Abstract
Human handedness has been described and measured from two perspectives: handedness inventories rate hand preferences, whereas other tests examine motor performance asymmetries. These two measurement approaches reflect a major controversy in a literature that defines handedness as either a preference or an asymmetry in sensorimotor processing. Over the past decade, our laboratory has developed a model of handedness based on lateralization of neural processes. This model attributes distinct control processes to each hemisphere, which in turn lead to observable interlimb sensorimotor performance asymmetries. We now hypothesize that arm preference, or choice, may depend on the interaction between sensorimotor performance asymmetries and the given task. The purpose of this study is to examine whether arm selection is linked to interlimb performance asymmetries during reaching. Right-handed subjects made choice and nonchoice reaches to each of eight targets (d = 3.5 cm) arranged radially (r = 13 cm) around a midline starting position. We displaced each cursor (one associated with each hand) 30 cm to the midline start circle to ensure that there were no hemispace-related geometric, mechanical, or perceptual biases to use either arm for the two midline targets. The three targets on each side of the midline received mostly reaches from the ipsilateral arm, a tendency previously described as a “hemispace bias.” However, the midline targets, which were equidistant from each hand, received more dominant arm reaches. Dominant arm hand paths to these targets were straighter and more accurately directed. Inverse dynamics analyses revealed a more proficient dominant arm strategy that exploited intersegmental dynamics to a greater extent than did the nondominant arm. These findings suggest that sensorimotor asymmetries in dynamic coordination might explain limb choices. We discuss the implications of these results for theories of action selection, models of handedness, and models of neural lateralization.
Keywords: neural lateralization, handedness, hand selection, motor control
some of the earliest research on human brain lateralization emphasized a left hemisphere dominance for motor functions in most humans. For example, Broca (1865) described a left hemisphere specialization for processes that subserve speech and language, including speech motor control. Liepmann (1905) showed that left hemisphere damage tends to produce greater movement impairment than does right-hemisphere damage, defining apraxia as a key example of such impairment (see Allen 1983 and Geschwind 1975 for reviews). However, Sperry and Gazzaniga's seminal research on split-brain patients provided definitive evidence that each hemisphere can be dominant for different neurobehavioral processes (see Gazzaniga 2000 for review). Although this evidence inspired a departure from Liepmann's view of a comprehensively dominant left hemisphere, this change was only recently incorporated into theories of handedness (Carson 1993; Sainburg 2002). The view that each hemisphere may be specialized for different aspects of motor control has led to the understanding that unilateral hemisphere damage produces unique motor deficits that depend on the side of the lesion (Haaland and Flaherty 1984; Mani et al. in press; Mutha et al. 2011). Perhaps more importantly, hemisphere-specific deficits also occur in the ipsilesional arm of stroke patients, demonstrating that each hemisphere contributes different processes to control of each arm (Schaefer et al. 2007, 2009).
The evidence that hemispheric asymmetries correspond to performance asymmetries in the arms suggests that these asymmetries might give rise to handedness. However, handedness is most often described and measured as a “preference” for performing select motor tasks with the dominant arm. This view of handedness is supported by the fact that most tasks can be accomplished with either limb, regardless of asymmetries in performance measures. In addition, one clearly can alter one's arm preference for performing a task under different environmental constraints. For example, one might reach for a coffee cup with the left nondominant arm if the table supporting the cup is situated on the left side of one's chair. It is therefore easy to understand why handedness has traditionally been characterized as a preference (Bryden 1977; Oldfield 1971), rather than as an asymmetry in performance (Carson 1993; Sainburg 2002). However, it remains unclear whether one's choice to use a particular hand in a given task results from performance asymmetries in motor control.
There has been surprisingly little previous research that has examined both arm selection and arm performance together in the same study. By combining the results of studies on limb selection with results from studies on limb performance, one might conclude that the two factors are, in fact, related. For example, it has been shown repeatedly that right-handed subjects tend to reach the nondominant limb to targets on the left side of the workspace and the dominant limb to targets located on the right side (Bryden et al. 2000; Bryden and Roy 2006; Gabbard and Helbig 2004; Gonzalez and Goodale 2009; Mamolo et al. 2004, 2006; Peters 1995). In addition, studies of interlimb motor performance have shown that reaches to the ipsilateral workspace often show advantages in reaction time, peak velocity, duration, final position accuracy, and movement trajectory relative to contralateral reaches (Carey et al. 1996; Carson et al. 1992, 1993; Chua et al. 1992; van Der Staak 1975; Elliott et al. 1993; Fisk and Goodale 1985; Ingum and Bjorklund 1994; Prablanc et al. 1979). Thus the tendency to avoid reaching across midline with each arm might depend on sensorimotor performance advantages for ipsilateral reaches. It should, however, be noted that the mechanisms that drive these hemispace biases in both preference and performance are controversial. Some researchers have argued that hemispace biases reflect cognitive effects of attention and stimulus-response compatibility (Gabbard and Helbig 2004; Gabbard et al. 1998; Hommel 1993; Verfaellie and Heilman 1990), a suggestion further supported by the finding that the dominant hand receives more attention during bimanual reaches (Buckingham and Carey 2009; Peters 1981). However, others have suggested that hemispace effects result from an intrahemispheric information processing advantage, an argument based on the fact that visual stimuli in each hemispace are initially processed in the hemisphere that controls the ipsilateral limb (Bradshaw et al. 1990; Fisk and Goodale 1985). Thus, although different underlying processes might give rise to the relations between limb selection patterns and limb performance patterns, we hypothesize that interlimb performance asymmetries associated with handedness can predict interlimb selection asymmetries.
The goal of the current study is to directly test this hypothesis. We first assessed which arm people chose to reach toward different targets and then examined whether those choices were associated with performance asymmetries observed in the same participants. We controlled for hemispace biases in the present study by providing right-handed subjects the choice of limb to each of eight targets in a center-out reaching task that was symmetrically centered in the workspace. This design allowed us to control for potential visual-spatial biases by assuring symmetry in midline start positions and in the eight radially arranged targets. There were three targets left of body midline, two targets on the midline, and three targets right of the midline. However, if both hands started in the same central location within such a center-out design, each arm would be an obstacle for contralateral reaches with the other arm. We eliminated this potential confound by presenting a virtual reality display in which cursors representing left and right hand positions were displaced 30 cm to the center of the workspace. Because these cursor displacements allowed each hand to remain in a symmetric configuration in its own motor hemispace, we assured biomechanical symmetry for both the start positions and for the two midline targets.
METHODS
Participants.
Ten right-handed Penn State undergraduate students (7 males) participated in this study for course credit. We used a 13-item version of the Edinburgh Handedness Inventory (EHI; Oldfield 1971) to confirm that all participants were right-handed. The mean number of EHI items that participants endorsed as right-limb tasks was 12.70 (SD 0.48). The mean height of the participants was 176.28 cm (SD 10.73 cm). Their mean weight was 76.61 kg (SD 11.84 kg), and their mean age was 20.50 yr (SD 1.41 yr). All participants signed an informed consent form approved by the Penn State Institutional Review Board.
Apparatus.
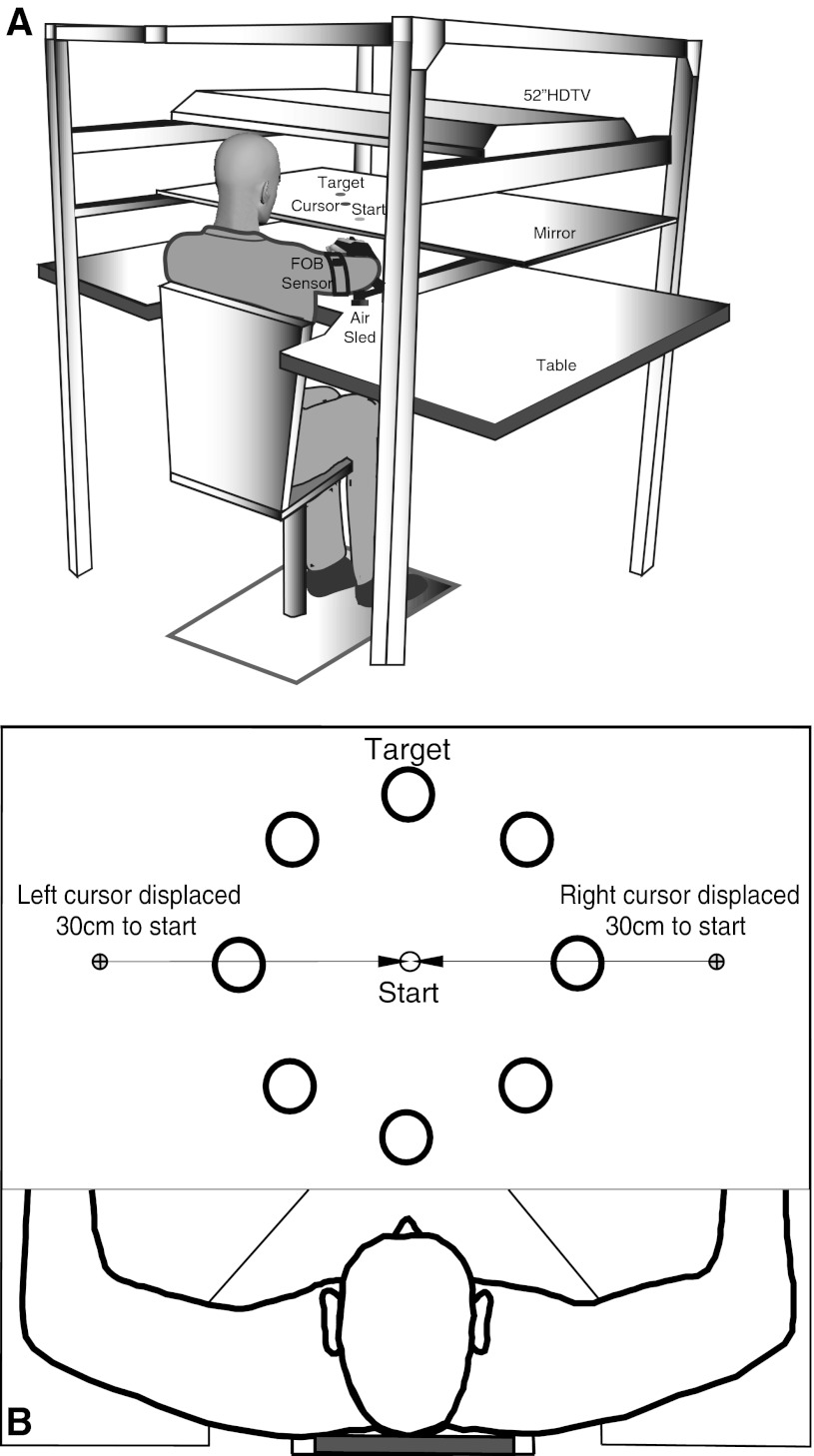
Figure 1A shows the experimental setup. Participants sat in a dentist-type chair and faced a horizontal workspace. An air sled attached to each arm minimized the effects of friction and fatigue as participants moved their arms across the workspace. A splint on each arm immobilized joints distal to the elbow. A mirror, positioned above the workspace, reflected stimuli projected by an overhead 55-in. high-definition television (Sony Electronics). The mirror prevented participants from seeing their arms. The stimuli were displayed with custom software written in REALbasic (REAL Software).
Fig. 1.
Schematics of the experimental apparatus (A) and task (B). The distance between the start circle (diameter d = 2.5 cm) and each target (d = 3.5 cm) was 13 cm. Only 1 target was visible in each trial. There were 8 potential targets that were spaced at 45° intervals. FOB, Flight of Birds sensor.
A six-degree-of-freedom (6-DOF) Flock of Birds (Ascension-Technology) magnetic tracking system sampled limb positions and orientations at a rate of 130 Hz. For motion tracking, we digitized the following bony structures in each limb: 1) index fingertip, 2) metacarpal-phalangeal joint, 3) lateral and medial epicondyle of the humerus, and 4) acromion process. Each arm had two 6-DOF sensors securely affixed to it (see Fig. 1).
Figure 1B shows that a 1.5-cm-diameter (d) circular cursor with crosshairs represented the two-dimensional (2-D) position of the index fingertip of each hand. The software used the 2-D position of the finger to project and update the cursor position at a rate of 60 Hz refresh. Each cursor (d = 1.5 cm) had a medial displacement of 30 cm. The mirror reflected the following stimuli: 1) two cursors, 2) a thermometer-style velocity feedback indicator located at the top of the workspace, 3) a green start circle (d = 2.5 cm) located at the body midline, and 4) a bull's-eye-style target (d = 3.5 cm) located 13 cm away from the start circle. In each trial, the target appeared at one of 8 possible positions. A 45° interval separated each possible target location.
Procedure.
We tested participants in individual sessions. After arriving at the laboratory, each participant signed an informed consent form and filled out demographic and handedness forms. The experimenter then set up the participant in the apparatus. The setup process lasted about 10 min. The experimenter gave the participant instructions and asked whether the participant had any questions. The session began when the experimenter was confident that the participant understood the procedure. The entire experiment took ∼1 h.
Experimental design.
We used a fully within-subjects design. In two choice conditions, participants could reach either hand to the target. In four nonchoice conditions, participants reached a specified hand to the target. In both types of trials, participants were asked to reach the target as quickly and accurately as possible and to minimize their corrective movements. Table 1 describes the progression of all the conditions.
Table 1.
Order of experimental conditions
| Order | Condition/Block | No. of Trials | Limb |
|---|---|---|---|
| 1 | Practice Choice | 32 | Both |
| 2 | Choice | 80 | Both |
| 3 | Nonchoice | 32 | Nondominant |
| 4 | Nonchoice | 32 | Dominant |
| 5 | Nonchoice | 32 | Nondominant |
| 6 | Nonchoice | 32 | Dominant |
The experimental design was within-subjects (n = 10).
Participants first received practice on 32 choice trials (4 choices to each of 8 targets) for familiarization with the dissociation between the visual feedback of the cursors and the proprioceptive feedback of the hands. To this end, we also encouraged participants to choose both the left and the right hands during different practice trials. In the second choice condition, they performed 80 more choice trials. In each of the last four conditions, they made 32 nonchoice reaches (4 reaches to each of 8 targets). We blocked the nonchoice trials on the basis of limb and fixed the order of all conditions; participants first performed 32 nonchoice trials with the nondominant limb and then 32 nonchoice trials with dominant limb. They repeated this sequence once. We randomized the order of target presentation within each condition so that the target location was unpredictable and fixed that order for all participants.
Experimental task.
To provide visual stimuli that were symmetric relative to the hand locations and the body midline, we centered the eight-direction center-out task at the body midline (see Fig. 1B). By displacing the cursor from the hand 30 cm toward the midline, the arms remained geometrically symmetric at the starting position. This design also separated the hands in space so that they could not interfere with each other during the task. In the choice conditions, we asked participants to reach only one cursor to the displayed target in each trial. Each cursor remained visible throughout the trial. In the nonchoice conditions, the setup was the same except that subjects were instructed to reach with either the left or the right hand for a full block of trials.
In the choice conditions, each trial began after participants moved both cursors into the midline start circle and kept them there for 0.7 s. Because the target for that trial appeared immediately after the preceding trial, the target was visible while the participants positioned the cursors in the start circle. After the participants held the cursors in the start circle for 700 ms, they received an audiovisual go cue consisting of a tone and color change of the start circle.
Participants had 1 s to complete each 13-cm-long reach. They completed all reaches well before this 1-s time limitation expired. To help ensure maximum tangential hand velocities were similar across targets and across participants, we instructed participants to move as quickly and accurately as possible. We required them to monitor the velocity feedback indicator, which provided feedback of the maximum velocity following each reach. To motivate participants to be both quick and accurate, we awarded points for accuracy only when the maximum tangential velocity exceeded 0.8 m/s. We showed at the top of the screen a running total of earned points.
Participants always had full visual feedback of the cursors. In addition, participants saw a brief feedback display (2-s interval) of the hand path following each reach. The hand path display then disappeared and participants began the next trial at their own pace. The only difference between the choice trials and the nonchoice trials was that each nonchoice trial began after participants moved only a single cursor into the midline start circle.
Data processing and analysis.
We processed the data with custom programs written in IgorPro 6.0 (WaveMetrics). We low-pass filtered the displacement data at 8 Hz with a third-order dual-pass Butterworth filter before differentiation to obtain velocity and acceleration profiles. Because there were minor oscillations of the cursors in the start circle, we defined the start of each reach as the first minimum in tangential velocity that was under 8% of the maximum velocity for that trial. Likewise, we defined the end of each reach as the first minimum following peak velocity that was below 8% of maximum velocity.
Limb choice analysis.
To assess whether limb choices varied by workspace, we first arcsine-transformed the proportion of right-hand reaches to each target. We did so because the variance of proportions derived from binomial data depends on the values of those proportions. This dependence violates the homogeneity of variance assumptions of traditional inferential statistics. By contrast, the variance of arcsine-transformed proportions is independent of the proportion values (Hogg and Craig 1995). It should be noted that the arcsine transformation yields the inverse sine of the square root of each proportion, which is expressed in radians. Thus some of the transformed values were >1.
We then grouped targets according to their visual workspace location relative to the body midline and pooled the transformed values accordingly (see Fig. 2). Hereafter, the three targets left of the body midline are simply called “left” targets, the two targets at the midline are called “midline” targets, and the three targets right of the midline are called “right” targets.
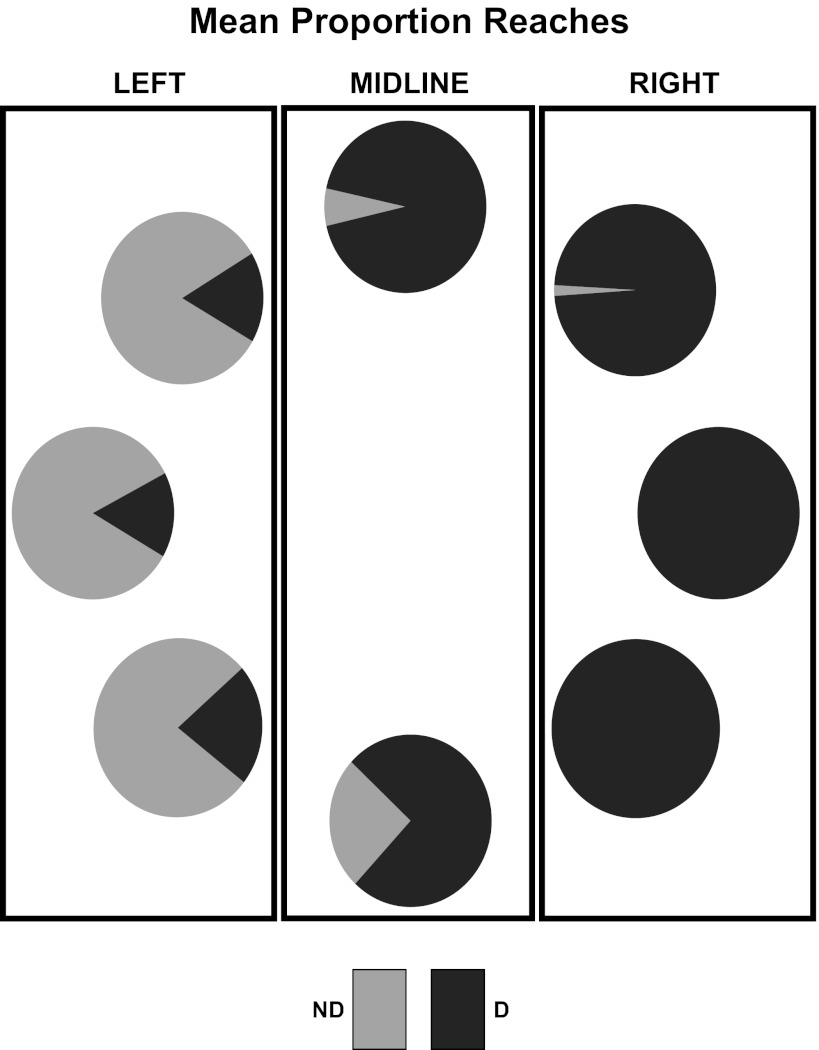
Fig. 2.
Mean proportions of nondominant and dominant reaches to each target. Means were computed across participants (n = 10). Rectangles show how targets were grouped into the left workspace, midline workspace, and right workspace. ND, nondominant; D, dominant.
Movement kinematics analysis.
We report three kinematic measures to characterize performance: hand path deviation from linearity, final position error, and maximum tangential hand velocity (Vmax). Previous work has shown that for reaches with similar maximum velocities, linearity deviation, and final position error systematically vary across the arms (Sainburg 2002; Sainburg and Kalakanis 2000; Wang and Sainburg 2007).
We defined hand path deviation from linearity as the minor axis of the path divided by the major axis of the path. The major axis was the longest distance between any two points on the hand path, whereas the minor axis was the longest distance between any two points in the hand path, measured perpendicular to the major axis. We computed final position error as the Euclidean distance between the cursor center (index of fingertip) at the end of the reach and the target center.
Movement dynamics analysis.
The Appendix details the method we used to quantify joint torques. We modeled each limb as a set of three rigid, planar links with 8 DOF (see Fig. A1, Appendix). We then partitioned the equations of motion into three components: 1) net torque, which equaled the sum of muscle and interaction torque components; 2) interaction torque, which reflected the effects of the motion of the other limb segments; and 3) muscle torque, which estimated the rotational forces from muscle contraction. This segmentation of the equations of motion was inspired by Schneider and Zernicke (1990) and has previously been reported by our group (Sainburg et al. 1999). Here we focus on the torques observed during the time interval between reach onset and Vmax because this interval largely reflects the effects of movement planning processes (Latash 2007; Sainburg et al. 1999).
Statistical analysis.
We analyzed the transformed choice data using a one-way, repeated measures ANOVA with visual workspace (left, midline, and right) as the factor. We performed planned comparisons between nondominant and dominant limb distributions (for choice, kinematic, and dynamic analyses) with paired-samples t-tests and Bonferroni-corrected the significance threshold for multiple comparisons. Bonferroni-corrected alpha was defined as αBon = α/n, where α = 0.05 and n was the number of comparisons (Abdi 2007).
Nonchoice data came from conditions in which participants made an equal number of reaches to each target. We analyzed each dependent measure using a 2 × 2 × 2 repeated-measures ANOVA with the following factors: limb (nondominant vs. dominant), target (90° vs. 270°), and nonchoice condition order (first vs. second).
RESULTS
Limb choice.
Previous research has shown that right-handed subjects tend to reach the nondominant limb to left targets and the dominant limb to right targets (see Peters 1995 for review). We therefore expected our participants to reach the dominant limb to left targets less often than to right targets. Indeed, a repeated-measures ANOVA on the arcsine-transformed values revealed a significant effect of target location on dominant reaches [F(2,18) = 42.935, P < 0.001 (see Fig. 2)]. The size of this effect, estimated by partial eta-squared (ηp2), was 0.827. Post hoc tests confirmed that left targets received fewer dominant reaches (mean 0.319, SE 0.153) than right targets (mean 1.549, SE 0.014) [t(9) = 7.622, P < 0.0001].
Previous work has also shown that the tendency for people to reach each limb to its own hemispace is asymmetric such that people reach the dominant limb to targets in the nondominant (left) space more often than they reach the nondominant limb to targets in the dominant (right) space (Bryden and Roy 2006; Bryden et al. 2000; Gabbard and Helbig 2004; Mamolo et al. 2004, 2006; Peters, 1995). Figure 2 shows that our participants displayed a similar pattern. There was a trend for the rate of nondominant reaches to left targets (mean 1.252, SE 0.153) to be lower than the rate of dominant reaches to right targets, although this trend did not reach statistical significance (mean 1.549, SE 0.014) [t(9) = 2.044, P = 0.071].
The main aim of this study was to determine whether limb performance asymmetries can predict limb choices in the absence of hemispace and proximity effects. Thus we focused on the midline targets because they were equidistant from each hand. Post hoc tests showed that the midline targets (mean 1.239, SE 0.075) received a larger proportion of dominant reaches than left targets [t(9) = 5.74, P < 0.001] but a lower proportion of dominant reaches than right targets [t(9) = 4.186, P = 0.002].
If limb choices depended entirely on hemispace effects on performance and preference, then one would expect equal rates of nondominant and dominant reaches to midline targets. We tested these predictions by assessing whether the midline targets received more dominant reaches than would have been expected by chance alone. A one-sample t-test showed that midline targets received an above-chance number of dominant reaches [t(9) = 6.061, P < 0.001]. We observed this effect at both the 90° target (mean 1.374, SE 0.074) [t(9) = 7.938, P < 0.001] and at the 270° target (mean 1.104, SE 0.095) [t(9) = 3.347, P = 0.009 (αBon = 0.05/3 = 0.017)].
Finally, to ensure that these effects were not due to differences across targets in the same visual workspace, we conducted seven pairwise t-tests: three for the left targets, one for the midline targets, and three for the right targets. These tests confirmed that there were no significant differences in dominant reaches across left [t(9)s < 1.539, Ps > 0.158] or right targets [t(9)s < 1.500, Ps > 0.168]. There was a trend suggesting that the 90° midline target received more dominant reaches (mean 1.374, SE 0.074) than the 270° midline target (mean 1.104, SE 0.095), but this effect did not survive the Bonferroni-corrected significance threshold (αBon = 0.05/7 = 0.007) [t(9) = 3.347, P = 0.009]. It should be noted that despite this trend, both midline targets received an above-chance number of dominant reaches.
Kinematics.
Having confirmed that left targets received mostly nondominant reaches and that right targets received mostly dominant reaches, we asked whether limb performance asymmetries can explain the preponderance of dominant reaches to midline targets. To address this issue, we compared movements made under nonchoice conditions with the left and right arms. The nonchoice conditions were necessary for drawing statistical comparisons because they allowed us to have equal numbers of observations in each condition of interest.
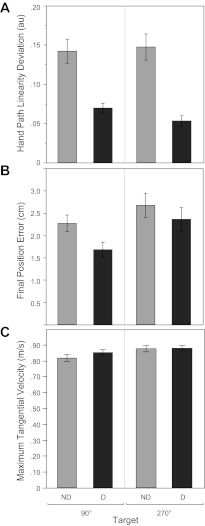
Below we first detail kinematic differences between nondominant and dominant reaches to each of the two midline targets. We then relate these kinematic results to interlimb differences in dynamic coordination. As shown in Fig. 3A, there was a main effect of limb on linearity deviation [F(1,9) = 20.397, P = 0.001, ηp2 = 0.694]; nondominant reaches [mean 0.145 arbitrary units (au), SE 0.022 au] displayed greater deviation than dominant reaches (mean 0.061 au, SE 0.007 au).1 There was also a main effect of order [F(1,9) = 12.035, P = 0.007, ηp2 = 0.572] such that linearity deviation was greater in the first nonchoice condition (mean 0.108 au, SE 0.014 au) than in the second (mean 0.099 au, SE 0.013 au). This order effect was the same for each limb and for each target because there were no interactions (Fs < 2.639, Ps > 0.130), so we collapsed across order and performed planned comparisons. These tests confirmed that nondominant linearity deviation was greater (mean 0.142 au, SE 0.015 au) than dominant linearity deviation (mean 0.070 au, SE 0.006 au) to the 90° target [t(9) = 3.819, P = 0.004]. The same pattern between nondominant linearity deviation (mean 0.147 au, SE 0.017 au) and dominant linearity deviation (mean 0.054 au, SE 0.007 au) was seen at the 270° target [t(9) = 4.380, P = 0.002].
Fig. 3.
Mean hand path deviation from linearity (minor axis divided by major axis; see methods), final position error (absolute distance between the center of the target and the center of the cursor), and maximum tangential hand velocity as a function of nondominant and dominant limbs and midline targets (90° and 270°). Error bars represent ±1SE; au, arbitrary units.
Figure 3B shows a main effect of limb on final position error [F(1,9) = 8.640, P = 0.017, ηp2 = 0.490] such that the nondominant limb (mean 2.50 cm, SE 0.20 cm) showed larger final position errors than the dominant limb (mean 2.00 cm, SE 0.20 cm). This is consistent with previous findings for reaching under visual feedback conditions (Carson et al. 1990). There was a main effect of target [F(1,9) = 5.515, P = 0.043, ηp2 = 0.380]; reaches to the 270° target (mean 2.00 cm, SE 0.20 cm) showed more final position error than reaches to the 90° target (mean 2.5 cm, SE 0.20 cm). There was no main effect of order, and there were no interactions (Fs < 3.278, Ps > 0.100).
Planned comparisons showed no difference between nondominant final position error (mean 2.673, SE 0.273) and dominant final position error (mean 2.359, SE 0.264) at the 270° target [t(9) = 1.378, P = 0.201]. At the 90° target, however, the nondominant limb showed greater final position error (mean 2.269 cm, SE 0.185 cm) than the dominant limb (mean 1.680 cm, SE 0.164 cm) [t(9) = 5.163, P = 0.001].
Figure 3C confirms that none of these interlimb differences could be attributed to differences in maximum velocity. There was only a main effect of target on maximum velocity [F(1,9) = 7.494, P = 0.023]; reaches were slower to the 90° target (mean 0.844 m/s, SE 0.026 m/s) than to the 270° target (mean 0.875 m/s, SE 0.020 m/s). There were no other main effects, and there were no interactions (Fs < 3.519, Ps > 0.050).
Inverse dynamics.
Results presented above established that limb choice varied by workspace and that midline workspace received mostly dominant reaches. They also showed that the dominant limb produced straighter hand paths to midline targets relative to the nondominant limb. By contrast, it was not clear that interlimb differences in final position accuracy could explain reaches to both midline targets.
These results indicate that asymmetries in rates of nondominant and dominant reaches to midline targets might be attributable to limb performance asymmetries. More specifically, the preponderance of dominant midline target reaches might be attributable to a dominant-limb coordination advantage. This notion is consistent with previous work showing that the dominant limb/hemisphere system appears specialized for the coordination of intersegmental dynamics (Sainburg 2002; Sainburg and Kalakanis 2000). Thus we now examine how the observed asymmetries in linearity deviation related to interlimb differences in dynamic control.
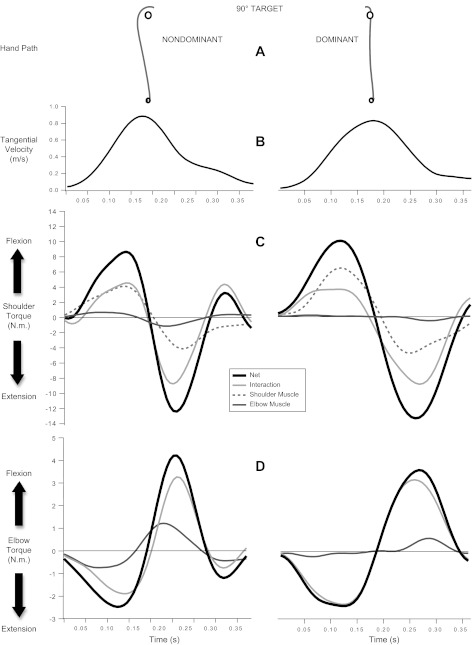
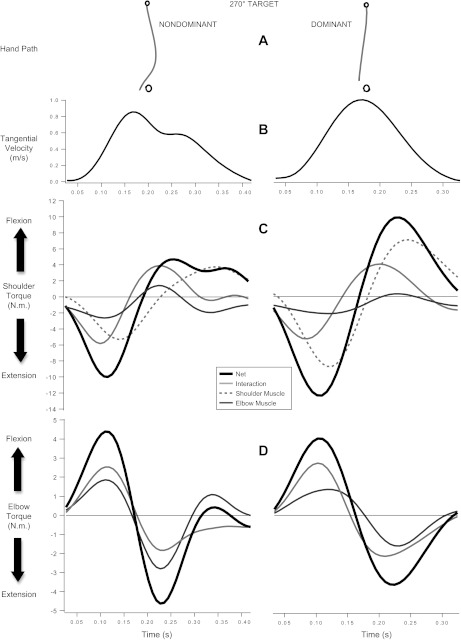
Figure 4, C and D, shows representative shoulder and elbow joint torques, respectively. Two torque components contributed to the shoulder (or elbow) net torque: muscle torque and interaction torque (see methods). The torque profiles in Fig. 4 show that each limb produced similar peak net torque magnitudes at both the elbow and shoulder. For both limbs, these magnitudes were higher at the shoulders than at the elbows (see y-axis scales) because the shoulders carry greater inertial loads. Overall, there were no significant interlimb differences between absolute peak net torques at the shoulders (Fs < 4.504, Ps > 0.050). There was only a statistical trend for a main effect of limb [F(1,9) = 4.503, P = 0.063]. The dominant limb tended to display slightly greater peak net torques (mean 13.229, SE 1.102) compared with the nondominant limb (mean 11.594, SE 1.167). However, this effect did not reach traditional levels of statistical significance. There were also no significant interlimb differences between absolute net torques at the elbows (Fs < 2.508, Ps > 0.147).2
Fig. 4.
Representative hand paths for each limb (A), tangential hand velocity profiles (B), shoulder torque components (C), and elbow torque components (D) toward the 90° target. Example data were taken from the same participant. Torque components include net torque (thick black line), interaction torque (solid light gray line), elbow muscle torque (solid dark gray line), and shoulder muscle torque (dashed gray line). Elbow muscle torque is equal in amplitude but opposite in sign at the elbow and shoulder.
Although initial peak net torques were similar across the limbs, closer inspection of peak muscle and peak interaction torques revealed substantial interlimb differences in dynamic coordination. Figure 4C shows that the nondominant arm generated smaller shoulder peak muscle torques (mean 6.635 N·m, SE 0.634 N·m) than the dominant arm (mean 8.838 N·m, SE 0.850 N·m). Across subjects, this difference was borne out as a main effect of limb [F(1,9) = 35.425, P < 0.001, ηp2 = 0.797]. There were no other main effects and or interactions (Fs < 4.462, Ps > 0.050).
Figure 4, C and D, shows that shoulder flexion in each limb was accompanied by elbow extension as the hand moved toward the target. However, interaction and muscle torque made different contributions to net torque at the shoulder and at the elbow in each limb. At the nondominant shoulder, interaction and muscle torque made similar contributions to net torque. By contrast, interaction torque at the dominant shoulder contributed less to net torque and muscle torque contributed more.
These asymmetries in shoulder torque components were associated with clear differences in elbow torque components. As shown in Fig. 4D, nondominant elbow muscle torque made a substantial contribution to net torque. By contrast, dominant elbow muscle torque made very little contribution to net torque, because interaction torque arising from motion of the upper arm accounted for most of the net torque and remained near zero throughout the trial. As a result, the dominant elbow displayed very little extensor muscle torque while driving the hand toward the target. Indeed, there was a main effect of limb on peak elbow muscle torque [F(1,9) = 10.082, P = 0.011, ηp2 = 0.582] such that nondominant reaches showed higher peak elbow muscle torques (mean 1.618 N·m, SE 0.228 N·m) than dominant reaches (mean 1.002 N·m, SE 0.091 N·m). There was also a main effect of target [F(1,9) = 40.194, P < 0.001, ηp2 = 0.817]; participants generated less elbow muscle torque to reach to the 90° target (mean 1.105 N·m, SE 0.136 N·m) than to the 270° target (mean 1.515 N·m, SE 0.158 N·m). There were no other main effects, and there were no interactions (Fs < 3.580, Ps > 0.050).
These results indicate that the coordination patterns displayed by the nondominant and dominant limbs were different. Relative to the dominant limb, coordination of the nondominant limb was characterized by a strategy that generated smaller peak muscle torques at the shoulder and greater peak muscle torques at the elbow. By contrast, coordination of the dominant limb was characterized by greater peak muscle torques at the shoulder, which produced elbow interaction torques that were efficiently coordinated with small elbow muscle torques.
These results are consistent with our previous findings that the movement of the shoulder joint creates large interaction torques at the elbow that are proficiently used to reduce muscle torque requirements at the elbow (Sainburg and Kalakanis 2000). However, nondominant arm movements in the current study were characterized by greater elbow and lower shoulder muscle torques. This strategy resulted in lower interaction torques from the shoulder acting at the elbow, which resulted in greater elbow muscle torque requirements. Furthermore, increases in muscle torques at the nondominant elbow resulted in greater interaction torques at the shoulder, an effect that required active compensation at the shoulder. That is, muscle torques and interaction torques acted “antagonistically,” suggesting that the nondominant arm uses a less efficient coordination strategy than does the dominant arm.
Figure 5 shows that these coordination patterns also characterized reaches to the 270° target, even though those reaches were made in the opposite direction from the 90° target. Both limbs tended to move the hand medial to the 270° target. However, linearity deviation was again greater for the left limb, which generated greater flexor elbow muscle torque.
Fig. 5.
Representative hand paths for each limb (A), tangential hand velocity profiles (B), shoulder torque components (C), and elbow torque components (D) toward the 270° target. Example data were taken from the same participant; these data and previous example data (Fig. 4) came from different participants. The signs of the torque components are the opposite of those shown in Fig. 4 because movements to the 270° target were made in the opposite direction.
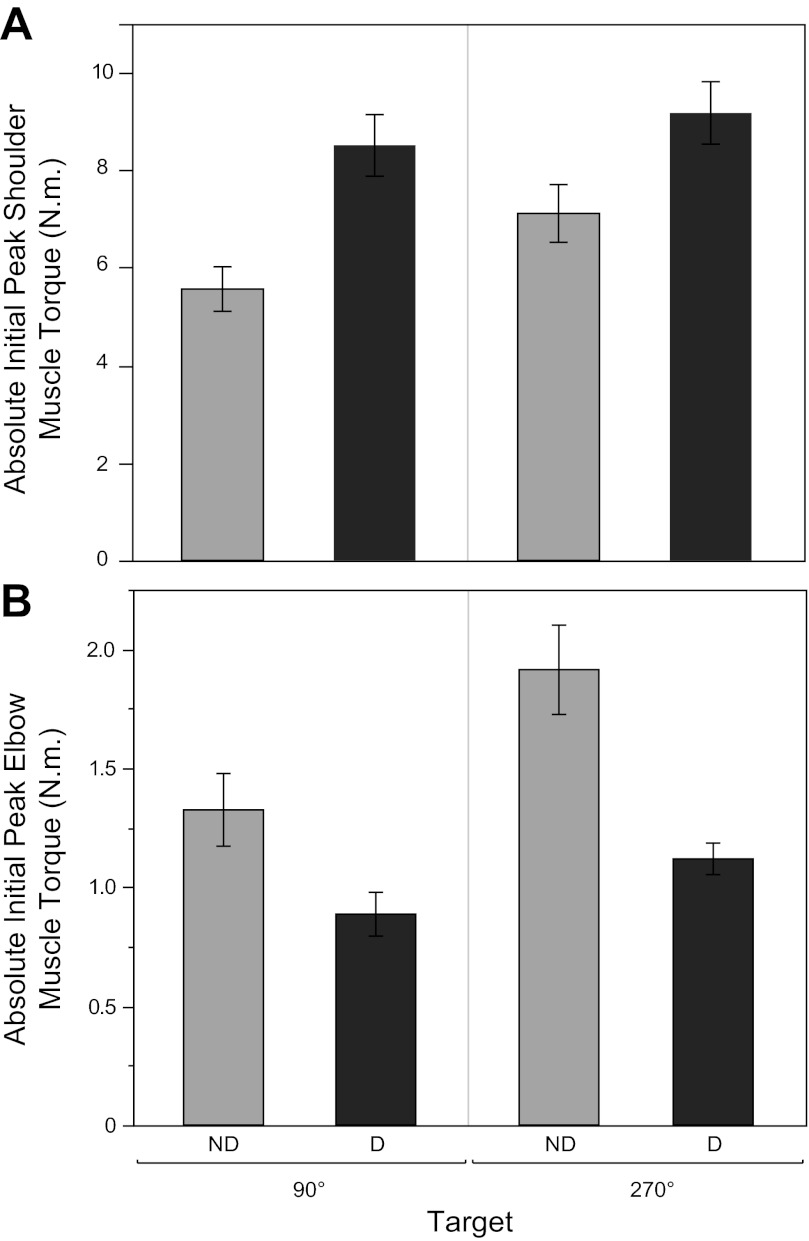
Figure 6 confirms that the patterns of shoulder and elbow muscle torques were relatively consistent across targets and participants. More specifically, Fig. 6A shows that initial peak muscle torques were smaller at the shoulder of the nondominant arm compared with the shoulder of the dominant arm. Figure 6B shows that this pattern was reversed at the elbow, where peak muscle torques were greater for the nondominant arm compared with the dominant arm.
Fig. 6.
Mean absolute initial peak shoulder (A) and elbow (B) muscle torque, averaged across subjects. Data are plotted as a function of nondominant and dominant limbs and midline targets (90° and 270°). Error bars represent ±1SE.
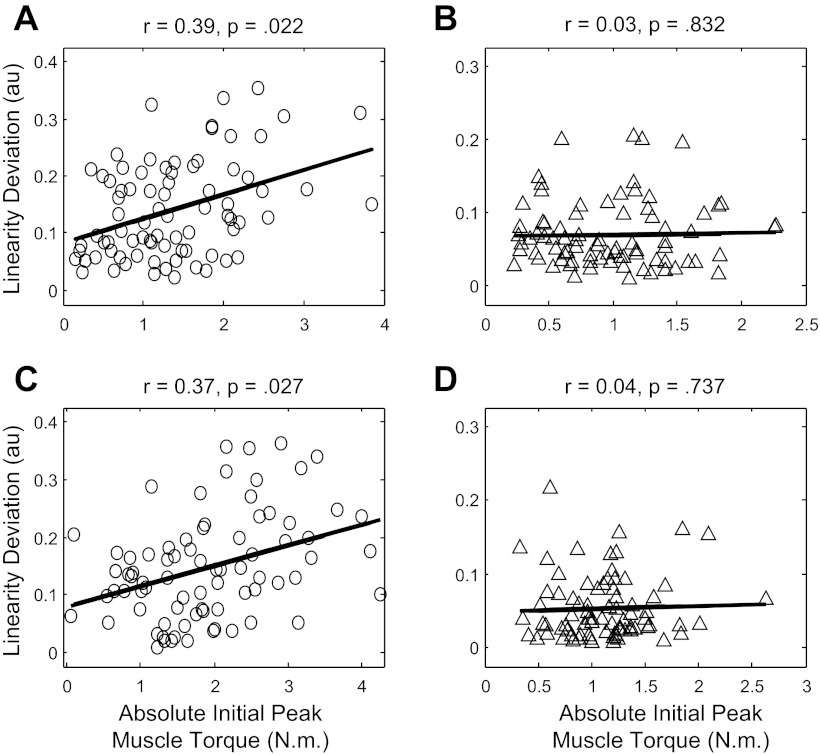
To further support the idea that interlimb differences in dynamic coordination may have led to interlimb differences in linearity deviation, we analyzed the relation, across participants, between initial peak elbow muscle torque magnitude and linearity for nondominant and dominant reaches. If higher peak elbow muscle torques led to increases in linearity deviation, then one would expect them to be positively correlated with linearity deviation for the nondominant limb but not for the dominant limb. Indeed, magnitudes of nondominant peak elbow muscle torques positively correlated with nondominant linearity deviation at the 90° target [r(77) = 0.39, R2 = 0.15, P = 0.022] and at the 270° target [r(77) = 0.37, R2 = 0.14, P = 0.027] (see Fig. 7, A and C). By contrast, dominant peak elbow muscle torques did not correlate with dominant linearity deviation at the 90° target [r(78) = 0.03, R2 < 0.01, P = 0.832] or at the 270° target [r(76) = 0.04, R2 < 0.01, P = 0.737] (see Fig. 7, B and D).
Fig. 7.
Hand path deviation from linearity (minor axis divided by major axis; see methods) as a function of absolute initial peak elbow muscle torque on each of 80 nonchoice trials. A and B: data from nondominant (circles) and dominant (triangles) reaches to the 90° target. C and D: data from nondominant (circles) and dominant (triangles) reaches to the 270° target.
The non-zero regression slopes in Fig. 7, A and C, indicate that nondominant peak elbow muscle torque explained a moderate but significant amount of variance in nondominant linearity deviation at each target. The moderate nature of these relations may be explained by the fact that for each limb, elbow muscle torque contributed less to elbow net torque than did elbow interaction torque. Variations in the shape of the torque profiles also contributed significantly to the relative displacement profiles of the joints, and thus ultimately to hand path linearity. These points notwithstanding, the non-zero slopes for the nondominant limb contrast the near-zero slopes for the dominant limb (see Fig. 7, B and D).
Finally, we analyzed the relation between initial peak shoulder muscle torque magnitude and linearity for dominant and nondominant reaches. If interlimb differences in linearity deviation were based on interlimb differences in dynamic coordination across the elbow and shoulder, then linearity deviations should also be more strongly negatively correlated with peak shoulder muscle torques in the dominant arm. We found some support for this prediction, because dominant peak shoulder muscle torques negatively correlated with dominant arm linearity deviation at both the 90° target [r(77) = −0.23, R2 = 0.05, P = 0.011] and at the 270° target [r(77) = −0.24, R2 = 0.06, P = 0.042]. By contrast, nondominant peak shoulder muscle torques only correlated with nondominant linearity deviation at the 270° target [r(76) = −0.30, R2 = 0.09, P = 0.010] and not at the 90° target [r(78) = 0.09, R2 < 0.01, P = 0.477].
DISCUSSION
Early research on brain lateralization proposed that the left hemisphere plays a special role in motor processes (Broca 1865; Geschwind 1975; Liepmann 1905). Later work focusing mainly on higher cognitive and perceptual asymmetries established that both hemispheres are specialized for different functions (Corballis 1998; Gazzaniga 2000; Sperry 1982). Recent work from our laboratory has extended these ideas to the motor system. We have proposed that the two hemisphere/limb systems are specialized for distinct, but complementary, motor control processes: the dominant system for coordination of limb and task dynamics and the nondominant system for stabilizing position through impedance control mechanisms (Bagesteiro and Sainburg 2002, 2003; Przybyla et al. 2012; Sainburg 2002; Sainburg and Kalakanis 2000; Wang and Sainburg 2003, 2007). The idea of distinct and complementary control systems was previously proposed by Guiard (1987), who described a similar division of labor between the arms during bimanual actions. Our research emphasizes the complementary division of labor between the two hemispheres during unimanual actions (Schaefer et al. 2007). This characterization of handedness provides a unique opportunity to study the link between functional lateralization and the decisions that people make about how to move. In fact, handedness has traditionally been characterized by the arm choices that people make when they approach a given task (Bryden 1977; Oldfield 1971). We now ask whether and how limb choice is related to, and may therefore depend on, sensorimotor asymmetries in dynamic coordination.
To address these questions, we had right-handed participants reach either limb to a single target in an eight-direction center-out task. We centered the task in the workspace so there were three targets left of body midline, three targets right of midline, and two targets on the midline. We displaced both cursors to a midline start circle so that each limb remained in its own hemispace (see Fig. 1). This design assured geometric symmetry between the arms for the midline targets and eliminated potential mechanical conflicts between the limbs.
Our main question was whether asymmetries in limb choice for symmetric targets correspond to asymmetries in performance. To address this question, we assessed whether the two midline targets would receive more dominant reaches than would be expected by chance. These targets were equidistant from the actual start locations of each hand and were neither in the left nor right hemispaces. Therefore, if limb selection were dictated solely by a hemispace bias, one would expect an equal distribution of dominant and nondominant reaches. However, data from the choice conditions showed that the midline targets received more dominant than nondominant reaches (see Fig. 2). Data from our nonchoice conditions clarified the relations between this pattern of limb choice and performance asymmetries. In these conditions, the dominant hand paths were substantially straighter (see Fig. 3A); they reflected more proficient utilization of limb dynamics (shoulder interaction torques) compared with the nondominant arm (Bagesteiro and Sainburg 2002, 2003; Sainburg 2002; Sainburg and Kalakanis 2000). Although both arms had similar initial peak elbow and shoulder net torques, the dominant arm strategy was characterized by higher shoulder muscle torque that drove motion at both the shoulder and elbow joints. Critically, nondominant initial peak elbow muscle torques positively correlated with linearity deviations, whereas no such correlations occurred for the dominant arm (see Fig. 7). Together, these findings suggest that in the absence of hemispace effects, sensorimotor asymmetries in dynamic coordination may contribute to limb choice.
As predicted by hemispace biases, left targets received mostly nondominant reaches and right targets received mostly dominant reaches (see Fig. 2). Our data confirmed a trend for the dominant (right) arm to reach to left targets more than the nondominant (left) limb reached to right targets. Previous research has reported that the distribution of reaches is asymmetric across the workspace such that right-handed subjects prefer dominant reaches to targets located in the middle of the workspace and to targets just left of the body midline (Bryden and Roy 2006; Bryden et al. 2000; Gabbard and Helbig 2004; Mamolo et al. 2004; Peters 1995). To the best of our knowledge, however, the current study is the first to show that the dominant limb reaches into contralateral space more than the nondominant arm even when only the visual representation of the hand (i.e., the cursor) moves across the body midline while the hand does not. This finding suggests a strong influence of vision on the dominant-arm preference.
Consistent with this idea, Przybyla et al. (in press) reported that people choose significantly fewer contralateral reaches with the dominant limb when visual feedback is not available. Moreover, this decrease appears to be driven by a corresponding change in sensorimotor performance that is consistent with the dynamic dominance hypothesis. This hypothesis states that dominant reaches rely largely on predictive mechanisms that specify mechanically efficient movement patterns. Such predictive control has been shown to depend on visual feedback mechanisms to update predictive mechanisms on a trial-to-trial basis (Sainburg 2002; Yadav and Sainburg 2011). Thus, if removing vision decreases the relative proficiency of dominant reaches, and if limb choice depends on sensorimotor asymmetries, then the dominant limb should reach less. The link between limb choice and the dynamic dominance model that has emerged in this study and in the study of Przybyla et al. has implications for theories of action selection, for our understanding of handedness, and possibly for models of neural lateralization. We address these issues next.
A popular approach to the problem of action selection has been to identify linkages within the neuromuscular system and then to model behavior as optimizations performed under those constraints (Bernstein 1967; Todorov 2004). With the use of this approach, a number of factors have been suggested to govern action selection (see Rosenbaum et al. 1990 for reviews). Although some 90% of all humans prefer the right limb for most unimanual actions (Annett 1972; Corballis 1997, 2003), researchers have been reluctant to characterize handedness as an important constraint on action selection. This reluctance has likely been due to the fact that it has proven difficult to establish whether sensorimotor performance asymmetries underlie handedness (see Carson 1993 and Sainburg 2002 for discussions). Indeed, it would be difficult to argue that choosing the dominant arm is optimal without knowing if there is a performance advantage for the dominant arm in that particular instance. A related problem is the absence of studies that have examined limb choice and sensorimotor asymmetries together in the same experiments.
The current study addresses these issues by assessing both limb choice and limb performance in the same participants and by linking limb choice to a reliable sensorimotor asymmetry in dynamic coordination. However, we do not suggest that asymmetries in dynamic coordination fully explain limb choice. By contrast, we acknowledge that limb choice likely depends upon multiple factors. In the current study, for example, it is clear that limb choice to targets that were visually located to the left and right strongly depended on hemispace biases, but where hemispace biases could not predict limb choice we found evidence for a link between limb choice and dynamic coordination differences. This case was one in which the mechanical requirements for the two possible actions, a dominant or nondominant reach, were symmetric and equivalent. Thus the current findings suggest that the hemispace bias may act as the most useful constraint on limb choice to lateralized targets, but also because dynamic coordination asymmetries may facilitate action selection in the face of geometric and mechanical symmetry. This perspective is consistent with the idea that action selection in general likely depends on a hierarchy of multiple constraints whose relative priority may depend on task demands (Rosenbaum et al. 2001).
In conclusion, our current results establish a clear relation between sensorimotor asymmetries in dynamic coordination and the decisions people make about how to move. However, we do not argue on the basis of this association that differences in dynamic coordination caused particular patterns of arm choices. Our aim in this study is simply to demonstrate a link between arm selection and reliable interlimb performance asymmetries. We can speculate, however, that although the interlimb differences in energetics we observed may be small in the current task, the choices we observed could reflect a well-established strategy based on dynamics. Moreover, that difference could be substantial when stressed by either the need for continuous action (fatigue) or strenuous action (approximating maximal output). The choices we observed in this submaximal task may reflect such rules that may have been developed to deal with more taxing conditions under the stress of evolutionary adaptation processes. We suggest that limb selection may in fact be driven by a lifetime (ontogenetic) and by many generations (phylogenetic) of movement choices that involve more energetically costly movements, such as those required during tool making, tool use, hunting, and fighting. Indeed, we have recently verified that interlimb differences in dynamic coordination persist during more stressful vertical reaching movements performed in a gravitational field (Tomlinson and Sainburg 2012). It will be important for future work to test whether hand choice corresponds to interlimb coordination asymmetries in this and other energetically demanding tasks.
This link between arm selection and arm performance may also have a more general implication for models of neural lateralization. It has been suggested that lateralization of neural function reflects an adaptive “no-cost extension” whereby more cortical tissue can be devoted to expansion of function while restricting control to local circuits, a factor that is beneficial in terms of both speed and energetics of neural processing (Corballis 2009; Gazzaniga 2000; Tommasi 2009). Consistent with this view, Sainburg and Eckhardt (2005) have argued that handedness first reflected adaptation at the individual level that resulted from the demand for skilled behavior and later became conserved genetically through the process of natural selection. Our current findings emphasize the link between hemispheric specializations in dynamic coordination and action selection, thereby supporting this skill-based evolutionary perspective. Moreover, Gazzaniga (2000) has pointed out that specialization of a given hemisphere for a particular function implies that the other hemisphere is functionally limited for that function. Thus a primary benefit of lateralization is that new abilities can emerge over the course of evolution through the expansion of intrahemispheric circuitry, while evolutionarily older abilities are sustained. It follows, then, that lateralization should also underlie the ability to make adaptive behavioral decisions that are based on lateralized skills. Future work can test this idea further by asking whether people use the nondominant limb more in tasks that match that arm's specialization for position stabilization via impedance-based control mechanisms (e.g., Wang and Sainburg 2007).
GRANTS
This research was supported by National Institute of Child Health and Human Development Grants R01 HD39311 and R01 HD059783.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.C., A.P., and R.L.S. conception and design of research; C.J.C. performed experiments; C.J.C., A.P., V.Y., and R.L.S. analyzed data; C.J.C., A.P., V.Y., and R.L.S. interpreted results of experiments; C.J.C., V.Y., and R.L.S. prepared figures; C.J.C. and R.L.S. drafted manuscript; C.J.C., A.P., V.Y., and R.L.S. edited and revised manuscript; C.J.C., A.P., V.Y., and R.L.S. approved final version of manuscript.
APPENDIX A
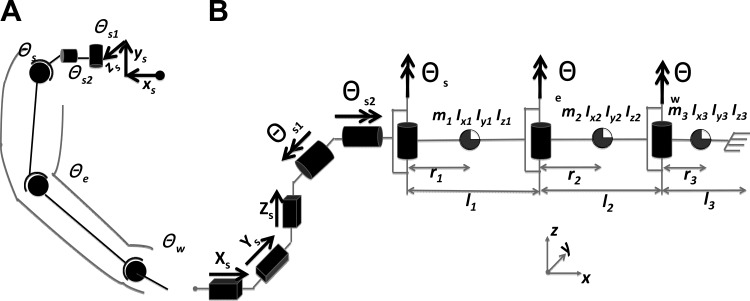
We modeled each limb as a serial three-link manipulator with eight DOFs (see Fig. A1A). The eight DOFs came from three translational joints and five rotational joints. The first three translational (x, y, and z) DOFs at the shoulder described movement of the shoulder that resulted from movement of the trunk. The next three DOFs at the shoulder corresponded to shoulder rotation. The first two DOFs at shoulder defined the orientation of the plane containing the shoulder, elbow, and wrist joints; the third described the orientation of the upper arm in this plane. The last two DOFs were intersegment joint angles at the elbow and wrist. We did not include pronation and wrist flexion/extension in the model because we immobilized the wrist and allowed movement only in the horizontal plane. Therefore, pronation and wrist flexion/extension movements had negligible contributions to the dynamic effects at the elbow and shoulder.
Fig. A1.
A: the human arm modeled as a 3-link system with 3 translational degrees of freedom (DOFs) and 5 rotational DOFs. B: the mechanical equivalent of the human arm model in zero configuration.
In Fig. A1B, i = 1, 2, and 3 represent the three links corresponding to upper arm, forearm, and hand, respectively. The terms mi, Ixi, Iyi, and Izi represent masses and moments of inertia of each limb; we computed these variables with the method described by Zatsiorsky (2002). The terms li and ri represent the length and center of mass location for the ith link, respectively.
As shown in Fig. A1A, we described the configuration of the limb model in generalized coordinates, q, as follows:
| (A1) |
We used a screw theory-based approach to compute these joint angles and joint torques from the position data of the Flock of Birds motion sensors. Screw theory describes motion of a rigid body as rotation about an axis and translation along that axis (Ball 1876). When combined with Lie algebra, screw theory provides tools that can be used to derive equations of motions for complex mechanical systems. In turn, these equations can be used to study mechanical properties of the system and to develop control algorithms (Murray et al. 1994). Below, we present the related inverse kinematics and inverse dynamics method. We computed joint angles and derived equations of motion using screw theory as follows.
First, we described a velocity twist. For a purely rotational or a revolute joint, the velocity twist is defined by the orientation of the screw axis and a point on the screw axis by
| (A2) |
where w is the unit vector along the axis of rotation and r is any point on the screw axis, the axis of rotation. For example, the velocity twist corresponding to the elbow joint is given by
| (A3) |
For a purely translational or prismatic joint, the velocity twist is defined as the direction of movement:
| (A4) |
where h is the unit vector along the direction of motion. For example, the velocity twist corresponding to translation degree of freedom along the x-axis is given by
| (A5) |
Therefore, the matrix of twists defining the arm model in Fig. A1B is
| (A6) |
Second, we computed inverse kinematics. To obtain equations for forward kinematics using screw theory, we needed the velocity twists that defined the mechanical system (Eq. A6) and the forward kinematic map in zero configuration,
| (A7) |
where I3×3 is the three-dimensional identity matrix.
We then computed the forward kinematics as
| (A8) |
where we computed matrix exponents as described in Murray et al. (1994). Once we had the forward kinematic mapping between joint angles and marker positions, we computed the inverse kinematics using analytical methods, when possible, or by using Newton-Raphson root-solving methods.
Third, we computed inverse dynamics. We calculated the joint torques that generated movement using the joint angles computed in the previous step. We did so by first deriving equations of motion for the arm model in Fig. A1B. The general form of the equations of motion of the arm model in a zero-gravity condition is
| (A9) |
where M is the mass-inertia matrix of the human arm and C is the matrix corresponding to Coriolis and centrifugal contributions from movement of joints. We therefore computed M and C matrices for our arm model.
Computing equations of motions for complex mechanical systems is straightforward with this screw theory-based approach in conjunction with methods from Lie algebra (Murray et al. 1994). To derive equations of motion using screw theory, we needed the velocity twist that defined the pose of the center of mass of each link. For example, in the model of human arm in Fig. A1B, the twists that defined movement of the center of mass and forward dynamics in zero configuration of the fore arm are given by
| (A10) |
and
| (A11) |
The twists and forward kinematics in zero-configuration for other links were computed in a similar manner. We next used these formulations to compute M and C in Eq. A9.
To compute M, we first computed the mass-inertia matrix. The kinetic energy of the arm model in Fig. A1 is
| (A12) |
where Jb is the body Jacobian of the mechanical system. Note that there are different types of manipulator Jacobian for different applications. We used the body Jacobian as defined by Murray et al. (1994). The mass-inertia matrix was then simply M(q) in Eq. A12.
To calculate C, we computed the matrix corresponding to Coriolis and centrifugal terms from M using Christoffel symbols:
| (A13) |
Fourth, we partitioned joint torques into interaction, muscle, and net torques. We defined muscle torque as the torques injected into the mechanical system at the joints:
| (A14) |
Net torque was defined as the joint torques corresponding to the acceleration of the ith joint:
| (A15) |
where Dj,k = 1 if j = k = i, otherwise Dj,k = 0.
Once we obtained the net and muscle torques, we computed the interaction torques. Noting that τi,net = τi,muscle + τi,interaction, we rewrote Eq. A8 as
| (A16) |
Rearranging terms in Eq. A16, we obtained interaction torques:
| (A17) |
Equations A14, A15, and A17 give muscle, net, and interaction torques, respectively, for the human arm model described in Fig. A1.
Footnotes
Three trials (0.94%) were excluded from the linearity analysis because they yielded values that were more than 3 SD above the mean. Nine trials (2.8%) were excluded from the final position error analysis, and four trials (1.3%) were excluded from the elbow muscle torque analysis for the same reason.
The ANOVAs that yielded these results also included target as a factor. Thus there were no differences in shoulder or elbow net torque across the two midline targets.
REFERENCES
- Abdi H. The Bonferonni and Šidák corrections for multiple comparisons. In: Encyclopedia of Measurement and Statistics, edited by Salkind N. Thousand Oaks, CA: Sage, 2007 [Google Scholar]
- Allen M. Models of hemispheric specialization. Psychol Bull 93: 73–104, 1983 [PubMed] [Google Scholar]
- Annett M. The distribution of manual asymmetry. Br J Psychol 63: 343–358, 1972 [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88: 2408–2421, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol 90: 1503–1513, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball RS. The Theory of Screws: A Study in Dynamics of a Rigid Body. Dublin, Ireland: Hodges and Foster, 1876 [Google Scholar]
- Bernstein N. The Coordination and Regulation of Movements. London: Pergamon, 1967 [Google Scholar]
- Bradshaw JL, Bradshaw JA, Nettleton NC. Abduction, adduction and hand differences in simple serial movements. Neuropsychologia 28: 917–931, 1990 [DOI] [PubMed] [Google Scholar]
- Broca P. Sur le siège de la faculté du langage articulé. Bull Soc Anthropol Paris 6: 493–494, 1865 [Google Scholar]
- Bryden MP. Measuring handedness with questionnaires. Neuropsychologia 15: 617–624, 1977 [DOI] [PubMed] [Google Scholar]
- Bryden PJ, Pryde KM, Roy EA. A performance measure of the degree of hand preference. Brain Cogn 44: 402–414, 2000 [DOI] [PubMed] [Google Scholar]
- Bryden PJ, Roy EA. Preferential reaching across regions of hemispace in adults and children. Dev Psychobiol 48: 121–132, 2006 [DOI] [PubMed] [Google Scholar]
- Buckingham G, Carey DP. Rightward biases during bimanual reaching. Exp Brain Res 194: 197–206, 2009 [DOI] [PubMed] [Google Scholar]
- Carey DP, Hargreaves EL, Goodale MA. Reaching to ipsilateral or contralateral targets: within-hemisphere visuomotor processing cannot explain hemispatial differences in motor control. Exp Brain Res 112: 496–504, 1996 [DOI] [PubMed] [Google Scholar]
- Carson RG. Manual asymmetries: old problems and new directions. Hum Mov Sci 12: 479–506, 1993 [Google Scholar]
- Carson RG, Elliott D, Goodman D, Dickinson J. Manual asymmetries in the reproduction of a 3-dimensional spatial location. Neuropsychologia 28: 99–103, 1990 [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, Chua R, Elliott D. Asymmetries in the regulation of visually guided aiming. J Mot Behav 25: 21–32, 1993 [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, Elliott D. Asymmetries in the discrete and pseudocontinuous regulation of visually guided reaching. Brain Cogn 18: 169–191, 1992 [DOI] [PubMed] [Google Scholar]
- Chua R, Carson RG, Goodman D, Elliott D. Asymmetries in the spatial localization of transformed targets. Brain Cogn 20: 227–235, 1992 [DOI] [PubMed] [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychol Rev 104: 714–727, 1997 [DOI] [PubMed] [Google Scholar]
- Corballis MC. Cerebral asymmetry: motoring on. Trends Cogn Sci 2: 152–157, 1998 [DOI] [PubMed] [Google Scholar]
- Corballis MC. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav Brain Sci 26: 199–208, 2003 [DOI] [PubMed] [Google Scholar]
- Corballis MC. The evolution and genetics of cerebral asymmetry. Phil Trans R Soc Lond B Biol Sci 364: 867–879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D, Roy EA, Goodman D, Chua R, Carson RG, Maraj BK. Asymmetries in the preparation and control of manual aiming movements. Can J Exp Psychol 47: 570–589, 1993 [Google Scholar]
- Fisk JD, Goodale MA. The organization of eye and limb movements during unrestricted reaching to targets in contralateral and ipsilateral visual space. Exp Brain Res 60: 159–178, 1985 [DOI] [PubMed] [Google Scholar]
- Gabbard C, Helbig CR. What drives children's limb selection for reaching in hemispace? Exp Brain Res 156: 325–332, 2004 [DOI] [PubMed] [Google Scholar]
- Gabbard C, Rabb C, Gentry V. Attentional stimuli and programming hand selection: a developmental perspective. Int J Neurosci 96: 205–215, 1998 [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123: 1293–1326, 2000 [DOI] [PubMed] [Google Scholar]
- Geschwind N. The apraxias: neural mechanisms of disorders of learned movement. Am Sci 63:188–195, 1975 [PubMed] [Google Scholar]
- Gonzalez CLR, Goodale MA. Hand preference for precision grasping predicts language lateralization. Neuropsychologia 47: 3182–3189, 2009 [DOI] [PubMed] [Google Scholar]
- Guiard Y. Asymmetric division of labor in human skilled bimanual action: the kinematic chain as a model. J Mot Behav 19: 486–517, 1987 [DOI] [PubMed] [Google Scholar]
- Haaland KY, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn 3: 370–384, 1984 [DOI] [PubMed] [Google Scholar]
- Hogg RV, Craig AT. Introduction to Mathematical Statistics (5th ed.). Englewood Cliffs, NJ: Prentice Hall, 1995 [Google Scholar]
- Hommel B. Inverting the Simon effect by intention: determinants of direction and extent of effects of irrelevant spatial information. Psychol Res 55: 270–279, 1993 [Google Scholar]
- Ingum J, Bjorklund R. Effects of flunitrazepam on responses to lateralized visual stimuli: evidence for cerebral asymmetry of execution of manual movements to targets in contralateral and ipsilateral visual space. Psychopharmacology 114: 551–558, 1994 [DOI] [PubMed] [Google Scholar]
- Latash ML. Neurophysiological Basis of Movement (2nd ed.). Champaign, IL: Human Kinetics, 2007 [Google Scholar]
- Liepmann H. Die linke Hemisphäre und das Handeln. Munch Med Wochenschr 49: 2375–2378, 1905 [Google Scholar]
- Mamolo CM, Roy EA, Bryden PJ, Rohr LE. The effects of skill demands and object position on the distribution of preferred hand reaches. Brain Cogn 55: 349–351, 2004 [DOI] [PubMed] [Google Scholar]
- Mamolo CM, Roy EA, Rohr LE, Bryden PJ. Reaching patterns across working space: the effects of handedness, task demands, and comfort levels. Laterality 11: 465–492, 2006 [DOI] [PubMed] [Google Scholar]
- Mani S, Mutha PK, Przyblya A, Haaland K, Good D, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Li Z, Sastry SS. A Mathematical Introduction to Robotic Manipulation. London: CRC, Taylor and Francis, 1994 [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Left parietal regions are critical for adaptive visuomotor control. J Neurosci 31: 6972–6981, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- Peters M. Attentional asymmetries during concurrent bimanual performance. Q J Exp Psychol 33: 95–103, 1981 [Google Scholar]
- Peters M. Handedness and its relation to other indices of cerebral lateralization. In: Brain Asymmetry , edited by Davidson RJ, Hugdahl K. Cambridge, MA: MIT Press, 1995, p. 183–214 [Google Scholar]
- Prablanc C, Echallier JF, Komilis E, Jeannerod M. Optimal response of eye and hand motor systems in pointing at a visual target 1: spatio-temporal characteristics of eye and hand movements and their relationships when varying the amount of visual information. Biol Cybern 35: 113–124, 1979 [DOI] [PubMed] [Google Scholar]
- Przybyla A, Good D, Sainburg RL. Dynamic dominance varies with handedness: reduced interlimb asymmetries in left-handers. Exp Brain Res 216: 419–431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A, Coelho C, Akpinar S, Kirazci S, Sainburg RL. Sensorimotor performance asymmetries predict hand selection. Neurosci 228C: 349–360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA, Marchak F, Barnes HJ, Vaughan J, Slotta J, Jorgensen M. Constraints for action selection: overhand versus underhand grips. In: Attention and Performance XIII: Motor Representation and Control, edited by Jeannerod M. Hillsdale, NJ: Lawrence Erlbaum Associates, 1990, p. 321–342 [Google Scholar]
- Rosenbaum DA, Meulenbroek RG, Vaughan J, Jansen C. Posture-based motion planning: applications to grasping. Psychol Rev 108: 709–734, 2001 [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Eckhardt RB. Optimization through lateralization: the evolution of handedness. Behav Brain Sci 28: 575–633, 2005. 16209828 [Google Scholar]
- Sainburg RL, Ghez C, Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81: 1045–1056, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83: 2661–2675, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain 130: 2146–2158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia 47: 2953–2966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Zernicke RF. A Fortran package for the planar analysis of limb intersegmental dynamics from spatial coordinate-time data. Adv Eng Software 12: 123–128, 1990 [Google Scholar]
- Sperry R. Some effects of disconnecting the cerebral hemispheres. Science 217: 1223–1226, 1982 [DOI] [PubMed] [Google Scholar]
- Todorov E. Optimality principles in sensorimotor control. Nat Neurosci 7: 907–915, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi L. Mechanisms and functions of brain and behavioral asymmetries. Phil Trans R Soc Lond B Biol Sci 364: 855–859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson T, Sainburg RL. Dynamic dominance persists during unsupported reaching. J Mot Behav 44: 13–25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Staak C. Intra- and interhemispheric visual-motor control of human arm movements. Neuropsychologia 13: 439–448, 1975 [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Heilman KM. Hemispheric asymmetries in attentional control: implications for hand preference in sensorimotor tasks. Brain Cogn 14: 70–80, 1990 [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp Brain Res 178: 565–570, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Motor Lateralization is characterized by a serial hybrid control scheme. Neuroscience 196: 153–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsiorsky V. Kinetics of Human Motion. Champaign, IL: Human Kinetics, 2002 [Google Scholar]