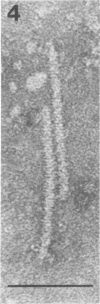
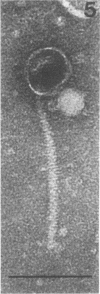
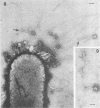
Abstract
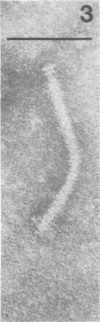
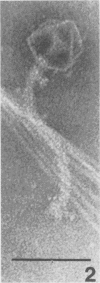
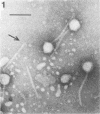
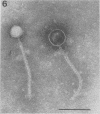
Bacteriophage PO4 has been found to depend on the presence of pili for the infection of its host organism, Pseudomonas aeruginosa. Unlike other pilus phages, which either contain RNA and are “spherical” or contain single-stranded DNA and are filamentous, PO4 has a head and a long noncontractile tail. This paper describes its basic characters, and a quantitative study is made of its adsorption to exponential-phase cells of piliated and nonpiliated strains of P. aeruginosa. PO4 is found to contain double-stranded DNA and appears to be virulent towards its two host strains.
Full text
PDF


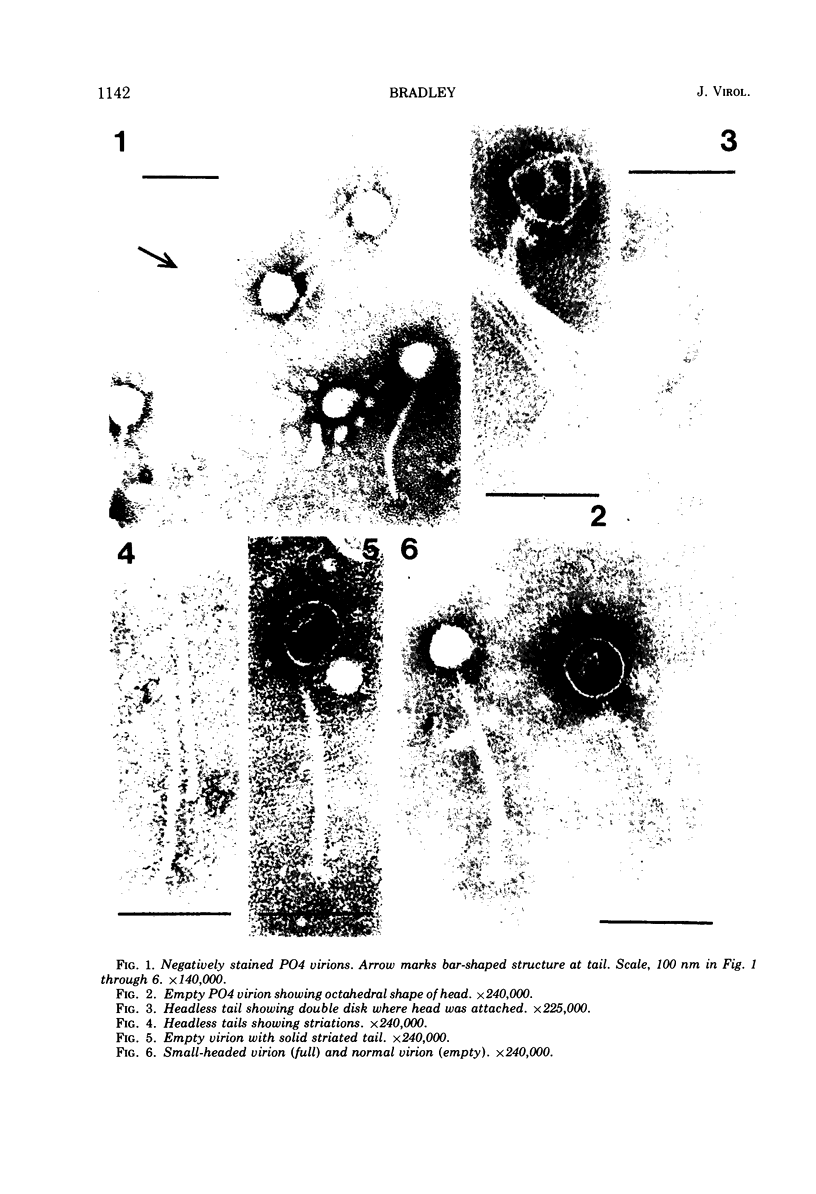




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. A pilus-dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology. 1973 Feb;51(2):489–492. doi: 10.1016/0042-6822(73)90448-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem Biophys Res Commun. 1972 Apr 14;47(1):142–149. doi: 10.1016/s0006-291x(72)80021-4. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Robertson D. The structure and infective process of a contractile Pseudomonas aeruginosa bacteriophage. J Gen Virol. 1968 Sep;3(2):247–254. doi: 10.1099/0022-1317-3-2-247. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. J Gen Microbiol. 1972 Sep;72(2):303–319. doi: 10.1099/00221287-72-2-303. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Stimulation of pilus formation in Pseudomonas aeruginosa by RNA bacteriophage adsorption. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1080–1087. doi: 10.1016/0006-291x(72)90944-8. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The fluorescent staining of bacteriophage nucleic acids. J Gen Microbiol. 1966 Sep;44(3):383–391. doi: 10.1099/00221287-44-3-383. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. T., Yamammoto T. Properties of a Pseudomonas aeruginosa bacteriophage phi-MC. Can J Microbiol. 1969 Oct;15(10):1179–1186. doi: 10.1139/m69-214. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Role of F pili in the penetration of bacteriophage fl. J Virol. 1972 Oct;10(4):835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Amako K. A rod-shaped Pseudomonas phage. Virology. 1966 Jan;28(1):163–165. doi: 10.1016/0042-6822(66)90317-5. [DOI] [PubMed] [Google Scholar]
- Voelz H., Burchard R. P. Fine structure of bacteriophage-infected Myxococcus xanthus. 1. The lytic cycle in vegetative cells. Virology. 1971 Jan;43(1):243–250. doi: 10.1016/0042-6822(71)90242-x. [DOI] [PubMed] [Google Scholar]
- Weppelman R. M., Brinton C. C., Jr The infection of Pseudomonas aeruginosa by RNA pilus phage PP7: the adsorption organelle and the relationship between phage sensitivity and the division cycle. Virology. 1971 Apr;44(1):1–17. doi: 10.1016/0042-6822(71)90147-4. [DOI] [PubMed] [Google Scholar]