Abstract
Dysfunction in sensorimotor synapses is one of the earliest pathological changes observed in a mouse model [spinal muscular atrophy (SMA)Δ7] of spinal muscular atrophy. Here, we examined the density of proprioceptive and cholinergic synapses on calbindin-immunoreactive interneurons ventral to the lateral motor column. This population includes inhibitory Renshaw interneurons that are known to receive synaptic input from muscle spindle afferents and from motoneurons. At postnatal day (P)13, near the end stage of the disease, the somatic area of calbindin+ neurons in the L1/L2 and L5/L6 segments was reduced in SMAΔ7 mice compared with controls. In addition, the number and density of terminals expressing the glutamate vesicular transporter (VGLUT1) and the vesicular acetylcholine transporter (VAChT) were increased on calbindin+ cells in the L1-L2 but not in the L5-L6 segments of SMAΔ7 mice. In addition, the isolated spinal cord of SMA mice was able to generate locomotor-like activity at P4-P6 in the presence of a drug cocktail or in response to dorsal root stimulation. These results argue against a generalized loss of proprioceptive input to spinal circuits in SMA and suggest that the loss of proprioceptive synapses on motoneurons may be secondary to motoneuron pathology. The increased number of VGLUT1+ and VAChT+ synapses on calbindin+ neurons in the L1/L2 segments may be the result of homeostatic mechanisms. Finally, we have shown that abnormal locomotor network function is unlikely to account for the motor deficits observed in SMA mice at P4–6.
Keywords: SMA, interneurons, spindle afferents, locomotion, Renshaw cells
spinal muscular atrophy (SMA) is the most common congenitally lethal disease of infants and is caused by mutation of the smn1 gene (Pearn 1978; Lefebvre et al. 1995). Several mouse models have been developed to investigate the effects of the disease on neuromuscular and synaptic function (Ruiz et al. 2010; Kong et al. 2009; Kariya et al. 2008; Ling et al. 2010; Mentis et al. 2011). One commonly used model, the SMAΔ7 mouse, lacks the smn gene but expresses two copies of the human smn2 gene (Le et al. 2005). These mice live up to 14 days and exhibit severe paralysis, slow righting reflexes, and weight loss. Mentis et al. (2011) demonstrated that proprioceptive synapse density is decreased on motoneurons as early as postnatal day (P)4. The very early expression of proprioceptive dysfunction raises the possibility that the primary defect in the SMAΔ7 mouse is in sensory afferents and that the motoneuron pathology is secondary to the loss of synaptic function or inputs. If this hypothesis were true, we might expect a similar loss of synaptic function at the other, nonmotoneuronal targets of muscle spindle afferents. Recently, it has been demonstrated that inhibitory Renshaw cells receive direct monosynaptic input from muscle spindle afferents in mice (Mentis et al. 2010). Renshaw cells can be identified morphologically by their location ventromedial to the motor column, their expression of the calcium binding protein calbindin, and the presence of cholinergic terminals on their somata and dendrites. To investigate if there is a general dysfunction of muscle spindle afferents, we asked if vesicular glutamate transporter 1 (VGLUT1) terminals on calbindin immunoreactive neurons ventral to the motor nucleus are reduced in SMAΔ7 mice.
In SMAΔ7 mice, hindlimb motoneuron loss is quite limited at 14 days and hindlimb neuromuscular junctions remain innervated although their quantal content is reduced (Kong et al. 2009). These changes probably cannot account for the severe weakness exhibited by these animals raising the possibility that abnormal proprioceptive function or more widespread synaptic dysfunction of spinal circuits might be responsible for the motor deficits. Consistent with this idea, it is known that L3–L5 lateral motoneurons experience a loss (∼16%) of VGLUT2 excitatory boutons at the end stage of the disease in SMAΔ7 mice (Ling et al. 2010) raising the possibility that interneuronal circuits and other afferents might also exhibit synaptic defects. For this reason, we also examined the ability of the isolated spinal cord in SMAΔ7 mice to generate locomotor-like activity initiated by drugs or by electrical stimulation of the dorsal roots.
MATERIALS AND METHODS
All experiments were performed in accordance with the National Institutes of Health Guidelines on the Care and Use of Animals and approved by the National Institute of Neurological Disorders and Stroke (NINDS) Animal Care and Use Committee. Wild-type mice (Swiss Webster) were obtained from Charles River Laboratories (Wilmington, MA) or Taconic Farms (Hudson, NY). SMAΔ7 mice were generated by breeding smn+/−; smn2+/+; smnΔ7+/+ parents (originally gift from A. Burghes, Ohio State University, Columbus, OH). SMAΔ7 pups were identified by the lack of the mouse smn gene and presence of the β-Gal insert using the following primers: mouse-specific smn forward: 5′-GTGTCTGGGCTGTAGGCATTGC-3′; mouse-specific smn reverse: 5′-GGCTGTGCCTTTTGGCTTATCTG-3′; β-Gal forward: 5′- GCCTGCGATGTCGGTTTCCGCGAGG-3′; and β-Gal reverse: 5′-CCAGCGCGGATCGGTCAGACG-3′.
Electrophysiology.
P4 to P6 mice were anesthetized by halothane exposure and then quickly decapitated. The torso was then pinned onto a large Sylgard dish ventral side down, and the entire vertebral column was quickly dissected free of the animal. The column was placed in a clear Sylgard dish over which chilled oxygenated artificial cerebrospinal fluid (aCSF) was circulated. The aCSF contained the following (in mM): 128.35 NaCl, 4 KCl, 0.58 NaH2PO4·H20, 21 NaHCO3, 30 d-glucose, 1.5 CaCl2·H20, and 1 MgSO4·7H20. Under a dissecting microscope, the ventral side of the vertebral column was slowly cut away exposing the underlying spinal cord. The entire spinal cord spanning thoracic to sacral segments together with the dorsal and ventral roots was then carefully dissected free and pinned in a recording chamber, superfused with oxygenated aCSF at room temperature. Custom-made suction electrodes were applied to various dorsal and ventral roots. To evoke locomotor-like activity, the dorsal roots were stimulated using a stimulus isolator (A.M.P.I., Jerusalem, Israel) for 10–12.5 s at 4 Hz (Whelan et al. 2000). Signals from the ventral roots were amplified ×10,000 using a differential amplifier (A-M Systems, Carlsborg, WA) and then digitized at 5 KHz using a Digidata 1440 data acquisition board (Molecular Devices, Sunnyvale, CA). Data were stored on a computer and analyzed offline using custom scripts written in MATLAB (The Mathworks, Natick, MA). Seven SMAΔ7 mice were used for the electrophysiology experiments.
Immunohistochemistry.
P12/P13 pups were anesthetized with halothane and then decapitated. The spinal cord was dissected out from the thoracic to sacral regions as described above. The cord was then fixed in 4% formaldehyde at 4°C overnight. After several washes in PBS, the L1-L2 region and the L5-L6 region were cut out and embedded in 5% agar and sectioned into 70-μm transverse sections on a Leica Vibratome (VT 1000S). The slices were placed in Netwell mesh inserts (Corning, Lowell, MA) placed inside a multiwell tissue culture dish whose bottoms were coated with blackened Sylgard (Dow Corning, Midland, MI). The slices were incubated in PBST (0.01% Triton-X) for 10 min. Following this, slices were blocked in 10% normal donkey serum for 90 min and then placed in appropriate dilutions of the primary antibodies in the presence of 10% normal donkey serum, overnight with regular shaking. The slices were then washed several times in PBST and incubated in 10% normal donkey serum and secondary antibodies of appropriate dilutions for 3 h. Slices were washed several times in PBS and then mounted on glass slides without air bubbles with Prolong Gold (Invitrogen) mounting medium and coverslipped. All incubations were done at room temperature. The antibodies and dilutions used were as follows: rabbit anti-calbindin D28k (Swant, Bellinzona, Switzerland), 1:2,000; guinea pig anti-VGLUT1 (Synaptic Systems), 1:2,000; guinea pig anti-vesicular acetylcholine transporter (anti-VAChT; Millipore), 1:250; Cy5 donkey anti-rabbit IgG (Jackson Immunoresearch), 1:200; Cy3 donkey anti-guinea pig (Jackson Immunoresearch), 1:200; Alexa Fluor 488 donkey anti-rabbit (Invitrogen), 1:200; Dylight 488 donkey anti-Guineapig (Jackson Immunoresearch), 1:200.
Confocal imaging and analysis.
Samples were imaged on a Zeiss 510 Meta Confocal Microscope using a ×20 air objective and a ×60 oil-immersion objective. Laser lines at 488, 543, and 633 nm were used for exciting the fluorophores. Images were acquired and stored on a computer and analyzed offline using Fiji and the Zeiss LSM Image Browser. We imaged with an optical resolution of 0.2 μm in the XY plane and 0.54 μm in the Z plane using a high numerical aperture ×60 objective. Numbers indicated in the text are means ± SE. Statistical significance was tested using Kruskal-Wallis (KW) test, followed by Tukey's honestly significant difference multiple comparisons. Slices were made from four SMA pups and three wild-type pups. All data have been plotted in box and whisker plots using the median and the interquartile range. Data points outside 1.5 times the interquartile range are treated as outliers. Cycle periods for dorsal root evoked locomotor-like activity were compared with the Wilcoxon rank test, and the phase values were compared using a circular statistics toolbox written in Matlab (Berens 2009). In the text, data are expressed as means ± SE.
RESULTS
Somatic area of calbindin+ neurons.
We first examined the somatic area of calbindin+ neurons in the putative Renshaw region of the lumbar spinal cord (Fig. 1) ∼200 μm from the ventral white matter boundary in laminae VII and IX (Sapir et al. 2004). In this region, a substantial fraction of the calbindin+ neurons are Renshaw cells (Sapir et al. 2004).
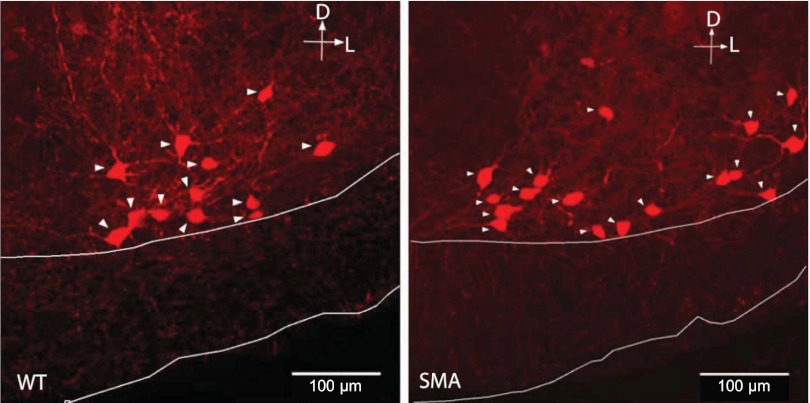
Fig. 1.
Immunochemical identification of calbindin+ cells in the “Renshaw area” of the ventral spinal cord of wild-type (WT; left) and [spinal muscular atrophy (SMA)Δ7; right] mice. Arrowheads point to neuronal somata. White lines delineate the boundaries of the ventral white matter.
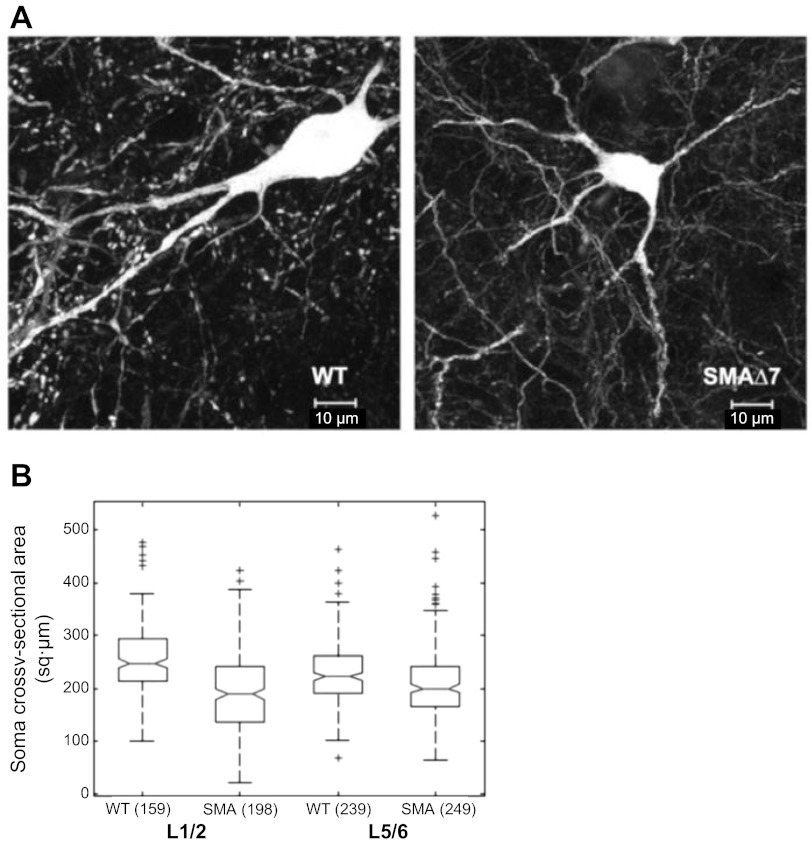
As illustrated in Fig. 2, the somatic area of calbindin+ cells was reduced in SMAΔ7 mice compared with wild-type in both the L1–L2 and L5–L6 regions (wild-type L1–L2: 258.1 ± 5.3; SMAΔ7 L1–L2: 194.7 ± 5.3; wild-type L5–L6: 227.2 ± 3.7; SMA L5–L6: 209 ± 4.1 sq μm; P < 0.001, KW test). The reduction in somatic area was greater in the L1–L2 segments compared with the L5–L6 segments (75 vs. 92% of the wild-type somatic area). We also observed that the somatic area of calbindin+ neurons in the L1–L2 region was significantly larger than those in the L5–L6 region of wild-type mice (P < 0.001, L5–L6 somatic area was 88% of that in ventral calbindin+ cells L1–L2).
Fig. 2.
Somatic area of calbindin+ neurons is reduced in SMAΔ7 mice. A: calbindin+ somata visualized by their calbindin immunoreactivity in wild-type (left) and SMAΔ7 (right) mice at the L1/L2 segmental level. B: median and interquartile range (IQR) of somatic area in wild-type and SMAΔ7 mice in L1–L2 and L5–L6 regions.; + symbols denote outliers. Numbers in parentheses indicate the number of somata that were counted.
Changes in synaptic inputs to calbindin+ cells.
Calbindin+ Renshaw cells receive glutamatergic inputs from muscle spindle afferent terminals that express the VGLUT1 (Mentis et al. 2006) and motoneuronal cholinergic terminals that express VAChT (Alvarez and Fyffe 2007). To establish if the synaptic input from VGLUT1+ afferents is reduced on calbindin+ cells in the Renshaw area of SMAΔ7 mice, we counted the number of VGLUT1+ terminals on the somata and dendrites of these neurons (Fig. 3). We observed VGLUT+ terminals apposed to somata and dendrites of calbindin+ cells in the Renshaw area in single optical sections (Fig. 3A). The number of somatic VGLUT1+ puncta in SMAΔ7 mice was not significantly different from that seen in wild-type mice in both the L1–L2 and L5–L6 regions. However, because of the decreased area of the L1/2 somata, the somatic VGLUT1 puncta density was increased in SMAΔ7 mice compared with wild-type in the L1–L2 region (Fig. 3B; P < 0.001, KW test).
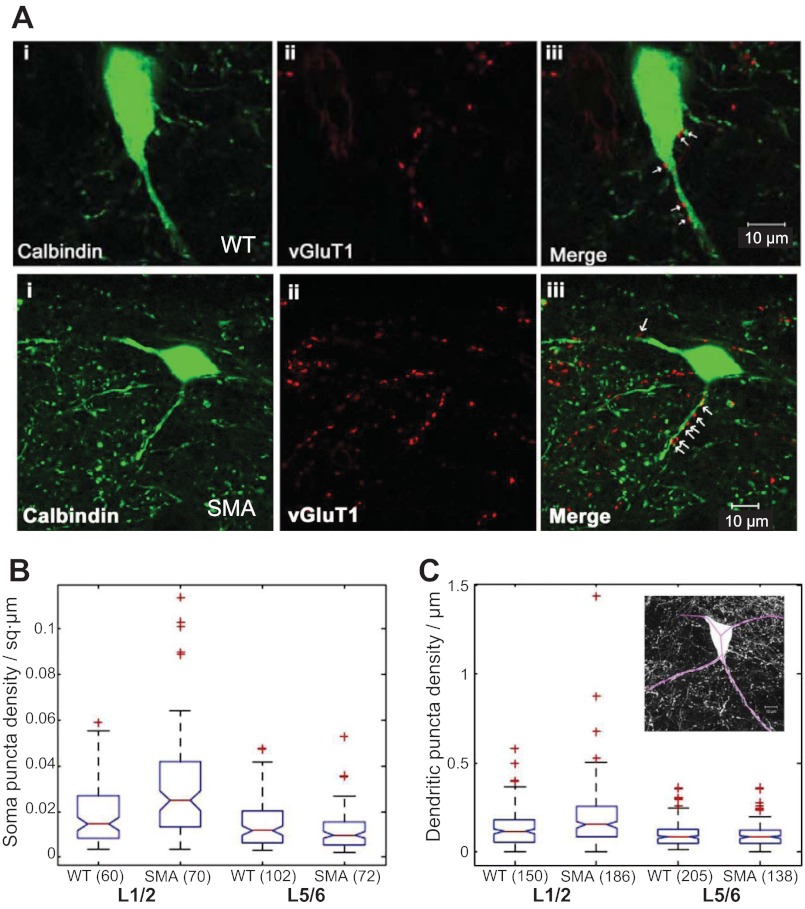
Fig. 3.
Number of glutamate vesicular transporter (VGLUT1+) terminals is not reduced on calbindin+ cells in SMAΔ7 mice at P12–13. A, top: calbindin+ cell (i), VGLUT1+ terminals (ii), and a merged image (iii) at the L1/L2 segmental level of a wild-type mouse at P12–13. A, bottom: the same immunocytochemistry for a calbindin+ cell from an SMAΔ7 mouse at postnatal day (P)12–13. Arrows in iii show VGLUT1 puncta apposed to the calbindin+ somata and dendrites. Images are generated from a single optical section (0.54 μm). B: Box plots showing medians and IQRs of somatic VGLUT1 puncta in wild-type and SMAΔ7 mice in the L1–L2 and L5–L6 regions. C: box plots showing medians and IQRs of dendritic VGLUT1 puncta in wild-type and SMAΔ7 mice in the L1–L2 and L5–L6 regions. Inset: maximal projection of the wild-type neuron shown in A with the traced dendrites (purple lines) superimposed. Only the calbindin channel is shown for clarity. Numbers in parentheses indicate the number of somata (B) or dendrites (C) that were used to generate the graph. In both B and C, + symbols denote outliers.
To quantify dendritic VGLUT1 puncta, we traced Renshaw neuron dendrites using their cytoplasmic calbindin immunoreactivity to the extent possible within a section (Fig. 3C, inset). The dendritic lengths traced were similar in wild-type and SMAΔ7 mice (wild-type dendrite length: 72.8 ± 1.9 μm; SMAΔ7 dendrite length: 71.7 ± 2.1 μm; P = 0.57, Mann-Whitney test). However, in contrast to the somatic measurements, we found that both the number and density of VGLUT1+ terminals were increased on the proximal dendrites of calbindin+ cells in SMAΔ7 mice in the L1–L2 region but not in the L5–L6 region (Fig. 3D; P < 0.001, KW test). The mean VGLUT1 puncta numbers and densities in the soma and dendrites of wild-type and SMAΔ7 mice are shown in Table 1.
Table 1.
Number and density of VGLUT1+ terminals on the soma and dendrites of SMAΔ7 and wild-type mice at P13
| Measurement | WT L1–L2 | SMAΔ7 L1–L2 | WT L5–L6 | SMAΔ7 L5–L6 |
|---|---|---|---|---|
| Somatic VGLUT1 puncta number | 4.7 ± 0.4 | 6 ± 0.5 | 3.2 ± 0.2 | 2.9 ± 0.3 |
| Somatic VGLUT1 puncta density | 0.019 ± 0.002 | 0.031 ± 0.003* | 0.014 ± 0.001 | 0.012 ± 0.001 |
| Dendritic VGLUT1 puncta number | 9.2 ± 0.6 | 11.6 ± 0.7* | 7.4 ± 0.5 | 7.9 ± 0.6 |
| Dendritic VGLUT1 puncta density | 0.14 ± 0.008 | 0.19 ± 0.011* | 0.1 ± 0.004 | 0.1 ± 0.006 |
Somatic densities are expressed as number of puncta/square micrometer of somatic area and dendritic densities are number of puncta/linear micrometer of dendrite. VGLUT1, glutamate vesicular transporter; P13, postnatal day 13.
P < 0.001 Kruskal-Wallis test comparing spinal muscular atrophy (SMA)Δ7 to wild-type (WT) in the L1/2 segments.
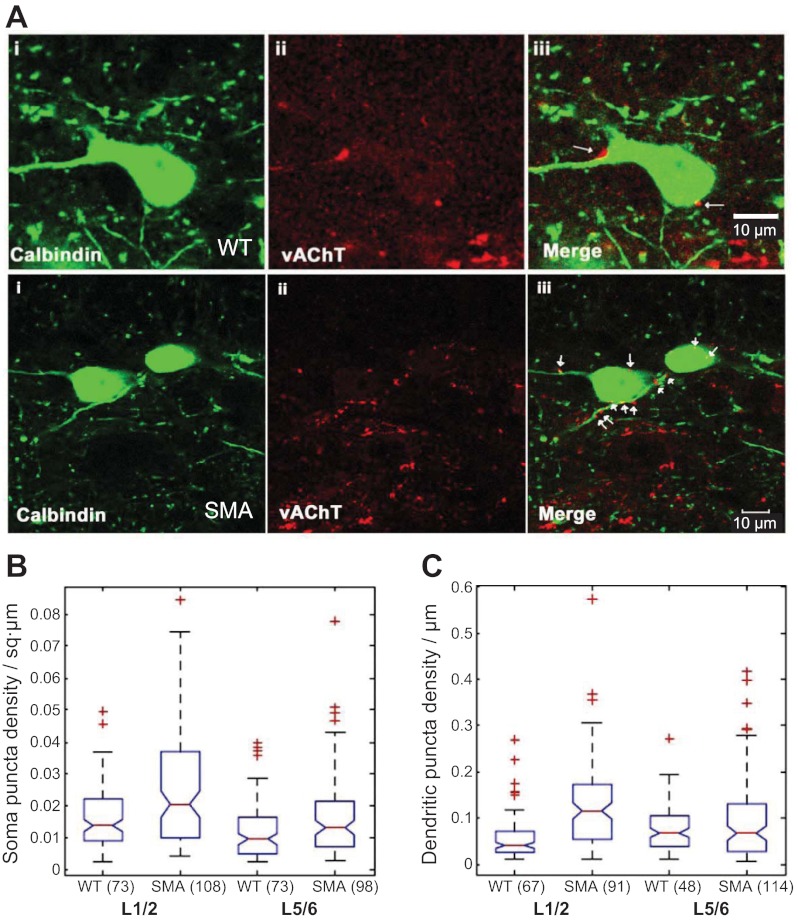
We then examined the density of cholinergic terminals on calbindin+ cells in the Renshaw area. For this purpose, we double-immunostained transverse sections of lumbar spinal cord for calbindin D28k and for VAChT (Fig. 4A) and measured somatic and dendritic puncta density (Fig. 4, B and C). The number of somatic VAChT+ puncta was similar in SMAΔ7 and wild-type mice in both the L1/2 and the L5/6 segments. However, because of the reduced size of calbindin+ neurons in the L1/2 segments, the somatic density of VAChT puncta was increased in this region. As with VGLUT1 terminals, both the number and density of dendritic VAChT+ were significantly higher in SMAΔ7 mice compared with wild-type in the L1–L2 region (P < 0.001, KW test), while the L5–L6 region was similar to the wild-type. The mean VAChT puncta numbers and densities in the soma and dendrites of wild-type and SMAΔ7 mice are shown in Table 2.
Fig. 4.
Vesicular acetylcholine transporter (VAChT+) terminals are not reduced on calbindin+ putative Renshaw cells in SMAΔ7 mice at P12–13. A, top: calbindin+ cell (i), VAChT+ terminals (ii), and a merged image (iii) at the L1/L2 segmental level of a wild-type mouse at P12–13. A, bottom: the same immunocytochemistry for a calbindin+ cell from an SMAΔ7 mouse at P12–13. Cholinergic synapses (arrows) are identified by the apposition of VAChT immunoreactive boutons to calbindin+ dendrites and somata in the merged images (iii). Images are generated from a single optical section (0.54 μm). B: box plots showing medians and IQRs of somatic VAChT puncta in wild-type and SMAΔ7 mice in the L1–L2 and L5–L6 regions. C: box plots showing medians and IQRs of dendritic VAChT puncta in wild-type and SMAΔ7 mice in the L1–L2 and L5–L6 regions. In both B and C, + symbols denote outliers. Numbers in parentheses indicate the number of somata (B) or dendrites (C) that were used to generate the graph.
Table 2.
Number and density of VAChT+ terminals on the soma and dendrites of SMAΔ7 and wild-type mice at P13
| Measurement | WT L1–L2 | SMAΔ7 L1–L2 | WT L5–L6 | SMAΔ7 L5–L6 |
|---|---|---|---|---|
| Somatic VAChT puncta number | 4.4 ± 0.4 | 4.7 ± 0.4 | 2.7 ± 0.27 | 3.3 ± 0.3 |
| Somatic VAChT puncta density | 0.017 ± 0.001 | 0.026 ± 0.002* | 0.013 ± 0.001 | 0.017 ± 0.001 |
| Dendritic VAChT puncta number | 4.2 ± 0.5 | 7.1 ± 0.8* | 4.7 ± 0.5 | 5.1 ± 0.4 |
| Dendritic VAChT puncta density | 0.059 ± 0.006 | 0.129 ± 0.01* | 0.076 ± 0.008 | 0.095 ± 0.008 |
Somatic densities are expressed as number of puncta/square micrometer of somatic area and dendritic densities are number of puncta/linear micrometer of dendrite. VAChT, vesicular acetylcholine transporter.
P < 0.001, Kruskal-Wallis test comparing SMAΔ7 toWT in the L1/2 segments.
Locomotor-like activity can be induced in SMAΔ7 mice spinal cords.
In the last set of experiments, we investigated the ability of isolated spinal cords from SMAΔ7 mice to generate locomotor-like activity. This is known to depend on networks of excitatory and inhibitory interneurons within the spinal cord (Kiehn 2011). Moreover, stimulation of the dorsal roots can activate locomotor-like activity in the spinal cord (Whelan et al. 2000) mediated by the concerted action of low and high threshold afferents (Blivis et al. 2007). Therefore, these experiments also allowed us to establish the extent to which afferents other than muscle spindles might be compromised in the SMAΔ7 mice.
We first examined the ability of the lumbar cord to generate a locomotor-like rhythm in the presence of a cocktail of drugs (10 μM serotonin, 5 μM N-methyl-d-aspartate, and 50 μM dopamine). This allowed us to test the functionality of the locomotor circuitry independently of afferent function. In wild-type mice, this cocktail induces rhythmic alternation between the right and left ventral roots and also between the ipsilateral L1/L2 and L5/L6 ventral roots at ∼0.2 Hz depending on the concentration of the drugs (Whelan et al. 2000). Because the L1/2 activity is dominated by flexor motoneurons and the L5/6 activity by extensor motoneurons, the phasing of discharge between these roots reflects flexor/extensor alternation (Whelan et al. 2000). In SMAΔ7 mice, the drugs induced a rhythmic locomotor pattern that was recorded from the L1/L2 and L5/L6 ventral roots. Figure 5A shows the locomotor pattern recorded from the isolated lumbar spinal cord of an SMAΔ7 mouse at P4. The power spectrum of the rhythmic activity shown in Fig. 5B revealed a peak frequency of 0.18 Hz across all four recorded ventral roots. We compared the peak frequencies from SMAΔ7 mice and wild-type mice and found these to be similar (wild-type peak frequency: 0.19 ± 0.01 Hz; SMAΔ7 peak frequency: 0.19 ± 0.02 Hz; P = 0.69; n = 5 animals in each group; Mann-Whitney test).
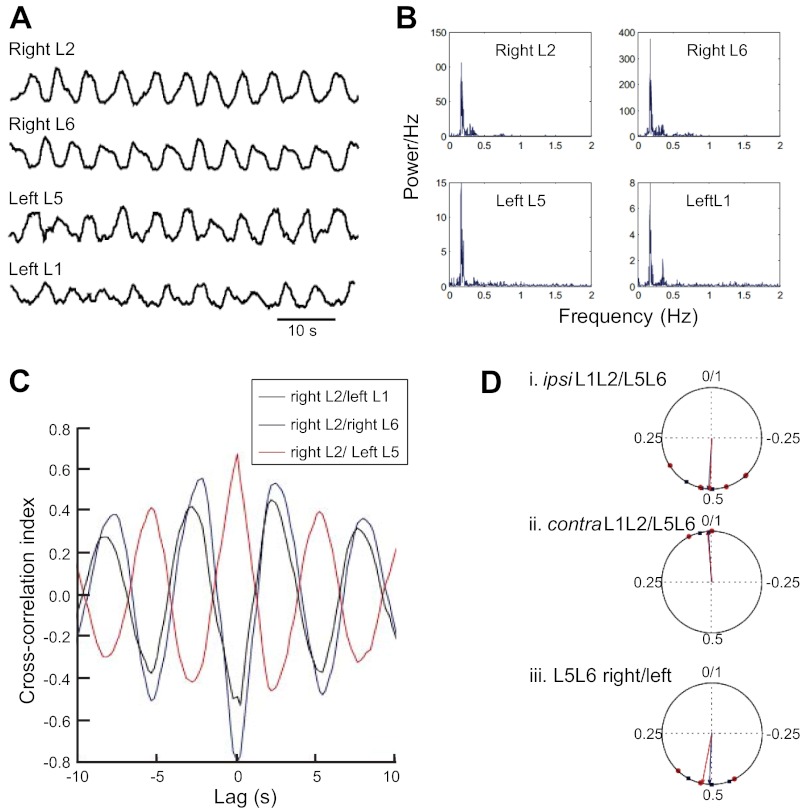
Fig. 5.
Locomotor output of the lumbar pattern generator appears normal in SMAΔ7 mice. A: locomotor pattern showing alternation between right and left sides of the cord and alternation between ipsilateral L1–L2 and L5–L6 regions evoked by the application of 10 μM 5-HT, 5 μM N-methyl-d-aspartate, and 50 μM DA. Ventral root activity was high-pass filtered at 50 Hz and then rectified and low pass filtered at 2 Hz. A moving average filter over 1,000 samples was then applied to smooth the data. B: power spectra computed using Welch's method for the data shown in B reveal a peak frequency at 0.18 Hz. C: black, cross-correlation index between right L2 and left L1 ventral roots; blue, between right L2 and right L6: red, between right L2 and left L5. D: phase values and mean direction plotted for wild-type (blue) and SMAΔ7 mice (red). Data from 4 mice in each group are shown. Some points lie on top of each other.
We next calculated the phase difference between the L1/L2 and L5/L6 ventral roots from the cross-correlogram. Figure 5C illustrates a cross-correlogram of the ventral root activity recorded in an SMAΔ7 mouse at P4. The cross-correlation index between the right and left roots is minimum at zero lag and is maximum with a lag of ∼2.5 s. The ipsilateral L2 and L6 roots also show a similar pattern indicating that these roots alternate with a period of roughly 5s or ∼0.2 Hz. The right L2 and left L5 roots are synchronized and are maximally correlated at zero-lag.
Using circular analysis (Fig. 5D), we determined that the ipsilateral L1/L2 and L5/L6 roots alternated with a mean phase difference of 0.49 ± 0.1 and the right and left L5/L6 roots alternated with a mean phase difference of 0.47 ± 0.07. The diagonal roots had a mean phase difference of 0.01 ± 0.03, and these phase values were similar to those observed in wild-type mice (wild-type L1/L2 vs. ipsilateral L5/L6: 0.49 ± 0.06; right/left L5/L6: 0.49 ± 0.05; diagonal roots: 0.01 ± 0.02; P = 0.97, KW test for circular data). These findings indicated that the locomotor circuitry was functional in SMAΔ7 mice and that the timing and phasing of the ventral root activity were statistically indistinguishable from wild-type mice.
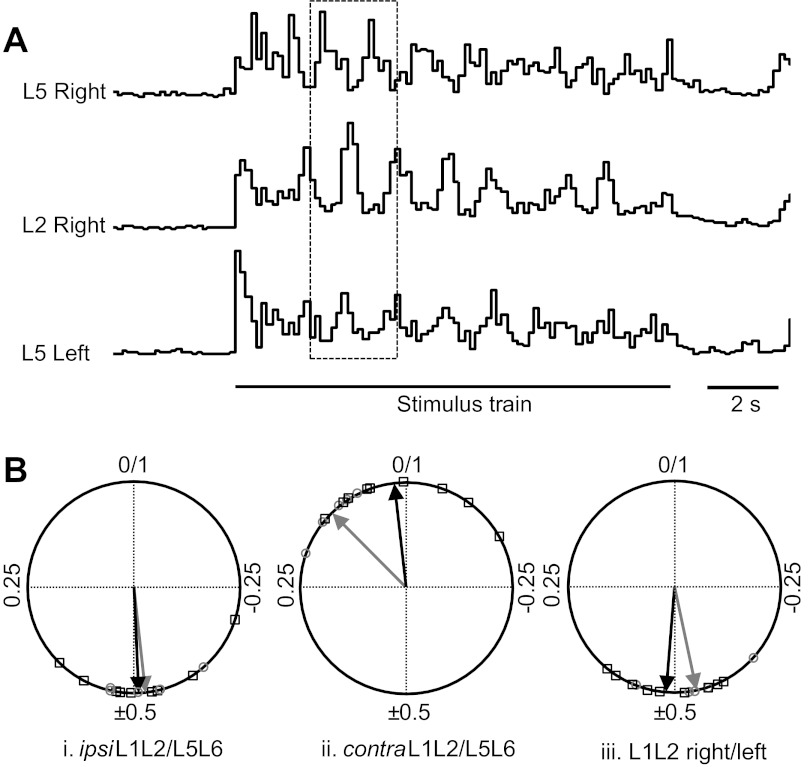
We then examined the ability of dorsal root stimulation to activate a locomotor-like rhythm. Stimulation of the L6 or S1 dorsal roots (4 Hz for 10s) in SMAΔ7 mice resulted in a locomotor pattern similar to that seen in wild-type animals (Fig. 6A; mean cycle period 1.22 ± 0.05 s; n = 5 SMA animals). In nine control animals, the cycle period was 1.23 ± 0.04 s, which was not significantly different from the value in the SMA animals (P = 0.3). The phase relationships between the ipsilateral L1/L2 and L5/L6 activity were conserved between wild-type and SMAΔ7 mice (Fig. 6Bi; mean phase 0.47 ± 0.02 SMA; 0.49 ± 0.01 wild-type; P = 0.17, Wilcoxon rank test). The contralateral L1/L2 and L5/L6 roots had a mean phase difference of 0.13 ± 0.01 and 0.06 ± 0.01 for the SMA and wild-type animals, respectively (Fig. 6Bii; P = 0.11). Finally, the right and left L1/L2 roots alternated with a mean phase difference of 0.48 ± 0.02 for the SMA and 0.48 ± 0.01 for the wild-type animals (Fig. 6Biii; P = 0.26). These results indicate the synaptic projections of afferents onto interneurons in the spinal cord remain sufficient to activate the locomotor central pattern generator (CPG) and that the output of the CPG onto motoneurons is functional at least up to P4-P6 when the experiments were performed.
Fig. 6.
Stimulating the left sacral dorsal root (S2) activates a rhythmic locomotor pattern in SMAΔ7 mice. A: integrated ventral root activity recorded from the right L2 and L5 ventral roots and from the left L5 ventral root. S2 dorsal root was stimulated with 50 pulses at 4 Hz and 15 mA (1.5× threshold for rhythmic activity indicated by the stimulus train). B: phase relationships between the L1–L2 and L5–L6 roots on the same side (i), L1–L2 and L5–L6 roots on opposite sides (ii), and L1–L2 roots on opposite sides (iii) of SMAΔ7 mice (grey circles and arrow) are similar to wild-type (black squares and arrow). Data are phase values taken from 5 SMA and 9 wild-type mice.
DISCUSSION
We have shown that the number of VGLUT1+ synapses on calbindin+ neurons in the Renshaw region is not reduced at the end stage of the disease in SMAΔ7 mice. This stands in contrast to the decreased number of VGLUT1+ synapses on motoneurons at the same stage of the disease. In addition, we have demonstrated that the spinal circuitry controlling locomotor-like activity is functional at P4-P6 in the SMAΔ7 mice and can be activated by stimulation of the dorsal roots. Collectively, these findings suggest that the loss of muscle spindle input to motoneurons that occurs early in the disease (Mentis et al. 2011) is probably not due to a primary defect of afferent connectivity but rather is secondary to motoneuron pathology.
The number of VGLUT1+ terminals on the somata of calbindin+ neurons was similar to that in wild-type animals. However, because the cell bodies of L1/2 calbindin+ neurons were smaller than wild-type controls, the somatic terminal density on the SMAΔ7 cells was almost double the control values. By comparison, at P13 in SMAΔ7 mice, the number of VGLUT1 synapses on the soma of motoneurons is ∼75% lower than on wild-type L1 motoneurons (Mentis et al. 2011). In contrast to the somatic observations, both the dendritic VGLUT1+ number and density were increased in SMAΔ7 mice compared with wild-type controls.
This increased proprioceptive innervation of calbindin+ neurons could reflect a compensatory sprouting of muscle spindle afferents in the ventral spinal cord in response to the de-afferentation of motoneurons. Whether or not muscle spindle afferents can sprout in response to denervation of their targets is not clear. Some classes of primary afferent, particularly nociceptors expressing calcitonin gene-related peptide, are known to sprout after a spinal cord lesion (McNeill et al. 1990; Krenz and Weaver 1998). Moreover, a recent study has shown that mice deficient for γ-protocadherin molecules experience a loss of ventral interneurons but not of motoneurons. In these animals, there is a 70% loss in the afferent collaterals projecting to ventral interneurons and a 2.5 increase in the area of terminals in the motor nucleus (Prasad et al. 2008; Prasad and Weiner 2011). Thus a loss of afferent input to ventral interneurons is accompanied by an increase in the number of inputs to motoneurons. Similarly, although less directly, Fouad and Pearson (1997) examined the effects of cutting an extensor muscle nerve on group I afferent field potentials recorded in the L6-L7 intermediate nucleus. They showed that the potentials evoked by stimulation of an uncut synergist nerve and were enhanced. This could occur if the synergist group I afferents sprouted onto denervated interneurons in the intermediate nucleus. Collectively, these findings, together with those reported in the present work, could be explained if primary afferent fibers intrinsically regulate the number of afferent synapses they make with their postsynaptic targets.
Another possibility that does not invoke homeostasis of muscle-afferent terminal number, posits that VGLUT1+ terminals from other spinal or descending neurons sprout to account for the increased number of VGLUT1+ terminals on calbindin+ neurons. It is known that corticospinal (Du Beau et al. 2012) and dorsal spinocerebellar (Hantman and Jessell 2010) neurons express VGLUT1 at their synapses. To our knowledge, there is no evidence that either class of neuron synapses with calbindin+ Renshaw cells. Nevertheless, we cannot eliminate the possibility that the increased VGLUT1+ synapses on calbindin+ cells might be derived from nonafferent sources.
It is interesting to note that the number of VAChT puncta was increased on the dendrites of L1/L2 SMA calbindin+ neurons compared with wild-type, despite the significant (∼60%) loss of L1/2 motoneurons at P13 (Mentis et al. 2011). Indeed, the loss of target motoneurons and their associated trophic support may be responsible for the reduced somatic area of the calbindin+ Renshaw cells. Since the majority of cholinergic synapses on Renshaw cells are believed to originate from motoneurons, this suggests one of two possibilities: either the remaining motoneurons have sprouted and generated new terminals or alternatively the terminals lost due to motoneuron cell death have been replaced by terminals from other cholinergic neurons. The present results do not allow us to distinguish these possibilities which are not mutually exclusive. However, it is known that motoneurons make synaptic connections with each other (Nishimaru et al. 2005) so that a loss of motoneurons will result in the remaining motoneurons losing some of their postsynaptic targets. Under these conditions, it is possible that motoneuron collaterals will sprout to innervate Renshaw cells thereby maintaining an intrinsically determined number of synaptic contacts. However, given the increased number of cholinergic terminals on calbindin+ neurons in SMAΔ7 mice, this is unlikely to be the only mechanism operating. One caveat to these considerations is that most of the cholinergic terminals on Renshaw cells likely originate from lateral motor neurons in which cell death is much less prominent than in medial motoneurons (Mentis et al. 2011). At present, very little is known about the relative innervation of Renshaw cells from lateral and medial motoneurons.
The increased number of VGLUT1 and VAChT synapses on calbindin+ neurons was observed only in the L1/2 segments. The most likely explanation for this difference is the greater severity of motoneuron dysfunction and afferent denervation in the rostral segments. At P13, L1 motoneurons (medial and lateral) in SMAΔ7 mice have lost ∼70% of their somatic VGLUT1 terminals whereas the lateral motoneurons in L5/6, which greatly outnumber the medial motoneurons, have lost only 46% of their VGLUT1 terminals. Similarly, 60% of the motoneurons in L1 are lost at P13, whereas L5 lateral motoneuron numbers are similar to wild-type controls at P13 (Mentis et al. 2011). The fact that no changes were observed in the number of VGLUT1 synapses on L5/6 calbindin+ neurons despite loss of 46% of the VGLUT+ terminals on motoneurons in the same segments suggests that there may be a threshold level of afferent loss before compensatory mechanisms occur. Alternatively, because motoneuron pathology in L1 occurs earlier than in L5, more time is available to manifest any compensatory changes in the innervation of calbindin+ cells.
Our results are consistent with recent experiments in which expression of the SMN protein was selectively increased in motoneurons (Gogliotti et al. 2012; Martinez et al. 2012). Under such conditions, most of the pathological changes in motoneurons, including the loss of afferent synapses, were reversed. We conclude, therefore, that the loss of afferent input to SMAΔ7 motoneurons is secondary to motoneuron pathology. Indeed, “synaptic stripping” of primary afferent synapses is a well-documented consequence of motoneuronal damage due to axotomy, which leads to a permanent loss of primary afferent synapses (Mendel et al. 1976). Another similarity between the synapse loss in SMA and synaptic stripping is the selective loss of glutamatergic synapses in both conditions (Lindå et al. 2000; Park et al. 2010; Ling et al. 2010, Mentis et al. 2011) and the preferential loss of somatic and proximal dendritic synapses (Ling et al. 2010; Mentis et al. 2011; Alvarez et al. 2011).
It is important to emphasize that the persistence of VGLUT1+ synapses on calbindin+ neurons does not imply that they are functional. This caveat also applies to the studies in which VGLUT1 synaptic loss was abrogated by the selective expression of the SMN protein in motoneurons (Gogliotti et al. 2012; Martinez et al. 2012). Previous work in mutant mice has demonstrated that structurally intact VGLUT1 synapses may exhibit functional deficits. For example, in mice lacking the neuregulin 1 receptor ErbB2, muscle spindle development is severely abnormal and the amplitude of monosynaptic excitatory potentials recorded in motoneurons is reduced by 80% despite a loss of only 30% of VGLUT1 synapses on motoneurons (Shneider et al. 2009).
We also determined that the central pattern generator for locomotion appears to operate normally in the mutant animals at least up to P4-P6. Although it would have been desirable to monitor locomotor function at the end stage of the disease, when the synaptic counts were performed, this is complicated by the fact that the increasing size of the cord precludes adequate oxygenation thereby compromising complex network operation in vitro. However, even at this early stage of the disease (P4), there is significant pathology of L1 motoneurons because the dorsal root-evoked monosynaptic reflex recorded from the L1 ventral root is reduced by 85% (Mentis et al. 2011). If the locomotor CPG inputs to L1 motoneurons were also disrupted at this time, it would likely be manifest as abnormal locomotor-like activity because the rostral lumbar segments are believed to exhibit the greatest rhythmogenic capacity (Cazalets et al. 1995; Kjaerulff and Kiehn 1996; Bertrand and Cazalets 2002).
Given the apparent normality of the locomotor circuitry at P4-P6 in the SMA mice, the question arises as to the source of the profound weakness exhibited by these animals. The loss of muscle spindle input appears to be the major deficit on motoneurons although there is also a loss (∼16%) of VGluT2 inputs (Ling et al. 2010). Could the loss of spindle input account for the weakness exhibited by the SMAΔ7 animals? One difficulty with this hypothesis is that group Ia synapses on motoneurons exhibit profound frequency-dependent synaptic depression in the neonatal period (Lev-tov and Pinco 1992; Li and Burke 2002), with the consequence that dynamic information transfer from spindles to motoneurons is likely to be compromised. However, little is known about the static inputs from group Ia and group II fibers. Indeed, it is possible that low frequency spindle input maintains a tonic excitatory drive to wild-type motoneurons that is absent in SMAΔ7 animals. If so, then the motoneurons might be difficult to recruit in natural motor behaviors. Of course, any change in the synaptic inputs to motoneurons will be compensated to some degree by the increased motoneuronal excitability. However, at least with regard to the group Ia input to motoneurons, the increased motoneuronal excitability does not compensate for the loss of afferent input because the amplitude of the monosynaptic reflex recorded from the L1 ventral roots is reduced by 85% at P4. For this reason, a useful therapeutic strategy might be to elevate motoneuronal excitability further using pharmacological methods. Several studies have demonstrated by that monoamines, including serotonin and dopamine, can increase motoneuronal excitability and the efficacy of muscle spindle inputs to motoneurons by upregulating excitatory inward currents in motoneuron dendrites (Lee and Heckman 2000; Hultborn et al. 2004; Harvey et al. 2006). It would therefore be of great interest to establish if such interventions could ameliorate some of the motor defects in spinal muscular atrophy.
GRANTS
This work was supported by the NINDS Intramural Program (to M. J. O'Donovan) and by a Wellcome Trust-DBT India Alliance Fellowship (to V. Thirumalai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.T. and M.J.O. conception and design of research; V.T., R.M.B., and D.B. performed experiments; V.T., R.M.B., S.B., W.L., and D.B. analyzed data; V.T. and M.J.O. interpreted results of experiments; V.T. and M.J.O. prepared figures; V.T. and M.J.O. drafted manuscript; V.T. and M.J.O. edited and revised manuscript; V.T., R.M.B., S.B., W.L., D.B., and M.J.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank George Mentis for comments on the manuscript.
REFERENCES
- Alvarez FJ, Fyffe RE. The continuing case for the Renshaw cell. J Physiol 584: 31–45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. CircStat: A Matlab toolbox for circular statistics. J Stat Software 31: 1–21, 2009 [Google Scholar]
- Bertrand S, Cazalets JR. The respective contribution of lumbar segments to the generation of locomotion in the isolated spinal cord of newborn rat. Eur J Neurosci 16: 1741–50, 2002 [DOI] [PubMed] [Google Scholar]
- Blivis D, Mentis GZ, O'Donovan Lev-Tov A MJ. Differential effects of opioids on sacrocaudal afferent pathways and central pattern generators in the neonatal rat spinal cord. J Neurophysiol 97: 2875–2886, 2007 [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15: 4943–4951, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Beau A, Shrestha SS, Bannatyne BA, Jalicy SM, Linnen S, Maxwell DJ. Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience 227C: 67–79, 2012 [DOI] [PubMed] [Google Scholar]
- Fouad K, Pearson KG. Modification of group I field potentials in the intermediate nucleus of the cat spinal cord after chronic axotomy of an extensor nerve. Neurosci Lett 236: 9–12, 1997 [DOI] [PubMed] [Google Scholar]
- Gogliotti RG, Quinlan KA, Barlow CB, Heier CR, Heckman CJ, Didonato CJ. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J Neurosci 32: 3818–3829, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 13: 1233–1239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004 [DOI] [PubMed] [Google Scholar]
- Kariya S, Park GH, Maeno-Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS, Landmesser LT, Monani UR. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet 17: 2552–2569, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21: 100–109, 2011 [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marcé M, Griffin JW, Rich MM, Sumner CJ. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J Neurosci 29: 842–851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience 85: 443–458, 1998 [DOI] [PubMed] [Google Scholar]
- Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AH. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet 14: 845–857, 2005 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80: 155–165, 1995 [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol 447: 149–169, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Burke RE. Developmental changes in short-term synaptic depression in the neonatal mouse spinal cord. J Neurophysiol 88: 3218–3231, 2002 [DOI] [PubMed] [Google Scholar]
- Lindå H, Shupliakov O, Ornung G, Ottersen OP, Storm-Mathisen J, Risling M, Cullheim S. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J Comp Neurol 425: 10–23, 2000 [PubMed] [Google Scholar]
- Ling KK, Lin MY, Zingg B, Feng Z, Ko CP. Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLos One 5: e15457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez TL, Kong L, Wang X, Osborne MA, Crowder ME, Van Meerbeke JP, Xu X, Davis C, Wooley J, Goldhamer DJ, Lutz CM, Rich MM, Sumner CJ. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J Neurosci 32: 8703–8715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill DL, Carlton SM, Coggeshall RE, Hulsebosch CE. Denervation-induced intraspinal synaptogenesis of calcitonin gene-related peptide containing primary afferent terminals. J Comp Neurol 296: 263–268, 1990 [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O'Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci 26: 13297–13310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, Alvarez FJ, Sumner CJ, O'Donovan MJ. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron 69: 453–467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci USA 102: 5245–5249, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GH, Maeno-Hikichi Y, Awano T, Landmesser LT, Monani UR. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J Neurosci 30: 12005–12019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn J. Incidence, prevalence, and gene frequency studies of chronicchildhood spinal muscular atrophy. J Med Genet 15: 409–413, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad T, Wang X, Gray PA, Weiner JA. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: insights from genetic analyses of the protocadherin-gamma gene cluster. Development 135: 4153–4164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad T, Weiner JA. Direct and indirect regulation of spinal cord ia afferent terminal formation by the γ-protocadherins. Front Mol Neurosci 4: 1–12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz R, Casañas JJ, Torres-Benito L, Cano R, Tabares L. Altered intracellular Ca2+ homeostasis in nerve terminals of severe spinal muscular atrophy mice. J Neurosci 30: 849–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Pax6 and engrailed 1 regulate two distinct aspects of Renshaw cell development. J Neurosci 24: 1255–1264, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider NA, Mentis GZ, Schustak J, O'Donovan MJ. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: the role of spindle-derived neurotrophin 3. J Neurosci 29: 4719–4735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84: 2821–2833, 2000 [DOI] [PubMed] [Google Scholar]