Abstract
OBJECTIVE
The purpose of this study was to describe antidepressant medication use patterns during pregnancy and pregnancy outcomes.
STUDY DESIGN
We evaluated a cohort of 228,876 singleton pregnancies that were covered by Tennessee Medicaid, 1995–2007.
RESULTS
Of 23,280 pregnant women with antidepressant prescriptions before pregnancy, 75% of them filled none in the second or third trimesters of pregnancy, and 10.7% of them used antidepressants throughout pregnancy. Filling 1, 2, and ≥3 antidepressant prescriptions during the second trimester was associated with shortened gestational age by 1.7 (95% confidence interval [CI], 1.2–2.3), 3.7 (95% CI, 2.8–4.6), and 4.9 (95% CI, 3.9–5.8) days, when controlled for measured confounders. Third-trimester selective serotonin reuptake inhibitor use was associated with infant convulsions; adjusted odds ratios were 1.4 (95% CI, 0.7–2.8); 2.8 (95% CI, 1.9 –5.5); and 4.9 (95% CI, 2.6–9.5) for filling 1, 2, and ≥3 prescriptions, respectively.
CONCLUSION
Most women discontinue antidepressant medications before or during the first trimester of pregnancy. Second-trimester antidepressant use is associated with preterm birth, and third-trimester selective serotonin reuptake inhibitor use is associated with infant convulsions.
Keywords: adverse outcome, maternal antidepressant, preterm birth
Depression is very common among pregnant women, with a prevalence of 10–20% in various survey studies.1–3 The prevalence of medication therapy for depression during pregnancy has been reported recently to be 4–10% in the United States and Canada.4–7 Antidepressant use has increased nationally, and the prevalence of antidepressant use during pregnancy has increased from the 1990s through 2007.5,6 To date, most studies have found that exposure to selective serotonin reuptake inhibitors (SSRIs) during pregnancy may be associated with preterm birth,8–11 lower birthweight,10,11 heart defects,12 and a neurologic withdrawal syndrome among infants who have been exposed to SSRIs in the third trimester; more extreme cases may include neonatal convulsions.13,14 Some notable studies have failed to confirm associations with gestational age15 and birthweight after adjustment for gestational age.8 Several larger studies failed to confirm the association between SSRIs and heart defects.4,11,16,17 Thus, more information from large, population-based studies is needed. The objective of this study was to estimate the independent effects of antidepressant medications on pregnancy outcomes by trimester after adjustment for available depression covariates. This study draws on the strengths of administrative Medicaid data, including the very large size of the population, the ability to describe patterns of antidepressant medication use, the ascertainment of some depression covariates before and during pregnancy, and the classification of antidepressant exposure by trimester.
Materials And Methods
Study population
We conducted a retrospective cohort study of 228,876 singleton pregnancies among women aged 15–44 years who were enrolled in the Tennessee Medicaid program from 1995 to 2007, with 180 days of continuous enrollment before their last menstrual period (LMP) through 90 days after delivery. All the data were anonymized, and the protocol was approved to be exempt by the institutional review boards of Vanderbilt University and the Tennessee Department of Health.
Data were obtained from the Medicaid database and birth certificates, which were developed for studying medication use and pregnancy outcomes among pregnant women who are enrolled in the Tennessee Medicaid. Birth certificates include the date of the maternal LMP, which was used to estimate the week of pregnancy for 85.3% of subjects. When LMP was not available on the birth certificate, LMP was set to median gestational age in weeks for infants of the same race, birthweight, and birth year (14.6%) or assigned a gestation period of 273 days (0.2%). Trimesters of pregnancy were defined as LMP at 91 days, 92–182 days, and 183 days through the date of delivery.
Women were classified as depressed if (1) an International Classification of Diseases, 9th Revision, diagnosis code of 296.2, 296.3, 300.4, or 311 was recorded in any diagnostic field on an inpatient or outpatient professional claim from 180 days before LMP to LMP or (2) they filled a prescription for at least 1 antidepressant medication from 180 days before LMP to the date of delivery. Filled prescriptions of antidepressant medications (Supplementary Table 1) were counted for the 180 days before LMP, during each trimester of pregnancy, and for the 90 days after delivery. Prescriptions are typically for a 30-day supply of medication. Mothers who met the definition of depression were further classified as depressed before pregnancy (before LMP), during pregnancy (from LMP to date of delivery), or both.
Infant sex, birthweight, and gestational age and maternal age, education, race, adequacy of prenatal care,17 parity, and smoking status were abstracted from birth certificates; the remaining maternal and infant adverse outcomes and potential confounders were determined with the use of International Classification of Diseases, 9th Revision diagnostic codes (Supplementary Table 2). Common and serious maternal comorbidities were defined as in-patient or outpatient claims for asthma, chronic cardiac disease, chronic obstructive pulmonary disease, malignancy, diabetes mellitus, immunodeficiency, renal disease, mental retardation, anxiety disorder, bipolar disorder, obsessive compulsive disorder, schizophrenia, and substance abuse during 180 days before LMP to LMP. Markers of depression severity were defined as depression diagnosis before LMP, prepreg-nancy use of antidepressants, and psycho-tropic polytherapy.
Statistical analysis
Descriptive statistics were presented as number (percentage) and in mean (±SD), as appropriate. Multivariable linear or logistic regression models were performed for continuous or categoric pregnancy outcomes. The main exposure of interest was number of filled antidepressant prescriptions during each trimester and throughout pregnancy. SSRI prescriptions or both SSRI and non-SSRI prescription filling were further analyzed separately. The continuous outcomes included birth-weight and gestational age in days. The categoric outcomes included infant respiratory distress, preterm labor, early preterm labor, and neonatal convulsions in the first 14 days. To better understand the relationship between antidepressant medication filling and gestational age, we further applied a proportional odds model with categoric gestational age as outcome (<32, 32–36, and ≥37 weeks). In this model, the odds ratios for having a gestational age <32 weeks or <37 weeks in women with a specific medication history is calculated with respect to women without this history. This model makes the proportional odds assumption that we assume the odds ratios for early gestational age that are associated with any risk factor are the same for a gestational age <32 weeks as for a gestational age <37 weeks. The proportional odds assumption was assessed graphically and by logistic regression to ensure the proportional odds model was reasonable.18–20 Because previous preterm delivery is the single biggest risk factor for preterm birth, we further conducted subgroup analyses of gestational age and pre-term labor that included only nulliparous women. All multivariable models were adjusted for gestational age (unless gestational age, preterm labor, or early preterm labor were the outcome), maternal age, smoking during pregnancy, maternal race, education, comorbidity, adequacy of prenatal care, maternal parity (unless limited to primiparous women), infant sex, year of delivery, depression diagnosis before LMP, anxiety disorder, substance abuse diagnosis, filling antidepressant prescriptions before LMP, psychiatric medication poly-therapy, and co-existing psychiatric diagnosis (bipolar disorder, obsessive compulsive disorder, or schizophrenia). All statistical tests were 2-tailed; the probability values of all tested models are presented. R-software (version 2.9.2; www.r-project.org) and SAS software (version 9.1; SAS Institute Inc, Cary, NC) were used for data analyses.
Results
There were 228,876 singleton pregnancies in women enrolled in the Tennessee Medicaid Program over the 13 study years, 1995–2007 (Table 1). Among the pregnancies that were studied, 13,593 women (5.9%) had a diagnosis of depression before pregnancy; 23,280 women (10.2%) filled at least 1 prescription of antidepressant medication before pregnancy, and 6340 women (2.8%) initiated antidepressant therapy during pregnancy, although they filled on average only 2 antidepressant prescriptions during pregnancy. Compared with women who were not depressed and never filled antidepressant prescriptions, women who were classified as depressed were older, more educated, and more likely to be white, suburban, or rural, to be married, to have a comorbid illness, and to have more children at home (P < .001; Table 1).
TABLE 1.
Maternal characteristics by depression and antidepressant exposure status during pregnancy
| Depressed pregnant women |
|||||
|---|---|---|---|---|---|
| Maternal characteristics | Entire cohort (n = 228,876) | Not classified as depressed (n = 195,079) | No prescription (n = 16,901) | 1–2 prescriptions (n = 10,700) | ≥3 prescriptions (n = 6196) |
|
| |||||
| Age, ya | 23.2 ± 5.2 | 22.9 ± 5.1 | 23.8 ± 5.3 | 24.6 ± 5.5 | 26.5 ± 5.9 |
|
| |||||
| Race, n (%) | |||||
|
| |||||
| White | 127,592 (55.7) | 100,340 (51.4) | 13,317 (78.8) | 8552 (79.9) | 5383 (86.9) |
|
| |||||
| Black | 95,503 (41.7) | 89,816 (46.0) | 3164 (18.7) | 1903 (17.8) | 620 (10.0) |
|
| |||||
| Other | 5781 (2.5) | 4923 (2.5) | 420 (2.5) | 245 (2.3) | 193 (3.1) |
|
| |||||
| Residence: 228,395 women, n (%) | |||||
|
| |||||
| Urban | 113,890 (49.9) | 101,755 (52.3) | 5996 (35.6) | 3911 (36.6) | 2228 (36.0) |
|
| |||||
| Standard metropolitan statistical area (suburban) | 51,316 (22.5) | 41,633 (21.4) | 4835 (28.7) | 3020 (28.3) | 1828 (29.6) |
|
| |||||
| Rural | 63,189 (27.7) | 51,283 (26.3) | 6032 (35.8) | 3745 (35.1) | 2129 (34.4) |
|
| |||||
| Education: 228,350 women, n (%) | |||||
|
| |||||
| <12 | 96,170 (42.1) | 82,577 (42.4) | 7258 (43.1) | 4206 (39.4) | 2129 (34.5) |
|
| |||||
| 12 | 98,224 (43.0) | 83,896 (43.1) | 6997 (41.5) | 4676 (43.8) | 2655 (43.0) |
|
| |||||
| >12 | 33,956 (14.9) | 28,174 (14.5) | 2597 (15.4) | 1792 (16.8) | 1393 (22.6) |
|
| |||||
| Smoking in pregnancy: 228,490 women, n (%) | 64,248 (29.9) | 52,513 (27.0) | 7528 (44.6) | 5044 (47.2) | 3163 (51.1) |
|
| |||||
| Parity: 228,365 women, n (%) | |||||
|
| |||||
| Primiparous | 67,265 (29.5) | 58,116 (29.9) | 5067 (30.1) | 2711 (25.4) | 1371 (22.2) |
|
| |||||
| 1 | 79,742 (34.9) | 68,162 (35.0) | 5765 (34.2) | 3661 (34.3) | 2154 (34.9) |
|
| |||||
| 2 | 45,813 (20.1) | 38,328 (19.7) | 3551 (21.1) | 2463 (23.1) | 1471 (23.8) |
|
| |||||
| ≥3 | 35,545 (15.6) | 30,051 (15.4) | 2471 (14.7) | 1843 (17.3) | 1180 (19.1) |
|
| |||||
| Married: 228,775 women, n (%) | 74,805 (32.7) | 60,876 (31.2) | 6751 (40.0) | 4331 (40.5) | 2847 (46.0) |
|
| |||||
| Any comorbidity, n (%)b | 21,232 (9.3) | 15,366 (7.9) | 2691 (15.9) | 1926 (18.0) | 1249 (20.2) |
|
| |||||
| Diagnosed depression before pregnancy, n (%) | 13,593 (5.9) | 0 | 8397 (49.7) | 2901 (27.1) | 2295 (37.0) |
|
| |||||
| Substance abuse, n (%) | 34,353 (15.0) | 23,592 (12.1) | 4934 (29.2) | 3516 (32.9) | 2311 (37.3) |
|
| |||||
| Anxiety disorder, n (%) | 17,958 (7.8) | 7064 (3.6) | 4897 (29.0) | 3313 (31.0) | 2684 (43.3) |
|
| |||||
| Bipolar disorder diagnosis, n (%) | 4897 (2.1) | 2937 (1.5) | 845 (5.0) | 602 (5.6) | 513 (8.3) |
|
| |||||
| Schizophrenia diagnosis, n (%) | 638 (0.3) | 214 (0.1) | 162 (1.0) | 122 (1.1) | 140 (2.3) |
|
| |||||
| Personality disorder, n (%) | 1369 (0.6) | 331 (0.2) | 414 (2.4) | 299 (2.8) | 325 (5.2) |
|
| |||||
| Antipsychotic in pregnancy, n (%) | 2756 (1.2) | 603 (0.3) | 365 (2.2) | 855 (8.0) | 933 (15.1) |
|
| |||||
| Anxiolytic in pregnancy, n (%) | 13,966 (6.1) | 7073 (3.6) | 2010 (11.9) | 2590 (24.2) | 2293 (37.0) |
|
| |||||
| Sedative in pregnancy, n (%) | 3172 (1.4) | 1608 (0.8) | 422 (2.5) | 569 (5.3) | 573 (9.2) |
|
| |||||
| Narcotic in pregnancy, n (%) | 65,504 (28.6) | 49,491 (25.4) | 7183 (42.5) | 5464 (51.1) | 3366 (54.3) |
|
| |||||
| Using antidepressants 180 days before last menstrual period through last menstrual period, n (%) | 23,280 (10.2) | 0 | 12,724 (75.3) | 5859 (54.8) | 4697 (75.8) |
|
| |||||
| Used antidepressants on the date of delivery through date of delivery + 90 days, n (%) | 17,773 (7.8) | 7340 (3.8) | 2782 (16.5) | 3389 (31.7) | 4262 (68.8) |
|
| |||||
Data are given as mean ± SD;
Includes asthma, chronic cardiac disease, malignancy, diabetes mellitus, immunodeficiency, renal disease, mental retardation, and chronic obstructive pulmonary disease.
Hayes. Maternal antidepressants and adverse outcomes. Am J Obstet Gynecol 2012.
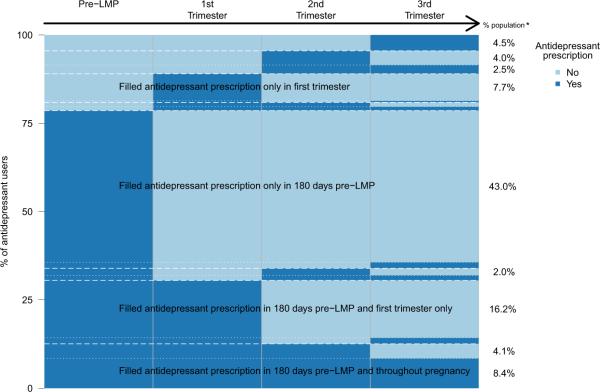
Antidepressant medication use was inconsistent; only 8004 of 23,280 women (34%) who filled a prescription before LMP filled a prescription ≥4 times before and during pregnancy. Seventy-five percent of women who filled antidepressant prescriptions before pregnancy discontinued use before or during the first trimester. Only 2161 women (0.9%) consistently filled prescriptions before and throughout pregnancy (Figure 1). Of the 13,593 women with diagnosed depression in the 180 days before LMP, 4874 (35%) did not fill any antidepressant prescriptions 180 days before LMP. Among all antidepressant users, women were 2.7 times more likely to fill prescriptions for SSRIs (n = 12,386) than for non-SSRIs (n = 4510).
FIGURE 1. Antidepressant medication prescription pattern before and during pregnancy.
*% population in each pattern of antidepressant prescription filling from pre-LMP to 3rd trimester. Categories with less than 2% were not labeled.
Antidepressant medication prescription patterns of the 33,797 women who ever filled an antidepressant prescription during 180 days before last menstrual period (LMP) to date of delivery. Each woman is represented by a single horizontal line; women with identical use patterns are grouped to enable visualization. The height of each distinct row represents the proportion of women in the cohort with each pattern of antidepressant use.
Antidepressant prescription filling was positively and significantly associated with lower birthweight in the univariate analysis (P < .001; Table 2). However, this significant relationship between antidepressant use and birthweight disappeared after we controlled for confounders that included gestational age, maternal race, age, education, smoking during pregnancy, comorbidity, parity, previous depression diagnosis, anxiety disorder, bipolar disorder, obsessive compulsive disorder, schizophrenia, substance abuse, prepregnancy use of antidepressants, psychotropic polytherapy, infant sex, and birth year (Figure 2, A).
TABLE 2.
Adverse pregnancy outcomes by maternal depression and antidepressant exposure status
| Pregnancy outcome | Entire cohort (n = 228,876) | Not classified as depressed (n = 195,079) | Depressed pregnant women |
||
|---|---|---|---|---|---|
| 0 prescriptions (n = 16,901) | 1∓2 prescriptions (n = 10,700) | >≥ prescriptions (n = 6196) | |||
|
| |||||
| Birthweight: 228,813 babies, ga | 3163 ± 587.2 | 3165 ± 587.5 | 3159 ± 577.9 | 3136 ± 588.2 | 3139 ± 598.7 |
|
| |||||
| Gestation age, da | 270.7 ± 17.5 | 270.8 ± 17.7 | 270.5 ± 16.5 | 270.6 ± 16.3 | 269.7 ± 16.2 |
|
| |||||
| <32 wk, n (%) | 5491 (2.4) | 4843 (2.5) | 340 (2.0) | 190 (1.8) | 118 (1.9) |
|
| |||||
| 32 to <37 wk, n (%) | 25,481 (11.2) | 21,524 (11.1) | 1939 (11.5) | 1231 (11.5) | 787 (12.7) |
|
| |||||
| ≥37 wk, n (%) | 197,519 (84.4) | 168,377 (86.5) | 14,595 (86.5) | 9271 (86.7) | 5276 (85.4) |
|
| |||||
| Preterm labor: 224,362 women, n (%) | 59,606 (26.6) | 50,094 (26.2) | 4682 (28.1) | 3064 (29.1) | 1766 (29.0) |
|
| |||||
| Early preterm labor: 224,362 women, n (%) | 2975 (1.3) | 2542 (1.3) | 209 (1.3) | 129 (1.2) | 95 (1.6) |
|
| |||||
| Delivery method: 228,821 deliveries, n (%) | |||||
|
| |||||
| Normal, spontaneous vaginal delivery | 160,171 (70.0) | 138,009 (70.8) | 11,211 (66.3) | 7027 (65.7) | 3924 (63.3) |
|
| |||||
| Cesarean section delivery | 53,407 (23.3) | 43,951 (22.5) | 4490 (26.6) | 3065 (28.7) | 1901 (30.7) |
|
| |||||
| Assisted | 15,243 (6.7) | 13,070 (6.7) | 1199 (7.1) | 603 (5.7) | 371 (5.9) |
|
| |||||
| Respiratory distress n (%) | 9981 (4.4) | 8358 (4.3) | 774 (4.6) | 516 (4.8) | 333 (5.4) |
|
| |||||
| Convulsions n (%) | 548 (0.2) | 429 (0.2) | 47 (0.3) | 31 (0.3) | 41 (0.7) |
|
| |||||
Data are given as mean ± SD.
Hayes. Maternal antidepressants and adverse outcomes. Am J Obstet Gynecol 2012.
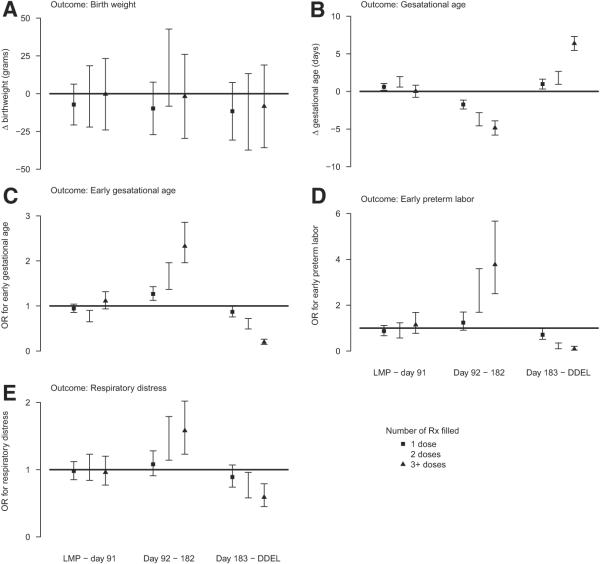
FIGURE 2. Maternal antidepressant medication and pregnancy outcomes.
Estimates of independent effect (95% CI) of antidepressant medication use during pregnancy (by duration of medication use and trimester of exposure) on A, birthweight, B, gestational age, C, early gestational age, D, early preterm labor, and E, respiratory distress after control for available maternal and psychiatric confounders. The reference group in these panels is the babies of women with no prescriptions that were filled in the indicated trimester. For panel C, the outcome is either a gestational age <32 or <37 weeks. We used a proportional odds model that forced these odds ratios (OR) that were associated with early gestational age to be equal.
CI, confidence interval; DDEL, day of delivery; Rx, prescription.
In multivariable models, filling more antidepressant prescriptions by women in the second trimester was associated with progressively shorter gestational age after we controlled for confounders (P < .001; Figure 2, B). This strong, significant, relationship between duration of exposure to second trimester antidepressant prescription and shorter gestational age was also observed when odds ratios for gestational age <32 weeks and <37 weeks were considered (Figure 2, C). The relationship between antidepressant use and early preterm labor and results were consistent (Figure 2, D). Because previous preterm delivery is a strong risk factor for preterm birth, we conducted a subanalysis of gestational age and preterm labor in 68,007 nulliparous women. The significant relationship between second trimester antidepressant prescription filling and shorter gestational age persisted; filling 1, 2, and ≥3 antidepressant prescriptions during the second trimester was associated with gestational age that was shorter by 2.6 (95% confidence interval [CI], 1.3–3.9), 5.8 (95% CI, 3.9 –7.8), and 6.6 (95% CI, 4.6–8.6) days, respectively. Both SSRI and non-SSRI prescriptions in the second trimester were similarly and independently associated with shorter gestational age (P < .0001 for both SSRIs and non-SSRIs).
Conversely, filling antidepressant prescriptions in the third trimester was associated positively with longer gestational age after we controlled for confounders; gestational age was 0.9 (95% CI, 0.3–1.6), 1.8 (95% CI, 0.9–2.7), and 6.4 (95% CI, 5.5–7.3) days longer, respectively, when women filled 1, 2, or ≥3 antidepressant prescriptions during the third trimester (Figure 2, B). Similar trends were observed between third-trimester antidepressant use and longer gestational age in proportional odds regression analysis (Figure2,C) and between third trimester antidepressant use and early preterm labor (Figure 2, D).
There were also significant associations between any antidepressant use and infant respiratory distress parallel to the association with preterm labor (Figure 2, E). Respiratory distress was 1.1 (95% CI, 0.9 –1.3), 1.4 (95% CI, 1.1–1.8), and 1.6 (95% CI, 1.2–2.0) times more common among infants who were born to women who filled 1, 2, and ≥3 prescriptions during the second trimester and 0.9 (95% CI, 0.7–1.1), 0.8 (95% CI, 0.6 –1.0), and 0.6 (95% CI, 0.5– 0.8) times as common among antidepressant users in the third trimester.
The relationships between gestational age and several potential important confounders were also estimated in the multivariable regression model. After we controlled for maternal race, age, education, smoking during pregnancy, parity, substance abuse, anxiety disorder, infant sex, and birth year, diagnosed maternal depression increased gestation by 0.8 days (95% CI, 0.5–1.1; P < .001). Previous antidepressant use (P = .87), maternal anxiety (P = .91), and psychiatric polytherapy (P = .57) had no independent effect on gestational age. Maternal substance abuse decreased gestation by 1.4 days (95% CI, 1.2–1.6; P < .001) and maternal comorbid psychiatric diagnosis (bipolar disorder, obsessive compulsive disorder, or schizophrenia) decreased gestation by 4.3 days (95% CI; 3.8–4.7; P < .001). Ever antidepressant use, cumulative pregnancy count of antidepressant prescriptions, and first-trimester use of antidepressants were also not associated with preterm delivery.
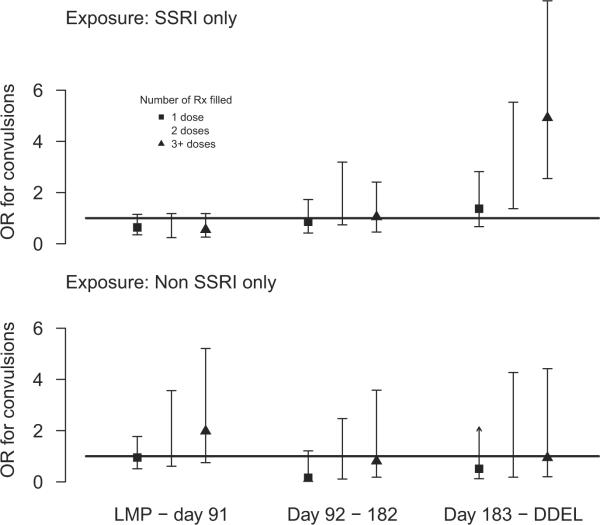
SSRI use, but not non-SSRI use in the third trimester, was associated positively with convulsions in the infant, with a strong relationship between risk of convulsions and duration of SSRI use (Figure 3); the model presented included terms for SSRI and non-SSRI medication use by a count of prescriptions filled and trimester. The odds ratios for filling 1, 2, and ≥3 SSRI prescriptions in the third trimester were 1.4 (95% CI, 0.7–2.8), 2.8 (95% CI, 1.4 –5.5), and 4.9 (95% CI, 2.6 –9.5), respectively.
FIGURE 3. Maternal selective- and non-SSRI medication and infant convulsion.
Estimates of independent effect (95% CI) of selective serotonin reuptake inhibitor medication and nonselective serotonin reuptake inhibitor antidepressant medication use during pregnancy, by duration of medication use and trimester of exposure on infant convulsions after control for available maternal and psychiatric confounders. The reference group in these panels is the babies of women with no prescriptions that were filled in the indicated trimester.
CI, confidence interval; DDEL, day of delivery; LMP, last menstrual period; OR, odds ratio; Rx, prescription; SSRI, serotonin reuptake inhibitor.
Comment
We studied adverse pregnancy outcomes that were associated with both diagnosed maternal depression and the filling anti-depressant prescriptions among 228,876 pregnancies that were covered fully by Tennessee Medicaid from 1995–2007. Detailed medication-dispensing records allowed the classification of medication exposure by trimester of pregnancy and number of prescriptions filled. In this population, 15% of pregnancies were affected by diagnosed maternal depression before LMP and/or prescribed antidepressant medications; 21% of pregnancies were in white women, and 6% of pregnancies were in black women. These rates are consistent with other reports; minority women are less likely to be diagnosed and treated for depression.
Most women (75%) who filled an antidepressant prescription before pregnancy did not fill a prescription for an antidepressant in the second or third trimesters of pregnancy. Women who were diagnosed as depressed before pregnancy, but who were not filling antidepressant prescriptions before LMP, were unlikely to initiate pharmaco-therapy during pregnancy. Women may fill prescriptions and not take all the medicine; however, filling a prescription is the first step in taking the medicine, and refilling a prescription very likely indicates consistent medication use. Many women fill antidepressants prescriptions in the absence of a coded diagnosis of depression.
There were large and highly significant associations between SSRI and non-SSRI antidepressant use in the second trimester and preterm labor and delivery. Because both SSRIs and non-SSRIs were associated similarly with preterm labor and shorter gestational age, these results are presented with all antidepressants grouped. Filling more antidepressant prescriptions in the second trimester was associated strongly with increased risks of both early preterm and late preterm delivery, even among nulliparous women. By contrast, third-trimester use of antidepressants was associated with longer gestations, especially among women who filled ≥3 prescriptions. We define third trimester as LMP + 183 days to date of delivery and medication use during the third trimester only through the actual date of delivery, thus, filling an additional prescription during the third trimester requires that pregnancy continues well into the third trimester. At that point, mothers who are at risk for preterm delivery because of antidepressant medication use may have already delivered. These associations were found in multivariable models that controlled for potential confounders. Markers of depression severity were not associated with shorter gestational age or early preterm labor (P > .6). If depression, rather than antidepressants, was the cause of shorter gestations (confounding by indication), these severity markers would also be associated with shorter gestations. The associations were strongest among women who filled multiple antidepressant medications; refilling prescriptions suggests actual consumption of medications. Conversely, the associations were not specific to 1 class of antidepressant medication. The specified models were complex, but the data were robust; many women used antidepressant medications during pregnancy and patterns of antidepressant medication use were variable. After we controlled for gestational age and other confounders, there was no association between antidepressant medication use and birthweight.
Although the association with preterm labor and delivery is consistent with many earlier findings, Oberlander et al21 reported that that the association was not observed after propensity score matching that was controlled for maternal characteristics, and the report on the management of depression during pregnancy by the American Psychiatric Association and the American College of Obstetricians and Gynecologists suggest associations between antidepressants and shorter gestational age may not be clinically significant.1 Estimating the severity of depressive illness from administrative data is daunting; diagnosis and treatment of depression do not correlate necessarily with severity of illness. A small cohort study of depressed women (n = 90) who were or were not exposed to antidepressants during pregnancy, in which the women were regularly assessed for depression severity found that antidepressant use (SSRIs and non-SSRIs), but not depression severity, was associated with preterm birth; 14% of users and none of nonusers of antidepressants delivered prematurely. In a Canadian pregnancy registry, Ramos et al22 reported that only second-trimester use of antidepressants was associated with an increased risk of small-for-gestational-age infants. Larger studies that used administrative data have reported no association between depression and preterm delivery,21,23 although smaller, nonmedical studies that actively look for symptoms3,24–26havere ported that depressed women deliver early. The observed effect in this study on preterm labor and delivery is clinically meaningful; the estimated average decrement in gestational age is 4.9 days, and infant morbidity decreases as gestational age increases by even 1 week, especially among white women.26 Filling each additional antidepressant prescription in the second trimester increased the risk of preterm labor by 2.3. Preterm delivery was common in this Medicaid cohort; 13.7% of infants were delivered at <37 weeks' gestation. African American women and smokers are known to be more likely to deliver an infant preterm. Maternal antidepressant use is more common among white women and smokers. There is substantial confounding, and the risk-adjusted odds ratios for the relationship between antidepressant use and preterm delivery are much stronger than the crude associations. Therefore, the crude estimate of the number needed to harm is 91, and the multivariable-adjusted estimate of the number needed to harm is 7. One percent of preterm deliveries may be attributable to the consistent use of antidepressants during the second trimester, despite the fact that 75% of women discontinued prescription filling before the second trimester.
There was also a large, highly significant association between the duration of SSRI use in the third trimester and neonatal convulsions that were diagnosed within 14 days of birth, which was described previously as an extreme component of an SSRI withdrawal syndrome. Convulsions are rare; in these data, 1 additional case of infant convulsions would be expected for every 117 women who use SSRI medications consistently in the third trimester. Although neonatal convulsions can be associated with serious central nervous system disease, so-called benign neonatal convulsions typically are not associated with serious adverse outcomes.27
These administrative data are not suited to the estimation of the severity of maternal depression. Co-existing mental illness and severity of depression could be identified when it was coded, but diagnoses are not always recorded. The classification of women as “not depressed” during pregnancy is also difficult with the use of administrative data, as is suggested by the number of women who fill antidepressants but for whom there is no coded diagnosis of depression. Women who sought treatment for depression may have had other health behaviors that are associated with adverse pregnancy outcomes. Administrative data also do not permit complete ascertainment of potential confounders, such as comorbid illness, maternal smoking, or substance abuse.
In summary, depression and the prescribing antidepressants are common in women of childbearing age. In this Medicaid pregnancy cohort, 75% of women who filled antidepressant prescriptions before pregnancy discontinued antidepressant medication during pregnancy. Ten percent of the women who filled antidepressant prescriptions before pregnancy filled prescriptions consistently throughout their pregnancy, and the remaining 15% of women had other medication dispensing patterns. Antidepressant use was associated significantly with both preterm birth and infant convulsions.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, and the Tennessee Department of Health, Office of Policy, Planning & Assessment, for providing the data.
Supported by research grants R03 MH 088902 (R.M.H.), K12 scholar HD 043483 and 1RC4MH092755-01 (P.W.), and K24 AI 77930 (T.V.H.) and by Vanderbilt CTSA grant UL1 RR024975-01 from the National Institutes of Health.
R.C.S. received research funding from Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Forest Pharmaceuticals, Janssen Pharmaceutica, Novartis Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab, Pfizer, Repligen, and St. Jude Medical. R.C.S. has consulted for Eli Lilly and Company, Cyberonics, Evotec AG, Forest Pharmaceuticals, Gideon Richter PLC, Janssen Pharmaceutica, Medronic, Otsuka Pharmaceuticals, Pamlab, Inc, Pfizer, Repligen, and Sierra Neuropharmaceuticals.
Footnotes
The first 2 authors contributed equally to this article.
The remaining authors report no conflict of interest.
REFERENCES
- 1.Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114:703–13. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seguin L, Potvin L, St-Denis M, Loiselle J. Chronic stressors, social support, and depression during pregnancy. Obstet Gynecol. 1995;85:583–9. doi: 10.1016/0029-7844(94)00449-N. [DOI] [PubMed] [Google Scholar]
- 3.Dayan J, Creveuil C, Herlicoviez M, et al. Role of anxiety and depression in the onset of spontaneous preterm labor. Am J Epidemiol. 2002;155:293–301. doi: 10.1093/aje/155.4.293. [DOI] [PubMed] [Google Scholar]
- 4.Reefhuis J, Rasmussen SA, Friedman JM. Selective serotonin-reuptake inhibitors and persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:2188–90. doi: 10.1056/NEJMc060602. [DOI] [PubMed] [Google Scholar]
- 5.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196:544–5. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194–5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 7.Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164:1515–20. doi: 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- 8.Lund N, Pedersen LH, Henriksen TB. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med. 2009;163:949–54. doi: 10.1001/archpediatrics.2009.164. [DOI] [PubMed] [Google Scholar]
- 9.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159:2055–61. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 10.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335:1010–5. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 11.GlaxoSmithKline . Use of Paroxetine in the first trimester of pregnancy may have a small increased risk of birth defects, compared to other antidepressants. GlaxoSmithKline; 2005. [Accessed May 14, 2012]. Study EPIP083-Preliminary report. Available at: http://ctr.gsk.co.uk/Summary/paroxetine/studylist.asp. [Google Scholar]
- 12.Kulin NA, Pastuszak A, Sage SR, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279:609–10. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- 13.Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:482–7. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- 14.Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol. 2005;106:1289–96. doi: 10.1097/01.AOG.0000187302.61812.53. [DOI] [PubMed] [Google Scholar]
- 15.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotoninreuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684–92. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 16.Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–83. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 17.Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am J Public Health. 1994;84:1414–20. doi: 10.2105/ajph.84.9.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrell FE., Jr. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 19.Walker SH, Duncan DB. Estimation of the probability of an event as a function of several independent variables. Biometrika. 1967;54:167–79. [PubMed] [Google Scholar]
- 20.Agresti A. Categorical data analysis. 2nd ed. John Wiley & Sons; Hoboken, NJ: 2002. [Google Scholar]
- 21.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. Br J Psychiatry. 2008;192:338–43. doi: 10.1192/bjp.bp.107.037101. [DOI] [PubMed] [Google Scholar]
- 22.Ramos E, St-Andre M, Berar A. Association between antidepressant use during pregnancy and infants born small for gestational age. Can J Psychiatry. 2010;55:643–52. [PubMed] [Google Scholar]
- 23.Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: a population-based study. Am J Epidemiol. 2004;159:872–81. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- 24.Orr ST, James SA, Blackmore PC. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156:797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 25.Steer RA, Scholl TO, Hediger ML, Fischer RL, Bixo M. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45:1093–9. doi: 10.1016/0895-4356(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 26.Loftin R, Chen A, Evans A, Defranco E. Racial differences in gestational age-specific neonatal morbidity: further evidence for different gestational lengths. Am J Obstet Gynecol. 2012;206:259.e1–6. doi: 10.1016/j.ajog.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hrastovec A, Hostnik T, Neubauer D. Benign convulsions in newborns and infants: occurrence, clinical course and prognosis. Eur J Paediatr Neurol. 2012;16:64–73. doi: 10.1016/j.ejpn.2011.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.