Abstract
Subacute and chronic changes in tonic GABAergic inhibition occur in human and experimental epilepsy. Less is known about how tonic inhibition is modulated over shorter time frames (seconds). We measured endogenous tonic GABA currents from cultured rat hippocampal neurons to evaluate how they are affected by 1) transient increases in extracellular GABA concentration ([GABA]), 2) transient postsynaptic depolarization, and 3) depolarization of presynaptic cells. Transient increases in [GABA] (1 μM) reduced tonic currents; this reduction resulted from GABA-induced shifts in the reversal potential for GABA currents (EGABA). Transient depolarization of postsynaptic neurons reversed the effects of exogenous GABA and potentiated tonic currents. The voltage-dependent potentiation of tonic GABA currents was independent of EGABA shifts and represented postdepolarization potentiation (PDP), an intrinsic GABAA receptor property (Ransom CB, Wu Y, Richerson GB. J Neurosci 30: 7672–7684, 2010). Inhibition of vesicular GABA release with concanamycin A (ConA) did not affect tonic currents. In ConA-treated cells, transient application of 12 mM K+ to depolarize presynaptic neurons and glia produced a persistent increase in tonic current amplitude. The K+-induced increase in tonic current was reversibly inhibited by SKF89976a (40 μM), indicating that this was caused by nonvesicular GABA release from GABA transporter type 1 (GAT1). Nonvesicular GABA release due to GAT1 reversal also occurred in acute hippocampal brain slices. Our results indicate that tonic GABA currents are rapidly regulated by GABA-induced changes in intracellular Cl− concentration, PDP of extrasynaptic GABAA receptors, and nonvesicular GABA release. These mechanisms may influence tonic inhibition during seizures when neurons are robustly depolarized and extracellular GABA and K+ concentrations are elevated.
Keywords: tonic inhibition, γ-aminobutyric acid receptor type A, γ-aminobutyric acid transporter type 1, seizure
high-affinity, extrasynaptic GABAA receptors are tonically activated by ambient GABA concentrations ([GABA]) in many areas of the brain, including cerebellum, thalamus, hippocampus, cortex, and striatum (Ade et al. 2008; Cope et al. 2005; Farrant and Nusser 2005). This tonic form of GABAergic inhibition occurs in vivo (Chadderton et al. 2004) and influences neuronal firing properties and network behavior (Glykys and Mody 2006; Mitchell and Silver 2003; Pavlov et al. 2009), thereby affecting many physiological and pathophysiological processes, including learning and memory, anxiety, and epilepsy (Maguire et al. 2005; Martin et al. 2010; Shen et al. 2007). In epilepsy, tonic inhibition is affected by changes in neurosteroid levels, altered expression/localization of GABAA receptor subunits, and decreased function of GABA transporters (Maguire et al. 2005; Patrylo et al. 2001; Peng et al. 2004; Zhang et al. 2007). The development of these changes occurs over days to weeks. Relatively less is known about how tonic inhibition is affected by ongoing neural activity over shorter time periods (seconds).
Tonic inhibition can be rapidly regulated by factors that influence ambient [GABA], including vesicular release of GABA at synapses (Glykys and Mody 2007b; Tang et al. 2011), both uptake and release by GABA transporters (Scimemi et al. 2005; Wu et al. 2006, 2007), and GABA release via glial anion channels (Lee et al. 2010). Extrasynaptic GABAA receptors that mediate tonic inhibition are also subject to direct voltage-dependent modulation (Pavlov et al. 2009; Ransom et al. 2010). All of these mechanisms may be influenced by ongoing neural activity. The intense neural activity that occurs during seizures is accompanied by elevations of extracellular GABA and depolarization of pre- and postsynaptic neurons (During and Spencer 1993). How these rapid and transient changes in [GABA] and membrane potential affect tonic inhibition is not known.
To investigate the rapid regulation of tonic inhibition, we measured tonic GABA currents in cultured hippocampal neurons and assessed how they are affected by 1) transient increases in extracellular [GABA], 2) transient postsynaptic depolarization, and 3) transient depolarization of presynaptic neurons and glia. Our results indicate that tonic inhibition can be rapidly regulated by multiple mechanisms, including changes in intracellular anion concentration, voltage-dependent modulation of GABAA receptor function, and nonvesicular GABA release mediated by GABA transporter type 1 (GAT1). These mechanisms may influence the effects of tonic inhibition during seizures.
MATERIALS AND METHODS
Cell culture.
Primary hippocampal cell cultures were prepared as previously described (Gaspary et al. 1998). In brief, 0- to 2-day-old Sprague-Dawley rat pups of both sexes were decapitated and the hippocampi dissected. The tissue was minced in sterile-filtered, HEPES-buffered solution and then treated with a digestion solution containing papain (10 U/ml), 0.5 mM EDTA, and cysteine (0.2 mg/ml) for 15 min. The enzyme-treated tissue was triturated in complete minimum essential medium (MEM), trypsin inhibitor (1.5 mg/ml), and bovine serum albumin (1.5 mg/ml). Triturated cells were added to culture medium (MEM-10% FBS) and plated directly on poly-l-ornithine-coated round glass coverslips (Erie Scientific, Portsmouth, NH) in 12-well culture dishes at a density of 2.5–5 × 105 cells/ml. After 1 h, medium was changed to 70% MEM-30% Neurobasal with B27 supplement (GIBCO, Carlsbad, CA). Cells were maintained in an incubator (model 3110; Forma Scientific, Marietta, OH) with a humidified environment containing 5% CO2 in room air at 37°C. Medium was changed on day 5–6 to Neurobasal medium with B27 supplement and 1 μM cytosine arabinoside (Ara-C), followed by half medium changes (without Ara-C) every 7 days. All animal use protocols were approved by the local Institutional Animal Care and Use Committee (VA Puget Sound).
Acute brain slices.
Hippocampal brain slices were prepared from 4-to 6-wk-old Sprague-Dawley rats of both sexes. Rats were anesthetized with 4% isoflurane, decapitated, and the brain dissected free. Transverse slices were made of hippocampus with a thickness of 300 μm using a vibratome (Leica VT1200). Slices were cut and stored in a solution containing (in mM) 125 NaCl, 3 KCl, 26 NaHCO3, 1.2 NaH2PO4, 0.5 CaCl2, 4 MgCl2, 20 dextrose, and 1 kynurenic acid. Solutions were continuously gassed with 95% O2-5% CO2. Slices were allowed to recover at room temperature for 1 h before recording began.
Electrophysiology.
Conventional whole cell patch-clamp techniques were used to record membrane currents from neurons aged 14–60 days in vitro. Cultured cells were visualized with an Axiovert 200 inverted microscope with differential interference contrast (DIC) optics (Carl Zeiss, Thornwood, NY). Dentate gyrus granule cells in brain slices were visualized with an Axioskop 2 upright microscope with a fixed stage using infrared DIC optics (Carl Zeiss). Recordings were made using a Multiclamp 700B or Axopatch 200B amplifier, a Digidata 1200 series analog-to-digital converter, and pClamp 10 software (Molecular Devices, Redwood City, CA). Data were acquired at 2–5 kHz and low-pass filtered at 1 kHz. Series resistance and whole cell capacitance were determined by compensating the current transients produced by a −10-mV voltage step and compensated 50–70% online; if series resistance exceeded 20 MΩ or changed by more than 25%, the experiment was discarded. Drug applications were made via a microperfusion device (SF-77B; Warner Instruments, Hamden, CT) connected to manifolds that allowed up to five test solutions to be applied in a single experiment. Flow through the manifolds was controlled with electronic solenoid valves (Parker Hannifin, General Valve Division, Cleveland, OH), and step application of test solutions was controlled using pClamp software. The microperfusion device produced 90% solution exchange times of 50–55 ms across open pipette tips. The recording chamber (RC-26; Warner Instruments) had a volume of ∼0.3 ml and was continuously superfused at a rate of ∼1–2 ml/min with bath solution that contained (in mM) 134 NaCl, 3 KCl, 1.4 NaH2PO4, 24 NaHCO3, 10 dextrose, 2 MgCl2, 2 CaCl2, 1 kynurenic acid, and 0.001 CGP-55845. The pH was 7.35–7.4 when bubbled with 95% O2-5% CO2. Osmolarity was adjusted to 300–305 mosM with H2O. In acute brain slices, local application of elevated K+ solution was made by pressure ejection from a patch pipette. A Picospritzer II was used for pressure ejection (General Valve). The baseline picospritzer solution contained (in mM) 150 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 10 dextrose, and 10 HEPES with pH adjusted to 7.4 with NaOH. For experiments with high K+ (12–48 mM), KCl was substituted for an equimolar amount of NaCl.
Patch electrodes were made with borosilicate glass without filament (593400; A-M Systems, Carlsborg, WA) using a micropipette puller (P-97; Sutter Instruments, Novato, CA). Pipettes had resistances of 2.5–3.5 MΩ when filled with an intracellular solution containing (in mM) 125 CsCl, 10 QX-314 chloride salt, 10 HEPES, and 1 EGTA with pH corrected to 7.25 with CsOH. Osmolarity was adjusted to 275–285 mosM with H2O as needed. All chemicals were purchased from Sigma except SKF89976a, SNAP-5114 (Tocris-Cookson, Ellisville, MO), and QX-314 (Alomone Labs, Tel Aviv, Israel). Data acquisition was begun 3–5 min after a whole cell recording was established. Experiments were performed at room temperature, typically 23°C.
Analysis.
Data analysis was performed with Clampfit (pClamp 10; Molecular Devices, Mountain View, CA) and Origin software (version 6.1; Microcal Software, Northhampton, MA). Tonic current amplitudes were measured as the difference in mean holding current in the absence and presence of the GABAA receptor antagonist bicuculline methiodide (10–20 μM). The mean holding current values were obtained from Gaussian fits to all-point current amplitude histograms (see Fig. 4A). Histograms were constructed from 5–10 s of data with a bin width of 1 pA. Gaussian fits were performed using a Levenberg-Marquardt curve-fitting algorithm provided in Clampfit software; fits were constrained to exclude the portion of the histogram skewed by synaptic currents beyond 20 pA from the peak (Glykys and Mody 2006). The slope conductance was determined by fitting current-voltage (I-V) plots with a linear equation. Tonic conductance, g, was calculated from the following equation:
where I is tonic current amplitude, Vm is membrane potential, and EGABA is the reversal potential for GABA currents (a composite of the equilibrium potential for permeant anions including Cl− and HCO3−). The measured reversal potential of currents evoked with exogenous GABA was used as EGABA for calculations of chord conductance in Fig. 3B. We used the chord conductance equation above to calculate the theoretical shifts in EGABA required to cause the tonic current changes observed in response to exogenous GABA alone or GABA with depolarization (i.e., data in Fig. 1), assuming no change in conductance. The theoretical Nernst potential for Cl− was 0 mV under our experimental conditions, and this value was used as the baseline value of EGABA for calculations of theoretical EGABA shifts presented in Fig. 3C. Charge transfer, Q, was determined by integrating baseline-subtracted currents over the period of high [K+] application.
Fig. 4.
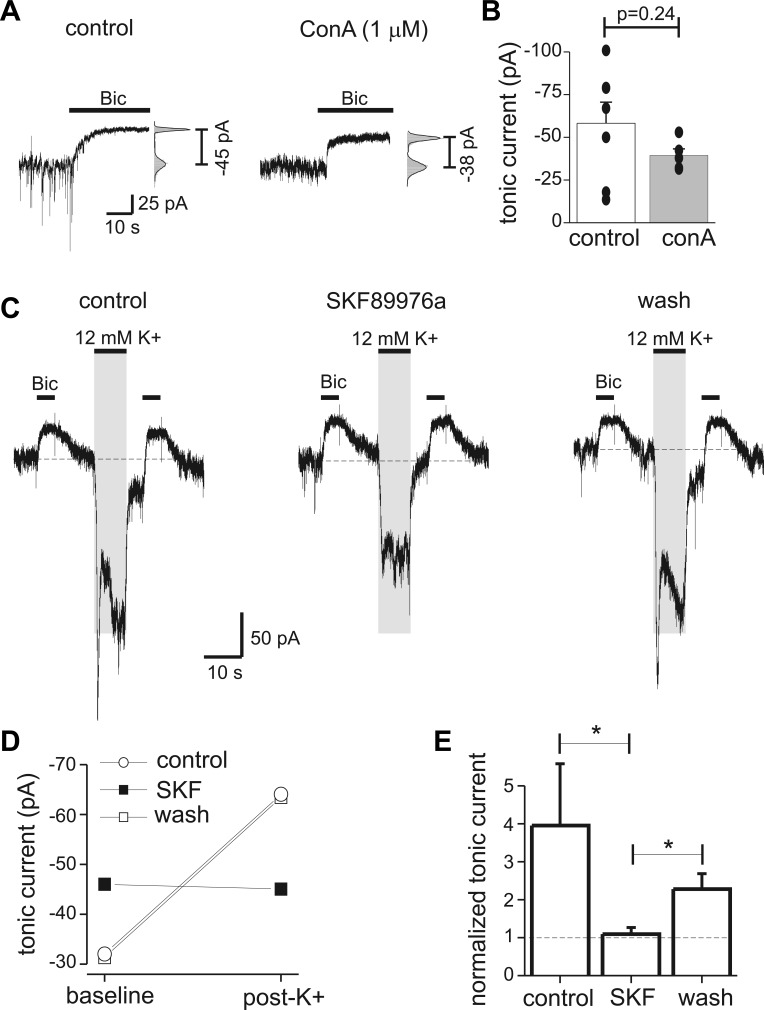
Nonvesicular GABA release potentiated tonic currents. A: endogenous tonic currents measured with Bic in a control neuron and a neuron pretreated with concanamycin A (ConA; 1 μM for 2 h). At right, an all-points histogram shows fit with a Gaussian function used to determine tonic current amplitude. B: mean tonic current from control neurons and neurons treated with ConA. Filled circles represent individual measurements (n = 5–7 cells for each condition). ConA treatment greatly reduced spontaneous synaptic currents but did not significantly affect tonic currents. C: measurement of tonic currents before and after depolarization of presynaptic neurons with 12 mM K+. Tonic currents measured 5 s after the K+ application were increased, and this potentiation was reversibly inhibited by the GABA transporter type 1 (GAT1) antagonist SKF89976a (SKF; 40 μM). Shaded area indicates timing of K+ application; solution exchange times were fast, so it is expected that the wash of high-K+ solution was complete before tonic current measurement. Dashed horizontal lines indicate baseline holding current for reference. Vesicular GABA release was inhibited by pretreatment with ConA; holding potential was −60 mV. D: tonic current amplitudes before (pre-K) and after (post-K) application of 12 mM K+ from experiment in C. GAT1 antagonism increased baseline tonic current amplitude but also prevented the increase in tonic currents by K+-induced nonvesicular release. E: mean values for normalized tonic current under control conditions, with SKF, and after wash (n = 4). *P < 0.05.
Fig. 3.
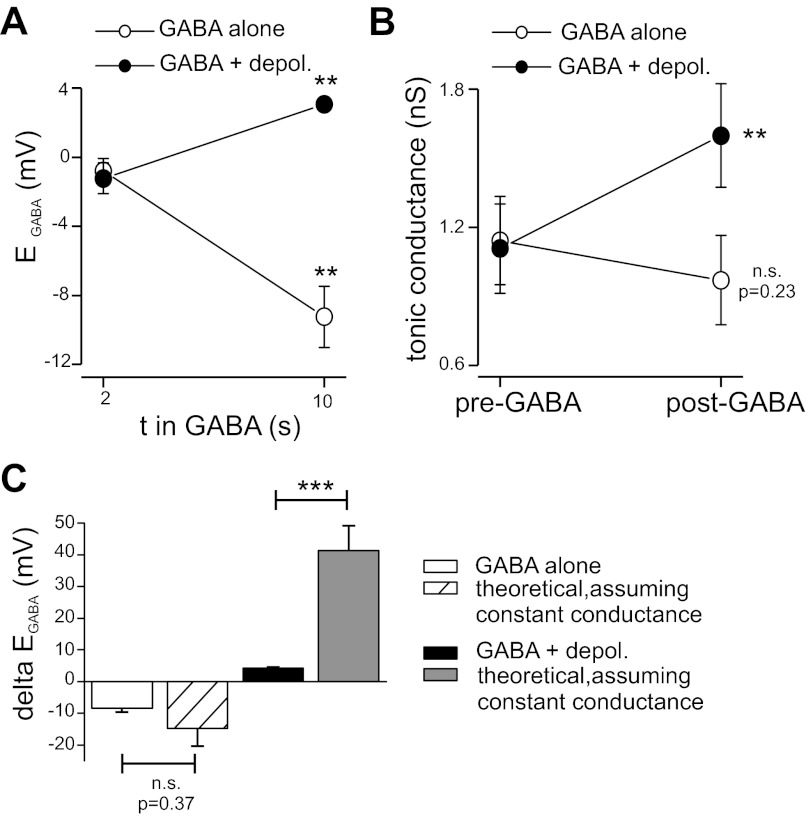
Tonic conductance was not affected by GABA alone but was increased by GABA plus depolarization. A: average GABA- and depolarization-induced changes in EGABA determined from 8 neurons. As in Fig. 2, EGABA was measured after 2 and 10 s of GABA application. Depol., depolarization. B: tonic conductance before and after GABA application. Tonic conductance was calculated using measurements of tonic current from experiments as in Fig. 1 and mean values for EGABA (see materials and methods). GABA alone did not significantly affect tonic conductance. GABA plus depolarization produced an increase in tonic conductance. C: comparison of observed shifts in EGABA (delta EGABA) with theoretical shifts that would be required to produce the observed tonic current changes, assuming no change in tonic conductance (i.e., assuming tonic current change was solely due to driving force alterations from shifts in EGABA). With GABA application alone, there was no difference between the observed shift in EGABA and the theoretical value, indicating that tonic current reduction by GABA was caused by reduced driving force. With GABA plus depolarization, the theoretical EGABA shift was significantly larger than experimental observation, indicating that depolarization increased tonic conductance independently of driving force changes. n.s., Not significant. **P < 0.01; ***P < 0.001.
Fig. 1.
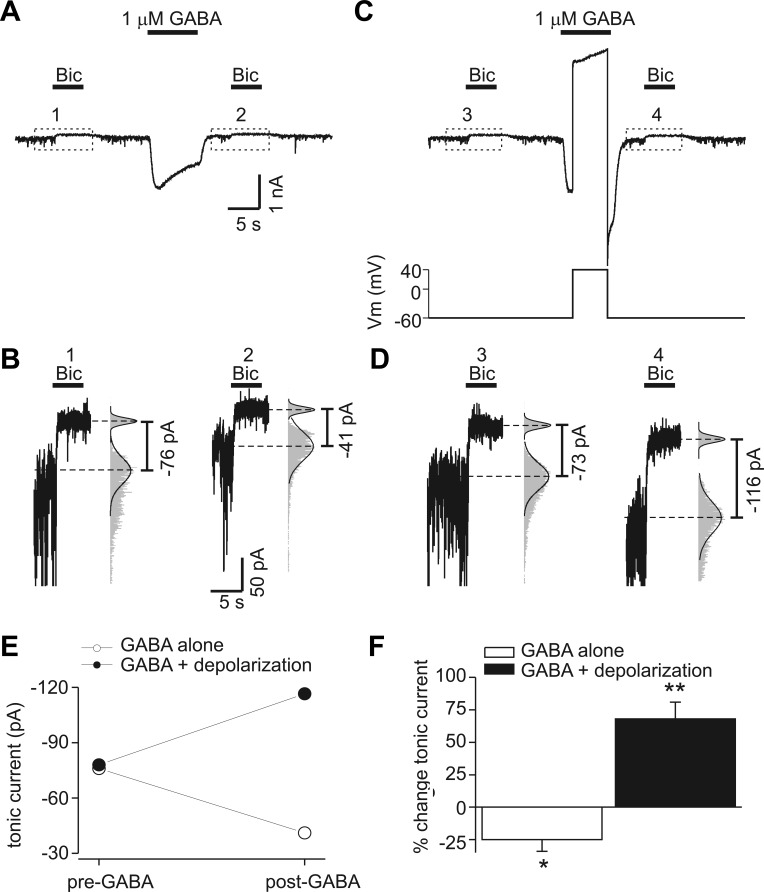
Endogenous tonic GABA currents are decreased by transient increases in GABA concentration ([GABA]) and increased by transient depolarization. A: membrane current recorded at −60 mV. Tonic currents were measured with bicuculline application (Bic; 10 μM) before and 5 s after exposure to exogenous GABA (1 μM). Horizontal bars indicate period of drug application in this and subsequent figures. B: data enclosed in dashed boxes in A displayed on an expanded scale. The numbers correspond to labeled time points in A. The histograms (gray bars) and Gaussian fits (solid line) used to determine the mean holding current before and after bicuculline are illustrated; histogram amplitudes were normalized to allow comparison of the distributions. Exogenous GABA reduced tonic current amplitude. C: tonic current measurement before and after exogenous GABA application paired with transient depolarization to +40 mV (same neuron as in A). Bottom trace shows voltage-clamp protocol (Vm, membrane potential). D: data enclosed in dashed boxes in C displayed on an expanded scale. The numbers correspond to time points in C. Transient depolarization potentiated tonic current in contrast to the reduction seen with GABA alone. E: tonic current amplitudes from experiment in A–D at baseline (pre-GABA) and after GABA application (post-GABA). F: %change in tonic current produced by exogenous GABA alone or GABA plus depolarization. Data are means from 11 neurons; error bars represent SE. *P < 0.05; **P < 0.01.
Data values are means ± SE, and all error bars represent SE. Statistical analyses were performed using Microsoft Excel (Bellevue, WA). A two-tailed paired or unpaired Student's t-test was used with a P value of 0.05 considered as significant. Data in Fig. 3 were analyzed with a nonparametric test (Wilcoxon signed rank test). Where appropriate, the actual P values are reported.
RESULTS
Transient changes in extracellular GABA and postsynaptic depolarization modulate tonic GABA currents.
To evaluate the effects of transient increases in extracellular GABA on tonic inhibition, tonic currents due to endogenous GABA were measured before and after application of exogenous GABA (1 μM, 10 s). Neurons were held at −60 mV. Transient application of exogenous GABA reduced subsequently measured endogenous tonic currents (measured 5 s after wash of GABA) (Fig. 1, A and B). We next examined the effects of transient depolarization during GABA application. In contrast to effects of exogenous GABA alone, tonic currents were increased when neurons were transiently depolarized during exogenous GABA application (Fig. 1, C and D). For the cell illustrated in Fig. 1, A–D, exogenous GABA alone reduced tonic current from −76 to −41 pA, whereas exogenous GABA paired with depolarization increased tonic current from −73 to −116 pA (Fig. 1E). On average, exogenous GABA alone reduced tonic currents by 25 ± 9% (n = 11 cells, P < 0.05), and exogenous GABA paired with transient depolarization potentiated tonic currents by 68 ± 13% (n = 11, P < 0.01) (Fig. 1F). These results indicate that transient increases in [GABA] with or without transient depolarization can rapidly (within seconds) cause a persistent modulation of endogenous tonic GABA currents.
Determination of GABA induced shifts in EGABA.
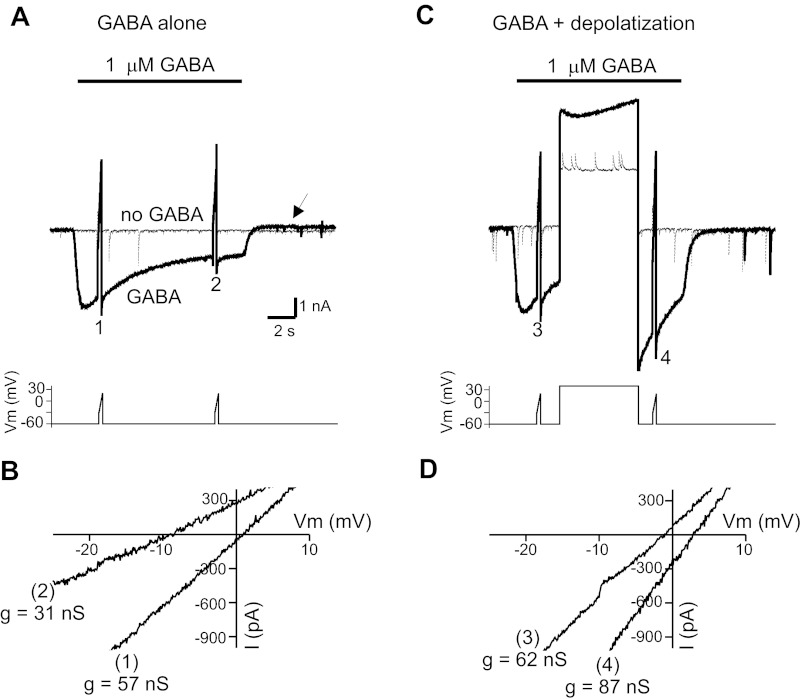
The changes in tonic current amplitudes produced by exogenous GABA and depolarization could be due to altered intracellular anion concentrations and changes in driving force (i.e., EGABA). To evaluate shifts in EGABA, we measured currents in response to ramp voltage commands under baseline conditions and during application of exogenous GABA (Fig. 2A). Ramp voltages were from −30 to +10 mV over 250 ms. A point-by-point subtraction of currents recorded in the presence and absence of GABA was performed to yield the GABA-induced ramp current. These data were used to determine reversal potential (i.e., EGABA) and slope conductance (linear fit to ramp current data). GABA application caused a shift in EGABA toward more negative potentials (indicating a decrease in intracellular anion concentration) and a reduction in slope conductance (Fig. 2B). This procedure was repeated in the same neurons with a transient depolarization to +40 mV (5 s) during the period of GABA application (Fig. 2C). Depolarization during GABA application shifted EGABA toward more positive voltages (indicating intracellular anion accumulation) and increased slope conductance, in contrast to the reduction of conductance seen with GABA alone (Fig. 2D). On average, application of exogenous GABA alone reduced conductance from 23.7 ± 5 to 14.0 ± 2 nS (n = 8, P < 0.01); because our measurement of slope conductance is independent of EGABA, this conductance change likely represents desensitization of receptors. Exogenous GABA paired with depolarization increased conductance from 22.1 ± 4 to 27 ± 8 nS (n = 8, P < 0.05). In the case of exogenous GABA paired with depolarization, the changes in conductance likely represent postdepolarization potentiation (PDP), an intrinsic voltage-dependent property of GABAA receptors in cultured hippocampal neurons (Ransom et al. 2010). Exogenous GABA application alone shifted EGABA from −0.8 ± 0.7 mV at baseline to −9.3 ± 1.8 mV (P < 0.01, n = 8), whereas exogenous GABA paired with depolarization shifted EGABA from −1.2 ± 0.9 mV at baseline to 3.1 ± 0.3 mV (P < 0.01, n = 8) (Fig. 3A). We did not characterize the recovery time course for EGABA shifts in detail, but they were fully recovered within 2 min (the time interval between GABA applications).
Fig. 2.
Determination of GABA-induced shifts in reversal potential for GABA currents (EGABA). A: GABA-evoked ramp currents. Ramp voltage commands were given at 2 time points in the absence (thin line) and presence of exogenous GABA (thick line). Point-by-point subtraction of these records was performed to obtain the GABA-evoked ramp currents. Note the reduction in holding current after application of GABA alone (arrow). Inset illustrates command potentials. B: GABA-evoked ramp currents from data in A. EGABA was +1.1 mV after 2 s in GABA and shifted to −9.2 mV after 10 s in GABA, indicating a reduction of intracellular anions. The slope conductance changes (g) are indicated; 1 and 2 correspond to labeled time points in A. C: GABA-evoked currents with transient depolarization. Experiment is similar to A except a 5-s depolarization was given during GABA application between the 2 voltage ramps. D: GABA-evoked ramp currents before and after transient depolarization. EGABA shifted from −1.4 to +2.8 mV following transient depolarization. Slope conductances are indicated; 3 and 4 correspond to labeled time points in C.
Relationship between EGABA shifts and changes in tonic current.
Data presented above show that currents evoked with exogenous GABA are associated with changes in EGABA and changes in slope conductance. Because the receptors mediating endogenous tonic currents are already activated by ambient GABA, they may represent a population of receptors distinct from those activated by exogenous GABA. These two receptor populations are expected to have different properties (Farrant and Nusser 2005) and could be affected differently by transient changes in [GABA] and transient depolarization. For example, the change in slope conductance (a measure independent of EGABA) shown in Fig. 2, A and B, is attributed to receptor desensitization, whereas the extrasynaptic receptors mediating tonic currents are believed to desensitize very little (Bianchi et al. 2002; Glykys and Mody 2007a; Mtchedlishvili and Kapur 2006; Saxena and Macdonald 1996).
We used our measured changes in EGABA (Fig. 3A) to estimate changes in tonic conductance produced by exogenous GABA alone or exogenous GABA paired with transient depolarization (using data presented in Fig. 1). For GABA application alone, the calculated tonic conductance was 1.1 ± 0.2 nS at baseline and 0.97 ± 0.2 nS following the GABA application; this change of −12 ± 11% was not significant (P = 0.22, n = 11) (Fig. 3B). Exogenous GABA paired with transient depolarization increased tonic conductance from 1.1 ± 0.2 nS at baseline to 1.6 ± 0.2 nS, a significant change of 57 ± 12% (P < 0.001, n = 11) (Fig. 3B). This analysis indicates that the reduction of tonic currents by exogenous GABA alone was primarily due to driving force changes caused by reductions in intracellular anions (i.e., changes in EGABA). In contrast, depolarization led to absolute increases in tonic conductance independent of EGABA shifts. Our calculations will overestimate the influence of EGABA on changes of tonic current, because changes in EGABA will begin to recover during the 5-s interval between washout of GABA and measurement of tonic current.
We also calculated the theoretical shift in EGABA (i.e., the change in driving force, ΔEGABA) required to produce the measured changes in tonic current amplitudes (assuming no change in conductance). These theoretical calculations were then compared with our observed changes in EGABA. For GABA application alone, there was no significant difference between the observed (−8.5 ± 1 mV) and theoretical ΔEGABA (−14.7 ± 6 mV; P = 0.37, n = 8) (Fig. 3C). With GABA plus depolarization, the theoretical EGABA change needed to produce the observed increases in tonic current was +41.3 ± 8 mV, much larger than the experimentally determined ΔEGABA of +4.3 ± 0.3 mV (P < 0.001, n = 8) (Fig. 3C). These considerations are consistent with our conclusions that changes in EGABA (and reduced driving force) were the primary cause of tonic current reduction produced by exogenous GABA alone and that depolarization produced absolute increases of tonic conductance independent of driving force changes.
Presynaptic depolarization enhanced tonic currents via nonvesicular GABA release.
During seizures there is widespread neuronal depolarization, and extracellular K+ concentrations can rise to 12–20 mM (Hablitz and Heinemann 1987). This depolarization and increased K+ concentration alters the thermodynamics of GAT1 function, causing GAT1 to operate in reverse and release GABA (nonvesicular GABA release) (Richerson and Wu 2003). Because we were interested in determining whether nonvesicular GABA release can produce persistent changes in tonic currents, these experiments were performed on neurons pretreated with the H+-ATPase inhibitor concanamycin A (ConA; 1 μM for 2 h) to inhibit vesicular GABA release (Rossi et al. 2003; Wu et al. 2007). ConA by itself did not significantly affect tonic current amplitude compared with neurons on untreated coverslips; average tonic current was −58 ± 12 pA under control conditions and −39 ± 4 pA following ConA treatment (P = 0.24, n = 5–7 neurons under each condition) (Fig. 4, A and B).
As previously shown, fast application of 12 mM K+ (8 s) rapidly activated inward currents that were sensitive to the GAT1 antagonist SKF89976A (SKF; 40 μM) (Fig. 4C) and bicuculline (data not shown), indicating that depolarization of presynaptic neurons and glia caused nonvesicular GABA release by GAT1 (Gaspary et al. 1998; Wu et al. 2001). Using our fast solution exchange system, we next tested whether nonvesicular GABA release triggered by high K+ had any persistent effects on endogenous tonic currents after the depolarizing stimulus was washed off. The effects of transient presynaptic depolarization on tonic currents was studied by voltage clamping the postsynaptic neuron to −60 mV and then measuring tonic currents at baseline and 5 s after application of high K+ (Fig. 4C). Depolarization of presynaptic neurons and glia with 12 mM K+ increased tonic current amplitude measured 5 s after wash of high K+ (Fig. 4C). This increase was sensitive to bath application of the GAT1 antagonist SKF (40 μM), indicating that a component of it was caused by nonvesicular GABA release from reverse transport of GAT1 (Fig. 4C, middle). GAT1 antagonism increased baseline tonic current amplitude but also prevented the increase in tonic currents induced by high K+ (Fig. 4D). The effects of transient presynaptic depolarization with high K+ on tonic current amplitude are summarized in Fig. 4E. The K+-induced increase in tonic current was significantly reduced by GAT1 antagonism with SKF, and this effect was partially reversible (P < 0.05, n = 5). This result indicates GAT1 function is dynamic, limiting ambient [GABA] (and tonic currents) under basal conditions and increasing ambient [GABA] during periods of intense presynaptic depolarization with persistent enhancement of tonic currents.
Nonvesicular GABA release occurred in acute brain slices.
We next evaluated whether GAT1 functions similarly in acute hippocampal brain slices. We made whole cell recordings from dentate gyrus granule cells and locally applied high K+ by pressure ejection from a pipette positioned 60–80 μm from the recorded cell. Vesicular GABA release was inhibited by treating slices with 1 μM ConA for 2 h. Local pressure ejection of high K+ for 10 s induced an inward current that was inhibited by bicuculline (10 μM), indicating that this current was due in part to GABA release and activation of GABAA receptors (Fig. 5A). The bicuculline-insensitive current likely represents a change in leak current due to a shift in EK. On average, bicuculline inhibited the total charge movement (Q) produced during application of high K+ by 63 ± 7% (−14 ± 6 nC under control conditions and −5.2 ± 2 nC in the presence of bicuculline) (P < 0.05, n = 5) (Fig. 5B).
Fig. 5.
Nonvesicular GABA release occurred in acute hippocampal slices. A: membrane currents of dentate gyrus granule cell in response to local pressure ejection of high-K+ solution under control conditions and in the presence of Bic. B: mean charge movement (Q) induced by K+ under control conditions and with Bic (n = 5). C: currents evoked with high K+ during bath application of SKF (40 μM). K+-induced currents were inhibited by SKF, and this was partially reversible. D: time course of holding current and K+-induced peak currents for experiment in C. GAT1 antagonism with SKF produced an increase in holding current (signifying an increase in tonic inhibition) simultaneously with a reduction in the K+-induced current. These data indicate that in acute brain slices, similar to cultured neurons, GAT1 serves to limit ambient GABA concentration under basal conditions but also operates in reverse to release GABA during high-K+ stimulation. E: mean holding current (I) under control conditions and with SKF (n = 8 neurons). Because tonic currents were not directly measured with Bic application in these experiments, holding current is used as a surrogate measure of tonic current. F: mean charge transfer Q produced by high K+ under control conditions and with SKF (n = 8 neurons). *P < 0.05; **P < 0.01.
Bath application of the GAT1 antagonist SKF (30 μM) had different effects on holding current (a surrogate measure of tonic currents) than it did on K+-induced currents. Holding current was increased by SKF, reflecting an increase in ambient GABA and tonic inhibition (Fig. 5, C and D). However, SKF simultaneously reduced the K+-induced currents, indicating that a large component of the K+-induced GABA currents was caused by nonvesicular GABA release. On average, SKF increased holding current from −68 ± 15 pA at baseline to −188 ± 35 pA (P < 0.01, n = 8) (Fig. 5, D and E). The charge movement Q produced by high K+ was −10.1 ± 2 nC (peak current of −1,450 ± 236 pA) under control conditions and −4.6 ± 1 nC (peak current of −813 ± 172 pA) in the presence of SKF, a reduction of 55 ± 5% (P < 0.01, n = 8) (Fig. 5, D and F). The GAT3-selective inhibitor SNAP-5114 (SNAP; 30 μM) did not significantly affect holding currents or K+-induced currents. Peak K+-induced currents were −1,054 ± 393 pA under control conditions and −1,005 ± 350 pA in the presence of SNAP (P = 0.18, n = 3, data not shown). These results in acute brain slices are similar to those obtained in cell culture and indicate that GAT1 functions differently under basal conditions (net uptake of GABA) than during periods of robust depolarization (i.e., high K+), when GAT1 reverses and releases GABA.
Induction of nonvesicular GABA release in acute brain slices required use of pressure ejection solutions with higher [K+] than was required in cell culture experiments, between 30 and 48 mM. Because of the distance of the pressure ejection pipette from recorded neurons (60–80 μM), the small volume of solution ejected, K+ buffering by cellular uptake mechanisms (Ransom et al. 2000), and the diffusion barriers presented by tissue (Nicholson and Sykova 1998), it is expected that the actual [K+] reaching the recorded neuron and adjacent cells/presynaptic terminals was considerably lower than that of the pressure ejection solution itself.
DISCUSSION
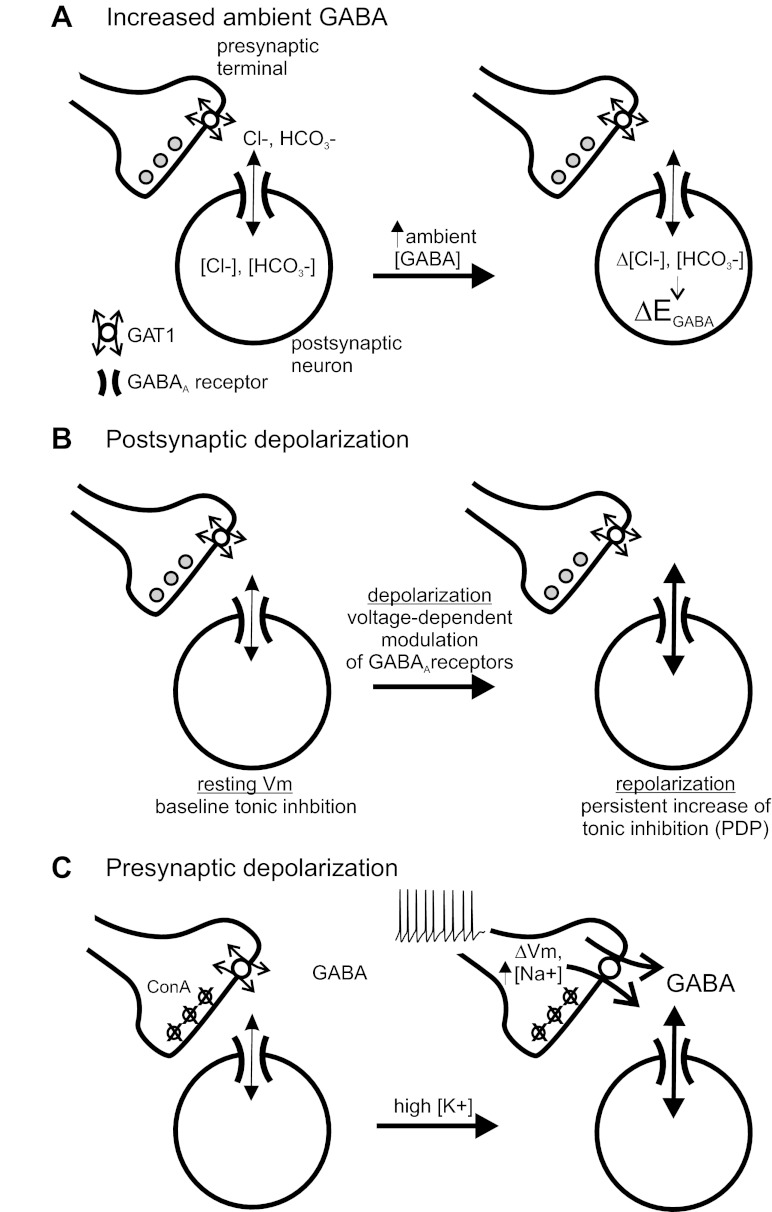
Pathophysiological events in the brain such as ischemia and epileptic seizures are associated with significant depolarization of pre- and postsynaptic neurons and elevations of ambient [GABA] (Allen et al. 2004; During and Spencer 1993). Our results suggest that tonic GABA currents will be rapidly (within seconds) affected by these conditions via multiple mechanisms; specifically, our data show rapid modulation of tonic GABA currents caused by GABA-induced anion shifts, intrinsic voltage-dependent modulation of GABAA receptors and PDP (Birnir et al. 1994; Gray and Johnston 1985; Pavlov et al. 2009; Ransom et al. 2010), and GABA release via reverse transport of GAT1 (Fig. 6). These mechanisms are predicted to influence the level of tonic inhibition during ongoing neural activity; other factors may also rapidly regulate tonic inhibition in addition to those described here (Glykys and Mody 2007b).
Fig. 6.
Schematic of mechanisms rapidly regulating tonic GABA currents. A: increased ambient [GABA] leads to GABAA receptor activation and anionic shifts, causing changes in EGABA (ΔEGABA) that will alter the polarizing influence of tonic inhibition without changing tonic conductance. B: postsynaptic depolarization increases tonic conductance via intrinsic voltage-dependent modulation of GABAA receptors. This voltage-dependent increase in tonic conductance, or “postdepolarization potentiation” (PDP), outlasts the period of depolarization (Ransom et al. 2010). C: presynaptic depolarization and intracellular Na+ accumulation affect the thermodynamics of GAT1, shifting the operation of GAT1 to cause nonvesicular GABA release and increase tonic conductance (Richerson and Wu 2003). In these experiments, vesicular GABA release was inhibited with ConA.
Anion shifts and tonic inhibition.
It is well established that activation of GABAA receptors by synaptically released GABA or exogenous GABA can produce appreciable changes in ionic gradients (i.e., Cl− and HCO3− ions) (Farrant and Kaila 2007; Foldy et al. 2010; Kaila 1994; Staley et al. 1995). This has been demonstrated electrophysiologically as a shift in the reversal potential or altered polarity of GABA currents (DeFazio and Hablitz 2001; Huguenard and Alger 1986; Staley et al. 1995; Thompson and Gahwiler 1989a), and experiments using ratiometric imaging techniques have directly measured changes in intracellular [Cl−] due to GABAA receptor activation or antagonism (Isomura et al. 2003; Kuner and Augustine 2000; Lee et al. 2010). Shifts in intracellular [Cl−] due to GABAA receptor activation occur in CA1 pyramidal neurons under current clamp (when membrane potential is not controlled) (Isomura et al. 2003), in cultured hippocampal neurons during gramicidin perforated patch-clamp experiments (when intracellular [Cl−] and the operation of Na+- and K+-coupled Cl− cotransporters are not disturbed) (Chabwine et al. 2004), and in undisturbed cerebellar granule cells during imaging experiments (Lee et al. 2010). These results indicate that GABAA receptor activation can alter intracellular [Cl−] (and [HCO3−]) at resting membrane potentials and with intact regulation of intracellular Cl−.
Our data indicate that tonic currents are reduced by transient application of exogenous GABA (1 μM). Exogenous GABA also produced changes in EGABA, and the magnitude of the measured EGABA shifts were sufficient to explain the reduction of tonic currents based solely on driving force considerations, without any change in tonic conductance (Fig. 6A). EGABA changes are unlikely to have recovered in the 5-s interval between wash off of exogenous GABA and measurement of tonic currents. Prior studies using our preparation showed GABA-induced changes in EGABA persist for >30 s with either 15 or 135 mM intracellular Cl− (Ransom et al. 2010). The time course of EGABA changes depends on both the duration of GABAA receptor activation and the recording conditions. In cultured hippocampal neurons studied with K+-based pipette solutions, brief GABA pulses (25 ms, 1 mM GABA) produced changes in intracellular Cl− that recovered over 10–15 s (Kuner and Augustine 2000). This may be a lower limit of recovery time for intracellular [Cl−] shifts, because in this situation Cl− recovery will be mediated by both KCC2 extrusion and diffusion from patch pipette (DeFazio et al. 2000; Thompson and Gahwiler 1989b). In gramicidin perforated-patch experiments, GABA-induced Cl− accumulation recovers much more slowly with a time constant of 2.5 min (Chabwine et al. 2004). The rate of recovery was faster after GABA-induced Cl− depletion (i.e., after GABA application to hyperpolarized neurons), but this still recovered over minutes (τ = 1.2 min) (Chabwine et al. 2004). To the best of our knowledge, ours is the first description of endogenous tonic GABA currents being affected by anion redistribution. Although this result may not be surprising, it highlights that tonic inhibition (similar to phasic inhibition) can be affected in a potentially maladaptive manner during recurrent seizures, when intracellular Cl− accumulation could convert the inhibitory, shunting function of tonic inhibition to an excitatory, depolarizing current (Dzhala et al. 2010; Thompson and Gahwiler 1989b).
Because extrasynaptic GABAA receptors that mediate tonic currents in the hippocampus (α5- and δ-subunit-containing receptors) are believed to desensitize very little, even in the presence of high GABA concentrations (i.e., 1 mM), exogenous GABA was not expected to reduce tonic currents by desensitizing extrasynaptic receptors (Bianchi et al. 2002; Glykys and Mody 2007a; Mtchedlishvili and Kapur 2006; Saxena and Macdonald 1996). However, recent results indicate that δ-subunit-containing receptors expressed in tsA201 cells undergo significant desensitization during prolonged exposure to low GABA concentrations (i.e., 1–3 min) (Bright et al. 2011). Our data on GABA-induced EGABA shifts quantitatively explain the reduction of tonic currents caused by exogenous GABA, suggesting that desensitization of extrasynaptic receptors in our experiments was not significant.
Voltage-dependent modulation of GABAA receptors.
Tonic inhibition and phasic inhibition both exhibit voltage-dependence (Collingridge et al. 1984; Mellor and Randall 1998; Pavlov et al. 2009; Ransom et al. 2010). Extrasynaptic GABAA receptors that mediate tonic inhibition in hippocampal neurons are strongly modulated by membrane potential, with a twofold increase in tonic conductance when neurons are depolarized from −80 to −40 mV (Ransom et al. 2010). This voltage dependence augments tonic inhibition and offsets action potential threshold (Pavlov et al. 2009). Our results show that voltage-dependent modulation of tonic inhibition is independent of anion accumulation and outlasts the period of depolarization, a phenomena termed postdepolarization potentiation (PDP) (Fig. 6B). Although we used large depolarizations in this study, PDP occurs following smaller depolarizations (i.e., −30 mV) and in response to epileptiform patterns of depolarization (Ransom et al. 2010). The mechanism of PDP at the single-channel level is not known, but it may relate to the increased open probability or unitary conductance of GABAA receptors seen with depolarization (Curmi et al. 1993; Fatima-Shad and Barry 1993; Gray and Johnston 1985). Voltage-dependent increases of tonic conductance are expected to affect the behavior of hippocampal neurons and networks (Glykys and Mody 2006; Pavlov et al. 2009). This would typically be an inhibitory effect, but if it occurred during periods of intracellular Cl− accumulation, voltage-dependent potentiation could enhance an excitatory influence of GABA currents (Dellal et al. 2012; Dzhala et al. 2010).
Nonvesicular GABA release by GAT1.
GAT1 is expressed by GABAergic neurons and exists at high density in presynaptic terminals, up to 800–1,200 molecules per terminal (Chiu et al. 2002). It is a Na+-dependent cotransporter that functions to uptake GABA (translocating 2 Na+ ions, 1 Cl− ion, and 1 GABA molecule), thereby constraining the time course and spatial distribution of synaptically released GABA (Keros and Hablitz 2005; Thompson and Gahwiler 1992). GAT1 can also operate in the reverse direction to release GABA under appropriate thermodynamic conditions (Gaspary et al. 1998; Ransom and Richerson 2009). This type of nonvesicular GABA release has been demonstrated with physiological and pathophysiological depolarizations and with alterations in ion gradients (Allen et al. 2004; Pin and Bockaert 1989; Wu et al. 2006). It can also mediate direct inhibitory neurotransmission between recorded pairs of hippocampal neurons (Wu et al. 2007). The present results provide new information on the dynamic function and temporal aspects of nonvesicular release mediated by GAT1. Our rapid solution exchange allowed us for the first time to detect enhanced tonic inhibition that outlasted the duration of a depolarizing stimulus (i.e., high K+); this enhancement lasted at least 5 s (see Fig. 4A). GAT1 antagonism with SKF prevented this increase of tonic inhibition, indicating that nonvesicular GABA release by GAT1 can rapidly and persistently modulate tonic inhibition (see Fig. 6C). GAT1 antagonism not only prevented nonvesicular GABA release but also increased baseline tonic inhibition. These results indicate that GAT1 limits ambient GABA (and tonic inhibition) under basal conditions but that GAT1 can operate in reverse to release GABA during periods of depolarization and intracellular Na+ accumulation (such as occur during seizures and ischemia), leading to transient enhancement of tonic inhibition. The temporal profile of ambient GABA elevations caused by nonvesicular release is likely determined by both diffusion and a shift in the thermodynamic operation of GAT1 back to net uptake as intracellular Na+ accumulation recovers; the rapid solution exchange times make direct effects of K+ on membrane potential unlikely. We also showed that similar effects occur in dentate gyrus granule cells in acute hippocampal slices, indicating that reversal of GAT1 is not an artifact of cell culture preparations (see also Allen et al. 2004).
The mechanisms described in this report that rapidly regulate tonic GABA currents will not work in isolation; for example, nonvesicular GABA release will increase ambient GABA causing alterations in anion concentrations and EGABA that will affect tonic currents. In our experiments, however, the increase in tonic conductance produced by nonvesicular GABA release overshadowed any effect of EGABA shifts (i.e., changes in driving force), allowing us to detect increased tonic current amplitudes. Increased ambient GABA will have other effects as well, including changes in glutamate uptake by astrocytes (Unichenko et al. 2012). GABA released by reverse transport of GAT1 is unlikely to reach the peak concentrations produced by GABA released from synaptic vesicles, but it is predicted to act in a wider spatiotemporal pattern, with the potential to affect many physiological processes of neurons and glia.
Summary and conclusions.
Tonic inhibition, by name, implies a static, invariant level of GABAergic signaling. This feature is likely advantageous under some conditions, allowing neurons to function on a stable background tone of inhibition. However, our results indicate that tonic inhibition is not really tonic, but instead that the level of tonic inhibition can rapidly change due to factors associated with intense neural activity, including increased ambient GABA, extracellular K+ accumulation, and neuronal depolarization. These factors modulate tonic inhibition via changes in ion gradients, GAT1 thermodynamics, and GABAA receptor behavior. Activity-dependent fluctuations in tonic inhibition may serve a negative feedback function for neurons, providing moment-to-moment adjustment of inhibitory tone in response to changes in the prevailing level of neural activity. In addition to the slower types of regulation of tonic inhibition due to changes in receptor expression or neurosteroid levels, our results establish that tonic inhibition can be rapidly and dynamically regulated.
GRANTS
This work was supported by the Department of Veterans Affairs Health Administration (C. B. Ransom, W. J. Spain), the National Institutes of Health (G. B. Richerson), and the National Epifellows Foundation (C. B. Ransom).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B.R., Y.W., and G.B.R. conception and design of research; C.B.R. and W.T. performed experiments; C.B.R. and W.T. analyzed data; C.B.R., W.T., W.J.S., and G.B.R. interpreted results of experiments; C.B.R. prepared figures; C.B.R. drafted manuscript; C.B.R., W.J.S., and G.B.R. edited and revised manuscript; C.B.R., W.T., Y.W., W.J.S., and G.B.R. approved final version of manuscript.
REFERENCES
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci 28: 1185–1197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Rossi DJ, Attwell D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J Neurosci 24: 3837–3849, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the delta subunit. Neuropharmacology 43: 492–502, 2002 [DOI] [PubMed] [Google Scholar]
- Birnir B, Everitt AB, Gage PW. Characteristics of GABAA channels in rat dentate gyrus. J Membr Biol 142: 93–102, 1994 [DOI] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKenzie G, Hosie AM, Farrant M, Brickley SG. Profound desensitization by ambient GABA limits activation of delta-containing GABAA receptors during spillover. J Neurosci 31: 753–763, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabwine JN, Van Damme P, Eggermont J, De Smedt H, Missiaen L, Van Den Bosch L, Parys JB, Robberecht W, Callewaert G. Long-lasting changes in GABA responsiveness in cultured neurons. Neurosci Lett 365: 69–72, 2004 [DOI] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature 428: 856–860, 2004 [DOI] [PubMed] [Google Scholar]
- Chiu CS, Jensen K, Sokolova I, Wang D, Li M, Deshpande P, Davidson N, Mody I, Quick MW, Quake SR, Lester HA. Number, density, and surface/cytoplasmic distribution of GABA transporters at presynaptic structures of knock-in mice carrying GABA transporter subtype 1-green fluorescent protein fusions. J Neurosci 22: 10251–10266, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Gage PW, Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol 356: 551–564, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci 25: 11553–11563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curmi JP, Premkumar LS, Birnir B, Gage PW. The influence of membrane potential on chloride channels activated by GABA in rat cultured hippocampal neurons. J Membr Biol 136: 273–280, 1993 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Hablitz JJ. Chloride accumulation and depletion during GABAA receptor activation in neocortex. Neuroreport 12: 2537–2541, 2001 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J Neurosci 20: 8069–8076, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellal SS, Luo R, Otis TS. GABAA receptors increase excitability and conduction velocity of cerebellar parallel fiber axons. J Neurophysiol 107: 2958–2970, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341: 1607–1610, 1993 [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Kuchibhotla KV, Glykys JC, Kahle KT, Swiercz WB, Feng G, Kuner T, Augustine GJ, Bacskai BJ, Staley KJ. Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J Neurosci 30: 11745–11761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Prog Brain Res 160: 59–87, 2007 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- Fatima-Shad K, Barry PH. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proc R Soc Lond B Biol Sci 253: 69–75, 1993 [DOI] [PubMed] [Google Scholar]
- Foldy C, Sang-Hun L, Morgan RJ, Soltesz I. Regulation of fast-spiking basket cell synapses by the chloride channel ClC-2. Nat Neurosci 13: 1047–1049, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspary HL, Wang W, Richerson GB. Carrier-mediated GABA release activates GABA receptors on hippocampal neurons. J Neurophysiol 80: 270–281, 1998 [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56: 763–770, 2007a [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J Neurophysiol 95: 2796–2807, 2006 [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol 582: 1163–1178, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Johnston D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J Neurophysiol 54: 134–142, 1985 [DOI] [PubMed] [Google Scholar]
- Hablitz JJ, Heinemann U. Extracellular K+ and Ca2+ changes during epileptiform discharges in the immature rat neocortex. Brain Res 433: 299–303, 1987 [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Alger BE. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol 56: 1–18, 1986 [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sugimoto M, Fujiwara-Tsukamoto Y, Yamamoto-Muraki S, Yamada J, Fukuda A. Synaptically activated Cl− accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J Neurophysiol 90: 2752–2756, 2003 [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol 42: 489–537, 1994 [DOI] [PubMed] [Google Scholar]
- Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol 94: 2073–2085, 2005 [DOI] [PubMed] [Google Scholar]
- Kuner T, Augustine GJ. A genetically encoded ratiometric indicator for chloride: capturing chloride transients in cultured hippocampal neurons. Neuron 27: 447–459, 2000 [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science 330: 790–796, 2010 [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8: 797–804, 2005 [DOI] [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, MacDonald JF, Roder JC, Jackson MF, Orser BA. α5 GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci 30: 5269–5282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor JR, Randall AD. Voltage-dependent deactivation and desensitization of GABA responses in cultured murine cerebellar granule cells. J Physiol 506: 377–390, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38: 433–445, 2003 [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol 69: 564–575, 2006 [DOI] [PubMed] [Google Scholar]
- Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci 21: 207–215, 1998 [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Spencer DD, Williamson A. GABA uptake and heterotransport are impaired in the dentate gyrus of epileptic rats and humans with temporal lobe sclerosis. J Neurophysiol 85: 1533–1542, 2001 [DOI] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci 29: 15341–15350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the d subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci 24: 8629–8639, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Bockaert J. Two distinct mechanisms, differentially affected by excitatory amino acids, trigger GABA release from fetal mouse striatal neurons in primary culture. J Neurosci 9: 648–656, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, Ransom BR, Sontheimer H. Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 3: 427–442, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, Richerson GB. GABA transporters: regulation of tonic inhibition. In: Encyclopedia of Epilepsy , edited by Schwartzkroin PA. London: Elsevier, 2009 [Google Scholar]
- Ransom CB, Wu Y, Richerson GB. Postdepolarization potentiation of GABAA receptors: a novel mechanism regulating tonic conductance in hippocampal neurons. J Neurosci 30: 7672–7684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol 90: 1363–1374, 2003 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol 548: 97–110, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol 49: 567–579, 1996 [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci 25: 10016–10024, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at a4b2d GABAA receptors triggers anxiety at puberty. Nat Neurosci 10: 469–477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269: 977–981, 1995 [DOI] [PubMed] [Google Scholar]
- Tang ZQ, Hoang Dinh E, Shi W, Lu Y. Ambient GABA-activated tonic inhibition sharpens auditory coincidence detection via a depolarizing shunting mechanism. J Neurosci 31: 6121–6131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol 61: 501–511, 1989a [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on ECl− in hippocampal CA3 neurons. J Neurophysiol 61: 512–523, 1989b [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. J Neurophysiol 67: 1698–1701, 1992 [DOI] [PubMed] [Google Scholar]
- Unichenko P, Myakhar O, Kirischuk S. Intracellular Na+ concentration influences short-term plasticity of glutamate transporter-mediated currents in neocortical astrocytes. Glia 60: 605–614, 2012 [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 56: 851–865, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurosci 21: 2630–2639, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. J Neurophysiol 96: 2425–2436, 2006 [DOI] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci 27: 7520–7531, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]