Abstract
Acute radiation syndrome is a life-threatening condition that has the potential to affect large populations of humans. Although several animal models of this syndrome are available, the total-body–irradiated mouse has emerged as an important tool to evaluate the efficacy of prospective prophylaxis, mitigation, and treatment compounds. Despite the widespread use of this model, humane endpoints have not been clearly identified. To address this issue, we developed a cageside observation-based scoring system specifically for total-body–irradiated mice to assess the progression of clinical signs associated with acute radiation syndrome. Male C57BL/6 mice (n = 175; age, 8 to 9 wk) received an anticipated LD50 dose of radiation and were observed for progression of clinical signs of acute radiation syndrome for 30 d. All mice were scored individually through cageside observation of their body posture (score, 0 to 3), eye appearance (0 to 3), and activity level (0 to 3). Retrospective analysis of the score data indicated that death could be predicted accurately by using increasing cumulative scores (0 to 9). Total scores of 6, 7, 8, and 9 were associated with mortality rates of 78.6%, 86.4%, 93.3%, and 100%, respectively. Furthermore, scores of 6, 7, and 8 predicted death within 3, 1.5, and 0.5 d, respectively. The use of this scoring system provides investigators and IACUCs with predictive humane, surrogate endpoints for total-body–irradiated mice. This system allows preemptive euthanasia of mice before they become moribund, thereby minimizing pain and distress associated with acute radiation syndrome and improving animal welfare.
Abbreviations: ARS, acute radiation syndrome; LD50/30, the dose of radiation that is lethal for 50% of the test subjects within 30 d; TBI, total body irradiation
Acute radiation syndrome (ARS) due to either accidental radiation exposure or nuclear attack is a life-threatening condition that has the potential to affect a large population of people. The severity of ARS is dependent on the overall dose, dose rate, radiation quality, and proportion of the body that is irradiated. ARS typically progresses through 4 clinical phases: prodrome, latency, illness, and either recovery or death.1,7 The prodromal period is characterized by nausea, vomiting, and fatigue and can include autonomic instability and loss of consciousness at high doses in humans.1 After the latent phase, which can be absent with high doses of radiation, the illness phase manifests in various organ systems as particular syndromes of the hematopoietic, gastrointestinal, skin, and neurovascular systems.1,8 The hematopoietic system is the most sensitive to radiation, and decreased blood cell counts can be detected even in asymptomatic patients.1 Increasing radiation doses cause complete destruction of bone marrow, sloughing of the mucosal layer of the gastrointestinal system, skin burns, and breakdown of the neurologic and cardiovascular systems, ultimately resulting in death.1,8
Preparatory planning for the medical management of ARS is essential and requires the use of total body irradiation (TBI) models in animals to evaluate the efficacy of prospective prophylaxis, mitigation, and treatment compounds.8,28 The 2 body systems most sensitive to TBI are the hematopoietic and gastrointestinal systems, and mice are an excellent model for both; the C57BL/6 and C3H/He strains are used most often.28 To study the hematopoietic syndrome, the most widely used TBI condition for acute radiation damage is the LD50, because of the temporal predictability of the development of subsequent clinical signs. The temporal sequence and outcome of the hematopoietic syndrome occur considerably more rapidly in mice than in humans, and the experimental outcome is defined as the LD50/30 (50% population death within 30 d of irradiation) in mouse models.28 In addition, because mice have been deemed an appropriate species for testing radioprotectors or mitigators, the clinical efficacy of various drugs can be evaluated easily in a mouse TBI model.28
Although mice are an exceedingly common model for ARS, there are currently no established humane endpoint criteria for these types of studies. Similar to sepsis models, TBI and ARS studies typically use LD50/30 as the standard of comparison, and this practice has resulted in the frequent and widespread use of death or moribund state as the experimental endpoint.5,7,18,26 The moribund condition, an unresponsive and immobile animal, is a commonly used endpoint for a variety of research protocols associated with high mortality or progressive and severe disease states.23,24 Using this criterion requires the animal to progress through all potential phases of pain and distress associated with the chosen model to a near-death state before the animal is euthanized.17
The ability to predict death with a high probability and high accuracy in TBI studies would allow for preemptive euthanasia, with the intent to (1) ameliorate terminal pain and distress associated with ARS and (2) improve animal welfare. To this end, the current study sought to establish an observation-based scoring system to assess the health status of irradiated mice. Specifically, mice that received a targeted LD50/30 TBI dose were evaluated by using daily cageside observational scoring of body posture, eye appearance, and activity level. This observation-based study was performed in conjunction with an approved IACUC study of the effects of synthetic parathyroid hormone in irradiated mice. The results were analyzed to identify the predictive nature of these criteria of impending death, with the goal of establishing useful endpoint criteria for future TBI studies.
Materials and Methods
Animals.
All mice used during this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals10 at the University of Illinois at Chicago (Chicago, IL), an AAALAC-accredited institution. All procedures were reviewed and approved by the University of Illinois at Chicago Animal Care Committee. Male C57BL/6NCrl (n = 175; age, 6 wk) mice were purchased from Charles River Laboratories (Kingston, NY). Mice were maintained in facilities in which dirty-bedding–contact sentinel mice tested negative on a quarterly basis for Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Theiler murine encephalomyelitis virus, reovirus, rotavirus, mouse adenovirus, polyoma virus, K virus, mouse cytomegalovirus, mouse thymic virus, lymphocytic choriomeningitis virus, hantavirus, ectromelia virus, lactate dehydrogenase elevating virus, mouse norovirus, Mycoplasma pulmonis, and Helicobacter spp. In addition, sentinel mice were free of helminth and external parasites. There were approximately 50 cages of research mice per sentinel cage. On arrival, mice were housed 5 per cage in static autoclaved (sterilized) polysulfone microisolation cages (Ancare, Bellmore, NY) with irradiated diet (7912, Harlan Teklad, Madison, WI), HCl-acidified municipal water in bottles, and autoclaved hardwood bedding (Sani-Chips, Harlan Teklad, Madison, WI) with a 14:10-h light:dark cycle. In the current study, the internal measured cage temperature and humidity were 20 to 25 °C and 56% to 88%, respectively. Mice were acclimated for 2 to 3 wk prior to irradiation. Once the mice were irradiated, they were housed individually under the same housing specifications. Beginning 5 d after irradiation and for the duration of the study period, the acidified drinking water was supplemented with 0.67 mg/mL ciprofloxacin. At the time of total body irradiation, mice were 8 to 9 wk of age and weighed an average of 22.4 g. Mice were weighed on days 1, 7, 14, and 31 after TBI.
All mice were irradiated by using a 6-MV LINAC photon source (model EX21, Varian Medical Systems, Palo Alto, CA). They received a uniform, total-body, midline tissue dose of 80 ± 2.5 cGy/min at a total dose of 845 cGy for an expected LD50/30. Mice were allocated into 3 cohorts, with TBI administered at 2-wk intervals.
Animals received treatment with a synthetic parathyroid hormone (Parathyroid Hormone, Human, Acetate, PolyPeptide Laboratories, Torrance, CA). Synthetic parathyroid hormone (300, 400, or 600 μg/kg) or saline (vehicle-only control) was administered subcutaneously. Treatment was initiated 24 h after radiation exposure and was repeated daily for 14 d. Each of the 4 groups comprised 42 or 43 mice. Six additional mice did not receive any injections. Any mouse that became moribund (see description following) during the study was euthanized by CO2 asphyxiation followed by cervical dislocation. All mice that survived to day 31 were euthanized in this manner also.
Cageside observational scoring system.
Cageside observations by the veterinary staff were made in parallel with research staff studying the effects of synthetic parathyroid hormone in irradiated mice. A veterinarian observed the mice on days 1 through 30 after TBI to score them. There was a graded observation schedule according to the progression and subsequent remission of ARS clinical signs in all mice that received TBI. The mice were scored once daily during days 1 through 6 and 19 through 30 and twice daily during days 7 through 18. One veterinarian performed 90% of the observations; 5 other veterinarians received one-on-one training to score mice on some weekends. Training was completed in one or two 30-min periods as needed to ensure independent scoring consistency within 1 total score unit (0 to 9) between veterinarians.
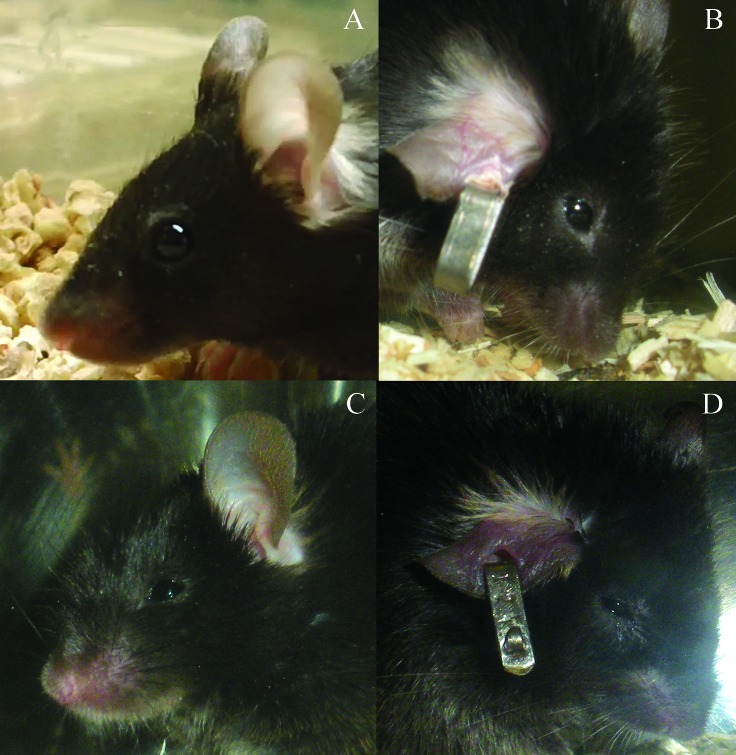
The scoring system used in the current study to assess irradiated mice was adapted from those developed for distressful rodent studies and according to behaviors exhibited in previous mouse TBI studies.14,16,19 Rodent cages were removed from racks to facilitate improved visualization of mice and to stimulate their movement around their cage, but cages were not opened at any point during the scoring process. Mice received a score of 0 to 3 for each of the criteria of posture, eye appearance, and activity level. Posture was scored based on a hunched appearance (Figure 1). A score of 0 indicated normal body posture; 1indicated a slightly hunched posture; 2 indicated a moderately hunched posture; and 3 indicated a severely hunched posture. Eye appearance was scored according to how open the eyes were (Figure 2). A score of 0 indicated that eyes open more than 75%; 1 indicated eyes 50% to 75% open; 2 indicated eyes 25% to 49% open; and 3 indicated eyes that were open less than 25%. Activity level was scored according to the amount each mouse moved in its cage. A score of 0 indicated an animal that moved around the cage normally and was very active; 1 indicated a slightly reduced activity level or a mild gait abnormality; 2 indicated a mouse that was moving very slowly or a severely altered gait; 3 indicated an animal that was reluctant to (or did not) move, taking no more than 3 or 4 steps. Moribund animals had a total score of 9, receiving a score of 3 for each of the criteria, and were euthanized as described. In addition, all mice were observed for morbidity by the primary investigator's staff between the veterinarian's observation periods but were not scored according to the current system. During the investigator's observations, any mouse deemed moribund was euthanized, thereby precluding final scoring by the veterinarian prior to euthanasia.
Figure 1.
Scoring of posture according to cageside observations. (A) The normal mouse posture received a score of 0. (B) A slightly hunched posture scored a 1. (C) A mouse that was moderately to severely hunched but was still able to rear up or stretch out to access food and water received a score of 2. (D) A mouse that was severely hunched and unable to rear up was scored as 3.
Figure 2.
Scoring of eye appearance according to cageside observations. (A) Normal mouse eyes that were open greater than 75% received a score of 0. (B) Mouse eyes that were open 50% to 75% were scored as 1. (C) Mice whose eyes were open 25% to 49% receive the score of 2. (D) Mouse eyes that were open less than 25% were scored as 3.
Statistics.
From the cageside observational scoring data for each mouse, the first day that each mouse received a total score of 5, 6, 7, 8 and 9 was identified. Subsequently, the number of days between the time that they were first observed at a particular score and their death (whether observed or euthanized) or the end of the study was calculated. For mice that skipped a score, the time of the observed higher score was recorded for both the observed score and the skipped score. For each of these initial score events, Kaplan–Meier survival curves were constructed to indicate the probability of survival over time, and the survival time and its corresponding 95% confidence interval were determined (version 9.2, SAS, Cary, NC). The median survival times and the estimated 25th percentile and 75th percentile survival times, after a specific score was achieved, were reported.
Results
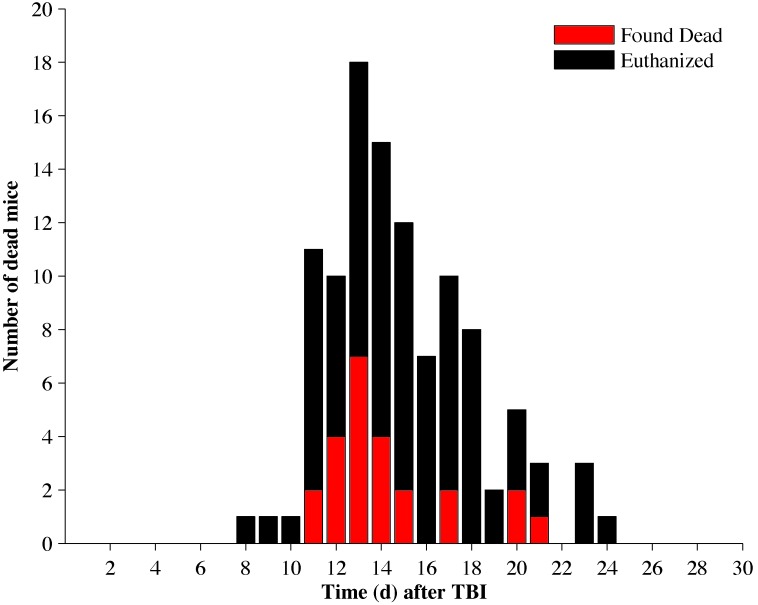
Mouse deaths were distributed between days 8 and 24 after TBI (Figure 3). Of the 109 mice that died during the 30-d period, 25 were found dead and 84 were euthanized. The overall mortality rate for the study was 62%. There were no statistical differences in mortality between treatment groups.
Figure 3.
Total mouse death due to TBI with 845 cGy. Mice that were euthanized are represented by black bars, and mice that died spontaneously are represented by red bars. The stacked value of the red and black bars represent the total number of animals that died on a given day. A total of 109 mice died between days 8 and 24 after TBI.
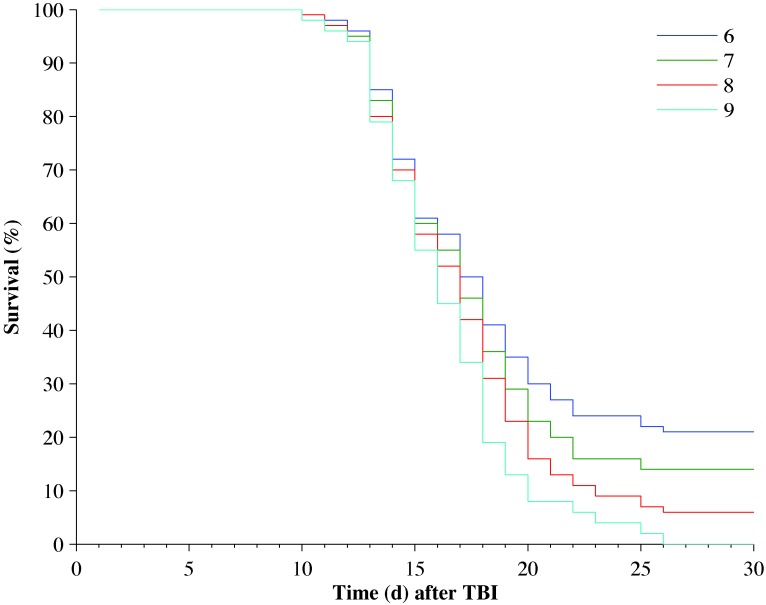
Observational scores were positively correlated with animal death; that is, mortality increased with increasing observational scores (Table 1 and Figure 4). Five mice died spontaneously between observation points that had scores of 5 or less at their last observation. Only mice with a score of 9 or animals determined to be moribund by the primary investigator staff were euthanized. Observational scores of 6, 7, and 8 were associated with mortality rates of 78.6%, 86.5%, and 93.4%, respectively (Figure 4). Observational scores of 6, 7, and 8 were associated with median subsequent survival times of 3.0, 1.5, and 0.5 d, respectively (Table 1).
Table 1.
Survival times for all mice after they attained a score of 5, 6, 7, 8, or 9 after TBI
| No. of mice |
||||||
| Score | Total | Died or were euthanized | Alive at the end of the 30-d period | Median survival (d) | 75% Survival (d) | 25% Survival (d) |
| 5 | 150 | 108 | 42 | 5.5 | 2.0 | 9.5 |
| 6 | 131 | 103 | 28 | 3.0 | 1.5 | 9.0 |
| 7 | 111 | 96 | 15 | 1.5 | 0.5 | 4.5 |
| 8 | 91 | 85 | 6 | 0.5 | 0.0 | 1.5 |
| 9 | 53 | 53 | 0 | 0.0 | 0.0 | 0.5 |
For example, a survival rate of 75% indicates that 25% of the mice died within the reported number of days after attaining the given score.
Figure 4.
Kaplan–Meier survival curves for all mice that amassed minimal scores of 6 (n = 131), 7 (n = 111), 8 (n = 91), and 9 (n = 53).
Irradiated mice lost weight during the first 2 wk after TBI (Table 2). The mice that survived the entire 30 d after TBI typically started gaining weight after day 14, whereas mice that died continued to lose body weight after day 14. Twelve (21.5%) of the animals that survived for the entire study and 58 (55.8%) of those that died during the study had lost at least 15% of their body weight when the last body weight was collected. Eight (12.3%) of the mice that survived for the entire study and 45 (43.3%) of those that died during the study had lost at least 20% of their body weight by the time body weight was measured for the last time.
Table 2.
Change in body weight of mice after TBI
| Day 1 | Day 7 | Day 14 | Day 21 | Day 31 | |
| Alive | −2.9% ± 0.3% (−18.0%, 2.1%) 65 | −4.6% ± 0.7% (−19.1%, 5.8%) 65 | −6.8% ± 1.2% (−33.9%, 5.8%) 65 | 0.3% ± 1.3% (−27.8%, 22.3%) 65 | 6.4% ± 1.1% (−27.0%, 24.2%) 65 |
| Died or euthanized | −3.5% ± 0.4% (−13.1%, 22.6%) 104 | −8.3% ± 0.7% (−26.6%, 2.9%) 104 | −24.1% ± 0.9% (−39.1%, -3.2%) 47 | −27.8% ± 5.9% (−35.0%, -16.2%) 3 | — |
Change in body weight (mean ± SEM [minimum, maximum] n) is represented as the percentage lost (indicated by −) or gained relative to the baseline weight before TBI.
Discussion
The use of morbidity as a surrogate for mortality requires the identification of criteria that can be used to define morbidity and verify applicability as a surrogate for death in survival endpoint studies.4,25 Clinical signs can provide an objective judgment of animal pain and distress if the observer is skilled, knowledgeable of the normal behavioral and physical parameters of the subject, and has good clinical observation skills.16 Behavioral monitoring has been suggested as a promising and unobtrusive way to monitor pain and distress.15,17,19 Recommended behavioral indicators include grooming, appetite, activity, aggression, facial expression, vocalization, appearance, posture, and response to handling.3,9 Scoring systems based on observations of unprovoked behavior and general physical appearance have been used previously to allow semiquantitative measurement of ‘sickness behavior’.17 These indicators also have been used successfully to generate surrogate humane endpoints, and examples include locomotion in chemotherapy fatigue studies;21 anorexia, labored breathing, and hindlimb paralysis in cancer models;29 labored breathing in aging studies;20 phenotypic monitoring in transgenic animals;6,27 and ambulation in sepsis models.13
Assigning scores to a series of observations of clinical signs provides a mechanism for tracking the deterioration or resolution of an animal's clinical condition. In addition, documenting a subject's progression of clinical signs facilitates discussion between veterinary and research staff to assist in decisions about euthanasia of that animal as a study progresses. The advantages of an observation-based score system have been acknowledged: there is close observation of the animals by all personnel involved, especially during the critical timeframe; subjective assessments of pain and distress are avoided, and evidence-based opinion becomes possible based on the documented and scored clinical signs; limited scoring options lead to an increased consistency of scoring; score sheets reveal patterns of deterioration or recovery over time; and the score sheet encourages all personnel involved to observe and recognize normal and abnormal behaviors in response to the experimental parameters.16 The current study limited the scoring to 3 specific observed clinical signs (body posture, eye appearance, and activity level) and provided guidance to assigning scores for those 3 parameters. C57BL/6 mice must first be assessed for anophthalmia and microphthalmia,12 because these conditions have the potential to affect the score for eye appearance. In developing the score sheet for TBI mice, other clinical signs including decreased grooming, focal swelling of face or limbs, and ocular discharge were inconsistent and thus not included in the numeric scoring.
Observational scoring was more predictive of death than was body weight in the current study. Specifically, scores of 7 and 8 in the current study were correlated to mortality rates of 86.5% and 93.4%, respectively. Furthermore, mice that scored 7 or 8 had median survival times of 1.5 and 0.5 d, respectively, before becoming moribund and requiring euthanasia. Preemptively euthanizing a mouse once an observational score of 7 or 8 is reached has the potential to mitigate animal pain and distress associated with ARS. Euthanasia of animals at a set score of 7 or 8 would have minimized pain and distress for up to 88.1% of all of the mice in the current study that died naturally or were euthanized due to ARS, only missing those animals that progressed to death quickly and received a score of 6 or lower on their last veterinarian observation. In future TBI studies, the initial number of mice placed on study could be increased to accommodate the potential confounding effects of animals that are euthanized but that might have gone on to live (13.5% at a score of 7 and 6.6% at a score of 8).
A body weight loss of 15% to 20% is a common endpoint criterion for a variety of studies; however, body weight is difficult to include in the evaluation of animals for TBI studies. Because of a long-standing implied correlation between animal handling and increased mortality in TBI studies, the frequency of weight measurement generally is limited to a maximum of every 2 to 3 d.11,22 In the current study, weight was measured only weekly and, as a result, the positive predictive value of either a 15% or 20% body weight loss for death (80.6% and 84.9%, respectively) was not as strong as it might otherwise have been. Weighing animals more frequently may increase the predictive value of body weight loss as a surrogate, humane endpoint; however, the increased handling necessary to do so might also increase the mortality rate.
A potential drawback with the use of observation-based endpoint criteria is interobserver variability,2 which we did not evaluate as part of the current study. Although multiple veterinarians scored the mice, they were trained to look at the specific criteria, and scoring consistency between veterinarians was ensured before any individual veterinarian scored mice independently. Interobserver variation likely could be a problem if score sheets are provided to observers without sufficient training or spot-checking to ensure consistency in observational scores. A future consideration for observation-based scoring for endpoint criteria is to evaluate and verify interobserver variability. Ultimately, training of and dialog between research staff and veterinary staff are essential for successful implementation of euthanasia criteria based on observational scores.
Other limitations of the current study include the use of a single strain, sex, animal source, radiation dose, and radiation source. Although a large population of mice was used in the current study to evaluate the observation-based scoring system's ability to accurately predict death due to TBI, whether mice with different genetic backgrounds, sources, or sex will develop a similar predictable ARS pattern of response to TBI is unknown. The relevance of these endpoints for other radiation doses also is unknown. Further evaluation is necessary to determine the broader applicability of these refined, humane endpoints in other radiation studies.
The results of the current study suggest that an observation-based scoring system can be an effective tool for mitigating pain and distress associated with ARS in mice through preemptive euthanasia. Using a cut-off score of 7 or 8 in TBI studies would accurately predict death 0.5 to 1.5 d in advance, providing for euthanasia of mice before they became moribund. IACUCs could use this information to establish behavior-based criteria for TBI studies based on the specific scientific objective of the protocol. Although these endpoint criteria may be useful for LD50/30 studies, there are limitations in using these same criteria for studies identifying mean survival times. In addition, IACUC could apply the observation-based scoring sheet to engage investigators to look more closely at their animals and to identify subtle changes in their behavior as ARS progresses. This use could be augmented with effective training and communication between all parties to ensure successful implementation as surrogate endpoint criteria in TBI mice.
Acknowledgments
We thank Dr Amelia Bartholomew for allowing us to do our observational study in parallel with her ongoing total-body–irradiation study. We also thank Drs Cynthia Adams, Kelly Garcia, Lisa Halliday, and Jeanette Purcell for their assistance in scoring the mice.
References
- 1.Anno GH, Baum SJ, Withers HR, Young RW. 1989. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5–30 Gy. Health Phys 56:821–838 [DOI] [PubMed] [Google Scholar]
- 2.Beynen AC, Baumans V, Bertens AP, Havenaar R, Hesp AP, Van Zutphen LF. 1987. Assessment of discomfort in gallstone-bearing mice: a practical example of the problems encountered in an attempt to recognize discomfort in laboratory animals. Lab Anim 21:35–42 [DOI] [PubMed] [Google Scholar]
- 3.Carstens E, Moberg GP. 2000. Recognizing pain and distress in laboratory animals. ILAR J 41:62–71 [DOI] [PubMed] [Google Scholar]
- 4.Clarke R. 1997. Issues in experimental design and endpoint analysis in the study of experimental cytotoxic agents in vivo in breast cancer and other models. Breast Cancer Res Treat 46:255–278 [DOI] [PubMed] [Google Scholar]
- 5.Davis TA, Landauer MR, Mog SR, Barshishat-Kupper M, Zins SR, Amare MF, Day RM. 2010. Timing of captopril administration determines radiation protection or radiation sensitization in a murine model of total body irradiation. Exp Hematol 38:270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis MB., Jr 2000. Humane endpoints for genetically engineered animal models. ILAR J 41:94–98 [DOI] [PubMed] [Google Scholar]
- 7.DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. 2011. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep 5 Suppl 1:S32–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorr H, Meineke V. 2011. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Med 9:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Laboratory Animal Research 2009. Recognition and alleviation of pain in laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 10.Institute of Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 11.Kallman RF, Silini G. 1964. Recuperation from lethal injury by whole-body irradiation. I. Kinetic aspects and the relationship with conditioning dose in C57Bl mice. Radiat Res 22:622–642 [PubMed] [Google Scholar]
- 12.Kalter H. 1968. Sporadic congenital malformations of newborn inbred mice. Teratology 1:193–199 [DOI] [PubMed] [Google Scholar]
- 13.Krarup A, Chattopadhyay P, Bhattacharjee AK, Burge JR, Ruble GR. 1999. Evaluation of surrogate markers of impending death in the galactosamine-sensitized murine model of bacterial endotoxemia. Lab Anim Sci 49:545–550 [PubMed] [Google Scholar]
- 14.Lloyd M, Wolfensohn S.1999. Practical use of distress scoring systems in the application of humane endpoints, p 48–53. In: Hendriksen CFM, Morton DB. Humane Endpoints in animal experiments for biomedical research. Proceedings of the International Conference, 22–25 November 1998. Zeist (The Netherlands): Royal Society of Medicine Press.
- 15.Lloyd M, Wolfensohn S, Thornton P.2000. Quantitative assessment of welfare in experimental animals: the development and use of scoring systems, p 1107–1117. In: Balls M, Zeller AMv, Halder ME. Progress in the reduction, refinement, and replacement of animal experimentation: Proceedings of the 3rd World Congress on Alternatives and Animal Use in the Life Sciences, held in Bologna, Italy, from 29 August to 2 September 1999. Amsterdam (The Netherlands): Elsevier Science BV.
- 16.Morton DB. 2000. A systematic approach for establishing humane endpoints. ILAR J 41:80–86 [DOI] [PubMed] [Google Scholar]
- 17.Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress, and discomfort in experimental animals and a hypothesis for assessment. Vet Rec 116:431–436 [DOI] [PubMed] [Google Scholar]
- 18.Nemzek JA, Hugunin KM, Opp MR. 2008. Modeling sepsis in the laboratory: merging sound science with animal wellbeing. Comp Med 58:120–128 [PMC free article] [PubMed] [Google Scholar]
- 19.Paster EV, Villines KA, Hickman DL. 2009. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med 59:234–241 [PMC free article] [PubMed] [Google Scholar]
- 20.Ray MA, Johnston NA, Verhulst S, Trammell RA, Toth LA. 2010. Identification of markers for imminent death in mice used in longevity and aging research. J Am Assoc Lab Anim Sci 49:282–288 [PMC free article] [PubMed] [Google Scholar]
- 21.Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. 2011. Development of a mouse model for assessing fatigue during chemotherapy. Comp Med 61:119–130 [PMC free article] [PubMed] [Google Scholar]
- 22.Rugh R, Castro V, Balter S, Kennelly EV, Marsden DS, Warmund J, Wollin M. 1963. X-rays: are there cyclic variations in radiosensitivity? Science 142:53–56 [DOI] [PubMed] [Google Scholar]
- 23.Stokes WS. 2002. Humane endpoints for laboratory animals used in regulatory testing. ILAR J 43 Suppl:S31–S38 [PubMed] [Google Scholar]
- 24.Toth LA. 1997. The moribund state as an experimental endpoint. Contemp Top Lab Anim Sci 36:44–48 [PubMed] [Google Scholar]
- 25.Toth LA. 2000. Defining the moribund condition as an experimental endpoint for animal research. ILAR J 41:72–79 [DOI] [PubMed] [Google Scholar]
- 26.Travis EL, Peters LJ, McNeill J, Thames HD, Jr, Karolis C. 1985. Effect of dose rate on total body irradiation: lethality and pathologic findings. Radiother Oncol 4:341–351 [DOI] [PubMed] [Google Scholar]
- 27.van der Meer M, Rolls A, Baumans V, Olivier B, van Zutphen LF. 2001. Use of score sheets for welfare assessment of transgenic mice. Lab Anim 35:379–389 [DOI] [PubMed] [Google Scholar]
- 28.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, Moulder JE, Okunieff P, Otterson MF, Robbins ME, Smathers JB, McBride WH. 2010. Animal models for medical countermeasures to radiation exposure. Radiat Res 173:557–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA. 2010. Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102:1555–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]