Abstract
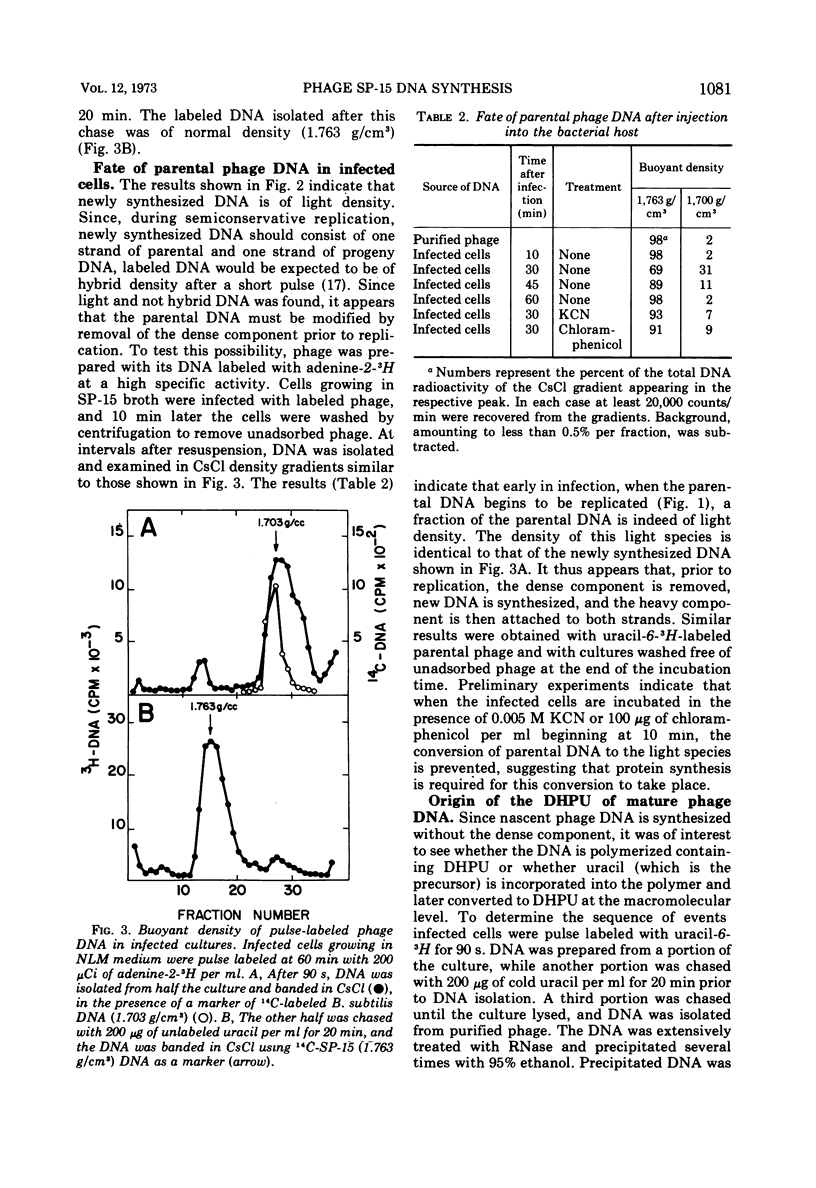
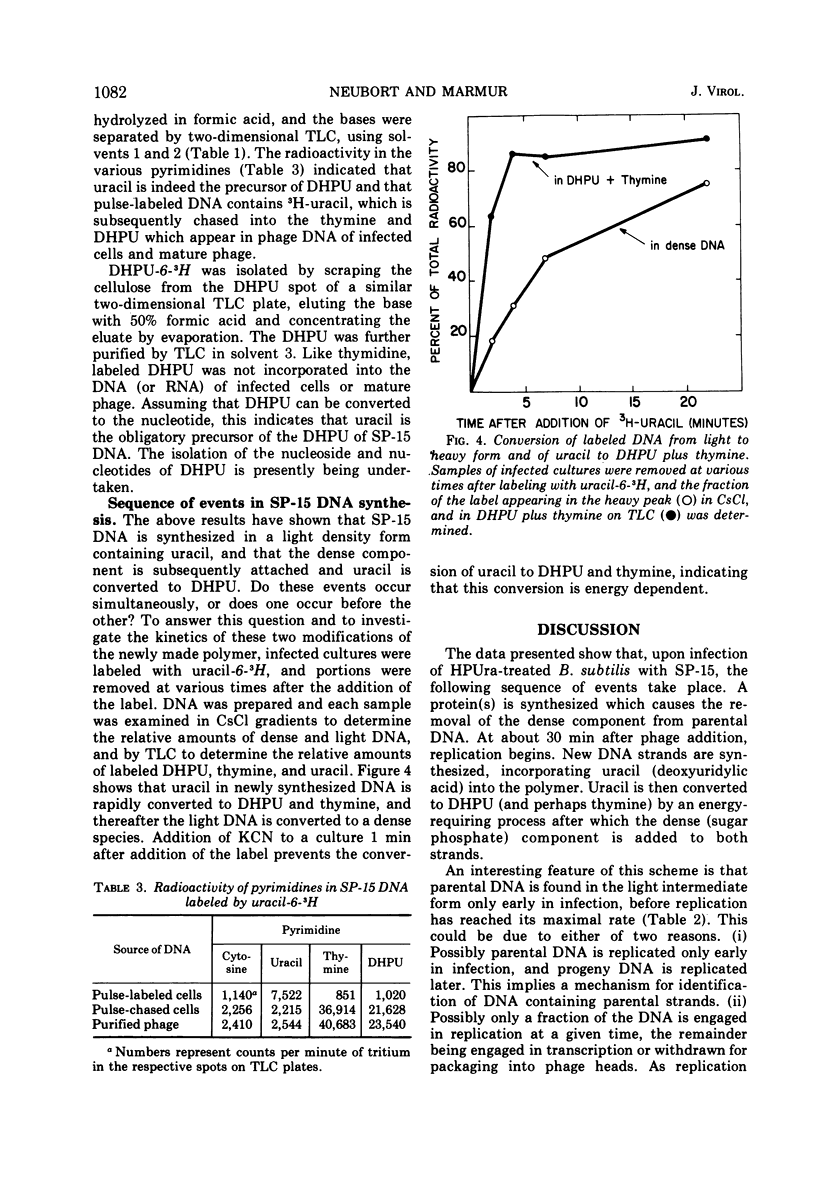
Cultures of Bacillus subtilis infected with phage SP-15 were examined to investigate the metabolic origin of two of the unique components of the phage DNA: the component responsible for the unusually high buoyant density in CsCl and the unusual pyrimidine, 5-(4′, 5′-dihydroxypentyl) uracil (DHPU). Newly synthesized pulse-labeled DNA was light in buoyant density and shifted to the high density of mature phage DNA upon further incubation. Parental DNA was converted to a light-density intermediate form prior to replication. When labeled uracil, thymidine, or DHPU were added to infected cells, it was found that only uracil served as the precursor to DHPU and thymine in phage DNA. Analysis of the bases from hydrolyzed DNA of labeled phage or infected cells indicated that the uracil was incorporated into the DNA as such (presumably via deoxyuridine triphosphate) and later converted to DHPU and thymine at the macromolecular level. The sequence of events after phage infection appeared to be: (i) injection of parental DNA; (ii) conversion of parental DNA to a light form; (iii) DNA replication, yielding light DNA containing uracil; (iv) conversion of uracil to DHPU and thymine; and (v) addition of the heavy component.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V. A DTMPASE FOUND AFTER INFECTION OF BACILLUS SUBTILIS WITH PHAGE SP5C. Biochem Biophys Res Commun. 1965 Jan 18;18:230–235. doi: 10.1016/0006-291x(65)90745-x. [DOI] [PubMed] [Google Scholar]
- Brandon C., Gallop P. M., Marmur J., Hayashi H., Nakanishi K. Structure of a new pyrimidine from Bacillus subtilis phage SP-15 nucleic acid. Nat New Biol. 1972 Sep 20;239(90):70–71. doi: 10.1038/newbio239070a0. [DOI] [PubMed] [Google Scholar]
- Brown N. C. 6(p-Hydroxyphenylazo)-uracil: a reversible, selective inhibitor of the replication of deoxyribonucleic acid of staphylococcal bacteriophage P11-M15. J Virol. 1971 Nov;8(5):759–765. doi: 10.1128/jvi.8.5.759-765.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Low R. L. Mutational alteration of Bacillus subtilis DNA polymerase 3 to hydroxyphenylazopyrimidine resistance: polymerase 3 is necessary for DNA replication. Biochem Biophys Res Commun. 1973 Mar 5;51(1):151–157. doi: 10.1016/0006-291x(73)90521-4. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. Virus-induced acquisition of metabolic function. I. Enzymatic formation of 5-hydroxymethyldeoxycytidylate. J Biol Chem. 1959 Jun;234(6):1501–1506. [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- JESAITIS M. A. Differences in the chemical composition of the phage nucleic acids. Nature. 1956 Sep 22;178(4534):637–637. doi: 10.1038/178637a0. [DOI] [PubMed] [Google Scholar]
- KORNBERG S. R., ZIMMERMAN S. B., KORNBERG A. Glucosylation of deoxyribonucleic acid by enzymes from bacteriophage-infected Escherichia coli. J Biol Chem. 1961 May;236:1487–1493. [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Biological effects of substituting cytosine for 5-hydroxymethylcytosine in the deoxyribonucleic acid of bacteriophage T4. J Virol. 1969 Oct;4(4):439–453. doi: 10.1128/jvi.4.4.439-453.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J. M., Neville M. M., Wright G. E., Brown N. C. Hydroxyphenylazopyrimidines: characterization of the active forms and their inhibitory action on a DNA polymerase from Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):512–516. doi: 10.1073/pnas.70.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Newlon M. C. Control of DNA synthesis in Bacillus subtilis by phage phi e. Virology. 1971 Apr;44(1):83–93. doi: 10.1016/0042-6822(71)90155-3. [DOI] [PubMed] [Google Scholar]
- Marmur J., Brandon C., Neubort S., Ehrlich M., Mandel M., Konvicka J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat New Biol. 1972 Sep 20;239(90):68–70. doi: 10.1038/newbio239068a0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara M., Friedman N., Vasken Aposhian H. Biological activity of 5-hydroxymethyluracil and its deoxynucleoside in noninfected and phage-infected Bacillus subtilis. J Virol. 1969 Feb;3(2):164–170. doi: 10.1128/jvi.3.2.164-170.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Price A. R., Warner H. R. Bacteriophage T4-induced deoxycytidine triphosphate-deoxyuridine triphosphate nucleotidohydrolase: its properties and its role during phage infection of Escherichia coli. Virology. 1969 Dec;39(4):882–892. doi: 10.1016/0042-6822(69)90024-5. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Luria S. E. DNA-glucosylation in T-even phage: genetic determination and role in phagehost interaction. Annu Rev Genet. 1970;4(0):177–192. doi: 10.1146/annurev.ge.04.120170.001141. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H., Tucker R. G. The biosynthesis of 5-hydroxymethyldeoxyuridylic acid in bacteriophage-infected Bacillus subtilis. Virology. 1966 May;29(1):157–166. doi: 10.1016/0042-6822(66)90205-4. [DOI] [PubMed] [Google Scholar]
- SINSHEIMER R. L. Nucleotides from T2r+ bacteriophage. Science. 1954 Oct 8;120(3119):551–553. doi: 10.1126/science.120.3119.551. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Taylor M. J., Lawton W. D., Goldberg I. D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1969 Nov;100(2):1027–1036. doi: 10.1128/jb.100.2.1027-1036.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Amber mutants of bacteriophage T4 defective in deoxycytidine diphosphatase and deoxycytidine triphosphatase. On the role of 5-hydroxymethylcytosine in bacteriophage deoxyribonucleic acid. J Biol Chem. 1967 Dec 25;242(24):5824–5829. [PubMed] [Google Scholar]



