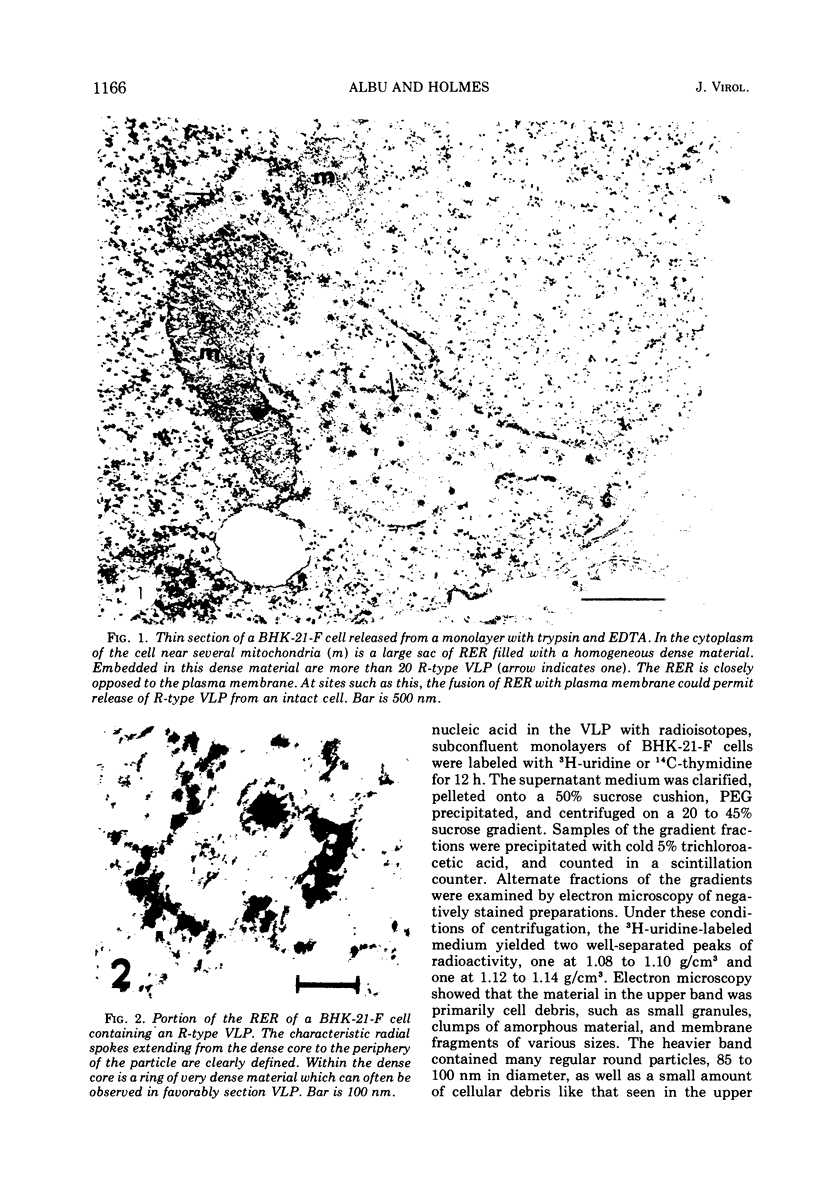
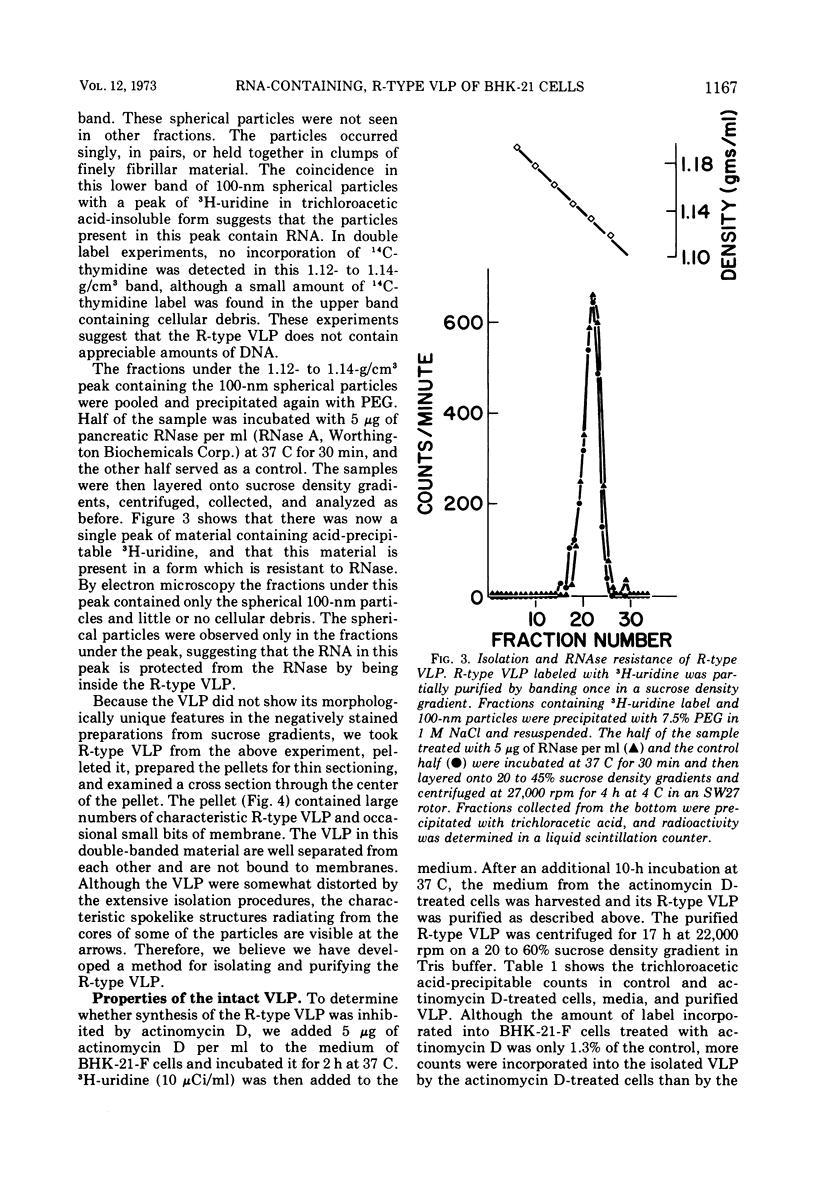
Abstract
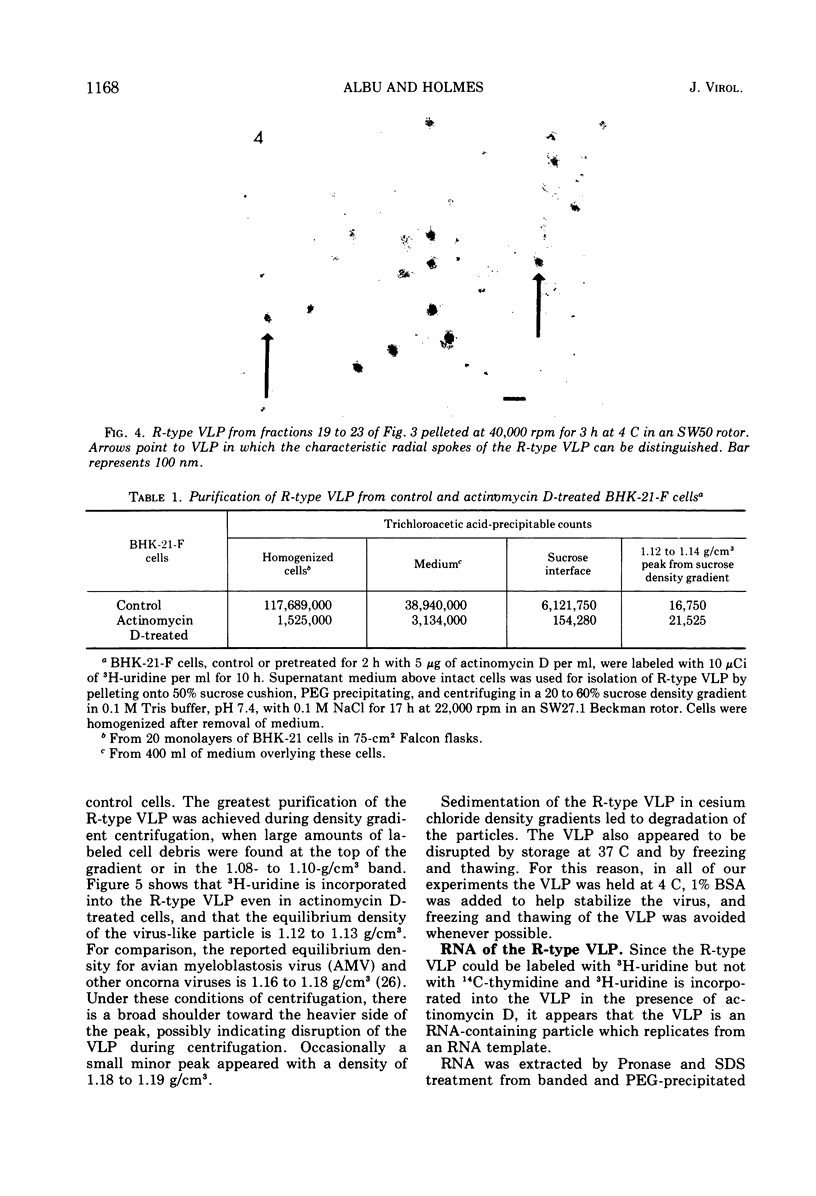
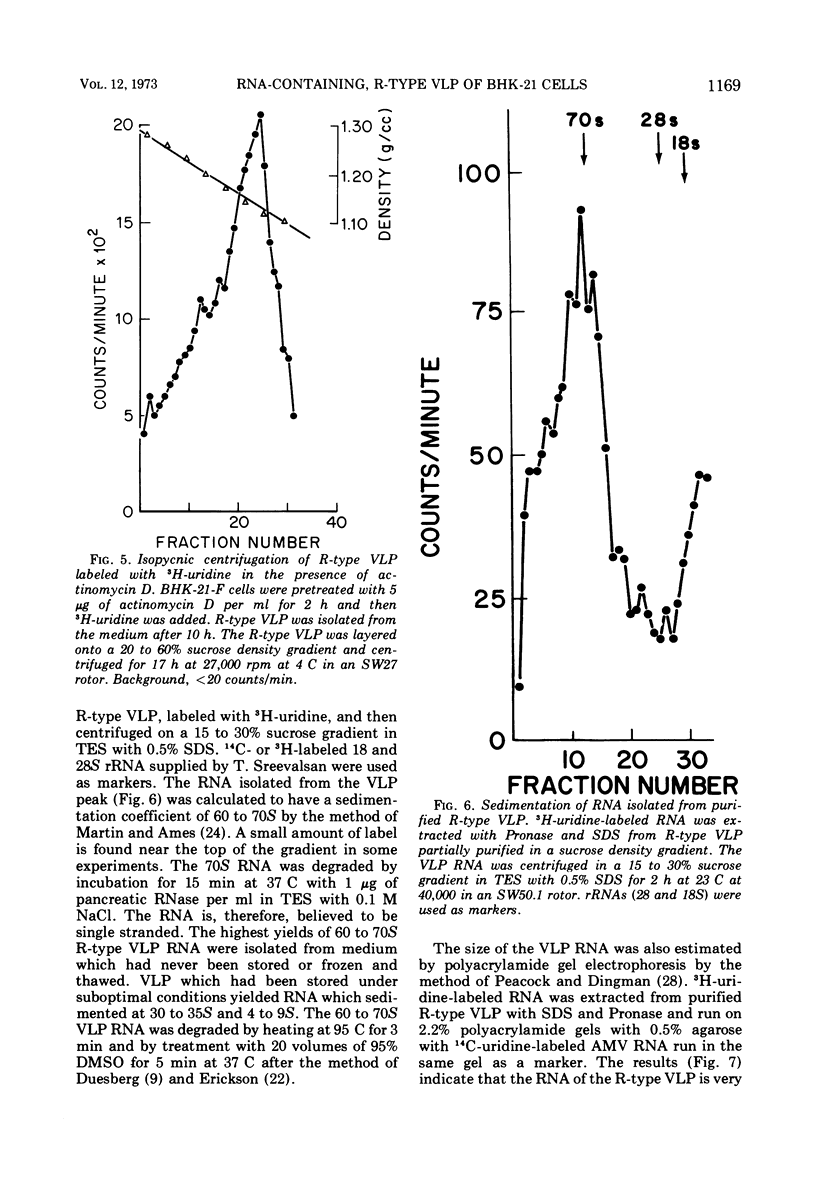
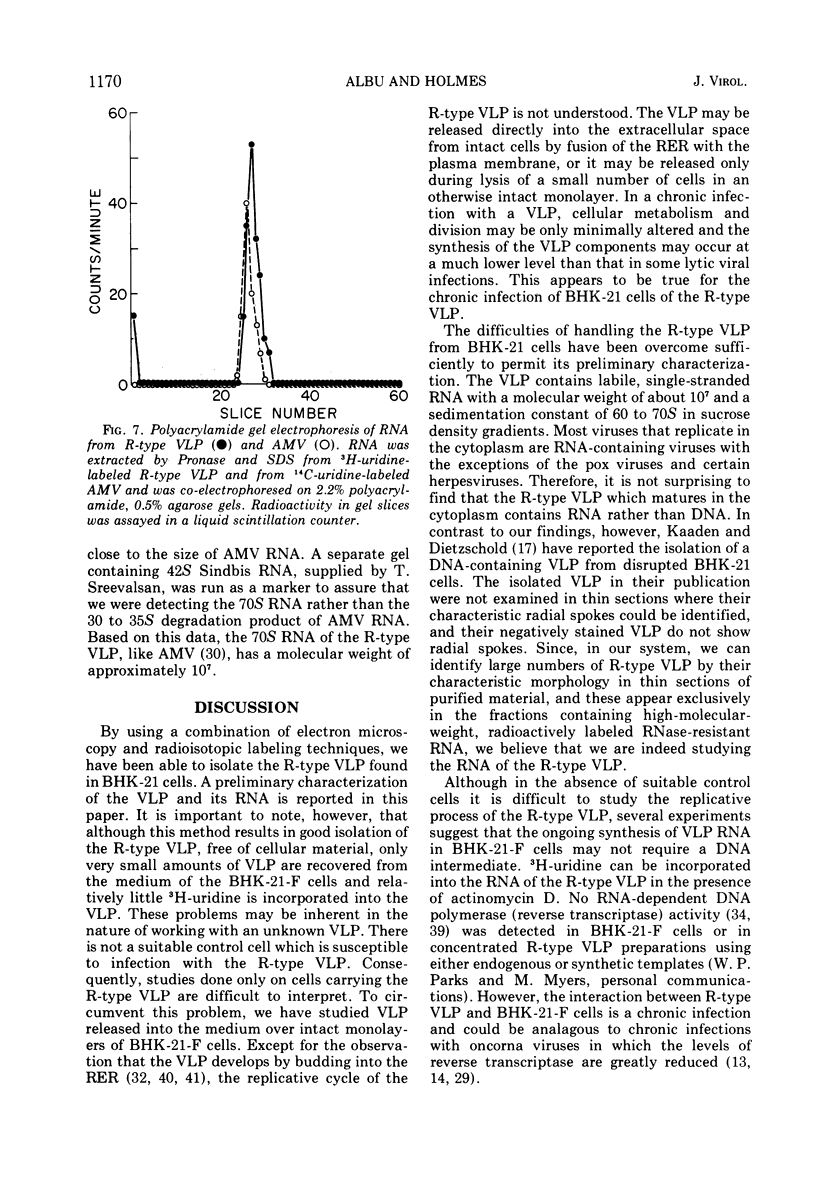
An R-type virus-like particle (VLP) has been isolated from the medium of BHK-21-F cells by ultracentrifugation and polyethylene glycol precipitation. The R-type VLP contains RNA which sediments at 60 to 70S in sucrose density gradients and has a molecular weight of approximately 107, as estimated by gel electrophoresis. The R-type VLP can be labeled with 3H-uridine in the presence of actinomycin D. On the basis of morphology, site of maturation, and preliminary biochemical characterization, the R-type VLP does not appear to fit into any of the major groups of animal viruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker W. B., McIntosh K., Dees J. H., Chanock R. M. Morphogenesis of avian infectious bronchitis virus and a related human virus (strain 229E). J Virol. 1967 Oct;1(5):1019–1027. doi: 10.1128/jvi.1.5.1019-1027.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmayer G. D., Miller F., Balda B. R. Inhibition of high molecular weight RNA synthesis in a hamster melanoma by ethidium bromide in vivo. Hoppe Seylers Z Physiol Chem. 1972 Nov;353(11):1749–1754. doi: 10.1515/bchm2.1972.353.2.1749. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- DAVID-FERREIRA J. F., MANAKER R. A. AN ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF A MOUSE HEPATITIS VIRUS IN TISSUE CULTURE CELLS. J Cell Biol. 1965 Jan;24:57–78. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. L., Fukuyama K., Benn M., Keston A. S., Brandt R. B. Transmission of a pigmented melanoma in golden hamsters by a cell-free ultrafiltrate. Nature. 1968 Aug 31;219(5157):979–980. doi: 10.1038/219979a0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Baltimore D., Smoler D., Watson K. F., Yaniv A., Spiegelman S. Absence of polymerase protein in virions of alpha-type rous sarcoma virus. Science. 1972 Sep 29;177(4055):1188–1191. doi: 10.1126/science.177.4055.1188. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of microtubules in movement and alignment of nuclei in virus-induced syncytia. J Cell Biol. 1968 Dec;39(3):526–543. doi: 10.1083/jcb.39.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaden O., Dietzschold B. Isolation and characterization of extracellular virus-like particles from BHK cells. Brief report. Arch Gesamte Virusforsch. 1971;35(2):317–321. doi: 10.1007/BF01249728. [DOI] [PubMed] [Google Scholar]
- Kisch A. L., Kelley R. O., Eberle B. J. Differential enhancement of R-type virus particles in polyoma-transformed BHK-21 cells by dimethyl sulfoxide. J Natl Cancer Inst. 1972 Sep;49(3):911–914. [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Plasma membrane lipids and parainfluenza virus assembly. Virology. 1970 Apr;40(4):939–947. doi: 10.1016/0042-6822(70)90140-6. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Martin S. J., Brown F. Synthesis of ribonucleic acid in baby-hamster kidney cells in the presence of actinomycin D. Biochem J. 1967 Dec;105(3):979–985. doi: 10.1042/bj1050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro L. S., Schieble J. H., Lennette E. H. Electron microscopic studies of coronavirus. J Gen Virol. 1971 Aug;12(2):161–168. doi: 10.1099/0022-1317-12-2-161. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Peebles P. T., Haapala D. K., Gazdar A. F. Deficiency of viral ribonucleic acid-dependent deoxyribonucleic acid polymerase in noninfectious virus-like particles released from murine sarcoma virus-transformed hamster cells. J Virol. 1972 Mar;9(3):488–493. doi: 10.1128/jvi.9.3.488-493.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP D. G., BEARD J. W. Virus of avian erythromyeloblastic leukosis. IV. Sedimentation, density and hydration. Biochim Biophys Acta. 1954 May;14(1):12–17. doi: 10.1016/0006-3002(54)90124-9. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr, Vander Weide G. C., Ma B. I. Prevalence of type R virus-like particles in clones of BHK-21 cells. Virology. 1969 Aug;38(4):707–710. doi: 10.1016/0042-6822(69)90192-5. [DOI] [PubMed] [Google Scholar]
- Sikes R. K., Peacock G. V., Acha P., Arko R. J., Dierks R. Rabies vaccines: duration-of-immunity study in dogs. J Am Vet Med Assoc. 1971 Dec 1;159(11):1491–1499. [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Trávnícek M., Watson K. Synthetic DNA-RNA hybrids and RNA-RNA duplexes as templates for the polymerases of the oncogenic RNA viruses. Nature. 1970 Oct 31;228(5270):430–432. doi: 10.1038/228430a0. [DOI] [PubMed] [Google Scholar]
- Stenback W. A., Van Hoosier G. L., Jr, Ferguson D. B., Trentin J. J. Significance of virus particles observed in spontaneous and induced tumors of the syrian hamster. Bibl Haematol. 1970;(36):559–565. doi: 10.1159/000391752. [DOI] [PubMed] [Google Scholar]
- Stenback W. A., Van Hoosier G. L., Jr, Trentin J. J. Virus particles in hamster tumors as revealed by electron microscopy. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1219–1223. doi: 10.3181/00379727-122-31365. [DOI] [PubMed] [Google Scholar]
- Stone L. B., Takemoto K. K., Martin M. A. Physical and biochemical properties of progressive pneumonia virus. J Virol. 1971 Oct;8(4):573–578. doi: 10.1128/jvi.8.4.573-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Delain E., Hollande E. Morphogenèse d'un virus du hamster associé à la souche BHK, ou à des tumeurs. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jan 30;264(5):785–788. [PubMed] [Google Scholar]
- Thomas J. A., Henry M., Hollande E., Vilain C. Nouvelles observations sur le virus du Hanster associé aux cellules BHK et aux tumeurs. C R Acad Sci Hebd Seances Acad Sci D. 1968 May 13;266(20):2129–2132. [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A. Growth of rubella virus in BHK21 cells. II. Enhancing effect of DEAE-dextran, semicarbazide and low doses of metabolic inhibitors. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1092–1098. doi: 10.3181/00379727-125-32284. [DOI] [PubMed] [Google Scholar]
- Van Hoosier G. L., Jr, Stenback W. A., Parker J. C., Burke J. G., Trentin J. J. The effects of cesarean derivation and foster nursing procedures on enzootic viruses of the LSH strain of inbred hamsters. Lab Anim Care. 1970 Apr;20(2):232–237. [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Analysis of high-molecular-weight ribonucleic acid associated with intracisternal A particles. J Virol. 1973 Feb;11(2):287–298. doi: 10.1128/jvi.11.2.287-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigel R. F., Rabotti G., Smith M. V. Electron microscopic observations on the association of viruses with membrane systems in hamster tumor cells propagated in tissue culture. J Natl Cancer Inst. 1969 Sep;43(3):653–669. [PubMed] [Google Scholar]