Abstract
One of the most common medical interventions to reopen an occluded vessel is the implantation of a coronary stent. While this method of treatment is effective initially, restenosis, or the re-narrowing of the artery frequently occurs largely due to neointimal hyperplasia of smooth muscle cells. Drug eluting stents were developed in order to provide local, site-specific, controlled release of drugs that can inhibit neointima formation. By implementing a controlled release delivery system it may be possible to control the time release of the pharmacological factors and thus be able to bypass some of the critical events associated with stent hyperplasia and prevent the need for subsequent intervention. However, since the advent of first-generation drug eluting stents, long-term adverse effects have raised concerns regarding their safety. These limitations in safety and efficacy have triggered considerable research in developing biodegradable stents and more potent drug delivery systems. In this review, we shed light on the current state-of-the-art in drug eluting stents, problems related to them and highlight some of the ongoing research in this area.
Keywords: drug eluting stent, stent, controlled release, restenosis, sirolimus
1. Introduction
Atherosclerosis, the hardening of arteries due to build-up of lipoproteins, is one of the leading causes of death in the United States (Massaro et al., 1979). One of the most common treatments for vessel occlusion due to atherosclerotic lesion formation is stent implantation. Stents are hollow cylinders that are implanted in a collapsed state and then guided into place and opened using the assistance of an angioplasty balloon (Burt and Hunter, 2006). While stenting has become a widely used procedure, it is not without complications.
Principally, many patients experience restenosis, or the re-narrowing of the arteries. This is due to the body’s own wound healing response to the mechanical injury associated with stent implantation. This wound healing response can be broken down into two separate processes: neointimal hyperplasia and vessel remodeling. The placement of the stent initializes a variety of reactions including de-endothelialization, crushing of the plaque, and stretching of the artery (Costa and Simon, 2005). In addition to these initial reactions to the mechanical damage initiated by stent implantation, a series of cellular events are triggered, beginning with platelet and fibrin deposition at the injury site which leads to a cascade of events leading ultimately to leukocyte recruitment and increased cellular proliferation, specifically increased proliferation of vascular smooth muscle cells as well as monocytes and macrophages (Costa and Simon, 2005; Welt and Rogers, 2002).
Ultimately, it is this increase in cellular proliferation that leads to restenosis. It is for this reason that those looking to eliminate restenosis focus on doing so by attempting to halt increased cellular division. In stent restenosis rates are typically reported to occur in 15–20% of patients receiving a bare metal stent for the treatment of simply coronary lesions, but may occur in up to 30–60% of patients with complex lesions. Thus, despite widespread stent use restenosis continues to pose significant problems, and is still the greatest drawback associated with stent implantation over long periods of time.
Drug eluting stents are biomaterials capable of delivering drugs locally and in a controlled manner, to eradicate the increased cellular proliferation ensuing metallic stent implantation. Clinical and long-term outcomes of implanting drug eluting stents have justified their role in curbing undesirable neointimal hyperplasia and even identified thrombosis related risks (Luescher et al., 2007; Joner et al., 2006; Van der Hoeven et al., 2005; Kukreja et al., 2008; Nakazawa, 2011a). This review lays emphasis on the current developments in drug eluting stents while providing mechanistic insight into the problems associated with them, and recognizing benchmarks for future research. A profound understanding of the underlying biological phenomena leading to in-stent restenosis and stent thrombosis is critical towards appreciating the key principles driving innovation in this field.
2. Bare metal stents
2.1 Initial stent design
Work began on therapies for occluded arteries in 1965, using a procedure called transluminal recanalization (Dotter and Judkins, 1964). This process involved gradually reopening the occluded artery by the addition of a series of wider sets of catheters, with each additional catheter forcing the region of occlusion open slightly more. Once the occlusion had been remedied they suggested that perhaps repeated dilation or the use of a splint of some sort could be used to hold the lumen open. The splint, they hypothesized, would act as a false lumen, forcing open the previously occluded region while the body was allowed to heal itself, suggesting that the re-intimalization would be just as likely to occur on their splint as it would on the patient’s own tissue.
Balloon angioplasty, a procedure later pioneered by Andreas Gruntzing, is currently one of the most popular treatments for heart attacks and has been recommended as the standard of care in the Journal of the American Medical Association. Balloon angioplasty uses, with the help of a guide wire, a balloon-tipped catheter to force through stenotic lesions. Once in the desired location, the balloon is inflated, forcing open the occluded region.
Coronary angioplasty does not necessarily provide a permanent fix to an occluded artery. When first being used, coronary angioplasty was often accompanied with a number of risks, most notably abrupt coronary closure. Although effective initially, restenosis occurs in anywhere from 32 to 50% of patients (King, 1997; Serruys et al., 1994).
Four years after Dotter’s landmark publication, he began experimenting with the usage of the prosthetic devices described previously to hold open the lumen of a canine artery. It was not until the 1980’s, however, that extensive experimentation with stent designs really began.
By using catheters, and more specifically balloon tipped catheters, scientists were able to develop new procedures to non-surgically implant a stent device to effectively hold open arteries. Investigations began on using a wire comprised of a heat-sensitive memory alloy, nitinol. This material could be made into the desired shape and then, after cooling, the wire could be straightened in order to be introduced into the body via a catheter. Once inside the body, the temperature of the body will cause the nitinol to warm, causing the material to return to its’ original shape. Using a guide wire, it was possible successfully implant nitinol wire coils into the aortas of canines (Cragg et al., 1983). They found that the nitinol coils they used appeared to have a low tendency of thrombosis, which they directly attributed to the devices ability to refrain from altering vessel wall and lumen interactions. More promising then the nitinol’s possible anti-thrombolytic response was the usage of a catheter and guide wire to implant a device that would effectively open within the artery, thus providing the possible application to be used as stent to maintain vessel patency.
A combinatorial method of a stent implantation along with angioplasty was soon developed. A stainless steel graft was designed that would have a high resistance to radial collapse as well as the ability to retain a desired diameter after balloon inflation. In this fashion, an expandable graft was implanted by mounting the device onto an angioplasty catheter. When the catheter is expanded within the occluded artery, the stent is opened and able to remain within the vessel after the balloon is deflated and removed. Initial studies completed with a canine provided somewhat mixed results. For shorter time periods, little to no stenosis was seen. At longer time points, however, there appeared to be a slight reduction in the diameter of the lumen and partial thrombosis was observed in two of the animals. Grafts removed and examined revealed the body’s surface modification of the implanted stent. The stent, over time, became coated with fibrin suggesting evidence of early stages of organization with what they seem to hope is re-endothelialization of the region. The stent used in this particular system had a large number of drawbacks. The material itself was fairly stiff, and thus, could only really be implemented in a linear region of the artery. Even in their limited studies they found this to be an issue, having the stent cause a kink within the artery leading to thrombosis. They also suggested that at the point of transition between the artery and the stent an increase in sheer stress could exist which may ultimately lead to neointimal proliferation (Palmaz et al., 1985). Similarly, a delivery system comprising a guide wire and an invaginated balloonlike structure that houses the stent has also been developed. Low hydraulic pressure effects stent release from the device and enables its placement within the artery. These stents were composed of a stainless steel alloy that allowed the device to be both radially self-expanding as well as longitudinally flexible (as compared to the Palmaz stent). (Rousseau et al.,1987).
Long-term studies on restenosis after placement of stents could not truly be completed until a significant amount of time had passed from the first ground-breaking procedures. Long-term results of patients who had received the Palmaz-Schatz stents provided some surprising results. Briefly this stent is comprised of a meshwork of steel and deployed by a coaxial balloon system. The incidence of restenosis in patients who received a single-stent implantation was found to be 30.2% while studies indicate that the incidence of restenosis after receiving angioplasty alone ranges from 28 to 41% (Ellis et al., 1992). Although stents were able to effectively prevent the immediate re-closing of the artery often seen after balloon angioplasty, they do not appear to be very effective in ultimately reducing restenosis development. Up until this point, most work primarily focused on designing a stent based on the mechanical properties it could afford, which although important, does not appear to help restenosis in long-term applications. It was therefore necessary to change focus on restenosis as a process, on the cellular level in order to prevent further occlusion of the arteries.
2.2 Stent improvement
Neointimal proliferation, as described briefly previously, is considered to be a component of normal vascular healing in response to injury. Because balloon angioplasty and stent implantation causes an actual physical injury in the artery, the body’s inflammatory response is the natural result of any stent implantation. With a greater understanding of the cellular processes involved in restenosis, it is possible to alter stent therapy to account for this problem. Several approaches have been suggested to improve the procedure and reduce tissue proliferation, these include: improving implantation techniques, stent design, coating the stent with a pharmacological factor, and gene therapy. Because many different biological and cellular mechanisms contribute to restenosis, drugs that target only a single pathway may have limited value in ultimately relieving the problem. By coating the stent with a drug, it may be possible to deliver the drug directly to a site-specific area without having to be concerned about possible interactions of the drug in other areas of the body. With the addition of a time release mechanism, it may also be possible to deliver this pharmacological factor over a given, perhaps prolonged, time period. In this situation, it is also possible to release a number of pharmacological factors a single time, allowing for more comprehensive targeting of restenosis. The drug or drugs can be incorporated into the stent in a number of ways; it can be linked to the stent surface, embedded and released from within polymer materials, or surrounded by and released through a carrier (Fattori and Piva, 2003).
Before beginning any type of design of a drug delivery system, the efficacy of delivering a drug to the artery after stent implantation must be determined. If, for example, delivery is altered in any way, this can be factored into the design of the drug delivery system, i.e. perhaps a higher dosage of drug is necessary to achieve the same arterial delivery as seen prior to stent implantation. Because the artery may be damaged by implantation of stents, altered delivery of the drug may be of concern. Therefore, the effect of stent implantation on drug deposition within the artery was examined (Baumbach et al., 1999). Their results indicated that stent implantation prior to drug delivery did not cause any alterations in drug deposition of paclitaxel, a commonly used drug for the reduction of neointimal growth. Also of note, the amount of drug delivered to the system actively (via a high pressured injection) or passively (via lower pressured infusion) did result in different drug deposition within the artery. These two facts will be of the utmost importance when choosing design parameters for drug delivery via stents.
3. Biological mechanisms
While atherosclerosis involves a fibroproliferative response to excessive amounts of lipids within blood vessels, restenosis due to angioplasty or implantation of metallic stents is mainly due to the arterial vessel wall’s response-to-injury mechanism. This section reviews the biological mechanisms that lead up to the undesirable luminal narrowing that necessitates the use of drug eluting stents and helps better understand the logic behind the use of commonly used anti-restenotic drugs.
3.1 Atherosclerosis
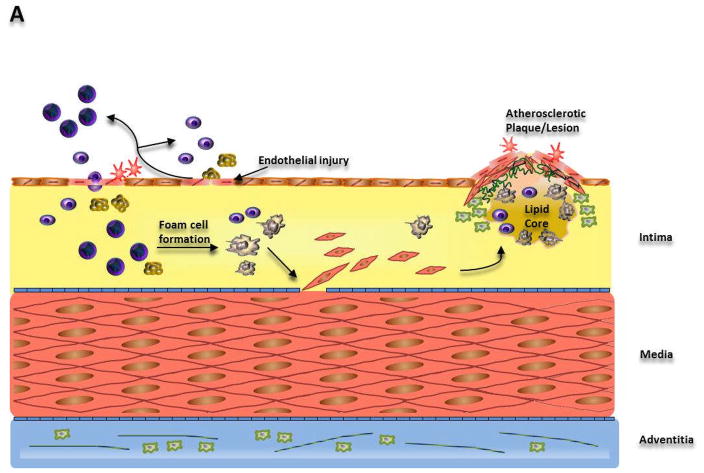
One of the most common causes of injury to the arterial epithelium is the formation of the oxidized low density lipoproteins (oxLDL). oxLDL dysfunctionalizes the endothelium by inducing genetic changes within the endothelial cell that result in increased permeability and augmented adhesiveness. Increased adhesion of the endothelial cells to monocytes in the blood flow facilitates monocyte-endothelial cell association and transport of the monocyte into the endothelium (Stary et al., 1994; Feig et al., 1982). Further, endotheilial cells produce several monocyte activators in response to oxLDL. Monocytes along with T-cells from the blood flow elicit an inflammatory response by encouraging adherence of monocytes and T-cells to the site of injury. Such a dysfunctional endothelium is also known to attract blood platelets. Monocytes localizing the “injured” endothelium from the blood flow eventually result in the formation of macrophages (Fig. 1A).
Fig. 1.
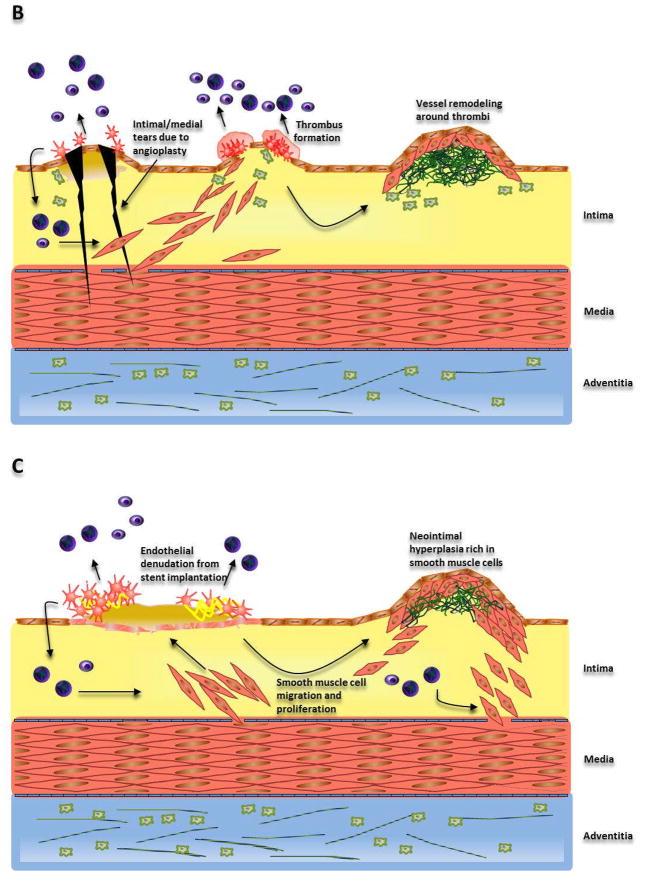
Illustration of the mechanisms leading to (A) Atherosclerosis, (B) Restenosis due to angioplasty and (C) in-stent restenosis.
(A) Dysfunction of endothelial cells triggers a response-to-injury mechanism increasing monocyte and lymphocyte adherence to the endothelium. Interaction of these cells with the endothelium leads to recruitment of more monocytes/macrophages, lymphocytes, platelets, and smooth muscle cells. Lipid accumulation produces foam cells, which along with lymphocytes and smooth muscle cells drives plaque/lesion formation. (B) Angioplasty causes intimal and medial tears which attract platelets and result in thrombi formation. Smooth muscle cells and collagen then synthesized as a result of the response-to-injury mechanism lead to vessel remodeling. (C) Stent implantation causes endothelial denudation and crushing of the plaque triggering an inflammatory response. Eventually, smooth muscle cell migration and proliferation leads to formation of neointima.
Macrophages formed subendothelially consume oxLDL, which itself acts as a chemoattractant to monocytes and release cytokines and growth signals to further their cause and call upon other inflammatory species. Macrophages ingest the oxLDL, becoming lipid-laden foam cells and become the growing atherosclerotic lesion, in addition to encumbering unfortunate oxidation of other LDL in the intima (Frostegard et al., 1990). This unfortunate oxidation amplifies the vicious cycle of regulatory and proliferative molecule production triggering further macrophage, endothelial cell and smooth muscle cell replication and migration. Smooth muscle cells activated as a result of these inappropriate signals migrate from their original site of residence in the media to the intima, exacerbating the inchoate fatty lesions. Similarly, other smooth muscles that do not themselves migrate, produce chemotactic/mitogenic signals effecting/causing migration of monocytes furthering the progression of the atherosclerotic plaque (Cushing et al., 1990). Smooth muscle cells covering lipid-filled macrophages create a connective tissue matrix constituting of collagen, elastic fibers and proteoglycans.
T-lymphocytes called into action mainly due to the interleukin-2 produced by activated macrophages, can also release IFN-γ, GM-CSF, or TNF-α, thereby further accentuating the deleterious effects of an excess of regulatory factors in the exacerbating environment. Lipid-filled monocytes upon further encapsulation by connective tissue such as collagen, elastic fibers and proteoglycans culminate into the formation of fibrous plaque or an advanced lesion signifying the advent of atherosclerosis. Once the lesion has developed, platelets can adhere to it and can become a part of the atherosclerotic vicious circle by releasing the array of the aforementioned growth-regulatory molecules such as PDGF, TGFα, TGFβ, and IGF-I (Stary et al., 1994).
Chronologically speaking, in response to oxLDL production, the monocytes and T-lymphocytes are the first to be summoned inside the arterial intima. Interactions amongst these T-lymphocytes and the macrophages derived from the monocytes appearing at the outset of lesion formation, in addition to the interaction between these macrophages and the endothelium, may be responsible for both replication and growth signal production, as well as cytokine related gene expression. Endothelial cells activated by the macrophages through chemotactic signals, along with the macrophages themselves, call upon the smooth muscle cells from the media into the intima. Subsequent interaction between the species present in the milieu of the potentially atherosclerotic microenvironment result in advancement of lesion progression (Berliner and Heinecke, 1996).
The products released by macrophages, smooth muscle cells, platelets, and endothelial cells are responsible for recruitment of other macrophages, T-cells and smooth muscle cells from the media to the site of injury. The net mass of lipid accumulated macrophages (foam cells), T-cells and smooth muscle cells result in the formation of a rudimentary fatty streak within the endothelium of the artery. Accumulation of cellular species ensue the release of growth signals and cytokines by inflammatory cells around the fatty lesion. Foam cells devoured during the inflammatory attack may relocate to the blood stream by passing through the endothelium, thereby injuring it in transit. This new injury becomes thrombogenic by as like a seed to blood platelets. Blood platelets execute the job of releasing growth-regulatory molecules and cytokines to activate other platelets, macrophages, T-cells, smooth muscle cells and even endothelial cells. Thus, the cycle continues and fatty streaks advance to form fibrous plaques that hinder blood flow to critical organs. A protective response to a hyperlipidemic endothelium culminates into the formation of a fibrous lesion covering the interior lipid core and cellular debris (Ross, 1995).
3.2 Restenosis
The presence of atherosclerotic plaque compromises the integrity of the lumen of coronary arteries, thereby hindering blood flow to the heart and lungs. Balloon angioplasty, the technique most commonly used to combat atherosclerosis involves inflating a balloon attached to the distal end of a catheter tube at the site of the plaque. Implications of mechanically dilating arteries included the possibility of over-distension of vessels lined with plaque in intimate contact with the endothelium. Consequently, the compressive force exerted by the balloon catheter led to reduction of plaque, axial dispersion and extrusion of the fibrous plaque, and more importantly vessel expansion.
Angioplasty procedures may undergo early failure primarily due to the elastic recoil of the arteries in response to forced dilation by the balloon catheter (Haude et al., 1993). On the other hand, late failure post-angioplasty is mainly because of thrombosis derived vessel remodeling by vascular smooth muscle cell migration and proliferation in the thrombi. Mechanical stress encountered by arterial walls during the angioplasty procedure can tear the intima and media and injure the endothelium (Fig. 1B). Over a period of time, the endothelial injury becomes thrombogenic and attracts blood platelets to it (Castanedazuniga et al., 1980).
Moreover, the process of trying to expand the vessel wall, typically results in denudation of endothelial cells underlying the plaque being subjected to compressive and extrusive forces. Dislodgment of endothelial cells leads to extrication of subintimal species that effect platelet adherence and aggregation initiating the response-to-injury mechanism commonly held accountable for atherosclerotic vessel thickening. Prominently, tissue factor exposure following injury has been implicated for enticing platelets and activating them to commence a response-to-injury mechanism. Platelet aggregation is promoted by the IIb/IIIa membrane glycoprotein receptors present on platelets activated by endothelial denudation. A conformational change in the receptor makes it susceptible for fibrinogen binding, eventually resulting in the crosslinking of platelets, which in turn aids in the aggregation of platelets (Ross, 1993).
Not only does platelet aggregation release thrombogenic factors that cooperate and complement platelet adhesion and aggregation, but also releases mitogenic and chemotactic factors for vascular smooth muscle cell migration and activation such as PDGF, FGF, TGF-β, as is described for the response-to-injury mechanism atherosclerosis. These fibrin/erythrocyte rich thrombi accommodate migration of VSMCs from the media so that they can neovascularize around the injury and lead to stenosis of the lumen. In comparison with atherosclerotic plaque lesions, restenotic injuries, angioplasty or stent-related, contain more of collagen and extracellular matrix components produced by proliferating cells than cellular material itself. The proportion of fibrous/extracellular matrix material grows as the restenosis advances. As a result, anti-restenotic therapies aiming to contain cellular proliferation alone have not really been successful (Faxon et al., 1987).
The inflammatory response to restenotic injury is also predicated upon leukocyte recruitment to the wounded site and their migration through the accumulated platelet thrombus into the intima. Leukocytes attach to the platelets already adhering to the wound. P-selectin presented by activated platelets plays an important role in facilitating the initial attachment and subsequent strong association (Marx et al., 2011). After securing the leukocytes to P-selectin, transplatelet migration is then achieved by integrin class of adhesion molecules. Employment of neutrophils and monocytes by the inflammatory machinery intensifies the leukocyte recruitment through upregulation of cell adhesion molecule expression, integrin activation and synthesis of chemokines.
In addition to platelets attracting leukocytes and other inflammatory cells, other cellular entities present in the restenotic lesion such as smooth muscle cells, endothelial cells and macrophages release molecules that further partake in generating a more prolific inflammatory response. Monocyte chemoattracnt cytokines such as MCP-1 released by these cells ensure recruitment of monocytes, basophils and T cells. Similarly, interleukin-8 (IL-8), another chemokine secreted by somatic cells amplifies neutrophil recruitment (Cushing et al., 1990).
3.3 In-stent restenosis
The re-narrowing of arteries after placing stents is called in-stent restenosis and is dominated by neointimal growth, from smooth muscle cell proliferation, covering the stent surface. Metallic stents are effective in opposing the acute elastic recoil of the blood vessel and the subsequent negative remodeling in response to the stretching caused by the stents. Long-term institution of stents in contact with the arterial walls can incite a prolonged, severe inflammatory response, in addition to neointimal hyperplasia (Lowe et al., 2002).
Placement of conventional, bare metals stents immediately results in deendothelialization of the surface subjected to stent-based trauma, crushing of the plaque lining the vessel wall causing deep, local and prolonged injuries to the media and adventitia, along with stretching the arteries to serve the primary purpose of increasing luminal blood flow (Fig. 1C). Growth factors released because of arterial injuries in the media and/or adventitia drive vascular smooth muscle cells into the media. Vascular smooth muscle cells are stirred into the G1/S cell cycle phase, enhancing their replication and migration into the intima. Deendothelialization also triggers an inflammatory response that is bolstered by the cytokines released from active vascular smooth muscle cells and leukocytes drawn towards the injury in a manner similar to that observed as a consequence of atherosclerotic and angioplasty-based injuries.
A collaborated paracrinal response from leukocytes, platelets, and VSMCs further augments the proliferation of active VSMCs and migration of more VSMCs from the media, culminating in a sizeable amount of new intimal cell growth (neointimal hyperplasia) over a period of weeks (Ramcharitar et al., 2007). With the passage of time, as the stent surface becomes endothelialized, the cellular and inflammatory response wanes. However, there is still a significant quantity of extracellular matrix components such as collagen, proteoglycans been synthesized by VSMCs in the intima. These components primarily constitute the neointimal hyperplasia engulfing the stent surfaces and blocking blood flow (Marx et al., 2011).
Surface de-endothelialization attracts platelets and activates them to produce adhesion molecules such as P-selectin. P-selectin binds to the inflammatory system agents such as leukocytes that are patrolling the circulation system, via P-selectin glycoprotein receptors present on their cell surface. P-selectin bound leukocytes can roll along the injured surface and firmly latch on to it through integrins (e.g. Mac-1 attaches to endothelial counterligands such as intercellular adhesion molecule-1) or platelet/firbrinogen receptors such as GP I and GPIIb/IIIa.
Chemokines released from activated VSMCs are purported to enable absorption of the leukocytes into the arterial tissue. Growth factors are simultaneously released by already activated platelets, leukocytes, and VSMCs, encouraging further activation, migration from the media into the intima and replication of VSMCs. Once activated, the VSMCs proliferate to generate a neointima replete with extracellular components such as collagen and proteoglycans. Formation of collagen and other ECM entities presumably leads to vessel shrinkage and negative remodeling in restenotic injuries, while also assisting smooth muscle cell migration. However, the permanent fixture of robust, metallic stents against a shrinking vessel has been largely successful at thwarting such negative remodeling of the vessel (Marx et al., 2011).
Advances in understanding the biological mechanisms underlying in-stent restenosis have helped us arrive at suitable drugs that can inhibit restenosis. A summary of some commonly used drugs for preventing in-stent restenosis and their mechanism of action are presented in Table 1.
Table 1.
Summary of mechanism of action and resulting outcome for anti-restenotic pharmaceutics currently used in drug eluting stents
| Sr. no. | Anti-restenotic drug | Binding target | Mode of action | Final result |
|---|---|---|---|---|
| 1 | Sirolimus | FK-506 Binding Protein 12 |
|
Smooth muscle cell cycle arrest in G1-S phase |
| 2 | Biolimus A9 | Smooth muscle cell and T-lymphocyte cell cycle arrest in G1-S phase | ||
| 3 | Zotarolimus | |||
| 4 | Everolimus | |||
| 5 | Novolimus | |||
| 6 | Tacrolimus |
|
T-lymphocyte cell cycle arrest in G1-S phase | |
| 7 | Pimecrolimus | Macrophilin-12 |
|
|
| 8 | Paclitaxel | Microtubules |
|
|
| 9 | Dexamethasone | Specific steroid-binding protein receptor |
|
|
| 10 | Curcumin | Microtubules |
|
|
4. First-generation drug eluting stents
4.1. Sirolimus eluting stents
Sirolimus (Rapamycin), an immunosuppressive agent/drug with anti-migratory and anti-proliferative effects on vascular smooth muscle cells has been used to prevent rejection in organ transplantation and was the first drug to be used in the first-ever US FDA approved drug eluting Cypher stent in April 2003. Prior to being approved by the FDA, sirolimus-eluting stents went through a number of clinical trials that endorsed the anti-proliferative and anti-restenotic ability of sirolimus. Before becoming popular as the commonly used agent in drug eluting stents, this antibiotic isolated from the bacterium streptomyces hygroscopicus was used primarily as an immunosuppressant in prophylaxis of organ transplant rejection (Vasquez, 2000). It has also been investigated as an anti-cancer agent owing to its anti-proliferative abilities (Muthukkumar et al., 1995).
4.1.1. Cypher sirolimus eluting stent
The CYPHER™ stents have been implanted in more than 3 million patients globally since their approval in 2003. The Cypher stents comprises of two layers of polymer coated on a balloon expanding BX Velocity™ stent which is made up of stainless steel 316 L, that has been laser cut and electropolished. The conventional bare metal stent is then coated with Parylene C, known for its increased dielectric constant, used conventionally for improving moisture resistance and biocompatibility of biomedical devices. The Cypher stent utilizes poly(ethylene-co-vinyl acetate) (PEVA) and poly(n-butyl methacrylate) (PBMA) as the non-erodible polymer coating that carries the sirolimus. A coating of a blend of PEVA and PBMA copolymer, mixed with the sirolimus (in a ratio of 67% polymer to 33% drug) is created by first dissolving the copolymer in THF, followed by the addition of lipophilic sirolimus to the copolymer/THF mixture. The first layer of the drug-polymer mix is then spray-coated with PBMA in THF to form an enclosing topcoat which acts as a rate controlling membrane (Acharya and Park, 2006) (Fig. 2). This rate controlling membrane is necessary to prevent the initial drug burst which can be associated with a constant release membrane reservoir type system and also further elongate the release of the sirolimus. Clinical trials implementing the Cypher indicate that sirolimus is released slowly over four to six weeks with 80% released by the fourth week, and 100% released by the sixth week. Complete elimination of restenosis with these stents was a result of the reduction in hyperplasia, and thus, no additional treatment for these patients was needed (Fattori and Piva, 2003).
Fig. 2.
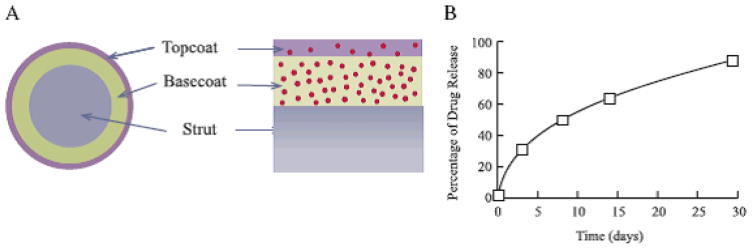
Schematic of sirolimus eluting Cypher stent: (A) cross-sectional and side views (B) in vitro sirolimus release profile
Adapted from Acharya and Park (2006) by permission.
4.1.2. Limitations
Inspite of the remarkable decrease in restenosis rates offered by sirolimus eluting stents, their long-term success has been marked by the incidence of late stent thrombosis due to hypersensitivity and inflammatory reactions. Stent thrombosis usually occurs very late (>12 months) or late (>30 days) after the implantation of the drug eluting stent. However, there have been reports confirming the occurrence of acute/subacute stent thrombosis within 24 hours or 30 days as well (McFadden et al., 2004; Joner et al., 2006). Stent-related coronary death has been attributed to the delayed arterial healing response patented by deficient re-endotheliazation and strut coverage of the stents that eventually leads to thrombosis and severe inflammatory reactions especially after discontinuance of dual antiplatelet therapy (Virmani et al., 2004). Pathological studies related to clinical trials and autopsies have shed light into the late-thrombosis associated with these stents. Local hypersensitivity to the sirolimus eluting stents characterized by eosinophils, lymphocytes, and giant cells throughout the stented segment was found to greatly contribute to the late-stent thrombosis. Stent malposition caused by positive arterial remodeling has also been observed in stents that evoke considerable inflammatory response.
Recent work has suggested that, while malapposition alone does not cause thrombogenicty, overall size of clots increased and appears to be related to blood recirculation patterns associated with the malappositioned stent (Kolandaivelu et al., 2011). When compared to bare metal stents malapposed to the same degree, drug eluting stents continued to reduce thrombogenicity.
These undesirable hypersensitivity responses associated with stent devices implanted for more than a year are believed to be caused by a combination of effects related to the non-erodible polymer coating, sirolimus, drug load and drug pharmacokinetics.
PEVA-PBMA, the polymeric carrier used in these stents, is guaranteed to elicit a chronic, local inflammatory reaction of CD45-positive lymphocytes and eosinophils, after a period of 4 months, resulting in the release of various growth factors that activate the smooth muscle cell proliferation cycle, thereby explaining the presence of late neointimal growth in most late-stent thrombosis related deaths (Finn et al., 2005; Wilson et al., 2009). The role of a non-biocompatible permanent polymeric coating in late thrombosis has given impetus to research in the area of biocompatible and biodegradable coatings that endow stents with the ability to control drug release in addition to reducing late stent thrombosis.
Apart from the stent and the polymeric coating, other innate factors escalating the risk of thrombosis include poor left ventricular function, diabetes mellitus, increasing age, acute coronary syndrome at presentation, renal failure, and presence of bifurcational lesions (Iakovou et al., 2005a; Park et al., 2006).
While the polymeric coating may be culpable in late stent thrombosis, sirolimus itself may be deemed responsible for acute/subacute stent thrombosis. The very phosphatidylinositol-3 kinase pathway that plays an important role in smooth muscle cell proliferation, is also known to downregulate tissue factor expression in endothelial cells and monocytes (Steffel et al., 2006). Inhibition of mTOR by the sirolimus-FKBP12 complex in endothelial cells, thus, leads to thrombin and tumor necrosis factor-α prompted increased tissue factor expression and activity (Guha and Mackman, 2002). Penetration of sirolimus in the endothelial cells of the arterial intima could possibly be the source of thrombosis within 30 days of stent placement, wherein almost 80% of sirolimus is eluted.
4.2. Paclitaxel eluting stents
The National Cancer Institute’s plant extract screening program for antitumor activity led to the discovery of paclitaxel, which was approved by the FDA for its antineoplastic use for ovarian cancer treatment in 1992. It is currently used for the intravenous treatment of lung (FDA approved-1998), breast (FDA approved-1994) and ovarian cancer. This Pacific Yew tree bark extract has been substantiated to wield its anti-proliferative abilities in order to minimize in-stent restenosis (Wani et al., 1971; Schiff et al., 1979). A flurry of clinical trials soon after the FDA approval of sirolimus-eluting stents in 2003 fronted the way for the FDA approval of paclitaxel-eluting TAXUS stents in 2004.
4.2.1. Taxus® paclitaxel eluting stents
The Taxus® stent developed by Boston Scientific comprises of a triblock copolymer that includes a hydrophobic moiety in the form of isobutylene, which can better associate with the hydrophobic drug paclitaxel and enhance loading and release kinetics of the same. This polymer matrix, called the Translute™ polymer, uses a poly(styrene-b-isobutylene-b-styrene) terpolymer for superior paclitaxel delivery. The styrene layer on the top acts as a rate controlling layer allowing controlled release of paclitaxel over a prolonged period of time (Fig. 3) (Acharya and Park, 2006).
Figure 3.
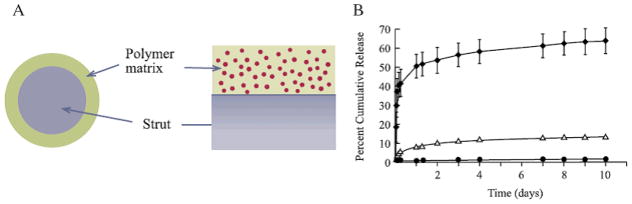
Schematic of paclitaxel eluting Taxus stent: (A) cross-sectional and side views (B) in vitro paclitaxel release profile
Adapted from Acharya and Park, 2006 by permission.
4.2.2. Limitations
Despite the promising antineoplastic talents of paclitaxel, incidence of in-stent thrombosis with long term usage of the Taxus stents has been a major cause of concern from the standpoint of their utility as anti-restenotic devices. Clinical trials, long term assessment of performance and pathological investigation of autopsies have contributed towards gaining further insight into the mechanisms of late stent thrombosis in paclitaxel eluting stents. The antiproliferative qualities of paclitaxel also end up hampering the chances of re-endothelialization of stents. Moreover, the hypersensitivity reactions from the non-erodible nature of the Taxus polymer coating of poly(styrene-isobutylene-styrene) caused inflammation contributed by eosinophils, lymphocytes, and giant cells (Nakazawa et al., 2011b). In contrast to sirolimus eluting stents, however, the Taxus stents were found to be positively remodeled mainly as a consequence of stent malapposition originating from the excessive accumulation of fibrin on the stent abluminal surface.
It is believed that the fibrin resulting from the use of paclitaxel, undergoes degradation to form fibrin fragment E, which is known to activate smooth muscle cell proliferation and migration. Along with the detrimental pro-apoptotic action, the release kinetics of paclitaxel has been found to be inferior to sirolimus. While both the drugs are found to infiltrate the arterial wall owing to their lipophilic nature, paclitaxel tends to accumulate preferentially in the adventitia rather than distributing itself among the arterial layers (Ishida and Tanaka, 1982; Naito et al., 2000). Since, smooth muscle cells are predominant in the arterial media; the prevailing presence of paclitaxel in the adventitia instead of the media/intima is not as effective at checking neointimal hyperplasia as sirolimus. The transmural diffusivity of paclitaxel is also not as high as that of sirolimus and is further diminished by the presence of red blood cell count/thrombus adhering to the site of stent-related vascular injury.
In sum, the combinatorial effects of paclitaxel loading, pharmacokinetics and polymer coating are considered responsible for the late-stent thrombosis and long term prevention of neointima formation in the case of Taxus stents. Use of vascular diagnostic techniques such as late lumen loss measurement via intravascular ultrasound along with quantitative angiography have illustrated the better efficacy of sirolimus eluting stents as compared to paclitaxel eluting stents. Iakovou and colleagues showed the presence of a diffuse restenosis pattern in 50% of Taxus stents in addition to 21% of severe restenotic lesions resulting in total vessel occlusion Iakovou et al., 2005b).
5. Second-generation drug eluting stents
5.1. Zotarolimus eluting stents
Better awareness and understanding of the inadequacies of sirolimus-eluting and paclitaxel-eluting stents set off the search for more efficacious and selective anti-proliferative agents that have lower systemic toxicity and do not delay endothelial healing. Endeavor® (Medtronic, Inc., Minneapolis, MN) was approved by the FDA in February 2008. The Endeavor® stent uses Abbott Vascular’s zotarolimus drug and a cobalt alloy stent coated with a biocompatible phosphorylcholine coating on Medtronic Vascular’s cobalt-chromium based Driver™ metallic stent platform (Sketch et al., 2005).
Zotarolimus is the first ever drug synthesized exclusively for treatment of in-stent restenosis. Zotarolimus is produced by the tetrazole ring substitution of the hydroxyl group at the C42 position. The presence of a tetrazole ring instead of a hydroxyl group makes zotarolimus extremely lipophilic. This hydrophobicity restricts the solubilization of zotarolimus in the luminal blood flow; leading to an immense decline in the systemic exposure risk and the negligible concentrations of the anti-proliferative agent also tends to be conducive to stent re-endothelialization. As expected, the lipophilic nature of zotarolimus helps the greatly required slow, dissolution-limited kinetics of release along with increased transport across cell membranes. Preliminary indications are that Zotarolimus is even more potent than its sirolimus counterpart, with only very slight concentrations necessary to achieve comparable results in terms of reducing the incidence of adverse vascular events associated with restenosis (Buellesfeld and Grube, 2004; Rogers, 2005).
Zotarolimus is a sirolimus-analog and an immunosuppressant that acts by binding itself to the protein FKBP-12 (Buellesfeld and Grube, 2004; Rogers, 2005) like sirolimus. The FKBP-12-bound zotarolimus blocks mTOR’s phosphorylation ability thereby halting the progress of cell proliferation in the G1 phase. The phosphorylcholine coating on the stent mimics the cell membrane of red blood cells in the plasma, thereby avoiding hypersensitivity and inflammatory reactions. They are actually purported to utilize a blend of three polymers comprising a ten carbon hydrophobic polymer that controls drug release, a polyvinyl pyrrolidone for fast release and the biocompatible phosphorylcholine coating; to form the BioLinx polymer.
While the use of the sirolimus eluting Cypher stent was recently discontinued, the sirolimus-analog bearing Endeavor® stents are still preferred in cases where there is a substantial thrombosis risk or with complex lesions and for the overall purpose of better anti-restenotic action..
5.2. Everolimus eluting stents
Unlike the extremely lipophilic zotarolimus, everolimus is a relatively polar immunosuppressant macrolide with a 2-hydroxyethyl chain at the C40 position of sirolimus. The everolimus eluting stent Xience V™ (Abbott Laboratories) is another second generation drug eluting stent which got approved by the FDA in July 2008. In addition to a change in inhibitor and different polymeric platform, these stents also have some changes in their stent design that may add to their therapeutic utility and minimize late/very late endothelial response generator.
Everolimus like sirolimus and zotarolimus, also binds to FKBP-12 protein upon cellular uptake. The mTOR protein kinase inhibited by the FKBP-12-everolimus blocks expression of p70 S6 kinase pathway and halts ribosomal protein production and subsequently the smooth muscle cell proliferation cycle at the G1/S phase. Everolimus also suppresses T-cell mediated immune response, thus reducing the inflammatory/hypersensitivity response to the polymer used. Combination therapies of everolimus with tacrolimus hold great promise for the future of stent technology (Terada et al., 1992; Kuo et al., 1992). The more polar nature of everolimus helps lower the tissue concentration of anti-proliferative drugs.
The Xience V®stent (Abbott Laboratories) has a L-605 Co-Cr balloon expandable stent with better stent design characteristics such as high flexibility and deliverability, acceptable recoil and better compliance (Hagemeister et al., 2005; Tanimoto et al., 2007). The low strut thickness and the resulting lower surface area in contact with luminal blood flow allows the smaller exposed stent surface to be covered with endothelial cells more rapidly. Also, the lower surface area minimizes the risk of hypersensitivity reaction due to the polymer coating. The Xience V uses a hydrophilic poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) coating for better biocompatibility of the adluminal surface, while the poly(butyl methacrylate) (PBMA) allows for slow, sustained drug release (Carter et al., 2006).
The major limitations of sirolimus-eluting stents were mainly due to platelet aggregation resulting from a lack of endothelial surface coverage of the stent, mainly due to adequate blood plasma concentration of sirolimus which resulted in a thrombogenic response to the stents. The possibility of such an adverse eventuality is significantly minimized with everolimus eluting stents. Similarly, the biocompatible, hydrophilic fluoropolymer coating instead of the PEVA/PBMA or SIBS coating in the first generation drug eluting stents, drastically reduce the hypersensitivity reactions to the polymer. Further, everolimus eluted from these has also demonstrated macrophage clearance by autophagy in rabbit arteries (Verheye et al., 2007).
6. Future research
6.1. Biocompatible stents
The second-generation of drug eluting stents focused on increasing the efficacy of the anti-restenotic effect expected from drug release by means of formulating more potent sirolimus analogues. Future research approaches in this field lay more emphasis on tending to the safety concerns posed by the polymeric stent coatings.
Instead of completely disqualifying the use of polymeric coatings on stents, another way to increase the overall hemocompatibility of the stent is by way of enriching the stent surface coating with inert or bioactive components. Non-thrombogenic coatings that may aid re-endothelialization are an encouraging alternative to conventional drug-eluting stents. Meanwhile, it is important to note that stent thrombus formation is triggered by adsorption of plasma proteins such as fibrinogen, fibronectin, tissue and complement factors, which in turn, is determined by stent surface hydrophobicity, morphology, roughness, and electrical charge (Sydow-Plum and Tabrizian, 2008).
Initial attempts towards the generation of biocompatible materials were relegated to the use of inert materials that avoided any sort of response from the body. However, further evaluation of the performance of such “inert” biomaterials shed light into changes that take place when a material is implanted into the arteries. This information, along with an enhanced understanding of blood-biomaterials interactions led us to a point where we could identify and synthesize materials that could proactively encourage endothelializaiton of the implant within the arteries.
Phosphorylcholine, a naturally occurring lipid group that constitutes the cell membrane has been celebrated for its biocompatibility in blood (Babapulle and Eisenberg, 2002). However, phosphorylcholine coated stents have not shown promising results despite the favorable characteristics of phosphorylcholine such as biocompatibility, non-allergenicity as well as lower inflammatory response. In fact, except stents coated with anti-proliferative drugs, there has been no significant clinical success in the area of favorable stent coatings. As previously mentioned, Phosphorylcholine is already a part of the coating used for the zotarolimus eluting Endeavor® stents.
Heparin, a negatively charged glycosaminoglycan and commonly used anticoagulant has been known to confer biomaterials with non-thrombogenic faculties. It therefore, seemed rational to coat stents with heparin to contend the thrombus formation commenced as a result of the injury incurred by stent implantation. Heparin can be attached to biomaterials by ionic bonding with cationic polymers, physically attached to surface by dip coating, chemically connected by covalent bonds or even eluted from polymer-heparin dispersions. Heparin hampers thrombin formation by forming a ternary complex with the cofactor antithrombin III which inhibits thrombin and thus, arrests progression of the coagulation cascade preventing thrombosis (Serruys et al., 1998). Despite the attractive utility of heparin in the long-term safety and efficacy of stents, there has been no major, perceptible benefit with heparin coated stents.
Heparin was first applied to Palmaz-Schatz stents coated with a three layered polymeric coating (comprising of a dextran sulfate layer between two polyamine layers) with heparin covalently linked to the polyamine backbone of the polymer. However, these stents were ineffective at preventing thrombus formation (Hardhammar et al., 1996). A stainless steel stent heparin consisting of hydrophobic binding agent was coated with with Duraflo II that allowed effective heparin binding to stent surface without altering the anti-coagulant activity of heparin (DeScheerder et al., 1997). The lack of inhibition of neoinitimal hyperplasia in vivo, despite presence of heparin coating was believed to be attributable to the antiplatelet therapy with ticlopidine. The long term human trials of heparin-coated stents performed by Serruys et al. in the BENESTENT II trial indicated the reduction in stent thrombosis; however, it was unclear if it was in fact the dual antiplatelet therapy with aspirin and ticlopidine that resulted in the non-thrombogenicity. In addition, coating with heparin alone did not demonstrate any antiproliferative effects.
Similarly, heparin coated Wiktor stent had heparin covalently bonded to a tantalum stent surface, in the international randomized multicenter trial MENTOR. Again, despite the presence of low thrombosis rates initially, the heparin was unable to inhibit smooth muscle cell proliferation and thus, restenosis (Vrolix et al., 2000). These clinical studies in fact, identified the importance of neointimal hyperplasia in restenosis and shifted the focus towards the more potent inhibitors of smooth muscle cell proliferation that presently dominate the drug eluting stent market.
Apart from heparin and phosphorylcholine, other strategies undertaken to improve the biocompatibility of drug eluting stents include coating the surface with endothelial cells/endothelial progenitor cells (Garg et al., 2010; Luo et al., 2011), cluster of differentiation 34 antibodies (CD-34) known to attract endothelial cells (Ako et al., 2007), poly(bis(trifluoroethoxy)phosphazene) (Satzl et al., 2007), and even corticosteroids (Babapulle and Eisenberg, 2002). Similarly, the Jostent coronary stent graft coated with a poly(tetrafluoroethylene) (PTFE) layer has shown promising results in clinical trials owing to the inherent ability of PTFE to prevent neointimal in-growth by shielding the potentially thrombogenic surface of the stent from blood components (Gercken et al., 2002).
6.2. Biodegradable stents
Polymers remaining behind after complete elution of drug pose a significant thrombogenic risk and are accountable for the subsequent hypersensitivity and inflammatory reactions (Luescher et al., 2007). Fortunately, developments in the synthesis of natural and synthetic materials that can be degraded biologically have brought the idea of degradable stents to fruition.
Biodegradable stents represent an area of marked interest, since they can accomplish diverse objectives such as being able to dilate arteries for a stipulated period of time, release anti-restenotic drugs and can be tailored to undergo biological degradation with kinetics that can be appropriately tuned by modifying the materials used. Biodegradable drug-eluting stents are defined to be stents that either have a completely biodegradable platform or are coated with a biodegradable polymer on a conventional bare metal stent. In either case, the goal is to avoid an inflammatory response to the polymer coating that culminates into thrombosis for a conventional drug eluting stent.
Synthetic biodegradable polymers that have been investigated for stent coating purposes include but are not limited to poly(L-lactic acid) (PLLA), poly(D,L-lactide) (PDLLA) and poly(lactic-co-glycolic acid) (PLGA). These polymers typically undergo ester bond degradation to form products such as lactic acid and glycolic acid, which can then be cleared from the system without triggering an undesirable inflammatory response.
The utility of blending biodegradable polymers to achieve the requisite mechanical strength and degradation rate has been investigated previously (Ye et al., 1998). The microporous PLLA/PCL blended stents afforded a distinctive advantage by offering to act as a biodegradable scaffold and providing the βGal reporter gene directly to rabbit carotid arteries. Although, concerns regarding complement-based inflammatory responses to PLLA as well as extremely short gene elution times persisted. One way to eliminate an inflammatory response to PLLA involved using PLLA just as the stent platform, while coating it with a PLGA layer that can further act as a sirolimus reservoir (Wang et al., 2006). In such a study, issues related to the rates of drug elution can be overcome by varying the composition of the layers, the drug loaded, and PLGA degradation rate. Other strategies include replacing PLLA with other natural, degradable biopolymers such as collagen, alginate, chitosan, or an appropriate combination of these so as to avoid an inflammatory response completely (Chen et al., 2005; Peng et al., 2007; Chen et al., 2009).
Covering metallic stents with a biodegradable polymeric layer that can elute an anti-restenotic drug also serves to accomplish an analogous intention, while being able to provide the requisite radial force and be able to restrict recoil expected from a typical drug eluting stent. The polymer coating can be arranged to degrade after serving its anti-restenotic purpose, thereby precluding any adverse effects attributable to the polymers. Elution of an anti-restenotic drug addresses concerns associated with smooth muscle cell related neointimal growth that stems from the procedure itself and is believed to cease within 6 months (Bennett, 2003). Also, metallic stents implanted within the arteries subsequent to inhibition of any neointimal proliferation are accepted to have fewer inflammatory/stenosis related detrimental effects incidents (Colombo and Karvouni, 2000). Presence of a metallic stent that can offer strong radial support and permit use of several coating techniques for more diverse polymer coatings that may not be compatible with the use of a biodegradable polymer based stent platform (Raval et al., 2011).
The BioMatrix and BioMatrix II (Biosensors International, Newport Beach, California) are a couple of prominent drug eluting stents with a biodegradable poly (D,L-lactic acid) (PLA) coating releasing a macrolide, immunosuppressive sirolimus-analog Biolimus A9. The BioMatrix stents have been approved by the Conformité Européenne (CE). The PLA-biolimus A9 formulation (in 1:1 ratio) coated on the stent surfaces in contact with the vessel wall by an automatic micro-pipette coating process. Presence of drug on the abluminal surfaces of the stent in addition to the more lipophilic nature of biolimus A9 are presumed to increase arterial wall uptake of the anti-proliferative drug while reducing systemic side-effects, while the presence of a non-permanent PLA coating is used to minimize risk of stent thrombosis (Ostojic et al., 2008).
Other noteworthy biodegradable stents that have been approved by the CE include the Infinnium and Supralimus stents manufactured by the Sahajanand Medical Technologies Pvt. Ltd., India), which deliver paclitaxel and sirolimus, respectively. Both these stents comprise of PDLLA-co-PGA, PNVP, PLLA-co-PCL polymeric coatings that are degraded in vivo after having served the purpose of acting as a drug barrier to the eluting drug and have exhibited their safety and efficacy in the SIMPLE II clinical trials for the treatment of de novo coronary lesions. LUC-Chopin2™ (Balton, Poland), another CE approved drug eluting stent, displayed moderate inflammatory reactions to paclitaxel release in addition to focal thrombus formation with fibrin deposits and incomplete re-endotheilalization despite degradation of the PLGA coating on the stent. Some other CE approved drug eluting stents with a PLGA biodegradable coat include EucaTax, which has a permanent endothelium-compatible natural coating of glycocalyx (Camouflage™) top-coated with paclitaxel eluting biodegradable PLGA. (Grabow et al., 2010).
It is during the six months after the procedure that the scaffolding effect of stents is important and it is also during this time period that restenosis therapy is crucial (Saito, 2005). As a result, after this period any polymer remaining behind can only exacerbate inflammatory responses. Most of the issues related to biodegradable stents can be attributed to adverse responses occurring during the first six months of implantation when the polymer has not been degraded completely or the oligomer type degradation products that can elicit undesirable responses the extent of which can vary from polymer to polymer (Erne et al., 2006).
6.3. Non-polymer stents
Taking cue from the fact that the polymers coated on the metallic stent stir up hypersensitivity and inflammatory reactions which promote stent thrombosis, researchers have worked on strategies to eliminate the use of polymers in stents. Polymers were primarily used as a diffusional barrier to release the anti-proliferative drug over a sufficiently long period of time. Since most anti-restenotic drugs target the smooth muscle cell proliferation at a particular stage of the cell cycle (For e.g. Sirolimus: G1/S; Paclitaxel : G2/M), it is important to ensure uninterrupted delivery of these drugs during the established SMC proliferation and migration time period of 2–3 weeks. The use of a permanent, durable polymer coating on stents allows for finer engineering and tailoring of the drug release kinetics by instituting a physical diffusional barrier that allows control by changing the thickness of polymer membrane, type of polymer used, and surface area. It is important to note that delivery can also be achieved by loading these drugs into reservoirs present in the metallic stent surface (Acharya and Park, 2006).
6.3.1 Microfabricated reservoirs in drug eluting stents
Loading anti-restenotic drugs into grooves and holes present on bare metal stent struts, which act as drug reservoirs for such non-polymer stents, can also help attain the above qualities of a good controlled release system. The Janus Carbostent™ (Sorin Biomedica, Italy) is one of the first reservoir-based, non-polymer stent, which obtained a CE mark in 2004. In the Janus stent, the sirolimus-analog tacrolimus is loaded into a groove/cut, which acts a drug reservoir on the stent surface and about 50% of the loaded tacrolimus is released within 30 days of implantation. In order to avert thrombogenic reactions associated with the metallic stent itself, the stainless steel 316L stent had a passive turbostatic carbon film coating (Bartorelli et al., 2003).
The BioFreedom™ (Biosensors International, Newport Beach, California) involve Biolimus A9 coating onto mechanically created microscale reservoirs on the textured abluminal stent surface of a BioFlex II 316L stainless steel S-stent. The presence of reservoirs allows drug to be eluted from the stent without concerns regarding polymer-associated adverse tissue responses, thereby reducing the duration of dual antiplatelet therapy as well. The abluminal reservoirs provide for rapid transfer of the lipophilic agent into the vessel wall tissues, where Biolimus A9 can exert its anti-proliferative therapy over the smooth muscle cell multiplication cycle. While the use of the more lipophilic Biolimus A9 itself reduces systemic exposure, the existence of micro-textures on the vessel wall side curb drug release in the luminal space. The presence of polymer in the luminal space, known to instigate hypersensitivity or use of a drug that inhibits proliferation of endothelial cells that increase the chances of stent thrombosis is avoided by use of such a stent. As a result, stent platforms with abluminal reservoirs may allow for the stent to be re-endothelialized and may even be successful in achieving timely healing of the arterial vessel (Tada et al., 2010).
Better control can also be obtained by coating the stents with micro-reservoirs with a series of polymers that degrade at rates that can be tailored according to the desired pharmacokinetics. The clinical use of Conor and CoStar stents that had a degradable PLGA membrane coat was studied for eluting paclitaxel over a sufficiently long time so as to prevent in-stent restenosis; thereby averting the need to take up high doses of paclitaxel that can be detrimental to the vascular system (Finkelstein et al., 2003).
The reservoirs in such stents are carved using laser machining techniques wherein a laser beam pulse capable of removing 0.1–0.5 um of polymer/metal facilitates the creation of micro channels <100 μm thick in the stent material. Microelectrodischarge machining, a technique applying electrical pulses using an electrode, provides the merit of allowing high-throughput batch processing of manifold stents at the same time, while offering to craft smaller size microscale features with great accuracy and reliability (Murali and Yeo, 2004). Not only do these stents have the same radial strength as conventional bare metal stents, but they can also be instilled with microsystems that detect the blood pressure and flow rate, thereby holding the potential to behave like biosensors (Takahata and Gianchandani, 2004).
Stents entrenched with microneedles penetrating dense, advanced atherosclerotic plaques in addition to delivering therapeutics to the internal elastic lamina of the arterial wall have also been studied (Reed et al., 1998). While there are much debate over how conventional photolithography and chemical etching techniques may be used to formulate microneedles on the cylindrical stent surfaces, there is no doubt that these pioneering stents will shepherd the field of drug eluting stents to newer directions.
6.3.2 Nanofabricated reservoirs in drug eluting stents
Extensive progress in nanofabrication techniques can allow us to further hone the surface characteristics of metallic stents in addition to utilize them for loading anti-restenotic drugs. Recent work integrating the nanoscale features on the stent surface produced by sol-gel processes has successfully demonstrated ability for enhanced cellular attachment as well as drug release via the nanoscale features. Such stents were fabricated by dipping bare metal stents or spraying upon them with hydroxyapatite and titania colloidal suspensions to fashion the surface with nanoscale textures (Liu et al., 2001; Liu et al., 2002).
Reservoir-based metal stents can either have a polymeric coating/membrane through which the drug diffuses or may have the drug directly loaded into the reservoirs within the metallic stent itself. A stent prominent in this class of drug eluting stents is the CoStar stent (Conor Medsystems, Inc, Menlo Park, Calif) where presence of these reservoirs allows more drug quantity to be loaded in the stent itself as compared to the amount of drug that needs to be dispersed in the polymer matrix coating of conventional drug eluting stents (Krucoff et al., 2008). Lack of the need to employ polymers that can efficiently solubilize hydrophobic antirestenotic agents such as sirolimus and paclitaxel as well as toxic adhesives and lubricants, permits more flexibility in choosing a biocompatible, biodegradable polymer that can act as an effective barrier to burst-release, while possessing the required drug permeation properties at the same time.
6.4 Drug carrier eluting stents
Evolution in methods of particle synthesis has allowed us to modulate sizes and properties of drug carriers so as to achieve effectual delivery of drugs. Nano- or micro-carriers help overcome some of the inherent limitations of stents eluting drug molecules by introducing desirable capabilities such as the ability to target diseased cells, responsiveness to external stimuli and superior tunability of drug pharmacokinetics. Furthermore, improvements in electrodeposition techniques have made possible the fabrication of stents that can be efficiently coated with nano-/micro-particles. These particles containing anti-restenotic drugs are then released from the stents over time and in turn, release drugs in a controlled manner.
Furthermore, knowledge of the inflammatory nature of permanent polymers used in “conventional” drug eluting stents, prompted researchers to explore the possibility of achieving the same therapeutic success with biodegradable polymers. Stents coated with nanoparticles/microparticles loaded with anti-restenotic can successfully combine a localized and targeted controlled release system, in addition to eliminating any deleterious effects that may arise from the use of a permanent polymeric coating on the stent. Moreover, simply releasing sirolimus from a biodegradable polymeric coating on the stent can lead to degradation of sirolimus into a less potent chemical entity (Banai et al, 2005). This necessitates the protection of sirolimus in its active, anti-proliferative form from the degradative agents in blood serum and can be achieved by using polymeric nanocarriers with a lipophilic core that preferentially associates with sirolimus.
Particles studied for their utility as nanovehicles for anti-restenotic drug delivery are mainly polymeric nanoparticles such as PLGA (Joo et al., 2009), copolymeric colloidal aggregates of phosphorylcholine and n-butyl methacrylate (Kim et al., 2009), and poly(ethylene oxide)-modified poly(epsilon caprolactone) (PEO-PCL) (Deshpande et al., 2008). PLGA nanoparticles are by far the most investigated, mainly, due to their quality of being biodegradable. PLGA nanoparticles prepared by an o/w emulsion polymerization technique have shown promising results with the release of a variety of anti-restenotic drugs such as paclitaxel (Deshpande et al., 2008) and curcumin (Nam et al., 2007). However, a comprehensive in vivo study highlighting the efficacy of such nanoparticles to load and release hydrophobic anti-restenotic drugs is yet to be seen. Other nanoparticle formulations incorporate a hydrophobic moiety such as n-butyl methacrylate that can preferentially associate with sirolimus and thus, holds great promise for this application (Kim et al., 2009). Similarly, PEO-PCL particles made by a solvent displacement technique have shown efficient paclitaxel uptake with smooth muscle cells and can be coated onto stents to further enhance their therapeutic effect (Deshpande et al., 2008).
These nanoparticles can be coated onto conventional bare metal stents through a cationic deposition method, while other novel methods of coating onto stents such as the ring-shaped surface tension method, wherein capillary forces coat suspensions of nanoparticles onto stents, have also been reported recently (Joo et al., 2009).
Random copolymers containing phosphorylcholine groups have been known to be capable of possessing the amphiphilic nature required to ferry lipophilic drugs such as sirolimus (Kim et al.,2009). It was proposed to make use of a biocompatible amphiphilic polymer poly (2-methacryloyloxyethyl phosphorylcholine (MPC)-co-n-butyl methacrylate (BMA) (PMB30W) to be able to enhance delivery of sirolimus eluted from PLCG films that can serve as biodegradable coatings on stents. Sirolimus was found to be retained in a stable, potent form in the interior of the “nanovehicle” formed by the colloidal aggregation of the self-assembling PMB30W chains leading to colloidal aggregation. The lipophilic interior of the PMB30W nanoparticles comprised of n-butyl methacrylate units augmented the solubilization of sirolimus while protecting it from the outer hydrophilic environment. The hydrophilic phosphorylcholine units of the copolymeric nanoparticles are involved in forming small sized (~20 nm) nanoparticles in an aqueous medium that can passively penetrate the vascular wall up to the internal elastic lamina. The sirolimus can then be delivered to the smooth muscle cells of the media through the fenestral openings in the internal elastic lamina. This carrier-mediated approach to deliver sirolimus from biodegradable films protects degradation of sirolimus while protecting the red blood cells from its adverse effects and preventing thrombus formation due to deposition on the arterial endothelium.
Lower concentration of cytotoxic drugs such as paclitaxel can be exercised by using drug loaded nanoparticles for targeted, localized and controlled release, thereby considerably lowering systemic toxicity. Liposomal nanoparticles based on phospholipids and cholesterol are known for their ability to effectively transport lipophilic drugs like sirolimus and rapamycin owing to the presence of both hydrophobic and hydrophilic moieties. Systemically administered 1:3 distearoyl phosphatidylglycerol, 1, 2 distearoly-sn-gylcero-3-phosphcholine liposomal nanoparticles for the delivery of clodronate were developed to inhibit neointimal prolierfation in a rabbit carotid artery injured by balloon angioplasty (Danenberg et al., 2002). The circulation time/targeting ability of such liposomal nanocarriers can be enhanced by surface modification or conjugation to antibodies/ligands. Morimoto and associates used pegylated 3,5-dipentadecyloxybenzamidine hydrochloride (TRX-20) liposomes to load the glucocorticoid prednisolone phosphate (Joner et al., 2008). The endocytosis-mediated cellular uptake of the cationic lipid nanoparticles was shown to allow for the actively targeted release of the drug in the cells.
The nanoparticle eluting stent technology developed is of great prospect and involves coating nanoparticles onto metallic stents via cationic electrodeposition coating technology. Biodegradable PLGA nanoparticles are typically used because of their ability to encapsulate water-soluble, next-generation drugs (DNAs, oligonucleotides, proteins as antimonocyte chemoattractant protein-1), improve cell membrane penetration via endocytosis and increase intracytoplasmic release. In a recent study, these nanoparticles were prepared by an emulsion solvent diffusion method with surface modification with chitosan (Nakano et al., 2009). Furthermore, PLGA is known to not increase the thrombosis risk (in porcine coronary artery models), or work against endothelial regeneration, in addition to its biodegradability and biocompatibility, thereby indicating that PLGA nanoparticle eluting stents can be safely used in humans (Nakano et al., 2009).
Similarly, paclitaxel and C6 ceramide have been incorporated into polymeric nanoparticles of poly(ethylene oxide)-modified poly(epsilon caprolactone) and may be considered for the role of nanoparticles to be eluted from coated stents (Deshpande et al., 2008). PLGA nanoparticles loaded with the angiotensin-converting-enzyme inhibitor, lisinopril that have been used with local delivery by catheters and polylactide nanoparticles delivering the PDGF receptor inhibitor AG-1295 post balloon angioplasty injuries are potential candidates for nanoparticles that can be released from stents and implement controlled release of anti-restenotic drugs (Varshosaz and Soheili, 2008).
The use of another drug delivery system that combines localized drug delivery with controlled drug release was demonstrated by using curcumin loaded PLGA nanoparticles. Curcumin, a natural ingredient known for its anti-proliferative effects, was loaded into PLGA nanoparticles using a spontaneous emulsion method (Nam et al., 2007). The electrophoretic deposition method was found to be a highly reproducible, fast, flexible and reasonably economical coating method while the PLGA nanoparticles provided for an effective biodegradable barrier to anti-proliferative drug release.
Similarly, stainless steel 316L stent can be uniformly coated with a poly(2-hydroxy-ethyl-methacrylate) based hydrogel via an air spray technique and PLGA (50:50) microspheres incorporated with a model hydrophilic molecule such as Rhod-dex can be prepared by a double emulsion-solvent evaporation technique. These microsphere-integrated drug eluting stents can be used to achieve localized, site-specific delivery of hydrophilic antiproliferative drugs like gemcitabine and fludarabine (Indolfi et al., 2011). They allow for better control of release kinetics, unlike direct release of conventional lipophilic drugs which results in faster release kinetics and rapid uptake by the cell membranes.
6.5. Gene eluting stents
Technological advancements have given us the opportunity to understand the mechanisms leading to in-stent restenosis. This in addition to the enormous progress made in nucleic-acid based drug discovery, recombinant DNA technology and gene transfer have served to provide us strong if not superior, remedial alternatives to conventional therapeutics. These nucleic acid based therapeutics, usually a RNA/DNA molecule, if locally delivered can block a specific regulatory gene that plays a significant role in the pathophysiological restenosis mechanism (Santiago and Khachigian, 2001). Genes coding for the FK506 binding protein 12 that are upregulated in in-stent restenosis can be targeted using this mode of therapy and can thus help intrinsically mediate the cascade of reactions leading to in-stent restenosis (DeYoung and Dicheck, 1998).
To test this hypothesis, a plasmid DNA-eluting gene was formulated and coated with a biodegradable PLGA polymer via micro-emulsification (Klugherz et al., 2000). The functionality of such a revolutionary stent was demonstrated by using a GFP-DNA whose uptake, transfection and subsequent expression could be observed by fluorescence microscopy. It was hypothesized that the DNA eluted from the PLGA coated stainless steel stent diffused through microscopic injuries produced by stent deployment. These superficial tears aided the encapsulated GFP-DNA to diffuse through the arterial cell walls into the smooth muscle cells of the media. The supplementary use of another gene that expresses an apoptosis-inducing cell surface protein that could combat the inflammatory response induced by PLGA was also hypothesized (Klugherz et al., 2000).
Similarly, the merits of gene-eluting stents were reinforced by locally delivering a plasmid DNA containing the coding sequence for the human vascular endothelial growth factor (phVEGF-2) (Walter et al., 2004). A 15mm electropolished and phosphorylcholine coated version of the previously mentioned stent BiodivYsio (Abbott Vascular Devices, Galway, Ireland) was used for the local delivery of this plasmid DNA. The VEGF expressed as a result of gene transfection enhanced endothelial recovery by serving to activate endothelial cell proliferation pathways, unlike other anti-proliferative drugs that inhibit them.
The ability to deliver genes/plasmid DNA opened a window of opportunity in the realm of local nucleic acid therapeutic delivery for in-stent restenosis, which was further explored by Juan and colleagues. Delivery of siRNA in order to inhibit mRNAs from producing proteins that play a key role in the pathophysiological mechanisms of in-stent restenosis was studied. Delivery of siRNA was achieved by means of a pullulan hydrogel solution dip coated onto stainless steel stents. Apart from being a highly biodegradable polysaccharide, the pullulan can be chemically modified and cationized for increased cellular uptake in vivo. The stents were immersed in a 10 μm aqueous solution of siRNA for an hour to load the nucleic acid therapeutic (San Juan et al., 2009). The target for the siRNA delivery was the MMP-2 gene sequence that codes for the matrix metalloproteinase 2, a zymogen which hydrolyzes extracellular matrices in its active form, permitting migration of smooth muscle cells from the media into the intima (Hlawaty et al., 2007). Similarly, SS316L stents coated with hyaluronic acid have displayed effective gene transfection of plasmid DNA/polyethyleneimine (PEI) polyplexes fortifying claims related to stents being efficacious platforms for gene delivery.
In contrast to the use of a plasmid DNA for gene delivery, gene transfer therapy operates on the basis of the principle of gene transduction mediated by a viral vector such as the adenovirus. The delivery of an adenoviral vector inhibiting metalloproteinase-3 by use of PC-coated BiodivYsio stents has also been demonstrated (Abbott Vascular Devices, Galway, Ireland) (Johnson et al., 2005). Similarly, the efficacy of adenoviral and adeno-associated viral vectors for delivering a reporter lacZ gene to the smooth muscle cells was compared using the same PC-coated BiodivYsio stents (Abbott Vascular Devices, Galway, Ireland), which ensures good hemocompatibility without eliciting unfavorable tissue responses and can carry viral vectors (Sharif et al., 2006). It was noted that adenovirus mediated delivery evinced shorter transgene expression owing to protein response to adenoviral vectors while the adeno-associated viral vector showed better and durable transgene expression. While their previous research article used a lacZ reporter gene to study transgene expression, a more recent article by that group illustrates the use of non-viral vectors, specifically, liposomes instead of adenoviruses to deliver a gene with therapeutic effects in order to tackle restenosis in stented arteries of New Zealand White rabbits (Sharif et al., 2012).
Both Co-Cr metal stents and PC-coated BiodivYsio stents (Abbott Vascular Devices, Galway, Ireland) were used to successfully accomplish “lipoplex-mediated” eNOS gene delivery to the stented vessel wall. The “lipoplex” was a complex between a plasmid DNA containing the eNOS gene and lipofectin liposomes and were coated onto the aforementioned stents using pipetting and air-drying technique. eNOS (endothelial Nitric Oxide synthase) expression increases the nitric oxide production in the arterial vasculature. Nitric oxide is purported to enhance re-endothelialization by preventing platelet aggregation and reducing neointimal hyperplasia by inhibiting vascular smooth muscle cell proliferation and migration. It was also noted that the liposomal particles themselves have a lower immunogenic response as compared to viral vectors and can carry larger payloads.
Along with gene delivery and gene transfer, recent work advocates the use of surface functionalized siRNA that can be coated onto stent surfaces and can help reduce inflammatory reactions to stent surface by effecting E-selectin receptor knockdown (Nolte et al., 2011). In this study, the negatively charged siRNA was complexed with the positively charged amino groups of PEI (polyethylenimine) to form a gelatinous coating. However, studies with animal models need to be done with these “gene silencing surfaces” to substantiate the safety of these coatings with respect to adverse reactions to PEI and efficacy of the surface for reducing neointimal hyperplasia.
7. Conclusions
Substantial clinical data from the use of drug eluting stents in patients since 2003 has given us a better understanding of the merits and demerits of first generation drug eluting stents. Instead of simply targeting smooth muscle cells to inhibit neointima formation, the need to restore the arterial endothelium at the site of injury has also gained paramount importance. It is evident that strategies which aim at re-endothelialization of the stent struts should be combined effectively with approaches preventing smooth muscle cell proliferation and migration. This can be achieved in a variety of ways considering the gamut of technological research and development that this field has witnessed. Numerous anti-proliferative drugs available including novel drugs such as nucleic acid drugs which can elicit endothelial regrowth, biodegradable polymers that can avoid hypersensitivity have given us a holistic perspective of the progress in this field. Improved stent design and polymeric coatings that allow for targeted delivery of superior drugs to the arterial wall in combination with drugs that bring about re-endothelialization and can be released after all of the anti-proliferative drug has been released, can work as a potent solution to the problem of in-stent restenosis. It is therefore, necessary to synergize these research and development efforts to arrive at the optimum treatment option and develop the next-generation of drug eluting stents for treating in-stent restenosis.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (EB 1R01-000346-18 and by a Fellowship (to ERD)) from the Cancer Prevention and Research Institute of Texas (CPRIT).
References
- Acharya G, Park K. Mechanisms of controlled drug release from drug-eluting stents. Adv Drug Deliv Rev. 2006;58:387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ako J, Bonneau HN, Honda Y, Fitzgerald PJ. Design criteria for the ideal drug-eluting Stent. Am J Cardiol. 2007;100:3M–9M. doi: 10.1016/j.amjcard.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Babapulle MN, Eisenberg MJ. Coated stents for the prevention of restenosis: Part I. Circulation. 2002;106:2734–2740. doi: 10.1161/01.cir.0000038982.49640.70. [DOI] [PubMed] [Google Scholar]
- Banai S, Chorny M, Gertz SD, Fishbein I, Gao JC, Perez L, Lazarovichi G, Gazit A, Levitzki A, Golomb G. Locally delivered nanoencapsulated tyrphostin (AGL-2043) reduces neointima formation in balloon-injured rat carotid and stented porcine coronary arteries. Biomaterials. 2005;26:451–461. doi: 10.1016/j.biomaterials.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Bartorelli AL, Trabattoni D, Fabbiocchi F, Montorsi P, de Martini S, Calligaris G, Teruzzi G, Galli S, Ravagnani P. Synergy of passive coating and targeted drug delivery: the tacrolimus-eluting Janus CarboStent. J Interv Cardiol. 2003;16:499–505. doi: 10.1046/j.1540-8183.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- Baumbach A, Herdeg C, Kluge M, Oberhoff M, Lerch M, Haase KK, Wolter C, Schroder S, Karsch KR. Local drug delivery: impact of pressure, substance characteristics, and stenting on drug transfer into the arterial wall. Catheter Cardiovasc Interv. 1999;47:102–106. doi: 10.1002/(SICI)1522-726X(199905)47:1<102::AID-CCD22>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Bennett MR. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart. 2003;89:218–224. doi: 10.1136/heart.89.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radical Bio Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Buellesfeld L, Grube E. ABT-578-eluting stents- the promising successor of sirolimus- and paclitaxel-eluting stent concepts? Herz. 2004;29:167–170. doi: 10.1007/s00059-004-2557-5. [DOI] [PubMed] [Google Scholar]
- Burt HM, Hunter WL. Drug-eluting stents: an innovative multidisciplinary drug delivery platforms. Adv Drug Deliv Rev. 2006;58:345–346. doi: 10.1016/j.addr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Carter AJ, Brodeur A, Collingwood R, Ross S, Gibson L, Wang CA, Haller S, Coleman L, Virmani R. Experimental efficacy of an everolimus eluting cobalt chromium stent. Catheter Cardiovasc Interv. 2006;68:97–103. doi: 10.1002/ccd.20769. [DOI] [PubMed] [Google Scholar]
- Castanedazuniga WR, Formanek A, Tadavarthy M, Vlodaver Z, Edwards JE, Zollikofer C, Amplatz K. The mechanism of balloon angioplasty. Radiology. 1980;135:565–571. doi: 10.1148/radiology.135.3.7384437. [DOI] [PubMed] [Google Scholar]
- Chen MC, Chang Y, Liu CT, Lai WY, Peng SF, Hung YW, Tsai HW, Sung HW. The characteristics and in vivo suppression of neointimal formation with sirolimus-eluting polymeric stents. Biomaterials. 2009;30:79–88. doi: 10.1016/j.biomaterials.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Chen MC, Liang HF, Chiu YL, Chang Y, Wei HJ, Sung HW. A novel drug-eluting stent spray-coated with multi-layers of collagen and sirolimus. J Control Release. 2005;108:178–189. doi: 10.1016/j.jconrel.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Colombo A, Karvouni E. Biodegradable stents-fulfilling the mission and stepping away. Circulation. 2000;102:371–373. doi: 10.1161/01.cir.102.4.371. [DOI] [PubMed] [Google Scholar]
- Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- Cragg A, Lund G, Rysavy J, Castaneda F, Castanedazuniga W, Amplatz K. Non-surgical placement of arterial endoprosthesis- a new technique using nitinol wire. Radiology. 1983;147:261–263. doi: 10.1148/radiology.147.1.6828742. [DOI] [PubMed] [Google Scholar]
- Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low-density-lipoprotein induces monocyte chemotactic protein-1 in human endothelial-cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danenberg HD, Fishbein I, Gao JC, Monkkonen J, Reich R, Gati I, Moerman E, Golomb G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- DeScheerder I, Wang K, Wilczek K, Meuleman D, VanAmsterdam R, Vogel G, Piessens J, VandeWerf F. Experimental study of thrombogenicity and foreign body reaction induced by heparin-coated coronary stents. Circulation. 1997;95:1549–1553. doi: 10.1161/01.cir.95.6.1549. [DOI] [PubMed] [Google Scholar]
- Deshpande D, Devalapally H, Amiji M. Enhancement in anti-proliferative effects of paclitaxel in aortic smooth muscle cells upon co-administration with ceramide using biodegradable polymeric nanoparticles. Pharm Res. 2008;25:1936–1947. doi: 10.1007/s11095-008-9614-3. [DOI] [PubMed] [Google Scholar]
- DeYoung MB, Dichek DA. Gene therapy for restenosis- are we ready? Circ Res. 1998;82:306–313. doi: 10.1161/01.res.82.3.306. [DOI] [PubMed] [Google Scholar]
- Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. Circulation. 1964;30:654–670. doi: 10.1161/01.cir.30.5.654. [DOI] [PubMed] [Google Scholar]
- Ellis SG, Savage M, Fischman D, Baim DS, Leon M, Goldberg S, Hirshfeld JW, Cleman MW, Teirstein PS, Walker C, Bailey S, Buchbinder M, Topol EJ, Schatz RA. Restenosis after placement of Palmaz-Schatz stents in native coronary-arteries–initial results of a multicenter experience. Circulation. 1992;86:1836–1844. doi: 10.1161/01.cir.86.6.1836. [DOI] [PubMed] [Google Scholar]
- Erne P, Schier M, Resink TJ. The road to bioabsorbable stents: reaching clinical reality? Cardiovasc Interv Radiol. 2006;29:11–16. doi: 10.1007/s00270-004-0341-9. [DOI] [PubMed] [Google Scholar]
- Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet. 2003;361:247–249. doi: 10.1016/S0140-6736(03)12275-1. [DOI] [PubMed] [Google Scholar]
- Faxon DP, Sanborn TA, Haudenschild CC. Mechanism of angioplasty and its relation to restenosis. Am J Cardiol. 1987;60:B5–B9. doi: 10.1016/0002-9149(87)90476-0. [DOI] [PubMed] [Google Scholar]
- Feig LA, Peppas NA, Colton CK, Smith KA, Lees RS. The effect of angiotensin-II on in vivo albumin transport in normal rabbit aortic tissue. Atherosclerosis. 1982;44:307–318. doi: 10.1016/0021-9150(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Finkelstein A, McClean D, Kar S, Takizawa K, Varghese K, Baek N, Park K, Fishbein MC, Makkar R, Litvack F, Eigler NL. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation. 2003;107:777–784. doi: 10.1161/01.cir.0000050367.65079.71. [DOI] [PubMed] [Google Scholar]
- Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112:270–278. doi: 10.1161/CIRCULATIONAHA.104.508937. [DOI] [PubMed] [Google Scholar]
- Frostegard J, Nilsson J, Haegerstrand A, Hamsten A, Wigzell H, Gidlund M. Oxidized low-density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell-line U937. Proc Natl Acad Sci USA. 1990;87:904–908. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Duckers HJ, Serruys PW. Endothelial progenitor cell capture stents: will this technology find its niche in contemporary practice? Eur Heart J. 2010;31:1032–1035. doi: 10.1093/eurheartj/ehp591. [DOI] [PubMed] [Google Scholar]
- Gercken U, Lansky AJ, Buellesfeld L, Desai K, Badereldin M, Mueller R, Selbach G, Leon MB, Grube E. Results of the Jostent coronary stent graft implantation in various clinical settings: procedural and follow-up results. Catheter Cardiovasc Interv. 2002;56:353–360. doi: 10.1002/ccd.10223. [DOI] [PubMed] [Google Scholar]
- Grabow N, Martin DP, Schmitz KP, Sternberg K. Absorbable polymer stent technologies for vascular regeneration. J Chem Technol Biotechnol. 2010;85:744–751. [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Hagemeister J, Baer FM, Schwinger RHG, Hopp HW. Compliance of a cobalt chromium coronary stent alloy- the COVIS trial. Curr Control Trials Cardiovasc Med. 2005;6:1–4. doi: 10.1186/1468-6708-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardhammar PA, vanBeusekom HMM, Emanuelsson HU, Hofma SH, Albertsson PA, Verdouw PD, Boersma E, Serruys PW, vanderGiessen WJ. Reduction in thrombotic events with heparin-coated Palmaz-Schatz stents in normal porcine coronary arteries. Circulation. 1996;93:423–430. doi: 10.1161/01.cir.93.3.423. [DOI] [PubMed] [Google Scholar]
- Haude M, Erbel R, Issa H, Meyer J. Quantitative-analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon-expandable Palmaz-Schatz stents. J Am Coll Cardiol. 1993;21:26–34. doi: 10.1016/0735-1097(93)90713-b. [DOI] [PubMed] [Google Scholar]
- Hlawaty H, San Juan A, Jacob MP, Vranckx R, Letourneur D, Feldman LJ. Inhibition of MMP-2 gene expression with small interfering RNA in rabbit vascular smooth muscle cells. Am J Physiol- Heart Circ Physiol. 2007;293:H3593–H3601. doi: 10.1152/ajpheart.00517.2007. [DOI] [PubMed] [Google Scholar]
- Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005a;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- Iakovou I, Schmidt T, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Cosgrave J, Gerckens U, Grube E, Colombo A. Angiographic patterns of restenosis after paclitaxel-eluting stent implantation. J Am Coll Cardiol. 2005b;45:805–796. doi: 10.1016/j.jacc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Indolfi L, Causa F, Giovino C, Ungaro F, Quaglia F, Netti PA. Microsphere-integrated drug-eluting stents: PLGA microsphere integration in hydrogel coating for local and prolonged delivery of hydrophilic antirestenosis agents. J Biomed Mater Res Part A. 2011;97A:201–211. doi: 10.1002/jbm.a.33039. [DOI] [PubMed] [Google Scholar]
- Ishida T, Tanaka K. Effects of fibrin and fibrinogen-degradation products on the growth of rabbit aortic smooth-muscle cells in culture. Atherosclerosis. 1982;44:161–174. doi: 10.1016/0021-9150(82)90111-3. [DOI] [PubMed] [Google Scholar]
- Johnson TW, Wu YX, Herdeg C, Baumbach A, Newby AC, Karsch KR, Oberhoff M. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol. 2005;25:754–759. doi: 10.1161/01.ATV.0000157582.33180.a9. [DOI] [PubMed] [Google Scholar]
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans-delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Joner M, Morimoto K, Kasukawa H, Steigerwald K, Merl S, Nakazawa G, John MC, Finn AV, Acampado E, Kolodgie FD, Gold HK, Virmani R. Site-specific targeting of nanoparticle prednisolone reduces in-stent restenosis in a rabbit model of established atheroma. Arterioscler Thromb Vasc Biol. 2008;28:1960–U1113. doi: 10.1161/ATVBAHA.108.170662. [DOI] [PubMed] [Google Scholar]
- Joo J-r, Nam HY, Nam SH, Baek I, Park JS. A novel deposition method of PLGA nanoparticles on coronary stents. Bull Korean Chem Soc. 2009;30:1085–1087. [Google Scholar]
- Kim HI, Takai M, Konno T, Matsuno R, Ishihara K. Biodegradable polymer films for releasing nanovehicles containing sirolimus. Drug Deliv. 2009;16:183–188. doi: 10.1080/10717540902757416. [DOI] [PubMed] [Google Scholar]
- King SB. A US perspective on the shortcomings of PTCA. Heart. 1997;78:6–6. doi: 10.1136/hrt.78.suppl_2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugherz BD, Jones PL, Cui XM, Chen WL, Meneveau NF, DeFelice S, Connolly J, Wilensky RL, Levy RJ. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich K, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucoff MW, Kereiakes DJ, Petersen JL, Mehran R, Hasselblad V, Lansky AJ, Fitzgerald PJ, Garg J, Turco MA, Simonton CA, III, Verheye S, Dubois CL, Gammon R, Batchelor WB, O’Shaughnessy CD, Hermiller JB, Jr, Schofer J, Buchbinder M, Wijns W, Grp CII. A novel bioresorbable polymer paclitaxel-eluting stent for the treatment of single and multivessel coronary disease. J Am Coll Cardiol. 2008;51:1543–1552. doi: 10.1016/j.jacc.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Kukreja N, Onuma Y, Daemen J, Serruys PW. The future of drug-eluting stents. Pharmacol Res. 2008;57:171–180. doi: 10.1016/j.phrs.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Kuo CJ, Chung JK, Fiorentino DF, Flanagan WM, Blenis J, Crabtree GR. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358:70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- Liu DM, Troczynski T, Tseng WJ. Water-based sol-gel synthesis of hydroxyapatite: process development. Biomaterials. 2001;22:1721–1730. doi: 10.1016/s0142-9612(00)00332-x. [DOI] [PubMed] [Google Scholar]
- Liu DM, Yang QZ, Troczynski T. Sol-gel hydroxyapatite coatings on stainless steel substrates. Biomaterials. 2002;23:691–698. doi: 10.1016/s0142-9612(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Lowe HC, Oesterle SN, Khachigian LM. Coronary in-stent restenosis: current status and future strategies. J Am Coll Cardiol. 2002;39:183–193. doi: 10.1016/s0735-1097(01)01742-9. [DOI] [PubMed] [Google Scholar]
- Luescher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis-biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- Luo C, Zheng Y, Diao Z, Qiu J, Wang G. Review: research progress and future prospects for promoting endothelialization on endovascular stents and preventing restenosis. J Med Biol Eng. 2011;31:307–316. [Google Scholar]
- Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4:104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro TA, Glatz CE, Peppas NA, Chisolm GM, Colton CK. Distribution of glycosaminoglycans in consecutive layers of the rabbit aorta. Artery. 1979;5:1–13. [PubMed] [Google Scholar]
- McFadden EP, Stabile E, Regar E, Cheneau E, Ong ATL, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- Murali M, Yeo SH. Rapid biocompatible micro device fabrication by micro electro-discharge machining. Biomed Microdevices. 2004;6:41–45. doi: 10.1023/b:bmmd.0000013364.71148.51. [DOI] [PubMed] [Google Scholar]
- Muthukkumar S, Ramesh TM, Bondada S. Rapamycin, a potent immunosuppressive drug, causes programmed cell death in B lymphoma cells. Transplantation. 1995;60:264–270. doi: 10.1097/00007890-199508000-00010. [DOI] [PubMed] [Google Scholar]
- Naito M, Stirk CM, Smith EB, Thompson WD. Smooth muscle cell outgrowth stimulated by fibrin degradation products: The potential role of fibrin fragment E in restenosis and atherogenesis. Thromb Res. 2000;98:165–174. doi: 10.1016/s0049-3848(99)00202-9. [DOI] [PubMed] [Google Scholar]
- Nakano K, Egashira K, Masuda S, Funakoshi K, Zhao G, Kimura S, Matoba T, Sueishi K, Endo Y, Kawashima Y, Hara K, Tsujimoto H, Tominaga R, Sunagawa K. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. J Am Coll Cardiol Intv. 2009;2:277–283. doi: 10.1016/j.jcin.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Nakazawa G. Stent thrombosis of drug eluting stent: pathological perspective. J Cardiol. 2011a;58:84–91. doi: 10.1016/j.jjcc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011b;57:390–398. doi: 10.1016/j.jacc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Nam SH, Nam HY, Joo JR, Back IS, Park JS. Curcumin-loaded PLGA nanoparticles coating onto metal stent by electrophoretic deposition techniques. Bull Korean Chem Soc. 2007;28:397–402. [Google Scholar]
- Nolte A, Walker T, Schneider M, Kray O, Avci-Adali M, Ziemer G, Wendel HP. Small-interfering RNA-eluting surfaces as a novel concept for intravascular local gene silencing. Mol Med. 2011;17:1213–1222. doi: 10.2119/molmed.2011.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostojic M, Sagic D, Jung R, Zhang YL, Nedeljkovic M, Mangovski L, Stojkovic S, Debeljacki D, Colic M, Beleslin B, Milosavljevic B, Orlic D, Topic D, Karanovic N, Paunovic D, Christians U, Behalf NPKI. The pharmacokinetics of biolimus A9 after elution from the nobori stent in patients with coronary artery disease: The NOBORI PK study. Catheter Cardiovasc Interv. 2008;72:901–908. doi: 10.1002/ccd.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmaz JC, Sibbitt RR, Reuter SR, Tio FO, Rice WJ. Expandable intraluminal graft– a preliminary study– work in progress. Radiology. 1985;156:73–77. doi: 10.1148/radiology.156.1.3159043. [DOI] [PubMed] [Google Scholar]
- Park DW, Park SW, Park KH, Lee BK, Kim YH, Lee CW, Hong MK, Kim JJ, Park SJ. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006;98:352–356. doi: 10.1016/j.amjcard.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Peng P, Voelcker NH, Kumar S, Griesser HJ. Nanoscale eluting coatings based on alginate/chitosan hydrogels. Biointerphases. 2007;2:95–104. doi: 10.1116/1.2751126. [DOI] [PubMed] [Google Scholar]
- Ramcharitar S, Louise G, Daemen J, Serruys P. MDrug-eluting stents, restenosis and revascularization. Herz. 2007;32:287–295. doi: 10.1007/s00059-007-2994-z. [DOI] [PubMed] [Google Scholar]
- Raval A, Parikh J, Engineer C. Mechanism and in vitro release kinetic study of sirolimus from a biodegradable polymeric matrix coated cardiovascular stent. Ind p Eng Chem Res. 2011;50:9539–9549. [Google Scholar]
- Reed ML, Wu C, Kneller J, Watkins S, Vorp DA, Nadeem A, Weiss LE, Rebello K, Mescher M, Smith AJC, Rosenblum W, Feldman MD. Micromechanical devices for intravascular drug delivery. J Pharm Sci. 1998;87:1387–1394. doi: 10.1021/js980085q. [DOI] [PubMed] [Google Scholar]
- Rogers CDK. Drug-eluting stents: clinical perspectives on drug and design differences. Rev Cardiovasc Med. 2005;6(Suppl 1):S3–12. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis – A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- Rousseau H, Puel J, Joffre F, Sigwart U, Duboucher C, Imbert C, Knight C, Kropf L, Wallsten H. Self-expanding endovascular prosthesis – An experimental-study. Radiology. 1987;164:709–714. doi: 10.1148/radiology.164.3.3303120. [DOI] [PubMed] [Google Scholar]
- Saito S. New horizon of bioabsorbable stent. Catheter Cardiovasc Interv. 2005;66:595–596. doi: 10.1002/ccd.20590. [DOI] [PubMed] [Google Scholar]
- San Juan A, Bala M, Hlawaty H, Portes P, Vranckx R, Feldman LJ, Letourneur D. Development of a functionalized polymer for stent coating in the arterial delivery of small interfering RNA. Biomacromolecules. 2009;10:3074–3080. doi: 10.1021/bm900740g. [DOI] [PubMed] [Google Scholar]
- Santiago FS, Khachigian LM. Nucleic acid based strategies as potential therapeutic tools: mechanistic considerations and implications to restenosis. J Mol Med (Berlin) 2001;79:695–706. doi: 10.1007/s001090100272. [DOI] [PubMed] [Google Scholar]
- Satzl S, Henn C, Christoph P, Kurz P, Stampfl U, Stampfl S, Thomas F, Radeleff B, Berger I, Grunze M, Richter GM. The efficacy of nanoscale poly bis(trifluoroethoxy)phosphazene (PTFEP) coatings in reducing thrombogenicity and late in-stent stenosis in a porcine coronary artery model. Investig Radiol. 2007;42:303–311. doi: 10.1097/01.rli.0000261439.90760.9d. [DOI] [PubMed] [Google Scholar]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Dejaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, Belardi J, Sigwart U, Colombo A, Goy JJ, Vandenheuvel P, Delcan J, Morel MA. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary-artery disease. New Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- Serruys PW, van Hout B, Bonnier H, Legrand V, Garcia E, Macaya C, Sousa E, van der Giessen W, Colombo A, Seabra-Gomes R, Kiemeneij F, Ruygrok P, Ormiston J, Emanuelsson H, Fajadet J, Haude M, Klugmann S, Morel MA, Benestent Study G. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II) Lancet. 1998;352:673–681. doi: 10.1016/s0140-6736(97)11128-x. [DOI] [PubMed] [Google Scholar]
- Sharif F, Hynes SO, McCullagh KJA, Ganley S, Greiser U, McHugh P, Crowley J, Barry F, O’Brien T. Gene-eluting stents: non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization but does not reduce restenosis in a hypercholesterolemic model. Gene Ther. 2012;19:321–328. doi: 10.1038/gt.2011.92. [DOI] [PubMed] [Google Scholar]
- Sharif F, Hynes SO, McMahon J, Cooney R, Conroy S, Dockery P, Duffy G, Daly K, Crowley J, Bartlett JS, O’Brien T. Gene-eluting stents: comparison of adenoviral and adeno-associated viral gene delivery to the blood vessel wall in vivo. Hum Gene Ther. 2006;17:741–750. doi: 10.1089/hum.2006.17.741. [DOI] [PubMed] [Google Scholar]
- Sketch MH, Ball M, Rutherford B, Popma JJ, Russell C, Kereiakes DJ, Driver I. Evaluation of the medtronic (Driver) cobalt-chromium alloy coronary stent system. Am J Cardiol. 2005;95:8–12. doi: 10.1016/j.amjcard.2004.08.055. [DOI] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial of fatty streak, and intermediate lesions of atherosclerosis–a report from the committee on vascular-lesions of the council on arteriosclerosis, Am -Heart Assoc Circ. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases-molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- Sydow-Plum G, Tabrizian M. Review of stent coating strategies: clinical insights. Mater Sci Technol. 2008;24:1127–1143. [Google Scholar]
- Tada N, Virmani R, Grant G, Bartlett L, Black A, Clavijo C, Christians U, Betts R, Savage D, Su SH, Shulze J, Kar S. Polymer-free biolimus A9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine sodel. Circ Cardiovasc Interv. 2010;3:174–183. doi: 10.1161/CIRCINTERVENTIONS.109.877522. [DOI] [PubMed] [Google Scholar]
- Takahata K, Gianchandani YB. A planar approach for manufacturing cardiac stents: design, fabrication, and mechanical evaluation. J Microelectromechan Syst. 2004;13:933–939. [Google Scholar]
- Tanimoto S, Serruys PW, Thuesen L, Dudek D, de Bruyne B, Chevalier B, Ormiston JA. Comparison of in vivo acute stent recoil between the bioabsorbable everolimus-eluting coronary stent and the everolimus-eluting cobalt chromium coronary stent: insights from the ABSORB and SPIRIT trials. Catheter Cardiovasc Interv. 2007;70:515–523. doi: 10.1002/ccd.21136. [DOI] [PubMed] [Google Scholar]
- Terada N, Lucas JJ, Szepesi A, Franklin RA, Takase K, Gelfand EW. Rapamycin inhibits the phosphorylation of p70 S6 kinase in IL-2 and mitogen-activated human T-cells. Biochem Biophys Res Commun. 1992;186:1315–1321. doi: 10.1016/s0006-291x(05)81549-9. [DOI] [PubMed] [Google Scholar]
- Van der Hoeven BL, Pires NM, Warda HM, Oemrawsingh PV, van Vlijmen BJ, Quax PH, Schalij MJ, van der Wall EE, Jukema JW. Drug-eluting stents: results, promises and problems. Int J Cardiol. 2005;99:9–17. doi: 10.1016/j.ijcard.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Varshosaz J, Soheili M. Production and in vitro characterization of lisinopril-loaded nanoparticles for the treatment of restenosis in stented coronary arteries. J Microencapsul. 2008;25:478–486. doi: 10.1080/02652040802054679. [DOI] [PubMed] [Google Scholar]
- Vasquez EM. Sirolimus: a new agent for prevention of renal allograft rejection. Am J Health Syst Pharm. 2000;57:437–448. doi: 10.1093/ajhp/57.5.437. [DOI] [PubMed] [Google Scholar]
- Verheye S, Martinet W, Kockx MM, Knaapen MWM, Salu K, Timmermans JP, Ellis JT, Kilpatrick DL, De Meyer GRY. Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol. 2007;49:706–715. doi: 10.1016/j.jacc.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- Vrolix MCM, Legrand VM, Reiber JHC, Grollier G, Schalij MJ, Brunel P, Martinez-Elbal L, Gomez-Recio M, Bar F, Bertrand ME, Colombo A, Brachman J, Investigators MT. Heparin-coated Wiktor stents in human coronary arteries (MENTOR trial) Am J Cardiol. 2000;86:385–389. doi: 10.1016/s0002-9149(00)00951-6. [DOI] [PubMed] [Google Scholar]
- Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB, Kirchmair R, Silver M, Curry C, Wecker A, Yoon YS, Heidenreich R, Hanley A, Kearney M, Tio FO, Kuenzler P, Isner JM, Losordo DW. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents - An alternative strategy for inhibition of restenosis. Circulation. 2004;110:36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- Wang X, Venkatraman SS, Boey FYC, Loo JSC, Tan LP. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials. 2006;27:5588–5595. doi: 10.1016/j.biomaterials.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. 6. Isolation and structure of Taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93:2325. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Welt FGP, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141–U175. doi: 10.1161/CIRCULATIONAHA.107.730010. [DOI] [PubMed] [Google Scholar]
- Ye YW, Landau C, Willard JE, Rajasubramanian G, Moskowitz A, Aziz S, Meidell RS, Eberhart RC. Bioresorbable microporous stents deliver recombinant adenovirus gene transfer vectors to the arterial wall. Ann Biomed Eng. 1998;26:398–408. doi: 10.1114/1.62. [DOI] [PubMed] [Google Scholar]