Abstract
The TREX complex couples nuclear pre-mRNA processing with mRNA export and contains multiple protein components, including Uap56, Alyref, Cip29 and the multi-subunit THO complex. Here, we have identified Chtop as a novel TREX component. We show that both Chtop and Alyref activate the ATPase and RNA helicase activities of Uap56 and that Uap56 functions to recruit both Alyref and Chtop onto mRNA. As observed with the THO complex subunit Thoc5, Chtop binds to the NTF2-like domain of Nxf1, and this interaction requires arginine methylation of Chtop. Using RNAi, we show that co-knockdown of Alyref and Chtop results in a potent mRNA export block. Chtop binds to Uap56 in a mutually exclusive manner with Alyref, and Chtop binds to Nxf1 in a mutually exclusive manner with Thoc5. However, Chtop, Thoc5 and Nxf1 exist in a single complex in vivo. Together, our data indicate that TREX and Nxf1 undergo dynamic remodelling, driven by the ATPase cycle of Uap56 and post-translational modifications of Chtop.
Keywords: ALY, C1ORF77, nuclear export, REF, TAP
Introduction
The TREX complex plays a central role in eukaryotic gene expression, integrating information from nuclear mRNA processing events to ensure the timely export of mRNA to the cytoplasm (Rodriguez-Navarro and Hurt, 2011). The metazoan core TREX complex contains multiple subunits including the RNA helicase Uap56 (Sub2 in yeast), Alyref (Aly/Thoc4/Ref/Bef; Yra1 in yeast), Cip29 (Tho1 in yeast), pDIP3, ZC11A and the THO subcomplex, which comprises Hpr1 (Thoc1/p84), Thoc2, Thoc3 (Tex1), Thoc5 (fSAP79), Thoc6 (fSAP35) and Thoc7 (fSAP24) (Dufu et al, 2010; Folco et al, 2012). The recruitment of human TREX to mRNA is splicing dependent (Masuda et al, 2005) and results in the loading of TREX near the 5′ end of mRNA through an interaction between Cbp80 and Alyref (Cheng et al, 2006). The assembly of Uap56, Cip29 and Alyref within TREX is dependent on Uap56 binding ATP (Dufu et al, 2010). The recruitment of metazoan Alyref to the TREX complex is probably coupled with 3′ end processing since Yra1 is recruited to Sub2 in yeast via the 3′ end processing factor Pcf11 and the Alyref:Pcf11 interaction is conserved (Johnson et al, 2009). Clp1 displaces Yra1 from Pcf11 as does Sub2 when loaded with RNA and ATP (Johnson et al, 2011). Moreover, recruitment of Clp1 to Pcf11 is important for generating an active cleavage-polyadenylation complex (Ghazy et al, 2012; Haddad et al, 2012). Thus, recruitment of Alyref to TREX via Pcf11 provides a means to couple mRNA 3′ end processing and export.
The mRNA export receptor Nxf1 (Mex67 in yeast) translocates mRNPs through the nuclear pore and GANP facilitates Nxf1 interaction with the nuclear pore (Wickramasinghe et al, 2010). The TREX components Alyref and Thoc5 bind the N-terminus and the NTF2-like (NTF2L) domain of Nxf1, respectively (Katahira et al, 2009). Hpr1 binds Nxf1’s UBA domain and recruits Nxf1’s orthologue, Mex67, to actively transcribed genes in yeast (Gwizdek et al, 2006). Thus, TREX acts as a binding platform for Nxf1 (Viphakone et al, 2012). Nxf1 has an N-terminal arginine rich RNA binding domain (RBD) (Hautbergue et al, 2008) which forms an intramolecular interaction with the NTF2L domain and this suppresses the RNA binding activity of Nxf1. TREX disrupts the Nxf1 intramolecular interaction, releasing the N-terminal RBD, which then binds mRNA (Viphakone et al, 2012). During export, mRNA is handed over from Alyref to Nxf1 (Hautbergue et al, 2008) and this process is facilitated by a decrease in Alyref RNA binding activity triggered by post-translational methylation of some of its arginines (Hung et al, 2010).
The loss of THO subunits in yeast leads to a rapid mRNA export block (Strasser et al, 2002) and TREX plays a genome-wide role in mRNP biogenesis in yeast (Gomez-Gonzalez et al, 2011). In contrast, knockdown of metazoan THO components leads to a less severe mRNA export phenotype (Rehwinkel et al, 2004). Similarly, Alyref knockdown in metazoans results in a modest mRNA export block. This may be accounted for by partial functional redundancy among mRNA export factors, for example, loss of Alyref triggers increased expression of Uif (Hautbergue et al, 2009). Uif is an mRNA export adaptor that associates with TREX components in vivo and binds Uap56 with a short peptide also found in Alyref. Uif and Alyref also bind in a mutually exclusive way to the N-terminal region of Nxf1. Moreover, both Uif and Alyref are found on the same mRNA (Hautbergue et al, 2009). Uif and Alyref appear to have overlapping functions since knockdown of either individually leads to a modest mRNA export block, yet knockdown of both leads to a rapid and severe mRNA export block. Despite these similarities, Uif is distinguished from Alyref, since only Uif requires the FACT chromatin remodelling complex for recruitment to the mRNP (Hautbergue et al, 2009). Functional overlap among export factors is not restricted to metazoans since in yeast, Nab2 also promotes recruitment of Mex67 to the mRNP (Iglesias et al, 2010). The combined loss of Alyref and Thoc5 in human cells leads to a severe mRNA export block, and a failure of Nxf1 to be recruited efficiently to the mRNP, consistent with the model whereby TREX acts as the binding platform for Nxf1 and stimulates its opening and direct interaction with mRNA (Viphakone et al, 2012). The loss of Uap56 in combination with its paralogue Ddx39 leads to a severe mRNA export block in humans (Hautbergue et al, 2009) and this is accompanied by accumulation of mRNA within nuclear speckles (Dias et al, 2010). This export block applies to both spliced and unspliced mRNAs and interestingly unspliced mRNAs require a specific coding region sequence to promote their export (Lei et al, 2011).
Here, we describe and characterise a new component of TREX, Chtop. We show that Chtop binds Uap56 and activates its ATPase and RNA helicase activities. Chtop also competes with Thoc5 for binding to the NTF2L domain of Nxf1 and yet both are found associated in a single complex in vivo. The discovery of Chtop indicates that TREX is likely to undergo substantial rearrangements during mRNP formation. In this respect, TREX may well act like other macromolecular machines such as the spliceosome, undergoing numerous conformational and compositional rearrangements in performing its cellular functions.
Results
Chtop binds Uap56 and associates with TREX
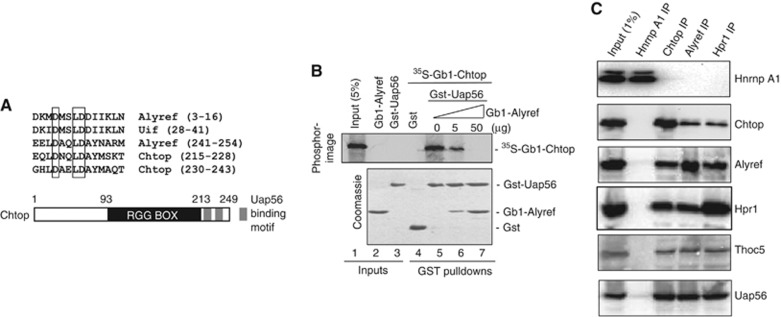
To identify novel Uap56 binding proteins, we took Alyref C-terminal Uap56 binding motif (UBM) (Hautbergue et al, 2009) and used this in a BLAST search which led to the identification of Chtop/Fop/C1orf77/Srag, a nuclear protein previously shown to be a substrate for the arginine methylase Prmt1 and required for ligand-dependent activation of oestrogen receptor target genes (van Dijk et al, 2010). Chtop contains two copies of a sequence similar to the Alyref UBM located towards the C-terminus. Alignment of metazoan UBMs reveals an invariant subsequence ΦDXXLD (Figure 1A), which forms part of a transient alpha helix in Alyref (Golovanov et al, 2006). Since Chtop and Alyref use a UBM to bind Uap56 we investigated whether they could simultaneously bind Uap56 in vitro. Pulldown assays confirmed that Chtop bound Gst-Uap56 (Figure 1B). Increasing concentrations of Gb1-Alyref displaced 35S-Gb1-Chtop from Gst-Uap56, indicating that Chtop and Alyref bind in a mutually exclusive manner to Uap56. Since Uap56 is a component of the TREX mRNA export complex, we investigated whether Chtop might also associate with TREX components. This analysis confirmed that Chtop co-immunoprecipitated (co-IPed) with multiple components of the TREX complex including Uap56 in ribonuclease A-treated extracts, but did not co-IP with a general mRNP binding protein, Hnrnpa1 (Figure 1C). Omitting ribonuclease treatment prior to co-IP only modestly increased the levels of TREX components that co-IP with Chtop (Supplementary Figure S1), indicating that the interactions are unlikely to be bridged by RNA. These data are consistent with the identification of Chtop by mass spectrometry in TREX immunopurified with antibodies to Thoc2, Cip29 and Uap56 TREX subunits (Dufu et al, 2010). Moreover, mass spectrometry analysis led to the identification of multiple proteins that co-purify in vivo with biotinylated Chtop, included TREX components (Fanis et al, 2012; Supplementary Table S1). In common with several other mRNA export factors, Chtop shows partial colocalisation with the splicing factor Srsf2 in nuclear speckles (Supplementary Figure S2).
Figure 1.
Chtop interacts with Uap56 and TREX. (A) Alignment of the Uap56-binding motifs (UBMs) present in Alyref, Uif and Chtop proteins. (B) Pulldown competition assay with Gst-Uap56 complexed with 35S-Gb1-Chtop and increasing amounts of Gb1-Alyref. Proteins were detected by Coomassie staining and Phosphorimaging. (C) Co-IP of Chtop with TREX subunits using RNAse A-treated 293T cell extract. HnrnpA1, Chtop, Alyref and Hpr1 were IPed using antibodies to the endogenous proteins and co-immunoprecipitating proteins were detected by western blotting with the indicated antibodies.
Source data for this figure is available on the online supplementary information page.
Chtop, Alyref and Cip29 stimulate the ATPase and helicase activities of Uap56
We examined the effects of both Alyref and Chtop on the ATPase and helicase activity for Uap56. Uap56 showed no ATPase activity in the absence of RNA, but significant activity in its presence (Figure 2A), consistent with an earlier report (Taniguchi and Ohno, 2008). Chtop, Alyref and Cip29 displayed no ATPase activity even in the presence of RNA. However, Alyref, Chtop and Cip29 individually stimulated both the RNA-dependent and RNA-independent ATPase activities of Uap56, although combinations of Alyref and Cip29 or Chtop and Cip29 did not further enhance the Uap56 ATPase activity. We also found that Alyref, Chtop and Cip29 all stimulated the Uap56 helicase activity, whereas the exon junction complex component Magoh did not (Figure 2B).
Figure 2.
Chtop activates the ATPase and helicase activity of Uap56. (A) ATPase activities for purified Uap56 in the presence of Alyref, Chtop, Cip29, RNA and ATP as indicated. Values are the average from three independent assays and error bars represent the standard deviation. Values are shown relative to the values observed for Uap56+ATP+RNA. (B) The helicase assay was performed at 37°C for 15 min with 1 μM Uap56, 2 μM Alyref, Chtop, Cip29 or Magoh. (C) In vitro protein:RNA UV crosslinking assay. 2 μg Gst-Uap56, Gb1-Alyref and Magoh purified from E. coli and Chtop purified from Baculovirus-infected cells were UV crosslinked with a 32P-radiolabelled RNA oligonucleotide in the presence or absence of ATP as indicated. Resulting complexes were analysed by SDS–PAGE and visualised by Coomassie blue staining and Phosphorimaging. (D) In vitro reconstitution of Uap56-RNA-Alyref complexes. Purified Uap56 expressed in E. coli was first incubated with continuously 32P-radiolabelled RNA and non-hydrolysable ATP. Recombinant Gst or Gst-Alyref was added to the reactions when indicated. Bound RNA was crosslinked (+) or not (−) to the proteins by UV irradiation, treated with RNAse A, and the resulting Uap56-RNA-Alyref complexes were purified using glutathione Sepharose. Eluted complexes were analysed by SDS–PAGE and visualised by Coomassie blue (left panel) and Phosphorimaging (right panel). (E) mRNP capture assay. Poly(A)+ RNA from stable Flp-In 293 cells, expressing Control or Uap56/Ddx39 miRNAs was purified on oligo(dT) beads in denaturing conditions after UV crosslinking (+) or not (−). Total extract (1% of input) and eluted proteins were analysed by western blotting with Chtop antibody.
Source data for this figure is available on the online supplementary information page.
Uap56 loads Chtop and Alyref onto mRNA.
Earlier work examined how Uap56 and Alyref bound RNA using immunoprecipitation (IP) of radiolabelled RNA (Taniguchi and Ohno, 2008). However, this study did not address which proteins were directly bound to RNA within ternary complexes. To examine how RNA associates with Uap56 and its binding partners, we carried out UV crosslinking experiments with proteins in solution (Figure 2C). In isolation, Alyref crosslinked well with RNA, irrespective of the presence of ATP whereas Uap56 showed a weak crosslink with RNA but only in the presence of ATP as reported previously (Taniguchi and Ohno, 2008). When Alyref and Uap56 were mixed, there was a dramatic stimulation of RNA crosslinked to Alyref and this was dependent on ATP (Figure 2C, left panel lanes 3, 4 and 7, 8). With Chtop, there was no distinct crosslink with RNA in the absence of Uap56 (Figure 2C, right panel lanes 3 and 4). In the presence of Uap56, there was weak RNA crosslinking to Chtop and this was enhanced by the addition of ATP (Figure 2C, right panel lanes 7 and 8). However, the level of RNA crosslink observed between Chtop and RNA was still significantly lower than that observed with Alyref under these conditions, indicating Chtop crosslinks with RNA less efficiently than Alyref in vitro. Together, these data indicate that in the presence of Uap56 and ATP, Chtop and Alyref are loaded onto mRNA. Given that both Alyref and Chtop stimulate the helicase and ATPase activity of Uap56 we also carried out RNA binding studies in the presence of the non-hydrolysable ATP analogue AMP-PNP. For these studies, we also ensured that all Uap56 present in the RNA UV crosslinking step was complexed with Alyref by isolating a Gst-Alyref:Uap56 complex by pulldown on glutathione sepharose beads (Figure 2D). Although Uap56 alone still cross-linked with RNA, when Uap56 is in complex with Gst-Alyref, RNA crosslinks to Gst-Alyref and not Uap56. Therefore, the RNA is normally likely to be handed over from Uap56 to Alyref. Since in the presence of the non-hydrolysable AMP-PNP, Uap56 failed to stimulate the loading of RNA onto Gst-Alyref above that seen with Gst-Alyref alone, this transfer is normally likely to take place concomitantly with Alyref- or Chtop-stimulated ATP hydrolysis (Figure 2D). We were unable to perform a similar experiment with Chtop, since Gst-Chtop expressed relatively poorly in E. coli. We further investigated the role of Uap56 and its paralogue Ddx39, in loading TREX factors onto the mRNP using in vivo UV crosslinking in a stable cell line where Uap56 and Ddx39 expression can be knocked down by RNAi (Hautbergue et al, 2009; Figure 2E). These experiments showed that both Chtop and Alyref loading onto the mRNP are dependent on Uap56/Ddx39. Since both Chtop and Alyref are found in the TREX complex and bind Uap56 in a mutually exclusive manner, these data suggest that Uap56 may go through more than one round of ATP hydrolysis during TREX assembly and this ATP hydrolysis drives loading of both TREX components onto the mRNP.
Methylation of Chtop regulates its interactions with Alyref, Nxf1 and RNA but not Uap56
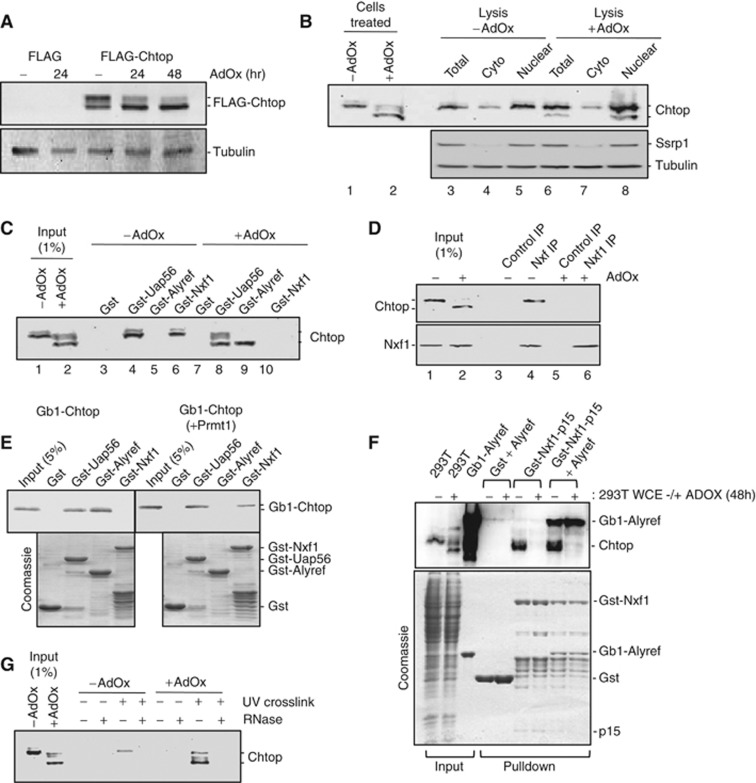
To generate Chtop protein for further analysis, we made a FLAG-Chtop cDNA expression vector and transfected it into 293T cells. Western analysis generated two discrete bands (Figure 3A). Since Chtop binds the arginine methylase Prmt1 (van Dijk et al, 2010), we investigated whether the two bands correspond to different methylation states. Cells expressing FLAG-Chtop were treated with the methylation inhibitor AdOx for varying times. At 48 h, the slower migrating Chtop band disappeared, suggesting that this band corresponded to a hyper-methylated Chtop form, whereas the faster migrating band corresponding to hypo-methylated Chtop remained (Figure 3A). We investigated the methylation status of endogenous Chtop in both nuclear and cytoplasmic fractions. In whole cell lysates, Chtop was predominantly methylated while growth of cells in AdOx shifted Chtop predominantly to a faster migrating hypo-methylated state (Figure 3B, lanes 1 and 2). However, we considered that Prmt1 may methylate some Chtop during cell lysis and preparation of subcellular fractions. To prevent this, we prepared fractions using lysis buffer containing AdOx from cells grown in the absence of AdOx. This revealed that a significant proportion of Chtop exists in the nuclear fraction in the hypo-methylated state (Figure 3B, lanes 3–8). We investigated whether Chtop associated with other mRNA export factors and what impact methylation might have on such interactions using Gst-pulldown assays with 293T cell extracts (Figure 3C). Gst-Uap56 bound both hyper- and hypo-methylated Chtop, whereas GST-Alyref only bound hypo-methylated Chtop. In contrast, Gst-Nxf1 only associated with hyper-methylated forms of Chtop. To confirm that Chtop associated with Nxf1, we IPed Nxf1 from cells and found that it did co-IP with Chtop but only in the absence of AdOx (Figure 3D). To establish that the interactions between Chtop and Nxf1/Alyref were direct and regulated by Chtop methylation, we generated and purified methylated Chtop in E. coli by coexpression with Prmt1 (Supplementary Figure S3). In pulldown assays, direct interactions were observed with Nxf1, Alyref and Uap56. Gst-Nxf1 interaction required Chtop methylation whereas Gst-Alyref only bound hypo-methylated Chtop and Gst-Uap56 interactions were not influenced by Chtop methylation state (Figure 3E). To further investigate whether hypomethylated Chtop could bind Alyref when Alyref was bound to Nxf1-p15, we carried out a pulldown assay (Figure 3F). Unmethylated Chtop failed to bind Nxf1 when Alyref was bound to Nxf1, thus Alyref cannot bridge the interaction between unmethylated Chtop and Nxf1. Finally, we found that hypo-methylated Chtop crosslinks with mRNA more efficiently than its methylated forms in vivo (Figure 3G), as is the case for Alyref (Hung et al, 2010). We conclude that Chtop exists in both hypo- and hyper-methylated states in the nucleus and that its methylation status governs its interactions with Alyref, Nxf1 and mRNA.
Figure 3.
Methylation of Chtop regulates its interactions with export factors. (A) FLAG-tagged Chtop was expressed in 293T cells grown in the presence or absence of the methylation inhibitor AdOx. At the indicated time points, 10 μg of total cell extracts was analysed by western blot using anti-FLAG antibody. (B) Western blot analysis of 293T cells incubated with 10 μg/ml cycloheximide for 8 h. Whole cell extracts of cells grown ±AdOx were analysed (lanes 1 and 2). Additionally, untreated cells were lysed in a buffer ±AdOx and nuclear and cytoplasmic fractions were analysed with the indicated antibodies (lanes 3–8). Ssrp1 western blotting was used to confirm that cytoplasmic fractions were not contaminated with nuclear material. (C) Gst-Uap56, Gst-Alyref and Gst-Nxf1 were used in pulldown assays with 293T cell extract with/without AdOx in the presence of RNase A. Proteins were detected by western blot using a Chtop monoclonal antibody. (D) IP of Nxf1 in the presence of RNAse A from 293T cells treated with AdOx as indicated. Proteins were detected by western blotting with anti-Chtop and anti-Nxf1. (E) Gst-Uap56, Gst-Alyref and Gst-Nxf1 were used in pulldown assays with recombinant Chtop alone or co-expressed with Prmt1 in the presence of RNase A. Proteins were detected via Coomassie staining and western blot (top panel). (F) Chtop only binds Nxf1 when methylated, even in the presence of Alyref. Gst-pulldown assays were carried out with the indicated fusion proteins using 293T cell lysate treated as indicated with AdOx. Chtop was detected using anti-Chtop monoclonal antibody (top panel). The secondary antibody used for western detection also detects the Gb1-6His-Alyref by virtue of the Gb1 tag. (G) mRNP capture assay. Poly(A)+ RNAs from 293T cells grown ±AdOx were purified on oligo-(dT) beads in denaturing conditions after UV crosslinking (+) or not (−). Total extract (1% of input) and eluted proteins were analysed by western blotting with Chtop antibody.
Source data for this figure is available on the online supplementary information page.
Chtop and Thoc5 bind Nxf1 in a mutually exclusive manner
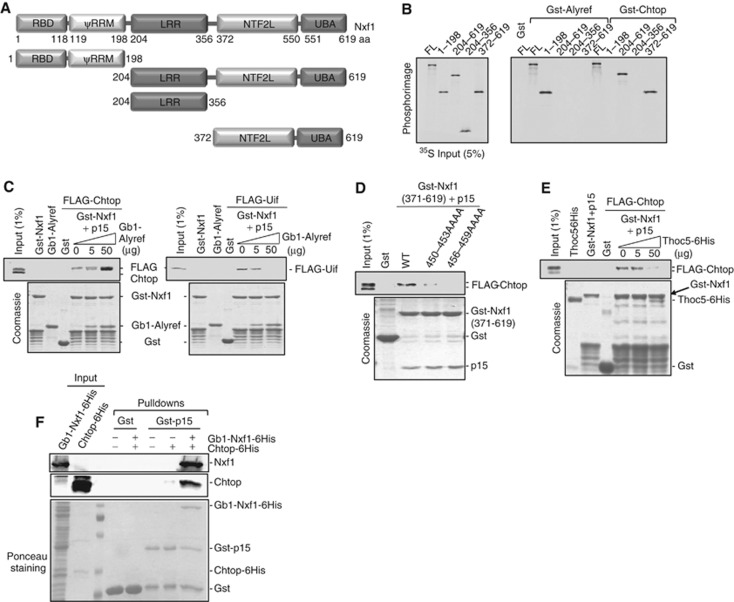
To examine the interaction of Chtop with Nxf1, we mapped the domain of Nxf1 responsible for interaction with Chtop using Gst pulldown assays (Figure 4A and B). This revealed that while Alyref bound amino acids (aa) 1–198 of Nxf1, corresponding to the N-terminal RBD and pseudo-RRM, Chtop bound aa 372–619, encompassing the NTF2L and UBA domains. We investigated what impact Alyref had on the interaction of Chtop with Nxf1 (Figure 4C) and found that increasing amounts of GB1-Alyref bound to Nxf1, stimulated the interaction of FLAG-Chtop with Nxf1. In contrast, FLAG-Uif, which binds aa 1–198 of Nxf1, was efficiently displaced by Gb1-Alyref in this assay. Together, these data suggest that Alyref stimulates Chtop binding to Nxf1, though we have not observed a reciprocal stimulation of Alyref binding to Nxf1 by Chtop. Since the NTF2L domain of Nxf1 binds Thoc5, we examined whether Chtop might recognise a similar surface of the NTF2L domain. Point mutations in the NTF2L domain which are known to block the Nxf1:Thoc5 interaction (Katahira et al, 2009) also block the Nxf1:Chtop interaction (Figure 4D). To confirm that Chtop and Thoc5 utilise a common binding site on Nxf1, we examined the effects of increasing amounts of Thoc5 on the Nxf1:Chtop interaction and found that Thoc5 efficiently displaces Chtop from Nxf1 (Figure 4E). p15 also binds the NTF2L domain on Nxf1, though on the face opposite to that which binds Thoc5 (Katahira et al, 2009). To establish whether Chtop competes with p15 for binding to Nxf1, we carried out a pulldown experiment using Gst-p15 (Figure 4F). Chtop did not bind Gst-p15 directly, but was pulled down in the presence of Nxf1, indicating that Nxf1, p15 and Chtop can form a ternary complex. We conclude that Chtop and Thoc5 bind in a mutually exclusive manner to a common binding site on Nxf1, which encompasses the NTF2L domain and this does not prevent the Nxf1:p15 interaction.
Figure 4.
Mutually exclusive binding of Thoc5 and Chtop to Nxf1. (A) Schematic representation of Nxf1 truncations used in this study. (B) Gst-Alyref and Gst-Chtop pulled down 35S-labelled Nxf1 full length and truncations. (C) Pull-down competition assay with Gst-Nxf1-p15, 293T overexpressed FLAG-Chtop or FLAG-Uif, and increasing amounts of purified Gb1-Alyref in the presence of RNase A. Proteins were detected by Coomassie staining or western blot. (D) Pull-down assays using Gst-Nxf1(aa 371–619)-p15 wild type or mutants with 293T cell extracts from cells transfected with FLAG-Chtop. Proteins were detected by Coomassie staining or western blot. (E) Pull-down competition assay with Gst-Nxf1-p15, with 293T cell extracts from cells overexpressed FLAG-Chtop and increasing amounts of purified Thoc5-6His. Proteins were detected via Coomassie staining or western blot. (F) Chtop does not bind p15 directly. Gst pulldowns with the indicated fusions were carried out in the presence of 4 μg baculovirus derived recombinant Chtop and Gb1-Nxf1 purified from E. coli as indicated. Chtop and Nxf1 were detected using monoclonal antibodies to each protein. The lower panel shows a ponceau stain of the western blot used to detect Nxf1 and Chtop. Experiments were performed in the presence of RNase A (10 μg/ml).
Source data for this figure is available on the online supplementary information page.
Chtop works with Alyref to enhance the RNA binding activity of Nxf1
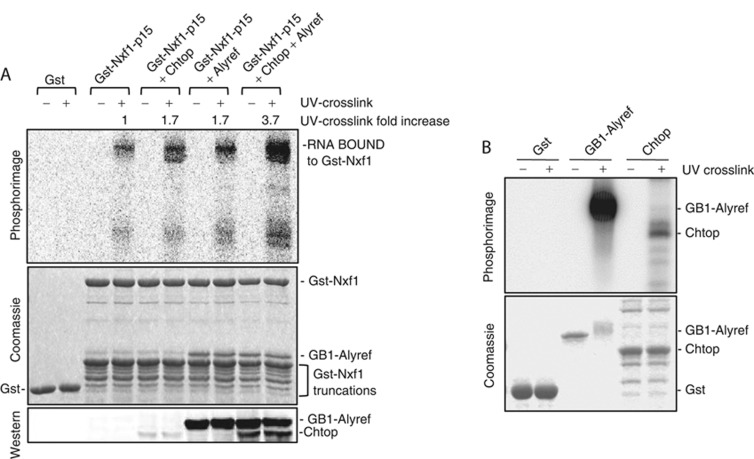
Nxf1 sequesters its RBD but Alyref binding triggers partial enhancement of Nxf1 RNA binding (Hautbergue et al, 2008). Maximal Nxf1 RNA binding requires binding of both Thoc5 and Alyref, which leads to complete exposure of the Nxf1 RBD (Viphakone et al, 2012). We investigated whether Chtop might also play a similar role to Thoc5 in enhancing the RNA binding activity of Nxf1. We incubated radiolabelled RNA with Gb1-Alyref or methylated recombinant Chtop purified from Baculovirus-infected cells (Supplementary Figure 3B) or both, then pulled down the protein:RNA complex using Gst-Nxf1. The complex was then eluted and the RNA subsequently UV crosslinked to proteins. Since Gst-Nxf1 produced a number of truncation products which masked where Chtop would migrate on the gel we confirmed the presence of Chtop in the pulldowns by western blotting (Figure 5A). In this assay, Gst-Nxf1 bound RNA weakly, but showed increased RNA crosslinking activity in the presence of Chtop or Alyref, an effect that was further enhanced when both Chtop and Alyref were bound (Figure 5A). In complex with Nxf1, no RNA binding to Alyref was detected and since Alyref crosslinks with RNA efficiently by itself (Figure 5B), it is clear that the RNA is handed over to Nxf1. In the case of Chtop, it is less clear whether Chtop retains RNA binding activity in the complex with Alyref and Nxf1 as it binds RNA poorly in isolation (Figure 5B). We conclude that the combined action of Chtop and Alyref binding to Nxf1 ensures maximal Nxf1 RNA binding activity.
Figure 5.
Chtop and Alyref modulate the RNA binding activity of Nxf1. (A) The indicated protein complexes were formed and UV crosslinked with a 5′-radiolabelled RNA. The top panel is a phosphorimage and the central panel is the corresponding gel Coomassie stained. The bottom panel is a western blot probed with the anti-Chtop antibody. The Gb1-Alyref is detected because the Gb1 domain binds the secondary antibody. (B) Purified Gst (5 μg), Gb1-Alyref (2 μg) or Baculovirus expressed and purified methylated Chtop (5 μg) was incubated with 32P-radiolabelled RNA with/without UV crosslinking. Protein:RNA complexes were analysed by Coomassie blue and Phosphorimaging.
Source data for this figure is available on the online supplementary information page.
Chtop is required for efficient mRNA export
To investigate the role of Chtop in vivo, we generated stable 293 cell lines expressing inducible miRNAs targeting Chtop, Alyref or Chtop+Alyref and examined the impact of their knockdown on the levels of other export factors by western blotting, 96 h post miRNA induction (Figure 6A). The levels of Uap56 were increased following RNAi of each export factor, with a particularly dramatic increase in the cell line where both Alyref and Chtop were depleted. The levels of Alyref did not increase in the Chtop RNAi cell line, whereas Chtop levels increased significantly following Alyref RNAi. Together, these data indicate that the cell mounts a compensatory response to loss of specific export factors by increasing the levels of other export factors. This has previously been observed in both Drosophila cells (Herold et al, 2003) and human cells where Uif and Thoc2 levels increase following Alyref and Nxf1 RNAi respectively (Hautbergue et al, 2009; Viphakone et al, 2012). We also investigated the effects of Chtop RNAi on cell growth (Figure 6B) and found that Chtop RNAi had a minor impact, whereas Alyref RNAi led to a significant growth defect. Strikingly, the combined RNAi of Alyref and Chtop led to cell death within 6 days, whereas the combination of Chtop and Thoc5 RNAi had a minor impact on cell growth which was only apparent after 12 days.
Figure 6.
Chtop is required for efficient mRNA export in vivo. (A) Total cell extracts from stable Flp-In 293 cells expressing Control, Alyref, Chtop and Alyref+Chtop RNAi were analysed by western blot 96 h post miRNA induction. (B) Growth of stable cell lines following induction of miRNAs targeting the indicated genes. Error bars represent the standard deviation of three independent experiments. (C) Localisation of Poly(A)+ RNAs following induction of miRNAs targeting export factors for 48, 72 and 96 h. All equivalent panels are shown at the same exposure. Examples of nuclear foci of poly(A)+ RNA are highlighted with white arrows in the Alyref RNAi overlay panel (96 h). The scale bar in the top left panel (control) corresponds to 10 μM. (D) Poly(A)+ RNA from stable Flp-In 293 cells, expressing Control RNAi, Alyref RNAi, or Chtop RNAi, was purified on oligo-(dT) beads in denaturing conditions after UV crosslinking (+) or not (−). Total extracts (1% of input) and eluted proteins were analysed by western blotting with Alyref, Chtop, Uap56 or Cbp80 antibodies.
Source data for this figure is available on the online supplementary information page.
We investigated the impact of Chtop RNAi on mRNA export using fluorescence in situ hybridisation with oligo(dT) (Figure 6C). As reported previously (Hautbergue et al, 2009), Alyref RNAi led to a modest mRNA export block, most apparent at 96 h post induction of the Alyref miRNA and was characterised by the appearance of clusters of poly (A)+ RNA in the nucleus (Figure 6C, highlighted with white arrows) as reported previously (Dias et al, 2010). In Chtop and Chtop+Thoc5 RNAi cells, there was a minor accumulation of poly (A)+ RNA in the nucleus in clusters, similar to that seen following Alyref RNAi, but the phenotype was less severe, even at 96 h, suggesting only a minor impact on bulk mRNA export. In contrast, the combined RNAi of Chtop+Alyref led to a very strong mRNA export block, with poly (A)+ RNA accumulating in prominent large clusters in the nucleus as early as 48 h post miRNA induction and was very severe by 72 h. This strong export block probably accounts for the death of the Chtop/Alyref RNAi cell line by 6 days (Figure 6B). Together, these data indicate that Chtop works with Alyref to ensure efficient mRNA export in vivo. We investigated the impact of Alyref and Chtop RNAi on the ability of mRNA export factors to associate with the mRNP in vivo using UV crosslinking in denaturing conditions (Figure 6D). Strikingly, Chtop RNAi led to a significant accumulation of Alyref on mRNA, despite Alyref levels remaining constant in the cell. This suggests that Alyref displacement from RNA which is normally accompanied by Nxf1 binding to Alyref (Hautbergue et al, 2008) is disrupted in these cells. We also observed increased levels of Chtop associated with the mRNP following Alyref RNAi, however, Chtop levels were also increased in the cell, so it is not clear whether Chtop is specifically blocked on the mRNP in these cells. Depletion of Alyref or Chtop had no impact on Uap56 association with the mRNP despite its upregulation in the cell, consistent with it lying upstream of these proteins in the mRNA export pathway.
The UAP56 binding motifs in Chtop are required for its function in vivo
To establish whether the UBMs within Chtop were required for its activity in vivo, we attempted a complementation assay. A Chtop cDNA, engineered to be resistant to Chtop RNAi (Supplementary Figure S4), was transiently transfected and overexpressed in 293 Chtop stable inducible RNAi cells. However, we noticed in these studies that wild-type Chtop overexpression led to a strong mRNA export block. We investigated this further using expression vectors for various mRNA export factors in 293T cells (Figure 7A). As reported previously, overexpression of E1b-ap5 which binds Nxf1 (Hautbergue et al, 2009) did not block mRNA export, whereas Alyref overexpression led to a strong nuclear accumulation of poly(A)+ material in distinct clusters within the nucleus. These clusters are reminiscent of the pattern observed following Alyref RNAi (Figure 6C) and consistent with the report that Alyref RNAi leads to accumulation of mRNA within nuclear speckles (Dias et al, 2010). The overexpression of Chtop also led to a strong nuclear accumulation of poly (A)+ RNA though the accumulation was more evenly distributed in the nucleus. Overexpression of Chtop lacking the two C-terminal UBMs did not lead to nuclear accumulation of poly (A)+ RNA, indicating that the UBMs within Chtop are required for the RNA export block observed following Chtop overexpression.
Figure 7.
The UAP56 binding motif of Chtop is required for function. (A) Overexpression of Chtop blocks mRNA export. The indicated FLAG-tagged expression vectors were transfected into 293T cells. Poly (A)+ RNA was detected using Cy3-oligodT (red) (left panels). The FLAG-tagged proteins were detected using FLAG antibodies and Alexa 488 secondary antibody (green) and DNA was detected using DAPI. The right panels show the overlay of all three channels. The white scale bar in the top right panel represents 10 μM. (B) Schematic of the inducible RNAi-resistant cDNA+miRNA expression cassette that was integrated into the chromosomal FLP site in the 293 T-REX Flp-In cell line. (C) Complementation analysis using stable cell lines. The indicated stable inducible RNAi cell lines were grown for 96 h in the presence of tetracycline and Cy3-labelled oligo (dT) used to detect poly (A)+ RNA. The left panels show the EmGFP fusion proteins. The white scale bar in the top left panel represents 10 μM.
To carry out the complementation assay, we generated stable cell lines in which an inducible expression cassette contained an RNAi-resistant Chtop cDNA (Supplementary Figure S4) fused to EmGFP with a control miRNA or miRNAs targeting Chtop, Alyref or both present in the 3′ untranslated region (Figure 7B and C). The lower expression of Chtop in the stable cell line with a single copy of the Chtop gene integrated did not lead to a block in mRNA export (Figure 7C, top panels). Thus, we were able to complement the strong mRNA export defect seen following combined Chtop/Alyref RNAi. However, Chtop lacking the UBMs failed to complement Chtop/Alyref combined RNAi (Figure 7C, bottom panels) therefore we conclude that the UBMs within Chtop are essential for its function in mRNA export in vivo.
Chtop, Thoc5 and Nxf1 exist in the same complex and are all required for export of mRNAs.
Chtop and Alyref bind to different regions of Nxf1 and the proteins work together to ensure efficient mRNA export. Moreover, Chtop and Thoc5 binding to Nxf1 are mutually exclusive. This raises the possibility that different combinations of Alyref with Chtop or Alyref with Thoc5 promote export of different groups of mRNAs in the cell. Alternatively but not mutually exclusively, Thoc5, Chtop and Alyref might exist in a single dynamic complex in which Nxf1 might exchange binding partners during mRNP biogenesis. To address this, we IPed FLAG-Nxf1 and FLAG-Alyref from cells and gently eluted the IP from beads using FLAG peptide. A proportion of the IP was subjected to western analysis for TREX components (Figure 8A). In the FLAG-Alyref IP, we detected multiple TREX components including Uap56. FLAG-Nxf1 associated with multiple TREX components except Uap56, as expected since Uap56 is displaced from Alyref by Nxf1 (Hautbergue et al, 2008). We then took the eluate from the FLAG-Nxf1 IP and subjected it to a second round of IP with control (anti-HA), Alyref, Chtop and Thoc5 antibodies and analysed these IPs for TREX components. The control antibody used in the secondary IP failed to immunoprecipitate with any TREX component. In contrast, secondary IPs with Chtop, Alyref and Thoc5 antibodies revealed interaction with multiple TREX components but no Uap56 was detected as expected. These data are consistent with Nxf1, Chtop, Thoc5 and other TREX components existing as a single complex in vivo.
Figure 8.
Chtop, Thoc5 and Alyref function in the same mRNA export pathway. (A) Primary IPs were carried out with anti-FLAG antibody followed by gentle elution with FLAG peptide and then secondary IPs with anti-HA (control), anti-Chtop, anti-Alyref or anti-Thoc5 antibodies. Proteins were detected by western blot. (B) Quantitative RT–PCR analysis was used on cytoplasmic and total mRNA to assess the levels of each gene relative to the U1 snRNA. mRNA levels for the cytoplasmic/total ratio are expressed relative to the values seen in the control RNAi which was set at 1.0. Error bars represent standard error of the mean from three experiments. (C) A model for assembly and maturation of the TREX mRNA export complex. Our studies do not differentiate whether Chtop or Alyref joins the assembling TREX complex first, nor the order in which Chtop and Thoc5 bind Nxf1. Me, arginine methylation.
Source data for this figure is available on the online supplementary information page.
To examine whether Chtop and Thoc5 loss affected export of common mRNAs, we analysed the export of specific spliced and intronless mRNAs in cell lines depleted for Alyref, Chtop, Thoc5 and combinations (Figure 8B). Alyref RNAi blocked export of all mRNAs tested with variable efficiencies ranging from 15 to 70% inhibition. Chtop and Thoc5 caused a much less severe block of export for the mRNAs tested, consistent with the modest effects seen using oligo(dT) FISH (Figure 6C). In fact several mRNAs showed no export block at all and for the spliced mRNAs CCNL2 and EPHX2, we even observed increased cytoplasmic RNA levels. The combined RNAi of Alyref/Chtop (40–90% export inhibition) or Alyref/Thoc5 (40–80% export inhibition) gave a robust mRNA export block for all mRNAs tested. Together, these data indicate that Chtop and Thoc5 exist in the same TREX complex and act in the same pathway to export mRNAs from the nucleus to the cytoplasm.
Discussion
We have defined Chtop as a component of TREX that interacts with Uap56 and Nxf1. Chtop has two repeats of a short peptide motif, we have named the UBM, found in other Uap56 binding proteins. Chtop competes with Alyref for association with Uap56, though both Chtop and Alyref are found in a single complex with Nxf1 in the cell. Alyref, Chtop and Cip29 are able to activate the ATPase and helicase activity of Uap56 and this in turn stimulates loading of Chtop and Alyref onto mRNA. This suggests a model in which Uap56 acts as an assembly factor for TREX, recruiting subunits in a sequential manner and loading them into an assembling TREX complex on mRNA (Figure 8C). Since Cip29 binds Uap56 simultaneously with Alyref it may be loaded into TREX in combination with Alyref or Chtop or may remain bound to Uap56 during subsequent ATPase cycles.
RNA helicases frequently drive assembly and conformational changes in protein complexes and associate with specific proteins at different stages in the ATPase cycle. For example, eIF4A associates with eIF4G heat repeat domain 1, Eif4H and RNA in an ATP-dependent manner and in the nucleotide-free state associates with eIF4G heat repeat domain 2 (Marintchev et al, 2009). Similarly, Dbp5, which is involved in a terminal step in mRNA export, makes interactions with other proteins and RNA which are coupled with its ATPase cycle (Folkmann et al, 2011). Gle1 together with IP6 stimulates ATP binding by Dbp5 which reciprocally stimulates Gle1 and IP6 binding to Dbp5 and in this respect the action of ATP parallels that observed previously for Alyref and Cip29 interacting with Uap56 (Dufu et al, 2010). ATP bound Dbp5 is then thought to associate with RNA. Similarly, Uap56 requires ATP to bind efficiently to RNA. Gle1 and IP6 stimulate ATP hydrolysis by Dbp5, which also mirrors what we observe with Chtop and Alyref. Subsequently, Nup159 promotes ADP release from Dbp5 and ATP hydrolysis, together with ADP release, reduces the affinity of Dbp5 for RNA. Whether Uap56 also requires an ADP release factor is undetermined. Uap56 associates with the THO complex and components of this complex may be involved in such an activity. Ultimately, the Dbp5 ATPase cycle is coupled with displacement of mRNA export factors such as Mex67 from the mRNP on the cytoplasmic face of the nuclear pore (Lund and Guthrie, 2005). Thus, while there are certainly similarities between Uap56 and Dbp5 in their ATPase cycles, Uap56 appears to drive assembly of a protein complex whereas Dbp5 appears to be disassembling complexes; therefore, the fine detail of how these two RNA helicases work may differ.
Chtop is a substrate for the arginine methylase Prmt1 (van Dijk et al, 2010) and here we have shown that methylation enhances its binding to Nxf1 and reduces its ability to bind mRNA. Chtop contains multiple repeats of the sequence RG and RGG, commonly known as the RGG box, an RNA binding and protein interaction motif (Kiledjian and Dreyfuss, 1992; Cartegni et al, 1996) found in proteins which are substrates for arginine methylation (Yu, 2011). Therefore, methylation of arginines within the RGG motifs of Chtop may cause the observed reduced RNA binding activity. Reduced RNA binding activity following arginine methylation has also been reported for a number of other proteins such as HnrnpA1 and Sam68 (Yu, 2011). However, arginine methylation of FMRP causes mRNA substrate-specific changes in RNA binding activity (Dolzhanskaya et al, 2006). Interestingly, Alyref is also arginine methylated by Prmt1 and this promotes handover of mRNA from Alyref to Nxf1 during mRNA export (Hung et al, 2010). Moreover, Alyref promotes binding of methylated Chtop to Nxf1 (Figure 4C) and loss of Chtop leads to accumulation of Alyref on mRNA in vivo (Figure 6D), suggesting that Alyref may be incapable of recruiting Nxf1 efficiently and handing mRNA over to it in this situation. Thus, the combined methylation of Chtop and Alyref is likely to be an important control step in the recruitment of Nxf1 to mRNA. Since Alyref also binds hypomethylated Chtop in vitro (Figure 3C), Chtop and Alyref may directly interact at an early stage in TREX assembly prior to arginine methylation and recruitment of Nxf1. The use of methylation in the regulation of mRNA export is conserved since methylation of mRNA export factors by the yeast Prmt1 orthologue, Hmt1, alters their interaction with other export factors. Moreover, Hmt1 is recruited to genes during transcription (Yu et al, 2004); therefore, methylation may provide an additional transcription coupled trigger for TREX assembly, together with splicing (Masuda et al, 2005) and polyadenylation (Johnson et al, 2011).
Chtop and Thoc5 bind in a mutually exclusive manner to the NTF2L domain of Nxf1 (Figure 4E). However, Chtop and Thoc5 are found in a single complex with Nxf1 in vivo. Together, these data suggest that the NTF2L domain of Nxf1 exchanges binding partners within TREX during mRNA export (Figure 8C), though the order of binding and stages of mRNA export where partner exchange occurs is unclear. Thoc5 bound to NTF2L domain of Nxf1 together with Alyref bound to the N-terminus, maintains Nxf1 in the open conformation which is required for full exposure of the Nxf1 RBD and its stable interaction with mRNA (Viphakone et al, 2012). Thus, it is likely that Chtop fufills a similar role to Thoc5 given that Chtop works together with Alyref to enhance the Nxf1 RNA binding (Figure 5A). Consistent with the NTF2L domain dynamically interacting with TREX components and other export factors, Rbm15 and the related Rbm15B also interact with the NTF2L domain of Nxf1 (Uranishi et al, 2009). Rbm15 associates with the nuclear pore and promotes the association Dbp5 with Nxf1-mRNP complexes (Zolotukhin et al, 2009). Thus, Rbm15 may be the terminal partner for the Nxf1 NTF2L domain during mRNA export. However, the combined RNAi of Chtop and Thoc5 does not cause a major mRNA export block (Figure 6C). Therefore, in this situation, Rbm15 may partially replace the functions of Thoc5 and Chtop at earlier stages in mRNA export. In yeast, the loss of Yra1 (Alyref) from the mRNP through ubiquitinylation by Tom1 occurs on the nuclear side of the nuclear pore complex (Iglesias et al, 2010). Such a mechanism, if conserved in metazoans would also destabilise the association of Chtop and Thoc5 that require Alyref for optimal interaction with Nxf1 (Katahira et al, 2009). Loss of both Alyref and Chtop/Thoc5 from Nxf1 would be predicted to switch Nxf1 back to its sequestered conformation with the RBD bound to the NTF2L domain and this together with the action of Dbp5 on the cytoplasmic side of the nuclear pore is predicted to trigger dissociation of Nxf1 from the mRNP.
Chtop is a chromatin-associated protein that affects expression of oestrogen responsive genes (van Dijk et al, 2010) and is part of a nuclear protein complex, 5FMC, which is recruited to and triggers desumoylation of the transcription factor Zbp-89, leading to transcription activation (Fanis et al, 2012). These data together with our present results suggest that Chtop may have dual functions in the cell but might also provide a link between transcriptional events and subsequent mRNA export. In this respect, Chtop would not be unique since Alyref was first described as a coactivator for the transcription factors Lef-1 and Aml-1, stimulating transcription associated with the TCRα enhancer in T cells (Bruhn et al, 1997). The early association of Alyref and Chtop with proteins involved in transcription may assist in their later recruitment to nascent RNA. Since Chtop levels increase significantly when Alyref is knocked down by RNAi (Figure 6A), this suggests that transcriptional targets of Chtop such as oestrogen responsive genes might show altered transcription in response to reduced levels of Alyref in cells, which naturally occurs in a range of cancer cells (Dominguez-Sanchez et al, 2011).
In addition to Chtop, four other putative new components of TREX have been identified (Dufu et al, 2010). Two of these, Pdip3 and Zc11a associate with TREX in an ATP-dependent manner (Folco et al, 2012) as do Alyref and Cip29 (Dufu et al, 2010). Thus, Pdip3 and Zc11A might also be assembled in TREX via Uap56. Pdip3 has a sequence with similarities to the UBM and may use this to directly associate with Uap56. Zc11A does not have a clear UBM and thus how it and other newly identified components of TREX assemble remains to be determined. The regulation of the Uap56 ATPase cycle by TREX components and establishing how Uap56 is first recruited to mRNA remain important questions for the future.
Materials and methods
Plasmid, antibodies, and cell cultures
FLAG-Nxf1, FLAG-Alyref, Gst-Nxf1 and 6His-Alyref full-length and truncations were described (Hautbergue et al, 2008). GB1-Alyref construct has been described (Hautbergue et al, 2009). The open reading frames of Chtop and Thoc5 were cloned into pGEX-6P1, pET24b, pET24b-Gb1 and p3XFLAG-myc-CMV-26 (Sigma) vectors. To generate a Gst-p15 expression plasmid, the p15 cDNA was PCR amplified using the following oligonucleotides 5′GGCCGGATTCATGGCATCTGTGGATTTCAAGACC-3′ and 5′-AGACGCGTCGACCTAGCTGGCCCAGTCCTG-3′ and cloned as an EcoRI-SalI fragment into pGEX6P1. Mutations in Nxf1 and Thoc5 were generated by Quikchange mutagenesis (Stratagene). Human inducible Flp-In T-REX 293 RNAi cell lines were constructed as described (Hautbergue et al, 2009) using the following target sequences: Chtop (GACAACCAATTGGATGCATAT). The target sequences used for Alyref and Thoc5 have been described (Viphakone et al, 2012). miRNA expression was induced with 1 μg/ml tetracycline (Sigma). The Nxf1, Cbp80 and Hpr1 antibodies were from Abcam. The HnrnpA1 antibody (9H10 monoclonal) was from Millipore. The Alyref and FLAG monoclonal antibodies, anti-6His antibody, FLAG-agarose and FLAG peptide were from Sigma. The Chtop monoclonal antibody (KT64) was described (van Dijk et al, 2010). Antibodies to Thoc5 (Hautbergue et al, 2009) and Thoc2 (Masuda et al, 2005) were described.
Complementation analysis
To generate a FLAG-Chtop cDNA resistant to RNAi, Quikchange mutagenesis was used to generate silent mutations in the Chtop cDNA resulting in the following sequence 5′-GATAATCAGCTTGACGCATAT-3′ at the site that the Chtop miRNA targets, with altered bases shown in bold. To create the p3XFLAG-Chtop(ΔUBMs) deletion mutant, site-directed mutagenesis was used to engineer an amber STOP codon in the ORF causing the translation of only the first 214 amino acids of the protein. To generate stable cell lines expressing both RNAi-resistant Chtop and miRNAs targeting Chtop and Alyref, the following strategy was used. Restriction sites EcoRI, NotI and a linker from pEGFPN1 were introduced by divergent PCR in pcDNA6.2-GW-EmGFP-miR-neg-control or pcDNA6.2-GW-EmGFP-miR-Chtop-miR-Alyref (backbone from the BLOCK-IT PolII miR RNAi/EmGFP kit, Invitrogen) using the following oligos: 5′-ATAAGAATgcggccgcgccaccggtcgccaccATGGTGAGCAAGGGCGAGGAGCTGTTC-3′ and 5′-GGCCGgaattcggttttaaagcctgcttttttgtacaaac-3′. RNAi-resistant Chtop or chtop(ΔUBMs) was amplified by PCR using fwd 5′-GGCCGgaattcgccaccATGGCTGCACAGTCAGCGCC-3′, rev FL 5′-ATAAGAATgcggccgcATCATTGGTTTCGGGATCTGTCTGCGCC-3′, rev ΔUBMs 5′-ATAAGAATgcggccgcCTTGGTCAATACAGGGCGAGCAAG-3′. The Chtop(ΔUBMs) constructs lacks the Chtop RNAi target region and is therefore RNAi resistant. PCR products were then cloned EcoRI/NotI into pcDNA6.2-GW-EmGFP-miR-neg-control or pcDNA6.2-GW-EmGFP-miR-Chtop-miR-Alyref. Therefore, these vectors produce an mRNA that encodes a fusion of Chtop-EmGFP with the indicated pre-miRNAs present in its 3′-UTR. The resulting (cDNA-EmGFP-pre-miRNA) constructs were then cloned as HindIII/XhoI PCR fragments into pcDNA5-FRT/TO/His to generate the corresponding Flp-In T-REX 293 (Invitrogen) stable cell lines cells as described (Hautbergue et al, 2009).
Gst-pulldown experiments and IPs
Gst-fusion constructs were expressed and purified as described (Hautbergue et al, 2009). Gb1-6His tagged proteins were expressed in E. coli and purified using Talon resin according to manufacturer’s instructions (Clontech). The binding reactions were performed in 1 ml of either PBST buffer (1 × PBS, 0.1% Tween) for GST-fusion proteins interaction or RB100 buffer (25 mM HEPES-KOH pH 7.5, 100 mM KOAc, 10 mM MgCl2, 1 mM DTT, 0.05% Triton X-100, 10% Glycerol) when purified proteins were present. Both buffers contained RNase A (10 μg/ml). The bound proteins were then washed with RB100 buffer, and then eluted with 50 mM Tris–HCl (pH 8.2), 40 mM reduced glutathione, 100 mM KOAc. For endogeneous protein co-IPs 8 μg KT64 anti-Chtop, 10 μg anti-Hnrnpa1, 12 μg anti-Alyref or 6 μg Hpr1 monoclonal antibodies were crosslinked to 30 μl Protein G-sepharose beads using DMP prior to IP. FLAG-Chtop was IPed using 30 μl FLAG-agarose (Sigma). RNAse A was used in IPs where indicated at 10 μg/ml.
Sequential IPs
In all, 6 μg of FLAG, FLAG-Nxf1 or FLAG-Alyref was transfected into each of 24 × 6 cm dishes (for FLAG and FLAG-Nxf1) or 2 × 6 cm dishes (FLAG-Alyref) of 293T cells. After 48 h, each dish was lysed in 0.5 ml of IP lysis buffer (50 mM HEPES pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.5% Triton X-100, 10% glycerol) containing protease inhibitors and 10 μg/ml RNase A. The supernatants of cell extracts were pooled and incubated with 300 μl FLAG-agarose beads slurry for FLAG and FLAG-Nxf1 or 60 μl FLAG-agarose beads slurry for FLAG-Alyref for 1 h. Beads were previously blocked overnight in IP lysis buffer supplemented with 1% BSA. The beads were then washed with 1 ml IP lysis buffer four times and the bound proteins were subsequently eluted in 1 ml IP lysis buffer with 100 ng/μl FLAG peptide. In all, 5 μg anti-HA, 5 μg anti-Chtop, 5 μg anti-Alyref or 100 μl anti-Thoc5 culture supernatant was each bound to 30 μl Protein G-Sepharose beads in IP lysis buffer with 1% BSA. Eluates from the first FLAG and FLAG-Nxf1 IPs were split into four and incubated with beads coupled to the various antibodies for 1 h. The beads were then washed with 900 μl IP lysis buffer three times. The bound proteins were finally eluted from the Protein G-Sepharose with 50 μl of (0.2 M glycine pH 2.8, 1 mM EDTA), and analysed by SDS–PAGE and western immunoblottings with the indicated antibodies.
mRNP capture assay
A 15-cm dish of 293T cells was UV crosslinked in 1 ml PBS with 300 mJ/cm2 and then lysed in 1 ml IP lysis buffer described above. The extracts were cleared by centrifugation at 16 100 g for 5 min, 2 mg of total protein was denatured in binding buffer (10 mM Tris–HCl pH 7.5, 0.5 M NaCl, 0.5% SDS, 0.1 mM EDTA) then incubated with 25 μl (bed volume) of oligo(dT)-cellulose beads (Sigma) for 1 h at room temperature. The beads were washed three times with 900 μl of binding buffer. The mRNPs were finally eluted for 30 min in elution buffer (10 mM Tris–HCl pH 7.5, 1 mM EDTA, 50 μg/ml RNase A) and analysed by 12% SDS–PAGE and western blot with the indicated antibodies.
UV-crosslinking experiments
A 5′-radiolabelled 15-mer RNA (5′-CAGUCGCAUAGUGCA-3′) described in Hung et al (2010) was incubated with 2 μg of each of the indicated proteins for 15 min on ice in 20 μl of RNA binding buffer (15 mM HEPES pH 7.9, 0.2 mM EDTA, 5 mM MgCl2, 0.05% Tween-20, 10% Glycerol, 100 mM NaCl). The reactions were UV irradiated for 15 min on ice and then analysed by SDS–PAGE. The results were visualised by Phosphorimaging.
Human Chtop expression and purification
Full-length Chtop was subcloned into a modified pFASTBAC (Invitrogen) vector. The corresponding Baculoviruses were made in SF9 cells according to the instructions of the Bac-to-Bac Baculovirus Expression System from Invitrogen. Full-length Chtop was purified on cobalt beads (TALON) from a 400-ml roller bottle culture of SF9 cells 3 days after infection. Purified Chtop was used in the ATPase, helicase, RNA UV crosslinking and RNA remodelling experiments.
Nxf1 remodelling assay
The RNA remodelling assay was described (Hautbergue et al, 2008). In all, 9 ng of 15-mer RNA was incubated for 10 min at room temperature with 50 μg of Alyref alone or 5 μg of Chtop alone, or a combination of both Alyref and Chtop before adding 2.5 μg of immobilised GST-Nxf1:p15. After pulldown, the beads were washed to remove unbound RNA and proteins, and eluted complexes were UV irradiated on ice. Complexes were analysed by SDS–PAGE, stained with Coomassie blue and Phosphorimaging. Western blotting was used to detect Chtop and Alyref as well.
Fluorescence in situ hybridisation and immunostaining
Fluorescence in situ hybridisation using Cy3-labelled oligo(dT) and immunostaining were carried out as described (Hautbergue et al, 2008, 2009).
ATPase assay
ATPase assays were performed as described in Cruz-Migoni et al (2011). Reactions of 50 μl were stopped by the addition of 10 μl 0.5 M EDTA and supplemented with 140 μl H2O before addition of 800 μl of Malachite green-Phosphomolybdenum reagent.
Helicase assay
The helicase assay was described in Cruz-Migoni et al (2011). Briefly, 2 μg recombinant Uap56-6His synthesised in E. coli was incubated in the presence or absence of recombinant Gb1-Alyref, Chtop or MAGOH-6His at a 2:1 molar ratio for 15 min at room temperature prior addition of duplex RNA and ATP. Products of reactions were run on 15% native polyacrylamide gels in TBE buffer before Phosphorimaging.
Quantitative analysis of total and cytoplasmic mRNA levels
Total and cytoplasmic RNA were extracted from indicated RNAi stable cell lines as described (Hautbergue et al, 2009). Dried RNA pellets were resuspended in H2O and 2 μg RNA was used for cDNA synthesis using poly(dN)6 random priming as described by the manufacturer (Bioscript kit from Bioline). In all, 35 μl H2O was added to 20 μl cDNA reactions and 1 μl diluted cDNA with 5 ng/μl primers was used in 10 μl quantitative PCRs (Quantace) run on a Rotorgene 6000 (Qiagen).
Supplementary Material
Acknowledgments
We thank Vicky Porteous for technical assistance and Robin Reed for critical reading of the manuscript. SW acknowledges support from the Wellcome Trust and Biotechnology and Biological Sciences Research Council (UK). TvD and SP were supported by the Dutch scientific organization (NWO) and the Landsteiner foundation for blood transfusion research.
Author contributions: CC, MW, GH, NV and TvD carried out the experiments and helped with data analysis. SP, GH, NV and SW designed the experiments and helped analyse the data. SP and SW wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bruhn L, Munnerlyn A, Grosschedl R (1997) ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRalpha enhancer function. Genes Dev 11: 640–653 [DOI] [PubMed] [Google Scholar]
- Cartegni L, Maconi M, Morandi E, Cobianchi F, Riva S, Biamonti G (1996) hnRNP A1 selectively interacts through its Gly-rich domain with different RNA-binding proteins. J Mol Biol 259: 337–348 [DOI] [PubMed] [Google Scholar]
- Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R (2006) Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 127: 1389–1400 [DOI] [PubMed] [Google Scholar]
- Cruz-Migoni A, Hautbergue GM, Artymiuk PJ, Baker PJ, Bokori-Brown M, Chang CT, Dickman MJ, Essex-Lopresti A, Harding SV, Mahadi NM, Marshall LE, Mobbs GW, Mohamed R, Nathan S, Ngugi SA, Ong C, Ooi WF, Partridge LJ, Phillips HL, Raih MF et al. (2011) A Burkholderia pseudomallei toxin inhibits helicase activity of translation factor eIF4A. Science 334: 821–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias AP, Dufu K, Lei H, Reed R (2010) A role for TREX components in the release of spliced mRNA from nuclear speckle domains. Nat Commun 1: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzhanskaya N, Merz G, Aletta JM, Denman RB (2006) Methylation regulates the intracellular protein-protein and protein-RNA interactions of FMRP. J Cell Sci 119: 1933–1946 [DOI] [PubMed] [Google Scholar]
- Dominguez-Sanchez MS, Saez C, Japon MA, Aguilera A, Luna R (2011) Differential expression of THOC1 and ALY mRNP biogenesis/export factors in human cancers. BMC Cancer 11: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufu K, Livingstone MJ, Seebacher J, Gygi SP, Wilson SA, Reed R (2010) ATP is required for interactions between UAP56 and two conserved mRNA export proteins, Aly and CIP29, to assemble the TREX complex. Genes Dev 24: 2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanis P, Gillemans N, Aghajanirefah A, Pourfarzad F, Demmers J, Esteghamat F, Vadlamudi RK, Grosveld F, Philipsen S, van Dijk TB (2012) Five Friends of Methylated Chtop, a complex linking arginine methylation to desumoylation. Mol Cell Proteomics 11: 1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco EG, Lee CS, Dufu K, Yamazaki T, Reed R (2012) The proteins PDIP3 and ZC11A associate with the human TREX complex in an ATP-dependent manner and function in mRNA export. PLoS One 7: e43804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkmann AW, Noble KN, Cole CN, Wente SR (2011) Dbp5, Gle1-IP6, and Nup159: A working model for mRNP export. Nucleus 2: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazy MA, Gordon JM, Lee SD, Singh BN, Bohm A, Hampsey M, Moore C (2012) The interaction of Pcf11 and Clp1 is needed for mRNA 3′-end formation and is modulated by amino acids in the ATP-binding site. Nucleic Acids Res 40: 1214–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovanov AP, Hautbergue GM, Tintaru AM, Lian LY, Wilson SA (2006) The solution structure of REF2-I reveals interdomain interactions and regions involved in binding mRNA export factors and RNA. RNA 12: 1933–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Garcia-Rubio M, Bermejo R, Gaillard H, Shirahige K, Marin A, Foiani M, Aguilera A (2011) Genome-wide function of THO/TREX in active genes prevents R-loop-dependent replication obstacles. EMBO J 30: 3106–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwizdek C, Iglesias N, Rodriguez MS, Ossareh-Nazari B, Hobeika M, Divita G, Stutz F, Dargemont C (2006) Ubiquitin-associated domain of Mex67 synchronizes recruitment of the mRNA export machinery with transcription. Proc Natl Acad Sci USA 103: 16376–16381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Maurice F, Viphakone N, Voisinet-Hakil F, Fribourg S, Minvielle-Sebastia L (2012) An essential role for Clp1 in assembly of polyadenylation complex CF IA and Pol II transcription termination. Nucleic Acids Res 40: 1226–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautbergue GM, Hung ML, Golovanov AP, Lian LY, Wilson SA (2008) Mutually exclusive interactions drive handover of mRNA from export adaptors to TAP. Proc Natl Acad Sci USA 105: 5154–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautbergue GM, Hung ML, Walsh MJ, Snijders AP, Chang C, Jones R, Ponting CP, Dickman MJ, Wilson SA (2009) UIF, a new mRNA export adaptor which works together with REF/ALY, requires FACT for recruitment to mRNA. Curr Biol 19: 1918–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold A, Teixeira L, Izaurralde E (2003) Genome-wide analysis of nuclear mRNA export pathways in Drosophila. EMBO J 22: 2472–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung ML, Hautbergue GM, Snijders AP, Dickman MJ, Wilson SA (2010) Arginine methylation of REF/ALY promotes efficient handover of mRNA to TAP/NXF1. Nucleic Acids Res 38: 3351–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias N, Tutucci E, Gwizdek C, Vinciguerra P, Von Dach E, Corbett AH, Dargemont C, Stutz F (2010) Ubiquitin-mediated mRNP dynamics and surveillance prior to budding yeast mRNA export. Genes Dev 24: 1927–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Cubberley G, Bentley DL (2009) Cotranscriptional recruitment of the mRNA export factor Yra1 by direct interaction with the 3′ end processing factor Pcf11. Mol Cell 33: 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Kim H, Erickson B, Bentley DL (2011) The export factor Yra1 modulates mRNA 3′ end processing. Nat Struct Mol Biol 18: 1164–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira J, Inoue H, Hurt E, Yoneda Y (2009) Adaptor Aly and co-adaptor Thoc5 function in the Tap-p15-mediated nuclear export of HSP70 mRNA. EMBO J 28: 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiledjian M, Dreyfuss G (1992) Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J 11: 2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Dias AP, Reed R (2011) Export and stability of naturally intronless mRNAs require specific coding region sequences and the TREX mRNA export complex. Proc Natl Acad Sci USA 108: 17985–17990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MK, Guthrie C (2005) The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol Cell 20: 645–651 [DOI] [PubMed] [Google Scholar]
- Marintchev A, Edmonds KA, Marintcheva B, Hendrickson E, Oberer M, Suzuki C, Herdy B, Sonenberg N, Wagner G (2009) Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell 136: 447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R (2005) Recruitment of the human TREX complex to mRNA during splicing. Genes Dev 19: 1512–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J, Herold A, Gari K, Kocher T, Rode M, Ciccarelli FL, Wilm M, Izaurralde E (2004) Genome-wide analysis of mRNAs regulated by the THO complex in Drosophila melanogaster. Nat Struct Mol Biol 11: 558–566 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro S, Hurt E (2011) Linking gene regulation to mRNA production and export. Curr Opin Cell Biol 23: 302–309 [DOI] [PubMed] [Google Scholar]
- Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, Hurt E (2002) TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417: 304–308 [DOI] [PubMed] [Google Scholar]
- Taniguchi I, Ohno M (2008) ATP-dependent recruitment of export factor Aly/REF onto intronless mRNAs by RNA helicase UAP56. Mol Cell Biol 28: 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranishi H, Zolotukhin AS, Lindtner S, Warming S, Zhang GM, Bear J, Copeland NG, Jenkins NA, Pavlakis GN, Felber BK (2009) The RNA-binding motif protein 15B (RBM15B/OTT3) acts as cofactor of the nuclear export receptor NXF1. J Biol Chem 284: 26106–26116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk TB, Gillemans N, Stein C, Fanis P, Demmers J, van de Corput M, Essers J, Grosveld F, Bauer UM, Philipsen S (2010) Friend of Prmt1, a novel chromatin target of protein arginine methyltransferases. Mol Cell Biol 30: 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viphakone N, Hautbergue GM, Walsh M, Chang CT, Holland A, Folco EG, Reed R, Wilson SA (2012) TREX exposes the RNA-binding domain of Nxf1 to enable mRNA export. Nat Commun 3: 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe VO, McMurtrie PI, Mills AD, Takei Y, Penrhyn-Lowe S, Amagase Y, Main S, Marr J, Stewart M, Laskey RA (2010) mRNA export from mammalian cell nuclei is dependent on GANP. Curr Biol 20: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MC (2011) The role of protein arginine methylation in mRNP dynamics. Mol Biol Int 2011: 163827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MC, Bachand F, McBride AE, Komili S, Casolari JM, Silver PA (2004) Arginine methyltransferase affects interactions and recruitment of mRNA processing and export factors. Genes Dev 18: 2024–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin AS, Uranishi H, Lindtner S, Bear J, Pavlakis GN, Felber BK (2009) Nuclear export factor RBM15 facilitates the access of DBP5 to mRNA. Nucleic Acids Res 37: 7151–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.