Abstract
Addition of newly generated neurons into mature neural circuits in the adult CNS responds to changes in neurotransmitter levels and is tightly coupled to the activity of specific brain regions. This postnatal neurogenesis contributes to plasticity of the olfactory bulb and hippocampus and is thought to play a role in learning and memory, context and odor discrimination, as well as perceptual learning. While acetylcholine plays an important role in odor discrimination and perceptual learning, its role in adult neurogenesis in the olfactory bulb has not been elucidated. In this study, I have examined the functional expression of nAChRs in progenitor cells of the rostral migratory stream (RMS) in the adult olfactory bulb of mice. I show that most of these cells in the RMS exhibit large nAChR-mediated calcium transients upon application of acetylcholine (ACh). Unlike in the hippocampus, the predominant functional nAChRs on progenitor cells are of α3β4 subtype. Interestingly, functional receptor expression is lost once progenitor cells mature, and are incorporated into the granule cell layer. Instead, nAChRs are now expressed on some presynaptic terminals and modulate glutamate release onto granule cells. My results imply that ACh is a part of the permissive niche and likely plays a role in development of progenitor cells.
Keywords: cholinergic, adult neurogenesis, rostral migratory stream, olfactory bulb, nAChRs
new neurons are continuously integrated into existing neuronal circuits in at least two regions of the adult central nervous system: the dentate gyrus and olfactory bulb. While the extent of adult neurogenesis in humans remains controversial (Bergmann et al. 2012; Macklis 2012; Sanai et al. 2011), a number of studies have shown that in rodents, adult neurogenesis contributes to the plasticity of the network in both these areas (Gao and Strowbridge 2009; Lledo et al. 2006; Nissant et al. 2009; Schmidt-Hieber et al. 2004). Functional relevance of this process, however, remains unresolved. Adult neurogenesis has been implicated in physiological processes such as learning and memory (Alonso et al. 2012; Deng et al. 2009; Imayoshi et al. 2008), odor (Gheusi et al. 2000) and context discrimination (Aimone et al. 2011; Kheirbek et al. 2012), but mechanistic information regarding progenitor regulation of these processes is not understood. Much of the focus has been on adult neurogenesis in the hippocampus and little is known regarding the significance and control of the process in the olfactory bulb (OB). Newborn cells in the subventricular (SVZ) region migrate along the rostral migratory stream (RMS) into the OB, where they form GABAergic granule cells (GCs) and periglomerular (PG) cells surrounding the glomerular neuropil (Lledo et al. 2008; Merkle et al. 2007). Olfactory perceptual learning is diminished in mice where neurogenesis is disrupted (Lazarini and Lledo 2011; Moreno et al. 2009), and mice with impaired neurogenesis show deficits in social recognition, although maternal recognition of pups remains intact (Feierstein et al. 2010; Mak and Weiss 2010). These results suggest an important role for neurogenesis in the adult mammalian OB, but the mechanisms that control the proliferation and differentiation of these cells have yet to be understood.
A key modulator of olfactory perceptual learning is acetylcholine (ACh). Studies indicate that blocking either muscarinic AChRs or nicotinic AChRs (nAChRs) can disrupt odor discrimination in mice (Hellier et al. 2012; Mandairon et al. 2006). Whether part of the effects of this transmitter could be via its modulation of neurogenesis in the OB is yet to be examined. Cholinergic fibers are present adjacent to newly generated neurons in the OB (Whitman and Greer 2007), but unlike receptors for GABA (Liu et al. 2005) and glutamate (Platel et al. 2010a, 2010b), presence of ACh receptors on progenitor cells has not been reported. There is evidence, from the hippocampus, that AChRs might play a role in neurogenesis where α7 and β2 subunit-based nAChRs have been shown to modulate progenitor survival (Kaneko et al. 2006; Kotani et al. 2008) and differentiation (Campbell et al. 2010; Lozada et al. 2012). Adult neuronal progenitors isolated from the hippocampus show increased apoptotic cell death when exposed to low levels of nicotine (Berger et al. 1998) by a mechanism mediated by changes in intracellular free calcium ([Ca]i). While cholinergic modulation of neurogenesis in the OB has not been examined systematically, it has been reported that increasing ACh levels by blocking ACh esterase can enhance the survival of progenitor cells in OB (Kaneko et al. 2006). Contrary to expectation from this study, a second report (Mechawar et al. 2004) shows that knocking out of β2 subunit of nAChRs, thus decreasing cholinergic influence, results in an increase in the number of granule cells.
The studies summarized above have raised a number of questions. Is the effect of ACh on progenitor cells cell-autonomous? What receptor subtypes mediate these effects? Are these effects mediated, in part, by the ability of nAChRs to alter [Ca]i and activate downstream calcium-dependent signaling cascades? How does functional nAChR expression change as progenitor cells migrate from the RMS into the OB cellular layers? A prerequisite to answering many of these questions is to know where functional nAChRs are expressed in the migratory pathway and whether there is a change in the receptor expression as incoming progenitor cells migrate into the GC layer of the OB.
Here I address this issue and show that functional nAChRs are expressed in the migrating progenitor cells. These receptors belong to the α3β4 subclass of nAChRs and are efficient at raising [Ca]i. I further show that once these cells form mature GCs, the functional receptor is lost, and a large fraction of cholinergic signals arises from presynaptic mechanisms via transmitter release.
METHODS
OB slices and identification of progenitor cells.
All experiments were performed under protocols approved by University of Colorado Anschutz Medical Center Institutional Animal Care and Use Committee. C57bl/6 mice, between the ages of 6 and 8 wk, were housed in ventilated cages in a 10:14 light:dark cycle and given food and water ad libitum. Progenitor cells were identified by either 1) position of RMS at the center (ependymal layer) of the bulb (we find that most cells in this area are progenitor cells and express doublecortin, a marker for immature neurons); or 2) specific labeling with GFP using replication-deficient Lentiviral particles (∼1010/ml from Viral Vector Core at Univ. of Pennsylvania). Virus of 0.5–1 μl was injected into the RMS using a Kopf Steretoxic instrument at coordinates 3.3A, 0.82L, −2.9 V (Lledo et al. 2008). Mice were euthanized after 8–10 days. GFP-expressing cells at the elbow, or further along the RMS, were identified and used for recordings.
Sagittal slices (300 μm) of the brain, including the OB and RMS, were made using standard protocols (Panzanelli et al. 2009; Sharma and Vijayaraghavan 2003) on a Leica VT1000S vibratome. Cutting solution contained (in mM) 72 sucrose, 83 NaCl, 1.25 NaH2PO4, 25 NaHCO3, 2.5 KCl, 10 glucose, 3 MgCl2, 0.5 CaCl2, 0.5 ascorbic acid, 2 sodium pyruvate. Slices were incubated in the same solution for an hour before the experiment.
Calcium imaging.
Slices were loaded with the calcium-sensitive dye fura 2-AM as described (Sharma et al. 2008). Briefly, slices were incubated in cutting solution containing 20 μM fura 2-AM and 2% Cremaphore ES for 30 min at room temperature. Slices were washed in recording solution and transferred to the recording chamber. Imaging was performed using Zeiss AxioExaminer fitted with a CCD camera (Cooke Sensicam). Sutter DG IV was used for the light source. Images were acquired and analyzed using SlideBook 5 software. Recording solution contained (in mM) 120 sodium chloride, 1.25 sodium monophosphate, 25 sodium bicarbonate, 10 glucose, 3.5 potassium chloride, 2.5 magnesium chloride, and 1 calcium chloride. Images were acquired as a time series at 1 Hz. Data were collected using a 510/80-nm filter and T400LP beam splitter (Chroma Technology). Individual regions of interest (ROI) were specified for each cell in the image by creating masks just large enough to cover an individual cell. Average fluorescence for each ROI/individual cell was measured along a 12-bit scale. Ratio of emission at 340 and 380 nm was normalized to the baseline and plotted as percentage increase over baseline. Experiments were performed on slices from four to seven animals for each condition. Data was pooled from different animals and are presented as means ± SE across cells. Statistical significance was evaluated by paired or independent Student t-tests.
All experiments were performed with 1 mM ACh+ 1 μM atropine (ACh/At) to block muscarinic ACh receptors and activate nicotinic receptors only. Drugs were applied using a Picospritzer. Antagonists were present in the bath as well as puffer pipette.
Immunohistochemistry.
Mice were perfused with 4% paraformaldehyde and brains postfixed for 2 h and cryoprotected in 20% sucrose. Section of 18 μm were cut on a cryostat, and free-floating sections were stained overnight at 4°C with goat polyclonal doublecortin antibody (SC8066 Santa Cruz, 1:500 dilution). A fluorescent donkey anti-goat secondary antibody (Jackson ImmunoResearch, 1:500 dilution) was used to visualize labeled cells. Control sections were processed using the same protocol but without the primary antibody to determine background staining and antibody specificity. Images were acquired using an Olympus spinning disc microscope.
RESULTS
Progenitor cells migrate from the SVZ along the RMS to the main olfactory bulb. After arriving at the bulb, they exhibit radial migration and stop either at the GC layer integrating into the circuit as GCs or at the glomerulus developing into PG cells. In this study, I focused on the cells in the subependymal layer of the OB of 6- to 10-wk-old mice. In agreement with previous studies (Darcy and Isaacson 2009), I find that the majority of the cells in the subependymal layer express doublecortin, a marker for migrating neural progenitor cells (Fig. 1, A and B). Dense doublecortin staining was observed in the RMS but not in other layers of the bulb. Based on this result, I conclude that most cells in the subependymal layer are migrating progenitor cells.
Fig. 1.
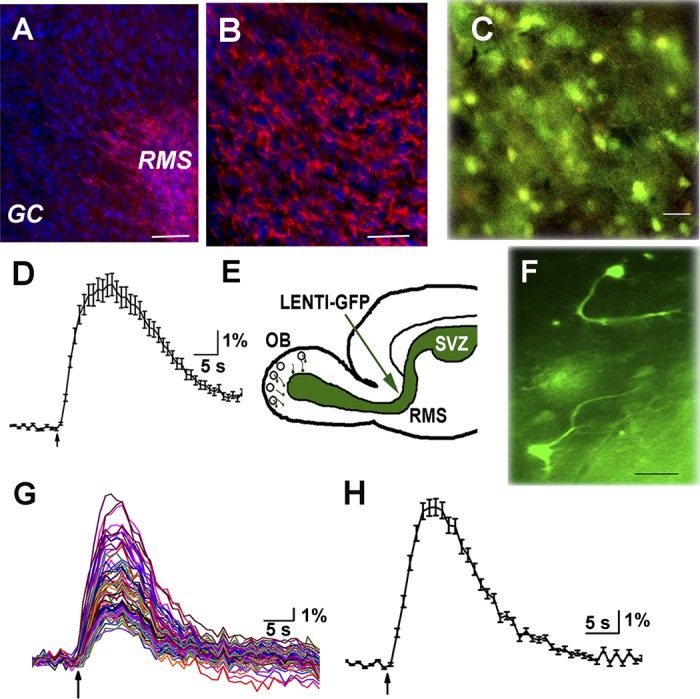
Progenitor cells in rostral migratory stream (RMS) express doublecortin and are depolarized by GABA and ACh. A: fixed frozen sections labeled with doublecortin antibody (red) showing dense labeling in RMS but not in other layers of the olfactory bulb (OB). Nuclei are stained with DAPI (blue). Scale bar, 100 μm. B: higher magnification of doublecortin labeling in RMS; scale bar, 10 μM. C: image of cells in the RMS loaded with fura 2-AM. Scale bar, 15 μm. D: calcium transients generated in a progenitor cell in the RMS upon a 10-s application of 1 mM GABA. E: cartoon of sagittal section of brain showing the site of injection. F: 2 cells in the RMS expressing GFP with processes pointing in the direction of migration. G: calcium transients in response to a 5-s puff application of 1 mM ACh and 1 μM atropine (ACh/AT). All cells in the field respond, but the response is variable among cells. H: average response from 36 cells shown in G.
I then examined for the presence of functional excitatory GABARs on these cells. I used calcium imaging arguing that depolarizing GABAergic signals should result in changes in intracellular calcium concentration ([Ca]i). Slices were loaded with fura 2-AM (Fig. 1C). Local application of 1 mM GABA resulted in rapid calcium transients in cells (n = 7 animals) (Fig. 1D). On average, the 340/380 ratio increased by 6.35 ± 0.47% (means ± SE, n = 88 cells) and decayed with a mean time constant of 58.2 ± 27.03 s.
I asked whether migrating cells in the RMS expressed functional nAChRs. A brief application of 1 mM ACh in the presence of 1 μM atropine (ACh/At) resulted in large but variable calcium transients with peak amplitudes ranging from 3 to 22% above baseline (Fig. 1, G and H). Cells in the subependymal zone responded with a mean of 8.03 ± 0.37% (n = 111) increase in intracellular calcium. The concentration of atropine used in these experiments was sufficient to block nAChR-mediated calcium signals (data not shown). My results indicate that migrating progenitor cells in the adult RMS show increase in cytosolic calcium levels via nAChRs.
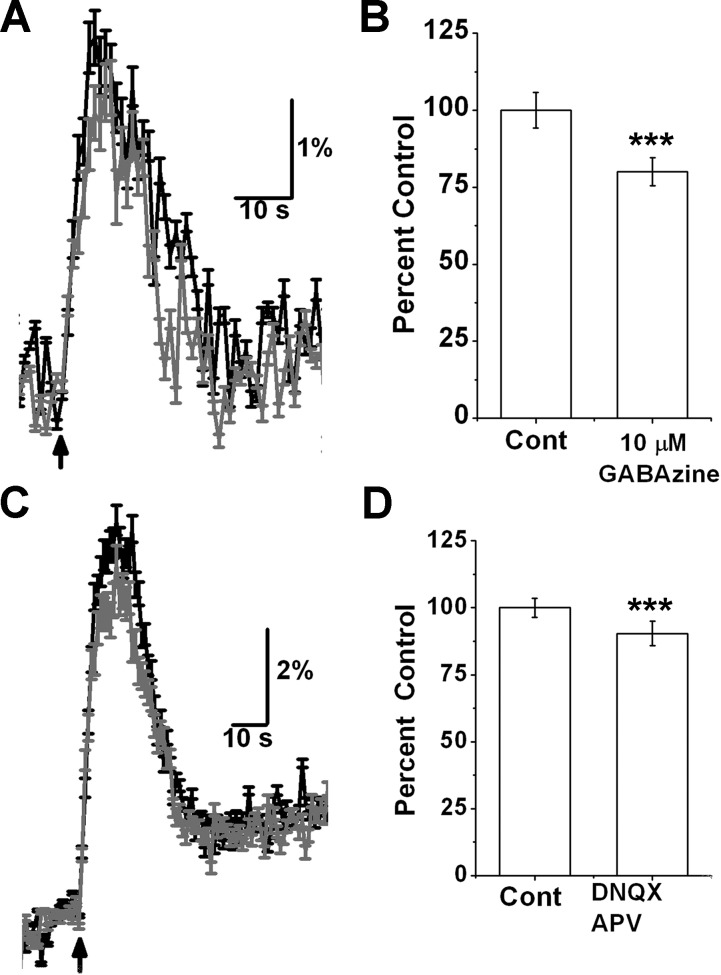
As progenitor cells exhibit depolarizing responses to both GABA and glutamate from surrounding cells (Platel et al. 2010a; Young et al. 2010), I asked what fraction of nAChR-mediated calcium signals were indirect, due to release of glutamate and/or GABA. When ACh/At-evoked responses were elicited in the presence of 10 μM GABAzine to block GABAA receptors, there was a 20 ± 4.5% decrease in peak amplitude of the response compared with control ACh/At response in the absence of the drug (n = 147 cells, 6 mice, P < 0.004; Fig. 2, A and B). Blocking ionotropic glutamate receptors with 50 μM DNQX + 100 μM APV resulted in a small but statistically significant reduction in the nAChR-mediated response by 9.6 ± 4.5% (n = 78, 5 animals; Fig. 2, C and D). These data suggest that the major component of the calcium signals arise from the activation of nAChRs expressed by migrating progenitor cells.
Fig. 2.
Response to ACh/AT is mainly due to nicotinic receptors expressed by progenitor cells. A: response from 1 field of cells. Black trace is control response to a 5-s puff of ACh/AT from 1 field. Gray trace is response from same cells in presence of 10 μM GABAzine. B: blocking GABA receptors with 10 μM GABAzine blocks only 20 ± 4.5% of the response; n = 147, 6 animals. C: response to a 10-s puff of ACh/AT from 1 field of cells before (black trace) and after (gray trace) treatment with 50 μM DNQX and 100 μM APV. D: ionotropic glutamate receptor blockers block 9.6 ± 4.5% of response; n = 78 cells, 5 animals. ***P < 0.004.
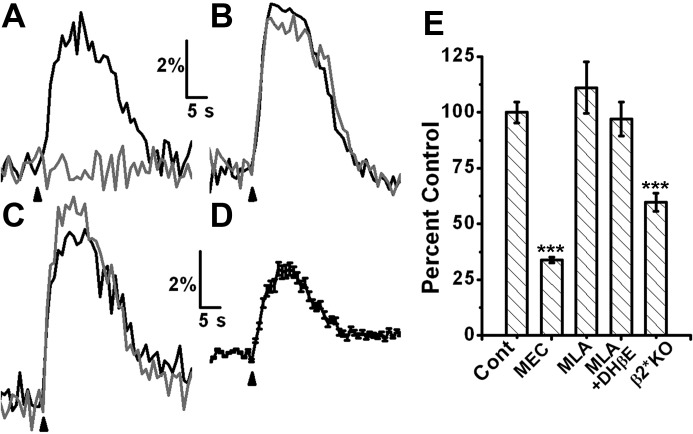
I then examined the pharmacology of functional nAChRs on progenitor cells. Incubation of slices with 5 μM mecamylamine, a general nAChR blocker, resulted in a large block of the nAChR-induced calcium signals (Fig. 3A; 64 ± 1.13% inhibition, n = 110, 4 animals, P < 0.001), confirming that these signals are indeed nicotinic in nature. The α7-nAChR-selective antagonist methyllycaconitine (MLA) (10 nM) failed to affect the nAChR-induced calcium signals, ruling out a role for this receptor subtype (n = 50, P < 0.29). Surprisingly, incubating the slices with 10 nM MLA and 10 μM dihydro-beta-erythroidine, a selective blocker of β2 subunit containing nAChRs, also failed to significantly block the calcium transients (P < 0.8 compared with control; n = 50, 5 animals; Fig. 3C). These results suggest that nAChR signals do not arise from either homomeric or heteromeric α7-nAChRs nor from α4β2 nAChRs, the other common nAChR subtype.
Fig. 3.
Nicotinic response is not due to activation of α7 or β2 subunit containing nAChRs. Responses from single cells. Black traces are control response and gray traces are after application of specific antagonists. A: response of a single progenitor cell is completely blocked by 5 μM mecamylamine (MEC). B: 10 nM MLA, an antagonist specific for α7nAChR, has no effect. C: further block by 10 μM DHBE (MLA+DHBE) also fails to block the calcium transient. D: response is present in progenitor cells from β2 nAChR knockout mice. Average response from 104 progenitor cells from 7 β2 nAChR knockout mice. E: bar graph comparing average responses under various conditions. ***P < 0.001.
As a role for β2-containing nAChRs can be inferred from a previous study (Mechawar et al. 2004), contrary to my pharmacological results, I examined nAChR signals from progenitors in a β2 knockout (KO) mouse. Progenitor cells from the β2 KOs showed robust calcium transients upon ACh/At application. These responses were significantly smaller (60 ± 6.7% of control response, 7 animals; n = 104) than those in wild-type mice. Similar to results from wild-type mice, these responses could be blocked by 5 μM mecamylamine (61.9 + 0.57% inhibition, n = 77, P < 0.001). However, as the responses are variable and comparisons were made across animals, it is not clear whether the differences arise due to disparities in calcium responses between the two mice. Combined with my pharmacology results, these data imply that β2-containing nAChRs play a small, if any, part in the nAChR-mediated calcium responses in progenitor cells in the RMS.
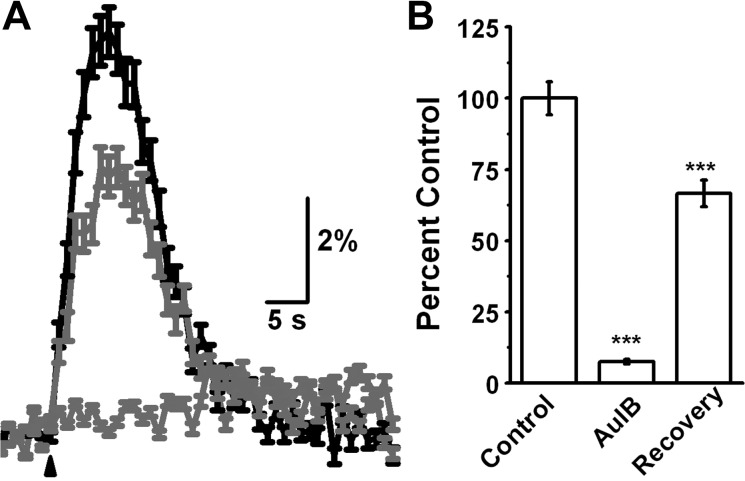
The absence of evidence implicating either α7- or β2-containing nAChRs motivated the examination of other possible receptor subtypes. Recent studies (D'Souza and Vijayaraghavan 2012; Mineur et al. 2011) have shown that the α3β4-nAChRs, initially thought to have roles mainly in the autonomic nervous system, might play important roles in CNS functions. I therefore examined the effects of incubating slices with conotoxin AuIB (5 μM), an antagonist specific for α3β4-nAChRs (Luo et al. 1998). The antagonist showed a large inhibition in ACh/At responses from the RMS progenitor cells (Fig. 4; 92.6 ± 0.76% inhibition, n = 78 cells, 4 animals, P < 0.001). A 15-min washout of the antagonist resulted in a recovery of the responses to 66.5 ± 4.6% of control responses (P < 0.001 compared with block). These results suggest that α3β4-nAChRs are the dominant functional nAChR subtype in progenitor cells.
Fig. 4.
Progenitor cells in the RMS express functional α3β4 nAChRs. A: average response from 116 cells from 4 animals. Response is inhibited by 5 μM conotoxin AuIB specific for α3β4 nAChR receptors (light gray). Response recovers after a 15-min washout of AuIB. B: on average, response was reduced by 92 ± 0.76% and recovered to 66.5 ± 6.8% of control. ***P < 0.001.
Last, I asked whether functional expression of nAChRs was maintained once the cells were incorporated into the GC circuit. Responses to local application of ACh/At were examined from fura 2-AM-loaded neurons in the GC layer. Only a small fraction of GCs (0–3%) responded to ACh/At application in the GC layer (Fig. 5). Surprisingly, however, the responses in this minor fraction were completely abolished upon application of 50 μM DNQX + 100 μM APV to block GluRs, while the ACh/At responses from the RMS cells were unaffected by this treatment (data from 4 animals).
Fig. 5.
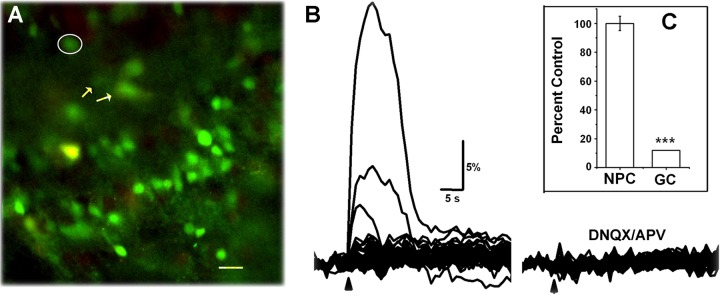
nAChRs are absent in mature granule cells (GCs). A: a field of GCs loaded with fura 2-AM. B: response from these cells to a 5-s puff of ACh/AT. Only 3 cells responded in this field. The cell with the largest response is circled in A. Two cells, marked with arrows in A, showed smaller responses. Traces at right are 15 min after application of DNQX/APV. Responses from the small fraction of GCs that respond to ACh/AT can be completely blocked by DNQX/APV, unlike that seen in NPCs (inset C). ***P < 0.001.
My results suggest that migrating progenitor cells in the RMS express functional nAChRs. Contrary to results from the hippocampus (Campbell et al. 2010; Liu et al. 2006) and expectation from one study in the OB (Mechawar et al. 2004), these cells predominantly express functional α3β4 nAChRs. Once migration stops and cells differentiate in the GC layer, the expression of this receptor subtype is suppressed, and nAChRs are expressed only on presynaptic terminals forming glutamatergic synapses on GCs.
DISCUSSION
In this study, I show that migrating progenitor neurons along the RMS express functional nAChRs in densities sufficient to significantly alter [Ca]i. The majority, if not all, of the progenitor cells in the subependymal layer express these receptors. While development of hippocampal progenitor cells appears to be modulated by the α7-nAChR subtype (Campbell et al. 2010; Liu et al. 2006), responses in the OB progenitors are mediated by the α3β4 subtype adding to the emerging importance of these receptors in CNS functions. Interestingly, functional nAChR expression is lost in differentiated GCs and partly replaced by presynaptic regulation of glutamate release by the receptors.
These data contribute to the growing realization that modulation of adult neurogenesis differs greatly between hippocampus and OB. In the hippocampus, knocking out α7-nAChR expression specifically in progenitor cells leads to delayed maturation and integration of dentate GCs (Liu et al. 2006), and β2 subunit-containing nAChRs modulate dendritic arborization of newly generated dentate GCs. Furthermore, presence of α3β4 nAChRs has not been reported on progenitor cells generated in the subgranular zone of dentate gyrus.
ACh has been shown to be neuroprotective as blockers of ACh esterase enhances progenitor cell survival in the RMS (Kaneko et al. 2006). However, a conflicting study (Mechawar et al. 2004) shows that endogenous stimulation of nAChRs can also lead to apoptosis. In this study, knockout of β2 nAChR subunits lead to a 46% increase in the number of new GCs generated in the OB while the number of PG cells remains unchanged.
Contrary to expectation from the studies mentioned above, my studies clearly demonstrate that the predominant functional subtype expressed in migrating progenitor cells in RMS contains α3β4 nAChR subunits. This receptor subclass modulates diverse processes. For example, α3β4 nAChRs in neurons expressing pro-opiomelanocortin in the hypothalamus participate in nicotine-dependent suppression of appetite (Mineur et al. 2011). In addition, α3β4 nAChRs modulate release of GABA in the hippocampus (Tang et al. 2011) and play a role in cholinergic modulation of OB output (D'Souza and Vijayaraghavan 2012). Their expression on progenitor cells in the RMS but not on mature GCs would imply that they likely modulate development of immature GCs.
My data show that functional α3β4 nAChRs are absent in the soma of mature GCs. This is in agreement with an earlier report that found that GCs do not exhibit detectable currents in response to application of nicotine (Castillo et al. 1999). It is, however, possible that in mature GCs, nAChRs (either α3β4 or other subtypes) are specifically localized at dendritic locations removed from the soma such that their contribution to calcium changes might have gone undetected.
I did see an increase in [Ca]i in a small fraction of mature GCs, but this was completely inhibited by ionotropic glutamate receptor blockers. This suggests that once neurons mature and are incorporated into the GC circuit, cholinergic control is ceded to presynaptic mechanisms. This would be consistent with Hebbian and other activity-dependent control mechanisms for synapse formation. Similar observation has been made in the hippocampus where nAChRs modulate hippocampal progenitor cell development by cell-autonomous calcium signaling, but this effect is lost once cells differentiate and acquire adequate calcium buffering capacities (Berger et al. 1998).
The role of the β2 subunit remains unclear. I have observed robust nicotinic responses from progenitor cells in slices from β2 KO mice. On average, the responses are smaller than those from control slices. My studies appear contradictory to results from Mechawar et al. (2004) where the number of newly generated GCs is higher in β2 KOs. This study does not address the step at which β2 subunit might act or whether there might be compensatory or reactive mechanisms. One plausible explanation is that β2-containing nAChRs are presynaptic and enhance glutamate release on to newly generate GCs, leading to excitotoxicity in some cells. If this is the case, knocking out β2 subunit will be neuroprotective.
Progenitor cells on the RMS have prominent cell bodies with relatively short processes. While it is clear that the majority of the response to ACh is mediated by α3β4-nAChRs, I cannot completely rule out contributions by nAChRs containing α7, β2, or other subunits that may be localized to the tips of the processes. Their responses may have gone undetected if they are small, fast, or localized at the tip alone. In addition, non-cell autonomous roles of nAChRs and the exact composition of these receptors on progenitor cells (whether they contain the β2 subunit as well) are yet to be determined.
GRANTS
This work was supported by Startup Funds from Univ. of Colorado, School of Medicine, and NIH/NCRR Colorado CTSI Grant UL1 RR-025780.
DISCLOSURES
The content of this manuscript is the author's sole responsibility and does not necessarily represent official NIH views. No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.S. conception and design of research; G.S. performed experiments; G.S. analyzed data; G.S. interpreted results of experiments; G.S. prepared figures; G.S. drafted manuscript; G.S. edited and revised manuscript; G.S. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank S. Vijayaraghavan, Diego Restrepo, Sue Kinnamon, and Bruce Appel for helpful discussions and feedback on the manuscript. β2 knockouts were a gift from M. Marks and J. Stitzel, Institute of Behavioral Genetics, Boulder, CO (P30DA015663).
REFERENCES
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70: 589–596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci 15: 897–904, 2012 [DOI] [PubMed] [Google Scholar]
- Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci 18: 6871–6881, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, Kutschera W, Johnson L, Landen M, Druid H, Spalding KL, Frisen J. The age of olfactory bulb neurons in humans. Neuron 74: 634–639, 2012 [DOI] [PubMed] [Google Scholar]
- Campbell NR, Fernandes CC, Halff AW, Berg DK. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci 30: 8734–8744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Carleton A, Vincent JD, Lledo PM. Multiple and opposing roles of cholinergic transmission in the main olfactory bulb. J Neurosci 19: 9180–9191, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza RD, Vijayaraghavan S. Nicotinic receptor-mediated filtering of mitral cell responses to olfactory nerve inputs involves the alpha3beta4 subtype. J Neurosci 32: 3261–3266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy DP, Isaacson JS. L-type calcium channels govern calcium signaling in migrating newborn neurons in the postnatal olfactory bulb. J Neurosci 29: 2510–2518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci 29: 13532–13542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Lazarini F, Wagner S, Gabellec MM, de Chaumont F, Olivo-Marin JC, Boussin FD, Lledo PM, Gheusi G. Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front Behav Neurosci 4: 176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci 12: 731–733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA 97: 1823–1828, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Arevalo NL, Smith L, Xiong KN, Restrepo D. alpha7-Nicotinic acetylcholine receptor: role in early odor learning preference in mice. PLoS One 7: e35251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11: 1153–1161, 2008 [DOI] [PubMed] [Google Scholar]
- Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 11: 1145–1159, 2006 [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci 32: 8696–8702, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact 175: 227–230, 2008 [DOI] [PubMed] [Google Scholar]
- Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci 34: 20–30, 2011 [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci 8: 1179–1187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 314: 1610–1613, 2006 [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7: 179–193, 2006 [DOI] [PubMed] [Google Scholar]
- Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci 31: 392–400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Induction of dendritic spines by beta2-containing nicotinic receptors. J Neurosci 32: 8391–8400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM. alpha-Conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci 18: 8571–8579, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklis JD. Human adult olfactory bulb neurogenesis? Novelty is the best policy. Neuron 74: 595–596, 2012 [DOI] [PubMed] [Google Scholar]
- Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci 13: 753–758, 2010 [DOI] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci 24: 3234–3244, 2006 [DOI] [PubMed] [Google Scholar]
- Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc Natl Acad Sci USA 101: 9822–9826, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science 317: 381–384, 2007 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR. Nicotine decreases food intake through activation of POMC neurons. Science 332: 1330–1332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci USA 106: 17980–17985, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci 12: 728–730, 2009 [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Bardy C, Nissant A, Pallotto M, Sassoe-Pognetto M, Lledo PM, Fritschy JM. Early synapse formation in developing interneurons of the adult olfactory bulb. J Neurosci 29: 15039–15052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron 65: 859–872, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Stamboulian S, Nguyen I, Bordey A. Neurotransmitter signaling in postnatal neurogenesis: the first leg. Brain Res Rev 63: 60–71, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478: 382–386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429: 184–187, 2004 [DOI] [PubMed] [Google Scholar]
- Sharma G, Grybko M, Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J Neurosci 28: 2563–2575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron 38: 929–939, 2003 [DOI] [PubMed] [Google Scholar]
- Tang AH, Karson MA, Nagode DA, McIntosh JM, Uebele VN, Renger JJ, Klugmann M, Milner TA, Alger BE. Nerve terminal nicotinic acetylcholine receptors initiate quantal GABA release from perisomatic interneurons by activating axonal T-type (Cav3) Ca2+ channels and Ca2+ release from stores. J Neurosci 31: 13546–13561, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Synaptic integration of adult-generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci 27: 9951–9961, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SZ, Platel JC, Nielsen JV, Jensen NA, Bordey A. GABA(A) increases calcium in subventricular zone astrocyte-like cells through L- and T-type voltage-gated calcium channels. Front Cell Neurosci 4: 8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]