Abstract
We focus here on the modulation of thin filament activity by cardiac troponin I (cTnI) phosphorylation as an integral and adaptive mechanism in cardiac homeostasis and as a mechanism vulnerable to maladaptive response to stress. We discuss a current concept of cTnI function in the A-band region of the sarcomere, and potential signaling to cTnI in a network involving the ends of the thin filaments at the Z-disk and the M-band regions. The cardiac sarcomere represents a remarkable set of interacting proteins that functions not only as a molecular machine generating the heartbeat, but also as a hub of signaling. We review how phosphorylation signaling to cardiac troponin I is integrated with parallel signals controlling excitation-contraction coupling, hypertrophy, and metabolism.
Keywords: Ca sensitization, Ca signaling, cardiac hypertrophy, ventricular myocytes, Z-disk
Introduction and Overview of the Structure and Function of Cardiac Thin Filament Proteins
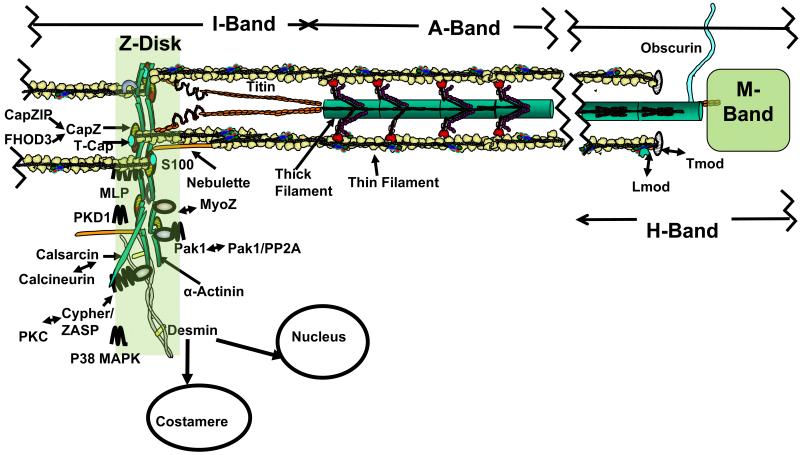
There are a number of relatively recent reviews addressing these topics and providing the detailed evidence for many of the ideas we present.1-4 Our intention here, as much as possible, is to update rather than repeat this information, Figure 1 shows a cartoon of the thin filaments in the sarcomere I-Z-I band and in the A-band. A-band operations of the thin filament are the ones that generally are thought of first, when considering structure/function and modulation of thin filaments. Yet the barbed (Z-disk) and pointed (M-band) ends of thin filaments operate in a realm in which function in the form of cross-bridge interactions is rare and function in the form of signaling is more common. Figure 1 illustrates this region of the thin filaments at and near the Z-disk with emphasis on some of the elements of the Z-disk network of proteins. These elements include CapZ, the protein capping the barbed end (Z-disk end) of the actin strand, α-actinin, titin (T-cap or telethonin), proteins kinases (PK) such as PKC (docked at ZASP), PKD, p38, p21-activated kinase (Pak-1), the phospho-diesterase PDE5, the phosphatase, calcineurin (docked at calsarcin), and transcription factors such as muscle lim proterin (MLP) and MyoZ that shuttle between the Z-disk and the nucleus. These are not all of the growing number of proteins with a Z-disk locus, but these examples serve to emphasize the significant role of thin filaments in this region of the sarcomere. There are a number of reviews providing a more detailed analysis and summary of the Z-disk protein network.5-7 Our purpose here is to point out the importance and relevance of considering this region when assessing the effects of thin filament protein phosphorylation with focus on cTnI. The extended interactions of the Z-disk proteins with costameres and integrins as well as nuclear proteins indicates the potential significance of altered interactions among thin filaments, Z-disk proteins, and the cytoskeletal network. Tropomodulin (Tmod) caps the pointed ends of the thin filaments as also illustrated in Figure 1. As discussed below, together with associated regulatory proteins, CapZ and Tmod operate at the ends of the thin filaments in the maintenance of thin filament functions by their control of the stability and length of thin filaments.
Figure 1.
Scheme illustrating the function of cardiac thin filaments in different regions of a half-sarcomere. The A-Band region depicts thin filaments reacting with thick filaments. At one end I-band regions interact with a complex network of proteins some of which are illustrated to emphasize the presence of kinases (PKC, p38 MAPK, PKD), phosphatases (calcineurin) and transcription factors (eg. MLP, MyoZ) that dock and engage in signaling in this region. at the function in Schematic illustration of a region of the I-Z-I region of the sarcomere also depicted in an electron micrograph. Desmin is depicted as connecting in a cytoskeletal signal network with the costamere as well as the nuclear envelope. CapZ caps the thin filaments at the barbed end at the Z-disk and is significant in signaling. CapZ is regulated by associated proteins, FHOD3 and CapZIP. At the pointed end (M-Band region), thin filaments are capped by Tmod, which is modulated by Lmod. Also in the M-band region, obscurin makes connections with membrane proteins. Titin is shown extending from Z-disk to M-band and is also engaged in signaling stress sensing. See text for details and further explanation.
Our consideration of thin filaments operating in the A-band includes new concepts of thin filament control of the force and shortening reaction of cross-bridges with actin. These new concepts include all of the major regulatory proteins – tropomyosin (Tm), cardiac troponin I (cTnI), cTnC and cTnT (Fig. 2). Extensive and reversible interactions among these regulatory proteins establish the diastolic state and provide an efficient mechanism for transition to the systolic state, maintenance of systolic elastance, and return to the diastolic state. As illustrated in Figure 2, an essential element in these mechanisms includes a movement of Tm on the actin backbone of the thin filament. There is also a likely modification of actin structure and reactivity by its interactions with Tm. Tm is a nearly 100% α-helical protein consisting of two alpha-helical polypeptide chains forming a stable coiled-coil with heptad repeats matching the stoichiometry of 1 Tm: 7 actins.8,9 Tm is thought to be a semi-flexible, mobile protein moving on surface of two stranded helix forming the thin filament backbone. Tm sterically hinders actin-cross-bridge reactions in one position and permits them in other positions.10 Speculation on the dynamics has been considered but definitive measurements, for example hydrogen/deuterium exchange measurements as has been carried out in the Tn complex11, have not been made in thin filaments. Evidence for flexibility with a bend in coils of Tm comes from a high resolution crystal structure.12 Strong evidence for a role of Tm flexibility as a determinant of function has come from studies of Tm in which an Asp at position 137, a highly conserved residue located in the sequence where one would expect a canonical hydrophobic residue.13. These studies provided evidence that Asp-137 endows Tm with flexibility, believed to be important in switching the thin filament-cross-bridge reaction on and off and in the spread of activation. A significance of variations in Tm flexibility has been inferred from studies of Tm mutants, which enhance myofilament response to Ca2+, and are linked to hypertrophic cardiomyopathy (HCM). 14, 15. These mutants demonstrate increased flexibility when investigated alone or bound to actin, and the theory is that the excess flexibility promotes cross-bridge interactions with the thin filaments. An important feature is an N-terminal to C-terminal overlap region between contiguous Tm proteins forming a specialized structure functionally significant in the steep relation between Ca2+ and steady state tension development.16 Disruption of the interaction between Tms on the thin filament reduces the cooperative activation of the reaction of cross-bridges with actin.17 Yet, there remains a residual cooperativity attributable to concerted changes in the state of neighboring actins.
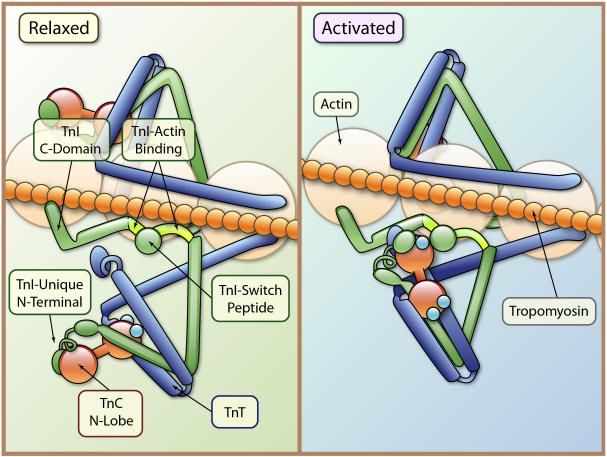
Figure 2.
Scheme illustrating thin filament structural changes in the transition from a relaxed to an active state. In relaxation Tm is immobilized and wedged between actin binding peptides of cTnI and the N-terminal tail of cTnT. There also may be an interaction between the C-terminal domain of cTnI and Tm. Activation occurs with Ca-binding to a single regulatory site on cTnC N-lobe, which promotes an interaction between this region of cTnC with SwP, a switch peptide that reacts with the Ca-bound cTnC N-lobe. Movement of the SwP promote a movement of the actin cTnI binding peptides and via reactions involving the intertwined arms of cTnT and cTnI together with the C-lobe of cTnC there is a release of Tm, exposing binding sites for cross-bridges on actin. Note the position of the dephosphorylated N-terminal extension of cTnI as bound to cTnC. This interactions promotes Ca-binding and Sw-P binding to cTnC (Illustration credit: Ben Smith).
As illustrated in Figure 2, the mobility and position of Tm is under the control of the heterotrimeric Tn complex. 1, 4, 18 Tm is held in a blocking position mainly by the action of the N-terminal tail of cTnT, and by two regions of cTnI, a highly basic inhibitory peptide (Ip) and a second actin-binding region. These cTnI peptides flank the switch peptide, SwP, poised to interact with regions of the N-lobe of cTnC, when exposed by Ca-binding to a single regulatory site. A relatively new concept is the idea that there are direct interactions between Tm and a C-terminal region of cTnI beyond the second actin binding region.19, 20 Another relatively new concept is that Tm is also held in a blocking position by the N-terminal tail of cTnT, and that the tail regulating a Tn on one strand of actins is from a Tn complex in register on the opposite strand of actins (Fig. 2).19 Interactions among C-terminal regions of cTnT, a near N-terminal region of cTnI and the C-terminal lobe of cTnC promote this action of cTnT in diastole. Thus, as illustrated in Figure 2, Tm is wedged between the cTnI actin binding peptides on one side and the cTnT tail on the other, and possibly also immobilized by interactions with a C-terminal region of cTnI.
Ca-binding to a single regulatory site, located in N-lobe of cTnC, results in a release of Tm from its immobilized state thereby allowing release of the sarcomere from its inhibited state and permitting the actin-cross-bridge reaction.21,22 Triggering of this release occurs with structural changes associated with cTnC Ca-binding, which induces the exposure of a hydrophobic patch that attracts an interaction with the SwP.22 Interaction of the SwP with TnC moves the TnI sites tethered to actin, thereby releasing Tm. It is also apparent that interactions between the C-domain of cTnI and Tm exist and are Ca-dependent.19, 20 Epitope mapping studies exploring structural changes in the last 23 amino acids of cTnI, which are highly conserved, indicates that the C-terminus of cTnI is essential to the Ca-switch.23 Truncation of the last 19 amino acids of cTnI at the C-terminus also depresses maximum tension and enhances cross-bridge cycling kinetics.24 The release of Tm is also related to transduction of the Ca-binding signal through to cTnT with induction of an altered interaction of the cTnT N-terminal tail. 25
Near neighbor interactions between thin filament regulatory units control cardiac function
In addition to these steric and allosteric regulatory mechanisms, the activation of thin filaments involves cooperative mechanisms in which the activation of one regulatory unit (RU), which is generally considered as one Tn-Tm complex in association with 7 actins, influences the activation of a near neighbor. The steep relation between pCa and steady-state tension development with Hill coefficients of the order of 4-6 provides strong evidence of a cooperative activation process occurring despite the control by a single regulatory Ca-binding sites on cTnC.16 This suggests that the RUs are in communication and not functioning independently; Tm overlap region as well as actin-actin interaction provides a means of this communication.17 There are opposing views on the mechanism of cooperative activation but both views involve near-neighbor interactions of RUs in the thin filament. One view is that strong, force generating cross-bridges increase the affinity of cTnC for Ca2+ and thus promote near neighbor activation.26-28 This is a classical view of cooperativity in which binding of a ligand promotes further binding of the ligand. Detailed balance dictates that protein-protein interactions, which constitute information flow from Ca-cTnC to actin-cross-bridge, also occur in the reverse direction i.e. also from actin-cross-bridge to Ca-cTnC. Energies of interaction in the steps of the flow of information determine the strength of the signal flow in either direction. Many experimental approaches have biased this information flow in favor of promoting the signaling from actin-cross-bridge to Tn. Thus, in the case of evidence supporting cross-bridge dependent activation of thin filaments, many of the studies have employed strong cross bridges in the form of rigor complexes or N-ethyl-maleimide modified heads of myosin (S-1) rather than cycling force generating cross-bridges. These strong cross-bridges are able to move Tm to a non-blocking position on the thin filament even without Ca-bound to the regulatory site of cTnC. Yet there are data indicating that cycling, force generating cross-bridges may not promote the same, intense level of energy of interaction to Tn as rigor cross-bridges. Experiments by Tobacman and Sawyer29 and Mehegan and Tobacman30 provided direct evidence that RU-RU interactions may be intrinsic to the thin filament. They reported that Ca2+ binds cooperatively to regulatory sites despite the absence of myosin and despite the presence of a single regulatory binding site. Cooperative binding of Ca2+ to thin filaments has been confirmed and demonstrated to be independent of cycling cross-bridges.31 To determine the influence of cycling cross-bridges on the thin filament cooperativity in the force generating lattice, Sun et al. 32 employed fluorescent probes attached to the E-helix in the I-T arm of Tn or to the C-helix next to the regulatory Ca-binding site. Both probes tracked the steep Ca-dependent increase in force in skinned trabeculae regulated by these modified Tn complexes. Although depletion of MgATP and generation of rigor complexes resulted in an increase in the Ca-affinity, inhibition of active force had little effect. Thus, these authors concluded that the cooperative activation of the myofilaments is likely to be intrinsic to the thin filaments, and that cooperative activation by cross-bridges is more likely to be extant in pathological conditions such as ischemia, where rigor cross-bridges may be present.
Studies addressing the molecular basis of length dependent activation, a mechanism fundamental to the Frank-Starling relation, also support a thin filament based cooperative mechanism. Farman et al. 33 reported that disruption of communication from RU to RU by replacing cTnC in skinned fibers with a variant of cTnC with a defunct regulatory Ca-binding site, had a bigger effect in reducing myofilament Ca-sensitivity and Hill n values at short sarcomere lengths compared to long sarcomere lengths. On the other hand reductions in the number of cross-bridges reacting with the thin filaments had similar effects at long and short sarcomere lengths. While the basis for the Frank-Starling relation length dependent activation remains controversial and likely to involve multiple mechanisms34, these data indicate that length dependent activation may not dependent cooperativity induced by strong, force generating cross-bridges.
Tm-Tm and actin-actin interactions are likely to couple an active RU to inactive near neighbor, but modulation of the cooperative spread of activation is likely to involve the Tn complex. The influence of cTnI-binding on actin and on cTnC Ca-affinity is well documented and modulation of these interactions may be an element in the communication between near neighbor RUs.21 The modulation could occur through cTnI phosphorylation, which is known to reduce cTnC Ca-affinity.35 Phosphorylation of cTnI affects length dependent activation 36, as does isoform switching of cardiac to slow skeletal TnI, the embryonic/neonatal isform.37 Length dependent activation is also critically dependent on cTnI-Thr144, a unique amino acid in the Ip and phosphorylation site in cTnI. There is also evidence that specific amino acid substitutions in cTnI are able to alter the effect of NEM-S1 on cooperative activation of cardiac myofilaments.38, 39 It remains to be determined whether interactions of a C-domain of cTnI with Tm affects cooperative spread of activation, but there is evidence for a role for modifications in cTnT.40 Apart from its implications in the Frank-Starling relation, cooperative control of myofilament response to Ca2+ is a feature of control of cardiac dynamics with relevance to the rate of rise of tension and pressure and stretch-dependent activation .27 We 41 have previously argued that the cooperative spread of activation is a significant and potentially dominant factor controlling the duration of systolic elastance and isovolumic relaxation in the heart beat. Here we address the question of the relative role of cTnI-cTnC interactions in the control of cooperative activation and the question of modulation of cooperative activation by cTnI phosphorylation. We consider these questions in the next section in discussions of the relevance of control mechanism at the level of the sarcomeres in regulation of cardiac function.
cTnI phosphorylation and integrated control of cardiac function by Ca-fluxes and myofilament response to Ca2+
There is no doubt that the amounts and rates of Ca2+ movements to and from the sarcomeres are significant controllers of the intensity and duration of tension (pressure) development and rates of contraction and relaxation. In the basal state estimates indicate that enough Ca is released to occupy about 20% of the Tn regulatory units (RU) on the thin filament.42 Thus one conceptualization of the cardiac inotropic reserve is the 80% of RUs available for recruitment and regulation of contractility. There is also a relaxation or lusitropic reserve. How these reserves are engaged physiologically, and what may go wrong with them in cardiac disorders are important questions. One mechanism is straight forward and involves variations in the load of Ca2+ in the SR for release and variations in the rate of return of Ca2+ to SR. The extent and complexity of the wealth of mechanisms for controlling Ca2+ fluxes to and from the myofilaments support the significance of this mechanism of control of inotropic and lusitropic reserve.43
A growing set of compelling data have brought support and clarity to the idea that control of cardiac dynamics and power is not solely dependent on cellular Ca-fluxes, but depend significantly on the response of the sarcomeres to Ca2+.1 The advantage of a regulatory process involving both Ca fluxes and Ca-response is that it provides a mechanism for control of inotropic and lusitropic reserve while limiting the liability of Ca2+ overload and the associated arrhythmias. In a previous paper, we set forth arguments and speculation that some aspects of the dynamics of the cardiac cycle are, in fact, dominated by control mechanisms at the level of the sarcomeres.41 Prominent mechanisms at the level of sarcomeres that can be rate limiting in the contraction/ relaxation cycle include the kinetics of the reaction of cross-bridges with actin, the kinetics of Ca-release from cTnC 31, 35, and the influence of the dynamics of cooperative processes within the myofilament lattice. There is strong evidence that protein phosphorylation of cTnI modifies each of these mechanisms in physiological control of the heartbeat.
There are many reports demonstrating that dynamics of cardiac myocytes may change with no change in dynamics of the Ca-transient. A common mechanism giving rise to this effect is a modification in cross-bridge cycling kinetics as for example occurs with isoform switching of myosin isoforms with differing ATPase rates. Moreover, it has been demonstrated by use of inotropic agents acting directly at the level of the myofilaments that force and shortening can be significantly enhanced by mechanisms specifically affecting myofilament response to Ca2+.44 Most recently data generated from experiments investigating the sarcomere activator, omecamtiv mecarbil, have demonstrated how an agent modifying cross-bridge duty cycle is able to increase contractility with no effect on cellular Ca-transients.45 Our studies have demonstrated that similar modification in actin myosin interactions occur with light chain phosphorylation 46 and with cTnI phosphorylation.47 Moreover, we have recently reported that ventricular myocytes from a model of diastolic heart failure demonstrates slowed relaxation but no change in Ca-fluxes.48 Linkage of DCM and HCM to sarcomeric mutations provides strong support for the significant role of modulation of myofilament response to Ca2+ in short and long term homeostasis of cardiac function. Mutations linked to HCM and DCM induce opposite alterations in myofilament Ca-sensitivity, and thus indicate that there exists a homeostatic zone of sarcomeric Ca-responsiveness.49 The idea is that when sarcomeres operate outside this zone for sustained periods, as occurs in a genetic defect, there is an induction of maladaptive responses. These may be mechanical stressors such as altered relaxation or altered force production or tension cost. There may be an induction of altered Ca2+ interactions with the TnC leading to arrhythmias.50 Moreover, as is evident in familial cardiomyopathies, there is induction of altered gene regulation resulting in a hypertrophic (DCM) or dilated cardiac phenotype (DCM).
Unique phosphorylation at Ser23/Ser24 by diverse kinases controls cardiac function
Phosphorylation of cTnI-Ser 23/Ser24 (Fig. 3) is the most well understood thin filament protein post-translational modification in control of sarcomeric response to Ca2+ and integration with Ca-fluxes controlling inotropic and lusitropic reserve.2-4, 18 Ser 23/Ser24 in the unique N-terminus of cTnI are substrates for PKA and were among the earliest phosphorylation sites to be identified. However, since these early studies more sites of phosphorylation, more kinases with cTnI as a substrate, and new insights into the role of multi-site phosphorylations have been identified. With the advent of high resolution mass spectrometry techniques such as “top down” approaches and multiple reaction monitoring, previously unappreciated sites have emerged and most likely will continue to emerge as the studies extend into more detailed investigations of specific myocardial regions and investigations of alterations in physiological and patho-physiological states of the hearts. In addition to PKA, Ser23/Ser24 sites are substrates for PKG, PKCβ, PKCδ, and PKD1.51
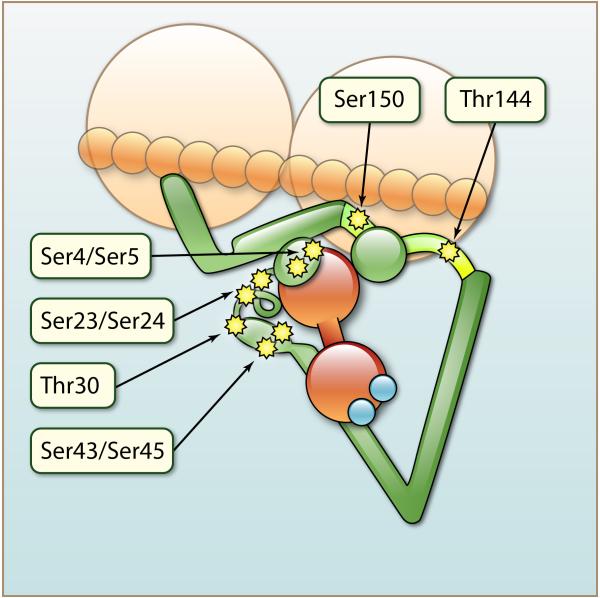
Figure 3.
Illustration of the potential phosphorylation sites of cTnI for which there are functional correlates as described in the text. Note the double arrow indicating that the N-terminal extension of cTnI shifts position upon phosphorylation at Ser23/Ser24 and thus promotes release of Ca2+ and the SwP from the N-lobe of cTnC. Also note the proximity of the acidic N-terminus of cTnI to the regulatory domain of cTnI containing the SwP. Functional correlates of the phosphorylation sites are discussed in the text. (Illustration credit: Ben Smith).
Ser23/Ser24 phosphorylation is well known and generally agreed to induce a desensitization of steady-state myofilament force generation to Ca2+. Although controversial52, 53, there are also data indicating that Ser23/Ser24 bis-phosphorylation is associated with an increase in cross-bridge kinetics.54 We think it is highly relevant that myofilaments controlled by constitutively phosphorylated cTnI at Ser23/Ser24 demonstrate increases power 55, and also show an enhanced force-frequency modulation and afterload relaxation sensitivity.56 Evidence also indicates that the bis-phosphorylation of these sites decreases the Ca-affinity of TnC regulatory sites and may also decrease the affinity of cTnC for the SwP independent of an effect on Ca-affinity.22, 35 The cTnI-Ser23/Ser24 sites occur in the highly flexible N-terminal domain, comprised of ~30 amino acids and unique to the cardiac variant. This region did not resolve in the core crystal structure of cTn 57, but data derived from solution NMR and neutron contrast variation studies, have provided an atomic model of Tn revealing the conformational transition with bis-phosphorylation of Ser23/Ser24.58 The atomic model revealed that without phosphorylation the region around the phosphorylation motif and an α-helix (residues 25-30) are less structured than with phosphorylation. In the absence of phosphorylation the N-extension interacts with the N-lobe of cTnC in a position to influence both Ca-binding and binding of the SwP by enhancing their affinity for the N-lobe relative to the phosphorylated state. The interactions are with cTnI acidic residues interacting with Arg22 and Arg 28 of cTnC and with hydrophobic residues Leu 29 and Pro12 of cTnC. Leu29 mutated to a Gly is linked to HCM, which induces a more open sub-state of the cTnC N-lobe and hinders the effect of phosphorylation of Ser23/Ser24 on myofilament Ca-sensitivity.59 Ser23/Ser24 phosphorylation induces an extension of helix (residues 21-30) and weakens the interaction of the N-extension with cTnC. This repositioning and bending alters the axial ratio of cTnI and appeared to be aided by a poly-proline helix (residues 11-19) forming a rigid linker. As illustrated in Figure 3, these conformational transitions place the acidic N-terminus cTnI (residues 1-10) close to the C-domain of cTnI containing the basic Ip, the second actin binding domain, and the SwP. To test for this intra-molecular interaction, we 60 employed cTnI-Ser5Cys and cTnI-Iso19Cys, labeled with the hetero-bifunctional cross-linker benzophenone-4-maleimide. Our studies identified novel cross-linking between these mutants at Met-154 (residue 19 mutant) and at Met-155 (residue 5 mutant) of cTnI and novel inter-molecular interactions at positions Met-47 and Met-80 of cTnC. The cross-linking between the acidic N-terminus of cTnI and these Met residues, which reside in the SwP, provide evidence supporting the possibility of an intra-molecular interaction controlling the Ca-responsiveness and power of the myofilaments with cTnI phosphorylated by PKA. Studies of myofilaments regulated by cTnI-Arg146Gly linked to HCM provide indirect evidence of interaction between the N-terminus of cTnI and the regulatory C-domain of cTnI surrounding the Ip.61 The loss of the basic residue in the Ip leads to enhanced Ca-sensitivity in the myofilaments, but importantly there is also a loss of the ability of PKA-phosphorylated cTnI to reduce Ca-sensitivity.62 This effect also indicates the possibility that the control of myofilament response to Ca2+ by cTnI phosphorylation involves an intra-molecular interaction. Studies investigating interactions between Thr144, a PKC substrate in the Ip and Ser23/Ser24 also support the potential significance of an interaction between the cTnI-N extension and the regulatory domain surrounding the Ip. These studies employed pseudo-phosphorylation variants of cTnI-Thr144Glu and cTnI-Ser23D, Ser24D. Whereas the presence of Ser23D/Ser24D or Thr144Glu both desensitized the myofilaments to Ca, there was reduction in Ca-affinity only in the myofilaments regulated by Ser23D/Ser24D. In other words, desensitization of the myofilaments to Ca2+ occurred independently of altered cTnC Ca-affinity in the case of myofilaments regulated by cTnI-Thr144Glu. Moreover, when compared to myofilaments regulated by either, wild-type cTnI, cTnI-Ser23D/Ser24D, or cTnI-Thr144E, myofilaments regulated by cTnI-Ser23D/Ser24D/Thr144E demonstrated a significantly depressed Hill n value for both the pCa-tension and Ca-binding relations. As discussed below there is also evidence of interactions between phosphorylation at Ser23/Ser24 and cTnI-Ser150, a site phosphorylated by Pak1, Pak3, and AMPK.
Troponin I phosphorylation as an integral mechanism in hypertrophic and mechano-signaling networks and in the progression to heart failure
While the functional significance of phosphorylation at Ser23/Ser24 is well accepted, the functional significance of the phosphorylation of other sites is either poorly understood or controversial3. Early studies identified Ser43/Ser45, and T144 as substrates for PKC, and eventually as substrates for specific PKC isoforms.63 Further studies employing transgenic mouse models with up and down regulation approaches with site specific modifications or expression of PKC isoforms further supported a functional role of these PKC sites.47, 64-66 The effect of A general conclusion is that when phosphorylated the Ser 43/Ser45 sites in contrast to phosphorylation of Ser23/Ser24 depress maximum tension and cross-bridge kinetics. In our hands, phosphorylation of T144 alone had little effect on tension or Ca-sensitivity, but also depresses cross-bridge kinetics 67. The effect of phosphorylation at Ser 43/Ser45/Thr144 dominates the effects of phosphorylation at Ser23/Ser24.55, 68 More recently, we 47reported results of extensive studies of a transgenic mouse model expressing pseudo-phosphorylated cTnI (cTnI-Ser43Glu, Ser45Glu,T144Glu). Although we determined that only ~7 per cent of the endogenous cTnI was replaced with the mutant, the experiments revealed an induction of a negative inotropic effect with significantly slowed relaxation in isolated hearts and in intact papillary muscle preparations. Ca-transients were unaffected in isolated cardiac myocytes, and there was no effect of myofilament Ca-sensitivity or tension cost. A mathematical model was employed to understand and analyze the integrative interpretation of the data. The model fit the data on the basis of a decrease in the rate of cross-bridges into the force generating state thereby producing negative inotropy, and an increased persistence of the myofilament active state producing negative lusitropy. Studies on animal models of heart failure produced by either pressure overload (P/O) or myocardial infarction (MI) also produced a decrease in maximum tension dependent on phosphorylation of cTnI 69. Analysis of the cTnI by non-equilibrium gel electrophoresis revealed a preponderance of highly charged cTnI in both the P/O and MI models of heart failure compared to controls. These changes could also be produced by treatment of the skinned myocytes with PKCα.70
A role for PKC activation in human hearts with ischemic failure was first made clear by studies reported by Bowling et al. 71, who reported an increased activation of PKCβII compared to control heart samples. Mouse hearts expressing an active form of PKCβII also demonstrated an decrease in contractility and an associated increase in cTnI phosphorylation 72. In a well-controlled study, Hwang et al. 73 infected adult cardiac myocytes with viral constructs expressing active PKCβII and observed an overall depression in rates and amplitudes of shortening with a parallel decrease in Ca2+ dynamics. Surprisingly while the activated PKCβII promoted dephosphorylation of phospholamban, there was an increase in phosphorylation of myofilament proteins including cTnI-Ser23/Ser24. Increases in activation of PKD1 in the myocytes expressing activated PKCβII may have accounted for the increased phosphorylation of cTnI-Ser23/Ser24. No further analysis of sites of cTnI phosphorylation was carried in the study by Hwang et al.
However despite all this evidence for the potential significance of phosphorylation at the PKC sites, most studies with human heart samples at end-stage failure showed only a reduction in phosphorylation at Ser23/Ser24 and no evidence for Ser43/Ser45 or Thr144 phosphorylation. Yet incubation of skinned myocytes from failing human hearts with PKCα and PKCε resulted in cTnI phosphorylation and desensitization to Ca2+. PKCα, but not PKCε, induced a small but significant depression in maximum tension. Dong et al.74 reported definitive evidence of an association of heart failure in the spontaneously hypertensive (SHR) rat model with phosphorylation on PKC sites on cTnI. Employing a “top down” proteomic approach they reported unambiguous evidence of an increase in cTnI-Ser43/Ser45 phosphorylation in samples from the failing hearts of the SHR rats compared to controls. Moreover, employing mutltiple reaction monitoring (MRM) in a mass spectrometry approach to compare and quantify cTnI phosphorylation in samples from donor and end stage DCM and HCM hearts, Zhang et al. 75 confirmed that both cardiac disorders show a depression in phosphorylation at cTnI-Ser23/Ser24, but an increase in phosphorylation of cTnI-Ser43/Ser 45. Zhang et al. also reported a depression in phosphorylation of sites identified at cTnI-Ser5/Ser6 associated with DCM and HCM. Other sites identified on cTnI await further functional analysis. In a separate set of studies, we had also found the existence of phosphorylation at cTnI-Ser5/Ser6 in mouse hearts.76 Pseudo-phosphorylation of the sites induced a decrease in maximum tension and ATPase rate. Thus, the decrease in phosphorylation of Ser5/Ser6 noted by Zhang et al. may have been an adaptive response. Whatever the case, the effects of phosphorylation in the acidic N-terminus of cTnI provides further evidence for the potential effect of intra-molecular interactions of this region of cTnI with the SwP 60, 77. Earlier studies had identified the acidic region of cTnI as a functionally significant domain in contractility and response to adrenergic stimulation.78 The significance of the acidic region of cTnI at amino acids 1-10 is also evident from linkage of an Ala5Val mutation to DCM.79 The mutation induced a depressed cooperative activation of the myofilaments but not in the presence of cTnI-Ser23/Ser 24 phosphorylation.76 With anticipated advances and more application of high resolution mass spectrometry, we expect that more new insights into phosphorylations and other post-translational modifications will be forthcoming. For example, there is evidence that mammalian sterile 20-like kinase 1 has sites of cTnI as a substrate, with Thr 30 at the hinge region of the N-extension (Fig. 3) as the most likely preferred site during hydrogen peroxide treatment. Further experiments are required to more fully understand the significance of this effect.80 The amplification of functional effects as a result of modest changes in phosphorylation makes the understanding of even small changes potentially significant.
Studies such as those described above emphasize the concept that homeostasis of cardiac function requires a balance of phosphorylations of cTnI and most likely other proteins such as MyBP-C, which also has multiple sites of phosphorylation. In addition to the disturbance of this balance by stresses such as MI or hypertension, there is an increase in appreciation of an influence of oxidative stress on the homeostatic balance of phosphorylation of myofilament proteins.81, 82 This is illustrated by a direct connection between oxidative stress, Src activation, and PKCδ with cTnI as a substrate. In the case of PKCδ activated by lipid co-factors phosphorylation occurs at cTnI occurs at Ser23Ser24. However, if the PKCδ is phosphorylated by Src at Tyr311 and Tyr 322 there is an enhanced auto-phosphorylation at Thr505 in the activation loop, and phosphorylation at both Thr144 and Ser23/Ser24. Studies with skinned myocytes demonstrated a mechanical correlate to this switch in substrate preferences. Moreover, treatment of heart muscle preparations with hydrogen peroxide as a mimic of oxidative stress also induced an activation of PKC and modification of myofilament83 response to Ca2+. 84
cTnI phosphorylation and signaling at the barbed and pointed ends of cardiac thin filaments
In previous studies, we have developed evidence indicating a modulation of cTnI phosphorylation by signaling associated with the modification of protein-protein interactions in the Z-disk protein network. Localization of kinases and phosphatases at the Z-disk provide one mechanism for this remote control of the A-band region of thin filaments by signals at the Z-disk regions. There are several reviews dealing with general signaling involving these thin filament regions.5, 6, 85 Regulation of protein phosphatase 2A (PP2A) activity by Pak1 with dephosphorylation of cTnI may also occur via a Z-disk related mechanism. We86, 87 reported that Pak1 has a Z-disk localization and moves to the cytoplasm with activation. Active Pak1 has anti-hypertrophic properties, but the role of Z-disk signaling in this mode of action remains unknown.88 In another line of experiments, we 89-91 and others 92, 93have reported that alterations in the interaction of CapZ at the barbed end of thin filaments sets into motion PKC mediated regulatory mechanisms involving phosphorylation of cTnI. We first identified a role for integrity of the CapZ-thin filament interaction in relocation of PKC isoforms to the myofilaments and in the phosphorylation of cTnI 90, and subsequent studies have developed evidence for control of the interaction by lipid signaling through PIP2 89, as well as induction of a protective effective in hearts stressed by cold cardioplegia, ischemia reperfusion and pre-conditioning92, 93. These results indicate that modulation of the CapZ-thin filament interaction by neighboring proteins is a significant factor in the modulation of cardiac function by cTnI phosphorylation. Inasmuch as our initial studies 90demonstrated that modification of the CapZ-thin filament interaction induced a significant increase in expression of Tmod at the pointed end, we also discuss regulatory proteins that affect Tmod.
Thin filament associated proteins positioned at the barbed (Z-disk) and pointed (M-line) ends of thin filaments are important in mechanisms controlling the assembly of thin filaments, which is critical to hypertrophic growth, as well the maintenance of thin filament stability and length. 90 Regulation of actin dynamics most likely requires coordination of control mechanisms at both ends of the thin filament, but Littlefield et al. 94 reported that actin exchange at the pointed end occurs significantly faster than actin exchange at the barbed end of actin filaments. The actin capping protein, CapZ, caps the barbed end of thin filaments and is tethered to the Z-disk via, alpha-actinin, which cross-links adjacent thin filaments (Fig. 1). At the pointed end, thin filaments are capped by the striated isoform of tropomodulin (Tmod-1) (Fig. 1). Tm is another actin-binding protein critical to the stability and maintenance of thin filament by inducing a resistance to de-polymerization. 95 As discussed below, Tmod binding to Tm is also an important element in the capping of the pointed ends of thin filaments. 96 Although more investigation is required, emerging evidence indicates that these control mechanisms may involve phosphorylation.
CapZIP, which is present in both cardiac and skeletal muscle, has been identified as a CapZ interacting protein, which is phosphorylated by several protein kinases, such as JNK, important in stress responses. 97, 98 The phosphorylation of CapZIP induces a dissociation of CapZ from thin filaments and is thus believed to regulate actin assembly. No detailed investigation of the role of CapZIP in thin filament assembly in the heart has been carried out. However, CapZIP phosphorylation has been reported to be modified in hearts stressed by hypertrophy. 99 Phosphorylation also appears to play a role in the control of CapZ binding to thin filament by FHOD3, which is a member of the formin family controlling the assembly of thin filament via FH2 (formin homology domain 2). There is disagreement as to the exact position of FHOD3 on the thin filaments. One set of studies 100 concluded that FHOD3 localizes at the A-band-I-band junction, but in a position permitting interaction of the FH2 domain with the barbed end of actin. Another set of studies 101 concluded that FHOD3 localizes to the Z-disk. Iskratsch et al. 101 reported evidence that FHOD3 is regulated by casein kinase 2 (CK2) dependent phosphorylation. The site of phosphorylation arises from tissue specific splicing generating a muscle specific isoform possessing an 8 amino acid C-terminal extension with the CK2 phosphorylation site. Phosphorylation targets FHOD3 to the thin filaments and releases it from p62, a protein which has multiple roles in kinase signaling, and functions in protosomal activity and autophagy.102 Compared to controls, hearts with a decrease in expression of FHOD3 (mouse model of DCM and human hearts in failure) show a loss of myofibrillar integrity. Future experiments will have to be carried out to determine whether, when, and how CK2 signaling is engaged in cardiac myocytes undergoing stresses such as stretch that induce increased actin assembly.
Interactions of Tmod at the ponted end of thin filaments provide a mechanism for control of filament length, stability, and assembly. Levels of Tmod are critical to myofibrillar stability. Sussman et al. 95 reported that over-expression of Tmod in a mouse model induced myofibrillar disorganization with short thin filaments, and a dilated cardio-myopathy, whereas Littlefield et al. 94 reported that a reduction in expression of Tmod leads to relatively long thin filaments. The interaction of Tmod with the pointed end of thin filaments occurs through interactions of an N-terminal region with both actin and Tm with two domains interacting with Tm and one domain interacting with actin. 96, 103 Tmod binding to the pointed end is antagonized by leiomodin-2 (Lmod), which also binds to the pointed end of thin filaments, but does not act as a capping protein. It is apparent that the competition of Lmod with Tmod for aTm binding site is most likely to account for the antagonism between the two regulatory proteins. It may be of significance that the human Lmod gene is located near the hypertrophic cardiomyopathy locus CMH6 on human chromosome 7q3. On the basis of this identification, Conley et al. 104suggested that Lmod may be implicated in HCM.
Whether phosphorylation is an important signaling mechanism to the set of proteins controlling actin dynamics at the pointed end of the thin filaments remains unclear and understudied. A role for Tm phosphorylation in modulating the interaction among these regulatory proteins at the pointed end has not been explored to our knowledge. However, there are data suggesting that phosphorylation of Tmod may modulate its interactions with actin and Tm105. Dorovkov et al. 105 reported that pseudo-phosphorylation of Tmod-1 mimicking phosphorylation byTRPM7 kinase induced a loss of Tmod-1 capping function in thin filaments. The phosphorylation was at a highly conserved Thr residue (T54), which appeared to alter only actin binding and not Tm binding. TRPM7 kinase is fusion of a Ser/Thr kinase with an ion channel.106 The channel is inactivated by PIP(2) hydrolysis 107, which may be of some interest inasmuch as PIP(2) has been reported to alter CapZ interactions with the barbed end of thin filaments 89, and one wonders if a similar mechanism may be occurring at the pointed end via a modification of TRPM7 activity. Whatever the case, there is a need to more thoroughly investigate a role for post-translational modification of thin filament regulatory proteins with a role in thin filament assembly, length, and stability.
cTnI phosphorylation as an integral homeostatic mechanism in metabolic signaling networks
A potentially significant and under investigated area of research is the coordination of signals controlling cardiac energy supply and signals controlling energy consumption by the myofilaments. As major consumers of energy this coordination at the level of myofilament proteins appears particularly important to understand. A strong case for this has been made in the case of mutations in myofilament proteins linked to familial hypertrophic cardiomyopathy, a disease that remains characterized as a disease of the sarcomere. One of theories related to mechanisms linking a sarcomere disease gene to hypertrophy and sudden death is related to the promotion of energy deficiency and altered Ca2+ fluxes. 108 There is evidence that the mechanism may be more than a simple energy demand/energy supply imbalance, but may involve signaling mechanisms that modify myofilament response to Ca2+. Moreover, metabolic links to altered gene expression are well described in the switch to a fetal gene program in hearts responding to a variety of stressors. 109 We have reported a similar switch to fetal metabolic phenotype by expression of slow skeletal TnI in the adult heart. 110 Here our focus is on coordinated signaling between metabolic demand and metabolic supply with cTnI phosphorylation as a relevant example.
A recent and excellent example of the integration of mechanical and metabolic signaling networks comes from studies, which have identified cTnI as a substrate for AMPK. 111-113 AMPK is well known to act as an energy sensor in cardiac myocytes 114, 115. Imbalances in AMP/ATP ratio resulting from metabolic stress, such as ischemia and reperfusion injury, shift this ratio and activate AMPK. The activation is generally considered to be protective as it offsets the metabolic stress. Apart from the metabolic enzymes demonstrated to be regulated by AMPK, there is evidence that other significant elements in function of cardiac myocytes may be substrates for AMPK. For example, there are data suggesting that Nchannels may be substrates for AMPK as indicated by arrhythmogenic activity in patients with mutations in the AMPK gene, PRKAG2 116. Along these lines, there is also evidence suggesting that Na+, Ca2+ exchanger may be a substrate for AMPK, but this has not been studied in the heart 117. Among the sarcomeric proteins, cTnI is the most well documented substrate for AMPK. In vitro studies 113 identified two regions of cTnI as substrates for AMPK, Ser22 in the unique N-terminal extension and Ser150 in the switch region (Fig. 1). Kinetic studies indicated Ser150, which was phosphorylated by AMPK at a much faster rate of phosphorylation than Ser22, as the more important and more likely in situ substrate. Two subsequent studies 111, 112 confirmed these initial finding with more direct evidence that cTnI-Ser150 is a substrate for AMPK. Oliveira 112 employed a yeast two-hybrid screen to identify cTnI as a protein interacting with AMPK. Hearts responding to promoters of AMPK activity (either Aicar or ischemia) showed an increase in cTnI-Ser150 phosphorylation, which was also, depressed by an inhibitor. Relaxation rate was slowed in association with activation of AMPK with no change in the dynamics of the Ca2+ transient 112. These results indicated that AMPK-dependent phosphorylation of cTnI might induce an increase in Ca-responsiveness of the myofilaments. This was confirmed by studies with skinned fiber preparations in which S-150 of cTnI was phosphorylated by incubation of reconstituted myofilament preparations with AMPK. Moreover, skinned fiber preparations regulated by a pseudo-phosphorylated mutant (cTnI-Ser150Asp) also demonstrated an increase in sensitivity to Ca2+ and an increased Ca-binding to the cTnC regulatory site.111 An important additional finding in this study was the demonstration that associated with phosphorylation of cTnI-Ser150 there was reduced desensitization by PKA dependent phosphorylation of cTnI-Ser23,Ser24 and a reduced effect of PKA-dependent phosphorylation to enhance length dependent activation. It is of interest that both these effects would tend to increase Ca-binding to cTnC, which may also be a factor in the arrhythmias. There are important implications of the findings on the effects of cTnI-S150 phosphorylation on mechanisms modulating sarcomere response to Ca2+. Data showing an interaction between effects of phosphorylation at Ser23/Ser24 in the N-helix and at Ser150 also support previous studies, as discussed above, in which we reported a close proximity between residues in the cTnI-N-terminal extension and Met residues at position 155 of cTnI 60. Moreover, Ouyang et al. 118 reported evidence from studies using steady-state Forster fluorescence resonance energy transfer that with pseudo-phosphorylation of cTnI at Ser150, there is a shortening of the inter-site distances between cTnI and cTnC. Interestingly, this modification in inter-site distance was similar to the effect of strong cross-bridges on the structural transitions in cTnI. This is a significant finding inasmuch it provides further evidence for a role for modulation of the cTnI-cTnC in thin filament related cooperative activation of the myofilaments.
Signaling via PKD1 (formerly known as PKCμ) also provides an example of the coordination of signaling to the myofilaments apparently coordinated with metabolic supply and demand, as well as nuclear signaling promoting hypertrophy.100, 119, 120 PKD1 is able to phosphorylate both cTnI and MyBP-C resulting in desensitization of the myofilament response to Ca2+. as well as increase in cross-bridge kinetics.119, 121. As with PKA, PKD also has effects on excitation-contraction coupling in form of altered L-type Ca-channel activity and Ca-loading of the sarcoplasmic reticulum.122 Studies with electrical stimulation of isolated cardiac myocytes demonstrated a stimulus dependent activation of PKD and dynamic regulation of cTnI phosphorylation and myofilament Ca-desensitization.122 Nuclear PKD1 also phosphorylates HDAC5, promoting its export from the nucleus and releasing MEF2 from inhibition thereby inducing hypertrophic growth. Active PKD1 is also critical in enhanced glucose uptake associated with contraction in adult cardiac myocytes by mechanism independent of AMPK activation.123 The mechanism is a PKD1 dependent increase in Glut4 translocation and glucose uptake. While AMPK activation can increase fatty acid uptake, PKD1 activation cannot. An interesting aspect of the experiments of Dirkx et al. 123 is the demonstration of the relative reactive oxygen species (ROS) sensitivity of PKD1 signaling and effects of glucose transport compared to ROS dependent effects on AMPK signaling and fatty acid uptake. The relative effects of ROS dependent modulation of PKD1 activity with regard to cTnI phosphorylation has not been investigated to our knowledge. As with many signaling networks, PKD1 signaling presents a puzzle to be solved in terms of why Nature has chosen to modify cTnI phosphorylation by different kinases. Localized signaling may provide the clues to solution of the puzzle. In the case of PKD1, localization at the Z-disk or related regions has been shown.122 There is also a regulatory complex consisting of AKAP-Lbc, which scaffolds PKA and PKD1. PKA activation is known to suppress PKD1 activity, and localized activity of PDE3 and PDE4 may control PKA activity. These mechanisms provide pathways whereby PKD1 activity may be directed toward various cellular functions via adrenergic receptors signaling through DAG/PKC for cAMP/PKA or via growth receptors such as endothelin receptors acting through PKC. This is but one example where more data are needed with regard to what happens, when and where following a stimulus of the cardiac myocytes relevant to a physiological adaptive response, or to pathophysiological maladaptive response.
Summary and Challenges
It is no surprise that in the nearly 40 years since the first identification of functionally significant covalent phosphorylation of sarcomeric proteins, major challenges remain, despite a steady increase in studies and the appreciation of these post-translational modifications in complex signaling networks. The discussion here on multi-site phosphorylation of cTnI in this network is an example of how little we know, despite the generation of much knowledge. We know or will soon know all the thin filament sites potentially phosphorylated and their relative abundance. We have a good idea of the functional consequences of the site specific phosphorylations at various levels of organization. We have knowledge strongly indicating that the phosphorylations provide an integrated response coordinating energy consumption and energy supply as well as excitation contraction coupling, and transcription and translation. We know that the sites of phosphorylation are modified in homeostatic, adaptive physiological control of cardiac output, and we know the sites of phosphorylation are modified in maladaptive responses to acquired and inherited disorders of the myocardium. We don’t know precisely the dynamics of the multi-site phosphorylations in the time course of the response to physiological or pathological signals and stressors. We don’t know precisely how the phosphorylations are coordinated with diverse signaling events and their site specific occupancy.. In the best of scientific worlds we would have an in situ probes providing readout of site-specific phosphorylations during a temporal change in cardiac function. Addressing these unanswered questions may be impossible for the near term; we have to make the best with the approaches we do have, and we have to continue to take lessons from advances made in network biology in much simpler systems than the heart, such as yeast. Moreover in the face of massive amounts of genomic and proteomic information, together with knowledge of spatial/temporal relations among proteins as well as kinase/phosphatase signaling networks we must devise ways of separating signal from noise.
Acknowledgements
The authors wish to thank their many colleagues for contributing to the studies cited in this review.
Sources of Funding
NIH RO1 HL 064035, RO1 HL 022231, PO1 062426 (RJS), T32 007692 (MH)
List of Non-standard Abbreviations
- AMPK
5′AMP Kinase
- CapZ
actin capping protein
- cTn
cardiac troponin
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- cTnC
cardiac troponin C
- DAG
diacyl glycerol
- DCM
dilated cardiomyopathy
- FH2
formin homology domain 2
- Ip
troponin I inhibitory peptide
- HCM
hypertrophic cardiomyopathy
- SwP
troponin I switch peptide
- NEM
n-ethyl maleimide
- MI
myocardial infarction
- Lmod
leiomodin 2
- MLP
muscle lim protein
- MRM
multiple reaction monitoring
- Pak1
p21-activated kinase 1
- PDE
phospho-diesterase
- PIP2
phosphoinositide bis-phosphate
- PK
protein kinase
- P/O
pressure overload
- PP2A
protein phosphatase 2A
- ROS
reactive oxygen specie
- RU
regulatory unit
- S-1
myosin head
- SR
sarcoplasmic reticulum
- Tcap
titin capping protein
- Tm
tropomyosin
- Tmod
tropomodulin
- TRPM7
transient receptor potential melastatin 7
Footnotes
Subject Codes: [105]; [145];[147] ;[148]
In November 2012, the average time from submission to first decision for all original research papers submitted to Circulation Research was 15.8 days.
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solaro RJ. Sarcomere control mechanisms and the dynamics of the cardiac cycle. J Biomed Biotechnol. 2010;2010:105648. doi: 10.1155/2010/105648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solaro RJ, Kobayashi T. Protein phosphorylation and signal transduction in cardiac thin filaments. J Biol Chem. 2011;286:9935–9940. doi: 10.1074/jbc.R110.197731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solaro RJ, van der Velden J. Why does troponin I have so many phosphorylation sites? Fact and fancy. J Mol Cell Cardiol. 2010;48:810–816. doi: 10.1016/j.yjmcc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: The essential role of troponin in cardiac muscle regulation. Circ Res. 2004;94:146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 5.Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: The role of z-discs in intracellular signaling and cardiac function. Circ Res. 2004;94:296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- 6.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solaro RJ. Remote control of A-band cardiac thin filaments by the I-Z-I protein network of cardiac sarcomeres. Trends Cardiovasc Med. 2005;15:148–152. doi: 10.1016/j.tcm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Wolska BM, Wieczorek DM. The role of tropomyosin in the regulation of myocardial contraction and relaxation. Pflugers Archiv: European journal of physiology. 2003;446:1–8. doi: 10.1007/s00424-002-0900-3. [DOI] [PubMed] [Google Scholar]
- 9.Hitchcock-DeGregori SE, Greenfield NJ, Singh A. Tropomyosin: Regulator of thin filaments. Adv Exp Med Biol. 2007;592:87–97. doi: 10.1007/978-4-431-38453-3_9. [DOI] [PubMed] [Google Scholar]
- 10.Li XE, Tobacman LS, Mun JY, Craig R, Fischer S, Lehman W. Tropomyosin position on f-actin revealed by em reconstruction and computational chemistry. Biophys J. 2011;100:1005–1013. doi: 10.1016/j.bpj.2010.12.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowlessur D, Tobacman LS. Troponin regulatory function and dynamics revealed by h/d exchange-mass spectrometry. J Biol Chem. 2010;285:2686–2694. doi: 10.1074/jbc.M109.062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JH, Kim KH, Jun G, Greenfield NJ, Dominguez R, Volkmann N, Hitchcock-DeGregori SE, Cohen C. Deciphering the design of the tropomyosin molecule. Proc Nat Acad Sci USA. 2001;98:8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumida JP, Wu E, Lehrer SS. Conserved asp-137 imparts flexibility to tropomyosin and affects function. J Biol Chem. 2008;283:6728–6734. doi: 10.1074/jbc.M707485200. [DOI] [PubMed] [Google Scholar]
- 14.Li XE, Suphamungmee W, Janco M, Geeves MA, Marston SB, Fischer S, Lehman W. The flexibility of two tropomyosin mutants, D175N and E180G, that cause hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2012 doi: 10.1016/j.bbrc.2012.06.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ly S, Lehrer SS. Long-range effects of familial hypertrophic cardiomyopathy mutations e180g and d175n on the properties of tropomyosin. Biochemistry. 2012 doi: 10.1021/bi3006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun YB, Irving M. The molecular basis of the steep force-calcium relation in heart muscle. J Mol Cell Cardiol. 2010;48:859–865. doi: 10.1016/j.yjmcc.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan BS, Gordon AM, Luo ZX. Removal of tropomyosin overlap modifies cooperative binding of myosin s-1 to reconstituted thin filaments of rabbit striated muscle. J Biol Chem. 1989;264:8495–8498. [PubMed] [Google Scholar]
- 18.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 19.Galinska-Rakoczy A, Engel P, Xu C, Jung H, Craig R, Tobacman LS, Lehman W. Structural basis for the regulation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2008;379:929–935. doi: 10.1016/j.jmb.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mudalige WA, Tao TC, Lehrer SS. Ca2+-dependent photocrosslinking of tropomyosin residue 146 to residues 157-163 in the c-terminal domain of troponin i in reconstituted skeletal muscle thin filaments. J Mol Biol. 2009;389:575–583. doi: 10.1016/j.jmb.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holroyde MJ, Robertson SP, Johnson JD, Solaro RJ, Potter JD. The calcium and magnesium binding sites on cardiac troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1980;255:11688–11693. [PubMed] [Google Scholar]
- 22.Baryshnikova OK, Li MX, Sykes BD. Modulation of cardiac troponin c function by the cardiac-specific n-terminus of troponin i: Influence of pka phosphorylation and involvement in cardiomyopathies. J Mol Biol. 2008;375:735–751. doi: 10.1016/j.jmb.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 23.Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved cooh terminus of troponin i forms a ca2+-modulated allosteric domain in the troponin complex. Biochemistry. 2001;40:2623–2631. doi: 10.1021/bi002423j. [DOI] [PubMed] [Google Scholar]
- 24.Tachampa K, Kobayashi T, Wang H, Martin AF, Biesiadecki BJ, Solaro RJ, de Tombe PP. Increased cross-bridge cycling kinetics after exchange of c-terminal truncated troponin I in skinned rat cardiac muscle. J Biol Chem. 2008;283:15114–15121. doi: 10.1074/jbc.M801636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda S, Kobayashi T, Taniguchi H, Hayashi H, Maeda Y. Structural and functional domains of the troponin complex revealed by limited digestion. Eur J Biochem. 1997;246:611–617. doi: 10.1111/j.1432-1033.1997.00611.x. [DOI] [PubMed] [Google Scholar]
- 26.Bremel RD, Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972;238:97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- 27.Fitzsimons DP, Moss RL. Cooperativity in the regulation of force and the kinetics of force development in heart and skeletal muscles: Cross-bridge activation of force. Adv Exp Med Biol. 2007;592:177–189. doi: 10.1007/978-4-431-38453-3_16. [DOI] [PubMed] [Google Scholar]
- 28.Moss RL, Fitzsimons DP. Regulation of contraction in mammalian striated muscles--the plot thick-ens. J Gen Physiol. 2010;136:21–27. doi: 10.1085/jgp.201010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobacman LS, Sawyer D. Calcium binds cooperatively to the regulatory sites of the cardiac thin filament. J Biol Chem. 1990;265:931–939. [PubMed] [Google Scholar]
- 30.Mehegan JP, Tobacman LS. Cooperative interactions between troponin molecules bound to the cardiac thin filament. J Biol Chem. 1991;266:966–972. [PubMed] [Google Scholar]
- 31.Davis JP, Norman C, Kobayashi T, Solaro RJ, Swartz DR, Tikunova SB. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin c. Biophys J. 2007;92:3195–3206. doi: 10.1529/biophysj.106.095406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun YB, Lou F, Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J Physiol. 2009;587:155–163. doi: 10.1113/jphysiol.2008.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farman GP, Allen EJ, Schoenfelt KQ, Backx PH, de Tombe PP. The role of thin filament cooperativity in cardiac length-dependent calcium activation. Biophys J. 2010;99:2978–2986. doi: 10.1016/j.bpj.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin i phosphorylation on the ca2+-binding properties of the ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982;257:260–263. [PubMed] [Google Scholar]
- 36.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin i in the murine myocardium: Influence on length-dependent activation and interfilament spacing. J Physiol. 2003;547:951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin i. J Physiol. 2000;526(Pt 3):541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engel PL, Kobayashi T, Biesiadecki B, Davis J, Tikunova S, Wu S, Solaro RJ. Identification of a region of troponin i important in signaling cross-bridge-dependent activation of cardiac myofilaments. J Biol Chem. 2007;282:183–193. doi: 10.1074/jbc.M512337200. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Patrick SE, Kobayashi M. Ala scanning of the inhibitory region of cardiac troponin i. J Biol Chem. 2009;284:20052–20060. doi: 10.1074/jbc.M109.001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandra M, Tschirgi ML, Rajapakse I, Campbell KB. Troponin t modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys J. 2006;90:2867–2876. doi: 10.1529/biophysj.105.076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinken AC, Solaro RJ. A dominant role of cardiac molecular motors in the intrinsic regulation of ventricular ejection and relaxation. Physiology (Bethesda) 2007;22:73–80. doi: 10.1152/physiol.00043.2006. [DOI] [PubMed] [Google Scholar]
- 42.Solaro RJ, Wise RM, Shiner JS, Briggs FN. Calcium requirements for cardiac myofibrillar activation. Circ Res. 1974;34:525–530. doi: 10.1161/01.res.34.4.525. [DOI] [PubMed] [Google Scholar]
- 43.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 44.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305–315. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- 45.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP, Geenen DL, Buttrick PM, Solaro RJ. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem. 2009;284:5097–5106. doi: 10.1074/jbc.M807414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirk JA, MacGowan GA, Evans C, Smith SH, Warren CM, Mamidi R, Chandra M, Stewart AF, Solaro RJ, Shroff SG. Left ventricular and myocardial function in mice expressing constitutively pseudophosphorylated cardiac troponin i. Circ Res. 2009;105:1232–1239. doi: 10.1161/CIRCRESAHA.109.205427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC., Jr. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alves ML, Gaffin RD, Wolska BM. Rescue of familial cardiomyopathies by modifications at the level of sarcomere and ca2+ fluxes. J Mol Cell Cardiol. 2010;48:834–842. doi: 10.1016/j.yjmcc.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J Biol Chem. 2008;283:26829–26833. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mamidi R, Gollapudi SK, Mallampalli SL, Chandra M. Alanine or aspartic acid substitutions at serine23/24 of cardiac troponin i decrease thin filament activation, with no effect on crossbridge detachment kinetics. Arch Biochem Biophys. 2012;525:1–8. doi: 10.1016/j.abb.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker JS, Walker LA, Margulies K, Buttrick P, de Tombe P. Protein kinase a changes calcium sensitivity but not crossbridge kinetics in human cardiac myofibrils. Am J Physiol Heart Circ Physiol. 2011;301:H138–146. doi: 10.1152/ajpheart.00838.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin c g159d mutation blunts myofilament desensitization induced by troponin i ser23/24 phosphorylation. Circ Res. 2007;100:1486–1493. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 55.Hinken AC, Hanft LM, Scruggs SB, Sadayappan S, Robbins J, Solaro RJ, McDonald KS. Protein kinase c depresses cardiac myocyte power output and attenuates myofilament responses induced by protein kinase a. J Muscle Res Cell Motil. 2012 doi: 10.1007/s10974-012-9294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004;94:496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- 57.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the ca(2+)-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 58.Howarth JW, Meller J, Solaro RJ, Trewhella J, Rosevear PR. Phosphorylation-dependent conformational transition of the cardiac specific N-extension of troponin I in cardiac troponin. J Mol Biol. 2007;373:706–722. doi: 10.1016/j.jmb.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 59.Schmidtmann A, Lindow C, Villard S, Heuser A, Mugge A, Gessner R, Granier C, Jaquet K. Cardiac troponin c-l29q, related to hypertrophic cardiomyopathy, hinders the transduction of the protein kinase a dependent phosphorylation signal from cardiac troponin I to c. Febs J. 2005;272:6087–6097. doi: 10.1111/j.1742-4658.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 60.Warren CM, Kobayashi T, Solaro RJ. Sites of intra- and intermolecular cross-linking of the n-terminal extension of troponin i in human cardiac whole troponin complex. J Biol Chem. 2009;284:14258–14266. doi: 10.1074/jbc.M807621200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomes AV, Potter JD. Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin i gene. Mol Cell Biochem. 2004;263:99–114. doi: 10.1023/B:MCBI.0000041852.42291.aa. [DOI] [PubMed] [Google Scholar]
- 62.Deng Y, Schmidtmann A, Redlich A, Westerdorf B, Jaquet K, Thieleczek R. Effects of phosphorylation and mutation R145G on human cardiac troponin i function. Biochemistry. 2001;40:14593–14602. doi: 10.1021/bi0115232. [DOI] [PubMed] [Google Scholar]
- 63.Noland TA, Jr., Guo X, Raynor RL, Jideama NM, Averyhart-Fullard V, Solaro RJ, Kuo JF. Cardiac troponin i mutants. Phosphorylation by protein kinases c and a and regulation of ca(2+)-stimulated mgatpase of reconstituted actomyosin s-1. J Biol Chem. 1995;270:25445–25454. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- 64.Goldspink PH, Montgomery DE, Walker LA, Urboniene D, McKinney RD, Geenen DL, Solaro RJ, Buttrick PM. Protein kinase cepsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res. 2004;95:424–432. doi: 10.1161/01.RES.0000138299.85648.92. [DOI] [PubMed] [Google Scholar]
- 65.Roman BB, Goldspink PH, Spaite E, Urboniene D, McKinney R, Geenen DL, Solaro RJ, Buttrick PM. Inhibition of pkc phosphorylation of ctni improves cardiac performance in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H2089–2095. doi: 10.1152/ajpheart.00582.2003. [DOI] [PubMed] [Google Scholar]
- 66.Scruggs SB, Walker LA, Lyu T, Geenen DL, Solaro RJ, Buttrick PM, Goldspink PH. Partial replacement of cardiac troponin I with a non-phosphorylatable mutant at serines 43/45 attenuates the contractile dysfunction associated with pkcepsilon phosphorylation. J Mol Cell Cardiol. 2006;40:465–473. doi: 10.1016/j.yjmcc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase c sites on cardiac troponin i differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003;278:11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 68.Sakthivel S, Finley NL, Rosevear PR, Lorenz JN, Gulick J, Kim S, VanBuren P, Martin LA, Robbins J. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem. 2005;280:703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- 69.Belin RJ, Sumandea MP, Kobayashi T, Walker LA, Rundell VL, Urboniene D, Yuzhakova M, Ruch SH, Geenen DL, Solaro RJ, de Tombe PP. Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol Heart Circ Physiol. 2006;291:H2344–2353. doi: 10.1152/ajpheart.00541.2006. [DOI] [PubMed] [Google Scholar]
- 70.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 71.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase c activity and expression of ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 72.Takeishi Y, Chu G, Kirkpatrick DM, Li Z, Wakasaki H, Kranias EG, King GL, Walsh RA. In vivo phosphorylation of cardiac troponin i by protein kinase C beta2 decreases cardiomyocyte calcium responsiveness and contractility in transgenic mouse hearts. J Clin Invest. 1998;102:72–78. doi: 10.1172/JCI2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hwang H, Robinson DA, Stevenson TK, Wu HC, Kampert SE, Pagani FD, Dyke DB, Martin JL, Sadayappan S, Day SM, Westfall MV. PKC beta(II) modulation of myocyte contractile performance. J Mol Cell Cardiol. 2012;53:176–186. doi: 10.1016/j.yjmcc.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong X, Sumandea CA, Chen YC, Garcia-Cazarin ML, Zhang J, Balke CW, Sumandea MP, Ge Y. Augmented phosphorylation of cardiac troponin i in hypertensive heart failure. J Biol Chem. 2012;287:848–857. doi: 10.1074/jbc.M111.293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang P, Kirk JA, Ji W, Dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henze M, Patrick SE, Hinken AC, Scruggs SB, Goldspink PH, de Tombe PP, Kobayashi M, Ping P, Kobayashi T, Solaro RJ. New insights into the functional significance of the acidic region of the unique n-terminal extension of cardiac troponin I. Biochimica et Biophysica Acta Mol Cell Res. 2012 doi: 10.1016/j.bbamcr.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howarth JW, Ramisetti S, Nolan K, Sadayappan S, Rosevear PR. Structural insight into the unique cardiac myosin binding protein-c motif: A partially folded domain. J Biol Chem. 2012;373:706–722. doi: 10.1074/jbc.M111.309591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadayappan S, Finley N, Howarth JW, Osinska H, Klevitsky R, Lorenz JN, Rosevear PR, Robbins J. Role of the acidic n’ region of cardiac troponin I in regulating myocardial function. Faseb J. 2008;22:1246–1257. doi: 10.1096/fj.07-9458com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy RT, Mogensen J, Shaw A, Kubo T, Hughes S, McKenna WJ. Novel mutation in cardiac troponin I in recessive idiopathic dilated cardiomyopathy. Lancet. 2004;363:371–372. doi: 10.1016/S0140-6736(04)15468-8. [DOI] [PubMed] [Google Scholar]
- 80.You B, Yan G, Zhang Z, Yan L, Li J, Ge Q, Jin JP, Sun J. Phosphorylation of cardiac troponin I by mammalian sterile 20-like kinase 1. Biochem J. 2009;418:93–101. doi: 10.1042/BJ20081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sumandea MP, Rybin VO, Hinken AC, Wang C, Kobayashi T, Harleton E, Sievert G, Balke CW, Feinmark SJ, Solaro RJ, Steinberg SF. Tyrosine phosphorylation modifies protein kinase c delta-dependent phosphorylation of cardiac troponin I. J Biol Chem. 2008;283:22680–22689. doi: 10.1074/jbc.M802396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sumandea MP, Steinberg SF. Redox signaling and cardiac sarcomeres. J Biol Chem. 2011;286:9921–9927. doi: 10.1074/jbc.R110.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abbott MB, Gaponenko V, Abusamhadneh E, Finley N, Li G, Dvoretsky A, Rance M, Solaro RJ, Rosevear PR. Regulatory domain conformational exchange and linker region flexibility in cardiac troponin C bound to cardiac troponin I. J Biol Chem. 2000;275:20610–20617. doi: 10.1074/jbc.M909252199. [DOI] [PubMed] [Google Scholar]
- 84.Avner BS, Hinken AC, Yuan C, Solaro RJ. H2O2 alters rat cardiac sarcomere function and protein phosphorylation through redox signaling. Am J Physiol Heart Circ Physiol. 2010;299:H723–730. doi: 10.1152/ajpheart.00050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frank D, Frey N. Cardiac z-disc signaling network. J Biol Chem. 2011;286:9897–9904. doi: 10.1074/jbc.R110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of p21-activated kinase-1 (pak1) in cardiac myocytes. Circ Res. 2004;94:194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 87.Sheehan KA, Ke Y, Wolska BM, Solaro RJ. Expression of active p21-activated kinase-1 induces ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am J Physiol Cell Physiol. 2009;296:C47–58. doi: 10.1152/ajpcell.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, Neyses L, Solaro RJ, Ke Y, Cartwright EJ, Lei M, Wang X. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation. 2011;124:2702–2715. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartman TJ, Martin JL, Solaro RJ, Samarel AM, Russell B. Capz dynamics are altered by endothelin-1 and phenylephrine via pip2- and pkc-dependent mechanisms. Am J Physiol Cell Physiol. 2009;296:C1034–1039. doi: 10.1152/ajpcell.00544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pyle WG, Hart MC, Cooper JA, Sumandea MP, de Tombe PP, Solaro RJ. Actin capping protein: An essential element in protein kinase signaling to the myofilaments. Circ Res. 2002;90:1299–1306. doi: 10.1161/01.res.0000024389.03152.22. [DOI] [PubMed] [Google Scholar]
- 91.Pyle WG, La Rotta G, de Tombe PP, Sumandea MP, Solaro RJ. Control of cardiac myofilament activation and pkc-betaii signaling through the actin capping protein, capz. J Mol Cell Cardiol. 2006;41:537–543. doi: 10.1016/j.yjmcc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Yang FH, Pyle WG. Cardiac actin capping protein reduction and protein kinase c inhibition maintain myofilament function during cardioplegic arrest. Cell Physiol Biochem. 2011;27:263–272. doi: 10.1159/000327952. [DOI] [PubMed] [Google Scholar]
- 93.Yang FH, Pyle WG. Reduced cardiac capz protein protects hearts against acute ischemia-reperfusion injury and enhances preconditioning. J Mol Cell Cardiol. 2012;52:761–772. doi: 10.1016/j.yjmcc.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 94.Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol. 2001;3:544–551. doi: 10.1038/35078517. [DOI] [PubMed] [Google Scholar]
- 95.Cuello F, Bardswell SC, Haworth RS, Yin X, Lutz S, Wieland T, Mayr M, Kentish JC, Avkiran M. Protein kinase d selectively targets cardiac troponin i and regulates myofilament ca2+ sensitivity in ventricular myocytes. Circ Res. 2007;100:864–873. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- 96.Kostyukova AS, Choy A, Rapp BA. Tropomodulin binds two tropomyosins: A novel model for actin filament capping. Biochemistry. 2006;45:12068–12075. doi: 10.1021/bi060899i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eyers CE, McNeill H, Knebel A, Morrice N, Arthur SJ, Cuenda A, Cohen P. The phosphorylation of capz-interacting protein (capzip) by stress-activated protein kinases triggers its dissociation from capz. Biochem J. 2005;389:127–135. doi: 10.1042/BJ20050387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hernandez-Valladares M, Kim T, Kannan B, Tung A, Aguda AH, Larsson M, Cooper JA, Robinson RC. Structural characterization of a capping protein interaction motif defines a family of actin filament regulators. Nat Struct Mol Biol. 2010;17:497–503. doi: 10.1038/nsmb.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dirkx E, Cazorla OF, Schwenk RW, Lorenzen-Schmidt I, Sadayappan S, Van Lint J, Carrier L, van Eys GJ, Glatz JF, Luiken JJ. Protein kinase d increases maximal ca2+-activated tension of cardiomyocyte contraction by phosphorylation of cMyBP-C-ser 315. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sin YY, Baillie GS. Protein kinase d in the hypertrophy pathway. Biochem Soc Trans. 2012;40:287–289. doi: 10.1042/BST20110626. [DOI] [PubMed] [Google Scholar]
- 101.Pyle WG. Searching for the missing link: A role for the actin capping protein in heart failure. Can J Cardiol. 2004;20:1429–1432. [PubMed] [Google Scholar]
- 102.Willard BB, Ruse CI, Keightley JA, Bond M, Kinter M. Site-specific quantitation of protein nitration using liquid chromatography/tandem mass spectrometry. Anal Chem. 2003;75:2370–2376. doi: 10.1021/ac034033j. [DOI] [PubMed] [Google Scholar]
- 103.Gokhin DS, Lewis RA, McKeown CR, Nowak RB, Kim NE, Littlefield RS, Lieber RL, Fowler VM. Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology. J Cell Biol. 2010;189:95–109. doi: 10.1083/jcb.201001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conley CA, Fritz-Six KL, Almenar-Queralt A, Fowler VM. Leiomodins: Larger members of the tropomodulin (tmod) gene family. Genomics. 2001;73:127–139. doi: 10.1006/geno.2000.6501. [DOI] [PubMed] [Google Scholar]
- 105.Bardswell SC, Cuello F, Kentish JC, Avkiran M. Cmybp-c as a promiscuous substrate: Phosphorylation by non-pka kinases and its potential significance. J Muscle Res Cell Motil. 2012;33:53–60. doi: 10.1007/s10974-011-9276-3. [DOI] [PubMed] [Google Scholar]
- 106.Runnels LW, Yue L, Clapham DE. Trp-plik, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 107.Runnels LW, Yue L, Clapham DE. The trpm7 channel is inactivated by pip(2) hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 108.Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res. 2011;109:86–96. doi: 10.1161/CIRCRESAHA.111.242974. [DOI] [PubMed] [Google Scholar]
- 109.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: A suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci. 2010;1188:191–198. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pound KM, Arteaga GM, Fasano M, Wilder T, Fischer SK, Warren CM, Wende AR, Farjah M, Abel ED, Solaro RJ, Lewandowski ED. Expression of slow skeletal tni in adult mouse hearts confers metabolic protection to ischemia. J Mol Cell Cardiol. 2011;51:236–243. doi: 10.1016/j.yjmcc.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nixon BR, Thawornkaiwong A, Jin J, Brundage EA, Little SC, Davis JP, Solaro RJ, Biesiadecki BJ. Amp-activated protein kinase phosphorylates cardiac troponin I at Ser-150 to increase myofilament calcium sensitivity and blunt PKA-dependent function. J Biol Chem. 2012;287:19136–19147. doi: 10.1074/jbc.M111.323048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oliveira SM, Zhang YH, Solis RS, Isackson H, Bellahcene M, Yavari A, Pinter K, Davies JK, Ge Y, Ashrafian H, Walker JW, Carling D, Watkins H, Casadei B, Redwood C. Amp-activated protein kinase phosphorylates cardiac troponinI and alters contractility of murine ventricular myocytes. Circ Res. 2012;110:1192–1201. doi: 10.1161/CIRCRESAHA.111.259952. [DOI] [PubMed] [Google Scholar]
- 113.Sancho Solis R, Ge Y, Walker JW. A preferred ampk phosphorylation site adjacent to the inhibitory loop of cardiac and skeletal troponin i. Protein Sci. 2011;20:894–907. doi: 10.1002/pro.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Horman S, Beauloye C, Vanoverschelde JL, Bertrand L. Amp-activated protein kinase in the control of cardiac metabolism and remodeling. Curr Heart Fail Rep. 2012 doi: 10.1007/s11897-012-0102-z. [DOI] [PubMed] [Google Scholar]
- 115.Nagendran J, Waller TJ, Dyck JR. AMPK signalling and the control of substrate use in the heart. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 116.Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107:1962–1965. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- 117.Nurbaeva MK, Schmid E, Szteyn K, Yang W, Viollet B, Shumilina E, Lang F. Enhanced ca2+ entry and Na+/Ca2+ exchanger activity in dendritic cells from amp-activated protein kinase-deficient mice. Faseb J. 2012;26:3049–3058. doi: 10.1096/fj.12-204024. [DOI] [PubMed] [Google Scholar]
- 118.Ouyang Y, Mamidi R, Jayasundar JJ, Chandra M, Dong WJ. Structural and kinetic effects of pak3 phosphorylation mimic of ctni(s151e) on the ctnc-ctni interaction in the cardiac thin filament. J Mol Biol. 2010;400:1036–1045. doi: 10.1016/j.jmb.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, Avkiran M. Protein kinase d is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 120.Haworth RS, Goss MW, Rozengurt E, Avkiran M. Expression and activity of protein kinase d/protein kinase c mu in myocardium: Evidence for alpha1-adrenergic receptor- and protein kinase c-mediated regulation. J Mol Cell Cardiol. 2000;32:1013–1023. doi: 10.1006/jmcc.2000.1143. [DOI] [PubMed] [Google Scholar]
- 121.Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem. 2010;285:5674–5682. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goodall MH, Wardlow RD, 2nd, Goldblum RR, Ziman A, Lederer WJ, Randall W, Rogers TB. Novel function of cardiac protein kinase d1 as a dynamic regulator of Ca2+ sensitivity of contraction. J Biol Chem. 2010;285:41686–41700. doi: 10.1074/jbc.M110.179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dirkx E, Schwenk RW, Coumans WA, Hoebers N, Angin Y, Viollet B, Bonen A, van Eys GJ, Glatz JF, Luiken JJ. Protein kinase d1 is essential for contraction-induced glucose uptake but is not involved in fatty acid uptake into cardiomyocytes. J Biol Chem. 2012;287:5871–5881. doi: 10.1074/jbc.M111.281881. [DOI] [PMC free article] [PubMed] [Google Scholar]