Abstract
Group B Streptococcus (Streptococcus agalactiae, GBS) is a gram-positive commensal and occasional opportunistic pathogen of the human vaginal, respiratory, and intestinal tracts that can cause sepsis, pneumonia, or meningitis in human neonates, infants, and immunosuppressed persons. We report here on a spontaneous outbreak of postnatal GBS-associated disease in rats. Ten of 26 (38.5%) 21- to 24-d-old rat pups died or were euthanized due to a moribund state in a colony of rats transgenic for the human diphtheria toxin receptor on a Munich–Wistar–Frömter genetic background. Four pups had intralesional coccoid bacteria in various organs without accompanying inflammation. GBS was isolated from the liver of 2 of these pups and from skin abscesses in 3 littermates. A connection with the transgene could not be established. A treatment protocol was evaluated in the remaining breeding female rats. GBS is a potentially clinically significant spontaneous infection in various populations of research rats, with some features that resemble late-onset postnatal GBS infection in human infants.
Abbreviations: GBS, Group B Streptococcus; MWF, Munich Wistar Frömter; hDTR, human diphtheria toxin receptor
Streptococci are gram-positive, coccoid bacteria that typically are classified according to their hemolytic capacity. α-hemolytic streptococci produce a zone of partial hemolysis that appears greenish on blood agar, whereas β-hemolytic streptococci produce a zone of complete hemolysis, and γ-hemolytic organisms produce no hemolysis on blood agar.24 The β-hemolytic streptococci are further subdivided into Lancefield groups (A through G), according to cell-wall carbohydrate antigens.24,29,39 The group B β-hemolytic Streptococcus (GBS) have been speciated as Streptococcus agalactiae.28,39 It was first isolated as a causative agent of mastitis in cattle.29 This organism has since been recognized as a cause of severe infection in human neonates.28,39 In humans, GBS is harbored asymptomatically in the maternal genitourinary tract.24,28 Infants can be infected and present with serious systemic disease in the first week of life (early-onset GBS) or from 1 wk to 3 mo of age (late-onset GBS).39 In laboratory animals, rats have been used experimentally as models for neonatal1,6,7,20,37,38,43,44,47,50,51 or adult45 GBS infection, but to our knowledge, GBS has not been associated with spontaneous disease in rats.
Case Report
Rats.
The rats in this case series consisted of offspring of the Munich Wistar Frömter strain (MWF; inhouse colony, original source unknown) crossed onto Fischer 344 rats (Harlan, Indianapolis, IN) transgenic for the human diphtheria toxin receptor (hDTR; University of Michigan Transgenic Core, Ann Arbor, MI) driven by a podocin promoter. These rats were in a breeding colony at an AAALAC-accredited animal research facility and on an IACUC-approved study regarding the mechanisms of glomerular injury. Rats were housed in static microisolation cages in a temperature-controlled room regulated with a 12:12-h light:dark cycle, fed a commercial diet ad libitum (Lab Diet 5008, PMI Nutrition International, Brentwood, MO), and provided filtered city water ad libitum by an automated watering system. No special treatment (for example, autoclaving, irradiation, sterilization) of the food, bedding, water, or caging for these rats was used. A rotating breeding system (2 male, 4 female rats) was used. Male rats usually were removed prior to parturition, but occasionally the female rat was bred again during the postpartum estrus. However, once pups were noted in the cage, the male rat was always removed. Colony health was monitored by use of a semiannual dirty bedding sentinel system. Three sentinel rats (Sprague–Dawley) were used for every 50 to 70 cages and these animals remained negative for the following infectious agents during the time period covered by this report: sialodacryoadenitis virus, rat parvovirus, Kilham rat virus, Toolan H1 virus, rat minute virus, Syphacia spp., Aspiculuris tetraptera, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus strain GDVII, reovirus type 3, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, and mouse adenovirus. Efforts were not made to screen for GBS, because it is not considered a primary pathogen in normal rats.
Culturing protocol.
Fresh tissue or samples taken by using a swab transport system (Remel Bactiswab, Thermo Fisher Scientific, Lenexa, KS) were shipped on ice for microbiologic testing at a diagnostic laboratory accredited by the American Association of Veterinary Laboratory Diagnosticians (Diagnostic Center for Population and Animal Health, Michigan State University, East Lansing, MI). The samples were grown and enriched in Todd– Hewitt broth. A CAMP test then was performed to establish whether GBS organisms were present. To further verify these results, a latex agglutination test was performed to determine the Lancefield group of the β-hemolytic Streptococcus organism (Remel PathoDx, Thermo Fisher Scientific, Lenexa, KS).
Index cases.
Six 21-d-old rat pups were presented to the veterinary staff. One was moribund and required euthanasia; the remaining 5 pups were smaller than their cohorts in the room but were otherwise healthy. We subsequently learned that a pup from this litter was found dead and another was euthanized on the day prior to presentation. The dam and sire were 4 and 6 mo old, respectively. Only the pup euthanized by the veterinary staff (case 1) was available for necropsy and histopathology.
The significant gross finding for case 1 was a focal white lesion (diameter, approximately 1 mm) in the left free wall of the heart. Histologically, this lesion corresponded to an area of myofibrillar degeneration (hypereosinophilia, swelling, loss of cross-striations) and necrosis (pyknosis, karyolysis) accompanied by large clusters of gram-positive, coccoid bacteria within the ventricular myocardium (Figure 1 A). Similar foci were seen in other areas of the myocardium. Very few inflammatory cells were seen in association with the bacteria. A similar focus of bacteria but without inflammation was present in the spleen (Figure 1 B). The spleen had minimal extramedullary hematopoiesis, and bone marrow from the femur, sternum, and skull bones was hypocellular, with mature erythrocytes but minimal myeloid or erythroid progenitors (Figure 1 C). All remaining major organs (brain, lung, gastrointestinal tract, liver, kidneys, adrenal glands, and testes) had no significant histologic lesions. Lung, spleen, and liver samples were submitted for bacterial culture. No organisms were isolated from the lung or spleen but numerous β-hemolytic GBS were isolated in pure culture from the liver.
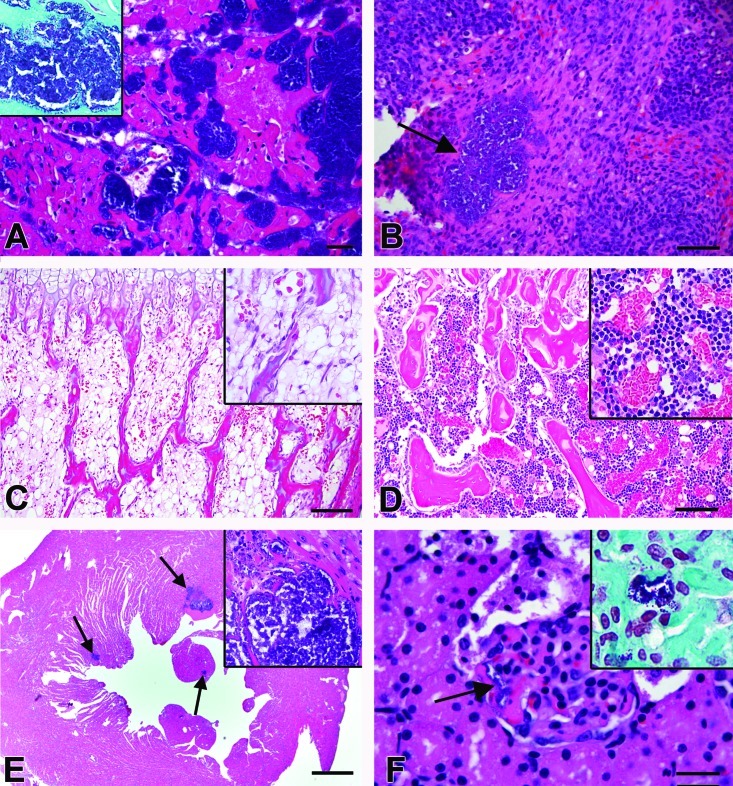
Figure 1.
Histopathology. (A) Heart case 1. Multifocal myofibrillar degeneration and necrosis accompanying large clusters of coccoid bacteria without accompanying inflammation. Hematoxylin and eosin stain; bar, 20 μm. Inset, Gram stain of heart showing gram-positive cocci within the ventricular myocardium. (B) Spleen, case 1. Focal aggregate of coccoid bacteria (arrow) without accompanying inflammation. Hematoxylin and eosin stain; bar, 50 μm. (C) Bone marrow, case 1. Marrow is hypocellular; bar, 100 μm. Inset, Higher magnification (600×) view of the bone marrow. Hematoxylin and eosin stain. (D) Normal age-matched rat bone marrow; case 2. Bar, 100 μm. Inset, Higher magnification (200×) view of the bone marrow. Hematoxylin and eosin stain. (E) Heart; case Number 9. Multifocal botryoid clusters of bacterial cocci (arrows) surrounded by degenerate and necrotic cardiac myocytes. Inflammatory cells were not associated with the cardiac lesions. Bar, 500 μm Inset, Higher magnification (600×) view of the heart. Multifocal clusters of bacteria are present within the ventricular myocardium. Hematoxylin and eosin stain. (F) Glomerulus, case 15. Clusters of coccoid bacteria (arrow) are present within the capillaries. Hematoxylin and eosin stain; bar, 20 μm. Inset, Gram stain of the kidney showing gram-positive cocci within the glomerular capillaries.
One of the 5 remaining rat pups (case 2) developed an abscess on the left hindlimb, and the right hindlimb became diffusely swollen. Numerous GBS were cultured from the abscess, along with moderate numbers of coagulase-negative Staphylococcus species that were considered to be skin-surface contaminants.
To prevent further disease dissemination, the remaining pups (cases 2 through 6), dam (case 7), and sire (case 8) were euthanized and submitted for necropsy. No significant gross or histologic lesions were noted for cases 3 through 6. In case 2, the abscess on the left hindlimb had resolved, but the right hindlimb remained diffusely swollen with a pale pink to slightly translucent subcutis. Histology showed mild subcutaneous and intramuscular edema and lymphatic dilation, but no causative agent was evident. The bone marrow for case 2 appeared normal (Figure 1 D). All remaining major organs (brain, lung, gastrointestinal tract, liver, kidneys, adrenal glands, and testes) had no significant histologic lesions. The dam and sire (cases 7 and 8, respectively) had end-stage kidney alterations that featured membranoproliferative glomerulonephropathy, tubular nephrosis, proteinosis, and cortical fibrosis; early-onset renal failure is a typical clinical manifestation for rats of this genetic background.19,34,41 Because these cases were considered to be an isolated event, no further action was taken at the time.
Second outbreak.
Six months after the initial presentation, three 24-d-old rat pups were found dead, and 2 required euthanasia due to their moribund condition. These rats were from a litter of 10 pups that were born to another dam and sire carrying the MWF-hDTR transgene. The 2 euthanized pups (cases 9 and 10) were submitted for necropsy. No gross lesions were noted. Case 9 had myocardial necrosis similar to that in case 1 (Figure 1 E). Multifocal clusters of coccoid bacteria accompanied by degenerate and necrotic cardiac myocytes were present in the left and right ventricular walls and within the left ventricular papillary muscle. There were minimal neutrophils or other inflammatory cells. All remaining major organs (brain, lung, gastrointestinal tract, liver, kidneys, adrenal glands, and testes) had no significant histologic lesions. Case 10 did not have histologically detectable bacteria or necrosis. Sternal and femoral bone marrow from cases 9 and 10 was hypocellular, with increased adipose tissue, few megakaryocytes, minimal small erythroid islands, decreased numbers of mature erythrocytes, and minimal mature or precursor myeloid cells. Histologically evident large clusters of gram-positive cocci were suggestive of Staphylococcus or Streptococcus spp. Given the previous identification of GBS and the identical signalment and histologic lesions in this subsequent presentation, bacterial cultures were not performed.
Transgene contribution.
Genotyping information was obtained for 9 of the 10 submitted pups and their parents (Table 1), due to concern that a transgene-related bone marrow defect was causing susceptibility to opportunistic infections. Genotyping was performed by PCR using established hDTR primers48 on DNA obtained from a tail snip taken at 14 to 21 d of age. Two rats (cases 9 and 10) with depleted bone marrow were homozygous for the transgene; however, 2 other homozygotes (cases 2 and 3) had normal bone marrow. For one rat with depleted bone marrow (case 1), genotyping information was not available. There were 5 heterozygotes, all of which had normal bone marrow.
Table 1.
Genotyping information for case nos. 1–10 and 14–17
| Case | Histologic lesions (nonmarrow) | Bone marrow histology | hDTR transgene status |
| 1 | Myocardial necrosis with intralesional bacteria | Depleted | Not available |
| 2 | Foot abscess (resolved), lymphedema | Normal | Homozygous |
| 3 | None | Normal | Homozygous |
| 4 | None | Normal | Heterozygous |
| 5 | None | Normal | Heterozygous |
| 6 | None | Normal | Heterozygous |
| 7 (dam) | Chronic glomerulonephritis | Normal | Heterozygous |
| 8 (sire) | Chronic glomerulonephritis | Normal | Heterozygous |
| 9 | Myocardial necrosis with intralesional bacteria | Depleted | Homozygous |
| 10 | None | Depleted | Homozygous |
| 14 | Renal microvascular thrombosis; coccoid aggregates in liver, kidney | Not examined | Not available |
| 15 | Renal microvascular thrombosis; coccoid aggregates in spleen, kidney, heart | Not examined | Not available |
| 16 | Left forelimb abscess (resolved) | Not examined | Homozygous |
| 17 | Abscess near left eye (resolved) | Not examined | Not available |
Treatment plan.
Based on the genotyping information, we considered it unlikely that the GBS infections were secondary to transgene-mediated bone marrow depletion. Instead, we theorized that an opportunistic infection with GBS caused secondary myeloid depletion. Depletion of the neutrophil storage pool is not infrequent in neonates of many species and has been reported in neonatal humans with bacterial infections, including GBS.15 Because the remaining transgenic breeding stock was extremely limited (3 female, 1 male rat) and of high value to the research project, a treatment protocol was developed in an effort to preserve and increase the colony. This protocol was designed based on current recommendations from the Centers for Disease Control regarding GBS prophylaxis in pregnant women.4,9,27,46 The intent of our protocol was to block disease transmission to pups by decreasing the likelihood of bacterial shedding by the dam during the postpartum and lactation period. Baseline (nonpregnant) vaginal and rectal cultures would be obtained from all 3 remaining female rats, which would be cultured again during late pregnancy. If positive for GBS, the dams were to be treated subcutaneously with 6000 IU penicillin G benzathine (0.03 mL Combi-Pen-48, Bimeda, Oakbrook Terrace, IL), around the time of parturition and every 10 d thereafter until the pups were weaned.31,46
Baseline (nonpregnant) rectal cultures from all 3 female rats (cases 11 through 13) were negative for GBS. The vaginal culture from case 12 yielded GBS, whereas the other 2 vaginal cultures (cases 11 and 13) were negative for GBS. Other organisms (E. coli, Enterococcus, α-hemolytic Streptococcus, nonhemolytic Streptococcus, Staphylococcus spp.) were present in low numbers and were considered to be commensal organisms. Cases 12 and 13 never became pregnant and eventually were culled from the colony. However, case 11 had 2 litters of pups. Although this dam had a GBS-negative baseline culture, a vaginal swab taken 1 wk prior to parturition of litter 1 yielded GBS; the corresponding rectal swab was negative for GBS. In light of the inability to isolate GBS from any of the rectal swab samples, this method of sampling was no longer used. For litter 1, penicillin injections were initiated at day 1 postpartum and continued every 10 d until the pups were weaned (21 d of age; 3 doses total). Genital (vaginal or preputial) cultures were taken from 9 of 10 pups at weaning (day 21) and one month later (day 51) to detect carriers; one pup was euthanized prior to the day-21 culture due to an unrelated ocular lesion. Genital (vaginal or preputial) cultures were negative for GBS in all 9 tested pups at both time points. A second litter of 10 pups was born to the same dam and, for this litter, the dam was not treated. All pups were healthy and survived through weaning. Shortly after weaning the second litter, the dam (case 11) was euthanized and was not available for follow-up culture or necropsy.
Final outbreak.
Due to the successful outcome observed in the second litter of case 11, the treatment protocol was discontinued for the breeding colony. However, 8 mo after the second outbreak, a litter of 21-d-old pups from this same colony was reported to the veterinary staff. Six of the 8 pups were hunched and unkempt, and the remaining 2 were dead (cases 14 and 15). Necropsies performed on cases 14 and 15 yielded no remarkable gross findings. Histologically, there was evidence of sepsis, with renal microvascular thrombosis and coccoid bacterial aggregates in the liver and kidney (case 14) and in the spleen, kidney, and heart (case 15; Figure 1 F). The coccoid bacterial aggregates noted in cases 14 and 15 did not have any accompanying inflammation. Numerous GBS organisms, along with very few colonies of E. coli, were isolated from liver in case no. 14. The remaining pups were weaned according to sex and were all clinically normal initially. However, 8 d after weaning, one male and one female rat (cases 16 and 17) developed subcutaneous abscesses adjacent to the left eye and on the left forelimb, respectively. All pups were started on the same antibiotic regimen as used previously for the dams. This therapy was discontinued for cases 16 and 17 due to the lack of clinical response, and trimethoprim–sulfamethoxazole was initiated in light of the sensitivity results from GBS-positive vaginal cultures from their dam. All of the 6 treated pups recovered. The bone marrow was not examined in cases 14 or 15. The genotypes of cases 14, 15, and 17 could not be obtained; case 16 was homozygous for the hDTR transgene (Table 1). Given the previous data and the fact that case 17 did not develop disseminated disease, we again presumed it unlikely that the hDTR transgene played a role in the pups’ susceptibility to GBS infection.
Colony follow-up.
Due to the sporadic occurrence of illness and the small colony size, we decided to treat every MWF-hDTR dam at the time of parturition with penicillin according to the same treatment protocol as mentioned earlier. Since beginning this antibiotic regimen, a total of 57 MWF-hDTR rat pups have been born over a period of 8 mo, and the colony currently has a total of 25 rats. No additional cases of GBS sepsis have occurred during this period. As of the writing of this report, follow-up vaginal cultures were performed on the 10 remaining female rats, and none was positive for GBS. Additional cultures will likely be taken at future time points to better establish the epidemiology of GBS in this rat colony.
Discussion
To our knowledge, our report includes the first cases of naturally occurring GBS infection in postnatal rats. However, neonatal rats are commonly used experimentally as animal models of human neonatal GBS infection. The clinical presentation in neonatal rats after experimental inoculation can include meningitis, bacteremia, pneumonia, and death.20,47 There are reports of naturally occurring GBS infections in other species, including dogs,30 cats,17 fish,18 and mice.21,42 In one of the reported outbreaks in mice, affected animals were 4- to 6-wk-old athymic nude mice that had lesions consistent with meningitis, encephalomyelitis, and rhinitis.42 The other reported outbreak involved DBA/2 mice of various ages, including adults, and multiple organ systems, including the heart, uterus, kidneys, lungs, liver, and brain, were affected.21 In comparison to these 2 case reports in mice, the lack of inflammation at the site of infection and the depletion of the bone marrow were features unique to our rats.
With regard to the hypocellularity and lesions seen within the bone marrow, the hDTR transgene44 and the MWF background do not appear to be involved directly. hDTR homozygotes and heterozygotes both showed lesions, as did pups with normal bone marrow on the MWF background. The genetics of the MWF background are not well understood,19,34,41 but the background is unlikely to be the cause of the lesions. The MWF background or hDTR transgene may have enhanced susceptibility to infection in these rats by a mechanism unrelated to the changes seen in the bone marrow.
Some studies report severe neutropenia and depletion of bone marrow in human neonates and infants with severe bacteremia or sepsis due to infection with GBS and other bacterial agents.3,13,14,23,36 This finding may be due to a decreased storage pool of neutrophils as well as to the functional immaturity of neutrophils in neonates and infants compared with adults.5,14,25 These differences have also been demonstrated in the neutrophils of neonatal, infant, and adult rats during severe bacteremia43 or sepsis12,16,51 with GBS. Therefore, the most likely explanation for the lack of inflammation at the sites of infection, along with the depleted bone marrow, is acute, overwhelming sepsis. Because our rats were relatively young (21 to 24 d of age) at presentation, their neutrophil function and storage pool most likely were inadequate to successfully respond to a severe, disseminated infection. This conclusion is further supported by the improvement in survival of both human15 and rat6,7,38 neonates and infants during sepsis or bacterial infection (due to GBS or other bacteria) when they have been treated with antibiotics and granulocytes or granulocyte colony-stimulating factor.
Another point of interest in the experience we present is the timing of infection. There are 2 common clinical presentations of GBS recognized in human neonates: early-onset and late-onset. In early-onset disease, neonates are infected during parturition or the first week of life, and typical findings can include respiratory distress due to pneumonia, bacteremia, lethargy, hypotension, and meningitis.28 Early-onset neonatal GBS disease has declined by 80% over the past 20 y due to the practice of culturing all pregnant women prior to parturition and implementing antibiotic therapy in those that test positive for GBS.9 In late-onset disease, infants usually are infected from 1 wk to 3 mo of age, and typical findings include meningitis, bacteremia, fever, lethargy, irritability, poor feeding, seizures, osteomyelitis, septic arthritis, and facial cellulitis with submandibular or preauricular adenitis.28 The transmission of GBS in late-onset cases is not well understood, and, unlike the scenario for early-onset cases, the Centers for Disease Control has not noted any change in the incidence of human late-onset GBS since the implementation of testing and treatment regimens.8,9
The age of our rat pups at the time of presentation (21 to 24 d of age, the time of weaning) is more consistent with late-onset than early-onset GBS. The treatment protocol used had clinical evidence of success, given that no further cases developed during treatment. However, whether bacterial clearance was achieved is unknown. In addition, the dam was treated throughout the entire postpartum and nursing period, an option that is unfeasible in humans, given that the nursing period can last 1 y or longer. However, the sporadic nature of the GBS infections in the breeding colony we describe also may mean that all pups exposed are not developing infections. This possibility is difficult to assess, because culture information was not available for all of the dams of affected pups. Performing additional studies on the epidemiology of GBS infection in rat colonies would be helpful.
The source of infection in our rats could not be established. In rats, GBS has not been documented as a pathogen but occasionally is cultured from healthy animals.11 Therefore, potential infection sources for these rat pups could either be the dam or human caretakers. No breach of personal protective equipment (PPE) was identified, and the correct and consistent use of PPE by laboratory and husbandry staff was emphasized strongly after the first incident. Although the potential for a human source of transmission cannot be ruled out, the repeated isolation of GBS from the vaginal tract of these rats at various time points suggests to us that this organism actually may be a commensal or opportunistic organism in rats. Of interest, examination of colony health reports from a commercial vendor (not the source of our rats) showed that GBS was isolated from rat colonies fairly commonly and was isolated in 33 of 58 rats tested over an 18-mo period.10 No specific action was taken when the organism was detected, likely because it was not considered a pathogen in normal rats. As in the current report, under certain circumstances or in certain strains, GBS can be associated with spontaneous disease, particularly in the postnatal period. We had limited opportunity to investigate host and bacterial factors contributing to disease, in light of the research needs of the investigator and the small colony size. However, additional investigations to establish sites of carriage of this organism in rats (vaginal tract, intestinal tract, nasopharynx), genetic comparison to human strains, and the likelihood of disease in other rat strains would be informative.
In humans, early-onset GBS disease is attributed to vertical transmission from mother to neonate during the birthing process.28 The source of infection for late-onset disease is unclear. Of interest, GBS has been found in breast milk and was thought to be a potential source of infection in several case reports.2,22,32,49 This route of infection has biologic precedent, given that GBS was first identified as an agent of mastitis in cattle.29 Other human case reports indicate potential nosocomial transmission35 and increased susceptibility due to concurrent viral infection.40 Infants born to immunocompromised mothers and infants of low birth weight are particularly susceptible to GBS. In our experience, although the rats had no known direct immunocompromise, the early-stage renal failure characteristic of the strain may contribute to poor immune response, increased rates of shedding, and decreased antibody production in dams. The organism was cultured from the vaginal tract of several female rats within the colony (cases 11 and 12), but milk was not available for culture. The rat pups remained healthy throughout the entire nursing period when the dam was treated with antibiotics, but further study of transmission routes and factors influencing susceptibility is warranted. Transmammary transmission is of particular interest, given that this route is under-investigated in humans and one that might contribute to our understanding of GBS transmission during the late postnatal period.
Unfortunately, the GBS isolates in this diagnostic series were not available to us for additional analysis beyond antibiotic sensitivity testing. In human medicine, several virulence factors (for example, surface antigens, invasins, antibiotic resistance genes) have been identified for GBS; however, the strongest predictive association with invasive disease is serotype, which is defined according to a polysaccharide component of the bacterial capsule.26,33 Serotype III is the predominant cause of late-onset invasive disease.26,33 Serotyping would be a useful addition to future investigations of laboratory rodent isolates from cases displaying clinical signs.
In conclusion, the current report presents the first known incidence of naturally occurring late-onset GBS infection in rats with apparent successful prevention of subsequent clinical cases in the colony. Although follow-up at 8 mo failed to culture GBS from any evaluated rats, further monitoring will be necessary to determine whether this finding actually represents clearance of GBS from this colony. In addition, genetic evaluation of rat-derived isolates and future studies in rats to determine potential routes of infection (milk, vaginal tract, anthropozoonosis), and factors influencing clinical disease would be informative in defining risk posed by this organism.
Acknowledgments
We thank Patrick Lester RPh, BCPS, DVM, DACLAM (Unit for Laboratory Animal Medicine, University of Michigan) for his assistance in devising an appropriate treatment plan for the GBS-positive rats. We also thank Paula Arrowsmith HT (ASCP); (Pathology Cores for Animal Research, University of Michigan) for her technical assistance with sample preparation and processing for histology.
References
- 1.Ancona RJ, Ferrieri P. 1979. Experimental vaginal colonization and mother–infant transmission of group B streptococci in rats. Infect Immun 26:599–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias-Camison JM. 2003. Late-onset group B streptococcal infection from maternal expressed breast milk in a very low-birth–weight infant. J Perinatol 23:691–692 [DOI] [PubMed] [Google Scholar]
- 3.Becker ID, Robinson OM, Bazán TS, López-Osuna M, Kretschmer RR. 1981. Bactericidal capacity of newborn phagocytes against group B β-hemolytic streptococci. Infect Immun 34:535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. 2000. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 106:244–250 [DOI] [PubMed] [Google Scholar]
- 5.Cairo MS. 1989. Neonatal neutrophil host defense. Prospects for immunologic enhancement during neonatal sepsis. Am J Dis Child 143:40–46 [DOI] [PubMed] [Google Scholar]
- 6.Cairo MS, Mauss D, Kommareddy S, Norris K, van de Ven C, Modanlou H. 1990. Prophylactic or simultaneous administration of recombinant human granulocyte colony-stimulating factor in the treatment of group B streptococcal sepsis in neonatal rats. Pediatr Res 27:612–616 [DOI] [PubMed] [Google Scholar]
- 7.Cairo MS, Plunkett JM, Mauss D, van de Ven C. 1990. Seven-day administration of recombinant human granulocyte colony-stimulating factor to newborn rats: modulation of neonatal neutrophilia, myelopoiesis, and group B Streptococcus sepsis. Blood 76:1788–1794 [PubMed] [Google Scholar]
- 8.Centers for Disease Control. [Internet]. 2009 GBS surveillance report—active bacterial core surveillance. [Cited 20 October 2010]. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/gbs09.html.
- 9.Centers for Disease Control. [Internet]. 2010 guidelines for the prevention of perinatal group B streptococcal disease. [Cited 20 October 2010]. Available at: http://www.cdc.gov/groupBstrep/guidelines/provisional-recs.htm.
- 10.Charles River Laboratories. [Internet]. Health reports indexed by area for Charles River animal colonies in North America. [Cited 8 March 2012]. Available at: http://www.criver.com/en-US/ProdServ/ByType/ResModOver/health/pages/indexbyarea.aspx.
- 11.Charles River Laboratories. [Internet]. Laboratory animal infectious agents and diseases. [Cited 8 March 2012]. Available at: http://www.criver.com/en-US/TrainEducation/RMS/InfectiousAgent/Pages/home.aspx.
- 12.Christensen RD, MacFarlane JL, Taylor NL, Hill HR, Rothstein G. 1982. Blood and marrow neutrophils during experimental group B streptococcal infection: quantification of the stem cell, proliferative, storage, and circulating pools. Pediatr Res 16:549–553 [DOI] [PubMed] [Google Scholar]
- 13.Christensen RD, Rothstein G. 1980. Exhaustion of mature marrow neutrophils in neonates with sepsis. J Pediatr 96:316–318 [DOI] [PubMed] [Google Scholar]
- 14.Christensen RD, Rothstein G. 1984. Pre- and postnatal development of granulocytic stem cells in the rat. Pediatr Res 18:599–602 [DOI] [PubMed] [Google Scholar]
- 15.Christensen RD, Rothstein G, Anstall HB, Bybee B. 1982. Granulocyte transfusions in neonates with bacterial infection, neutropenia, and depletion of mature marrow neutrophils. Pediatrics 70:1–6 [PubMed] [Google Scholar]
- 16.Christensen RD, Shigeoka AO, Hill HR, Rothstein G. 1980. Circulating and storage neutrophil changes in experimental type II group B streptococcal sepsis. Pediatr Res 14:806–808 [DOI] [PubMed] [Google Scholar]
- 17.Dow SW, Jones RL, Thomas TN, Linn KA, Hamilton HB. 1987. Group B streptococcal infection in 2 cats. J Am Vet Med Assoc 190:71–72 [PubMed] [Google Scholar]
- 18.Eldar A, Bejerano Y, Livoff A, Horovitcz A, Bercovier H. 1995. Experimental streptococcal meningoencephalitis in cultured fish. Vet Microbiol 43:33–40 [DOI] [PubMed] [Google Scholar]
- 19.Fassi A, Sangalli F, Maffi R, Colombi F, Mohamed EI, Brenner BM, Remuzzi G, Remuzzi A. 1998. Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J Am Soc Nephrol 9:1399–1406 [DOI] [PubMed] [Google Scholar]
- 20.Ferrieri P, Burke B, Nelson J. 1980. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect Immun 27:1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geistfeld JG, Weisbroth SH, Jansen EA, Kumpfmiller D. 1998. Epizootic of group B Streptococcus agalactiae serotype V in DBA/2 mice. Lab Anim Sci 48:29–33 [PubMed] [Google Scholar]
- 22.Godambe S, Shah PS, Shah V. 2005. Breast milk as a source of late-onset neonatal sepsis. Pediatr Infect Dis J 24:381–382 [DOI] [PubMed] [Google Scholar]
- 23.Gregory J, Hey E. 1972. Blood neutrophil response to bacterial infection in the first month of life. Arch Dis Child 47:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen AK.2000. Handbook of laboratory animal bacteriology. Boca Raton (FL): CRC Press.
- 25.Hill HR. 1987. Biochemical, structural, and functional abnormalities of polymorphonuclear leukocytes in the neonate. Pediatr Res 22:375–382 [DOI] [PubMed] [Google Scholar]
- 26.Jordan HT, Farley MM, Craig A, Mohle-Boetani J, Harrison LH, Petit S, Lynfield R, Thomas A, Zansky S, Gershman K, Albanese BA, Schaffner W, Schrag SJ. 2008. Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr Infect Dis J 27:1057–1064 [DOI] [PubMed] [Google Scholar]
- 27.Kanto WP, Baker CJ. 2003. New recommendations for prevention of early-onset group B streptococcal disease in newborns. Pediatr Rev 24: 219–221. [DOI] [PubMed]
- 28.Kasper DL, Braunwald E, Hauser S, Longo D, Jameson JL, Fauci AS. 2004. Harrison's principles of internal medicine, 16th ed. New York (NY): McGraw–Hill Professional. [Google Scholar]
- 29.Keefe GP. 1997. Streptococcus agalactiae mastitis: a review. Can Vet J 38:429–437 [PMC free article] [PubMed] [Google Scholar]
- 30.Kornblatt AN, Adams RL, Barthold SW, Cameron GA. 1983. Canine neonatal deaths associated with group B streptococcal septicemia. J Am Vet Med Assoc 183:700–701 [PubMed] [Google Scholar]
- 31.Korzhova VV, Lisitsina NT, Smirnova EI, Kiseleva LA. 1976. [Influence of antibiotics of the penicillin series on the fetal and newborn development of rats]. Biull Eksp Biol Med 82:864–866 [Article in Russian] [PubMed] [Google Scholar]
- 32.Lanari M, Serra L, Cavrini F, Liguori G, Sambri V. 2007. Late-onset group B streptococcal disease by infected mother's milk detected by polymerase chain reaction. New Microbiol 30:253–254 [PubMed] [Google Scholar]
- 33.Lin F, Sintchenko V, Kong F, Gilbert GL, Coiera E. 2009. Commonly used molecular epidemiology markers of Streptococcus agalactiae do not appear to predict virulence. Pathology 41:576–581 [DOI] [PubMed] [Google Scholar]
- 34.Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A. 2006. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168:42–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacFarquhar JK, Jones TF, Woron AM, Kainer MA, Whitney CG, Beall B, Schrag SJ, Schaffner W. 2010. Outbreak of late-onset group B Streptococcus in a neonatal intensive care unit. Am J Infect Control 38:283–288 [DOI] [PubMed] [Google Scholar]
- 36.Manroe BL, Rosenfeld CR, Weinberg AG, Browne R. 1977. The differential leukocyte count in the assessment and outcome of early-onset neonatal group B streptococcal disease. J Pediatr 91:632–637 [DOI] [PubMed] [Google Scholar]
- 37.Moore JC, Muckett PJ, Hughes MJ. 2001. Age-dependent presence of antibodies in rat dams, capable of conferring protection against group B Streptococcus infection in neonates. FEMS Microbiol Lett 202:125–127 [DOI] [PubMed] [Google Scholar]
- 38.Novales JS, Salva AM, Modanlou HD, Kaplan DL, del Castillo J, Andersen J, Medlock ES. 1993. Maternal administration of granulocyte colony-stimulating factor improves neonatal rat survival after a lethal group B streptococcal infection. Blood 81:923–927 [PubMed] [Google Scholar]
- 39.Onile BA. 1985. Review of group B streptococci and their infections. Afr J Med Med Sci 14:131–143 [PubMed] [Google Scholar]
- 40.Raymond J, Armengaud JB, Lambe C, Tchetchoua A, Moulin F, Lebon P, Poyart C, Gendrel D. 2007. Late-onset neonatal infections caused by group B Streptococcus associated with viral infection. Pediatr Infect Dis J 26:963–965 [DOI] [PubMed] [Google Scholar]
- 41.Remuzzi A, Puntorieri S, Alfano M, Macconi D, Abbate M, Bertani T, Remuzzi G. 1992. Pathophysiologic implications of proteinuria in a rat model of progressive glomerular injury. Lab Invest 67:572 –579. [PubMed]
- 42.Schenkman DI, Rahija RJ, Klingenberger KL, Elliott JA, Richter CB. 1994. Outbreak of group B streptococcal meningoencephalitis in athymic mice. Lab Anim Sci 44:639–641 [PubMed] [Google Scholar]
- 43.Schuit KE, DeBiasio R. 1980. Kinetics of phagocyte response to group B streptococcal infections in newborn rats. Infect Immun 28:319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran T-VP, Weisman LE. 2004. Dexamethasone effects on group B streptococcal infection in newborn rats. Pediatr Infect Dis J 23:47–52 [DOI] [PubMed] [Google Scholar]
- 45.Warejcka DJ, Goodrum KJ, Spitznagel JK. 1985. Toxicity of group B Streptococcus agalactiae in adult rats. Infect Immun 48:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weeks JW, Myers SR, Lasher L, Goldsmith J, Watkins C, Gall SA. 1997. Persistence of penicillin G benzathine in pregnant group B streptococcus carriers. Obstet Gynecol 90:240–243 [DOI] [PubMed] [Google Scholar]
- 47.Weisman LE, McKinney L, Villalobos R. 1990. Systemic group B streptococcal disease in the neonate: characterization of an oral colonization model using the suckling rat. Microbiol Immunol 34:755–764 [DOI] [PubMed] [Google Scholar]
- 48.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. 2005. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16:2941–2952 [DOI] [PubMed] [Google Scholar]
- 49.Widger J, O'Connell NH, Stack T. 2009. Breast milk causing neonatal sepsis and death. Clin Microbiol Infect 16:1796 –1798. [DOI] [PubMed]
- 50.Wittler RR, Harris RW, Paine DD. 1999. Failure of γ-interferon to decrease mortality from group B streptococcal sepsis in neonatal rats. Biol Neonate 76:125–128 [DOI] [PubMed] [Google Scholar]
- 51.Zeligs BJ, Armstrong CD, Walser JB, Bellanti JA. 1982. Age-dependent susceptibility of neonatal rats to group B streptococcal type III infection: correlation of severity of infection and response of myeloid pools. Infect Immun 37:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]