Abstract
Recent studies support roles for neurokinin-1 (NK-1) and gastrin-releasing peptide (GRP) receptor-expressing spinal neurons in itch. We presently investigated expression of substance P (SP) and GRP in pruritogen-responsive primary sensory neurons and roles for these neuropeptides in itch signaling. Responses of dorsal root ganglion (DRG) cells to various pruritogens were observed by calcium imaging. DRG cells were then processed for SP, GRP, and isolectin B-4 (IB4; a marker for nonpeptidergic neurons) immunofluorescence. Of pruritogen-responsive DRG cells, 11.8–26.8%, 21.8–40.0%, and 21.4–26.8% were immunopositive for SP, GRP, and IB4, respectively. In behavioral studies, both systemic and intrathecal administration of a NK-1 receptor antagonist significantly attenuated scratching evoked by chloroquine and a protease-activated receptor 2 agonist, SLIGRL, but not histamine, bovine adrenal medulla peptide 8-22 (BAM8-22), or serotonin. Systemic or intrathecal administration of a GRP receptor antagonist attenuated scratching evoked by chloroquine and SLIGRL but not BAM8-22 or histamine. The GRP receptor antagonist enhanced scratching evoked by serotonin. These results indicate that SP and GRP expressed in primary sensory neurons are partially involved as neurotransmitters in histamine-independent itch signaling from the skin to the spinal cord.
Keywords: dorsal root ganglion cell, itch, mouse, scratching
although chronic itch is a burdensome clinical problem that decreases the quality of life (Weisshaar et al. 2006), the neuronal mechanisms of itch are still not fully understood. Recent studies have implicated histamine-dependent and histamine-independent pathways in transmitting itch. One histamine-independent itch pathway involves a family of over 50 Mas-related G protein-coupled receptors (Mrgprs), including MrgprAs, MrgprB4-5, MrgprC11, and MrgprD, which are restricted to small-diameter dorsal root ganglion (DRG) neurons in mice (Dong et al. 2001). Chloroquine and the bovine adrenal medulla peptide 8-22 (BAM8-22) elicit itch-related scratching through MrgprA3 and MrgprC11, respectively, in mice (Liu et al. 2009), and BAM8-22 elicits itch in humans (Sikand et al. 2011). Another histamine-independent itch pathway is the protease-activated receptor (PAR)-2, one of a family of four PAR subtypes (Ossovskaya and Bunnett 2004). Both endogenous and exogenous agonists of PAR-2, tryptase, and PAR-2 agonist, SLIGRL-NH2, respectively, elicit scratching in a dose-dependent manner (Akiyama et al. 2009; Shimada et al. 2006; Ui et al. 2006).
Recent studies support roles for neurokinin-1 (NK-1) and gastrin-releasing peptide (GRP) receptor-expressing spinal neurons in itch (Carstens et al. 2010; Sun et al. 2009). Both NK-1 and GRP receptors are expressed in neurons in the spinal superficial dorsal horn (Carstens et al. 2010; Sun et al. 2009). Intrathecal injection of saporin-conjugated bombesin eliminated GRP receptor-expressing spinal neurons in mice and reduced scratching elicited by a variety of pruritogens. In addition, intrathecal injection of saporin-conjugated substance P (SP) eliminated the NK-1 receptor-expressing spinal neurons in rats and reduced scratching elicited by serotonin (5-HT). Nevertheless, the roles for SP and GRP in the spinal transmission of itch signals are not fully understood. A GRP receptor antagonist partially reduced scratching elicited by a PAR-2 agonist, compound 48/80, and chloroquine (Sun and Chen 2007), whereas GRP receptor knockout mice did not exhibit a reduction in scratching evoked by histamine, 5-HT, or endothelin-1 (Sun et al. 2009). It is reported that GRP is not essential to activate the GRP receptor, which heterodimerizes with the μ-opioid receptor isoform MOR1D in mouse superficial dorsal horn neurons and may mediate opioid-induced itch (Liu et al. 2011). A NK-1 receptor antagonist suppressed scratching elicited by trypsin (Costa et al. 2008), whereas deletion of the preprotachykinin A gene that encodes SP and NK-A in mice did not reduce 5-HT-evoked scratching (Cuellar et al. 2003). In this study, we determined the expression of SP and GRP in pruritogen-sensitive primary sensory neurons. We further investigated whether antagonists of the NK-1 or GRP receptor attenuated scratching evoked by different types of pruritogens in mice.
MATERIALS AND METHODS
Calcium imaging.
A total of 43 adult male C57/BL6 mice (7–9 wk old; 18–21 g; Simonsen Laboratories, Gilroy, CA) was used under a protocol approved by the University of California (UC), Davis, Institutional Animal Care and Use Committee. The animal was euthanized under sodium pentobarbital anesthesia, and upper- to midcervical DRGs were acutely dissected and enzymatically digested at 37°C for 10 min in HBSS (Invitrogen, Carlsbad, CA) containing 20 U/ml papain (Worthington Biochemical, Lakewood, NJ) and 6.7 mg/ml L-cysteine (Sigma, St. Louis, MO), followed by 10 min at 37°C in HBSS containing 3 mg/ml collagenase (Worthington Biochemical). The ganglia were then mechanically triturated using fire-polished glass pipettes. DRG cells were pelleted; suspended in MEM with Earle's balanced salt solution (Gibco, Life Technologies, Carlsbad, CA) containing 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco, Life Technologies), 1× vitamin (Gibco, Life Technologies), and 10% horse serum (Quad Five, Ryegate, MT); plated on poly-d-lysine-coated glass coverslips; and cultured for 16–24 h.
DRG cells were incubated in Ringer's solution (pH 7.4, 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 4.54 mM NaOH) with 10 μM Fura-2 AM and 0.05% of Pluronic F-127 (Invitrogen). Coverslips were mounted on a custom-made aluminum perfusion block and viewed through an inverted microscope (Nikon TS100, Technical Instruments, Burlingame, CA). Fluorescence was excited by UV light at 340 nm and 380 nm alternately, and emitted light was collected via a CoolSNAP camera attached to a Lambda LS lamp and a Lambda optical filter changer (Sutter Instrument, Novato, CA). Ratiometric measurements were made using Simple PCI software (Hamamatsu, Sewickley, PA) every 3 s.
Solutions were delivered by a solenoid-controlled eight-channel perfusion system (ValveLink, AutoMate Scientific, San Francisco, CA). Two of the following agents were delivered in randomized order: chloroquine (300 μM), BAM8-22 (10 μM), histamine (100 μM), and the PAR-2 agonist SLIGRL-NH2 (100 μM). Potassium, at a concentration of 144 mM, was always delivered at the end of each experiment. Stimulus duration was 30 s (10 s for capsaicin). Ratios were normalized to baseline. Cells were judged to be sensitive if the ratio value increased by >10% of the resting level following chemical application. Only cells responsive to high potassium were included for analysis. After the experiment, coverslips were marked with a diamond pen to provide landmarks for alignment with subsequent immunohistofluoresence labeling of the same cells.
Immunocytochemistry.
After calcium imaging, DRG cells in the culture dish were fixed in formalin, followed by 30% sucrose, and then incubated with 5% normal serum. They were immunostained with anti-GRP antibody (1:500; ImmunoStar, Hudson, WI), anti-SP antibody (1:500; Millpore, Billerica, MA), or biotinylated isolectin B-4 (IB4; 1:200; Vector Laboratories, Burlingame, CA) at 4°C overnight, followed by incubation with the corresponding secondary antibody conjugated with Alexa Fluor 488 (1:500; Life Technologies, Grand Island, NY) or avidin-Texas Red (1:100; Life Technologies) for 2 h. Images were captured using a fluorescence microscope (Nikon Eclipse Ti; Technical Instruments). Immunohistofluorescent images were aligned with images captured during calcium imaging to determine the percentages of pruritogen-responsive DRG cells that were double labeled for SP, GRP, or IB4.
Behavioral scratching studies.
Experiments were conducted using adult male C57/BL6 mice (19–26 g; Simonsen Laboratories), again under a protocol approved by the UC Davis Animal Care and Use Committee. The fur on the rostral back was shaved, and mice were habituated to the Plexiglas recording arena 1 wk prior to testing. In a first set of experiments, vehicle [saline, subcutaneously (sc)], the GRP receptor antagonist RC-3095 (0.3 mg/kg sc; Sigma), or the NK-1 antagonist L-733060 (10 mg/kg ip; Tocris Bioscience, Minneapolis, MN) was administered systemically, followed 30 min later by intradermal (id) injection (10 μl) of one of the following: vehicle (isotonic saline), histamine (271 nmol; Sigma), PAR-2 agonist SLIGRL-NH2 (76 nmol; Quality Controlled Biochemicals, Hopkinton, MA), serotonin (5-HT; 47 nmol; Alfa Aesar, Ward Hill, MA), chloroquine (193 nmol; Sigma), or BAM8-22 (50 nmol; Genemed Synthesis, San Antonio, TX). In a second set of experiments, vehicle (saline; 5 μl), RC-3095 (0.3 nmol/5 μl), or L-733060 (22.7 nmol/5 μl) was administered intrathecally via lumbar puncture, 5 min prior to id injection of one of the pruritogens noted above. In a third set of experiments, mice received an id microinjection of either 10 μl serotonin (47 nmol) or a combination of serotonin (47 nmol) and RC-3095 (0.6 nmol). Microinjections were made id in the nape of the neck using a 30G needle attached to a Hamilton microsyringe by PE-50 tubing. Immediately after the injection, the mouse was placed into the arena and videotaped from above for 30 min. Generally, three to four mice were injected and videotaped simultaneously. Immediately after commencing videotaping, all investigators left the room.
Videotapes were reviewed by investigators blinded to the treatment, and the number of scratch bouts was counted at 5-min intervals. A scratch bout was defined as one or more rapid back-and-forth hind-paw motions directed toward and contacting the injection site, ending with licking or biting of the toes and/or placement of the hind paw on the floor. Hind-paw movements directed away from the injection site (e.g., ear-scratching) and grooming movements were not counted. For analysis of the time course of scratching, two-way repeated-measures ANOVA followed by Student-Newman-Keuls test was used to compare the mean number of scratch bouts/5-min interval after pruritogen injection without vs. with pretreatment. One-way ANOVA followed by the Bonferroni post-test was used to compare the total number of scratch bouts across pretreatment groups. In all cases, P < 0.05 was considered to be significant.
RESULTS
Calcium imaging of DRG cells.
A total of 8,248 DRG cells was imaged for responsiveness to chloroquine, BAM8-22, histamine, and the PAR-2 agonist SLIGRL. The percentages of responsive DRG cells were as follows: chloroquine 9.8%, BAM8-22 3.4%, histamine 19.1%, and the PAR-2 agonist 2.5%. These values are consistent with previous studies (Akiyama et al. 2010; Liu et al. 2009; Wilson et al. 2011). Additional details regarding this DRG cell population have been published separately (Akiyama et al. 2012).
Immunofluorescent labeling of pruritogen-responsive DRG cells.
Following calcium imaging, the DRG cells were fixed and immunostained with an antibody directed against SP, GRP, or IB4. A total of 2,696 DRG cells was labeled. Fig. 1A shows a fluorescence image of a DRG cell that responded to chloroquine in calcium imaging, and Fig. 1B shows that the same cell was immunopositive for SP. Fig. 1, C–E, shows examples of DRG cells immunopositive for SP, GRP, and IB4, respectively.
Fig. 1.
Prutirogen-responsive dorsal root ganglion (DRG) cells double-labeled for substance P (SP), gastrin-releasing peptide (GRP), or isolectin B-4 (IB4) immunoreactivity. A: fluorscence microscopic image of calcium response following application of chloroquine (CQ; encircled DRG cell). Scale bar applies to A and B. B: same cell in A double-labeled for SP. C: example of DRG cells labeled for SP. D: example of DRG cells labeled for GRP. E: example of DRG cells labeled for IB4. Scale bar applies to C–E. F: summary of percentages of CQ-responsive DRG cells (assessed by calcium imaging) that were double labeled for SP (black bar), GRP (open bar), or IB4 (striped bar; n = 14–83/group). G: bovine adrenal medulla peptide 8–22 [BAM8-22 (BAM)]-sensitive cells (n = 15–44/group). H: histamine (His)-sensitive cells (n = 11–87/group). I: protease-activated receptor 2 agonist SLIGRL-NH2-sensitive cells (n = 10–29/group).
Overall, 33.2% of all DRG cells examined were immunopositive for SP, 27.2% for GRP, and 23.5% for IB4, consistent with previous studies (Caterina et al. 2000; Chen et al. 2006; Dirajlal et al. 2003; Sun and Chen 2007; Tominaga et al. 2009). Of the DRG cells that responded to chloroquine, 26.8%, 25.3%, and 21.4% were immunpositive for SP, GRP, and IB4, respectively (Fig. 1F). Of the BAM8-22-responsive cells, 15.9%, 40.0%, and 17.6% were immunpositive for SP, GRP, and IB4, respectively (Fig. 1G). Of the histamine-responsive cells, 18.1%, 21.8%, and 45.5% were immunpositive for SP, GRP, and IB4, respectively (Fig. 1H). Of the SLIGRL-NH2-responsive cells, 11.8%, 30.0%, and 17.2% were immunpositive for SP, GRP, and IB4, respectively (Fig. 1I).
Systemic injection of NK-1 and GRP receptor antagonists reduces scratching elicited by certain pruritogens.
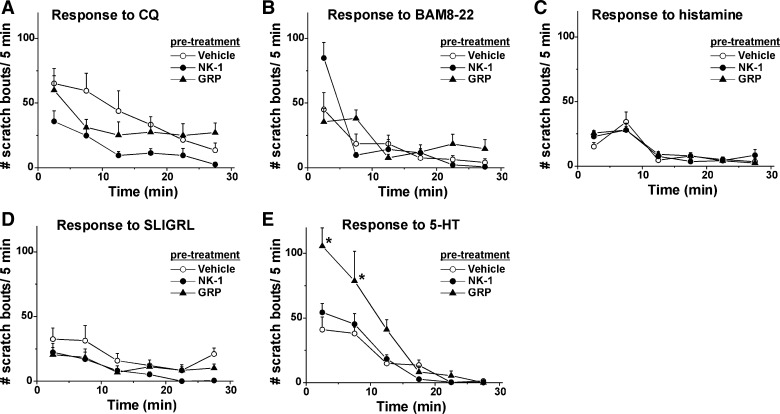
The time course of scratching elicited by id injection of each pruritogen is shown in Fig. 2, A–E, for chloroquine, BAM8-22, histamine, SLIGRL-NH2, and 5-HT, respectively. In all cases, maximal scratching occurred within the initial 5- to 10-min postinjection and decreased within 20–30 min.
Fig. 2.
Time course of pruritogen-elicited scratching. A: mean number of scratch bouts/5 min over the 30-min period following intradermal (id) pruritogen injection preceded by systemic injection of vehicle (saline; open circles), neurokinin-1 (NK-1) receptor antagonist L-733060 (10 mg/kg; filled circles), or GRP receptor antagonist RC-3095 (0.3 mg/kg; filled triangles). Error bars: SE; n = 6/group. B: as in A for BAM8-22. C: as in A for histamine. D: as in A for SLIGRL-NH2. E: as in A for 5-HT. *Significantly different from vehicle group; P < 0.05, post hoc Student-Newman-Keuls test, following 2-way repeated-measures ANOVA; F(10, 97) = 7.29; n = 6/group.
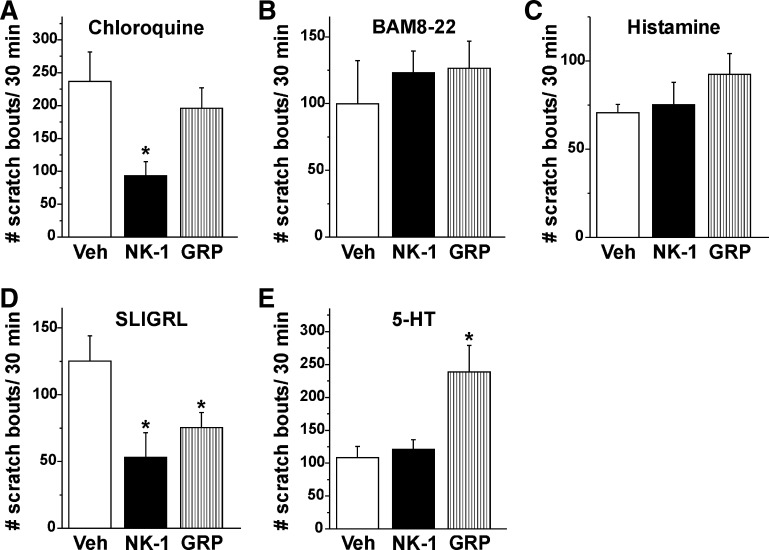
The NK-1 antagonist L-733060, but not the GRP receptor antagonist RC-3095, significantly attenuated chloroquine-evoked scratching (Figs. 2A and 3A; P < 0.05). Neither the NK-1 nor the GRP receptor antagonist had any significant effect on scratching elicited by BAM8-22 (Figs. 2B and 3B) or histamine (Figs. 2C and 3C). Both the NK-1 and GRP receptor antagonist (RC-3095) significantly attenuated SLIGRL-NH2-evoked scratching (Figs. 2D and 3D). The GRP receptor antagonist significantly enhanced 5-HT-evoked scratching (Figs. 2E and 3E; P < 0.05).
Fig. 3.
Summary of systemic effects of NK-1 and GRP receptor antagonist on pruritogen-elicited scratching. A: bar graph plots, from left to right, the mean number of scratch bouts/30 min elicited by id microinjection of CQ, 30 min after prior systemic injection of vehicle (Veh; saline; open bar), NK-1 antagonist L-733060 (10 mg/kg; filled bar), or GRP antagonist RC-3095 (0.3 mg/kg; striped bar). Error bars: SE. *Significantly different from vehicle group; P < 0.05, 1-way ANOVA, Bonferroni post-test, n = 6/group. B: as in A for BAM8-22. C: as in A for histamine. D: as in A for SLIGRL-NH2. *Significantly different from vehicle group; P < 0.05, 1-way ANOVA, Bonferroni post-test, n = 6/group. E: as in A for 5-HT. *Significantly different from vehicle group; P < 0.05, 1-way ANOVA, Bonferroni post-test, n = 6/group.
Intrathecal injection of NK-1 and GRP receptor antagonists reduce scratching elicited by certain pruritogens.
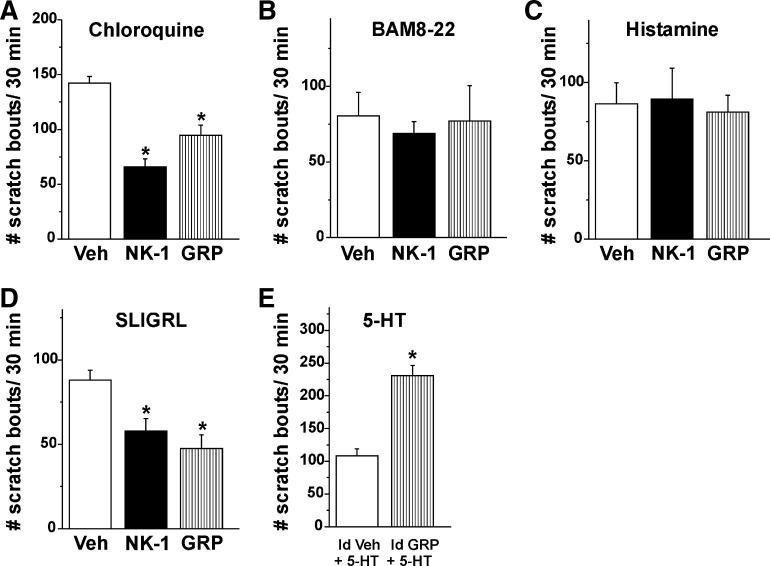
Consistent with the results following its systemic administration, intrathecal injection of the NK-1 antagonist L-733060 attenuated chloroquine-evoked scratching (Fig. 4A; P < 0.05), and intrathecal injection of the GRP receptor antagonist significantly attenuated chloroquine-evoked scratching (Fig. 4A; P < 0.05), in contrast to the lack of antagonist effect when administered systemically. Neither the NK-1 nor the GRP receptor antagonist had any significant effect on scratching elicited by BAM8-22 (Fig. 4B) or histamine (Fig. 4C). Both the NK-1 and GRP receptor antagonist significantly attenuated SLIGRL-NH2-evoked scratching (Fig. 4D).
Fig. 4.
Summary of effects of of NK-1 and GRP receptor antagonists on pruritogen-elicited scratching. Antagonists were injected intrathecally in A–D and id (Id) in E. A: bar graph plots, from left to right, the mean number of scratch bouts/30 min elicited by id microinjection of CQ, 30 min after prior intrathecal injection of vehicle (saline; open bar), NK-1 antagonist L-733060 (22.7 nmol/5 μl; filled bar), or GRP antagonist RC-3095 (0.3 nmol/5 μl; striped bar). Error bars: SE. *Significantly different from vehicle group; P < 0.05, 1-way ANOVA, Bonferroni post-test, n = 6/group. B: as in A for BAM8-22. C: as in A for histamine. D: as in A for SLIGRL-NH2. *Significantly different from vehicle group; P < 0.05, 1-way ANOVA, Bonferroni post-test, n = 6/group. E: a mixture of either vehicle and 5-HT (Id Veh + 5-HT; saline; 10 μl; open bar) or 5-HT and GRP receptor antagonist RC-3095 (Id GRP + 5-HT; 0.6 nmol/10 μl, striped bar) was injected id. *Significantly different from vehicle group; P < 0.05, paired t-test, n = 6/group.
Injection (id) of the GRP receptor antagonist significantly enhanced 5-HT-evoked scratching.
Injection (id) of the GRP receptor antagonist elicited a modest but statistically significant increase in scratching [15.8 ± 2.8 (SE) scratch bouts/15 min] compared with id saline (5.8 ± 3.3). Injection (id) of the GRP receptor antagonist also significantly enhanced 5-HT-evoked scratching (Fig. 4E; P < 0.05), similar to its effect when administered systemically.
DISCUSSION
A substantial proportion of pruritogen-responsive DRG cells expressed SP or GRP. Antagonists of the NK-1 and/or GRP receptor reduced scratching elicited by the SLIGRL-NH2 and chloroquine, implying roles for the neuropeptide transmitters SP and GRP in the spinal transmission of a nonhistaminergic itch.
Neuroanatomical and molecular characterization allows C fibers to be categorized as either peptidergic neurons that release neuropeptides, including SP and GRP, or nonpeptidergic neurons that bind IB4 (Basbaum et al. 2009; Sun and Chen 2007). It was reported originally that Mrgprs, including those that bind chloroquine or BAM8-22, were expressed predominantly in nonpeptidergic neurons (Dong et al. 2001; Zylka et al. 2003). However, Mrgprs have been reported more recently to also be expressed in peptidergic neurons (Liu et al. 2009, 2010). Injection (id) of chloroquine induced expression of Fos, a marker for neuronal activation, in neurons located in the superficial spinal dorsal horn dorsal to and within the region of IB4 labeling, implying that chloroquine activates both peptidergic and nonpeptidergic neurons (Han et al. 2012). Consistent with these findings, we showed that subpopulations of DRG cells responsive to chloroquine or BAM8-22 were also immunoreactive for SP and GRP and were labeled with IB4.
Approximately one-half of histamine-responsive DRG cells was immunoreactive for IB4. An earlier study similarly reported that approximately three-fourths of histamine-responsive DRG cells were IB4 positive (Han et al. 2006). In this latter study, a deficiency of PLCβ3 in IB4-positive DRG cells abolished their responses to histamine. We presently observed that the incidence of IB4 expression in histamine-sensitive DRG cells was much greater compared with that of SP or GRP (Fig. 1H). This is consistent with a previous report that id injection of histamine elicited Fos expression in superficial dorsal horn neurons located primarily within the region of IB4 labeling, suggesting that histamine activates mainly nonpeptidergic neurons (Nakano et al. 2008). SLIGRL-NH2-responsive DRG cells were immunoreactive for SP or GRP, consistent with previous reports (Nakano et al. 2008; Steinhoff et al. 2000). In the present study, a substantial proportion of SLIGRL-NH2-responsive DRG cells was immunoreactive for IB4, suggesting that the SLIGRL-NH2 also activated nonpeptidergic neurons.
The NK-1 antagonist partially inhibited scratching evoked by chloroquine and SLIGRL-NH2, and SP was expressed in subpopulations of chloroquine- and SLIGRL-NH2-responsive DRG cells. These findings are consistent with the report that a NK-1 antagonist suppressed scratching elicited by tryptase (Costa et al. 2008). NK-1 receptors are expressed in 30% of DRG neurons and may be responsible for itch signaling through a possible autocrine and paracrine action of SP (Zhang et al. 2007). However, the NK-1 antagonist failed to inhibit scratching elicited by histamine, 5-HT, or BAM8-22. In accordance with the present results, deletion of the preprotachykinin A gene in mice did not affect 5-HT-evoked scratching (Cuellar et al. 2003). 5-HT-evoked scratching was suppressed following neurotoxic destruction of NK-1 receptor-expressing neurons in the superficial dorsal horn (Carstens et al. 2010), implying that neurons expressing NK-1 receptors, rather than SP per se, are involved in certain types of itch signaling.
The GRP receptor antagonist partially suppressed SLIGRL-NH2-evoked scratching, and GRP was expressed in a subpopulation of SLIGRL-NH2-responsive DRG cells. These findings are largely consistent with recent reports that the GRP receptor is involved in itch (Sun and Chen 2007; Sun et al. 2009). Whereas systemic injection of the GRP antagonist only weakly inhibited chloroquine-evoked scratching, intrathecal injection of the GRP receptor antagonist significantly suppressed chloroquine-evoked scratching, consistent with earlier findings (Sun and Chen 2007; Sun et al. 2009). This difference might depend on the route of administration of the antagonist (intrathecal vs. systemic). Systemic injection of the GRP receptor antagonist RC-3095 presumably elicited weak scratching via an action at peripheral GRP receptors. The GRP receptor antagonist did not inhibit histamine-elicited scratching, which is also consistent with earlier findings (Sun and Chen 2007; Sun et al. 2009).
It is unexpected that systemic injection of the GRP receptor antagonist enhanced scratching elicited by 5-HT. Injection (id) of the GRP receptor antagonist also enhanced 5-HT-evoked scratching (Fig. 4E), suggesting that local GRP receptors participated in this effect. In addition, id injection of the GRP receptor antagonist itself elicited scratching. One possibility is that peripheral and central GRP receptors play different roles in itch. Speculatively, a peripheral GRP receptor may constitutively inhibit itch signaling, such that blocking the GRP receptor reduces local inhibition to elicit and enhance scratching. Another possibility is that RC-3095 may act as a partial agonist but not full antagonist, such that RC-3095 activates GRP receptors expressed at peripheral sites. This is supported by a recent study showing that id injection of GRP elicited scratching (Andoh et al. 2011). In either case, speculatively, 5-HT may sensitize this itch signaling pathway. The present results need to be taken into consideration in developing GRP receptor antagonists for the treatment of itch.
Of the pruritogen-responsive DRG cells, 19.1% and 25.1% were immunopositive for SP and GRP, respectively. These proportions of peptidergic sensory neurons may be sufficient to transmit certain types of itch signals. However, pruritogen-evoked scratching was reduced only partially by antagonists of the NK-1 or GRP receptor, suggesting that an additional mediator other than SP or GRP is also involved in spinal transmission of itch signaling. NK-B, encoded by the tachykinin-2 gene, is a member of the tachykinin peptides, along with SP, and is a possible candidate as a neuropeptide transmitter in spinal itch transmission. However, a recent study revealed that tachykinin-2 null mice exhibited normal scratch responses to compound 48/80, chloroquinine, SLIGRL-NH2, and methyl-serotonin, implying that NK-B is not involved in the spinal transmission of itch signaling (Mar et al. 2012). It is widely accepted that glutamate acts as an excitatory spinal neurotransmitter. Most peptidergic and nonpeptidergic DRG neurons express vesicular glutamate transporters, which are responsible for the uptake of the glutamate into synaptic vesicles (Brumovsky et al. 2007; Scherrer et al. 2010). GRP receptor-expressing spinal neurons are thought to be essential for transmission of itch signaling (Sun et al. 2009). It was recently reported that GRP receptor-expressing spinal neurons are activated by glutamate—rather than GRP—release from primary afferents (Koga et al. 2011; Sun et al. 2009). Thus glutamate is a good candidate as an additional spinal neurotransmitter for itch.
GRANTS
Support for this work was provided by grants from the National Institute of Dental and Craniofacial Research (DE013685) and National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR057194). T. Akiyama and M. Tominaga received postdoctoral fellowships from the Japan Society for the Promotion of Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.A., M.T., and E.C. conception and design of research; T.A., M.T., A.D., M.N., K.B., A.H., and M.I.C. performed experiments; T.A., M.T., A.D., M.N., K.B., A.H., and M.I.C. analyzed data; T.A., M.T., A.H., and E.C. interpreted results of experiments; E.C. prepared figures; T.A. and E.C. drafted manuscript; T.A., M.T., and E.C. edited and revised manuscript; T.A., M.T., A.D., M.N., K.B., A.H., M.I.C., and E.C. approved final version of manuscript.
REFERENCES
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain 151: 378–383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther 329: 945–951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Cross-sensitization of histamine-independent itch in mouse primary sensory neurons. Neuroscience 226: 305–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Kuwazono T, Lee JB, Kuraishi Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides 32: 2098–2103, 2011 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky P, Watanabe M, Hokfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience 147: 469–490, 2007 [DOI] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport 21: 303–308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000 [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49: 365–377, 2006 [DOI] [PubMed] [Google Scholar]
- Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, Quintao NL, Juliano L, Brain SD, Calixto JB. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol 154: 1094–1103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar JM, Jinks SL, Simons CT, Carstens E. Deletion of the preprotachykinin A gene in mice does not reduce scratching behavior elicited by intradermal serotonin. Neurosci Lett 339: 72–76, 2003 [DOI] [PubMed] [Google Scholar]
- Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB(4)-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol 89: 513–524, 2003 [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619–632, 2001 [DOI] [PubMed] [Google Scholar]
- Han N, Zu JY, Chai J. Spinal bombesin-recognized neurones mediate more nonhistaminergic than histaminergic sensation of itch in mice. Clin Exp Dermatol 37: 290–295, 2012 [DOI] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron 52: 691–703, 2006 [DOI] [PubMed] [Google Scholar]
- Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol Pain 7: 47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci 13: 1460–1462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, Chen ZF. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147: 447–458, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar L, Yang FC, Ma Q. Genetic marking and characterization of Tac2-expressing neurons in the central and peripheral nervous system. Mol Brain 5: 3, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Andoh T, Lee JB, Kuraishi Y. Different dorsal horn neurons responding to histamine and allergic itch stimuli. Neuroreport 19: 723–726, 2008 [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84: 579–621, 2004 [DOI] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci USA 107: 22296–22301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 530: 281–283, 2006 [DOI] [PubMed] [Google Scholar]
- Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci 31: 7563–7567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 6: 151–158, 2000 [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448: 700–703, 2007 [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science 325: 1531–1534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis model. J Invest Dermatol 129: 2901–2905, 2009 [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol 530: 172–178, 2006 [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Apfelbacher C, Jager G, Zimmermann E, Bruckner T, Diepgen TL, Gollnick H. Pruritus as a leading symptom: clinical characteristics and quality of life in German and Ugandan patients. Br J Dermatol 155: 957–964, 2006 [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14: 595–602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci 27: 12067–12077, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci USA 100: 10043–10048, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]