Abstract
Chronic diarrhea remains the principal burden in providing health care for nonhuman primates in biomedical research facilities. Although the exact etiology continues to puzzle nonhuman primate clinicians, recent research in humans has shown that restoring the indigenous microbial diversity may be successful in resolving cases of chronic diarrhea when other treatment modalities have failed. The process of restoring this microbial balance, known as fecal bacteriotherapy, uses the complete flora from a normal donor as a therapeutic probiotic mixture. In the current study, Indian-origin rhesus macaques were randomized into treatment (n = 7) and control (n = 6) groups to determine whether orally administered fecal bacteriotherapy would reduce the overall incidence of chronic diarrhea during a 60-d follow-up period in the treatment group compared with control macaques, which received a placebo. Although the treatment effect, determined by comparing the baseline fecal scores of the treatment and control groups, did not reach statistical significance, preprocedure and postprocedure fecal scores in the treatment group differed significantly. These findings are encouraging, and we hope that our study will motivate larger studies evaluating the use of fecal bacteriotherapy in nonhuman primates.
Chronic diarrhea is perhaps the most daunting clinical challenge of nearly every biomedical research facility that houses large numbers of nonhuman primates. Our facility, The Oregon National Primate Research Center (ONPRC), is no exception. As of 2008, approximately 14.7% of the total population at this center was reported to have diarrhea requiring medical attention each year, which constituted an average of 35.2% of the total clinical caseload.27 Our more recent analysis of the medical records from 2010 has confirmed these statistics: 16.2% of the total population was treated for diarrhea in 2010, which comprised 29.3% of the total clinical caseload for that year. The cost of chronic diarrhea to institutions such as ours in terms of veterinary staff time, diagnostics, and medications is profound. Consequently, colony management personnel and resources are taxed due to the care and maintenance of these patients, given that nonhuman primates with chronic diarrhea generally are in poor body condition, lag behind the growth rate of their peers, and require frequent medical intervention, thereby making them undesirable as research subjects and unproductive members of the breeding colony.8
Chronic diarrhea is undeniably the largest, most expensive problem in providing health care for nonhuman primate colonies. Nonhuman primates diagnosed with chronic diarrhea typically test negative for known fecal pathogens19,24,27 and are recalcitrant to common diarrhea treatment modalities. For these reasons, the underlying cause of chronic diarrhea has been elusive and is likely multifactorial. Over the years, numerous researchers and clinicians in this field have attempted to devise an effective treatment regimen for these patients, with little success.
Recent research in humans has shown that restoring the indigenous microbial diversity may be useful in resolving cases of chronic diarrhea when other treatment modalities have failed.10,18 A normal healthy digestive tract contains numerous bacterial inhabitants, which typically act to impede exogenous bacteria from establishing themselves as pathogens. After an episode of gastrointestinal disease that results in diarrhea, the population of indigenous bacteria often is disrupted, subsequently leading to decreased numbers and diversity of these organisms. This imbalance, or dysbiosis, may result from pathogenic diarrhea or may be nosocomial due to prescribed antibiotic therapy.7,10,18,21 Several publications have explored the idea that dysbiosis can be treated with an infusion of normal flora.1,4,16 Fecal bacteriotherapy uses the complete flora of a normal donor as a therapeutic probiotic mixture of living organisms.5 Because the bacterial components of the normal fecal flora that are the most important for host defense are unknown, reintroducing all flora is currently recommended.23 In addition to providing the complete bacterial flora from a normal donor, another possible advantage of this therapy is that it halts the cycle of antimicrobial use in these patients.1 The discontinuance of intestinal flora disruption through the use of antimicrobials, when combined with the probiotic effects of fecal bacteriotherapy, constitutes the philosophy of this therapeutic approach. Several case series in the human literature have demonstrated that this therapy is capable of resolving refractory cases of diarrhea, with very high success rates after single administrations.2,4,11,15,28 In addition, the transplantation of donor stool can dramatically change the recipient's intestinal flora in as little as 14 d.16 Furthermore, fecal bacteriotherapy has been an effective tool in veterinary medicine for the treatment of ruminants and horses with enteric disease.6,9,12,22 However, whether this treatment modality will be effective in nonhuman primates or whether successful cases will continue to be sporadic and species-specific remains unknown.
The goal of the current study was to test a new treatment modality, fecal bacteriotherapy, which if successful, would reduce the overall incidence of chronic diarrhea in rhesus macaques. Because the need for detailed information regarding techniques used to prepare and administer the fecal suspension has been recognized in the human literature,5,23,28 we here describe in detail the standardized treatment protocol that we developed. Our hypothesis was that the incidence and severity of diarrhea during the 60-d follow-up period would be decreased in macaques that received fecal bacteriotherapy compared with those that received placebo treatment.
Materials and Methods
Animals.
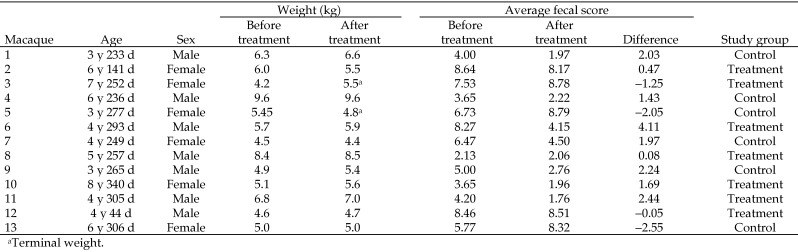
Indian-origin rhesus macaques in this study (7 male, 6 female; Figure 1) were singly housed (with the exception of one dam–infant pair) in standard indoor monkey cages at ONPRC (Beaverton, OR). All macaques were given enrichment, including cage toys, television, radio, and foraging manipulanda, on a rotating basis. Macaques were fed LabDiet 5000 (PMI Nutrition International, Brentwood, MO) twice daily and supplemental produce or other enrichment once daily. Water was available ad libitum, lighting was on a 12:12-h light:dark cycle, and temperature was maintained at 72 ± 5 °F (22.2 ± 1.2 °C). The ages of the study animals ranged from 3 to 8 y (mean, 5.52 y). Macaques were either reared in cages with their mothers or in outdoor breeding or social groups. This study was conducted between October 2010 and April 2011 and was approved by the ONPRC IACUC. The ONPRC is accredited by AAALAC.
Figure 1.
Demographics of study participants.
All macaques enrolled in this prospective study were diagnosed as having chronic diarrhea by meeting one or more of the following criteria: (1) evaluated as a clinical case and recorded as having diarrhea for at least 30 d within a 90-d period; (2) reported for multiple episodes of diarrhea that were not responsive to symptomatic medication; (3) permanently removed from a social group due to either an ongoing need for aggressive rehydration therapy; and (4) having 3 removals from a social group within a 1-y period for treatment associated with diarrhea. To verify that the diagnosed chronic diarrhea was idiopathic, all subjects were screened in the following manner: (1) 3 serial fecal parasite microscopic examinations via direct wet mount and fecal floatation (Fecasol, Vétoquinol USA, Fort Worth, TX) within 5 d (Balantidium coli, and trichomonads were considered to be intermittently shed opportunistic organisms, whereas Trichuris was treated on detection); (2) 3 serial fecal bacterial cultures within 5 d (negative for Shigella and Campylobacter jejuni; Campylobacter coli was considered to be an opportunistic organism); and (3) 2 immunofluorescent assays for Cryptosporidium spp. and Giardia spp. (Merifluor Cryptosporidium–Giardia Direct Immunofluorescent Assay, Meridian Bioscience, Cincinnati, OH) within 5 d. Macaques yielding positive test results for the pathogens described were excluded from the study. In general, all patients presenting for diarrhea at the ONPRC are prescribed symptomatic treatments, with additional medications as warranted by culture and ova and parasite screen. No treatments were given once subjects were identified as potential study participants. Macaques also were excluded when they exhibited weight loss of greater than 10% per month for 2 consecutive months during the immediate prestudy period, an indicator of deteriorating physical condition. No subjects in this experiment were assigned concurrently to another active experimental protocol.
Animals that met the inclusion and exclusion criteria were then randomly assigned to either the treatment group or the control group. As soon as macaques were enrolled into the study, they were relocated from their original location to a common housing room for the subjects in this study to control for variability between animal care staff and other factors. Macaques were allowed to acclimate for at least 1 wk to resolve any stress-related diarrhea that may have resulted from their new environment. In addition, they were randomly assigned to a top or bottom cage within the 2-over-2 racks (Britz, Wheatland, WY). Group assignment was random and determined prior to patient selection and therefore was not biased by the patient's prior history, physical examination, or medical record review. Stool consistency was scored (range, 1 to 10) daily (Figure 2) from the time of assignment to the protocol and was monitored for at least 2 mo after the trial intervention. A score of 1 indicated that the patient's stool was normal (that is, firm), whereas a score of 10 indicated that the patient's stool was liquid to watery. In addition, combinations of these consistencies were also possible in which the first letter of the score denoted the stool quality present in the greatest quantity. For example, a score of 6 indicated that liquid feces was the primary component of the stool observed, although some mounded and firm feces were noted as well. This fecal scoring system is currently in use at the ONPRC for daily clinical observations and has been published previously.27 Veterinary technicians scoring the daily fecal output were blind to group assignments and were dedicated to the care of macaques in this housing room for the duration of the study. Clinical care of the animals was managed by the area veterinarian, who was blind to group assignment.
Figure 2.
Scoring system for stool consistency.
Each subject received famotidine (0.5 mg/kg; 10-mg tablet, Teva Pharmaceuticals USA, Sellersville, PA) administered by mouth the evening before and the morning of the procedure to decrease gastric acidity, in an attempt to create a receptive environment for the newly instilled bacterial flora. All macaques, regardless of group assignment, received this treatment. In addition, macaques were fasted the morning of the procedure, to decrease the possibility of emesis during or after the procedure. Each macaque received a single treatment of the fecal bacteriotherapy (treatment group) or saline (control group), which began 1 to 3 wk after each animal's movement to the room.
Preparation of fecal suspension.
The donor was an 8 y-old Indian-origin male rhesus macaque that had been born in a breeding corral at the ONPRC. He had no history of diarrhea since birth and had not received any form of antibiotic therapy during the 6 wk prior to stool donation. The donor was serologically negative for SIV, simian T-lymphotrophic virus, simian retrovirus type D, and Macacine herpesvirus 1 and had been tested for the latter 2 viruses yearly since 1 y of age. In addition, the donor was screened for enteric pathogens via the same testing methods as were the study subjects and was fed the same diet, including enrichment. Fresh stool from the donor was used within 10 min of collection.
On each scheduled procedure day, 25 to 30 g of fresh stool was collected from the cage pan of the donor macaque, diluted in 50 mL saline (0.9% Sodium Chloride Irrigation USP, Hospira, Lake Forest, IL), placed in a household blender (Cyclone, Black and Decker, Towson, MD), and stirred for 2 to 4 min until the mixture was homogenized to a liquid slurry consistency. The resulting slurry was filtered through gauze into a sterile stainless steel bowl, to remove any particulate matter. We then transferred 25 mL of the liquid stool slurry to a 35-mL catheter-tipped syringe (Kendall Monoject, Tyco Healthcare Group, Mansfield, MA) for each subject in the treatment group. Subjects in the control group received 25 mL saline as a placebo.
Instillation procedure.
For the instillation procedure, macaques were sedated with ketamine hydrochloride (10 mg/kg IM; 100 Ketathesia, 100 mg/mL, Butler Animal Health Supply, Dublin, OH). An 18-French rubber gavage tube (Kendall Sovereign, Tyco Healthcare Group) was passed through the oropharynx into the stomach. Once correct placement of the orogastric tube was verified by means of saline flush and auscultation, the treatment or placebo syringe was attached and the contents slowly administered. After the entire 25 mL was administered, 10 mL of saline was flushed through the tube to ensure that the entire volume of the test substance had been administered. Throughout the procedure and for 10 min afterward, macaques were maintained in a sitting position and monitored via pulse oximetry and for mucous membrane color, coughing, and vomiting. At the completion of this monitoring period, macaques were returned to their home cages to recover from sedation.
Monitoring of clinical status.
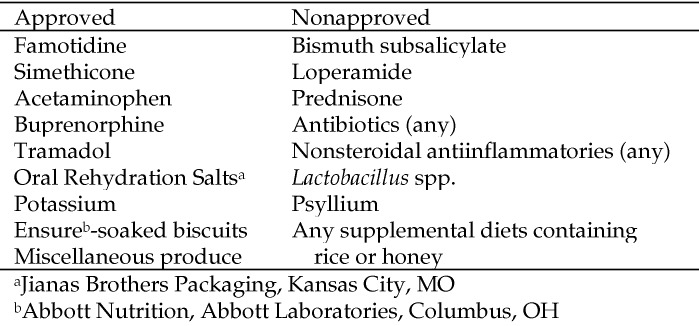
In addition to fecal scoring, macaques were assessed daily for appetite (good, fair, or poor), attitude (bright, quiet, or lethargic), hydration status (good, fair, or poor), and given a pain score according to a pain scale developed and routinely used at the ONPRC. Macaques were weighed weekly. If weight loss progressed to greater than 10% of the animal's baseline weight and was unresponsive to approved dietary supplementation (Figure 3), fluid therapy, and pain management, the macaque was removed from the study and euthanized. Hydration status noted as fair or poor was further assessed by veterinary technicians via a skin turgor test under ketamine sedation or by use of a handheld blood analyzer (i-Stat Portable Clinical Analyzer, Abbott Point of Care, East Windsor, NJ). When necessary, hydration was supplemented via oral supplements or intravenously. If more than 3 d of intravenous fluid therapy was deemed necessary, the macaque was removed from the study and euthanized. Additional humane endpoints included complete anorexia of more than 3 d duration, a total protein of less than 5.0 g/dL or albumin of less than 2.0 g/dL, or perceived pain that was not responsive to acetaminophen or buprenorphine. Famotidine was administered when an animal appeared to be experiencing pain that may have been associated with gastric acidity.
Figure 3.
Medications and supplements approved for or prohibited from use during the study.
Statistical analysis.
All statistical analyses were performed by using SAS (version 9.3, SAS Institute, Cary, NC). Repeated-measures ANOVA with treatment status as a between-subject factor and different time periods of housing (before acclimation, after acclimation to before procedure, and after procedure) were used to explore the effect of fecal bacteriotherapy as a treatment for chronic diarrhea. Preplanned comparisons were performed by using contrasts to address specific questions of interests. This analysis also was used to ensure that movement between housing rooms before treatment did not have a significant effect. Because in a typical experiment using repeated measures, 2 measurements taken at adjacent times are more highly correlated than are 2 measurements taken several time points apart, autoregressive order 1 was chosen as an optimal correlation within subjects by using a Bayesian information criterion.25,26
Results
Once macaques with chronic diarrhea that met the study criteria were identified, they were relocated to a room specifically dedicated to this study. This practice allowed some control over potential variability in diet, caretakers, temperature, humidity, lighting, and nonpharmacologic therapy for diarrhea. To determine any possible effect of this relocation on severity of diarrhea, the fecal scores of the macaques prior to relocation were compared with those after 1 wk of acclimation to the new environment. The effect of room change was not statistically significant (P = 0.2001). For this reason, fecal scores from these 2 time periods (prior to relocation and after acclimation) were combined into a single preprocedure time period for the remaining analyses.
Randomization was performed before group assignment. Unfortunately, chance variation during randomization resulted in an unequal distribution with respect to severity of diarrhea observed at the beginning of the study. The pretreatment fecal score (mean ± 1 SD) of the treatment group (7.00 ± 2.60) was higher (P = 0.0038) than that of the control group (4.57 ± 2.59). This difference suggests that the severity of diarrhea among the treatment group was greater at baseline than that of the control group. For this reason, it was necessary to use baseline-adjusted fecal scores for comparisons between treatment and control groups, such that the change in fecal scores from baseline values were used rather than the absolute fecal scores.
Comparing fecal scores before and after treatment revealed significant decreases in scores (that is, clinical improvement) after oral fecal bacteriotherapy in the treatment group (mean change, −1.55 ± 0.534, P = 0.0156) but not in the control group (mean change, −0.44 ± 0.58, P = 0.4629), which received saline as a placebo. However, the difference between the treatment and control groups was not statistically significant (P = 0.1869) when baseline-adjusted pretreatment fecal scores were used.
Of the 13 subjects, 2 macaques reached predetermined humane endpoints and were euthanized prior to the completing the 60-d follow up period. One macaque (treatment group) was euthanized 46 d after receiving bacteriotherapy and the other (control group) was euthanized at 14 d afterward. Data collected from these subjects before euthanasia were included in the analysis.
Discussion
Several animals in the treatment group showed considerable clinical improvement from their baseline diarrhea severity score during the 60-d follow-up period. While the positive treatment effect did not reach statistical significance (P = 0.1869) between groups, the comparison within the treatment group between preprocedure and postprocedure time periods appeared statistically significant (P = 0.0156). Therefore the trend demonstrated in the current study is encouraging in terms of prospective larger studies evaluating the use of fecal bacteriotherapy in nonhuman primates. Lack of statistical significance in the current study may have been affected by several factors, most notable of which was the study's small sample size. Similarly, temporal changes in fecal scores for each animal were extremely variable. The effect of this variability through time was moderated by averaging multiple fecal scores, a practice that might limit the validity of the comparison of overall fecal scores between the control and treatment groups. Another factor that potentially affected outcomes of the current study was the required movement of the study macaques during the study period. Housing space for nonhuman primates in a biomedical research setting is a valuable asset, and designated long-term housing is especially challenging to secure. Therefore, movement of animals within a facility is inevitable. Because some animals are prone to stress-associated diarrhea,27 the transfer of subjects cannot be ignored as a potential confounder. However, only 4 subjects changed housing room more than once, and their group distribution was equal (2 from the control group and 2 from the treatment group). All other subjects were moved only once—to the study room in which all follow-up observations were made. Furthermore, many of the macaques selected for this study perhaps already had reached the point of clinical decompensation, with little likelihood of improvement in their condition. Clinical deterioration necessitated euthanasia in 2 of the subjects prior to the completion of the study. Associated pathologic findings included chronic-active proliferative typhlocolitis, mesenteric lymphoid hyperplasia, and lean body condition. Therefore, although not proven to be effective as a sole treatment for macaques with chronic diarrhea in the current study, fecal bacteriotherapy may yet be useful as an adjunct therapy in patients that are affected less severely.
Potential complications that have been associated with fecal bacteriotherapy include perforation of the upper gastrointestinal tract by the orogastric tube,17 aspiration of fecal material, bacterial inactivation by stomach acid, and transmission of contagious agents via the donor stool.2 None of these complications occurred in any of the study animals. The donor underwent rigorous fecal testing and medical history review, above and beyond routine colony surveillance testing, prior to his selection for the study. In addition, considerable care was taken during each instillation procedure to ensure the correct placement of the orogastric tube, slow administration of the treatment material, and monitoring of subjects after instillation to circumvent complications associated with the procedure itself. Administration of famotidine was included as part of the protocol, in an attempt to reduce gastric acidity and improve the survival of the instilled bacterial flora. Similar drugs that decrease gastric acidity, such as proton pump inhibitors, have been administered prior to instillation in some human cases, and further study of their use, as well as other pretreatment practices, has been proposed.1-3,7,11 We excluded several common dietary supplements and medications from use in the current study to avoid their potentially confounding effects. For example, opioid analgesics and simethicone were allowed when subjects were noted to be uncomfortable, and oral electrolyte supplements were provided for those determined to be dehydrated. However, medications such as antibiotics that might disrupt the newly instilled flora were excluded, as were food items and medications that have possible or known antidiarrheal properties. Although specific analgesics were approved, their administration was not necessary during the study.
Several publications in the human literature have indicated the need for a randomized clinical trial to assess the efficacy of fecal bacteriotherapy through the development of a standardized treatment protocol.1,5,17,23,28 We hope that our documentation of the procedure we used in the current study will benefit future studies in both humans and nonhuman primates. Additional studies of fecal bacteriotherapy in nonhuman primates are warranted for numerous reasons. First, a dose–response relationship was not evaluated in this study. All subjects in the treatment group received the same dose of the prepared fecal suspension, and each macaque received a single dose. Higher doses or multiple doses may lead to a more positive treatment effect. In addition, long-term follow-up of the subjects was not possible due to housing constraints. Additional follow-up including characterization of the bacterial flora of subjects after treatment was not obtained. Such information may be valuable in future studies to determine the similarity between the flora of the donor and recipients. Finally, stratification of subjects between groups according to the severity of their chronic diarrhea may be worth investigating.
Future randomized clinical trials using nonhuman primates as recipients of fecal bacteriotherapy likely will benefit human research. The need to disguise the placebo treatment has been recognized as problematic in human studies,5 whereas blinding of nonhuman primate subjects is unnecessary. In addition, the challenge of overcoming the social concerns surrounding this therapy in human patients still exists.14,20 Despite the fact that recent human research has begun to realize the important relationship between fecal flora and overall health,12,13 nonhuman-primate–focused research in this area is minimal. Certainly, although numerous studies have endeavored to resolve the issue of chronic diarrhea among captive nonhuman primates, none has considered a formalized trial using fecal bacteriotherapy. Therefore, the current study represents the first randomized trial to assess the efficacy of orally administered fecal bacteriotherapy in macaques. The detailed account of the procedure we used here will allow clinicians at other facilities to incorporate this method as a potential tool for the treatment of persistent cases of diarrhea in nonhuman primates.
Acknowledgments
We thank Drs CJ Doane, Anne Lewis, Kristine Coleman, and Jennifer Wilk for the invaluable input that they provided during the preparation of this study and manuscript and Dr Byung Park for providing statistical analysis.
This publication was made possible with support from the ONPRC core grant award 5P51RR000163-51 and the ONPRC Nonhuman Primate Veterinary Clinical Education Program, grant 5R25RR024233-04.
References
- 1.Aas J, Gessert CE, Bakken JS. 2003. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 36:580–585 [DOI] [PubMed] [Google Scholar]
- 2.Bakken JS. 2009. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 15:285–289 [DOI] [PubMed] [Google Scholar]
- 3.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, Moore TA, Russel G, Surawicz C. 2011. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 9:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. 2003. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 37:42–47 [DOI] [PubMed] [Google Scholar]
- 5.Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. 2004. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol 38:475–483 [DOI] [PubMed] [Google Scholar]
- 6.Brandt LJ, Reddy SS. 2011. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 45:S159–S167 [DOI] [PubMed] [Google Scholar]
- 7.Duplessis CA, You D, Johnson M, Speziale A. 2012. Efficacious outcome employing fecal bacteriotherapy in severe Crohn's colitis complicated by refractory Clostridium difficile infection. Infection 40:469–472 [DOI] [PubMed] [Google Scholar]
- 8.Elmore DB, Anderson JH, Hird DW, Sanders KD, Lerche NW. 1992. Diarrhea rates and risk factors for developing chronic diarrhea in infant and juvenile rhesus monkeys. Lab Anim Sci 42:356–359 [PubMed] [Google Scholar]
- 9.Feary DJ, Hassel DM. 2006. Enteritis and colitis in horses. Vet Clin North Am Equine Pract 22:437–479 [DOI] [PubMed] [Google Scholar]
- 10.Fooks LJ, Gibson GR. 2002. Probiotics as modulators of the gut flora. Br J Nutr 88:S39–S49 [DOI] [PubMed] [Google Scholar]
- 11.Gough E, Shaikh H, Manges AR. 2011. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 53:994–1002 [DOI] [PubMed] [Google Scholar]
- 12.Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. 2010. Durable alteration of the colonic microbiota by the administration of donor fecal fora. J Clin Gastroenterol 44:551–561 [DOI] [PubMed] [Google Scholar]
- 13.Hansen R, Thomson JM, El-Omar EM, Hold GL. 2010. The role of infection in the aetiology of inflammatory bowel disease. J Gastroenterol 45:266–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn SA, Gorawara-Bhat R, Rubin DT.2011. Fecal bacteriotherapy for ulcerative colitis: Patients are ready, are we? Inflamm Bowel Dis 18: 676–684. [DOI] [PMC free article] [PubMed]
- 15.Kelly CR, de Leon L, Jasutkar N. 2012. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 46:145–149 [DOI] [PubMed] [Google Scholar]
- 16.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. 2010. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 44:354–360. [DOI] [PubMed] [Google Scholar]
- 17.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. 2009. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM 102:781–784 [DOI] [PubMed] [Google Scholar]
- 18.Marteau PR. 2002. Probiotics in clinical conditions. Clin Rev Allergy Immunol 22:255–273 [DOI] [PubMed] [Google Scholar]
- 19.McKenna P, Hoffman C, Minkah N, Aye PP, Lackner A, Liu Z, Lozupone CA, Hamady M, Knight R, Bushman FD. 2008. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathog 4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellow MH, Kanatzar A. 2011. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection—results and follow-up. J Okla State Med Assoc 104:89–91 [PubMed] [Google Scholar]
- 21.Pillai A, Nelson R.2008. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst RevCD004611.
- 22.Rager KD, George LW, House JK, DePeters EJ. 2004. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 225:915–920 [DOI] [PubMed] [Google Scholar]
- 23.Rohlke F, Surawicz CM, Stollman N. 2010. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 44:567–570 [DOI] [PubMed] [Google Scholar]
- 24.Russell RG, Rosenkranz SL, Lee LA, Howard H, DiGiacomo RF, Bronsdon MA, Blakley GA, Tsai CC, Morton WR. 1987. Epidemiology and etiology of diarrhea in colony-born Macaca nemestrina. Lab Anim Sci 37:309–316 [PubMed] [Google Scholar]
- 25.Schwarz GE.1978. Estimating the dimension of a model. Ann Statist 6:461–464.
- 26.Vonesh EF, Chinchilli VG.1997. Linear and nonlinear models for the analysis of repeated measurements. London (UK): Chapman and Hall.
- 27.Wilk JL, Maginnis GM, Coleman K, Lewis A, Ogden B. 2008. Evaluation of the use of coconut to treat chronic diarrhea in rhesus macaques (Macaca mulatta). J Med Primatol 37:271–276 [DOI] [PubMed] [Google Scholar]
- 28.Yoon SS, Brandt LJ. 2010. Treatment of refractory–recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 44:562–566 [DOI] [PubMed] [Google Scholar]