAM15 expression is increased in several inflammatory conditions, we suspected that ADAM15 could similarly be involved in lung inflammation. To test this notion, we examined ADAM15 expression in the lungs in a mouse model of LPS-induced endotoxicosis; LPS injection is known to cause systemic inflammation and ALI, similar to sepsis (2). Mouse lung tissues were examined for ADAM15 expression at 24 h after LPS treatment. Compared with vehicle (PBS) treatment, LPS treatment significantly increased ADAM15 expression in the lungs (Fig. 2, A and B). LPS induction of endothelial ADAM15 upregulation was confirmed by using cultured cells in vitro both by Western blotting and immunofluorescence (Fig. 2, C and D).
Fig. 2.
ADAM15 is upregulated in the lung tissue of mice receiving LPS. A: Western blot analysis of ADAM15 expression in mouse lung tissue. Bottom, representative blot from C57/BL6 WT mice 24 h after intraperitoneal injection of LPS (10 mg/kg body wt) or an equivalent volume of PBS. Top, quantification (n = 4; *P < 0.05 vs. PBS treated). B: immunofluorescence microscopy shows increased ADAM15 staining in LPS-treated compared with PBS-treated lungs. Lung sections were labeled with ADAM15 antibody, followed by phycoerythrin-conjugated second antibody. C: Western blotting and densitometry quantification (top, n = 3, *P < 0.01) of ADAM15 expressed by in vitro cultured endothelial cells with or without LPS stimulation (12 h.). D: immunocytochemistry of untreated (left) of LPS-treated cells (as in C). Green, ADAM15; red, VE-cadherin; blue, nuclei.
ADAM15 contributes to LPS-induced pulmonary edema and inflammatory injury.
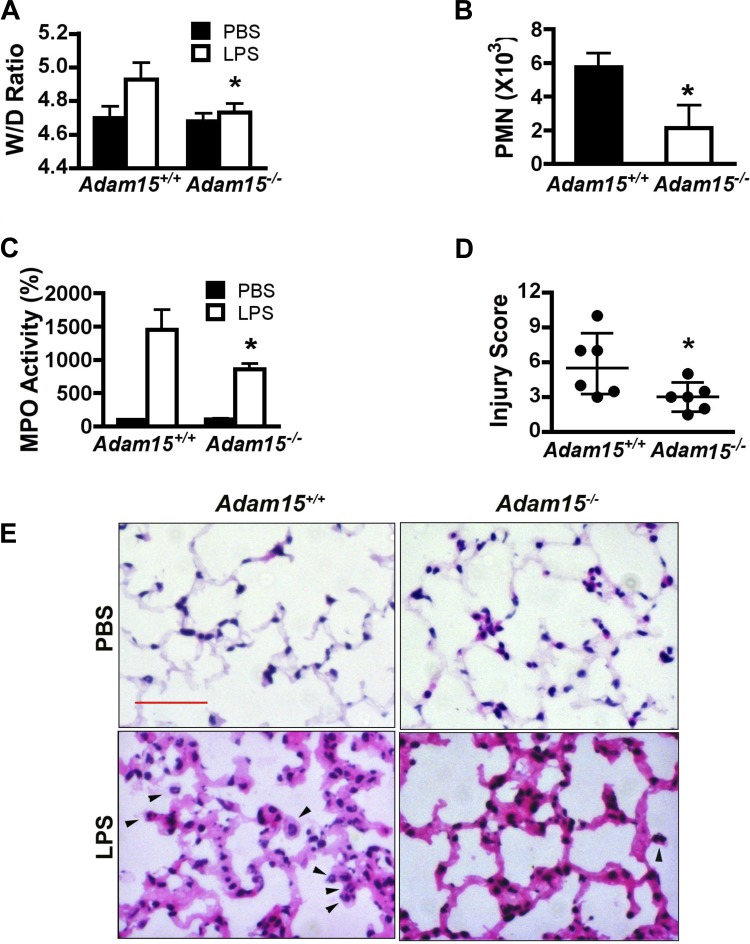
To evaluate the extent of lung injury and the functional role of ADAM15 in LPS-induced ALI, we performed quantitative assessments of ratios of wet to dry lung tissue weights (an indicator of edema), neutrophil accumulation in the BAL, and MPO activity in lung tissues, in parallel with visual examination of histopathology (Fig. 3) between Adam15−/− mice and their wild-type (WT) littermates at 24 h after LPS or PBS injection. Compared with PBS, LPS challenge induced a marked increase in the lung wet-to-dry weight ratios in WT mice (Fig. 3A), indicating fluid accumulation. This edema response was significantly attenuated in Adam15−/− mice. In the BALF, neutrophils were almost undetectable after PBS (data not shown), but significantly increased after LPS injection, a response attenuated in ADAM15 deficiency (Fig. 3B). MPO assays also indicated neutrophil accumulation in lung tissues after LPS; MPO levels were lower in Adam15−/− than in WT lungs (Fig. 2C). Similar results were observed for immunohistochemical staining with the neutrophil-specific marker Ly-6G (data not shown). Furthermore, WT and Adam15−/− mice showed similar peripheral neutrophil counts, indicating that differences in neutrophil abundance were due to barrier dysfunction rather than to increased abundance of leukocytes in the blood circulation (data not shown). Further histological examination of lung tissues showed plasma fluid leakage coupled with thickening of alveolus-capillary membrane in LPS-treated mice, indicative of diffuse inflammatory injury; pathologies were observed to a lesser extent in Adam15−/− compared with WT mice (Fig. 3E). To more objectively quantify overall injury severity, the results of these tests were compiled into a composite lung injury score, including histological assessment of alveolar congestion, neutrophil infiltration, and alveolus-capillary membrane pathology (described in materials and methods). The results of this score indicated that overall LPS-induced lung injury was significantly attenuated in ADAM15 deficient mice compared with WT mice (Fig. 3D), supporting a functional role for ADAM15 in LPS-induced ALI.
Fig. 3.
ADAM15 contributes to LPS-induced pulmonary edema and inflammatory injury in mice. Adam15−/− mice and their Adam15+/+ littermates were intraperitoneally injected with LPS (10 mg/kg body wt) or PBS vehicle. A: the ratio of lung tissue wet to dry weights (W/D ratio) is assessed as an indicator of edema (n = 12; *P < 0.05 vs. Adam15+/+). B: neutrophil counts in bronchial alveolar lavage (n = 6; *P < 0.05 vs. Adam15+/+). C: myeloperoxidase (MPO) activity in lung tissues (n = 6; *P < 0.05 vs. Adam15+/+). D: comparison of the lung injury score (n = 6, *P < 0.05 vs. Adam15+/+). E: compared with vehicle (PBS)-treated, LPS-treated lung shows thickened alveolus-capillary membrane and neutrophil infiltration as indicated with hematoxylin and eosin staining (arrowheads pointing to neutrophils in alveoli). The LPS-induced inflammatory injury is less pronounced in Adam15−/− lungs. Scale bar = 50 μm.
ADAM15 deficiency attenuates LPS-induced endothelial barrier dysfunction.
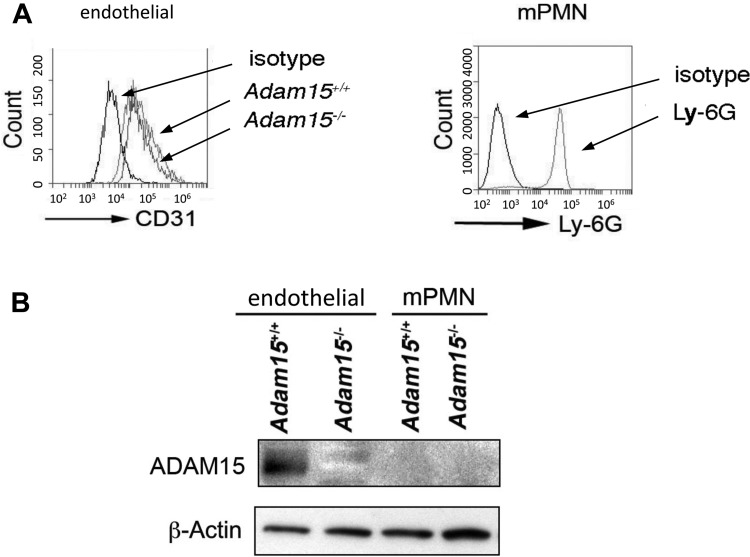
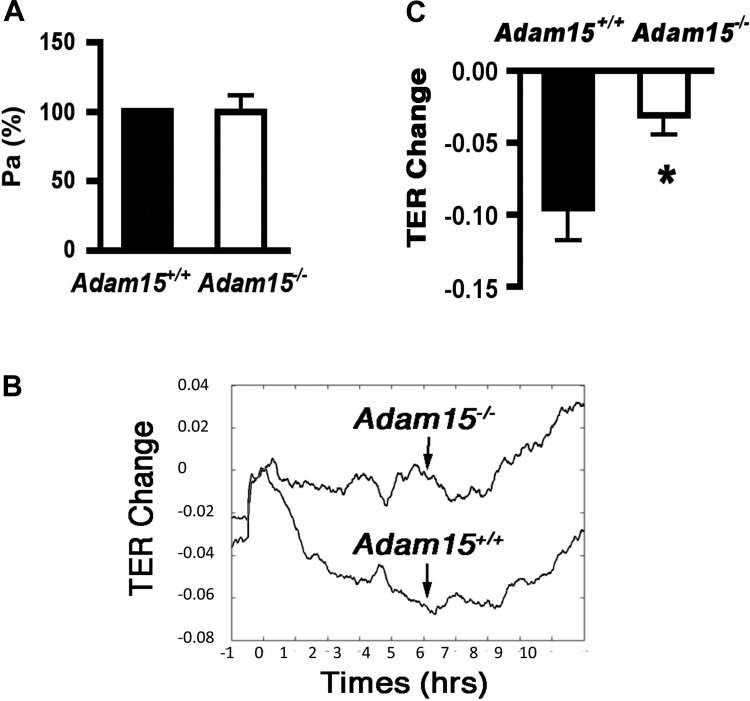
The direct effects of ADAM15 on cell function were examined in primary MLEC and in mPMNs. The identity and purity of these cells were confirmed by flow cytometric staining for CD31 and Ly-6G, respectively (Fig. 4A). Western blotting detected abundant ADAM15 protein expression in MLEC from WT mice (Fig. 4B). Interestingly, ADAM15 was not detectable in mPMN lysates. Confluent MLEC monolayers were used to assess endothelial permeability to albumin (Pa). ADAM15 deficiency did not significantly alter basal Pa compared with that of WT cells (Fig. 5A). We also assessed TER using ECIS, a much more sensitive measure of barrier integrity. ECIS measurements revealed a significantly attenuated endothelial hyperpermeability (decreased TER) response to LPS treatment in ADAM15-deficient cells compared with WT MLEC monolayers (Fig. 5, B and C).
Fig. 4.
ADAM15 expression is detectable in endothelial cells but not in neutrophils. A: flow cytometric evidence of cell identity and purity. Left, primary lung microvascular EC (MLEC) from Adam15+/+ or Adam15−/− or mice labeled with an anti-CD31 antibody or IgG isotype control. Right, neutrophils from mouse bone marrow (mPMN) are labeled with an anti-Ly-6G antibody, and granulocytes are gated. Isotype control is also shown. The data represent 3 independent experiments from different mice. B: ADAM15 expression in MLEC and mPMN. Shown are representative blots from 3 independent experiments demonstrating abundant ADAM15 in Adam15+/+ EC but not in neutrophils.
Fig. 5.
ADAM15 deficiency attenuates LPS-induced endothelial barrier dysfunction. A: Transwell assays that measure the flux of FITC-albumin across endothelial monolayers show no significant difference in basal albumin permeability (Pa) between Adam15−/− and Adam15+/+ MLEC monolayers (P > 0.05). B and C: electrical cell impedance sensing (ECIS) studies that measure the dynamic transepithelial resistance changes indicate ADAM15 deficiency in MLEC attenuates LPS-induced endothelial hyperpermeability (n = 3, *P < 0.05 vs. Adam15+/+ MLECs).
Endothelial ADAM15 mediates neutrophil transendothelial migration.
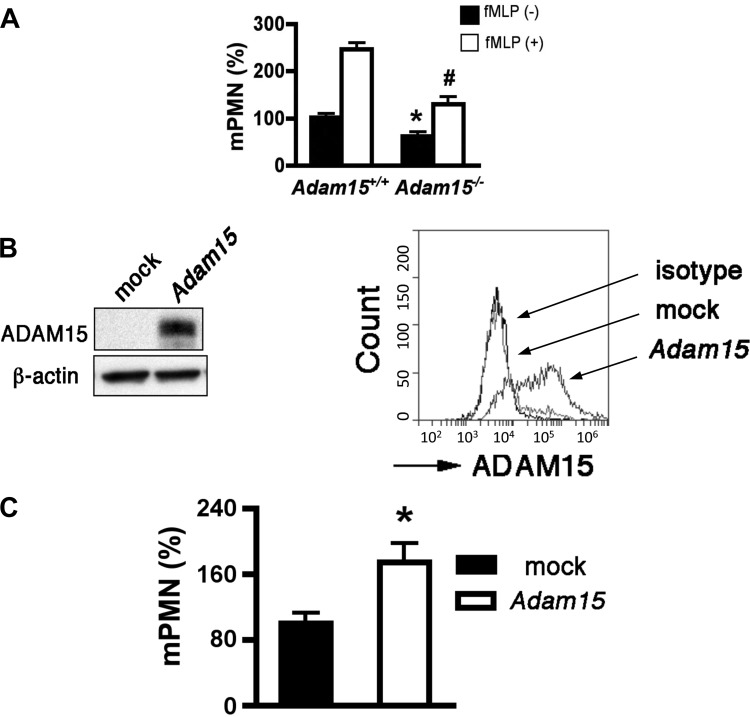
An important property of endothelial barriers is restriction of neutrophil transmigration across endothelium. To determine the role of ADAM15 in this aspect of barrier function, neutrophil transmigration assays were performed in the presence or absence of the chemoattractant fMLP. The addition of fMLP potently increased neutrophil transmigration across WT MLEC monolayers compared with the absence of fMLP. In both cases, ADAM15 deficiency significantly reduced neutrophil transendothelial migration; this effect was especially pronounced in the presence of fMLP (Fig. 6A). Because ADAM15 protein was not detected in neutrophils, we reasoned that endothelial ADAM15 plays an active role in regulating neutrophil transmigration by controlling endothelial barrier function rather than by controlling the migratory properties of neutrophils per se. This inference is supported by the observation that rescued expression of ADAM15 in Adam15−/− endothelial cells (Fig. 6B) restores neutrophil transendothelial migration (Fig. 6C).
Fig. 6.
Endothelial ADAM15 mediates neutrophil transendothelial migration. A: mPMN transmigration across Adam15−/− MLECs, especially in the presence of fMLP (10 nM), is significantly attenuated (n = 3; *P < 0.05 vs. Adam15+/+ without fMLP; #P < 0.05 vs. Adam15+/+ with fMLP). B: rescue expression of ADAM15 in Adam15−/− MLECs is confirmed with Western blotting (left) and flow cytometry (right). C: rescue expression of ADAM15 in Adam15−/− MLECs increased neutrophil transendothelial migration. Adam15−/− MLECs were transfected with pcDNA/Adam15 or mock. At 72 h posttransmigration, fMLP-induced neutrophil transendothelial migration was assayed as above (n = 3, *P < 0.05 vs. mock transfected).
DISCUSSION
Pulmonary microvascular endothelial barrier dysfunction plays a critical role in the development of ALI. The cellular mechanisms and signaling events responsible for this injurious process are not fully understood. Through this study we have identified a novel function of ADAM15 in pulmonary endothelial injury during acute inflammation. We first demonstrated that ADAM15 is upregulated in the lungs of septic mice. The functional impact of this molecule in regulating endothelial barrier function is supported by the studies with ADAM15 knockout mice and ADAM15-deficient primary cells. In WT mice, LPS injection induces significant upregulation of ADAM15 coupled with plasma leakage and neutrophil infiltration in the lungs. The inflammatory injury was also greatly attenuated in Adam15−/− mice. Consistent with these in vivo observations, cell experiments showed that ADAM15 deficiency reduced permeability and neutrophil transmigration across endothelial monolayers. These data provide direct evidence that ADAM15 contributes to acute lung inflammation.
Expression of ADAM15 in vascular endothelial cells was identified in 1997 (13, 17). More recently, ADAM15 has been implicated in cancer and chronic inflammatory processes, including atherosclerosis, rheumatoid arthritis, inflammatory bowel disease, and wound angiogenesis (1, 3–5). Although correlative analysis showing increased abundance in inflammatory tissues supports the pathophysiological function of this molecule, whether and how it contributes to the inflammatory response, especially that in vital organs such as the lungs, has not been evaluated. To the best of our knowledge, this study provides the first line of evidence for the upregulation of ADAM15 in mediating ALI. ADAM15 expression is markedly increased in the lungs of mice receiving LPS, a widely accepted animal model of sepsis and ALI (2). Because ADAM15-specific inhibitors have not been developed, we used genetically modified (Adam15−/−) mice for in vivo studies in conjunction with functional studies with primary cell culture. Given the results that ADAM15 deficiency did not significantly affect permeability in unstimulated endothelial cells but attenuated the LPS-induced hyperpermeability response, we suggest that constitutive expression of ADAM15 is not a determinant of basal barrier properties; however, it plays an active role in mediating endothelial hyperpermeability under inflammatory conditions. In support of this notion, our previous study with HUVEC cells demonstrates that ADAM15 overexpression augments thrombin-induced hyperpermeability (27). LPS treatment reportedly opens the endothelial paracellular permeability pathway by activating Src family kinases and subsequently phosphorylating tyrosine residues of junction proteins (10). Interestingly, our previous study with HUVEC cells indicates that ADAM15-induced endothelial hyperpermeability also involves Src activity (27). However, whether ADAM15 and TLR4 signaling have synergistic effects on endothelial barrier dysfunction remains to be investigated.
We also examined the effects of ADAM15 on neutrophil transendothelial migration, a process associated with endothelial barrier dysfunction and acute lung injury in response to infection or sepsis (6, 9). ADAM15 deficiency attenuated neutrophil infiltration into the lungs after LPS treatment. Likewise, ADAM15 knockdown reduced neutrophil transendothelial chemotaxis toward fMLP (27). Because neutrophil extravasation and infiltration into the alveolar space are controlled not only by neutrophil migration activity but also by endothelial/epithelial barrier properties (11, 25), it is difficult to discern the relative importance or specific contribution of these processes to the observed neutrophil response in the lungs. However, given the fact that ADAM15 expression is not detectable in neutrophils, we suggest that the attenuated transmigration across the endothelium with ADAM15 knockout or knockdown is due, at least in large part, to the change in expression endothelial ADAM15. In other words, endothelial ADAM15 plays an active role in mediating neutrophil transmigration. This is well supported by our evidence showing that rescued expression of ADAM15 in Adam15−/− endothelial cells restores the ability of neutrophils to transmigrate across endothelial monolayers.
In conclusion, we provide novel evidence that ADAM15 is upregulated in the lungs during inflammation and that increased expression of ADAM15 promotes pulmonary edema and neutrophil infiltration. ADAM15 disrupts endothelial barrier integrity and contributes to the LPS-induced endothelial hyperpermeability response. Although the present study contributes to a better understanding of the pathophysiology of acute lung injury, further investigation in this area may lead to the development of therapeutic targets for effective treatment and prevention of inflammatory diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL61507, HL84542, and HL96640.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.S., R.S.B., D.L.M., R.R.R., T.K., M.H.W., and S.Y.Y. analyzed data; C.S., T.K., M.H.W., and S.Y.Y. interpreted results of experiments; C.S. prepared figures; R.S.B. and D.L.M. performed experiments; R.R.R. drafted manuscript; R.R.R., M.H.W., and S.Y.Y. edited and revised manuscript; M.H.W. and S.Y.Y. conception and design of research; S.Y.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Chris D. Pivetti, Robert L. Pitts, Olesya P. Litovka, and Bert J. Frederich for excellent technical assistance.
REFERENCES
- 1. Al-Fakhri N, Wilhelm J, Hahn M, Heidt M, Hehrlein FW, Endisch AM, Hupp T, Cherian SM, Bobryshev YV, Lord RS, Katz N. Increased expression of disintegrin-metalloproteinases ADAM-15 and ADAM-9 following upregulation of integrins alpha5beta1 and alphavbeta3 in atherosclerosis. J Cell Biochem 89: 808–823, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med 29: S2–S6; discussion S6–S7, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bohm BB, Aigner T, Blobel CP, Kalden JR, Burkhardt H. Highly enhanced expression of the disintegrin metalloproteinase MDC15 (metargidin) in rheumatoid synovial tissue. Arthritis Rheum 44: 2046–2054, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Charrier L, Yan Y, Driss A, Laboisse CL, Sitaraman SV, Merlin D. ADAM-15 inhibits wound healing in human intestinal epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol 288: G346–G353, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Charrier-Hisamuddin L, Laboisse CL, Merlin D. ADAM-15: a metalloprotease that mediates inflammation. FASEB J 22: 641–653, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis 20: 345–352, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Czabanka M, Peter C, Martin E, Walther A. Microcirculatory endothelial dysfunction during endotoxemia—insights into pathophysiology, pathologic mechanisms and clinical relevance. Curr Vasc Pharmacol 5: 266–275, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122, 2008 [DOI] [PubMed] [Google Scholar]
- 9. DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol 30: 547–556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem 283: 13437–13449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol 55: 662–675, 1994 [PubMed] [Google Scholar]
- 12. Guo M, Breslin JW, Wu MH, Gottardi CJ, Yuan SY. VE-cadherin and β-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am J Physiol Cell Physiol 294: C977–C984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herren B, Raines EW, Ross R. Expression of a disintegrin-like protein in cultured human vascular cells and in vivo. FASEB J 11: 173–180, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie TA, Ludwig T, Chiusaroli R, Baron R, Preissner KT, Manova K, Blobel CP. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol 23: 5614–5624, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Curr Drug Targets 8: 509–514, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kratzschmar J, Lum L, Blobel CP. Metargidin, a membrane-anchored metalloprotease-disintegrin protein with an RGD integrin binding sequence. J Biol Chem 271: 4593–4596, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med 11: e19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langer H, May AE, Bultmann A, Gawaz M. ADAM 15 is an adhesion receptor for platelet GPIIb-IIIa and induces platelet activation. Thromb Haemost 94: 555–561, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61 hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol 133: 895–910, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucas N, Najy AJ, Day ML. The therapeutic potential of ADAM15. Curr Pharm Des 15: 2311–2318, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Mikawa K, Nishina K, Takao Y, Obara H. Efficacy of partial liquid ventilation in improving acute lung injury induced by intratracheal acidified infant formula: determination of optimal dose and positive end-expiratory pressure level. Crit Care Med 32: 209–216, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Najy AJ, Day KC, Day ML. ADAM15 supports prostate cancer metastasis by modulating tumor cell-endothelial cell interaction. Cancer Res 68: 1092–1099, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7: 803–815, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. ADAM10 regulates endothelial permeability and T-cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res 102: 1192–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun C, Wu MH, Guo M, Day ML, Lee ES, Yuan SY. ADAM15 regulates endothelial permeability and neutrophil migration via Src/ERK1/2 signalling. Cardiovasc Res 87: 348–355, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun C, Wu MH, Guo M, Day ML, Lee ES, Yuan SY. ADAM15 regulates endothelial permeability and neutrophil migration via Src/ERK1/2 signalling. Cardiovasc Res 87: 348–355, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 38: 1276–1283, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Wood KA, Angus DC. Pharmacoeconomic implications of new therapies in sepsis. Pharmacoeconomics 22: 895–906, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation 7: 395–403, 2000 [PubMed] [Google Scholar]