Abstract
Neuropathic pain is a debilitating condition for which the development of effective treatments has been limited by an incomplete understanding of its chemical basis. We show by using untargeted metabolomics that sphingomyelin-ceramide metabolism is altered in the dorsal horn of rats with neuropathic pain and that the up-regulated, endogenous metabolite N,N-dimethylsphingosine induces mechanical hypersensitivity in vivo. These results demonstrate the utility of metabolomics to implicate unexplored biochemical pathways in disease.
Millions of individuals suffer from neuropathic pain 1,2, a disabling condition that develops after damage to the nervous system 3. Treatment options for neuropathic pain are limited, associated with undesirable side effects, and rarely provide complete therapeutic relief. Alterations in gene transcription, protein expression, ion-channel organization, and trophic factors are all associated with the development of neuropathic pain symptoms 4,5, but the molecular etiology of the condition remains unclear and has limited the development of effective treatments.
To investigate the chemical basis of neuropathic pain, we performed mass spectrometry-based metabolomics on samples collected from Sprague-Dawley rats suffering from tibial-nerve transection (TNT). Transection of the tibial branch of the sciatic nerve is a well-established model of neuropathic pain that induces allodynia, a condition in which normally innocuous stimuli elicit a pain response. In the TNT model, allodynia persists for at least 9 weeks post-surgery 6, long after resolution of the initial peripheral injury (Supplementary Fig 1). To focus on the chronic phase of neuropathic pain, the most clinically problematic symptom, we analyzed tissues from rats 21 days after TNT and compared them to control rats undergoing a sham surgery. Metabolites were profiled from rat dorsal horn, dorsal root ganglia (DRG), tibial nerve, and plasma (Supplementary Fig 2).
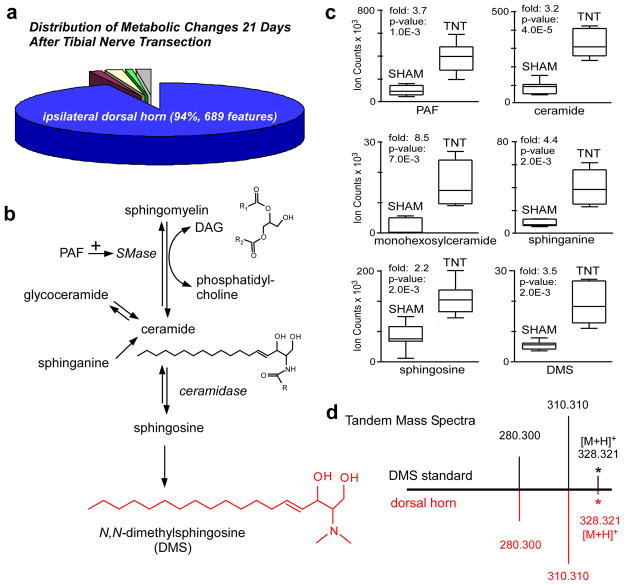
We separately compared each of the following in TNT rats to sham-operated rats twenty-one days after surgery: (i) the lumbar enlargement of the ipsilateral dorsal horn (L3-L5, where the sciatic nerve projects), (ii) the same region of the contralateral dorsal horn, (iii) the ipsilateral DRG, (iv) 1-mm segments of the tibial nerve proximal to site of transection, and (v) plasma. Out of the total 733 statistically significant (p<0.01, fold change>2) metabolic features that we detected as dysregulated between TNT and sham animals 21 days after injury, 94% were in the ipsilateral dorsal horn (Fig 1a). Metabolic features with fold changes greater than 3 were only detected in the ipsilateral dorsal horn, and not in the DRG, tibial nerve, or plasma after TNT. Therefore, during the later stages of neuropathic pain, metabolic alterations in the spinal cord appear to play a significant role in perpetuating pain sensitivity and were the focus of our investigation.
Fig. 1.
Untargeted metabolomics identifies the dysregulation of sphingomyelin-ceramide metabolism in the ipsilateral dorsal horn during chronic neuropathic pain. (a) Distribution of the 733 statistically significant molecular features (p<0.01) with changes greater than 2 fold that are altered in TNT relative to sham animals 21 days after injury (n=7 animals per group). The distribution of changes is: 94% ipsilateral dorsal horn (blue), 1% contralateral dorsal horn (purple), 2% ipsilateral dorsal root ganglia (yellow), 1% ipsilateral tibial nerve (green), and 2% plasma (grey). (b) The sphingomyelin-ceramide pathway, highlighting metabolites determined to be dysregulated. (c) Box-and-Whisker plots of altered sphingomyelin-ceramide metabolites in the ipsilateral dorsal horn after TNT. The intensities represent ion counts from extracted ion chromatograms. (d) Tandem mass spectra from DMS standard and m/z 328.321 from the dorsal horn show the same fragments and relative intensities.
We characterized dysregulated metabolites in the ipsilateral dorsal horn associated with the pain phenotype by using an untargeted workflow (Supplemental Methods). With this approach, we identified multiple alterations in sphingomyelin-ceramide metabolism 21 days after TNT injury (Fig 1b–d). Sphingomyelin-ceramide metabolism plays an important role in many cellular processes including myelin formation, apoptosis, and cell signaling 7. We therefore hypothesized that dysregulated metabolites in this pathway may be linked to the physiological changes underlying neuropathic pain and represent possible new targets for therapeutic intervention.
The first step in the degradation of sphingomyelin is the formation of ceramide through activation of sphingomyelinase (SMase) 8. This biochemical reaction is coupled with the conversion of diacylgylcerols to phosphatidylcholines (Fig 1b) 8. We detected that both ceramide (d18:1:16:0) and several phosphatidylcholines are up-regulated in the ipsilateral dorsal horn 21 days after TNT by more than 3 fold (Supplementary Fig 3–4, Supplementary Table 1). Consistent with the increased degradation of sphingomyelin, we found that several diacylglycerols are down-regulated by 2 to 3 fold in the dorsal horn of TNT rats (Supplementary Fig 5, Supplementary Table 1). Furthermore, several of the up-regulated phosphatidylcholines and the down-regulated diacylglycerols have the same fatty acid side chains. We also detected a 3.8-fold increase in TNT animals of platelet-activating factor (PAF), a metabolite that has been reported to activate sphingomyelinase (Supplemental Fig 6) 9,10. Additionally we found that monohexosylceramide (d18:1/24:1), sphinganine, sphingosine, and N,N-dimethylsphingosine (DMS) were each significantly up-regulated more than 2 fold (Fig 1c, Supplemental Fig 7–10).
Reorganization of the central termination areas of primary sensory neurons is known to occur following peripheral-nerve injury 11–13, suggesting that cellular membrane degradation after TNT may contribute to alterations in sphingomyelin catabolism. In support of this we found that the expression of acid ceramidase, the enzyme involved in ceramide catabolism, is significantly up-regulated by 2.3 fold in the ipsilateral DRG cell bodies 21 days after TNT injury (Supplemental Fig 11). These data suggest that persistence of metabolite components of the cell membrane may contribute to altered cell signaling and neuronal hypersensitivity during the development of neuropathic pain.
Among the dysregulated metabolites, endogenous DMS has not been previously investigated in the context of neuropathic pain. DMS is a catabolite of ceramide14 that has been shown to increase the concentration of intracellular Ca2+ in astrocytes as well as inhibit glutamate uptake15. Deficient glutamate uptake by glial cells in spinal sensory synapses results in the excessive activation of N-methyl-D-aspartate (NMDA) receptors that has been associated with neuropathic pain.16 The role and concentration of endogenous DMS, however, is largely unknown and a robust characterization of DMS as an endogenous metabolite has not been previously reported (Supplementary Results, Supplementary Fig 12).
We quantitated the physiological level of naturally occurring DMS in the ipsilateral dorsal horn of TNT animals 21 days after injury. By using selected reaction monitoring, we determined that there were 3.5 ± 1.2 fmol of DMS per mg of ipsilateral dorsal horn tissue 21 days after TNT. To determine if DMS at these physiological concentrations is sufficient for the development of neuropathic pain behavior in vivo, we intrathecally injected DMS into healthy rats and measured the development of mechanical allodynia in the hind paw. Within 24 hours following injections at concentrations of 0.25 μg/Kg, rats developed mechanical allodynia that persisted for at least 3 days (Supplementary Fig 13). In contrast, vehicle-treated rats displayed no evidence of abnormal sensation to mechanical stimuli. We determined the concentration of DMS in the dorsal horn to be 4.1 ± 1.4 fmol per mg of tissue 2 hours after injection with 0.25 μg/Kg, a level that approximates the amount of DMS detected in TNT animals after injury.
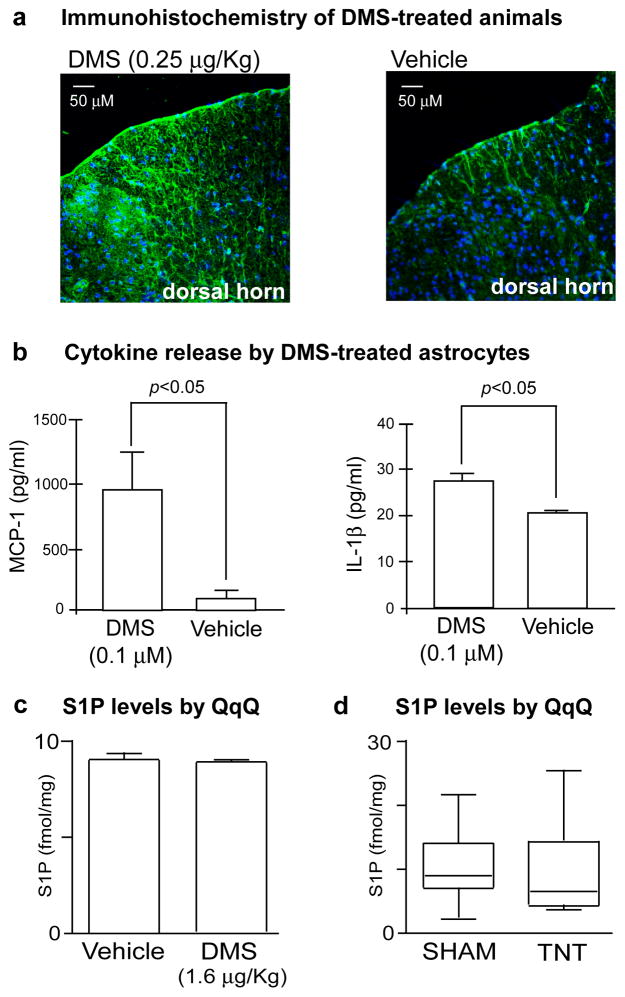
A number of pathological alterations are associated with the development of mechanical allodynia including abnormal astrocyte responses triggered by tissue damage or cellular dysfunction in the CNS 17. Therefore, we used immunohistochemistry to examine GFAP expression in the spinal cords of rats treated with DMS relative to vehicle-treated rats (Fig 2a). Following intrathecal DMS administration, increased GFAP staining was detected in the spinal cord relative to vehicle-treated controls, indicating an increase in astrocyte activation induced by DMS. Activated astrocytes release a variety of substances such as proinflammatory cytokines that have been shown to modulate neuronal hypersensitivity syndromes18. The cytokine interleukin 1β (IL-1β) is of special interest because it is known to be elevated in the cerebrospinal fluid of patients with chronic pain 19. Deletion of IL-1 receptor type 1 and transgenic overexpression of the naturally occurring IL-1 receptor antagonist delays the onset and severity of pain following peripheral nerve injury 20. To determine if DMS triggers IL-1β release, we treated cultured astrocytes with 0.1 μM of DMS and measured release of IL-1β by ELISA 24 hours later (Fig 2b). We observed a significant increase in IL-1β release from cells treated with DMS relative to vehicle-treated cultures. Based on our quantiation, we estimate that this concentration of DMS is in the range of physiological levels after TNT injury.
Fig. 2.
N,N-dimethylsphingosine (DMS) elicits neuropathic pain behavior and cytokine release. (a) Immunohistochemistry of the dorsal horn of rats intrathecally administered DMS at 0.25 μg/Kg (left) and vehicle (right) and stained with anti-glial fibrillary acidic protein (anti-GFAP) and a Alexa fluor 488 anti-rabbit IgG secondary antibody (green). Increased GFAP staining, a marker of astrocyte activation, is seen in the dorsal horn of DMS-treated rats relative to vehicle controls. (b) ELISAs of IL-1β and MCP-1 released into the supernatant of astrocyte cultures treated with DMS or vehicle. Cultures treated with 0.1 μM of DMS show a significant increase in IL-1β and MCP-1 release relative to vehicle controls. Data are expressed as mean ± SEM, with n=3 cultures per group. (c) Comparison of S1P levels in the dorsal horn of rats intrathecally administered DMS at 1.6 μg/Kg and vehicle control. S1P was measured by selective reaction monitoring triple quadrupole mass spectrometry (QqQ). S1P levels between the groups are not statistically different (n=4 animals per group). S1P levels are represented as fmol per mg of spinal cord tissue. (d) Comparison of S1P levels in the ipsilateral dorsal horn of sham control animals relative to animals suffering from TNT 21 days after injury. S1P levels between the groups are not statistically different (n=7 animals per group). S1P levels are represented as fmol per mg of spinal cord tissue.
Another inflammatory mediator that plays a role in nociceptive responses is the chemokine monocyte chemoattractant protein-1 (MCP-1). MCP-1 recruits inflammatory cells to sites of injury and is up-regulated in spinal cord astrocytes following nerve injury21. To determine if DMS increases MCP-1 production, we treated astrocyte cultures with 0.1 μM of DMS and measured release of MCP-1 by ELISA 24 hours later (Fig 2b). We observed a significant increase in MCP-1 release from cells treated with 0.1 μM DMS relative to vehicle-treated controls. Taken together, our results show that DMS induces pathological responses in the dorsal spinal cord that are associated with the development of pain behaviors and that the mechanism by which DMS mediates mechanical allodynia may be via production of inflammatory mediators such as IL-1β or MCP-1 in the CNS.
Previous studies have implicated another ceramide derivative, sphingosine-1-phosphate (S1P), in nociceptive processing 22. DMS is an inhibitor of the enzyme sphingosine kinase and treatment of cells with DMS blocks production of S1P 23. To investigate the possibility that DMS affects hypersensitivity by regulating S1P levels, we compared S1P in allodynic rats intrathecally treated with 1.6 μg/Kg of DMS to animals administered a vehicle control and found no statistically significant difference in S1P levels between groups (Fig 2c). Moreover, we determined that S1P levels were similarly not altered between TNT and sham animals 21 days after injury (Fig 2d). These results are consistent with the physiological concentrations of DMS being substantially lower than the 2.3–6.8 μM Ki values at which DMS has been reported to inhibit sphingosine kinase23 and support that DMS is sensitizing neurons in the CNS via an alternative mechanism that remains to be elucidated.
In summary, our results have shown that the majority of metabolic perturbations characterizing the chronic phase of neuropathic pain are localized to the spinal cord. The data presented here show that DMS, an endogenous metabolite that has not been previously implicated in nociception, induces mechanical allodynia in rats in vivo and elicits cytokine release from astrocytes in vitro. Further investigation is needed to determine the specific enzyme(s) responsible for DMS biosynthesis. The capacity of mammalian tissues to achieve N-methylation of sphingoid bases, however, has been demonstrated previously for the analog sphingolipid safingol 24 and it has been suggested that a SAM-dependent N-methyltransferase is active in mouse CNS tissue 25. Our results therefore suggest that inhibition of endogenous DMS production, with a methyltransferase or ceramidase inhibitor for example, may be an attractive therapeutic candidate to treat this debilitating condition.
Supplementary Material
Footnotes
Competing financial interests statement. The authors declare no competing financial interests.
Author contributions. G.J.P. and O.Y. contributed equally to this work. G.J.P., L.S., J.C., and R.T. performed animal work. G.J.P., O.Y., and L.S. performed analytical experiments. G.J.P., O.Y., L.S., J.C., R.T., M.M., and G.S. contributed to experimental design, performed data analysis, and wrote the manuscript.
References
- 1.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–9. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Warfield CA, Fausett HJ. Manual of Pain Management. In: Warfield CA, Fausett HJ, editors. Manual of Pain Management. Lippincott Williams & Wilkins; Philadelphia: 2002. [Google Scholar]
- 3.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 4.Singh OV, et al. Proteome of synaptosome-associated proteins in spinal cord dorsal horn after peripheral nerve injury. Proteomics. 2009;9:1241–53. doi: 10.1002/pmic.200800636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung YJ, Ambron RT. Pathways that elicit long-term changes in gene expression in nociceptive neurons following nerve injury: contributions to neuropathic pain. Neurol Res. 2004;26:195–203. doi: 10.1179/016164104225013761. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann HA, De Vry J, Siegling A, Spreyer P, Denzer D. Pharmacological sensitivity and gene expression analysis of the tibial nerve injury model of neuropathic pain. Eur J Pharmacol. 2003;470:17–25. doi: 10.1016/s0014-2999(03)01753-9. [DOI] [PubMed] [Google Scholar]
- 7.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 8.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 9.Latorre E, Aragones MD, Fernandez I, Catalan RE. Platelet-activating factor modulates brain sphingomyelin metabolism. Eur J Biochem. 1999;262:308–14. doi: 10.1046/j.1432-1327.1999.00358.x. [DOI] [PubMed] [Google Scholar]
- 10.Goggel R, et al. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10:155–60. doi: 10.1038/nm977. [DOI] [PubMed] [Google Scholar]
- 11.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–8. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 12.Knyihar-Csillik E, Rakic P, Csillik B. Transganglionic degenerative atrophy in the substantia gelatinosa of the spinal cord after peripheral nerve transection in rhesus monkeys. Cell Tissue Res. 1987;247:599–604. doi: 10.1007/BF00215754. [DOI] [PubMed] [Google Scholar]
- 13.Inoue M, et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–8. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi Y, et al. A specific enhancing effect of N,N-dimethylsphingosine on epidermal growth factor receptor autophosphorylation. Demonstration of its endogenous occurrence (and the virtual absence of unsubstituted sphingosine) in human epidermoid carcinoma A431 cells. J Biol Chem. 1990;265:5385–9. [PubMed] [Google Scholar]
- 15.Lee YK, Kim HL, Kim YL, Im DS. Multiple actions of dimethylsphingosine in 1321N1 astrocytes. Mol Cells. 2007;23:11–6. [PubMed] [Google Scholar]
- 16.Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. J Neurophysiol. 103:2570–80. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 18.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–5. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 19.Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116:213–9. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120:315–24. doi: 10.1016/j.pain.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Gao YJ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coste O, et al. Sphingosine 1-phosphate modulates spinal nociceptive processing. J Biol Chem. 2008;283:32442–51. doi: 10.1074/jbc.M806410200. [DOI] [PubMed] [Google Scholar]
- 23.Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–8. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- 24.Morales PR, et al. Safingol toxicology after oral administration to TRAMP mice: demonstration of safingol uptake and metabolism by N-acylation and N-methylation. Drug Chem Toxicol. 2007;30:197–216. doi: 10.1080/01480540701375018. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi Y, Hakomori S. Enzymatic synthesis of N,N-dimethyl-sphingosine: demonstration of the sphingosine: N-methyltransferase in mouse brain. Biochem Biophys Res Commun. 1989;164:1411–6. doi: 10.1016/0006-291x(89)91827-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.