Background: Heparin regulates mast cell proteases.
Results: Both HS6ST-1 and HS6ST-2 are involved in 6-O-sulfation of heparin. The contents of tryptase and CPA in mast cells depend on 6-O-sulfation of heparin but chymase does not.
Conclusion: The 6-O-sulfation pattern regulates differently the storage of MC-specific proteases.
Significance: The fine structure of heparin may be essential for MC homeostasis.
Keywords: Heparan Sulfate, Heparin, Mast Cell, Protease, Sulfotransferase, Carboxypeptidase-A, Chymase, Connective Tissue-type Mast Cell, Knock-out Mice, Tryptase
Abstract
Heparan sulfate 6-O-sulfotransferase (HS6ST) is an enzyme involved in heparan sulfate (HS) biosynthesis that transfers a sulfate residue to position 6 of the GlcNAc/GlcNSO3 residues of HS, and it consists of three isoforms. Heparin, the highly sulfated form of HS, resides in connective tissue mast cells and is involved in the storage of mast cell proteases (MCPs). However, it is not well understood which isoform(s) of HS6ST participates in 6-O-sulfation of heparin and how the 6-O-sulfate residues in heparin affect MCPs. To investigate these issues, we prepared fetal skin-derived mast cells (FSMCs) from wild type (WT) and HS6ST-deficient mice (HS6ST-1−/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−) and determined the structure of heparin, the protease activity, and the mRNA expression of each MCP in cultured FSMCs. The activities of tryptase and carboxypeptidase-A were decreased in HS6ST-2−/−-FSMCs in which 6-O-sulfation of heparin was decreased at 50% of WT-FSMCs and almost lost in HS6ST-1−/−/HS6ST-2−/−-FSMCs, which lacked the 6-O-sulfation in heparin nearly completely. In contrast, chymase activity was retained even in HS6ST-1−/−/HS6ST-2−/−-FSMCs. Each MCP mRNA was not decreased in any of the mutant FSMCs. Western blot analysis showed that tryptase (mMCP-6) was almost absent from HS6ST-1−/−/HS6ST-2−/−-FSMCs indicating degradation/secretion of the enzyme protein. These observations suggest that both HS6ST-1 and HS6ST-2 are involved in 6-O-sulfation of heparin and that the proper packaging and storage of tryptase, carboxypeptidase-A, and chymase may be regulated differently by the 6-O-sulfate residues in heparin. It is thus likely that 6-O-sulfation of heparin plays important roles in regulating MCP functions.
Introduction
Heparan sulfate (HS)2/heparin proteoglycans are composed of sulfated polysaccharides attached to the core protein. The backbone polysaccharides of HS/heparin are synthesized by the alternating addition of GlcUA and GlcNAc residues from their respective UDP-sugar precursors, then partially modified by C5-epimerization of GlcUA to IdoUA, and subsequently modified in sequence by N-deacetylation/N-sulfation of position 2 of the GlcNAc residues by sulfation of position 2 on HexA, by sulfation of position 6 of the GlcNAc/GlcNSO3 residues, and finally by sulfation of position 3 of GlcNAc/GlcNSO3. These modification reactions of HS/heparin are catalyzed by specific enzymes as follows: N-deacetylase/N-sulfotransferases, C5-epimerase (Hsepi), and 2-O-, 6-O-, and 3-O-sulfotransferases (1). HS/heparin interacts with a huge number of ligand proteins such as growth factors, morphogens, and proteases. These interactions regulate the activity, gradient formation, and stability of the corresponding ligands and thereby play important roles in various developmental, morphogenic, physiological, and pathogenic processes (2–7). HS6ST is present as three isoforms (HS6ST-1, -2, and -3) and one spliced form of HS6ST-2 (HS6ST-2S) (8, 9). Each isoform exhibits a characteristic in vitro substrate preference, although the substrate specificities of the three isoforms partially overlap (8, 10). Compared with other isoforms, HS6ST-2 preferentially catalyzes 6-O-sulfation of IdoUA (2SO4)-GlcNSO3 unit to form IdoUA(2SO4)-GlcNSO3(6SO4), a unit that is abundant in heparin. We have generated HS6ST-1- and HS6ST-2-null mice. Mice deficient in HS6ST-1 (HS6ST-1−/−) show HS abnormal structures and mostly die on E15.5 of the peritoneal stage. Mice that grow to adults exhibit aberrant morphology in placental labyrinthine microvessels (11) and lung (11, 12) and show erroneous axon navigation in the optic chiasm (13). In humans, Hs6st-1 is mutated in families with idiopathic hypogonadotropic hypogonadism (14). Other organisms, such as zebrafish (15, 16), Caenorhabditis elegans (17), Drosophila (18, 19), and chick (20), also have abnormal development due to defects in 6-O-sulfation in HS. In contrast, HS6ST-2-KO (HS6ST-2−/−) mice are born normal and fertile, with normal morphology. However, HS6ST-1−/−/HS6ST-2−/− mice die at a slightly earlier stage than HS6ST-1-null mice. Embryonic fibroblasts from these HS6ST-1−/−/HS6ST-2−/− mice have completely lost 6-O-sulfate in HS and exhibit differences in responses to FGF-1, -2, and -4 from wild type fibroblasts (21). Considering those observations, redundancy of the HS6ST-1 substrate preference might hide the actual functions of HS6ST-2. Thus, we could say that the physiological roles of HS6ST-2 remain unknown.
Mast cells (MCs) are classified into connective tissue-type mast cells (CTMCs) and mucosal mast cells (22). Each mast cell type is differentiated by the types of glycosaminoglycans and MC-specific proteases present and is involved in a variety of physiological and pathological processes (23–30). CTMCs mainly produce heparin, whereas mucosal mast cells primarily produce highly sulfated chondroitin sulfate (23, 31, 32). Biosynthesis of heparin in mast cells and the structure of heparin required for the interaction with granular proteins are largely unknown. NDST-2, C5-epimerase, and HS3ST-1 have been reported to be involved in the biosynthesis of heparin because NDST-2-null mice (33, 34), C5-epimerase-null mice (35), and HS3ST-1-null mice (36) generate abnormal heparin (heparin with low sulfate or low IdoUA, low anticoagulant activity); however, it has not been studied which isoform(s) of HS6ST catalyzes the addition of the 6-O-sulfate residues in heparin in vivo. From studies of NDST-2-null mice, it is evident that heparin is required for the storage of several proteases in the granules; however, it is currently unknown as to why different proteases require 6-O-sulfate residues in heparin for their stable packaging and storage in the granules. In this study, we isolated fetal skin-derived mast cells (FSMCs) from HS6ST-deficient mice (HS6ST-1−/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/− double KO), and we then investigated the role of these sulfotransferases in the biosynthesis of heparin. We also determined which MCPs were affected by deficient 6-O-sulfation of heparin. As the results, we demonstrated first that 6-O-sulfation of heparin was nearly abolished in HS6ST-1−/−/HS6ST-2−/−-FSMCs and then that although some chymase activities remained, tryptase (mMCP-6) and CPA activities were almost completely absent in HS6ST-1−/−/HS6ST-2−/−-FSMCs, suggesting important roles of HS6ST-2 not only in the heparin 6-O-sulfation but also in the CTMC functions.
EXPERIMENTAL PROCEDURES
Materials
Heparitinase-I (Flavobacterium heparinum, EC 4.2.2.8.), heparitinase-II (F. heparinum, no number assigned), heparinase (F. heparinum, EC 4.2.2.7), and an unsaturated glycosaminoglycan (GAG) disaccharide kit were purchased from Seikagaku Corp. (Tokyo, Japan). Sensyu Pak Docosil was purchased from Sensyu Scientific (Tokyo, Japan). DEAE-Sephacel was from GE Healthcare. An anti-mast cell tryptase (V-13) antibody against mouse MCP-6 was from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant mouse IL-3 was from R&D Systems (Minneapolis, MN), and recombinant mouse stem cell factor was from PeproTech (Rocky Hill, NJ). RPMI 1640 medium, HEPES buffer, nonessential amino acids, sodium pyruvate, DNase I, RNase A, safranin O, and Alcian blue were from Sigma. Potassium acetate, NaOH, NaCl, acetic acid, Triton X-100, and EDTA were from Nacalai Tesque (Kyoto, Japan). HS6ST-1 (11) and HS6ST-2 knock-out (HS6ST-1−/− and HS6ST-2−/−, respectively) mice were generated as described previously (21).
Isolation of Fetal Skin-derived Mast Cells
FSMCs were prepared from 15.5-day-old wild type (WT), HS6ST-1−/−, HS6ST-2+/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−embryos of the C57BL/6 mouse strain according to Yamada et al. (37) with a slight modification. Briefly, internal organs, limbs, tails, and heads were removed from the embryos, and the trunks were cut into small pieces that were digested with 0.25% trypsin in PBS containing 0.53 mm EDTA for 10 min at 37 °C. The residues were repeatedly digested, and then the supernatant was passed through a 100-μm mesh. The cells in the filtrate were collected by centrifugation at 120 × g for 10 min and were subjected to erythrolysis in ACK lysing buffer (0.15 m NH4Cl, 10 mm KHCO3, and 0.1 mm EDTA). After repeated washing in Hanks' balanced salt solution, the cell pellets were suspended at 5 × 104cells/ml in RPMI 1640 medium containing 10 ng/ml IL-3, 10 ng/ml stem cell factor, 10% heat-inactivated fetal bovine serum, 2 mm l-glutamine, 10 mm nonessential amino acids, 10 mm sodium pyruvate, 25 mm HEPES buffer, 50 μm 2-mercaptoethanol, and penicillin/streptomycin and cultured at 37 °C in a CO2 incubator for 4 weeks. After incubation, nonadherent cells were harvested and then layered on a Percoll density gradient and centrifuged. The cells that pelleted at the bottom were used as FSMCs.
Isolation and Structural Analysis of Glycosaminoglycans
The ears from 8-week-old WT and HS6ST-2−/− mice were cut into small pieces, defatted with acetone, and dried. The FSMCs and the ear samples were suspended in 0.2 m NaOH and incubated for 16 h at room temperature, neutralized with 4 m acetic acid, and then incubated at 37 °C for 2 h in 50 mm Tris-HCl (pH 7.5) containing DNase I, RNase A, and 10 mm MgCl2. Subsequently, the cells were subjected to proteinase (Actinase-E; Sigma) digestion at a final concentration of 1 mg/ml and incubated for 2 h at 37 °C. The reaction was stopped by heating at 100 °C for 5 min, and the samples were centrifuged at 14,000 × g for 10 min to remove any insoluble materials. The supernatants were diluted with an equal volume of 20 mm Tris-HCl buffer (pH 7.5) and loaded onto a 0.3-ml DEAE-Sephacel column equilibrated with the same buffer. The columns were washed with 10 column volumes of buffer containing 0.2 m NaCl and then eluted with 4 column volumes of 2 m NaCl in 20 mm Tris-HCl buffer (pH 7.5). Next, 3 volumes of cold 95% (v/v) ethanol containing 1.3% (w/v) potassium acetate and 1 mm EDTA were added to the eluates, and then the GAGs were recovered by centrifugation. An aliquot of the GAG was digested with a mixture of 0.2 milliunits of heparitinase I, 0.1 milliunits of heparitinase II, and 0.2 milliunits of heparinase in 50 μl of 50 mm Tris-HCl buffer (pH 7.5), 1 mm CaCl2, and 5 μg of bovine serum albumin at 37 °C for 2 h. After filtration of the digests in an Ultrafree MC (5-kDa molecular mass cutoff filtering unit; Millipore Corp., Bedford, MA), the unsaturated disaccharide products in the filtrates were analyzed by reverse phase ion pair chromatography using a Sensyu Pak Docosil column with a fluorescence detector (Model RF-10AxL, Shimadzu Co. Kyoto, Japan) according to Toyoda et al. (38) with slightly modified elution conditions, in which unsaturated disaccharide products after separation by the chromatography were reacted with 2-cyanoacetoamide (Wako, Osaka, Japan) as a post-labeling reagent.
Mono Q Column Chromatography
The crude GAGs from the ears of WT and HS6ST-2−/− mice, prepared as described above, were digested with 100 milliunits of chondroitinase ABC at 37 °C for 1 h to digest the chondroitin sulfate (CS). The chondroitinase ABC-resistant GAGs were precipitated with 3 volumes of cold 95% ethanol containing 1.3% potassium acetate and 1 mm EDTA and recovered by centrifugation at 14,000 × g for 30 min. The precipitates were dissolved in 0.5 ml of 0.1 m NaCl in 50 mm Tris-HCl (pH 7.2) (Buffer A) and were applied to a Mono Q column (GE Healthcare) equilibrated with Buffer A. The column was developed with Buffer A for 5 min (0–5 min), then with a linear gradient from 0.1 to 2.0 m NaCl in 50 mm Tris-HCl buffer (pH 7.2) for 40 min (5–45 min), and finally with 2.0 m NaCl in 50 mm Tris-HCl (pH 7.2) for 10 min (45–55 min). The flow rate was 0.5 ml/min, and the eluate was collected in tubes every 0.5 min. Under this elution program, HS (bovine liver), shark cartilage, and heparin (porcine intestinal mucosa) were eluted at 36.7, 42.5, and 45.6 min, respectively. The samples were divided into six fractions; fraction I (30.5–33.5 min), fraction II (34–36.5 min), fraction III (37–41 min), fraction IV (41.5–44 min), fraction V (44.5–46 min), and fraction VI (46.5–49 min). The HS/heparin contained in each fraction was precipitated by the addition of 3 volumes of cold 95% (v/v) ethanol containing 1.3% (w/v) potassium acetate and 1 mm EDTA, followed by centrifugation. The disaccharide composition of each fraction was analyzed as described above. The yield of each fraction was calculated from the total amount of disaccharides obtained from HPLC analysis.
Western Blot Analysis
FSMC proteins were extracted in PBS containing 2 m NaCl, 1% Triton X-100, 1 mm EDTA, and protease inhibitor mixture (1 tablet per 10 ml, complete Mini; Roche Applied Science) for 30 min on a rotary shaker, and the extracts were clarified by centrifugation (11,000 × g for 30 min). Protein concentration was determined using a Dc protein assay kit (Bio-Rad), and 60 μg of protein were separated by 10% SDS-PAGE. The separated proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were immunoblotted with anti-mouse tryptase (MCP-6) antibody. The blot was developed using a horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence (Western Lightning Plus; PerkinElmer Life Sciences). The bands were detected using luminescent image analyzer (Fuji Film, Tokyo, Japan).
Expression Levels of MCPs and HS6STs mRNAs by RT-PCR
Total RNA from WT, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs was isolated using TRIzol (Invitrogen) and purified (PureLink RNA mini kit; Invitrogen). The reverse-transcription reaction was performed using the high capacity cDNA archive kit (Applied Biosystems, Foster City, CA) and 1.0 μg of total RNA as the template. The expression levels of the genes were semi-quantitatively determined by RT-PCR using the primer pairs shown in Table 1. The data were obtained from two independent experiments. Quantitative RT-PCR was performed using specific primer pairs and SYBR Green (Takara, Shiga, Japan) according to the manufacturer's protocol. Each transcript was normalized to reactions using β-actin-specific primer pairs (Takara, Shiga, Japan). The PCR products were analyzed in real time using the ABI Prism 7700 system. The values were obtained in two independent experiments performed in triplicate.
TABLE 1.
Semiquantitative PCR primer sequences
Hdc, histidine decarboxylase.
| Target gene | Sequence |
|
|---|---|---|
| Forward | Reverse | |
| Hs6st-1 | 5′-AGGACCATGGTTGAGCG-3′ | |
| 5′-GCGGCGATTGGGCCGATA-3′ | ||
| Hs6st-2 | 5′-CCGGTGCCGGATCCGTA-3′ | 5′-GCGTCGCGCTTGCCATC-3′ |
| Hs6st-3 | 5′-CAACTTCGGGGAGCAGC-3′ | 5′-TCCACGAAGCGGGTCAG-3′ |
| SerGly | 5′-GAACTGCATCGAGGAGAAGG-3′ | 5′-TGAGGAAAGGGGTAACAGGA-3′ |
| Ndst-2 | 5′-TGCAACATTCCAGTGTG-3′ | 5′-GAGAGGCCAGGCCACAG-3′ |
| Chymase (mMcp-1) | 5′-ACGGACAGAGGTTCTGAGGA-3′ | 5′-CCCACACAGACCTGGAAGTT-3′ |
| Chymase (mMcp-2) | 5′-GGTTTCTCATAGCCCCACAA-3′ | 5′-TACCATGGGCCACACTATCA-3′ |
| Chymase (mMcp-4) | 5′-GCAAGATGCAGGCCCTACTA-3′ | |
| 5′-AGTCAGAAGGACGAGGCAGA-3′ | ||
| Chymase (mMcp-5) | 5′-CCCTACATGGCCTATCTGGA-3′ | |
| 5′-TACTTCCTGCAGTGTGTCGG-3′ | ||
| Tryptase (mMcp-6) | 5′-CTCCCACCTCCTTATCCTC-3′ | |
| 5′-ATGGCTAGGGACTCAAGAC-3′ | ||
| Cpa | 5′-ATGACCAAACTCTTGGACCG-3′ | |
| 5′-ATGCTATTGGGCCGTAGATG-3′ | ||
| Hdc | 5′-GCACTGTGCGGGAGAGGCAG-3′ | |
| 5′-ACGAGCCGAGCGTTCAGGGA-3′ | ||
| Hs3st-1 | 5′-AGATTCCTGAAGCTTTCTCCAC-3′ | 5′-AACAATACAAAACGCCCTCCA-3′ |
Assay of the Activities of Tryptase, Chymase, and Carboxypeptidase A
FSMCs were solubilized in lysis buffer (PBS containing 2 m NaCl and 0.5% Triton X-100), with 100 μl of lysis buffer, 1–2 × 105 FSMCs. In a final volume of 120 μl, the reaction mixtures contained 10-μl aliquot of the extracts containing 1 μg of protein, 20 μl (S-2586 and S-2288) or 40 μl (M-2245) of a 1.8 mm aqueous solution of chromogenic substrates. S-2586 (Chromogenix, Molndal, Sweden), S-2288 (Chromogenix, Milano, Italy), and M-2245 (Bachem AG, Bubendorf, Switzerland) were used to determine the activity of chymotrypsin-like proteases (chymase), trypsin-like proteases (tryptase), and CPA, respectively. The reaction mixtures were incubated at 37 °C. The activities were monitored as the absorbance at 405 nm. To examine the effects of exogenous heparin on protease activity, heparin was added to the reaction mixtures at three different concentrations (0.08, 0.8, and 8 μg/ml).
Histocytochemistry of Cultured Fetal Skin Mast Cells
Five-week-old cultured skin mast cells were placed into a 4-well slide glass and centrifuged at 30 × g for 5 min. The medium was carefully aspirated, and the cells were fixed with methanol, acetic acid, 10% formaldehyde (85:5:10) for 30 min at 4 °C (39). The cells were double-stained with Alcian blue and safranin O and reacted with naphthol AS-D chloroacetate to detect the chloroacetate esterase activity (34).
RESULTS
Effect of HS6ST-2 Null Mutations on 6-O-Sulfation of Ear Heparin
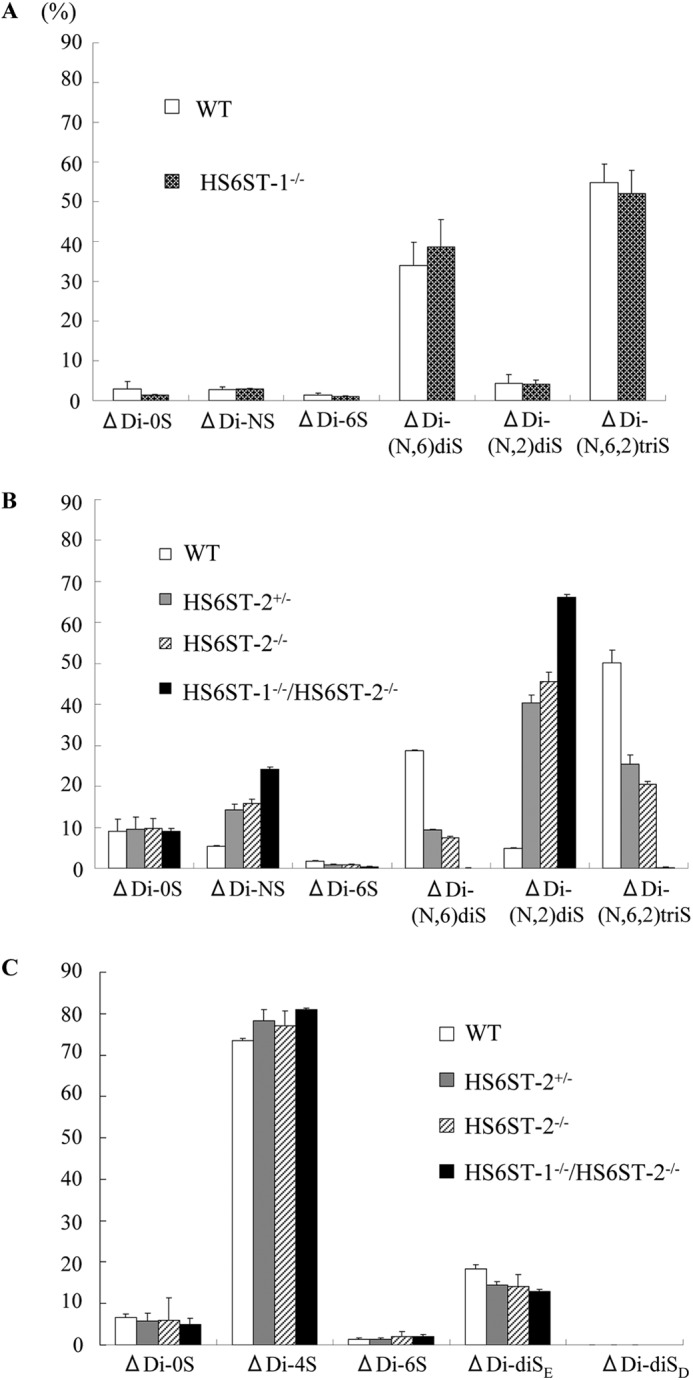
We previously showed that the IdoUA(2SO4)-GlcNSO3(6SO4) contents in HS/heparin from mouse ears of WT mice and HS6ST-1-deficient mice were nearly identical and that only the GlcNAc-6SO4 residues were significantly reduced in the ears of HS6ST-1−/− mice (11). Considering that CTMCs are abundant in the ear, these observations suggest that HS6ST isoform(s) other than HS6ST-1 may be involved in 6-O-sulfation of heparin. Alternatively, both HS6ST-1 and other isoform(s) may be involved in 6-O-sulfation of heparin in WT mice, and the other isoform(s) may compensate for the deletion of HS6ST-1. In this study, we examined whether or not HS6ST-2 contributes to the 6-O-sulfation of HS/heparin in the ear using HS6ST-2−/− mice. Disaccharide composition analysis of HS/heparin obtained from the ear showed that ΔDi-(N,6,2)triS derived from IdoUA(2SO4)-GlcNSO3(6SO4) and ΔDi-(N,6)diS derived from HexA-GlcNSO3(6SO4) were both markedly reduced in HS/heparin from HS6ST-2−/− mice, compared with those in wild type mice. In contrast, ΔDi-(N,2)diS derived from HexA(2SO4)-GlcNSO3 was increased in HS/heparin from HS6ST-2−/− mice (Fig. 1A). These data suggest that although HS6ST-2 is involved in 6-O-sulfation of heparin, other isoforms also contribute to 6-O-sulfation of heparin because IdoUA(2SO4)-GlcNSO3(6SO4) from HS/heparin of HS6ST-2−/− mice was about 50% of that from HS/heparin of WT mice. To further clarify the involvement of HS6ST-2 in the 6-O-sulfation of heparin, we tried to separate HS and heparin from each other by anion exchange chromatography (Mono Q column). Under the conditions described under “Experimental Procedures,” HS (bovine kidney), shark cartilage, and heparin standards eluted from the column at 36.7, 42.5, and 45.6 min, respectively. HS/heparin obtained from the ear of WT or HS6ST-2−/− mice were separated into six fractions by this column as follows: fraction I (30.5–33.5 min), fraction II (34–36.5 min), fraction III (37–41 min), fraction IV (41.5–44 min), fraction V (44.5–46 min), and fraction VI (46.5–49 min). Fractions I to VI were then subjected to disaccharide analysis. The disaccharide compositions of these fractions are shown in Fig. 1C. In fraction VI from WT mice, ΔDi-(N,6,2)triS and ΔDi-0S derived from the GlcUA-GlcNAc unit were 64% and less than 5%, respectively, of the total disaccharides. Such a disaccharide composition is characteristic of a typical heparin. In contrast, the ΔDi-(N,6,2)triS of fraction VI from HS6ST-2−/− mice was 39% of the total disaccharides. The decrease in the proportion of ΔDi-(N,6,2)triS was accompanied by an increase in the proportion of ΔDi-(N,2)diS. The total amounts of HS/heparin in these fractions were calculated from the yield of each disaccharide. As shown in Fig. 1B, fraction VI from WT mice accounted for 58% of the total HS/heparin, whereas fraction VI from HS6ST-2−/− mice accounted for less than 9% of the total HS/heparin. In addition, the proportions of fraction IV and V from HS6ST-2−/− mice were higher than those from WT mice. These observations support the idea that HS6ST-2 is mainly involved in 6-O-sulfation of heparin.
FIGURE 1.
Characterization of HS/heparin from WT and HS6ST-2−/− ears. GAGs were prepared from the ears of 8-week-old WT and HS6ST-2−/− mice as described under “Experimental Procedures.” A, aliquot of these GAGs was digested with a mixture of heparitinase-I, heparitinase-II, and heparinase and then subjected to reverse phase ion pair chromatography with postcolumn fluorescence labeling as described under “Experimental Procedures.” The histogram shows the percent composition of unsaturated disaccharides in HS/heparin from wild type (open bars) and HS6ST-2−/− ears (filled bars). ΔDi-0S, ΔDi-NS, ΔDi-6S, ΔDi-(N,6)diS, ΔDi-(N,2)diS, and ΔDi-(N,6,2)triS are unsaturated disaccharides derived from the following units in HS/heparin-HexA-GlcNAc-, -HexA-GlcNSO3-, -HexA-GlcNAc(6SO4)-, -HexA-GlcNSO3(6SO4)-, -HexA(2SO4)-GlcNSO3-, and -HexA(2SO4)-GlcNSO3(6SO4)-, respectively. The values shown are the means of triplicate analyses, and the bars represent the standard deviation. B, GAGs from wild type and HS6ST-2−/− ears were digested with chondroitinase ABC; the digested products were removed, and the remaining HS/heparin from wild type and HS6ST-2−/−ears were fractionated into the following six fractions by Mono Q column chromatography as described under “Experimental Procedures”: fraction I (30.5–33.5 min), fraction II (34–36.5 min), fraction III (37–41 min), fraction IV (41.5–44 min), fraction V (44.5–46 min), and fraction VI (46.5–49 min). The % yields of each fraction from WT and HS6ST-2−/− heparin/HS were calculated from the total amounts of unsaturated disaccharides. C, disaccharide composition of each fraction was analyzed as described under “Experimental Procedures.”
Because HS6ST-1−/−/HS6ST-2−/− mice were lethal at E14.5 to E15, we could not examine the in vivo effects of deletion of both HS6ST-1 and HS6ST-2 on the 6-O-sulfation of heparin. Therefore, we prepared FSMCs from WT, HS6ST-1−/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/− mice to clarify the roles of HS6ST-1 and HS6ST-2 on the 6-O-sulfation of heparin.
Heparan Sulfate 6-O-Sulfotransferases-1 and -2 Are Involved in 6-O-Sulfation of Heparin
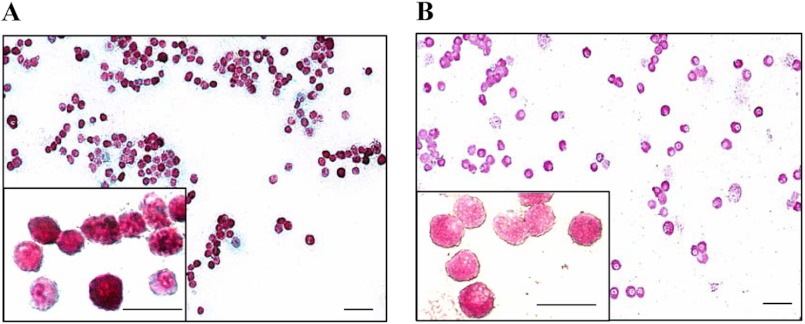
We first confirmed that the FSMCs prepared according to Yamada et al. (37) possessed the characteristics of CTMCs; WT-FSMCs were double-stained with safranin O and Alcian blue (Fig. 2A) and reacted with AS-D to detect chloroacetate esterase activity (Fig. 2B). Safranin O stains (in red) heparin, a marker of connective tissue-type mast cells, but it does not stain chondroitin sulfate. In contrast, Alcian blue stains both heparin and chondroitin sulfate. Most WT-FSMCs were strongly stained with safranin O, indicating that heparin is abundant in these cells. Chloroacetate esterase activity is a mast-cell marker. WT-FSMCs had high esterase activity. These results indicated that most FSMCs appear to be CTMCs, as described by Yamada et al. (37).
FIGURE 2.
Histocytochemical detection of heparin and chloroacetate esterase in WT-FSMCs. A, WT-FSMCs from C57BL/6 mice were cultured for 5 weeks, and then double-stained with Alcian blue and safranin O. Red staining in the FSMCs is heparin. B, WT-FSMCs were also reacted with naphthol AS-D chloroacetate to detect chloroacetate esterase activity. FSMCs containing chymotrypsin-like activity were positively stained (pink). Bar, 50 μm. The granular dot staining outside the cells shows the presence of heparin/CS due to the degranulation of some mast cells. Insets are the 4-fold magnification of some parts of respective stained FSMCs. Bar, 25 μm.
We then confirmed that the defect in HS6ST-1 did not alter the 6-O-sulfation of heparin/HS synthesized in FSMCs as was observed in the HS/heparin obtained from the ear. We isolated glycosaminoglycans from WT and HS6ST-1−/−-FSMCs and analyzed their disaccharide compositions by digestion with a heparanase-I, heparanase-II, and heparinase mixture (Fig. 3A). In the digested products from the glycosaminoglycans obtained from WT-FSMCs, the proportions of ΔDi-(N,6,2)triS, ΔDi-0S, and ΔDi-6S were 51–60%, 2–9%, and less than 2%, respectively, of total disaccharides. Undigested glycosaminoglycans, mainly consisting of chondroitin sulfate, accounted for only 4.7 ± 1.2% of the heparin/HS in the glycosaminoglycans, again indicating that the FSMCs were CTMCs (37, 39). The disaccharide composition of the glycosaminoglycans isolated from HS6ST-1−/−-FSMCs was nearly the same as that of the glycosaminoglycans obtained from WT-FSMCs, although the proportion of ΔDi-(N,6,2)triS was slightly lower. These results also indicated that, as was observed in the ear, either HS6ST-1 barely contributed to the 6-O-sulfation of heparin in the FSMCs or the absence of HS6ST-1 was compensated by other HS6ST isoforms.
FIGURE 3.
Disaccharide compositions of GAGs from WT, HS6ST-1−/−, HS6ST-2+/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs. FSMCs were prepared from WT, HS6ST-1−/−, HS6ST-2+/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/− embryos as described under “Experimental Procedures.” HS/heparin and chondroitin sulfate were isolated from FSMCs, digested with a mixture of heparitinase-I, heparitinase-II, and heparinase, and chondroitinase ABC, and subjected to reverse phase ion pair chromatography as described under “Experimental Procedures.” The histograms show the percentage compositions of the unsaturated disaccharides in the HS/heparin (A and B) and in chondroitin sulfate (C) isolated from the FSMCs of WT (open bar), HS6ST-1−/− (Crossed hatched bar), HS6ST-2+/− (gray bar), HS6ST-2−/− (hatched bar), and HS6ST-1−/−/HS6ST-2−/− (filled bar). A and B, the values (heights of the histograms) shown are the means of triplicate experiments, and the bars represent the standard deviation. ΔDi-0S, ΔDi-NS, ΔDi-6S, ΔDi-(N,6)diS, ΔDi-(N,2)diS, and ΔDi-(N,6,2)triS are the same as described in the legend for Fig. 1A. C, ΔDi-0S, ΔDi-4S, ΔDi-6S, ΔDi-diSE, and ΔDi-diSD are unsaturated disaccharides derived from -HexA-GalNAc-, -HexA-GalNAc(4SO4)-, -HexAGalNAc(6SO4)-, -HexA-GalNAc(4,6)SO4-, and -HexA(2SO4)-GalNAc(6SO4)- units of the GAGs, respectively.
Next, to determine the effects of HS6ST-2 on the 6-O-sulfation of heparin, we isolated glycosaminoglycans from FSMCs prepared from WT, HS6ST-2+/−, and HS6ST-2−/− embryos. The disaccharide composition analysis of these glycosaminoglycans suggested that 6-O-sulfation of HS/heparin in the FSMCs was markedly affected by the deletion of HS6ST-2; in the digested products from the glycosaminoglycans obtained from both HS6ST-2+/−-FSMCs and HS6ST-2−/−-FSMCs, ΔDi-(N,6,2)triS, ΔDi-(N,6)diS, and ΔDi-6S decreased to less than half of the respective disaccharides from WT-FSMCs (Fig. 3B). In contrast, the decrease in 6-O-sulfated disaccharides was accompanied by the increase in ΔDi-(N,2)diS; the proportions of ΔDi-(N,2)diS in those from the glycosaminoglycans obtained from HS6ST-2+/−-FSMCs and HS6ST-2−/−-FSMCs were 8.2- and 9.8-fold, respectively, higher than that in WT-FSMCs. The observed increase in the proportion of ΔDi-(N,2)diS in the HS6ST-2-deficient FSMCs appears to be consistent with the widely accepted biosynthesis pathway in which an IdoUA(2SO4)-GlcNSO3(6SO4) unit is produced by 6-O-sulfation of an IdoUA(2SO4)-GlcNSO3 unit. Because the heparin structure changed significantly due to the absence of HS6ST-2, as observed above, it is evident that this enzyme is significantly involved in heparin biosynthesis. However, HS6ST isoform(s) other than HS6ST-2 might be involved in 6-O sulfation of heparin, because even in HS6ST-2−/−-FSMCs, the 6-O-sulfation of heparin did not disappear completely. To address this issue, we developed HS6ST-1−/−/HS6ST-2−/− embryos and prepared FSMCs from 14.5-day-old HS6ST-1−/−/HS6ST-2−/−-embryos (designated HS6ST-1−/−/HS6ST-2−/−-FSMCs). Analysis of the disaccharide composition of heparin/HS isolated from HS6ST-1−/−/HS6ST-2−/−-FSMCs revealed drastic structural abnormalities in heparin/HS; ΔDi-(N,6,2)triS, ΔDi-(N,6)diS, and ΔDi-6S were barely detected in heparin/HS from HS6ST-1−/−/HS6ST-2−/−-FSMCs (Fig. 3B). These results clearly indicate that HS6ST-1 is indispensable for 6-O-sulfation of heparin when HS6ST-2 is absent.
To determine whether the defective 6-O-sulfation of heparin/HS affected CS biosynthesis in the FSMCs, we digested the glycosaminoglycans obtained from WT, HS6ST-2+/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs with chondroitinase ABC, and we analyzed the disaccharide composition of CS (Fig. 3C). In CS from WT and all three mutant FSMCs, the total ΔDi-4S, which was derived from GlcUA-GalNAc(4SO4), and ΔDi-diSE, which was derived from GlcUA-GalNAc(4,6-SO4), was nearly constant (92–94% of total disaccharides) among these glycosaminoglycans, indicating that the major parts of the repeating unit in these CSs bear a 4-O-sulfate group and that the degree of 4-O-sulfation was not altered in the three mutant FSMCs. In contrast, the ratio of ΔDi-diSE/(ΔDi-4S +ΔDi-diSE) varied slightly and was 0.18 for WT-CS and 0.13 for HS6ST-1−/−/HS6ST-2−/−-CS. These values are much lower than those observed in BMMCs (0.28) (40), which are thought to be immature mast cells. These results indicated that 6-O-sulfation of the GalNAc(4SO4) residues of chondroitin sulfate was only slightly affected by the defects in the 6-O-sulfation of heparin/HS.
In a parallel experiment, we analyzed the expression profiles of Hs6st-1, Hs6st-2, and Hs6st-3 mRNA using semi-quantitative RT-PCR (Fig. 4). It is evident that Hs6st-1 and Hs6st-2 are the predominant isoforms in WT-FSMCs. We previously showed that HS6ST-2 is present as the long form and the one spliced form (HS6ST-2S) (8, 9). In the RT-PCR used in this study, the transcript sizes for the long form and the short form of HS6ST should be 507 and 387 bp, respectively. The expression profiles of Hs6st-2 mRNA in WT-FSMCs suggested that more than 85% of the mRNA was a short form (see the major band corresponding to the short form and a faint band above the major one corresponding to the long form in Fig. 4A). The profiles also showed no expression of either form of Hs6st-2 mRNA in HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/−-FSMCs (Fig. 4B). The expression of Hs6st-3 mRNA was barely detected in WT and HS6ST-1−/−/HS6ST-2−/−-FSMCs, indicating that the expression level of heparan sulfate 6-O-sulfotransferase-3 gene (Hs6st-3) is very low in CTMCs. The expression level of 3-O-sulfotransferase-1 (Hs3st-1) did not differ among WT-, HS6ST-2−/−-, and HS6ST-1−/−/HS6ST-2−/−-FSMCs. In addition, N-deacetylase/N-sulfotransferase-2 (Ndst-2), another important heparin biosynthetic enzyme, which when disrupted leads to the production of abnormal heparin (33, 34), was expressed in mutant FSMCs nearly at the same level as in the WT-FSMCs. These data appear to be consistent with the observation that the N-sulfate residue content in the HS/heparin chains in the WT and mutant FSMCs was nearly equal. Serglycin transcript, to which heparin is attached, was also expressed in the mutant FSMCs at the same level as in the WT-FSMCs. Taken together, these results suggest that N-sulfation of heparin and the expression of serglycin core protein were not affected by deletion of HS6ST-1 and/or HS6ST-2. Because structural differences in heparin were barely detected between HS6ST-1−/−-FSMCs and WT-FSMCs, and the expression of serglycin was not altered by deletion of HS6ST-1 compared with WT,3 it is likely that the interaction of mast cell proteases with serglycin in the granules of HS6ST-1−/−-FSMCs is similar to that in the granules of WT-FSMCs. Therefore, the effects of deletion of HS6ST-1 on the expression and storage of mast cell proteases were not analyzed in this study.
FIGURE 4.
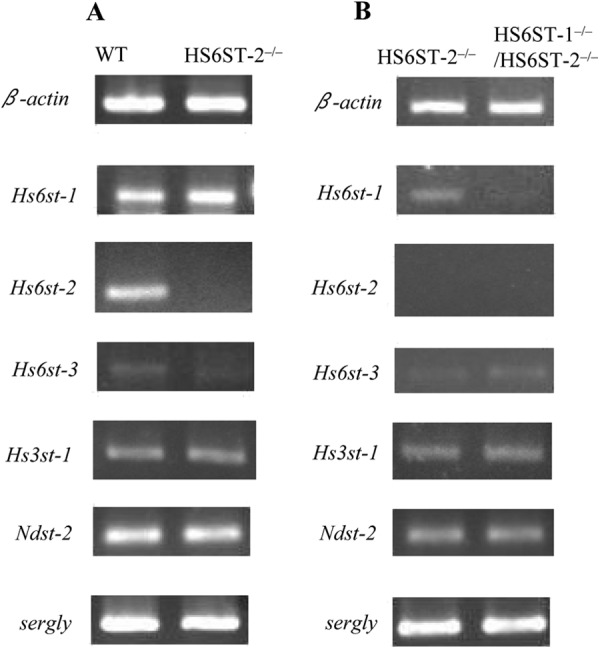
Expression of the mRNAs of various sulfotransferases and serglycin in WT, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs. The expression of Hs6st-1, Hs6st-2, Hs6st-3, Hs3st-1, Ndst-2, and serglycin mRNAs in WT, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs was measured by semi-quantitative RT-PCR using the primer pairs shown in Table 1. β-Actin was used as a control. A, expression of mRNA was compared between WT and HS6ST-2−/−-FSMCs. B, expression of mRNA was compared between HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/−-FSMCs. The expression levels for the left and right panels were normalized to the respective β-actin mRNA expression levels that were different between the left and right panels.
Activities of Various Mast Cell Proteases Are Regulated Differently by the 6-O-Sulfate Residues in Heparin
Previous in vivo studies using Ndst-2-deficient mice (33, 34) showed that the MC proteases interacted with heparin and that the mutant mice had very low sulfated heparin, suggesting the sequential reaction of heparin modification, namely that the N-deacetylation/N-sulfation of GlcNAc residues catalyzed by NDST-2 is the first step in the modification, followed by 2-O-sulfation of HexA residues and 6-O-sulfation of GlcNSO3 residues. Therefore, those studies using NDST-2-deficient mice suggested that the sulfation of heparin is important for the interaction between heparin and mast cell proteases but provided no evidence for the roles of O-sulfate residues in heparin. As indicated above, we found that deletion of HS6ST-2 or deletion of both HS6ST-1 and HS6ST-2 caused a decrease or disappearance of 6-O-sulfated disaccharide units in heparin, whereas deletion of HS6ST-1 alone did not. Therefore, we expected that deletion of HS6ST-2 or deletion of both HS6ST-1/HS6ST-2 would reveal the role of 6-O-sulfated residues of heparin in the protease-heparin interactions in mast cell granules.
Tryptase (mMCP-6) activity in HS6ST-2+/−-FSMCs and HS6ST-2−/−-FSMCs was 37 and 29%, respectively, of that in WT-FSMCs, whereas tryptase activity was barely detected in HS6ST-1−/−/HS6ST-2−/−-FSMCs (less than 5%) (Table 2). The decrease in tryptase (mMCP-6) activity appeared to be correlated with the decrease in the 6-O-sulfation of heparin. Of the two tryptases, mMCP-6 and mMCP-7, only mMCP-6 is expressed in the C57BL/6 mouse strain (41); therefore, these results indicate that the activity of a specific tryptase (mMCP-6) may require 6-O-sulfated disaccharide units in heparin.
TABLE 2.
Relative activities of MCPs in WT, HS6ST-2+/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs
The activity measurements are described under “Experimental Procedures.” The absorbance was measured at intervals of 5 min for 30 min. Activities were determined from the linear portion of the curve and normalized as per microgram of protein of the cell extract from each cell type. The relative activity of each protease was calculated when the activity in the WT-FSMC was denoted as 1.
| FSMC | Relative activity |
||
|---|---|---|---|
| Tryptase | Chymase | CPA | |
| WT | 1 | 1 | 1 |
| HS6ST2+/− | 0.37 ± 0.09 | 0.68 ± 0.02 | 0.74 ± 0.01 |
| HS6ST2−/− | 0.29 ± 0.07 | 0.56 ± 0.06 | 0.51 ± 0.025 |
| HS6ST-1−/−/HS6ST-2−/− | ≤0.05 | 0.46 ± 0.01 | ≤0.03 |
An almost identical tendency was observed for CPA activity. The CPA activity in HS6ST-2+/−-FSMCs and HS6ST-2−/−-FSMCs was 74 and 51%, respectively, of that in WT-FSMCs, while this activity was barely detected in HS6ST-1−/−/HS6ST-2−/−-FSMCs (less than 3%) (Table 2). These results clearly demonstrated that both tryptase (mMCP-6) and CPA activity are highly dependent on 6-O-sulfation of heparin. In contrast, chymase activity was not correlated with the degree of 6-O-sulfation in heparin. The chymase activity in HS6ST-2+/−-FSMCs and HS6ST-2−/−-FSMCs was 68 and 56%, respectively, of that of WT-FSMCs. Unlike tryptase (mMCP6) and CPA, chymase in HS6ST-1−/−/HS6ST-2−/−-FSMCs, where 6-O-sulfation in heparin was hardly detected, retained as much as 46% of the activity detected in WT-FSMCs. These observations indicate different regulation of chymase by 6-O-sulfation of heparin from that of tryptase (mMCP6) and CPA.
However, there are alternative explanations, for example, heparin with the altered structure synthesized in the mutant FSMCs might not be sufficient to support full protease activity. To determine such a possibility, we investigated the effects of normal heparin on the protease activities. Increasing concentrations of heparin (0.08, 0.8, and 8 μg/ml) were added to the reaction mixture containing the extracts from HS6ST-1−/−/HS6ST-2−/−-FSMCs, and no increase in the activities of tryptase, CPA, or chymase were observed (Fig. 5), suggesting that the abnormal structure of heparin did not cause a reduction of the protease activities in the extract of mutant FSMCs.
FIGURE 5.
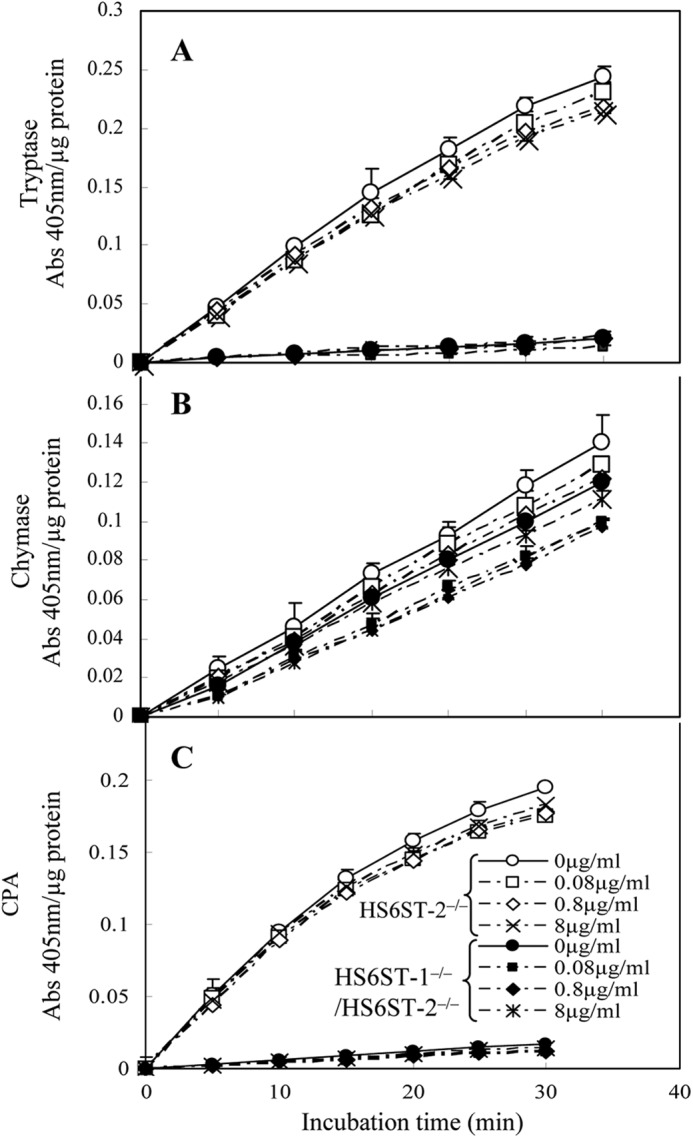
Activities of tryptase (A), chymase (B), and carboxypeptidase A (C) in extracts from HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/−-FSMCs and the effects of heparin addition. Extracts from HS6ST-2−/− (open symbols) and HS6ST-1−/−/HS6ST-2−/−-FSMCs (closed symbols) (1 μg of protein) were incubated with substrates (S-2288 for tryptase, S-2586 for chymase, and M-2245 for CPA) as described under “Experimental Procedures” in the presence and absence of three different concentrations of heparin (0.08, 0.8, and 8 μg/ml) at 37 °C, and their activities were monitored as absorbance at 405 nm as described under “Experimental Procedures.” The addition of heparin to these extracts did not affect their activities. The values shown represent the mean ± S.D. of triplicate determinations.
mRNA Expression of Mast Cell Proteases Was Unaffected in HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/−-FSMCs
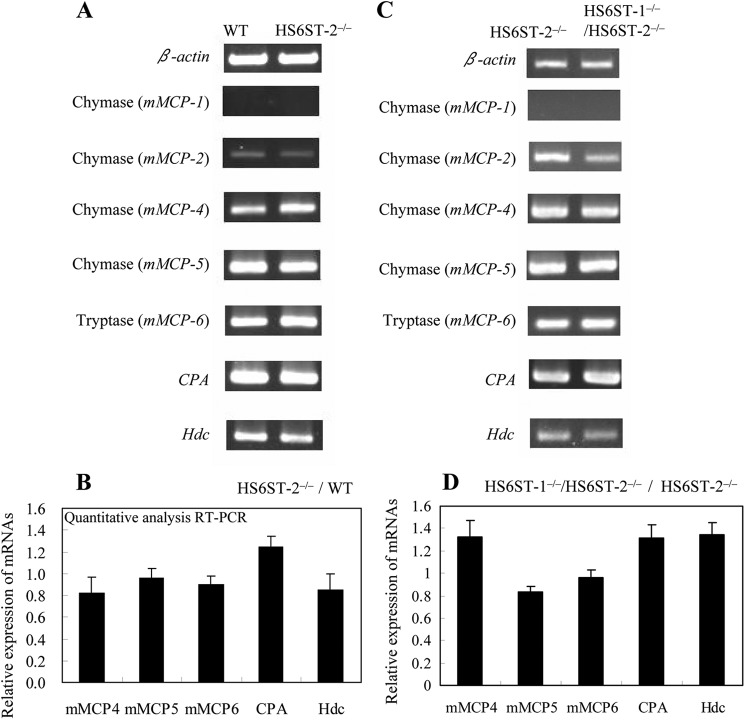
Next, we determined whether the decreased protease activities might be attributable to the mRNA expression levels of mast cell proteases. The expression of mast cell proteases, including chymases (mMCP-1, mMCP-2, mMCP-4, and mMCP-5), tryptase (mMCP-6), and CPA in HS6ST-2−/−-FSMCs and/or in HS6ST-1−/−/HS6ST-2−/−-FSMCs was examined by semi-quantitative RT-PCR. Because it was previously reported that C57BL/6 mice do not express mMCP-7 (41), we did not examine the expression of mMCP-7. Strong tryptase (mMCP-6) expression was observed in both the HS6ST-2−/−-FSMCs (Fig. 6A) and HS6ST-1−/−/HS6ST-2−/−-FSMCs (Fig. 6C), and the expression was the same as in the WT-FSMCs. Quantitative RT-PCR analysis also showed that the expression of tryptase (mMCP-6) was unaffected in HS6ST-2−/−-FSMCs (Fig. 6B) and in HS6ST-1−/−/HS6ST-2−/−-FSMCs (Fig. 6D). The expression of CPA in HS6ST-2−/−-FSMCs and HS6ST-1−/−/HS6ST-2−/−-FSMCs tended to increase compared with that in WT-FSMCs (Fig. 6). These results clearly indicate that the decreased activities of tryptase (mMCP-6) and CPA in HS6ST-1−/−/HS6ST-2−/−-FSMCs were not due to the reduced gene expression of these proteases. Analysis of different chymases showed that transcripts of mMCP-4 and mMCP-5 were strongly expressed and transcripts of mMCP-1 and mMCP-2 were weakly expressed in the WT-FSMCs (Fig. 6A). Such expression profiles of the chymase genes in WT-FSMCs are consistent with those in CTMCs (22, 24). Expression of the mRNAs of the major chymases in FSMCs, mMCP-4 and mMCP-5, was analyzed by quantitative RT-PCR (Fig. 6, B and D). The expression of mMCP-4 in HS6ST-2−/−-FSMCs and HS6ST-1−/−/HS6ST-2−/−-FSMCs was 0.8- and 1.0-fold that in WT-FSMCs, respectively. Expression of mMCP-5 in mutant FSMCs was almost identical to that in WT-FSMCs. These results indicate that the partial reduction of chymase activities in the mutant-FSMCs was not due to a reduction in the expression of the chymase genes. The histidine decarboxylase gene (Hdc), which encodes a protein that produces histamine from histidine and is a marker of differentiated mast cells, was nearly equally expressed in WT and mutant FSMCs (Fig. 6, B and D), suggesting that the defect in 6-O-sulfation of HS/heparin appears not to affect the maturation of mast cells.
FIGURE 6.
Expression levels of mMCPs, CPA, and Hdc mRNAs in WT, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs. Total RNA was isolated from the FSMCs of WT and HS6ST-2−/− littermates (A and B) or HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/− littermates (C and D). A and C, expression of each mRNA was measured by semi-quantitative RT-PCR using primer pairs as described under “Experimental Procedures.” The expression level of β-actin was used as a control. The mRNA expression was compared between WT and HS6ST-2−/− FSMCs (A) or between HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/−-FSMCs (C). B and D, mRNA expression was quantitatively measured using SYBR Green according to the manufacturer's protocol, and expression levels were normalized to β-actin expression as described under “Experimental Procedures.” The mRNA expression was compared between WT and HS6ST-2−/−-FSMCs (B) or between HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/−-FSMCs (D). Relative mRNA expression values were obtained from two independent experiments that were performed in triplicate, and error bars represent the standard deviation.
Tryptase (mMCP-6) Protein Is Substantially Lost in HS6ST-1−/−/HS6ST-2−/−-FSMCs
Next, we examined whether or not the loss of tryptase (mMCP6) activity in HS6ST-1−/−/HS6ST-2−/−-FSMCs was due to defective storage in mast cell granules. Immunoblot analysis of tryptase (mMCP-6) in HS6ST-1−/−/HS6ST-2−/−-FSMCs showed that the protease was nearly absent from HS6ST-1−/−/HS6ST-2−/−-FSMCs (Fig. 7). Together with the expression of tryptase (mMCP-6) mRNA described above, this observation suggests the possibility that tryptase (mMCP-6) mRNA may be fully transcribed in HS6ST-1−/−/HS6ST-2−/−-FSMCs; however, the protein may not be properly stored in the mast cell granules due to the lack of 6-O-sulfate residues in heparin. CPA was not examined by immunoblot analysis due to the lack of available antibodies. However, it is likely that CPA protein might also be substantially lost from HS6ST-1−/−/HS6ST-2−/−-FSMCs.
FIGURE 7.
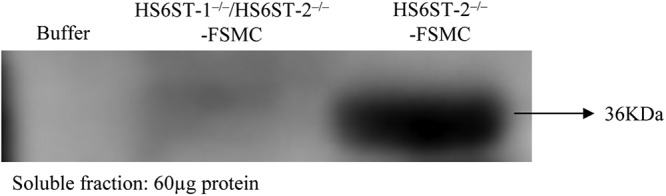
Immunoblot analysis of tryptase (mMCP-6) from HS6ST-1−/−/HS6ST-2−/−-FSMC. Proteins were extracted with a high salt buffer containing Triton X-100, EDTA, and a mixture of protease inhibitors. Sixty micrograms of protein was subjected to 10% SDS-PAGE, and the immunoblots were probed using an antibody against mMCP-6 as described under “Experimental Procedures.” The extraction buffer was loaded in the left lane as a control.
Considering these results together, it is likely that the 6-O-sulfate residues in heparin are essential for the proper packaging and/or storage of active tryptase (mMCP-6) and CPA in CTMCs and that different structures in heparin are required for the interaction with chymases.
DISCUSSION
We previously showed that the proportion of 6-O-sulfate-bearing units in HS extracted from the various tissues of HS6ST-1−/− mice was much lower than that in HS obtained from WT mice (11). In contrast, the structure of heparin from the ears of HS6ST-1−/− mice (HS6ST1-KO ear) was almost the same as that from the ear of WT mice (WT ear). In this study, we found that heparin from the ears of HS6ST-2−/− mice (HS6ST2-KO ear) contained much less IdoUA(2SO4)-GlcNSO3(6SO4) units than heparin from the WT ear. This repeating unit was 39% of that in heparin prepared from HS6ST-2−/− ear and 64% of that in the heparin prepared from WT ear. We previously reported that HS6ST-2 showed a higher preference for IdoUA(2SO4)-GlcNSO3 as the acceptor than HS6ST-1, although the substrate specificity of the three HS6ST isoforms (HS6ST-1, HS6ST-2, and HS6ST-3) overlapped (8, 10). The effects of the targeting destruction of HS6ST-1 or HS6ST-2 on heparin structure appear to be consistent with these enzymatic properties.
We prepared FSMCs from HS6ST-1−/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/− mice, and we found that the structure of heparin synthesized in HS6ST-1−/−-FSMCs was almost the same as that synthesized by WT-FSMCs and that heparin produced in HS6ST-2−/−-FSMCs had a partial decrease in 6-O-sulfated units in heparin. The results obtained from these FSMCs are consistent with the results obtained from the ear GAG analysis of WT and mutant mice, indicating that the in vitro culture systems using FSMCs may reflect the in vivo synthesis of heparin. We also found that heparin synthesized by the HS6ST-1−/−/HS6ST-2−/−-FSMCs was nearly devoid of 6-O-sulfate residues. Taken together, it strongly suggested that both HS6ST-2 and HS6ST-1 are involved in the 6-O-sulfation of heparin, although only a slight structural change was observed in HS6ST-1-deficient mice (11) and HS6ST-1-deficient FSMCs (Fig. 3A).
NDST-2-KO mice (33, 34) and serglycin-KO mice (42) clearly indicated that the disappearance of fully sulfated heparin in mast cells severely affected the storage of active mast cell-specific proteases in the granules. Present studies on HS6ST-2−/− and HS6ST-1−/−/HS6ST-2−/−-FSMCs also showed that differently 6-O-sulfated heparins synthesized by those FSMCs affected the storage and activity of tryptase, chymase, and CPA differently. Tryptase activity in HS6ST-1−/−/HS6ST-2−/−-FSMCs almost completely disappeared, although the expression of tryptase mRNA was nearly identical to that in WT and HS6ST-1−/−/HS6ST-2−/−-FSMCs. Because it has been reported that tryptase forms a tetramer with heparin, and tetramerization is a prerequisite for protease activity (43, 44), loss of tryptase activity in HS6ST-1−/−/HS6ST-2−/−-FSMCs might be caused by a decrease in the ability of heparin to assist in tetramer formation, due to fewer 6-O-sulfate residues. However, this does not seem to be a possibility because the levels of tryptase (mMCP-6) protein itself were lower in HS6ST-1−/−/HS6ST-2−/−-FSMCs, and the addition of commercial heparin to the tryptase reaction mixture did not recover the activity in the extracts of HS6ST-1−/−/HS6ST-2−/−-FSMCs at any concentration examined. Therefore, it is very likely that the defect in the 6-O-sulfation of heparin caused a failure in the packaging and storage of active tryptase (mMCP-6) into granules.
It has been shown that storage of tryptase in BMMCs containing serglycin bearing CS-E instead of heparin (39, 45) was not affected by the absence of NDST-2 (46), which proposed that the storage of tryptase in BMMCs was related to the presence of CS-E in these cells (45) and that CS-E could compensate for the lack of heparin in promoting the storage of tryptase (47). In addition, BMMCs derived from CS-E-deficient mice showed a decreased tryptase protein level and activity than WT-BMMCs (40), supporting that CS-E can contribute to the storage of tryptase in mast cell granules. We found that both WT-FSMCs and HS6ST-1−/−/HS6ST-2−/−-FSMCs produced CS, and the proportion of E units [GlcUA-GalNAc(4,6-SO4)] was 18% in WT-FSMCs and 13% in HS6ST-1−/−/HS6ST-2−/−-FSMCs, which is consistent with previous studies that showed that both human and mouse CTMCs contain both oversulfated chondroitin sulfate and heparin (48–50). It is not currently known why tryptase (mMCP6) activity was almost completely absent from HS6ST-1−/−/HS6ST-2−/−-FSMCs even though CS-containing E units were still synthesized in these cells. One explanation for this apparent discrepancy is that the content of the E units in CS synthesized by HS6ST-1−/−/HS6ST-2−/−-FSMCs might be below the hypothetical threshold for binding to tryptase (mMCP-6) protein, because the percentage of E units in CS synthesized by BMMCs was 28% (40). Alternatively, the intracellular concentration of CS in HS6ST-1−/−/HS6ST-2−/−-FSMCs might be too low (only 4.7% compared with heparin) to promote the storage of tryptase.
The mechanisms of tryptase (mMCP6) loss from HS6ST-1−/−/HS6ST-2−/−-FSMCs (Table 2 and Fig. 7) are currently unknown. It was suggested that transportation of tryptase (mMCP6) into the granules might not require serglycin (51). Therefore, it may be possible that heparin-unbound tryptase (mMCP6) after being transported into the granules is degraded by some proteases in the secretory granules, because some of the lysosomal proteases, e.g. cathepsin C, cathepsin D, and cathepsin E, were found in lysosomes and MC secretory granules, which are both kept acidic (51–54). Another possible mechanism for tryptase (mMCP6) loss would be that the transported protein may be secreted from HS6ST-1−/−/HS6ST-2−/−-FSMCs due to the failure of making complexes with 6-O-sulfation-deficient heparin. In relation to this possibility, it is interesting to note, on the Western blotting membrane, slightly but significantly stained bands with somewhat lower mobility in the protein sample from HS6ST-1−/−/HS6ST-2−/−-FSMCs (Fig. 7). It has been predicted, based on the rules of von Heijine (55), that proenzymes with 10-amino acid-long propeptides are produced after the cleavage of the signal peptides when tryptase (mMCP6) is synthesized (56) and the processing mechanisms for mature tryptase is critically dependent on acidic pH and heparin (57). Therefore, the slightly stained band with the lower mobility might correspond to the proenzyme. However, further studies will be needed to clarify the mechanisms.
Although similar levels of CPA mRNA expression were observed in both WT and HS6ST-1−/−/HS6ST-2−/−-FSMCs, CPA activity was nearly absent from HS6ST-1−/−/HS6ST-2−/−-FSMCs. These data suggest that, like tryptase, CPA also requires the 6-O-sulfate residues of heparin for packaging and storage into granules. However, other possibilities could not be excluded. The loss of CPA activity in HS6ST-1−/−/HS6ST-2−/−-FSMCs may be due to defective processing of pro-CPA, because CPA processing is defective in cathepsin-E KO mice, and the activity of cathepsin-E in mast cells is strongly dependent on fully sulfated heparin, possibly because the physical colocalization of cathepsin-E and CPA is mediated by heparin (52). Therefore, it is possible that such colocalization might be functionally hampered by the 6-O-sulfate-deficient heparin in HS6ST-1−/−/HS6ST-2−/−-FSMCs. However, such a possibility appears to be insufficient to explain the substantial loss of CPA in HS6ST-1−/−/HS6ST-2−/−-FSMCs because partial CPA activity was detected in cathepsin-E KO mice (52), and other proteases might be involved in the processing of pro-CPA.
Because HS6ST-1−/−/HS6ST-2−/−-FSMCs synthesize abnormal heparin almost lacking in 6-O-sulfate residues, those heparin chains are supposed to be lower in total sulfation than those in WT-FSMCs (Table 3). Even HS6ST-1−/−/HS6ST-2−/−-FSMCs still had heparin with about 30% reduction in total sulfation. In addition, the total amount of heparin produced by HS6ST-1−/−/HS6ST-2−/−-FSMCs did not differ from those of WT-FSMCs (data not shown). Considering those together, the net negative charge (total sulfation) may have unlikely observed effects on the storage of mast cell proteases. However, the possibility that the effects are due in part to a decrease in the net negative charge can hardly be excluded.
TABLE 3.
Total sulfation from the heparin of WT, HS6ST-1−/−, HS6ST-2+/−, HS6ST-2−/−, and HS6ST-1−/−/HS6ST-2−/−-FSMCs
GAGs were isolated from FSMCs and were analyzed by reverse phase ion-pair chromatography as described under “Experimental Procedures.” Total sulfation is the average number of sulfate groups per 100 disaccharides calculated from the disaccharide analysis.
| ΔDi-HS/Hep | Number of Δdisaccharides/100 disaccharides |
||||
|---|---|---|---|---|---|
| WT | HS6ST-1−/− | HS6ST-2+/− | HS6ST-2−/− | HS6ST-1−/−/HS6ST-2−/− | |
| ΔDi-0S | 4.9 ± 3.8 | 1.35 ± 0.2 | 9.5 ± 2.9 | 9.8 ± 2.4 | 9 ± 0.6 |
| ΔDi-NS | 3.7 ± 1.4 | 2.9 ± 0.1 | 14.3 ± 1.4 | 15.8 ± 1.0 | 24.2 ± 0.5 |
| ΔDi-6S | 1.5 ± 0.5 | 0.95 ± 0.07 | 0.9 ± 0.01 | 0.8 ± 0.1 | 0.3 ± 0.07 |
| ΔDi-(N, 6)diS | 32.2 ± 5.2 | 38.55 ± 6.8 | 9.4 ± 0.1 | 7.4 ± 0.3 | 0.05 ± 0.1 |
| ΔDi-(N,2)diS | 4.5 ± 1.7 | 4.1 ± 1.1 | 40.4 ± 1.9 | 45.6 ± 2.3 | 66.2 ± 0.7 |
| ΔDi-(N, 6, 2)triS | 53.3 ± 4.6 | 52.1 ± 5.7 | 25.5 ± 2.2 | 20.6 ± 0.7 | 0.1 ± 0.1 |
| Total number of sulfate residues per 100 disaccharides (percentage) | 238 ± 12.1 (100%) | 245 ± 5.7 (103%) | 191 ± 9.5 (80.3%) | 184 ± 7.5 (77.3%) | 157 ± 1.8 (70%) |
The mRNA levels of the mast cell proteases were largely unaffected by the loss of 6-O-sulfation of heparin. Therefore, the most likely explanation for the differences observed in the activities of tryptases (mMCP6) and CPA between WT-FSMCs and HS6ST-1−/−/HS6ST-2−/−-FSMCs is that the post-translational processes that enable the active proteases to be packaged and stored in secretory granules are highly dependent on heparin. Interestingly, substantial chymase activity remained in the HS6ST-1−/−/HS6ST-2−/−-FSMCs. In WT and mutant FSMCs, the transcripts of two chymases (mMCP-4 and mMCP-5) were expressed strongly, whereas the expression of mMCP-1 and mMCP-2 was much lower than that of mMCP-4 and mMCP-5 (Fig. 6), which was consistent with previous studies showing that CTMCs express mMCP-4 and mMCP-5 but not mMCP-1 and mMCP-2 (47, 58, 59). It has been shown that mMCP-5-null mice lack CPA (60) and CPA-null mice lack mMCP-5 (61). Because CPA activity was considerably abolished in HS6ST-1−/−/HS6ST-2−/−-FSMCs, the partial loss of chymase activity in HS6ST-1−/−/HS6ST-2−/−-FSMCs that was observed in this study might be due to the loss of mMCP-5. If this were the case, the retained chymase activity in HS6ST-1−/−/HS6ST-2−/−-FSMCs would likely be from mMCP-4. Although there are several possible explanations for less effect of the defect of the 6-O-sulfate residues in heparin on chymase activity, we did show that the heparin structures required for chymase binding are different from those required for binding to tryptase (mMCP-6) and CPA.
Mast cell proteases possess a number of pivotal and individually different roles in the immune system and in diseases. Chymase plays a role in Trichinella spiralis clearance (62) and bacterial infection (63) and has been implicated as a biomarker for cardiovascular diseases (64). CPA regulates sepsis (65) and functions in degrading certain snake venom toxins (65, 66). Tryptase (mMCP6) also contributes to the innate immune response toward T. spiralis (67) and Klebsiella pneumoniae infection (68). MC tryptase was also found to have attenuated arthritic response via tryptase-heparin complexes in tryptase-KO (mMCP-6−/−) animals that developed lower inflammation and bone/cartilage erosion than did WT mice (69). Therefore, it is possible that HS6ST activity might be involved in the specific regulation of some protease activities in CTMCs. For example, a possible up-regulation of HS6ST activity in patients during arthritic inflammation might affect the treatment of this disease. Therefore, some reagents that are able to regulate HS-6-O-sulfation might have therapeutic potential in diseases involving mast cell proteases.
This work was supported by Grants-in-aid for Scientific Research C 20570113 (to H. H.), 22590296 (to N. N. and K. K.), and 23570148 (to K. K.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, Grant-in-aid for Scientific Research on Priority Areas 14082206 (to K. K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and in part by Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (2011–2015), S1101027.
H. Habuchi, unpublished data.
- HS
- heparan sulfate
- GAG
- glycosaminoglycan
- CS
- chondroitin sulfate
- IdoUA
- iduronic acid
- GlcUA
- glucuronic acid
- HexA
- hexuronic acid
- GlcNSO3
- N-sulfoglucosamine
- HS6ST
- HS6-O-sulfotransferase
- MC
- mast cell
- CTMC
- connective tissue-type MC
- FSMC
- fetal skin-derived MC
- BMMC
- bone marrow-derived MC
- MCP
- mast cell protease
- CPA
- carboxypeptidase A
- CS-E
- chondroitin sulfate E type
- AS-D
- naphthol 3-hydroxy-2-naphthoic-o-toluidide chloroacetate.
REFERENCES
- 1. Esko J. D., Lindahl U. (2001) Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 [DOI] [PubMed] [Google Scholar]
- 3. Perrimon N., Bernfield M. (2000) Specificities of heparan sulphate proteoglycans in developmental processes. Nature 404, 725–728 [DOI] [PubMed] [Google Scholar]
- 4. Grobe K., Ledin J., Ringvall M., Holmborn K., Forsberg E., Esko J. D., Kjellén L. (2002) Heparan sulfate and development: differential roles of the N-acetylglucosamine N-deacetylase/N-sulfotransferase isozymes. Biochim. Biophys. Acta 1573, 209–215 [DOI] [PubMed] [Google Scholar]
- 5. Esko J. D., Selleck S. B. (2002) Order out of chaos. Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 6. Habuchi H., Habuchi O., Kimata K. (2004) Sulfation pattern in glycosaminoglycan. Does it have a code? Glycoconj. J. 21, 47–52 [DOI] [PubMed] [Google Scholar]
- 7. Bishop J. R., Schuksz M., Esko J. D. (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 8. Habuchi H., Tanaka M., Habuchi O., Yoshida K., Suzuki H., Ban K., Kimata K. (2000) The occurrence of three isoforms of heparan sulfate 6-O-sulfotransferase having different specificities for hexuronic acid adjacent to the targeted N-sulfoglucosamine. J. Biol. Chem. 275, 2859–2868 [DOI] [PubMed] [Google Scholar]
- 9. Habuchi H., Kimata K. (2010) Mice deficient in heparan sulfate 6-O-sulfotransferase-1. Prog. Mol. Biol. Transl. Sci. 93, 79–111 [DOI] [PubMed] [Google Scholar]
- 10. Habuchi H., Miyake G., Nogami K., Kuroiwa A., Matsuda Y., Kusche-Gullberg M., Habuchi O., Tanaka M., Kimata K. (2003) Biosynthesis of heparan sulphate with diverse structures and functions. Two alternatively spliced forms of human heparan sulphate 6-O-sulphotransferase-2 having different expression patterns and properties. Biochem. J. 371, 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habuchi H., Nagai N., Sugaya N., Atsumi F., Stevens R. L., Kimata K. (2007) Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J. Biol. Chem. 282, 15578–15588 [DOI] [PubMed] [Google Scholar]
- 12. Izvolsky K. I., Lu J., Martin G., Albrecht K. H., Cardoso W. V. (2008) Systemic inactivation of Hs6st1 in mice is associated with late postnatal mortality without major defects in organogenesis. Genesis 46, 8–18 [DOI] [PubMed] [Google Scholar]
- 13. Pratt T., Conway C. D., Tian N. M., Price D. J., Mason J. O. (2006) Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm. J. Neurosci. 26, 6911–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tornberg J., Sykiotis G. P., Keefe K., Plummer L., Hoang X., Hall J. E., Quinton R., Seminara S. B., Hughes V., Van Vliet G., Van Uum S., Crowley W. F., Habuchi H., Kimata K., Pitteloud N., Bülow H. E. (2011) Heparan sulfate 6-O-sulfotransferase 1, a gene involved in extracellular sugar modifications, is mutated in patients with idiopathic hypogonadotrophic hypogonadism. Proc. Natl. Acad. Sci. U.S.A. 108, 11524–11529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bink R. J., Habuchi H., Lele Z., Dolk E., Joore J., Rauch G. J., Geisler R., Wilson S. W., den Hertog J., Kimata K., Zivkovic D. (2003) Heparan sulfate 6-O-sulfotransferase is essential for muscle development in zebrafish. J. Biol. Chem. 278, 31118–31127 [DOI] [PubMed] [Google Scholar]
- 16. Chen E., Stringer S. E., Rusch M. A., Selleck S. B., Ekker S. C. (2005) A unique role for 6-O-sulfation modification in zebrafish vascular development. Dev. Biol. 284, 364–376 [DOI] [PubMed] [Google Scholar]
- 17. Bülow H. E., Hobert O. (2004) Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron 41, 723–736 [DOI] [PubMed] [Google Scholar]
- 18. Kamimura K., Fujise M., Villa F., Izumi S., Habuchi H., Kimata K., Nakato H. (2001) Drosophila heparan sulfate 6-O-sulfotransferase (dHS6ST) gene. Structure, expression, and function in the formation of the tracheal system. J. Biol. Chem. 276, 17014–17021 [DOI] [PubMed] [Google Scholar]
- 19. Kamimura K., Koyama T., Habuchi H., Ueda R., Masu M., Kimata K., Nakato H. (2006) Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J. Cell Biol. 174, 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi T., Habuchi H., Nogami K., Ashikari-Hada S., Tamura K., Ide H., Kimata K. (2010) Functional analysis of chick heparan sulfate 6-O-sulfotransferases in limb bud development. Dev. Growth Differ. 52, 146–156 [DOI] [PubMed] [Google Scholar]
- 21. Sugaya N., Habuchi H., Nagai N., Ashikari-Hada S., Kimata K. (2008) 6-O-Sulfation of heparan sulfate differentially regulates various fibroblast growth factor-dependent signalings in culture. J. Biol. Chem. 283, 10366–10376 [DOI] [PubMed] [Google Scholar]
- 22. Xing W., Austen K. F., Gurish M. F., Jones T. G. (2011) Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc. Natl. Acad. Sci. U.S.A. 108, 14210–14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Metcalfe D. D., Baram D., Mekori Y. A. (1997) Mast cells. Physiol. Rev. 77, 1033–1079 [DOI] [PubMed] [Google Scholar]
- 24. Pejler G., Rönnberg E., Waern I., Wernersson S. (2010) Mast cell proteases. Multifaceted regulators of inflammatory disease. Blood 115, 4981–4990 [DOI] [PubMed] [Google Scholar]
- 25. Welle M. (1997) Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukocyte Biol. 61, 233–245 [DOI] [PubMed] [Google Scholar]
- 26. Coussens L. M., Raymond W. W., Bergers G., Laig-Webster M., Behrendtsen O., Werb Z., Caughey G. H., Hanahan D. (1999) Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 13, 1382–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller H. R., Pemberton A. D. (2002) Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology 105, 375–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolset S. O., Prydz K., Pejler G. (2004) Intracellular proteoglycans. Biochem. J. 379, 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prieto-García A., Zheng D., Adachi R., Xing W., Lane W. S., Chung K., Anderson P., Hansbro P. M., Castells M., Stevens R. L. (2012) Mast cell-restricted mouse and human tryptase·heparin complexes hinder thrombin-induced coagulation of plasma and the generation of fibrin by proteolytically destroying fibrinogen. J. Biol. Chem. 287, 7834–7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNeil H. P., Adachi R., Stevens R. L. (2007) Mast cell-restricted tryptases. Structure and function in inflammation and pathogen defense. J. Biol. Chem. 282, 20785–20789 [DOI] [PubMed] [Google Scholar]
- 31. Enerbäck L., Kolset S. O., Kusche M., Hjerpe A., Lindahl U. (1985) Glycosaminoglycans in rat mucosal mast cells. Biochem. J. 227, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kolset S. O., Tveit H. (2008) Serglycin-structure and biology. Cell. Mol. Life Sci. 65, 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forsberg E., Pejler G., Ringvall M., Lunderius C., Tomasini-Johansson B., Kusche-Gullberg M., Eriksson I., Ledin J., Hellman L., Kjellén L. (1999) Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400, 773–776 [DOI] [PubMed] [Google Scholar]
- 34. Humphries D. E., Wong G. W., Friend D. S., Gurish M. F., Qiu W.-T., Huang C., Sharpe A. H., Stevens R. L. (1999) Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400, 769–772 [DOI] [PubMed] [Google Scholar]
- 35. Feyerabend T. B., Li J. P., Lindahl U., Rodewald H. R. (2006) Heparan sulfate C5-epimerase is essential for heparin biosynthesis in mast cells. Nat. Chem. Biol. 2, 195–196 [DOI] [PubMed] [Google Scholar]
- 36. HajMohammadi S., Enjyoji K., Princivalle M., Christi P., Lech M., Beeler D., Rayburn H., Schwartz J. J., Barzegar S., de Agostini A. I., Post M. J., Rosenberg R. D., Shworak N. W. (2003) Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. J. Clin. Invest. 111, 989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada N., Matsushima H., Tagaya Y., Shimada S., Katz S. I. (2003) Generation of a large number of connective tissue type mast cells by culture of murine fetal skin cells. J. Invest. Dermatol. 121, 1425–1432 [DOI] [PubMed] [Google Scholar]
- 38. Toyoda H., Kinoshita-Toyoda A., Selleck S. B. (2000) Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J. Biol. Chem. 275, 2269–2275 [DOI] [PubMed] [Google Scholar]
- 39. Stevens R. L., Adachi R. (2007) Protease-proteoglycan complexes of mouse and human mast cells and importance of their β-tryptase-heparin complexes in inflammation and innate immunity. Immunol. Rev. 217, 155–167 [DOI] [PubMed] [Google Scholar]
- 40. Ohtake-Niimi S., Kondo S., Ito T., Kakehi S., Ohta T., Habuchi H., Kimata K., Habuchi O. (2010) Mice deficient in N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase are unable to synthesize chondroitin/dermatan sulfate containing N-acetylgalactosamine 4,6-bissulfate residues and exhibit decreased protease activity in bone marrow-derived mast cells. J. Biol. Chem. 285, 20793–20805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hunt J. E., Stevens R. L., Austen K. F., Zhang J., Xia Z., Ghildyal N. (1996) Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J. Biol. Chem. 271, 2851–2855 [DOI] [PubMed] [Google Scholar]
- 42. Abrink M., Grujic M., Pejler G. (2004) Serglycin is essential for maturation of mast cell secretory granule. J. Biol. Chem. 279, 40897–40905 [DOI] [PubMed] [Google Scholar]
- 43. Hallgren J., Karlson U., Poorafshar M., Hellman L., Pejler G. (2000) Mechanism for activation of mouse mast cell tryptase. Dependence on heparin and acidic pH for formation of active tetramers of mouse mast cell protease 6. Biochemistry 39, 13068–13077 [DOI] [PubMed] [Google Scholar]
- 44. Hallgren J., Spillmann D., Pejler G. (2001) Structural requirements and mechanism for heparin-induced activation of a recombinant mouse mast cell tryptase, mouse mast cell protease-6. Formation of active tryptase monomers in the presence of low molecular weight heparin. J. Biol. Chem. 276, 42774–42781 [DOI] [PubMed] [Google Scholar]
- 45. Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. (1982) Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J. Biol. Chem. 257, 7229–7236 [PubMed] [Google Scholar]
- 46. Henningsson F., Ledin J., Lunderius C., Wilén M., Hellman L., Pejler G. (2002) Altered storage of proteases in mast cells from mice lacking heparin. A possible role for heparin in carboxypeptidase A processing. Biol. Chem. 383, 793–801 [DOI] [PubMed] [Google Scholar]
- 47. Pejler G., Abrink M., Ringvall M., Wernersson S. (2007) Mast cell proteases. Adv. Immunol. 95, 167–255 [DOI] [PubMed] [Google Scholar]
- 48. Thompson H. L., Schulman E. S., Metcalfe D. D. (1988) Identification of chondroitin sulfate E in human lung mast cells. J. Immunol. 140, 2708–2713 [PubMed] [Google Scholar]
- 49. Stevens R. L., Fox C. C., Lichtenstein L. M., Austen K. F. (1988) Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc. Natl. Acad. Sci. U.S.A. 85, 2284–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lidholt K., Eriksson I., Kjellén L. (1995) Heparin proteoglycans synthesized by mouse mastocytoma contain chondroitin sulphate. Biochem. J. 311, 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Henningsson F., Hergeth S., Cortelius R., Abrink M., Pejler G. (2006) A role for serglycin proteoglycan in granular retention and processing of mast cell secretory granule components. FEBS J. 273, 4901–4912 [DOI] [PubMed] [Google Scholar]
- 52. Henningsson F., Yamamoto K., Saftig P., Reinheckel T., Peters C., Knight S. D., Pejler G. (2005) A role for cathepsin E in the processing of mast-cell carboxypeptidase A. J. Cell Sci. 118, 2035–2042 [DOI] [PubMed] [Google Scholar]
- 53. Dragonetti A., Baldassarre M., Castino R., Démoz M., Luini A., Buccione R., Isidoro C. (2000) The lysosomal protease cathepsin D is efficiently sorted to and secreted from regulated secretory compartments in the rat basophilic/mast cell line RBL. J. Cell Sci. 113, 3289–3298 [DOI] [PubMed] [Google Scholar]
- 54. Wolters P. J., Laig-Webster M., Caughey G. H. (2000) Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am. J. Respir. Cell Mol. Biol. 22, 183–190 [DOI] [PubMed] [Google Scholar]
- 55. von Heijne G. (1984) How signal sequences maintain cleavage specificity. J. Mol. Biol. 173, 243–251 [DOI] [PubMed] [Google Scholar]
- 56. Reynolds D. S., Gurley D. S., Austen K. F., Serafin W. E. (1991) Cloning of the cDNA and gene of mouse mast cell protease-6. Transcription by progenitor mast cells and mast cells of the connective tissue subclass. J. Biol. Chem. 266, 3847–3853 [PubMed] [Google Scholar]
- 57. Sakai K., Ren S., Schwartz L. B. (1996) A novel heparin-dependent processing pathway for human tryptase. Autocatalysis followed by activation with dipeptidyl peptidase I. J. Clin. Invest. 97, 988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang R. Y., Blom T., Hellman L. (1991) Cloning and structural analysis of MMCP-1, MMCP-4, and MMCP-5, three mouse mast cell-specific serine proteases. Eur. J. Immunol. 21, 1611–1621 [DOI] [PubMed] [Google Scholar]
- 59. Serafin W. E., Reynolds D. S., Rogelj S., Lane W. S., Conder G. A., Johnson S. S., Austen K. F., Stevens R. L. (1990) Identification and molecular cloning of a novel mouse mucosal mast cell serine protease. J. Biol. Chem. 265, 423–429 [PubMed] [Google Scholar]
- 60. Pejler G., Knight S. D., Henningsson F., Wernersson S. (2009) Novel insights into the biological function of mast cell carboxypeptidase A. Trends Immunol. 8, 401–408 [DOI] [PubMed] [Google Scholar]
- 61. Feyerabend T. B., Hausser H., Tietz A., Blum C., Hellman L., Straus A. H., Takahashi H. K., Morgan E. S., Dvorak A. M., Fehling H. J., Rodewald H. (2005) Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol. Cell Biol. 25, 6199–6210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Knight P. A., Wright S. H., Lawrence C. E., Paterson Y. Y., Miller H. R. (2000) Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192, 1849–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orinska Z., Maurer M., Mirghomizadeh F., Bulanova E., Metz M., Nashkevich N., Schiemann F., Schulmistrat J., Budagian V., Giron-Michel J., Brandt E., Paus R., Bulfone-Paus S. (2007) IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat. Med. 13, 927–934 [DOI] [PubMed] [Google Scholar]
- 64. Sun J., Zhang J., Lindholt J. S., Sukhova G. K., Liu J., He A., Abrink M., Pejler G., Stevens R. L., Thompson R. W., Ennis T. L., Gurish M. F., Libby P., Shi G. P. (2009) Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation 120, 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schneider L. A., Schlenner S. M., Feyerabend T. B., Wunderlin M., Rodewald H. R. (2007) Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J. Exp. Med. 204, 2629–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Metz M., Piliponsky A. M., Chen C. C., Lammel V., Abrink M., Pejler G., Tsai M., Galli S. J. (2006) Mast cells can enhance resistance to snake and honeybee venoms. Science 313, 526–530 [DOI] [PubMed] [Google Scholar]
- 67. Shin K., Watts G. F., Oettgen H. C., Friend D. S., Pemberton A. D., Gurish M. F., Lee D. M. (2008) Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 180, 4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., Adachi R. (2007) The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 69. Shin K., Nigrovic P. A., Crish J., Boilard E., McNeil H. P., Larabee K. S., Adachi R., Gurish M. F., Gobezie R., Stevens R. L., Lee D. M. (2009) Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J. Immunol. 182, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]