Abstract
Changes in inhibition during development are well documented, but the role of inhibition in adult learning-related plasticity is not understood. In songbirds, vocal recognition learning alters the neural representation of songs across the auditory forebrain, including the caudomedial nidopallium (NCM), a region analogous to mammalian secondary auditory cortices. Here, we block local inhibition with the iontophoretic application of gabazine, while simultaneously measuring song-evoked spiking activity in NCM of European starlings trained to recognize sets of conspecific songs. We find that local inhibition differentially suppresses the responses to learned and unfamiliar songs and enhances spike-rate differences between learned categories of songs. These learning-dependent response patterns emerge, in part, through inhibitory modulation of selectivity for song components and the masking of responses to specific acoustic features without altering spectrotemporal tuning. The results describe a novel form of inhibitory modulation of the encoding of learned categories and demonstrate that inhibition plays a central role in shaping the responses of neurons to learned, natural signals.
Keywords: learning-dependent plasticity, inhibition, gabazine, iontophoresis
experience, including sensory deprivation during development and sensory-guided learning in adults, can profoundly alter neural encoding in the brain (Buonomano and Merzenich 1998; Feldman 2009; Galindo-Leon et al. 2009; Weinberger 1995; Woolley et al. 2010). In the auditory cortex, this experience-dependent plasticity plays a causal role in the ability to learn new associations and concepts (Reed et al. 2011). The circuit mechanisms that underlie experience-dependent changes to sensory encoding are only beginning to be understood but are thought to be tightly regulated by changes in inhibitory circuitry (Feldman 2009; Hensch 2005). During postnatal development, the maturation of inhibitory circuitry initiates the critical period for monocular deprivation plasticity in the visual cortex (Hensch et al. 1998), and responses to the deprived eye are weakened through strengthening of inhibitory synapses (Maffei et al. 2006). Changes in inhibition also shape the frequency tuning of primary auditory cortex during critical period development (Dorrn et al. 2010), and inhibition shapes the visually calibrated spatial tuning of neurons in the inferior colliculus of juvenile barn owls (Zheng and Knudsen 1999). In adults, inhibition mediated by GABA sharpens receptive fields (Dykes et al. 1984; Jacobs and Donoghue 1991; Ramoa et al. 1988), controls response gain (Ingham and McAlpine 2005; Katzner et al. 2011), and enhances neural selectivity (Wang et al. 2000). Moreover, learning influences the molecular regulation of GABA (Gierdalski et al. 2001) and GABA receptors (Lech et al. 2001), strengthens inhibitory synapses (Tokarski et al. 2007), and drives inhibitory synaptogenesis (Jasinska et al. 2010). However, how GABAergic inhibition shapes learning-dependent sensory encoding of natural signals in the adult cortex is not well understood.
Here, we examine the functional role of local inhibition in shaping learning-dependent sensory representations in adults. In songbirds, auditory experience changes the representations of conspecific songs in multiple regions throughout the auditory forebrain (Gentner and Margoliash 2003; Jeanne et al. 2011). Following song-recognition learning, neurons in the caudomedial nidopallium (NCM), a large forebrain area analogous to mammalian auditory cortex (Vates et al. 1996), show increasingly weaker responses to learned (compared with unfamiliar) songs along the dorsal-ventral axis (Thompson and Gentner 2010). This results in a functional response gradient across NCM, with learned and unfamiliar songs evoking similar responses at dorsal sites, whereas responses to unfamiliar songs are much stronger than those to learned songs at ventral sites. A large proportion of the neurons in NCM are inhibitory, and blocking GABAA receptors in NCM disrupts the temporal profile of the auditory response (Pinaud et al. 2004, 2008). To understand how GABAergic inhibition influences learning-dependent neural encoding, we manipulated local inhibition in NCM of European starlings trained to recognize controlled sets of conspecific songs. We found that inhibition masks an underlying excitatory preference for learned songs and enhances neural discrimination of behaviorally relevant song categories in dorsal NCM, while finding no major effects in ventral NCM. These effects arise, in part, by a reduction of responses to specific song features that contributes to neural selectivity but not from changes to average spectrotemporal tuning.
MATERIALS AND METHODS
Subjects.
Experiments used 10 adult European starlings (Sturnus vulgaris), wild-caught in southern California. Both sexes were included in these studies, as previous studies show that similar experience-dependent changes accompany song-recognition learning in both males and females (Gentner and Margoliash 2003; Thompson and Gentner 2010). Starlings were housed in a large, mixed-sex flight aviary with free access to food and water until behavioral training began. Light-dark cycles were synchronized to natural photoperiods in the aviary and during behavioral training. Prior to behavioral training, all song stimuli were unfamiliar to the subjects. All procedures were conducted in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Animals, under protocol number S05383, approved by the University of California, San Diego, Institutional Animal Care and Use Committee.
Behavioral training.
We trained starlings to recognize the songs of conspecifics using operant conditioning techniques, described in detail previously (Gentner and Margoliash 2003). Briefly, starlings were isolated in a sound attenuation chamber (Acoustic Systems, Austin, TX), equipped with an operant panel and food hopper (see Fig. 1A). Starlings learned to use the operant panel and food hopper through a series of successive shaping procedures. Custom software monitored peck responses and controlled the food hopper, lights, and stimulus presentation.
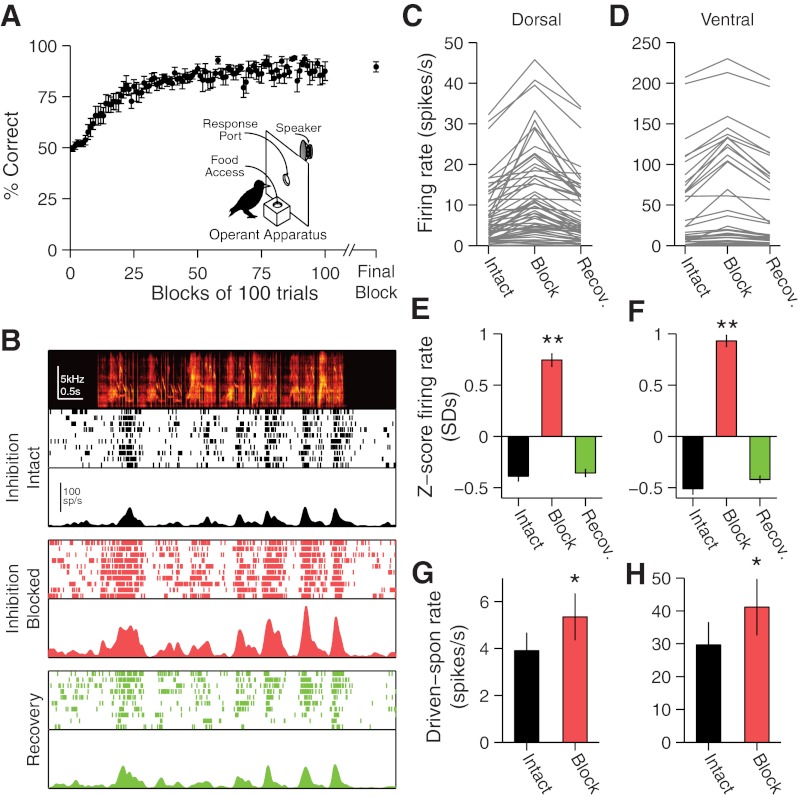
Fig. 1.
Behavioral training and gabazine iontophoresis. A: behavioral acquisition curve for the song-recognition task shows mean (±SE) percent correct (across subjects) for each consecutive 100-trial block during initial training and each subject's final 100 trials. Inset: schematic of behavioral training apparatus. B: effects of iontophoretic application of gabazine on neural responses at a multiunit (MU) caudomedial nidopallium (NCM) site. Top: song spectrogram, raster, and poststimulus time histogram (PSTH) of response with inhibition intact. Middle: raster and PSTH of response with inhibition blocked. Bottom: raster and PSTH of response during recovery from gabazine application. C and D: mean-driven firing rates when inhibition is intact and blocked and during recovery (Recov.) for all sites in dorsal (C) and ventral (D) NCM. E and F: normalized mean firing rates in intact, blocked, and recovery conditions for dorsal (E) and ventral (F) NCM sites. Repeated measures ANOVA: **P < 1 × 10−15. G and H: evoked response [song-driven − spontaneous (spon) firing rates] with local inhibition intact and blocked in dorsal (G) and ventral (H) NCM. Paired t-tests: *P < 5 × 10−4.
Each starling learned to recognize two songs from two different conspecific individuals (four songs total), performing trials freely throughout the day (from sunrise to sunset). A trial was initiated when a starling pecked the response port, triggering the presentation of a song from a speaker mounted inside of the chamber. Songs were chosen randomly (with replacement) on each trial from all of the training songs. After the song, the starling had to either peck the response port within 2 s for the songs of one individual (go trials) or withhold a peck response for the songs of the other individual (no-go trials). Responses on go trials were reinforced with 2 s of access to the food hopper. Responses on no-go trials were punished with a short timeout (10–60 s), in which the lights were extinguished, and food was unavailable. Starlings trained for a total of 70.3 ± 27.6 days, performing a total of 346.3 ± 151.8 100-trial blocks.
Song stimuli were 5-s segments of continuous singing from longer song bouts. We used songs from four different adult male starlings (eight total), which were captured and recorded in Maryland, ensuring that the songs were initially unfamiliar to the subjects in this study. For each subject, we used four songs for recognition training and saved four additional songs to be used as unfamiliar stimuli during electrophysiological testing. In some initial experiments (four subjects), we used longer segments of songs ranging from 8 s to 11 s. We found no differences in the results between starlings trained with shorter or longer songs. We counterbalanced the assignment of the same eight songs as either training or unfamiliar stimuli across individuals, such that the same song was used as a learned and unfamiliar song the same number of times. This design allows us to compare the responses of single neurons to multiple stimuli with different behavioral relevance. For any single neuron [or multiunit (MU) site], one cannot dissociate response differences due to acoustics from those due to learning. Across the population, however, our counterbalancing does permit this dissociation, because the same exact songs have different behavioral relevance for different animals and recording sites.
Electrophysiology and microiontophoresis.
In the days preceding electrophysiological recording, we anesthetized the starlings with isoflurane and attached a small pin stereotaxically to the surface of the skull, after which, starlings were allowed to recover. On the day of recording, we anesthetized the starlings with 20% urethane (7–8 ml/kg, in three to four intramuscular injections over 2–4 h). Starlings were then placed in a cloth jacket and secured via the attached pin to a stereotaxic apparatus inside of a sound attenuation chamber. A small craniotomy was made just dorsal to NCM, the dura removed, and electrodes advanced into NCM. All neural recordings and iontophoresis were performed with commercial multibarreled glass pipettes containing a carbon fiber electrode (5 μm diameter; 400–1,200 kΩ impedance) and six attached barrels (∼3 μm diameter) for drug microiontophoresis (Kation Scientific, Minneapolis, MN). Recordings were made over the course of 1 day, between 9 AM and midnight. In each experiment, we filled pipettes with NaCl (0.9%) for capacitance compensation and the GABAA receptor antagonist gabazine (SR95531, 3 mM, pH 3.2; Sigma Aldrich, St. Louis, MO). Through the microiontophoresis amplifier (NPI, Tamm, Germany), we applied retaining currents from −5 to −10 nA and ejection currents from 25 to 50 nA to control drug application. The gabazine concentration and iontophoresis parameters were chosen to match those in a previous study (Kurt et al. 2006). No bursting or epiletiform activity was induced at these ejection currents. In a subset of experiments, we also ejected GABA (1 M; Sigma Aldrich) and the GABAB receptor antagonist saclofen (20 mM; Sigma Aldrich). Application of GABA (n = 15 sites) significantly reduced evoked spike rates (P = 0.012, paired t-test) but had no effect on any of the learning-dependent measures reported here. Application of saclofen (n = 5 sites) similarly had no effect on any of these measures. The combined application of gabazine and saclofen (n = 12 sites) did not elicit responses different from those during application of gabazine alone. Spike2 software (Cambridge Electronic Design, Cambridge, UK) was used to play song stimuli, record extracellular activity, and sort spike waveforms offline following methods described previously (Jeanne et al. 2011; Thompson and Gentner 2010).
To search for responsive sites, we played the four songs that the starling learned to recognize and four songs that were unfamiliar. Unfamiliar songs were songs that the starlings had never heard before the electrophysiological experiment. Once an active site was located, we recorded responses to the four learned and four unfamiliar songs. We used the same set of eight songs at each recording site and each block. At each site, we began by recording a baseline block of responses to each song with inhibition intact and retaining current applied to the pipette. Blocks contained 10 repetitions of each song presented pseudorandomly, with a 4-s interstimulus interval. Block duration was 776 s or 1,187 s for short and long songs, respectively. Three sites contained only five repetitions of each song. Immediately after the initial block ended, we applied the ejection current to the pipette containing gabazine to initiate microiontophoresis of the antagonist. One minute later, we presented a second block of song stimuli identical to the baseline block (eight songs, 10 repetitions each, pseudorandom order). We then switched back to the retaining currents on the pipette to stop iontophoresis, waited 1 min, then began a third, “recovery” block with same stimuli and presentation protocol as in the first two blocks. Complete recovery (determined as the time after three consecutive recovery trials elicited firing rates within two SDs of the mean baseline rate) occurred after an average of 230 s (minimum: 98 s; maximum: 940 s). In experiments where we also recorded the responses during iontophoresis of GABA or saclofen, we also presented stimuli in blocks of 10 repetitions/stimulus with 1 min between blocks. In some initial experiments (four subjects), we did not wait 1 min between blocks. We found no major differences in the data collected from experiments with or without the 1-min interblock intervals. We matched the intensity of all songs at 68 dB peak root mean square at the center point of the subject's head and presented them free field. At the end of the recording experiment, we made a small electrolytic lesion to assist in postmortem histological placement of recording sites.
We note that the volume of drug released by current, even with our standardized pipettes and fixed concentrations, depends on a number of parameters that are very difficult to control in vivo (Lalley 1999). The randomly interleaved stimulus presentation, however, ameliorates the impact of any such differences because any variability in drug delivery is distributed evenly between learned and unfamiliar songs, on average. Thus each site serves as its own control for all effects described here except those comparing differences between dorsal and ventral NCM. In addition, because the iontophoresis pipette can become blocked while in the brain, we used a physiological assessment to ensure that drug delivery was successful. Delivery was considered successful at a particular site if the average song-driven firing rate during iontophoresis was significantly different (either higher or lower) from the firing rate prior to iontophoresis. Sites with no significant difference were included as long as subsequently recorded sites along the same penetration showed significant differences, indicating that the pipette was not blocked. Three of the 100 total sites were omitted because we could not ensure successful drug delivery. An additional six of 100 sites were omitted because firing rates did not recover (or partially recover) after drug delivery was stopped. Inclusion of these nine sites does not alter any of the results reported here.
Histology.
After the recording experiment, we injected the starlings with a lethal dose of Nembutal. The starlings were then decapitated, and the heads were fixed in 10% neutral-buffered formalin. After several days, we transferred the brains to 30% sucrose PBS for cryoprotection. We sectioned the brain into 50-μm sections and stained for Nissl. All sites reported here were determined to be within NCM by comparing their positions relative to the electrolytic lesion and to the anatomical boundaries of NCM. For purposes of comparison with previously published work (Thompson and Gentner 2010), we defined an operational border between dorsal and ventral NCM at 2,580 μm from the dorsal surface of the brain, roughly bisecting NCM at the midpoint of its longest dorso-ventral axis. The functional and anatomical significance of this division is explained in results and discussion
Data analysis.
All recordings were sorted offline into single-unit and MU sites using Spike2 software. A site was considered a single unit only if there was a high signal-to-noise ratio, and the waveform was clearly distinct from other spikes. Single-unit sites were sorted using template matching and had few interspike-interval (ISI) refractory-period violations (ISIs of <2 ms accounted for <0.5% of all ISIs for each unit). The same spike-sorting parameters were used for each microiontophoresis condition at each site. Most of our recordings did not meet the criteria for single units and were considered MU sites (dorsal: nine single units, 46 MUs; ventral: four single units, 32 MUs). These sites were typically made up of several waveforms that could not be separated confidently. For MU sites, we set a spike threshold that was above the noise floor and counted events above the threshold. We used the same threshold for spikes collected in all microiontophoresis conditions for each site. Spike times were exported to MATLAB (MathWorks, Natick, MA) for all subsequent analyses.
Spontaneous firing rates for each site were computed as the mean rate of spikes in the 2-s period before the onset of each song stimulus. Mean-driven firing rates for each song stimulus were computed as the mean number of spikes observed during presentation of that song divided by the song duration. For analyses where we consider differences in responses across stimuli (see Figs. 1–3), we normalized for the variability in the firing rates between different sites by converting the firing rates of each site to z-scores. In all cases, nearly identical effects were seen in both normalized and non-normalized firing rates.
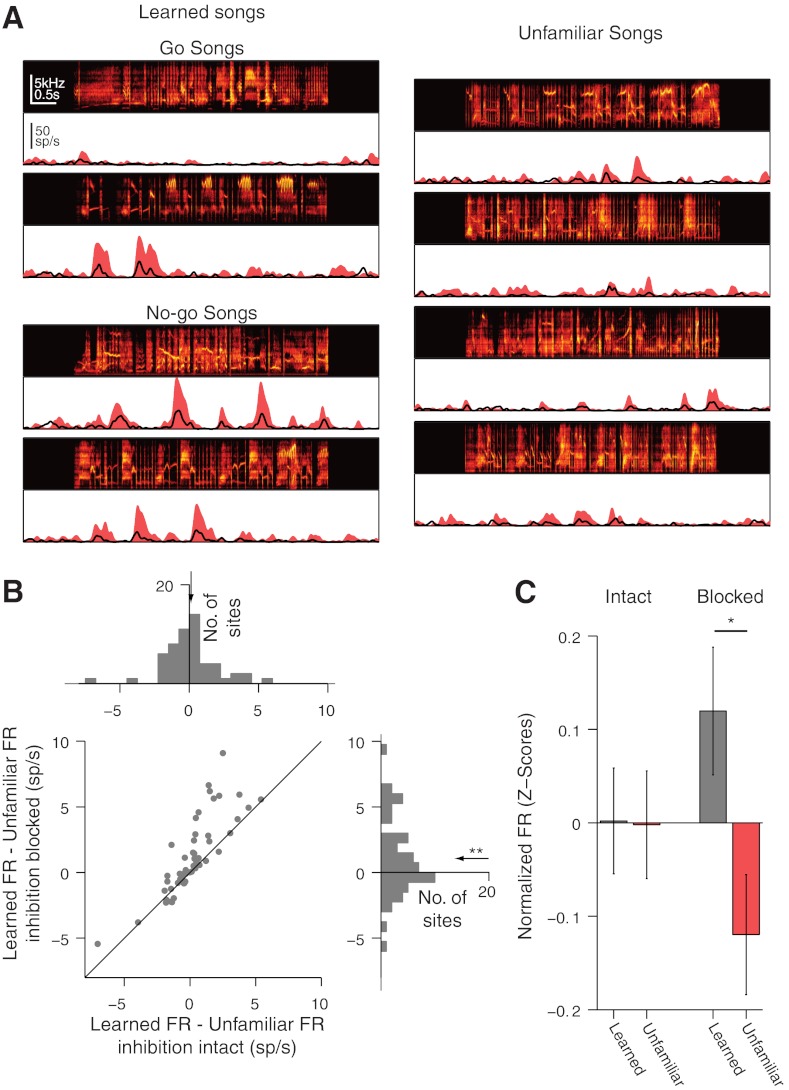
Fig. 2.
Blocking inhibition reveals an excitatory bias for learned songs in dorsal NCM. A: song spectrograms and response PSTHs for learned songs (left) and unfamiliar songs (right) for an example site in dorsal NCM. Solid red denotes PSTH with inhibition blocked. Black line denotes PSTH with inhibition intact. B: histograms of average firing-rate (FR) differences between learned and unfamiliar songs for all dorsal sites with inhibition intact (top) and inhibition blocked (bottom right). Scatter plot (bottom left) shows that inhibition increases the firing-rate difference between learned and unfamiliar songs for nearly all sites. C: mean (±SE) song firing rates in dorsal NCM normalized by the SD of firing rates to all songs (z-scores). The average effect of blocking inhibition has been subtracted from these numbers. Significant differences between learned and unfamiliar songs are only observed when inhibition is blocked. sp/s, spikes/s. Paired t-tests: *P < 0.05; **P < 0.01.
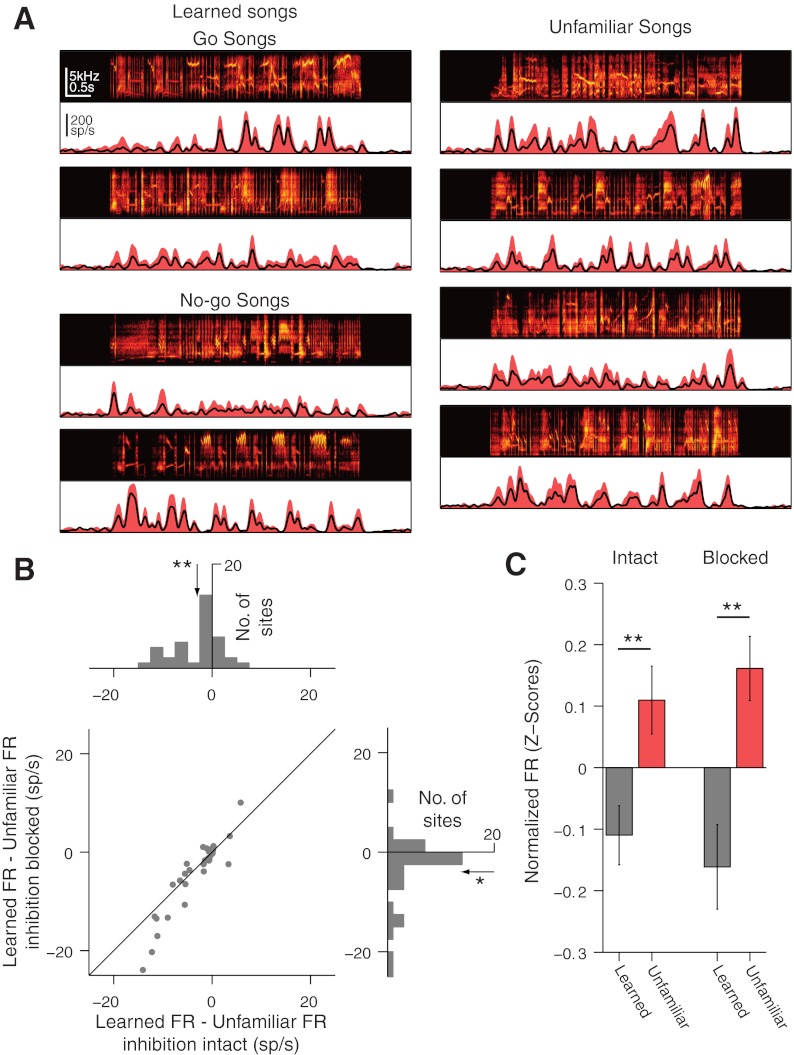
Fig. 3.
Blocking inhibition does not alter an excitatory bias for unfamiliar songs in ventral NCM. A: song spectrograms and response PSTHs for learned songs (left) and unfamiliar songs (right) for an example site in ventral NCM. Solid red denotes PSTH with inhibition blocked. Black line denotes PSTH with inhibition intact. B: histograms of average firing-rate differences between learned and unfamiliar songs for all ventral sites with inhibition intact (top) and inhibition blocked (bottom right). Scatter plot (bottom left) shows no clear trend for differential effects of inhibition between learned and unfamiliar songs. C: mean (±SE) song firing rates in ventral NCM normalized by the SD of firing rates to all songs (z-scores). The average effect of blocking inhibition has been subtracted from these numbers. Paired t-tests: *P < 0.005; **P < 0.001.
To measure the stimulus selectivity, we broke songs into their motifs (∼1 s) and calculated the firing rates to each motif with inhibition intact and with inhibition blocked. Selectivity was calculated for each site using a well-established measure, sometimes referred to as lifetime sparseness (Vinje and Gallant 2000)
where ri is the firing rate to the ith motif, and n is the total number of motifs. Selectivity values range from zero, in which a site responds equally to all motifs, to one, in which a site responds only to a single motif. Although this selectivity measure was developed for use with single-unit responses, it can still be applied to MU activity as an approximate lower bound for the selectivity of the constituent single units (Weliky et al. 2003).
To estimate how much of the song response was completely masked by inhibition, we binned all responses into 50-ms bins. We considered a bin to be unresponsive if the firing rate during the bin was zero during all repetitions of the stimulus. We then computed the fraction of all bins that were responsive under blockade of local inhibition and subtracted the fraction that was still responsive with local inhibition intact. The resulting fraction measures the proportion of the underlying excitatory response that is masked completely by local inhibition. A negative fraction indicates that blockade of inhibition reduced the number of bins eliciting positive responses. Changing the bin size or raising the threshold for a bin to be considered responsive did not qualitatively alter the effects reported here.
Receptive fields were modeled using the maximally informative dimension (MID) technique, which is described in detail elsewhere (Sharpee et al. 2004). Briefly, the MID filter was determined via gradient ascent optimization and simulated annealing to be the vector in the high-dimensional stimulus space (from the spectrogram) that maximized the mutual information between the spike response and the stimulus projected onto the vector. The nonlinearity was computed as
where P(x) is the distribution of stimulus-projection values onto the MID vector, P(x|spike) is the distribution of stimulus-projection values onto the MID vector that elicited spikes, and P(spike) is the overall probability of a spike. P(spike|x) thus gives the probability of observing a spike given a particular stimulus-projection value. The mutual information between the stimulus projected onto the MID filter and single spikes was evaluated as
where v is the stimulus projection. The total mutual information about single spikes was evaluated as
where the angled brackets denote an average overall stimuli (Sharpee et al. 2004). The performance of the MID model was then computed as the percentage of the total information/spike, which is accounted for by the MID: I(v)/Ispike × 100%. Due to limitations in dataset size, only one filter of the MID could be computed reliably here. Each MID model was computed separately for learned and unfamiliar songs and is the average over four jack-knife subsets of each dataset. Five sites contained too few spikes to estimate the MID model reliably and were not included in the analysis.
d′ between the responses to go songs and no-go songs was computed as the absolute value of the difference in mean firing rates divided by the square root of the average of the variance in the firing rates over repeated trials. d′ was computed separately for the inhibition-blocked and inhibition-intact conditions. Statistically similar results were obtained using a mutual information analysis and a receiver-operating characteristic analysis.
Statistical analysis.
All statistical tests and associated P values are reported in the text. In all cases, significance was evaluated at α = 0.05.
RESULTS
To investigate the role of local inhibitory circuit function on learning-dependent auditory forebrain neural encoding, we trained starlings to recognize conspecific songs and then recorded neural spiking activity in NCM under local blockade of GABAA receptor-mediated inhibition (see materials and methods). All subjects learned to recognize two sets of conspecific song segments using a go/no-go operant-training procedure (Fig. 1A). Starlings learned the song-recognition task quickly, performing above chance after an average of 2,200 ± 302 trials. The mean performance for all starlings on the final 100-trial block was 89.6 ± 2.5% correct (Fig. 1A). Following behavioral training, we recorded neural activity in dorsal and ventral NCM in response to the four training songs and to four unfamiliar songs. The selective blockade of GABAA receptors in a region around the recording site (see materials and methods) caused a large, reversible increase in the spontaneous and the song-evoked firing rates (Fig. 1B). On average, spontaneous spike rates increased from 3.52 ± 0.43 Hz to 7.31 ± 0.88 Hz in dorsal NCM (n = 55 sites) and from 14.6 ± 3.1 Hz to 20.6 ± 3.6 Hz in ventral NCM (n = 36 sites). Similarly, we observed significant increases in the mean song-driven spike rates in both dorsal (P = 1.4 × 10−7) and ventral (P = 6.3 × 10−6, paired t-tests for both regions; Fig. 1, C–F) NCM. These observations are consistent with the presence of local GABAergic inhibition throughout NCM. Moreover, blocking inhibition in both dorsal and ventral NCM leads to a greater change in firing rate during the song-evoked response than during spontaneous firing when no stimulus is presented (dorsal: P = 2.7 × 10−4; ventral: P = 3.0 × 10−5, paired t-tests; Fig. 1, G and H). This supports the conclusion that the effects of local GABAergic inhibition increase above tonic background levels when the network is processing songs.
Blocking local inhibition alters learning-dependent plasticity.
Learning modifies the neural activity in many regions of the songbird auditory forebrain (Gentner and Margoliash 2003; Jeanne et al. 2011; Thompson and Gentner 2010). In NCM, these effects vary along the dorsal-ventral axis. In dorsal NCM, learned and unfamiliar songs evoke similar responses, whereas in ventral NCM, learned songs elicit significantly lower spiking rates than unfamiliar songs (Thompson and Gentner 2010). Here, we ask whether local inhibition differentially influences neural responses to learned and unfamiliar songs in these two NCM regions. Figure 2A shows the response of an example MU site in dorsal NCM to learned and unfamiliar songs. Consistent with previous findings with inhibition intact, similar mean firing rates were elicited at this site by learned (3.4 ± 0.9 Hz) and unfamiliar (2.8 ± 0.3 Hz) songs. With inhibition blocked, however, the firing rate elicited by learned songs (12.1 ± 3.0 Hz) was substantially greater than the rate for unfamiliar songs (7.5 ± 0.6 Hz). Thus blocking local inhibition at this site reveals an underlying excitatory preference for learned over unfamiliar songs. This suggests that inhibition may serve to mask latent excitatory biases in NCM neurons.
On average, this masking effect was observed across our sample of dorsal NCM sites. With inhibition intact, learned and unfamiliar songs elicited similar firing rates in dorsal NCM (P = 0.65, paired t-test; Fig. 2B). With local inhibition blocked, however, learned songs elicited significantly higher mean firing rates (13.2 ± 1.5 Hz) than unfamiliar songs (12.1 ± 1.5 Hz; P = 0.0082, paired t-test; Fig. 2B). Consistent with the idea that inhibition blocks the effects of learning on dorsal NCM firing rates, directly comparing the data from both conditions in a single analysis revealed a significant interaction between learning and local inhibition [two-way repeated measures ANOVA; main effect of blocking inhibition: F(1, 54) = 45.45, P = 1.1 × 10−8; main effect of learning: F(1, 54) = 3.57, P = 0.064; learning × inhibition interaction: F(1, 54) = 17.77, P = 9.5 × 10−5; Fig. 2B]. Likewise, normalizing the responses of all sites to unit variance (see materials and methods) did not change these results (Fig. 2C). Therefore, the general increase in firing rates caused by blocking inhibition cannot explain the revealed response bias. Instead, our results suggest that local GABAergic inhibition masks a learning-dependent excitatory bias in dorsal NCM.
We next examined the role of inhibition on learning-dependent encoding in ventral NCM, where under normal conditions, most neurons elicit higher spike rates for unfamiliar songs than for learned songs (Thompson and Gentner 2010). Figure 3A shows the response of an example MU site in ventral NCM. With inhibition intact, lower mean firing rates were elicited at this site by learned (60.4 ± 5.7 Hz) rather than unfamiliar (72.6 ± 4.0 Hz) songs. Although blocking inhibition increased the overall firing rate, the learned songs still elicited a lower mean response rate (101.4 ± 6.4 Hz) than the unfamiliar songs (121.7 ± 5.2 Hz). Similar effects held across all of the ventral NCM sites in our sample. On average, learned songs elicited significantly lower firing rates (42.8 ± 9.0 Hz) than unfamiliar songs (45.8 ± 9.4 Hz) with inhibition intact (P = 5.5 × 10−4, paired t-test; Fig. 3B). With inhibition blocked, the mean firing rate evoked by learned songs (59.9 ± 10.9 Hz) was still significantly lower than the mean rate evoked by unfamiliar songs (63.8 ± 11.5 Hz; P = 0.0016, paired t-test; Fig. 3B). Considering the effects of learning and local inhibition together in a single analysis, we find that blocking inhibition did not alter the effect of learning on ventral NCM firing rates [two-way repeated measures ANOVA; main effect of learning: F(1, 35) = 13.23, P = 8.8 × 10−4; main effect of blocking inhibition: F(1, 35) = 29.71, P = 4.1 × 10−6; learning × inhibition interaction: F(1, 35) = 3.38, P = 0.075; Fig. 3B]. As with dorsal NCM, normalizing the responses of all sites to unit variance (see materials and methods) did not change these results (Fig. 3C). The persistence of stronger responses for unfamiliar songs with the removal of local inhibition suggests that in ventral NCM, the excitatory components of evoked spiking responses are stimulus specific and shaped by learning. In particular, song-recognition learning decreases the effective excitatory drive (i.e., the response that remains after blocking local inhibition) on neurons in ventral NCM—the opposite of the increase in effective excitatory drive elicited by learned songs on neurons in dorsal NCM.
To control for the possibility that our results might be due to song acoustics rather than learning, we re-sorted the empirical response data (Figs. 2C and 3C) by song identity rather than behavioral relevance. For example, instead of sometimes treating responses to a given song as “learned” and sometimes as “unfamiliar”, depending on how a bird was trained, we average all responses to that song regardless of the training. If acoustic differences between songs explain our results, then the effects shown in Figs. 2C and 3C should remain or even strengthen when the response data are sorted this way. This was not the case, however; re-sorting responses by stimulus acoustics abolished all of the significant differences shown in Figs. 2C and 3C (P > 0.62 all cases, paired t-tests).
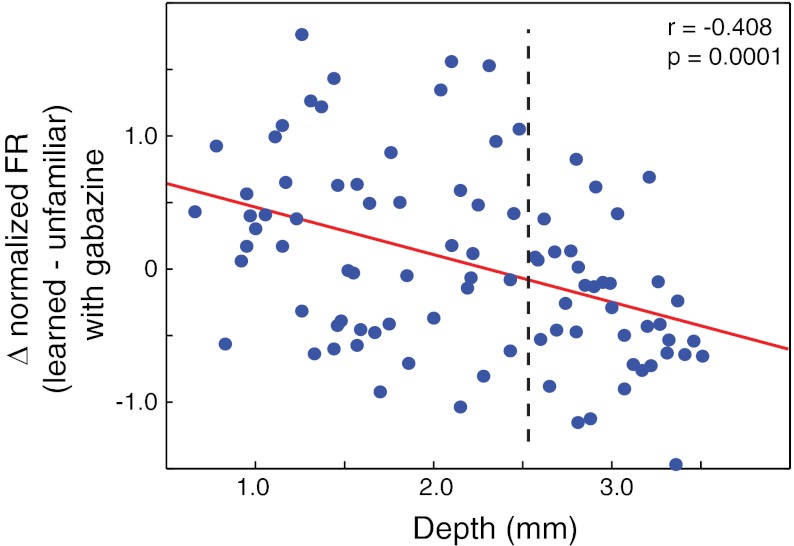
Overall, blocking local inhibition makes it apparent that learning induces substantial and diverse modulation of excitatory drive onto neurons throughout NCM. Whereas the cellular and circuit-level processes mediated by local GABAA receptors do not alter the net excitatory preference for unfamiliar songs in ventral NCM, they sharply attenuate the excitatory preference for learned songs in dorsal NCM to yield similar responses to both learned and unfamiliar songs. Although we operationally define the border between dorsal and ventral NCM at 2,580 μm (see materials and methods), there is likely a more continuous shift in response properties across the whole dorsal-ventral axis rather than a strict functional boundary (Thompson and Gentner 2010). Consistent with this idea, the present results show a significant, negative correlation between the learning-driven response bias (learned − novel z-score firing rates) and the recording depth of each site (Fig. 4; r = −0.408; P = 0.0001). Likewise, the change in this response bias produced by blocking inhibition (intact response bias − blocked response bias) is significantly correlated with depth (r = 0.321; P = 0.002), moving from large, negative values at dorsal sites to approximately zero in ventral sites.
Fig. 4.
Learning-driven response bias along dorso-ventral axis of NCM. Each point shows the difference between mean z-score firing rates for learned and unfamiliar songs at each site while gabazine is delivered plotted as a function of depth. Red line shows the best-fit linear regression, and values in the top right show the linear correlation coefficient and associated P value.
Blocking inhibition impairs discriminability of learned categories.
Because blocking inhibition altered firing rates in a learning-dependent manner, we hypothesized that it might also contribute to the neural encoding of behaviorally relevant song categories. To test this idea, we measured the discriminability of the average firing-rate responses to two sets of learned songs: those associated through operant training with go responses and those associated with no-go responses. During training, these two sets of songs required distinct behaviors and elicited distinct reward outcomes (see materials and methods). On average, the differences between mean firing rates for go songs and no-go songs were small in dorsal NCM (inhibition intact: P = 0.18; inhibition blocked: P = 0.12, paired t-tests) and only marginally significant in ventral NCM, with inhibition blocked (inhibition intact: P = 0.15; inhibition blocked: P = 0.042, paired t-tests). Such comparisons between mean firing rates, however, may obscure large differences at individual sites if the signs of these differences are not consistent across sites, even though a difference in either direction may be equally informative for discrimination. To quantify this discriminability, we computed d′, a measure analogous to the signal-to-noise ratio, between the distributions of firing-rate responses to go and no-go songs (see materials and methods). Figure 5A shows histograms of the distributions of firing rates to go and no-go songs for an example site. For this site, blocking inhibition substantially increased the overlap of response between these two song categories. To quantify this, we computed d′ (see materials and methods), a measure analogous to the signal-to-noise ratio. At this site, d′ was greater with inhibition intact (2.49) than with inhibition blocked (1.40). On average, blocking inhibition impaired the discriminability of the go and no-go songs, with a d′ of 1.07 ± 0.10 when inhibition was intact and 0.98 ± 0.10 when inhibition was blocked (Fig. 5B). We observed a significant increase in discriminability among dorsal NCM sites (P = 0.042, paired t-test; Fig. 5C) but not among ventral NCM sites (P = 0.89, paired t-test). This suggests that inhibition functions in dorsal NCM to enhance the firing-rate differences between songs associated with distinct behavioral responses and rewards.
Fig. 5.
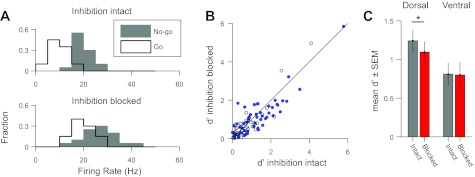
Blocking inhibition impairs the discrimination of learned songs in NCM. A: histograms of probability distributions of firing rates in response to songs associated with go and no-go behavioral responses for an example site in dorsal NCM with inhibition intact (top) and inhibition blocked (bottom). B: comparison of d′ for discrimination between go and no-go songs with inhibition intact and with inhibition blocked for each site. Blue dots denote dorsal sites; open circles denote ventral sites. C: comparison of mean (±SE) d′ values in dorsal and ventral NCM with inhibition intact and blocked. Paired t-test: *P < 0.05.
Blocking inhibition does not alter spectrotemporal tuning.
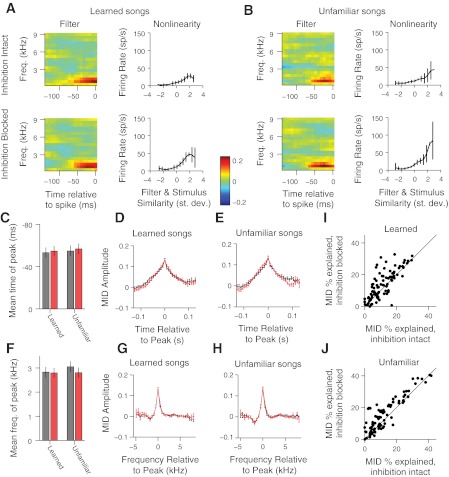
Because inhibition can alter the receptive-field properties of auditory cortical neurons (Andoni et al. 2007; Chen and Jen 2000; Dykes et al. 1984; Jacobs and Donoghue 1991; Muller and Scheich 1987; Ramoa et al. 1988; Sillito 1975; Thiele et al. 2004; Wang et al. 2002), we investigated whether blocking inhibition affects average spectrotemporal tuning in NCM and whether this could underlie the learning-dependent effects of inhibition. We modeled the relationship between the song stimulus and the spiking response using spectrotemporal receptive-field models fit with the technique of MIDs (Sharpee et al. 2004) (see materials and methods). Such models are composed of a dynamic linear filter that projects the high-dimensional stimulus onto a single dimension and a static nonlinearity that relates each projection value to a neural firing rate. Figure 6, A and B, shows a MID model of responses to learned songs (Fig. 6A) and to unfamiliar songs (Fig. 6B) for an example site from dorsal NCM. For this site and most others, blocking inhibition did not substantially alter the average spectrotemporal tuning. However, the MID model for this site accounted for a greater percentage of the information/spike with inhibition blocked (25.6% for learned songs; 17.2% for unfamiliar songs) than with inhibition intact (19.1% for learned songs; 12.3% for unfamiliar songs).
Fig. 6.
Blocking inhibition does not change the average spectrotemporal tuning properties of sites in NCM. A: spectrotemporal filter (left) and nonlinearity (right) for a maximally informative dimension (MID) model of an example site in dorsal NCM with inhibition intact (top) and blocked (bottom) for learned songs. Freq., frequency; st. dev., SD. B: same as A but for unfamiliar songs. C: mean (±SE) time of peak amplitude in MID filter for learned and unfamiliar songs. Gray bars denote values with inhibition intact; red bars denote values with inhibition blocked. D: comparison of average ± SE (across all sites) temporal profile of MID filter for learned songs at the frequency that yielded the highest magnitude. E: same as D but for unfamiliar songs. F–H: same as C–E but for the spectral profile of the filter. I: percentage of information explained by the MID model for learned songs with inhibition intact compared with the percentage explained with inhibition blocked. J: same as I but for unfamiliar songs.
The observation that blocking inhibition increased predictability of spiking without altering spectrotemporal tuning was consistent throughout NCM. Across all NCM sites, we observed no reliable trend for changes in spectrotemporal tuning due to blocking inhibition. In particular, blocking inhibition did not alter the time of the peak excitatory component in the filter (model for learned songs: P = 0.72; model for unfamiliar songs: P = 0.67, paired t-tests; Fig. 6C) or the temporal profile of the filter (Fig. 6, D and E). Likewise, blocking inhibition did not alter the frequency of the peak excitatory component in the filter (model for learned songs: P = 0.85; model for unfamiliar songs: P = 0.35, paired t-tests; Fig. 6F) or the spectral profile of the filter (Fig. 6, G and H). Thus differences in the average spectrotemporal tuning do not underlie the learning-dependent responses in NCM. However, blocking inhibition did increase the fraction of the information/spike, which could be accounted for by the MID models, both for learned songs (1.0 × 10−4, paired t-test; inhibition intact: 11.5 ± 0.8%; inhibition blocked: 14.0 ± 1.0%; Fig. 6I) and unfamiliar (1.8 × 10−7, paired t-test; inhibition intact: 12.7 ± 1.1%; inhibition blocked: 15.6 ± 1.1%; Fig. 6J) songs. These results suggest that local inhibition reduces the proportion of the neural response that can be explained by a single linear filter and that inhibition increases the complexity of NCM responses (e.g., by temporally specific reductions in firing rate), while not altering the average spectrotemporal tuning.
Inhibition shapes selectivity for complex sounds.
We next explored how inhibition affected NCM responses in ways not described by the average spectrotemporal response properties. In some sensory systems, local inhibition increases the selectivity for specific sensory features by suppressing responses to other stimuli (Muller and Scheich 1987; Sillito 1975; Wang et al. 2000). In songbirds, neurons in NCM respond preferentially to conspecific vocalizations and are selective for specific features of conspecific songs (Chew et al. 1996; George et al. 2008; Muller and Leppelsack 1985; Ribeiro et al. 1998; Stripling et al. 1997; Thompson and Gentner 2010). We examined whether the selectivity for complex features of conspecific songs in NCM is also altered by local inhibition. Starling songs are composed of strings of unique acoustic units called motifs. To investigate selectivity, we divided songs into their constituent motifs (Fig. 7A) and computed selectivity values (Vinje and Gallant 2000) across the firing rates to each motif with inhibition intact and blocked (see materials and methods; Fig. 7B). Motif selectivity values range from zero for sites that respond to all song motifs equally to one for sites that respond to only a single motif.
Fig. 7.
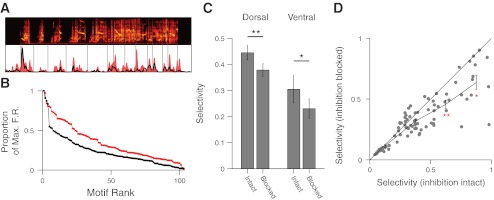
Blocking inhibition decreases selectivity in NCM. A: example firing rate response of a site in dorsal NCM. Red histogram denotes PSTH of response with inhibition blocked. Black line denotes PSTH of response with inhibition intact. Gray lines demarcate motif boundaries. Blocking inhibition reduces responses substantially to some motifs but has relatively little effect on other motifs. B: relative firing rates of rank-ordered motifs normalized to the maximum firing rate (Max. F.R.) for inhibition intact (black) and inhibition blocked (red). C: mean (±SE) selectivity in dorsal and ventral NCM with and without inhibition. In both regions, inhibition increases selectivity. D: comparison of selectivity with inhibition intact (abscissa) and selectivity with inhibition blocked (ordinate). Line plot represents mean (±SE) selectivity with inhibition blocked for 4 quadrants of selectivity with inhibition intact (boundaries: 0, 0.25, 0.5, 0.75, 1). Paired t-tests: *P < 0.005; **P < 5 × 10−5.
Local inhibition shaped selective representations in both dorsal and ventral NCM (Fig. 7C). In dorsal NCM, blocking inhibition decreased the mean selectivity from 0.44 ± 0.03 to 0.38 ± 0.02 (P = 4.0 × 10−5, paired t-test). In ventral NCM, blocking inhibition decreased the mean selectivity from 0.30 ± 0.05 to 0.23 ± 0.04 (P = 0.0039, paired t-test). Overall, selectivity with inhibition intact was significantly higher in dorsal sites than in ventral sites (P = 0.011, paired t-test; Fig. 7C). We observed similar effects within the subset of confirmed single units (see materials and methods) in dorsal NCM (P = 0.020, paired t-test; selectivity with inhibition intact: 0.76 ± 0.04; with inhibition blocked: 0.60 ± 0.07). A similar trend was seen in single units in ventral NCM, but the limited sample size precluded statistical analysis. Inhibition did not affect selectivity at all sites uniformly. On average, only those sites that were highly selective with inhibition intact saw a significant decrease in selectivity when inhibition was blocked (Fig. 7D). Moreover, even after blocking local GABAA-mediated inhibition, some (or at some sites, all) selectivity remained (Fig. 7D). This gabazine-resistant selectivity may reflect the contribution of GABAA-independent (including excitatory) processes and/or an incomplete GABAA receptor blockade. In either case, however, local inhibition plays a significant role in the generation of selective responses on average.
Blocking inhibition unmasks responses to specific song features.
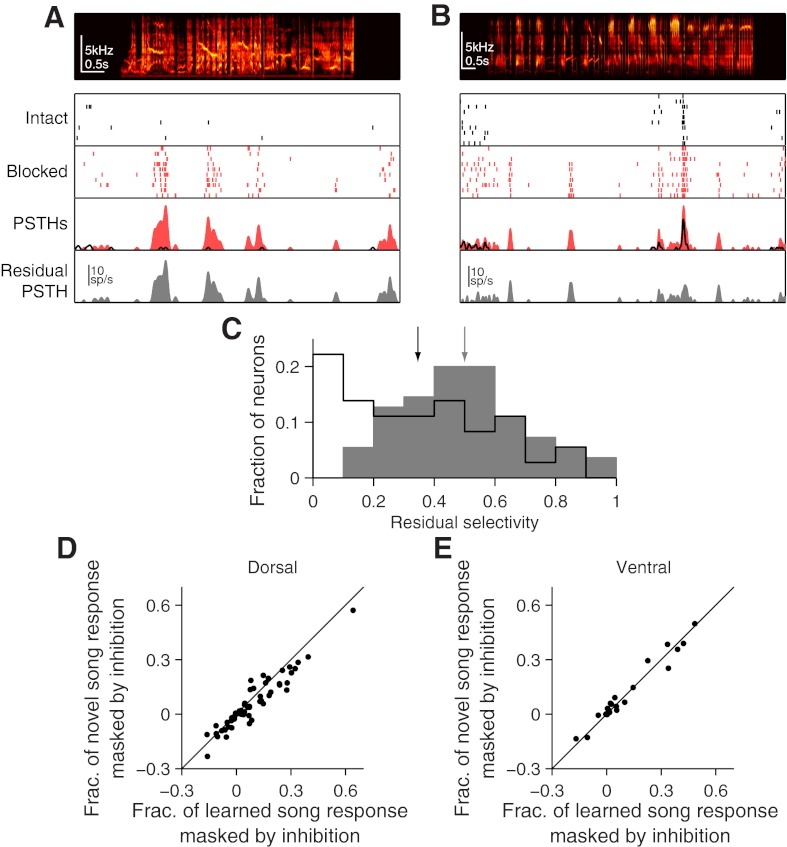
One way local inhibition could increase motif selectivity, while not affecting average spectrotemporal tuning, is by suppressing responses to specific song features. Consistent with this, we saw that blocking local inhibition revealed responses to portions of songs that evoked no response with inhibition intact. Figure 8A shows a MU site where blocking local inhibition reveals responses during several segments of the song. Similar effects are observed in single neurons (Fig. 8B) and thus are not likely caused by the recruitment of additional neurons to the MU response. As an initial analysis of these observations, we measured the selectivity of the net effectiveness of inhibition—the difference between firing rate with inhibition intact and with inhibition blocked (Fig. 8C). This residual selectivity was distributed broadly for both dorsal and ventral sites but was, on average, higher in dorsal NCM than ventral NCM (P = 0.0013, paired t-test, mean ± SE; dorsal: 0.50 ± 0.03; ventral: 0.35 ± 0.04). We further quantified these residual responses by computing the fraction of each song that evoked positive responses with inhibition intact and asked if that fraction changed during blockade of local inhibition (see materials and methods). We then asked whether responses were masked more often during learned or unfamiliar songs. In dorsal NCM, inhibition masked a greater fraction of responses during learned songs than during unfamiliar songs (Fig. 8D). On average, local inhibition masked responses to 9.14 ± 2.05% of the duration of each learned song but only 6.20 ± 1.87% of the duration of each unfamiliar song (P = 6.46 × 10−5, paired t-test). These measures mirror the differences in average firing rates reported above in dorsal NCM (Fig. 2) and suggest that local inhibition in this subregion abolishes the bias for learned songs, in part, by masking more responses to features of learned songs than to unfamiliar songs. In ventral NCM, however, a similar number of responses were revealed during learned and unfamiliar songs (Fig. 8E). Inhibition masked responses to 6.64 ± 2.47% of the duration of each learned song and 6.89 ± 2.42% of the duration of each unfamiliar song (P = 0.60, paired t-test). This is consistent with the lack of learning-dependent effects of inhibition in ventral NCM (Fig. 3).
Fig. 8.
Blocking inhibition reveals selective responses. A: example MU site where blocking inhibition uncovered selective responses. Intact panel displays spike raster with inhibition intact. Blocked panel displays spike raster with inhibition blocked. PSTH panel displays mean responses in the inhibition-blocked condition (red) and in the intact condition (black line). Residual PSTH panel displays the difference in spike rate between the inhibition blocked and inhibition intact conditions. B: same as A but for an example single-unit site. C: histograms of residual selectivity (selectivity of the firing-rate differences between the inhibition-blocked and inhibition-intact conditions) for dorsal (gray bars) and ventral (black line) NCM. Arrows denote means for each population. D: comparison of the fraction (Frac.) of the response masked by inhibition for learned songs with the fraction masked by inhibition for unfamiliar songs (see materials and methods for details) for sites within dorsal NCM. Negative values indicate that inhibition increased the fraction of song that elicits positive responses. E: same as D but for sites within ventral NCM.
DISCUSSION
Learning alters sensory cortical representations (Buonomano and Merzenich 1998; Galindo-Leon et al. 2009; Weinberger 1995), but how or even if GABAergic inhibition contributes to these changes is not known. Here, we show that local inhibition modulates song-evoked neural activity in NCM, a secondary auditory cortical region, in a learning-dependent manner. This modulation takes two forms. First, inhibition masks a latent excitatory preference for learned songs in dorsal NCM. The masking is phenomenologically similar to the effects of peripheral sensory disruption on inhibitory function in somatosensory (Lane et al. 1997) and visual (Mower et al. 1984) cortices and the tectum (Zheng and Knudsen 1999). This is consistent with the idea that multiple types of experience activate the same inhibitory mechanisms to reshape neural representations. Second, inhibition sharpens the encoding of learned song categories throughout NCM. Inhibitory modulation of categorical responses is novel and indicates that the effects of learning-dependent inhibition extend to complex representations that cannot be explained parsimoniously by the average spectrotemporal acoustics of the stimuli (George et al. 2008). Similar circuitry may contribute to the encoding of learned categories observed in other regions of the songbird forebrain (Jeanne et al. 2011).
The learning-dependent inhibitory modulation in NCM is explained, in part, by changes in neural selectivity. We find that the GABAergic suppression of responses to features within learned songs (Fig. 8), which clearly changes feature selectivity, underlies both the masking of the excitatory preference for learned songs (Fig. 2) and the overall selectivity for motifs (Fig. 7). Enhanced neural selectivity is one of several well-described roles for GABAergic inhibition in sensory encoding (Muller and Scheich 1987; Sillito 1975; Wang et al. 2000). Another reported role for inhibition is the sharpening of receptive fields (Dykes et al. 1984; Jacobs and Donoghue 1991; Ramoa et al. 1988). We observed no significant changes, however, in the average spectrotemporal tuning of neurons in NCM (Fig. 6). Thus learning modifies inhibitory function in NCM in ways that are consistent with known effects on selectivity but independent of measurable effects on receptive-field structure.
It is important that the observed changes in categorical encoding (Fig. 5) and selectivity (Figs. 7 and 8) are not caused by a saturation of the firing rates when gabazine is applied. In most cases (Figs. 1–3), the increase in response during gabazine administration is proportional to the baseline response, and many of the responses evoked under gabazine are lower than responses to other song features without gabazine. If gabazine were saturating the firing rate, we would expect a much more uniform amplitude over the regions of song that show a change with inhibition blocked. The observed changes in selectivity also argue against the saturation hypothesis, since we see that 1) gabazine lowers selectivity but does not abolish it (Fig. 7C), and 2) the shape of the rank-ordered, firing-rate curve (e.g., Fig. 7B) is largely preserved. If firing rates were saturating, we would predict a much larger drop in selectivity and a curve showing an obvious plateau at the maximum response.
The decrease in motif selectivity observed when local inhibition is blocked in NCM is particularly robust. Previous studies have reported that inhibition increases selectivity for complex objects (Wang et al. 2000) and sounds (Muller and Scheich 1987) but relied on bicuculline, which has known non-GABAergic effects, to block GABA receptors (Kurt et al. 2006). Our use of the specific GABAA receptor antagonist gabazine provides the first specific evidence that GABAA receptors contribute to the formation of selective forebrain responses to natural signals. Roughly 50% of the neurons in NCM are GABAergic, and most of the spontaneous currents observed in NCM whole-cell slice recordings are inhibitory (Pinaud et al. 2008). Thus all of the neurons recorded and manipulated in the present study are likely receiving strong inhibition.
Because the effectiveness of inhibition is itself selective (Fig. 8C), the actual inhibitory synaptic input is most likely selective as well. Blocking inhibition did not abolish selective NCM responses completely, however, so components of the excitatory input must also be selective, consistent with reports from the visual cortex when inhibition is blocked intracellularly (Nelson et al. 1994). This selective excitatory input may come from other brain regions that contain selective neural responses, such as caudomedial mesopallium (CMM) (Gentner and Margoliash 2003; Meliza et al. 2010). Intracellular studies in visual and auditory cortex show that inhibition and excitation have similar tuning in many neurons (Anderson et al. 2000; Marino et al. 2005; Wehr and Zador 2003); even in sites where inhibition and excitation are not similarly tuned, the inhibitory input can still be selective in its function (Monier et al. 2003). Although further experiments that make direct measurements of excitatory and inhibitory currents in single neurons are needed, the results suggest that the selective co-tuning of these two inputs can extend to complex stimulus properties, such as familiarity. More generally, the results demonstrate a system in which learning modifies both inhibitory and excitatory circuitry and where inhibition plays a key role in shaping behaviorally relevant neural representations.
Consistent with inhibition increasing selectivity in NCM, our receptive-field analysis shows that inhibition also increases the complexity of NCM responses. With inhibition blocked, the fraction of spike information explained by the MID models was greater than with inhibition intact. However, blocking inhibition did not change the spectrotemporal receptive-field properties of NCM neurons, similar to findings from a recent study of NCM (Pinaud et al. 2008) but distinct from effects in multiple other brain areas (Andoni et al. 2007; Chen and Jen 2000; Dykes et al. 1984; Jacobs and Donoghue 1991; Muller and Scheich 1987; Ramoa et al. 1988; Sillito 1975; Thiele et al. 2004; Wang et al. 2002). Our results show that without inhibition, the same average spectrotemporal feature becomes a better predictor of actual spike activity. This effect is mediated, in part, by the GABAergic masking of temporally specific responses (Fig. 8). GABAA-mediated inhibition plays a fundamental role in the circuitry that yields neurons with highly complex response properties, and learning modifies this role.
Given that learning selectively biases excitation throughout NCM, why would inhibition specifically mask one form of this plasticity at dorsal sites? One possibility is that this relates to the differential encoding of learned categories in NCM. Increasing the average excitatory drive for learned songs provides a greater suprathreshold range over which inhibition can function to encode behaviorally meaningful representations. Although inhibition counteracts the average increase in excitatory drive in dorsal NCM, it does so in a manner that sharpens the discrimination of songs associated with go responses from songs associated with no-go responses. Consistently, in ventral NCM, where learning reduces firing rates, and inhibition does not affect responses to learned songs differently from unfamiliar songs, the discriminability (i.e., d′) between go and no-go songs is weaker than in dorsal NCM.
The observed differences in response properties between dorsal and ventral NCM replicate previous results of encoding differences along the dorso-ventral axis of this region (Thompson and Gentner 2010). Both the past and the present study are consistent with the idea that there are fundamental shifts in the way that learned features of songs are processed along this axis. It is important that although both studies define an operational boundary between dorsal and ventral NCM, the reported processing differences likely reflect a more continuous change in processing across this axis rather than a categorical shift in function at a specific anatomical boundary (Fig. 4). The understanding of how this functional dimension corresponds to changes in the local circuitry of NCM requires further study. There is, however, some suggestion of a dorso-ventral topography to NCM in the pattern of projections from field L subregions (Vates et al. 1996). Primary NCM afferents arise from the nucleus ovoidalis shell and fields L2 and L3, and these are most dense in the rostro-ventral portion of NCM. CMM is also densely interconnected to NCM, but these projections ramify widely throughout NCM, which itself is densely interconnected. One possible (albeit overly simplistic) pathway may involve the integration of ascending information in the more rostro-ventral regions prior to processing within NCM proper, followed by additional convergence into CMM.
A second function of inhibition in dorsal NCM may be renormalization. Multiple studies of learning-dependent plasticity suggest that the number of cortical neurons that respond best to training stimuli increases substantially during the initial stages of learning but then normalizes back to the initial state, even though behavioral performance continues to remain high (Reed et al. 2011; Yotsumoto et al. 2008). This suggests that a cortical over-representation of all training stimuli is only present during the initial stages of learning and that other, perhaps more subtle, representations persist to support the animal's continued performance. Because of the masking of the firing-rate preference for learned songs that we observed in dorsal NCM, we speculate that inhibition may be a critical mechanism for renormalizing cortical responses after the initial stages of learning. Consistently, all of the starlings used in this study performed the behavioral task with high accuracy for many trials following initial learning, and recordings were performed at a time when cortical map plasticity would be expected to have renormalized (Reed et al. 2011). Confirmation of this idea, however, will require a carefully controlled comparison of the excitatory and inhibitory effects on experience-dependent plasticity at various time points during the learning process.
One limitation in the interpretation of our data is that most of our recording sites were MU clusters rather than single neurons. In principle, it is possible that individual neurons may drift in and out of the recorded voltage signal. Our analysis of firing rates during the recovery period (Fig. 1, E and F), however, shows nearly complete recovery in both dorsal and ventral sites, suggesting that the neuron clusters are stable over time. Another concern relates to the heterogeneous composition of the recorded clusters; roughly one-half of the neurons in NCM are GABAergic (Pinaud et al. 2008). Thus we cannot distinguish the local inhibitory effects on excitatory neurons from the effects on inhibitory neurons. This distinction will be important to further understand the function of the local inhibitory circuitry in processing learned signals.
The present study is also limited in that we cannot know exactly the spatial extent of gabazine diffusion. Published reports using gabazine with similar ejection currents and slightly longer durations, but in somatosensory cortex, suggest that a radius of detectable response changes of ∼350 μm around the recording site is a reasonable estimate (Foeller et al. 2005). Because the iontophoresis pipette was in close proximity to the recording electrode, gabazine likely influenced GABAA receptors most strongly near the soma, with a much weaker (or nonexistent) effect on the more distal dendrites. In addition, our iontophoresis protocol also likely enhanced the excitation of neighboring neurons by blocking some of their inhibitory input. If these neighbors are presynaptic to the recorded neurons, this may partially contribute to the observed increase in responsiveness. The effects of blocking local inhibition reported here are therefore likely to be the joint result of cellular and local network effects. Because the microcircuitry of NCM is not known, however, there could be physiological effects well beyond the spatial spread of gabazine. Such distant network effects could theoretically also contribute to the effects that we observe here. Because of the proximity between the iontophoresis pipette and the recording electrode, these effects are likely small. Determining the role of distant network effects in future work will help to delineate the nature of NCM microcircuitry. Importantly, because we randomized our presentation of learned and unfamiliar songs, the learning-related differences that we observe cannot be attributed to variability in the effectiveness of the GABAA receptor blockade over the duration of each experiment. Whereas the precise effects of learning on local inhibitory microcircuitry and the direct synaptic contribution of inhibition in single neurons will require further study, our results clearly show the importance of inhibition for the encoding of learned information in NCM.
By combining controlled behavioral experience with pharmacological manipulation of local inhibitory circuitry, we demonstrate that local inhibition plays an important role in NCM to enhance the encoding of behaviorally relevant songs. This occurs concurrently with some well-established effects of inhibition, including reducing response strengths and increasing selectivity but independently of another reported effect—the sharpening of spectrotemporal-receptive fields. Collectively, our results suggest that inhibition does more than enhance neural tuning functions: it can modify neural coding to better represent behaviorally important stimuli.
GRANTS
Support for this work was provided by a grant from the National Institute on Deafness and Other Communication Disorders (DC008358) to T. Q. Gentner, a predoctoral training fellowship from the Institute for Neural Computation at University of California, San Diego (MH020002) to J. M. Jeanne, and a National Science Foundation graduate research fellowship to J. M. Jeanne.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.V.T. and T.Q.G. conception and design of research; J.V.T. performed experiments; J.V.T., J.M.J., and T.Q.G. analyzed data; J.V.T., J.M.J., and T.Q.G. interpreted results of experiments; J.V.T., J.M.J., and T.Q.G. prepared figures; J.V.T. and J.M.J. drafted manuscript; J.V.T., J.M.J., and T.Q.G. edited and revised manuscript; J.V.T., J.M.J., and T.Q.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank T. Sharpee for assistance and advice with the MID calculations and R. Krauzlis for comments on an earlier version of the manuscript.
REFERENCES
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol 84: 909–926, 2000 [DOI] [PubMed] [Google Scholar]
- Andoni S, Li N, Pollak GD. Spectrotemporal receptive fields in the inferior colliculus revealing selectivity for spectral motion in conspecific vocalizations. J Neurosci 27: 4882–4893, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186, 1998 [DOI] [PubMed] [Google Scholar]
- Chen QC, Jen PH. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear Res 150: 161–174, 2000 [DOI] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci USA 93: 1950–1955, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature 465: 932–936, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes RW, Landry P, Metherate R, Hicks TP. Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J Neurophysiol 52: 1066–1093, 1984 [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci 32: 33–55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeller E, Celikel T, Feldman DE. Inhibitory sharpening of receptive fields contributes to whisker map plasticity in rat somatosensory cortex. J Neurophysiol 94: 4387–4400, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Leon EE, Lin FG, Liu RC. Inhibitory plasticity in a lateral band improves cortical detection of natural vocalizations. Neuron 62: 705–716, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature 424: 669–674, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I, Cousillas H, Richard JP, Hausberger M. A potential neural substrate for processing functional classes of complex acoustic signals. PLoS One 3: e2203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierdalski M, Jablonska B, Siucinska E, Lech M, Skibinska A, Kossut M. Rapid regulation of GAD67 mRNA and protein level in cortical neurons after sensory learning. Cereb Cortex 11: 806–815, 2001 [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888, 2005 [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504–1508, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham NJ, McAlpine D. GABAergic inhibition controls neural gain in inferior colliculus neurons sensitive to interaural time differences. J Neurosci 25: 6187–6198, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251: 944–947, 1991 [DOI] [PubMed] [Google Scholar]
- Jasinska M, Siucinska E, Cybulska-Klosowicz A, Pyza E, Furness DN, Kossut M, Glazewski S. Rapid, learning-induced inhibitory synaptogenesis in murine barrel field. J Neurosci 30: 1176–1184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne JM, Thompson JV, Sharpee TO, Gentner TQ. Emergence of learned categorical representations within an auditory forebrain circuit. J Neurosci 31: 2595–2606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Busse L, Carandini M. GABAA inhibition controls response gain in visual cortex. J Neurosci 31: 5931–5941, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABA(A)-antagonists bicuculline and gabazine in sensory cortex. Hear Res 212: 224–235, 2006 [DOI] [PubMed] [Google Scholar]
- Lalley P. Microiontophoresis and pressure injection. In: Modern Techniques in Neuroscience Research , edited by Windhorst U, Johansson H. Berlin: Springer-Verlag, 1999, p. 193–212 [Google Scholar]
- Lane RD, Killackey HP, Rhoades RW. Blockade of GABAergic inhibition reveals reordered cortical somatotopic maps in rats that sustained neonatal forelimb removal. J Neurophysiol 77: 2723–2735, 1997 [DOI] [PubMed] [Google Scholar]
- Lech M, Skibinska A, Kossut M. Delayed upregulation of GABA(A) alpha1 receptor subunit mRNA in somatosensory cortex of mice following learning-dependent plasticity of cortical representations. Brain Res Mol Brain Res 96: 82–86, 2001 [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature 443: 81–84, 2006 [DOI] [PubMed] [Google Scholar]
- Marino J, Schummers J, Lyon DC, Schwabe L, Beck O, Wiesing P, Obermayer K, Sur M. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat Neurosci 8: 194–201, 2005 [DOI] [PubMed] [Google Scholar]
- Meliza CD, Chi Z, Margoliash D. Representations of conspecific song by starling secondary forebrain auditory neurons: toward a hierarchical framework. J Neurophysiol 103: 1195–1208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Fregnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron 37: 663–680, 2003 [DOI] [PubMed] [Google Scholar]
- Mower GD, Christen WG, Burchfiel JL, Duffy FH. Microiontophoretic bicuculline restores binocular responses to visual cortical neurons in strabismic cats. Brain Res 309: 168–172, 1984 [DOI] [PubMed] [Google Scholar]
- Muller CM, Leppelsack HJ. Feature extraction and tonotopic organization in the avian auditory forebrain. Exp Brain Res 59: 587–599, 1985 [DOI] [PubMed] [Google Scholar]
- Muller CM, Scheich H. GABAergic inhibition increases the neuronal selectivity to natural sounds in the avian auditory forebrain. Brain Res 414: 376–380, 1987 [DOI] [PubMed] [Google Scholar]
- Nelson S, Toth L, Sheth B, Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science 265: 774–777, 1994 [DOI] [PubMed] [Google Scholar]
- Pinaud R, Terleph TA, Tremere LA, Phan ML, Dagostin AA, Leao RM, Mello CV, Vicario DS. Inhibitory network interactions shape the auditory processing of natural communication signals in the songbird auditory forebrain. J Neurophysiol 100: 441–455, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Velho TA, Jeong JK, Tremere LA, Leão RM, von Gersdorff H, Mello CV. GABAergic neurons participate in the brain's response to birdsong auditory stimulation. Eur J Neurosci 20: 1318–1330, 2004 [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Paradiso MA, Freeman RD. Blockade of intracortical inhibition in kitten striate cortex: effects on receptive field properties and associated loss of ocular dominance plasticity. Exp Brain Res 73: 285–296, 1988 [DOI] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron 70: 121–131, 2011 [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Cecchi GA, Magnasco MO, Mello CV. Toward a song code: evidence for a syllabic representation in the canary brain. Neuron 21: 359–371, 1998 [DOI] [PubMed] [Google Scholar]
- Sharpee T, Rust NC, Bialek W. Analyzing neural responses to natural signals: maximally informative dimensions. Neural Comput 16: 223–250, 2004 [DOI] [PubMed] [Google Scholar]
- Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol 250: 305–329, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. J Neurosci 17: 3883–3893, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Distler C, Korbmacher H, Hoffmann KP. Contribution of inhibitory mechanisms to direction selectivity and response normalization in macaque middle temporal area. Proc Natl Acad Sci USA 101: 9810–9815, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JV, Gentner TQ. Song recognition learning and stimulus-specific weakening of neural responses in the avian auditory forebrain. J Neurophysiol 103: 1785–1797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Urban-Ciecko J, Kossut M, Hess G. Sensory learning-induced enhancement of inhibitory synaptic transmission in the barrel cortex of the mouse. Eur J Neurosci 26: 134–141, 2007 [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol 366: 613–642, 1996 [DOI] [PubMed] [Google Scholar]
- Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science 287: 1273–1276, 2000 [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res 944: 219–231, 2002 [DOI] [PubMed] [Google Scholar]
- Wang Y, Fujita I, Murayama Y. Neuronal mechanisms of selectivity for object features revealed by blocking inhibition in inferotemporal cortex. Nat Neurosci 3: 807–813, 2000 [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci 18: 129–158, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Fiser J, Hunt RH, Wagner DN. Coding of natural scenes in primary visual cortex. Neuron 37: 703–718, 2003 [DOI] [PubMed] [Google Scholar]
- Woolley SM, Hauber ME, Theunissen FE. Developmental experience alters information coding in auditory midbrain and forebrain neurons. Dev Neurobiol 70: 235–252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 57: 827–833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science 284: 962–965, 1999 [DOI] [PubMed] [Google Scholar]