Background: APC is modified with Lys-63-linked polyubiquitin when bound to Axin in an assembled β-catenin destruction complex.
Results: HectD1 E3 ligase modifies APC with Lys-63-linked ubiquitin chains to facilitate the APC-Axin interaction.
Conclusion: HectD1 is a candidate E3 ligase for APC.
Significance: The identification of HectD1 could lead to a better understanding of APC function.
Keywords: Deubiquitylation, Signal Transduction, Tumor Suppressor Gene, Ubiquitin Ligase, Ubiquitylation, β-Catenin Destruction Complex, APC, Axin, HectD1 E3 Ligase, Lys-63-linked Ubiquitin Chains
Abstract
The adenomatous polyposis coli (APC) protein functions as a negative regulator of the Wnt signaling pathway. In this capacity, APC forms a “destruction complex” with Axin, CK1α, and GSK3β to foster phosphorylation of the Wnt effector β-catenin earmarking it for Lys-48-linked polyubiquitylation and proteasomal degradation. APC is conjugated with Lys-63-linked ubiquitin chains when it is bound to Axin, but it is unclear whether this modification promotes the APC-Axin interaction or confers upon APC an alternative function in the destruction complex. Here we identify HectD1 as a candidate E3 ubiquitin ligase that modifies APC with Lys-63 polyubiquitin. Knockdown of HectD1 diminished APC ubiquitylation, disrupted the APC-Axin interaction, and augmented Wnt3a-induced β-catenin stabilization and signaling. These results indicate that HectD1 promotes the APC-Axin interaction to negatively regulate Wnt signaling.
Introduction
The Wnt signaling pathway controls developmental cell fate decisions and adult tissue homeostasis (1). Aberrant activation of Wnt signaling is also linked to a variety of human cancers (2). Many core components of this important pathway have been identified, but the regulatory post-translational modifications that provide mechanistic insights to their function are still being uncovered. In this respect, it is increasingly apparent that ubiquitin modification regulates all steps of the Wnt pathway (3). Catalyzed by a cascade of E1, E2, and E3 enzymes, the ∼8-kDa protein ubiquitin is conjugated to protein substrates as a single moiety (monoubiquitin), as polyubiquitin chains linked through one of seven internal lysine residues of ubiquitin (Lys-6, Lys-11, Lys-27, Lys-29, Lys-33, Lys-48, and Lys-63), or as linear head to tail chains linked via the C-terminal glycine of one ubiquitin to the N-terminal methionine residue of the next. The array of different ubiquitin adducts reflects their diverse regulatory functions. These include the targeting of protein substrates for degradation, subcellular localization, or assembly within multiprotein complexes that promote signal transduction (4–6).
The involvement of ubiquitin in canonical Wnt signaling was first demonstrated for the transcriptional co-activator β-catenin (7). Conjugated with Lys-48-linked polyubiquitin by the β-TrCP E3 ubiquitin ligase, β-catenin is rapidly degraded by the proteasome in unstimulated cells (8, 9). The Lys-48 polyubiquitylation of β-catenin is coupled to its phosphorylation in a “destruction complex” that comprises the tumor suppressors adenomatous polyposis coli (APC)3 and Axin and the kinases GSK3β and CK1α (10–13). When bound to Axin in unstimulated cells, APC is modified with Lys-63-linked ubiquitin chains, which appears necessary for efficient turnover of β-catenin (14). Thus, both Lys-48- and Lys-63-linked chains are involved in the negative regulation of Wnt signaling, and the formation of these ubiquitin adducts on β-catenin and APC are Wnt-dependent (14). The stabilities of Axin and Dishevelled, an upstream positive component of the pathway, are regulated by the E3 ligase activities of RNF146 and KLHL12, respectively. The KLHL12-mediated proteasomal degradation of Dishevelled is a Wnt-regulated event, but it is unclear whether the same is true of RNF146-mediated Axin degradation (15–17). Like KLHL12, the recently described ZNRF3 represents another E3 ubiquitin ligase that negatively regulates proximal events in the Wnt pathway. ZNRF3 is a transmembrane ligase that targets the Wnt receptors Frizzled and LRP6 for ubiquitin-dependent endocytosis and degradation (18). Binding of canonical Wnt ligands to Frizzled and LRP5/6 inactivates the β-catenin destruction complex through APC-Axin dissociation (14, 19). This results in nuclear accumulation of β-catenin, which binds T cell factor/lymphoid enhancer factor transcription factors to activate the expression of Wnt target genes. The E3 ligases NARF and Jade-1 promote turnover of T cell factor/lymphoid enhancer factor and nuclear β-catenin, respectively (20–22), suggesting negative regulation of Wnt signaling in the nucleus by the ubiquitin-proteasome pathway.
In contrast to the several E3 ubiquitin ligases that function as negative regulators, a few deubiquitylating enzymes (DUBs) have been described that confer positive regulation of the Wnt pathway. Through its DUB activity, UBPY facilitates the recycling of ubiquitylated Frizzled receptors from endosomal compartments to the plasma membrane (23). Further downstream, Trabid is a DUB that appears to promote Wnt signaling in the nucleus, but its relevant target(s) in this compartment have not been identified (24). Interestingly, siRNA knockdown of Trabid caused accumulation of polyubiquitin chains on APC, implicating APC as an enzymatic target (24). We have established that APC is modified predominantly with Lys-63-linked polyubiquitin in cells, relative to Lys-11- and Lys-48-linked chains (14), but the functional significance of Lys-63 adducts on APC remains unclear. To demonstrate an important role for APC Lys-63 adducts in Wnt pathway regulation, we initiated a study to identify the E3 ligase responsible for the Lys-63 polyubiquitylation of APC.
EXPERIMENTAL PROCEDURES
Cells and Reagents
HEK293, HEK293T, and hTERT-RPE1 cells were cultured at 37 °C with 5% CO2 in appropriate media supplemented with 10% FBS and 2 mm l-glutamine (Invitrogen). The cells were transfected with siRNAs (50 nm total) using RNAiMAX as instructed (Invitrogen). Lipofectamine 2000 (Invitrogen) was used for plasmid transfection or co-transfection of plasmids and siRNAs in HEK293/T cells. FuGENE 6 HD (Roche Applied Science) was used for plasmid transfection in RPE cells. Epitope-tagged Trabid, HectD1, APC, Tankyrase (TNKS1) and RNF146 constructs have been described (17, 24, 25). Recombinant mouse Wnt3a was purchased from R & D Systems. Proteasome inhibitors MG132 and bortezomib were purchased from Tocris Bioscience and Fisher Scientific, respectively.
Immunoprecipitation
Immunoprecipitation with polyubiquitin linkage-specific antibodies and co-immunoprecipitation assays were performed as described (14). Immunoprecipitation of epitope-tagged HectD1 and Trabid from transfected cells was performed using 20 μl of anti-HA 3F10 (Roche Applied Science) or anti-FLAG M2-Sepharose (Sigma) slurry for every 1 mg of total cell lysate. Immunoprecipitation of FLAG-Trabid for mass spectrometry analysis: HEK293 cells in 100-mm dishes were transfected with 10 μg of the indicated expression vector (four dishes per construct) for 48 h. All subsequent steps were performed on ice or at 4 °C unless indicated otherwise. The cells were lysed with 1 ml of 1% Nonidet P-40 lysis buffer pH 8.0 (LB: 50 mm Tris-HCl, pH 8.0, 120 mm NaCl, 1% Nonidet P-40, 1 mm EDTA) per dish. The lysates were pooled (∼5 ml) and precleared for 1 h with 1 ml of protein G-Sepharose slurry (Invitrogen). Precleared lysates were mixed with 600 μl of 50% anti-FLAG M2 affinity gel (Ezview Red; Sigma) overnight. The beads were subjected to the following washing steps: (a) 10 ml of LB for 1 h; (b) 10 ml of LB + 250 mm NaCl for 30 min; (c) 10 ml of PBS for 30 min; and (d) 10 ml of PBS for 15 min. Immune complexes were eluted from beads with 30 μl of 2× LDS sample buffer (Invitrogen) at 90 °C for 10 min.
Protein Identification and Quantitative Analysis of Ubiquitin Linkages by Mass Spectrometry
FLAG-tagged and co-eluting proteins were separated on a 3–8% Tris-acetate SDS-PAGel (Invitrogen) and stained with Coomassie Brilliant Blue R-250 (Serva Electrophoresis). Gel regions were excised, processed for trypsin digestion, and combined with ubiquitin-AQUA peptides as described (26). Processed samples were analyzed on an LTQ-Orbitrap XL (ThermoFisher) equipped with a Michrom ADVANCE source (Auburn) in combination with a Waters nanoAcquity UPLC system (Milford) (26). Quantitation was performed at the MS level by integrating areas of peaks corresponding to heavy and light m/z values of the most abundant charge states of each peptide. Peaks for integration were obtained through application of extracted ion chromatograms over 10-ppm mass intervals using the Xcalibur QualBrowser v2.07 (ThermoFisher). Xcalibur raw files were converted to mzxml for loading into a relational database. MS/MS data were searched using Mascot (Matrix Sciences) against a concatenated target-decoy database of human proteins (Uniprot) and common contaminants. Linear discriminant analysis was used to filter peptide results from each run to 1% false discovery rate prior to grouping run data to compare protein identifications between each of the three immunoprecipitations.
Protein Purification and in Vitro Binding Assays
A PCR-amplified DNA fragment encoding residues 318–818 encompassing the ARD domain of human APC, incorporating an N-terminal Glu-Glu tag, was cloned into an in-house expression vector pST239. The protein was expressed in an in-house Escherichia coli strain 58F3. Cell pellets were resuspended in 50 mm Tris-HCl, pH 8.6, 5 mm EDTA, 0.5 mm TCEP, and Complete protease inhibitor mixture (Roche Applied Science) and then lysed with a Polytron homogenizer followed by three passes through a microfluidizer. The lysate was centrifuged at 40,000 rpm using a 45 Ti rotor (Beckman) at 4 °C for 1 h. The supernatant was loaded over a 5-ml HiTrap Q FF column (GE Healthcare). Bound protein was eluted with a 0–1 m NaCl gradient. APC ARD fractions were pooled and passed over a packed 1-ml anti-Glu-Glu affinity column (Covance), eluted with 0.1 m glycine, pH 2.8, and neutralized using 1:10 volume 1 m Tris-HCl, pH 8.6. The protein was then dialyzed into 25 mm Tris-HCl, pH 8.0, 140 mm NaCl, 2 mm TCEP. Other Glu-Glu-tagged APC proteins were expressed in Sf9 insect cells and purified as described (27). Purified proteins (1 μg) were combined with 1 mg of lysates from transfected HEK293 cells expressing the indicated epitope-tagged proteins in a total volume of 1 ml of Nonidet P-40 lysis buffer pH 8.0. Binding reactions were performed at 4 °C for 2 h, precipitated with 30 μl of anti-Glu-Glu affinity agarose, then washed, and eluted with 25 μl of 2× LDS sample buffer (Invitrogen) at 90 °C for 10 min.
Antibodies
Polyubiquitin linkage-specific antibodies have been described (28, 29). We generated rabbit polyclonal antibodies to HectD1 by immunizing rabbits with a 24-mer, C-terminal peptide sequence common to human and mouse HectD1: DDESRHVDLGGGLKPPGYYVQRSC. Anti-serum were affinity-purified prior to use (Yenzym). Monoclonal Trabid antibodies were generated in-house using a purified protein comprising the first 111 residues of mouse Trabid as the immunogen: clones 5B3 and 4F9 (mouse IgG2a/IgG3) were used for immunoprecipitation, and clones 2C9 and 6D4 (rat IgG2a) were used for Western blotting. Commercial antibodies: anti-APC ALi 12-28 (Santa Cruz), anti-β-catenin (Millipore), anti-Striatin (BD Biosciences), anti-Striatin 3 (SG2NA, Novus Biologicals), anti-Axin1 C95H11, anti-β-TrCP D13F10, anti-ubiquitin P4D1, anti-Glu-Glu (Cell Signaling), anti-IQGAP1, anti-CtBP1 (Bethyl Laboratories), anti FLAG M2, anti-β-actin-HRP AC-15, anti-β-tubulin (Sigma), and anti-HA 3F10 (Roche Applied Science).
siRNAs
Individual siRNA duplexes were purchased from Dharmacon. Control siRNA: nontargeting siRNA 1 (catalog no. D-001810-01-20). HectD1 siRNAs (only sense strand shown) were: 1, AGAUAAAGGUGGUGAUAUA; 2, GAGAACACUUGGAGAGAUU; 3, GUUAAUAGCUGUACUAGAA; and 4, GAAAGGGACAUGCAACUAA. Trabid siRNAs were: 2, AGAGGUGUCUCAACAAGCA; 3, CCAAAGACCUAGUGGAACA; 4, GGGAGAAACUUUAGGAUAU; 5, AGACCUAGUGGAACAAUUA; 6, CAACAAGCAGCAAAGUGUA; 7, GAAGUACGCUUGCUGAAUC; 8, GACCAAGGGUGAAAUCUUC; APC, GACAAGAGCUAGAAGAUAA; β-TrCP, AGAUAAUACCAGAGAAGAA; Striatin, GCACAGAGGCUGAAGUUAA; Striatin 3, GCAAAAGGGACAAGAAAUA; and Striatin 4, GAUCAAGAUGCUAGAGUAU. The oligonucleotides targeting Ubc13 and the three isoforms of UbcH5 have been described (30).
Wnt Reporter Assays and Quantitative RT-PCR
TOP-Flash luciferase reporter assays in HEK293 cells were performed as described (24). For quantitative RT-PCR analysis, RPE cells were grown in 6-well dishes (BD Biosciences) and harvested for RNA extraction (Qiagen RNeasy kit) following transfection and Wnt3a treatment where indicated. A one-step RT-PCR mix was used to quantify the expression of specific transcripts with TaqMan TAMRA probes in a 7900 HT real time PCR machine (Applied Biosystems). Target mRNA levels were normalized to β-actin mRNA.
Immunofluorescence Microscopy
Cells grown on glass coverslips or in Lab-Tek 4-well chamber slides were fixed with 4% paraformaldehyde in PBS, permeabilized with 0.2% Triton X-100 (Sigma), and blocked with 5% normal goat serum. The cells were incubated with primary antibodies diluted in 5% normal goat serum. To achieve greater signal fidelity for EGFP-APC, we incubated EGFP-APC-transfected cells with rabbit anti-GFP (Millipore), followed by Alexa Fluor-conjugated secondary antibodies (Invitrogen). The localization of HA-HectD1 in transfected cells was detected with anti-HA 3F10 (Roche Applied Science). ProLong Gold antifade reagent with DAPI (Invitrogen) was used to mount coverslips to microscope slides. Images were acquired with an Olympus BX61 upright wide field microscope or a Leica TCS SPE confocal microscope using Slidebook (Intelligent Imaging Innovations) or LAS AF (Leica) acquisition software.
RESULTS
Identification of E3 Ubiquitin Ligase HectD1 in a Trabid IP-MS Experiment
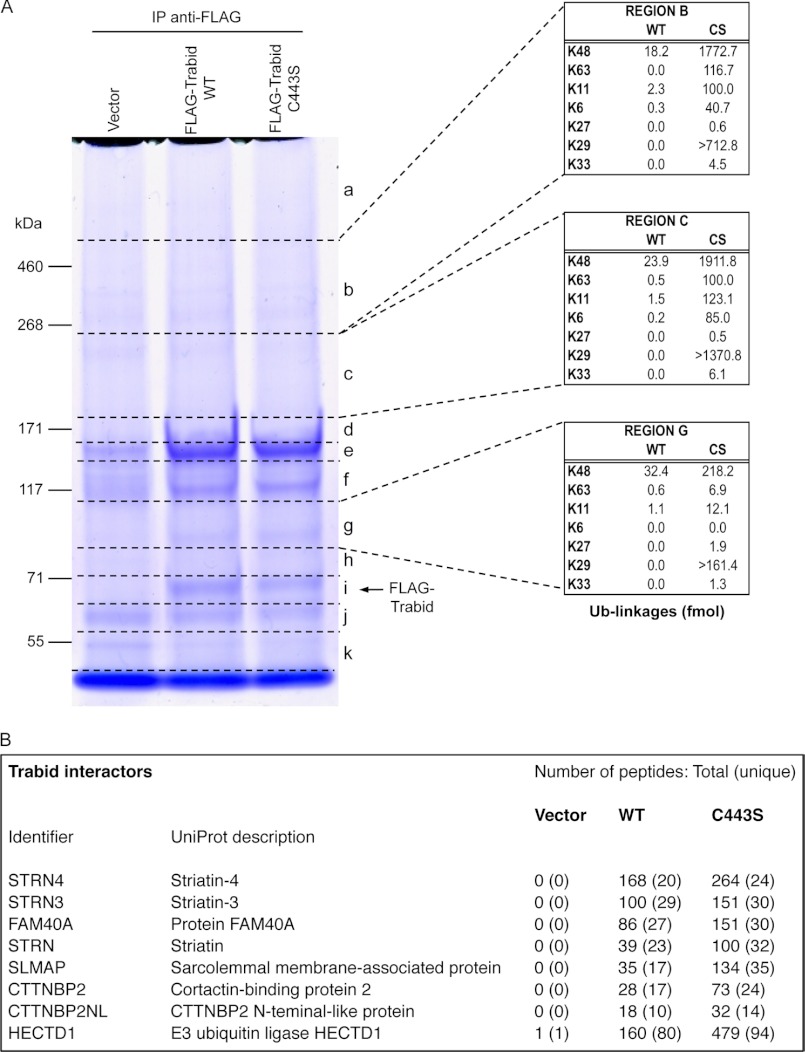
E3 ubiquitin ligases and DUBs often exist as pairs in cells to regulate, through (poly)ubiquitin addition and removal, the opposing activities of their respective substrates (31). Given that APC is a putative substrate of the DUB Trabid (24), we reasoned that a Trabid-associated E3 ligase might exhibit specific ubiquitin ligase activity toward APC. To identify new Trabid interactors and substrates, we performed an IP of FLAG-tagged, WT Trabid or a DUB-inactive version, C443S, from transfected HEK293 cells (Fig. 1A). MS analysis of discreet regions from the gel-resolved IPs (Fig. 1A, regions a–k) revealed several proteins that co-precipitated specifically with FLAG-Trabid WT and C443S, but not the vector control IP (Fig. 1B). These Trabid-interacting proteins are known components of the STRIPAK (Striatin-interacting phosphatase and kinase) complex and include the Striatin family of WD40 domain proteins that function as regulatory subunits of protein phosphatase PP2A; factors that regulate cortical actin cytoskeleton dynamics: cortactin-binding protein 2, CTTNBP2NL, and FAM40A; and the centrosome-associated protein SLMAP (32–34). Interestingly, we did not find any PP2A subunits in the Trabid IPs, suggesting mutually exclusive or transient association of subcomplexes of STRIPAK to Trabid and PP2A (33). All identified STRIPAK proteins were enriched in the C443S IP as indicated by the greater number of their respective total peptides relative to the WT IP (Fig. 1B). Mutant Trabid C443S has been shown to co-precipitate an abundance of endogenous polyubiquitylated proteins (24). This suggests that Trabid's interaction with one or more members of the STRIPAK complex is dependent, in part, on polyubiquitin.
FIGURE 1.
A, IP-MS identification of Trabid interactors and substrates. Immunoprecipitation (IP) of WT FLAG-Trabid and its C443S DUB-inactive version from transfected HEK293 cells. IPs, including an empty vector control, were resolved by SDS-PAGE and stained with Coomassie. Regions a–k of the gel were excised for MS and analyzed in the presence of isotopically labeled internal standards against various forms of polyubiquitin. The abundance of linkages measured (fmol) in select gel regions from WT and C443S (CS) IPs is displayed in the adjacent tables. The abundance reported for Lys-29 linkages represents the fraction of the signal detectable in the form of the K29-short (AK*IQDK) peptide and is an underestimate of the total Lys-29 signal. B, MS/MS analysis of the gel-resolved IPs identified peptide sequences matching several components of the STRIPAK complex, which co-eluted specifically with the Trabid IPs and not the vector control IP. The E3 ubiquitin ligase HectD1 was also identified as a specific Trabid-associated protein.
When the presence of different polyubiquitin linkages were examined, we found among the peptide spectral matches observed in the C443S Trabid IP several corresponding to an extended sequence of the Lys-29 GG peptide, AK*IQDKEGIPPDQQR (K29-long). No peptide spectral matches were identified for K29-long in the vector control or WT Trabid IP, nor were any observed for the adjacent K33-GG peptide. Previous studies have indicated that incomplete proteolysis often occurs adjacent to lysine 33 of ubiquitin because of the flanking acidic residues. Using an established method (26), we quantified the abundance of ubiquitin linkages that co-precipitated with Trabid in several regions of the Coomassie-stained gel (Fig. 1A). In regions b and c of the C443S IP, Lys-48-linked ubiquitin was ∼15-fold more abundant than Lys-63- and Lys-11-linked chains. We also found that region a (tabulated data not shown) was the most enriched with Lys-63 chains (253.7 fmol), but this was still ∼2.8-fold less than Lys-48 linkages (724.1 fmol) in this same region. The values from the K29-short peptide (AK*IQDK) represented between 40 and 70% the abundance of Lys-48 linkages across regions b, c, and g (Fig. 1A). The observed abundance of K29-long peptides and the expectation of incomplete proteolysis suggest that our quantification of K29-short underestimates the total amount of Lys-29 linkages in the sample. Together, our analysis indicates that Lys-29 chains are of equal or higher abundance than Lys-48 chains in the C443S Trabid IP. Appreciable amounts of Lys-11 and Lys-63 chains were also co-precipitated and, like Lys-29 and Lys-48 chains, could represent adducts conjugated either to FLAG-Trabid C443S or co-precipitating proteins.
Consistent with a previous IP-MS analysis of epitope-tagged WT Trabid in HEK293 cells (35), we identified the E3 ubiquitin ligase HectD1 as a specific Trabid-associated protein. The number of total HectD1 peptides was three times more abundant in the C443S IP compared with the WT Trabid IP, indicating that the Trabid-HectD1 interaction is also mediated partly through polyubiquitin chains (Fig. 1B). A member of the HECT superfamily of E3 ubiquitin ligases, HectD1 is required for neural tube closure during mouse embryonic development (36). Accordingly, HectD1 regulates the extracellular secretion of Hsp90, its only known substrate, to control the migration and organization of cranial mesenchyme cells (25). Ankyrin repeats in the N-terminal half of HectD1 and Sad1/UNC-like, Mib/Herc2 domains in its central region are predicted to mediate interactions with partner proteins and/or confer substrate specificity. A highly conserved, ∼350 residue HECT catalytic domain is present at the extreme C terminus (Fig. 2A).
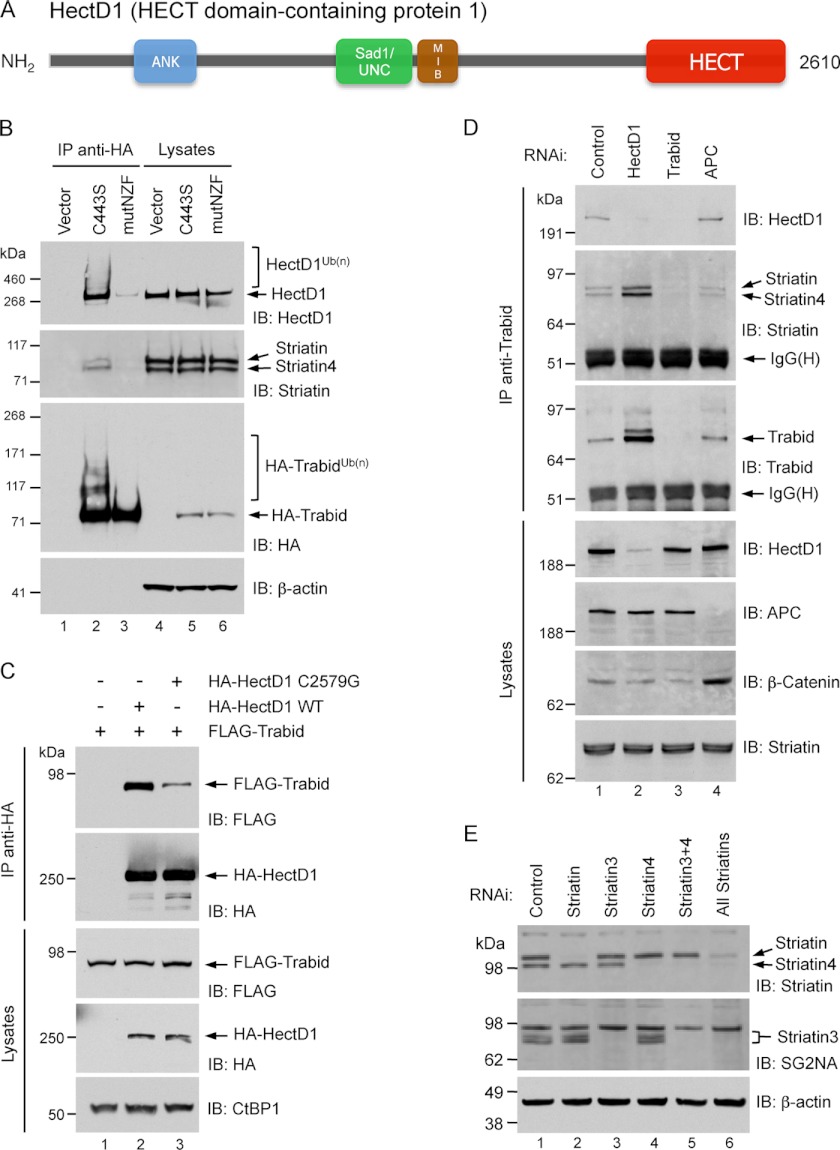
FIGURE 2.
A, domain structure of HectD1. ANK, ankyrin repeats. MIB, mind bomb. HECT, homologous to E6-AP C terminus. EMBL-EBI InterPro analysis predicted armadillo-like repeats encompassing the N-terminal third of HectD1. B, co-immunoprecipitation from transfected HEK293 cells between HA-Trabid C443S and endogenous HectD1 and Striatin. The cells were transfected with the indicated expression vectors for 48 h. The lysates were immunoprecipitated with anti-HA beads and analyzed by immunoblot (IB) as indicated. A Trabid C443S mutant that is also deficient in polyubiquitin-binding (mutNZF) exhibits minimal co-precipitation of HectD1 and Striatin. C, co-immunoprecipitation from transfected HEK293T cells between HA-HectD1 and FLAG-Trabid. The cells were co-transfected with the indicated expression vectors for 48 h. Lysates were immunoprecipitated with anti-HA beads and analyzed by immunoblot as indicated. D, co-immunoprecipitation from HEK293 cells between endogenous Trabid, HectD1, and Striatin. The cells were transfected with the indicated siRNAs for 72 h. The lysates were immunoprecipitated with anti-Trabid antibodies and analyzed by immunoblot as indicated. E, validating the specificity of siRNAs and antibodies to the three Striatin family members. HEK293 cells were transfected with the indicated siRNAs or their combinations (50 nm total) for 72 h. Lysates were analyzed by immunoblot with anti-Striatin (BD Biosciences; catalog no. 610838), which detects both Striatin and Striatin-4, as well as anti-SG2NA, clone S68 (Novus Biologicals), which is specific for Striatin-3 and detects several of its isoforms.
To validate the association between Trabid, HectD1, and Striatin in HEK293 cells, we performed co-immunoprecipitation assays. Immunoprecipitation of HA-tagged C443S Trabid or a C443S mutant that is also deficient in polyubiquitin-binding (mutNZF, TY>LV substitutions in the first two NZF zinc fingers) (24) revealed a strong requirement of this activity for Trabid's interaction with endogenous HectD1, Striatin, and Striatin-4 (Fig. 2B). Notably, HA-Trabid C443S, but not the C443S/mutNZF double mutant, migrated as a high molecular weight (HMW) species and co-precipitated HMW forms of endogenous HectD1, suggestive of ubiquitin-modified pools of these two enzymes (HA-TrabidUb(n) and HectD1Ub(n)). The absence of HMW species of the C443S/mutNZF protein suggests that the NZF domain of Trabid is important for its putative modification with ubiquitin. For the reciprocal co-IP experiment, we transfected cells with wild type HA-HectD1 or a catalytic cysteine mutant, C2579G (25), together with FLAG-Trabid. Immunoprecipitation of HA-HectD1 revealed robust co-precipitation of FLAG-Trabid with WT HA-HectD1, but the association between FLAG-Trabid and the C2579G ligase-deficient mutant was markedly diminished (Fig. 2C). This suggests that efficient interaction between these two enzymes is mediated through ubiquitin chains synthesized by HectD1. To confirm the existence of an endogenous Trabid-Striatin-HectD1 complex, we immunoprecipitated endogenous Trabid and found appreciable co-precipitation of HectD1, Striatin, and Striatin-4 (Fig. 2D). Although Striatin-3 was a specific Trabid-associated protein in our IP-MS analysis (Fig. 1B), we were unable to detect its co-precipitation with Trabid by Western blotting. We believe this is a technical issue with antibody detection of the stripped blots rather than exclusion of Striatin-3 from these subsequent Trabid co-IP experiments. Antibodies to HectD1, Trabid, and the three Striatins were validated for specificity by siRNA knockdown of the respective proteins (Fig. 2, D and E). We observed in five different human cell lines significantly elevated levels of endogenous Trabid protein following RNAi-mediated depletion of HectD1, for example in HEK293 cells (Fig. 2D), which is not due to increased ZRANB1 (TRABID) transcription (data not shown). This suggests that HectD1 suppresses the levels of Trabid post-translationally and in a manner that is independent of proteasomal degradation (see Fig. 6B). Interestingly, knockdown of APC did not affect the total levels of Trabid and HectD1 nor their interaction but did cause a slight decrease in the amount of Striatin and Striatin-4 associated with this complex (Fig. 2D). Together, these results suggest a heterotrimeric complex of Trabid, Striatin, and HectD1 in mammalian cells.
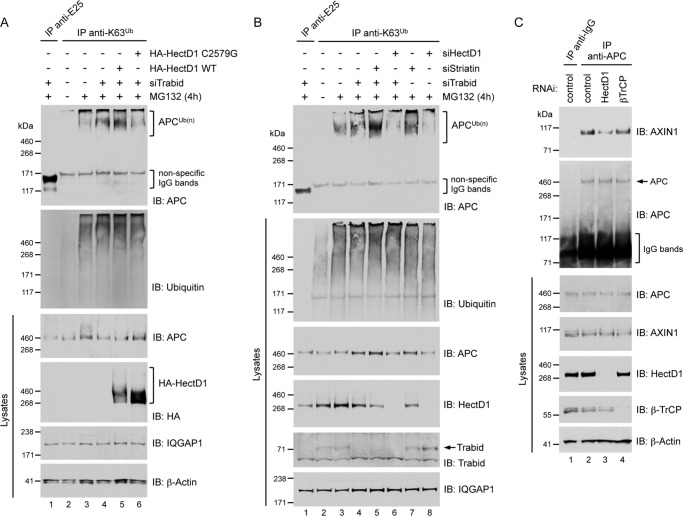
FIGURE 6.
A, overexpression of HA-HectD1 increased the pool of endogenous APC modified with Lys-63 polyubiquitin. HEK293 cells were transfected with Trabid siRNA (72 h) and expression vectors for WT or C2579G HA-HectD1 (48 h) as indicated. The cells were treated with vehicle (dimethyl sulfoxide, −) or 10 μm MG132 for 4 h. Polyubiquitin adducts in lysates were immunoprecipitated with Lys-63 polyubiquitin linkage-specific antibodies (control IgG immunoprecipitation, anti-E25). Immunoprecipitates and lysates were analyzed by immunoblot (IB) as indicated. B, RNAi-mediated depletion of HectD1 decreased the pool of endogenous APC modified with Lys-63 polyubiquitin. HEK293 cells were transfected (72 h) with siRNAs targeting individually or combinations of Trabid, HectD1, or all three Striatins as indicated, followed by treatment with vehicle (−) or 10 μm MG132 for 4 h. Lysates were immunoprecipitated as described in A and analyzed by immunoblot with the indicated antibodies. C, depletion of HectD1 decreased the APC-Axin interaction. HEK293 cells were transfected with the indicated siRNAs for 72 h. Lysates were immunoprecipitated using anti-APC or control mouse IgG antibody and analyzed by immunoblot as indicated.
HectD1 Is a Candidate Lys-63-Polyubiquitin-specific E3 Ligase
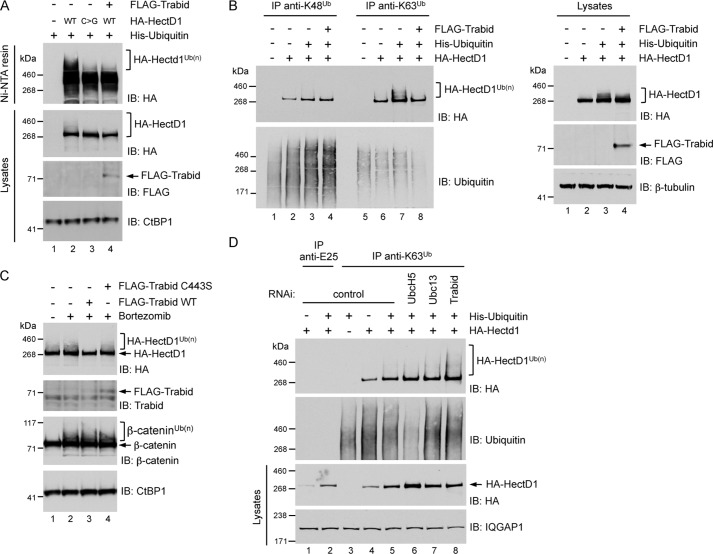
The HMW smear of endogenous HectD1 indicated possible polyubiquitin-modified species (Fig. 2B). To confirm that HectD1 is conjugated with polyubiquitin, we co-expressed HA-HectD1 with polyhistidine-tagged ubiquitin in HEK293 cells. Precipitating the His-ubiquitin pool with nickel-nitrilotriacetic acid affinity resin and immunoblotting with anti-HA revealed the presence of a HMW smear with WT HA-HectD1, but not with the C2579G mutant (Fig. 3A). This suggests that the catalytic cysteine of HectD1 is required for its E3 ligase activity, most likely for its ability to catalyze the self-transfer of polyubiquitin chains from a cognate E2 ubiquitin-conjugating enzyme. Notably, co-expression of FLAG-Trabid abrogated detection of the HMW smear of WT HA-HectD1 (Fig. 3A).
FIGURE 3.
A, WT HA-HectD1, but not its catalytic cysteine mutant counterpart (C2579G, C>G), is modified with polyubiquitin. HEK293 cells were transfected with the indicated epitope-tagged expression vectors for 48 h. The His-ubiquitin pool in lysates were precipitated with nickel-nitrilotriacetic acid affinity resin and analyzed by immunoblot (IB) as indicated. Co-expression of FLAG-Trabid abrogated the polyubiquitylation of HA-HectD1. B, HA-HectD1 is conjugated with Lys-63-linked polyubiquitin. HEK293 cells were transfected with the indicated expression vectors for 48 h. Polyubiquitin adducts in lysates were immunoprecipitated with Lys-48 or Lys-63 polyubiquitin linkage-specific antibodies and analyzed by immunoblot as indicated. Co-expression of FLAG-Trabid abrogated the Lys-63 polyubiquitylation of HA-HectD1. C, the DUB activity of Trabid is required for inhibition of HA-HectD1 polyubiquitylation. HEK293 cells were co-transfected with HA-HectD1 and the indicated Trabid expression vectors for 24 h, followed by treatment with 20 μm bortezomib (proteasome inhibitor) for 4 h. The lysates were analyzed by immunoblot as indicated. D, Trabid negatively regulates the Lys-63 polyubiquitylation of HA-HectD1. HEK293 cells were transfected with the indicated siRNA (72 h) and expression vectors (48 h). The lysates were immunoprecipitated with Lys-63 polyubiquitin linkage-specific antibodies or anti-E25 control IgG and analyzed by immunoblot as indicated.
In addition to the recently proposed Lys-63 E3 ligase activity of HectD1 (25), several observations prompted us to ask whether HectD1 might be conjugated with Lys-63-linked polyubiquitin. First, the interaction between HectD1 and Trabid is largely dependent on the Lys-63 polyubiquitin-binding NZF domain of Trabid (Fig. 2B) (24). Second, full-length, wild type Trabid hydrolyzes Lys-63-linked chains (24, 37–39). Third, although Lys-48 and Lys-29 chains were the most abundant ubiquitin polymers that co-precipitated with Trabid C443S, Lys-63 chains were also detected, and the majority of these were present in the HMW regions a and b (Fig. 1A) above the ∼290-kDa electrophoretic migration of HectD1 (Fig. 2B). Fourth, the availability of antibodies specific for Lys-63 polyubiquitin linkages (28) allowed us to examine whether HectD1 might assemble Lys-63-linked chains in vivo. Lysates from HEK293 cells co-transfected with HA-HectD1 and His-ubiquitin were used in immunoprecipitations with Lys-48 or Lys-63 polyubiquitin linkage-specific antibodies. Western blotting with anti-HA revealed distinct HMW species of HA-HectD1 in the anti-Lys-63 but not the anti-Lys-48 polyubiquitin immunoprecipitate (Fig. 3B). We found that His-ubiquitin co-transfection allowed for robust detection of polyubiquitin-modified HA-HectD1 species, possibly because the increased intracellular pool of ubiquitin enhanced a putative autoubiquitylase activity of HectD1. Co-expression of WT FLAG-Trabid, but not the C443S DUB-inactive version, negated the detection of Lys-63 polyubiquitylated species of HA-HectD1, suggesting that Trabid hydrolyzes Lys-63 adducts from HectD1 (Fig. 3, B and C). Consistently, RNAi-mediated depletion of Trabid, but not control depletions that included knockdown of the E2 ubiquitin-conjugating enzymes UbcH5 (all three isoforms) and Ubc13, significantly increased the pool of HA-HectD1 modified with Lys-63 polyubiquitin (Fig. 3D). Intriguingly, although Ubc13 is considered a Lys-63-specific E2 enzyme (40), we find that depletion of UbcH5, not Ubc13, strongly reduced the total pool of Lys-63 adducts in HEK293 cells. Notwithstanding, these results indicate that HectD1 possesses Lys-63-specific E3 ubiquitin ligase activity, and Trabid antagonizes this activity.
HectD1 Associates with the Armadillo Repeats of APC
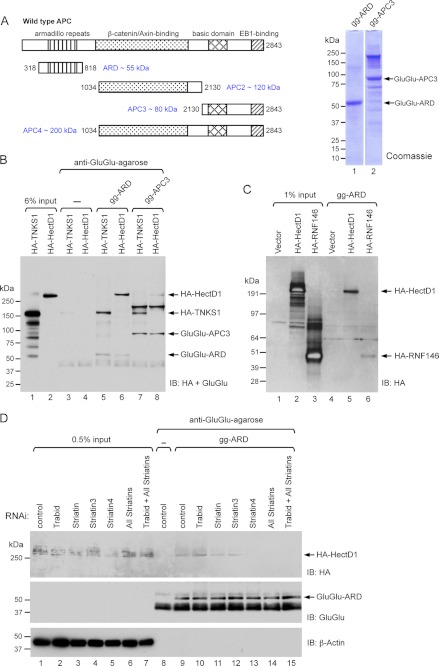
Given that Trabid and Striatin binds the armadillo repeat domain (ARD) of APC (24, 41) and that HectD1 is a component of the Trabid/Striatin complex (Figs. 1 and 2), we asked whether HectD1 might also associate with the APC ARD. For in vitro binding studies, we used a purified Glu-Glu-tagged protein encompassing the ARD domain (gg-ARD) of APC (Fig. 4A). This protein was incubated with lysates from HEK293 cells expressing HA-HectD1. To control for binding specificity, we used a Glu-Glu-tagged APC3 protein (gg-APC3) purified from insect cells (27) and lysates from HEK293 cells expressing HA-Tankyrase (TNKS1), a poly(ADP-ribosyl)ase that binds the E3 ligase RNF146 to cooperatively down-regulate Axin (17). Following pulldown with anti-Glu-Glu-agarose, we detected an interaction between HA-HectD1 and the APC ARD, but only weak association between HA-HectD1 and APC3 (Fig. 4B). Surprisingly, HA-TNKS1, which was expressed at significantly higher levels than HA-HectD1, bound both APC fragments, albeit with slightly lower affinity to APC3 (Fig. 4B). To further evaluate the specificity of these binding reactions, we tested the interaction between HA-RNF146 and the APC ARD but found only minimal interaction between these two proteins (Fig. 4C). This interaction could conceivably be mediated by endogenous TNKS. However, similarly weak, nondifferential interaction of HA-RNF146 to APC3 and to two other purified Glu-Glu-tagged APC proteins that span its middle portion (APC2) or its final two thirds (APC4), suggests nonspecific association between HA-RNF146 and the APC ARD (data not shown). Furthermore, HA-TNKS1 bound APC2 and APC4 fragments with an affinity comparable to its co-precipitation with APC ARD and APC3, whereas HA-HectD1 showed only weak binding to APC2 and APC4 (data not shown). Although these binding assays indicate relatively specific association between HA-HectD1 and the ARD domain of APC, we have not ruled out the possibility that TNKS and RNF146 could have physiologically relevant interactions with APC. Future studies are needed to test this hypothesis. We asked whether Trabid and/or Striatin was required for the HA-HectD1-ARD interaction. HEK293 cells were co-transfected with HA-HectD1 and siRNAs to deplete Trabid and either individual or all three Striatin family members. Incubation of lysates from these cells with the purified ARD revealed a requirement of all the Striatins, but not of Trabid, for the HA-HectD1-ARD association (Fig. 4D). Depletion of the Striatins did not significantly affect the HA-TNKS1-ARD interaction (data not shown). These results raise the prospect that HectD1 might be recruited to APC via Striatin, which binds directly to the ARD domain of APC (41).
FIGURE 4.
A, schematic representation of purified APC proteins. The purity of Glu-Glu-tagged (gg) APC ARD and APC3 fractions (5 μg) was evaluated by SDS-PAGE and Coomassie stain. B, HA-HectD1 associates with the ARD domain of APC. Lysates (1 mg) from HEK293 cells expressing HA-Tankyrase (TNKS1) or HA-HectD1 were combined with 1 μg of gg-ARD or gg-APC3 for 2 h. Binding reactions were precipitated with anti-Glu-Glu-agarose and analyzed by immunoblot (IB) as indicated. C, lysates (1 mg) prepared from HEK293 cells transfected with empty vector, HA-HectD1, or HA-RNF146 were combined with 1 μg of gg-ARD for 2 h. Binding reactions were precipitated with anti-Glu-Glu-agarose and analyzed by immunoblot as indicated. D, the Striatin family of WD40 domain proteins mediates the HectD1-APC ARD interaction. HEK293 cells were transfected with the indicated siRNAs (72 h) and HA-HectD1 (48 h). Lysates (1 mg) were incubated with 1 μg of gg-ARD for 2 h, precipitated with anti-Glu-Glu-agarose, and analyzed by immunoblot as indicated. Note that HA-HectD1 expression was reduced in Striatin 4-depleted cells (inputs), which could account for the absence of an HA-HectD1 signal following gg-ARD pulldown (lane 13).
HectD1 Negatively Regulates the Accumulation of APC at Cortical Protrusions
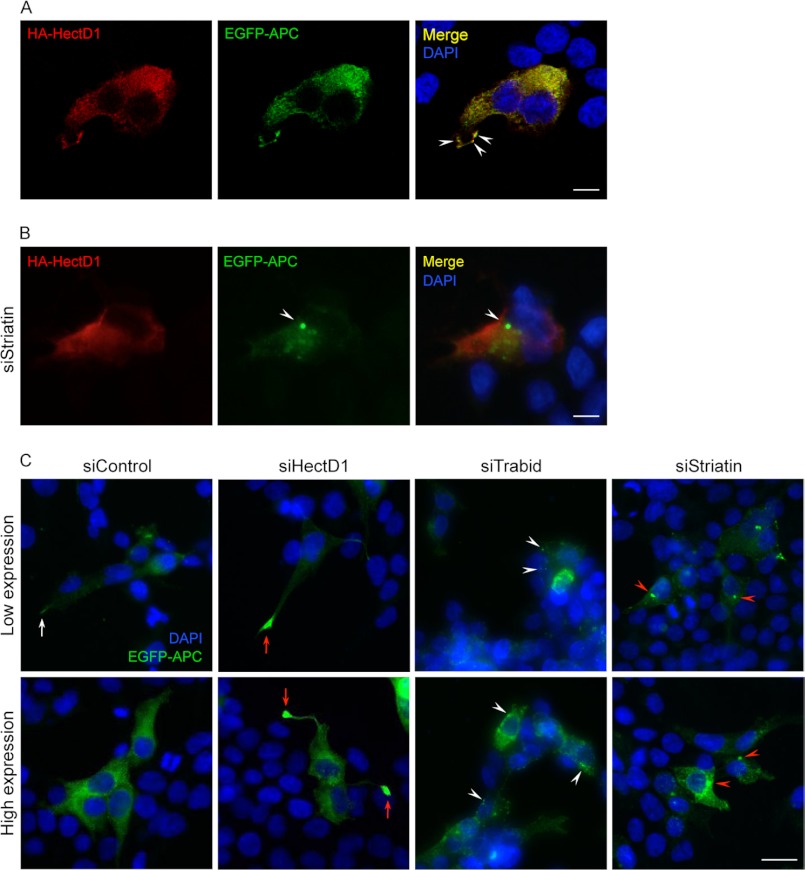
APC is predominantly localized diffusely throughout the cytoplasm but also accumulates in clusters at microtubule plus ends that terminate in the membrane protrusions of migrating cells (42). To support the in vitro binding studies, we examined whether APC and HectD1 co-localize in cells. Because of difficulties in reliably detecting endogenous APC and HectD1 in HEK293 cells, we evaluated the co-localization of epitope-tagged proteins: EGFP-APC and HA-HectD1. By confocal microscopy, we observed extensive cytoplasmic co-localization between HA-HectD1 and EGFP-APC in transfected HEK293 cells (Fig. 5A). Interestingly, conspicuous co-localization at several cortical sites decorating the leading edge of broad cell protrusions was also detected in ∼5% of transfected cells (Fig. 5A, arrowheads). We asked whether this co-localization was affected by Striatin knockdown. Strikingly, depletion of all three Striatins caused the redistribution of EGFP-APC to a pericentriolar region, without affecting the diffuse cytoplasmic localization of HA-HectD1 (Fig. 5B, arrowhead). Transfection with a control siRNA did not alter the co-localization of HA-HectD1 and EGFP-APC (data not shown). These results are consistent with our in vitro binding studies, supporting a role for the Striatins as mediators of the HectD1-APC interaction.
FIGURE 5.
A, co-localization between HA-HectD1 and EGFP-APC. Shown are confocal images of a single optical section through HEK293 cells co-transfected with HA-HectD1 and EGFP-APC. DAPI marks cell nuclei in the merged image. Scale bar, 10 μm. B, RNAi knockdown of all three Striatins affects the subcellular localization of EGFP-APC. Wide field microscope images of HEK293 cells co-transfected with HA-HectD1, EGFP-APC, and siRNA oligonucleotides targeting all three Striatins (72 h). DAPI marks cell nuclei in the merged image. Scale bar, 10 μm. C, RNAi knockdown of HectD1, Trabid, and Striatin affects the subcellular localization of EGFP-APC. Wide field microscope images of HEK293 cells co-transfected (72 h) with EGFP-APC and control siRNA or oligonucleotides targeting HectD1, Trabid, or all three Striatins. Cells judged to have low or high cytoplasmic expression of EGFP-APC were imaged. DAPI marks cell nuclei. Scale bar, 20 μm.
The redistribution of EGFP-APC upon Striatin knockdown prompted us to examine whether the depletion of HectD1 and Trabid might also perturb the localization of APC. In cells co-transfected with a control siRNA, EGFP-APC exhibited diffuse cytoplasmic distribution, and of the ∼5% of cells that protruded long, thin cell extensions, some clustering of EGFP-APC, although not strong, was discernible at the tips of these extensions (Fig. 5C, white arrow). These cells could represent a small number that are actively migrating. By contrast, following HectD1 knockdown, ∼60% of cells expressing EGFP-APC also projected long, thin cell extensions, and strikingly, in ∼25% of this cohort of cells, the cortical protrusions at the tips of their extensions were distended and engorged with EGFP-APC (Fig. 5C, red arrows). We often found cells that had low or no detectable cytoplasmic expression of EGFP-APC but nevertheless showed strong clustering of EGFP-APC in their protrusions. These results indicate that HectD1 negatively regulates cell membrane extensions and the accumulation of APC in the cortical protrusions of these extensions. In Trabid-depleted cells, long membrane extensions were never observed, and EGFP-APC accumulated in numerous small, irregular cytoplasmic puncta (see Fig. 5C, white arrowheads). As shown in Fig. 5B, Striatin knockdown caused accumulation of EGFP-APC in large aggregates, often as a single mass, at a pericentriolar region (Fig. 5C, red arrowheads). Together, these results indicate that the HectD1-Trabid-Striatin complex might regulate the distribution of APC between different functional pools.
HectD1 Modifies APC with Lys-63-linked Polyubiquitin
APC is modified with Lys-63-linked ubiquitin chains in unstimulated cells (14). To determine whether HectD1 is the E3 ligase responsible for this modification of APC, we monitored the effect of its overexpression and knockdown on APC ubiquitylation. Using the anti-Lys-63 polyubiquitin immunoprecipitation assay, we first confirmed that the Lys-63-ubiquitylated pool of endogenous APC increased after Trabid knockdown (Fig. 6A), as previously predicted (24). Overexpression of WT HA-HectD1 in Trabid-depleted cells further increased APC ubiquitylation, but not expression of the ligase-deficient C2579G mutant, which appears to have a dominant-negative effect and caused appreciable loss of polyubiquitylated APC (Fig. 6A). In contrast to the overexpression of HectD1, its RNAi-mediated depletion, either alone or together with Trabid knockdown, resulted in a significant decrease of the APC pool modified with Lys-63 adducts (Fig. 6B). These results indicate that HectD1 is a candidate E3 ligase responsible for catalyzing the formation of Lys-63-linked ubiquitin chains on APC. Depletion of all three Striatins, either alone or together with Trabid knockdown, increased the Lys-63 polyubiquitin-modified APC pool (Fig. 6B). This was surprising given that the Striatins appeared important for recruiting HectD1 to APC (Figs. 4 and 5). Moreover, co-depletion of Striatin and HectD1 did not mitigate the increased APC ubiquitylation in Striatin-depleted cells (data not shown). Because of the dramatic relocalization of APC to a centrosomal region following Striatin knockdown (Fig. 5) and the known enrichment of ubiquitin-associated activities in this subcellular location (43), it is plausible that APC is ubiquitylated by an unidentified E3 ligase in Striatin-depleted cells.
HectD1 Promotes the APC-Axin Interaction
Wnt3a stimulation leads to loss of Lys-63 adducts from APC, which coincides with a reduction in associated Axin (14). Because depletion of HectD1 resulted in the loss of Lys-63 adducts from APC in unstimulated cells (Fig. 6B), we asked whether this had any impact on the interaction between APC and Axin. Compared with the levels of Axin co-precipitated with APC in control- or β-TrCP-depleted cells, immunoprecipitation of endogenous APC from HectD1-depleted cells revealed a significant decrease in co-precipitated Axin (Fig. 6C). These results indicate that HectD1 promotes the APC-Axin interaction and suggests that APC Lys-63 adducts are important for this interaction.
HectD1 Negatively Regulates the Wnt3a-induced Stabilization of β-Catenin
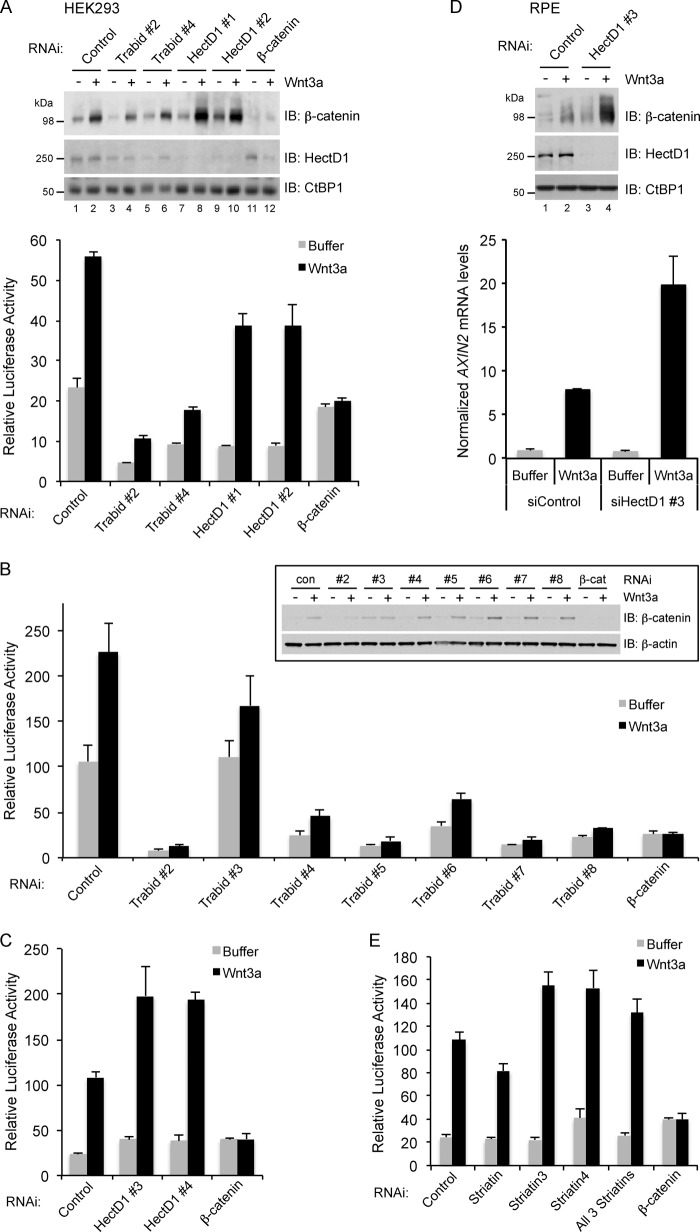
The significant loss of interaction between APC and Axin in HectD1-depleted cells is predicted to result in increased β-catenin levels and signaling. Surprisingly, compared with control siRNA transfection, little if any stabilization of β-catenin occurred in unstimulated HEK293 cells following HectD1 knockdown with two different siRNA oligonucleotides (Fig. 7A).
FIGURE 7.
A, depletion of HectD1 augmented Wnt3a-induced β-catenin stabilization and transcription. HEK293 cells were co-transfected with the indicated siRNAs and luciferase reporter plasmids (pTOP-Flash and pCMV-Renilla) for 48 h. The cells were then treated with vehicle (PBS + 0.2% BSA, −) or Wnt3a (100 ng/ml) for 24 h. Equal amounts of lysates were analyzed by immunoblotting with the indicated antibodies or assayed for TOP-Flash luciferase reporter activity (bottom panel). Knockdown of β-catenin served as a negative control. The anti-CtBP1 blot indicates loading equivalence. The error bars indicate standard deviation of triplicate samples. The data shown are representative of three independent experiments. B, depletion of Trabid suppresses β-catenin-mediated transcription. HEK293 cells were co-transfected with the indicated ON-TARGETplus-modified Trabid-specific siRNAs and TOP-Flash luciferase reporter plasmids as in A. The cells were treated with Wnt3a and processed for immunoblotting or TOP-Flash assays as described in A. After 72 h of transfection, all Trabid-specific siRNAs reduced ZRANB1 transcript levels by at least 90% (quantified by quantitative RT-PCR; data not shown). The anti-β-actin blot indicates loading equivalence. The error bars indicate standard deviation of triplicate samples. The data shown are representative of two independent experiments. C, HEK293 cells were co-transfected with two additional ON-TARGETplus-modified HectD1-specific siRNA oligonucleotides (#3 and #4) and TOP-Flash luciferase reporter plasmids, followed by Wnt3a treatment as described for A. The error bars indicate standard deviation of triplicate samples. The data shown are representative of three independent experiments. D, RPE cells were transfected with control or HectD1-specific siRNA 3 for 48 h and then treated with vehicle (−) or Wnt3a (100 ng/ml) for 24 h. The cells were harvested and divided into two equal portions to prepare lysates for immunoblotting with the indicated antibodies or processed for quantitative RT-PCR to quantify the expression of AXIN2 (lower panel). The error bars indicate standard deviation of triplicate samples. The data shown are representative of three independent experiments. E, HEK293 cells were co-transfected with the indicated siRNAs and TOP-Flash luciferase reporter plasmids, followed by Wnt3a treatment as described for A. The cells were harvested, and the lysates were used for TOP-Flash assays as described for A. The error bars indicate standard deviation of triplicate samples. The data shown are representative of two independent experiments.
Recently, small molecule inhibitors of Trabid DUB activity were identified, but these compounds, and several shRNA sequences targeting Trabid, were reported to have no effect on Wnt signaling (44). This contradicts our previous study where the use of siRNAs to suppress Trabid resulted in loss of Wnt/β-catenin signaling (24). We have revisited our siRNA studies with six different Trabid oligonucleotides unrelated in sequence from those we have used previously and also from the reported shRNAs (44). Although Trabid siRNA 2 caused some reduction of β-catenin levels in unstimulated HEK293 cells, which contrasts with the slightly increased β-catenin levels in our previous analysis using the same siRNA (24), evaluation of the additional six Trabid oligonucleotides revealed that, with the exception of oligonucleotide 3, the majority of these did not significantly affect β-catenin levels in unstimulated cells (Fig. 7, A and B). Despite minimal changes to β-catenin levels, both knockdown of HectD1 and Trabid caused a strong reduction of basal β-catenin-T cell factor-mediated transcription in unstimulated cells, as assayed with the TOP-Flash luciferase reporter (Fig. 7, A and B). Notably, differences between Trabid- and HectD1-depleted cells, with respect to β-catenin stabilization and TOP-Flash activity, were evident following Wnt3a stimulation. Compared with control cells, the Wnt3a-stimulated stabilization of β-catenin in Trabid-depleted cells was restricted (Fig. 7A). However, this effect was not observed with all Trabid siRNAs (Fig. 7B, inset). Irrespective of effects on β-catenin protein stability, we found strong inhibition of Wnt3a-stimulated TOP-Flash activity with five of the six additional Trabid siRNAs, compared with mock-depleted cells (Fig. 7, A and B). When the fold change between unstimulated and Wnt3a-stimulated conditions was evaluated for each siRNA, cells transfected with Trabid oligonucleotides 2, 4, and 6 generally did not show strong deviation from the ∼2.4-fold stimulation of TOP-Flash activity in control cells, but cells transfected with Trabid siRNAs 3, 5, 7, and 8 did exhibit appreciable loss of the Wnt3a-stimulated fold change in TOP-Flash activity (Fig. 7, A and B).
In contrast to Trabid depletion, HectD1 knockdown resulted in significant enhancement of β-catenin stabilization upon Wnt3a stimulation (Fig. 7A). Intriguingly, this was not reflected in an overall increase in TOP-Flash activity when compared with control-depleted cells. Significantly, however, the fold change in TOP-Flash activity between unstimulated and Wnt3a-stimulated HectD1-depleted cells was ∼4.3-fold: a 1.9-fold increase in Wnt responsiveness versus control siRNA-transfected cells (Fig. 7A). We also tested two additional siRNAs targeting HectD1 and found that these augmented Wnt3a-stimulated TOP-Flash activity compared with the control siRNA, without significant effects on transcription in unstimulated cells (Fig. 7C). These results were replicated in human RPE cells, where, following HectD1 knockdown with oligonucleotide 3 and Wnt3a treatment, we found both augmented β-catenin stabilization and an ∼2.8-fold increase in expression of the Wnt target gene AXIN2, compared with control cells (Fig. 7D). The requirement of Striatin for Wnt signaling in HEK293 cells was evaluated. Depleting individual or all three Striatins had largely negligible effects on β-catenin stability or signaling in unstimulated or Wnt3a-stimulated cells (Fig. 7E and data not shown). Thus, of the three components of the HectD1-Trabid-Striatin complex, HectD1 appears to have the strongest negative impact on Wnt3a-stimulated β-catenin stabilization and signaling.
DISCUSSION
We began this study with the premise that a specific E3 ubiquitin ligase might be associated with Trabid to modulate the Lys-63 polyubiquitylation of APC. We have described the identification of HectD1 as a candidate E3 ligase with these properties. Our evidence suggests that the attachment of Lys-63-linked ubiquitin adducts on APC, catalyzed by HectD1, mediate the efficient interaction of APC with Axin. Although we have not determined how this modification enforces the interaction, one possibility is that Lys-63 chains alter APC conformation in a manner that facilitates the association of its SAMP repeats with the RGS domain of Axin (45).
Analysis of the abundance of different ubiquitin linkages co-precipitating with Trabid (Fig. 1) and HectD1 (data not shown) revealed a near majority of Lys-29 linkages, suggesting that these enzymes are primarily associated with Lys-29 polyubiquitin-specific DUB and E3 ligase activities, respectively. This would be consistent with the potent DUB activity of an extended Trabid catalytic domain toward Lys-29-linked di-ubiquitin in vitro (38). Nonetheless, we also detected significant amounts of Lys-48 linkages co-precipitating with Trabid, and these chains do not appear to be conjugated to Trabid itself; a conclusion based on examining the ubiquitylation of HA-Trabid in an anti-Lys-48 immunoprecipitation assay (data not shown). Combined with previous evidence that Trabid does not bind or exhibit any DUB activity toward Lys-48 linkages (24, 38, 39), we suspect that these chains, and perhaps a majority pool of all the identified linkages, represent adducts attached to co-precipitating proteins. These may or may not be specific Trabid-associated proteins or substrates.
Despite the reported Lys-29 and Lys-33 DUB specificity of Trabid in vitro (38), our in vivo data show that full-length, wild type Trabid is capable of cleaving Lys-63 adducts from its putative physiological substrate HectD1 (Fig. 3). Moreover, an appreciable reduction of the total Lys-63-ubiquitylated pool in Trabid-overexpressing cells (Fig. 3B) indicates that Lys-63 adducts are robust Trabid substrates in vivo. Given that the NZF domain of Trabid binds both Lys-63-linked and linear ubiquitin chains (24, 37) and that several different ubiquitin linkages were co-precipitated with Trabid (Fig. 1), it is conceivable that additional factors determine the linkage specificity of Trabid's polyubiquitin-binding and DUB activities in cells.
The use of polyubiquitin linkage-specific antibodies provide good evidence that HectD1 is conjugated with Lys-63-linked chains, and manipulation of HectD1 levels produced the predicted changes in abundance of Lys-63 chains on APC (Fig. 6). Although these results support our conclusion that HectD1 mediates the assembly of Lys-63 chains on APC, we recognize that future tools, including the availability of Lys-29 and Lys-33 polyubiquitin linkage-specific antibodies, could reveal additional linkage specificities and/or substrates for HectD1.
Our results indicate that opposing E3 ligase and DUB activities of HectD1 and Trabid, respectively, modulate the Lys-63 ubiquitylation of APC. These activities, possibly regulated by the Striatin proteins, appear to influence APC localization (Fig. 5). Thus, the modification of APC with polyubiquitin could be a mechanism that regulates its distribution between different functional pools. How is HectD1 recruited to APC? Although we have not fully investigated the interactions, several observations indicate that the Striatins, a family of WD40 domain proteins (46), are integral components of the Trabid-HectD1 complex and could play a role in recruiting these enzymes to APC. Striatin was one of two proteins identified as specific binders to the ARD domain of APC in a yeast two-hybrid screen (24), and more comprehensive analysis of a direct interaction between Striatin and the APC ARD has been described (41). Efficient binding between Striatin and Trabid is facilitated by polyubiquitin (Figs. 1 and 2). Accordingly, WD40 domains are known to bind ubiquitin (47), and notably, WD40 proteins are associated with more than a third of all DUBs (35). Various WD40 proteins have been shown to function as co-factors that stimulate the ubiquitin hydrolase activity of their cognate DUBs (48–50). Therefore, it is conceivable that Striatin could bridge the Trabid-HectD1-APC interaction and may function as co-factors that stimulate the DUB activity of Trabid to cleave Lys-63 polyubiquitin from HectD1 and/or APC, thereby abrogating their polyubiquitylation and activity.
What is the role of HectD1 and Trabid in Wnt signaling? Consistent with our previous report (24), experiments employing six additional siRNA oligonucleotides to deplete Trabid show a severe negative impact on β-catenin-mediated transcription in HEK293 cells (Fig. 7). A recent study targeted Trabid with shRNAs and small molecule inhibitors of its DUB activity but failed to find an effect on Wnt target gene expression in HCT116 and SW480 colorectal cancer cells (44). These incongruous results are at present difficult to reconcile, and future studies, including the analysis of knock-out mice, are necessary to further evaluate the role of Trabid in Wnt signaling. Nonetheless, we are open to the possibility that the loss of β-catenin-dependent transcription after Trabid knockdown with siRNAs, especially in nonstimulated HEK293 cells, is an indirect effect of the putative requirement of Trabid for cell growth (44). Consistent with this possibility, our preliminary results support a positive function for Trabid in microtubule growth and stability.4 Moreover, we have found that HectD1-depleted cells exhibited increased Trabid protein levels, microtubule stability, and Wnt responsiveness (Figs. 2 and 7).4 It is conceivable that perturbations in ciliogenesis or basal body function, processes that are interconnected with microtubule growth dynamics, may account for the peculiar effects on β-catenin/Wnt readouts in HectD1- and Trabid-depleted cells (51, 52).4 The enzymatic activities of HectD1 and Trabid might be regulated by signals that inhibit or promote cell migration, which would be consistent with the proposed roles of these enzymes as regulators of cell motility (25, 34). Interestingly, HectD1 and Trabid are putative novel components of the STRIPAK complex (Fig. 1), which links these two enzymes to potential roles in Golgi polarization (53) and Hippo signaling (54).
We have previously established a negative correlation between APC Lys-63 ubiquitylation and β-catenin turnover in cells (14). Therefore, it was surprising to find minimal effects on β-catenin levels in unstimulated cells following the depletion of HectD1 and Trabid (Fig. 7). This was more intriguing given that the APC-Axin interaction was disrupted in HectD1-depleted cells. It is plausible that a sufficient pool of functional APC-Axin complexes was still available to effectively down-regulate β-catenin levels. Alternatively, APC-Axin dissociation is not sufficient, and a canonical Wnt signal, perhaps required to inhibit GSK3β activity, is necessary to fully promote β-catenin stabilization. Indeed, β-catenin stability and signaling in response to Wnt3a was significantly enhanced in HectD1-depleted cells (Fig. 7), which could be consistent with a deficit in the ability to reform the APC-Axin complex following Wnt pathway activation. Accordingly, it is conceivable that HectD1 modifies APC with Lys-63 polyubiquitin to “reset” the β-catenin destruction complex. This modification of APC would also synergistically cooperate with the increased levels of Axin2, which is a direct consequence of Wnt signaling (55), and thereby reinforce negative feedback to restore homeostasis in Wnt-activated cells.
Our previous siRNA screen targeting all predicted human ubiquitin E3 ligases (17) identified HectD1 as a negative regulator of Wnt signaling (data not shown). Together with current evidence, we postulate that HectD1 functions as a negative feedback regulator of Wnt signaling. The HectD1-mediated modification of APC with Lys-63 polyubiquitin likely precedes the binding of APC to Axin, and Lys-63 chains could serve as a targeting signal to direct the trafficking of APC to Axin. Thus, Lys-63 adducts on APC regulate assembly of the β-catenin destruction complex. Identification of the APC ubiquitin acceptor site(s) will enable mutational analysis and more conclusive determination of the importance of APC Lys-63 adducts in β-catenin degradation and other APC-regulated cellular processes.
Acknowledgments
We thank Vishva Dixit, Mike Costa, Marinella Callow, Ted Lau, Venita DeAlmeida, Ken Dong, Dirk Siepe, Laszlo Kumoves, Meredith Sagolla, Jeff Sfakianos, Marissa Matsumoto, Rowena Suriben, and Jasmin Dynek for discussion, reagents, and support.
H. Tran and P. Polakis, unpublished data.
- APC
- adenomatous polyposis coli
- DUB
- deubiquitylating enzyme
- RPE
- retinal pigmented epithelial
- IP
- immunoprecipitation
- HMW
- high molecular weight
- ARD
- armadillo repeat domain
- gg
- Glu-Glu-tagged.
REFERENCES
- 1. Reya T., Clevers H. (2005) Wnt signalling in stem cells and cancer. Nature 434, 843–850 [DOI] [PubMed] [Google Scholar]
- 2. Polakis P. (2013) Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 4, doi: 10.1101/cshperspect.a008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tauriello D. V., Maurice M. M. (2010) The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle 9, 3700–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hershko A., Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 5. Ikeda F., Dikic I. (2008) Atypical ubiquitin chains. New molecular signals. “Protein Modifications.” Beyond the Usual Suspects review series. EMBO Rep. 9, 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emmerich C. H., Schmukle A. C., Walczak H. (2011) The emerging role of linear ubiquitination in cell signaling. Sci. Signal. 4, re5. [DOI] [PubMed] [Google Scholar]
- 7. Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. (1997) β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart M., Concordet J. P., Lassot I., Albert I., del los Santos R., Durand H., Perret C., Rubinfeld B., Margottin F., Benarous R., Polakis P. (1999) The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9, 207–210 [DOI] [PubMed] [Google Scholar]
- 9. Kitagawa M., Hatakeyama S., Shirane M., Matsumoto M., Ishida N., Hattori K., Nakamichi I., Kikuchi A., Nakayama K., Nakayama K. (1999) An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18, 2401–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubinfeld B., Albert I., Porfiri E., Fiol C., Munemitsu S., Polakis P. (1996) Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272, 1023–1026 [DOI] [PubMed] [Google Scholar]
- 11. Behrens J., Jerchow B. A., Würtele M., Grimm J., Asbrand C., Wirtz R., Kühl M., Wedlich D., Birchmeier W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280, 596–599 [DOI] [PubMed] [Google Scholar]
- 12. Hart M. J., de los Santos R., Albert I. N., Rubinfeld B., Polakis P. (1998) Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr. Biol. 8, 573–581 [DOI] [PubMed] [Google Scholar]
- 13. Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 14. Tran H., Polakis P. (2012) Reversible modification of adenomatous polyposis coli (APC) with K63-linked polyubiquitin regulates the assembly and activity of the β-catenin destruction complex. J. Biol. Chem. 287, 28552–28563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-β-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y., Liu S., Mickanin C., Feng Y., Charlat O., Michaud G. A., Schirle M., Shi X., Hild M., Bauer A., Myer V. E., Finan P. M., Porter J. A., Huang S. M., Cong F. (2011) RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat. Cell Biol. 13, 623–629 [DOI] [PubMed] [Google Scholar]
- 17. Callow M. G., Tran H., Phu L., Lau T., Lee J., Sandoval W. N., Liu P. S., Bheddah S., Tao J., Lill J. R., Hongo J. A., Davis D., Kirkpatrick D. S., Polakis P., Costa M. (2011) Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PLoS One 6, e22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hao H. X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H., Mao X., Ma Q., Zamponi R., Bouwmeester T., Finan P. M., Kirschner M. W., Porter J. A., Serluca F. C., Cong F. (2012) ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 [DOI] [PubMed] [Google Scholar]
- 19. Valvezan A. J., Zhang F., Diehl J. A., Klein P. S. (2012) Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J. Biol. Chem. 287, 3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada M., Ohnishi J., Ohkawara B., Iemura S., Satoh K., Hyodo-Miura J., Kawachi K., Natsume T., Shibuya H. (2006) NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF). J. Biol. Chem. 281, 20749–20760 [DOI] [PubMed] [Google Scholar]
- 21. Chitalia V. C., Foy R. L., Bachschmid M. M., Zeng L., Panchenko M. V., Zhou M. I., Bharti A., Seldin D. C., Lecker S. H., Dominguez I., Cohen H. T. (2008) Jade-1 inhibits Wnt signalling by ubiquitylating β-catenin and mediates Wnt pathway inhibition by pVHL. Nat. Cell Biol. 10, 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borgal L., Habbig S., Hatzold J., Liebau M. C., Dafinger C., Sacarea I., Hammerschmidt M., Benzing T., Schermer B. (2012) The ciliary protein nephrocystin-4 translocates the canonical Wnt regulator Jade-1 to the nucleus to negatively regulate β-catenin signaling. J. Biol. Chem. 287, 25370–25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mukai A., Yamamoto-Hino M., Awano W., Watanabe W., Komada M., Goto S. (2010) Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J. 29, 2114–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran H., Hamada F., Schwarz-Romond T., Bienz M. (2008) Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev. 22, 528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sarkar A. A., Zohn I. E. (2012) Hectd1 regulates intracellular localization and secretion of Hsp90 to control cellular behavior of the cranial mesenchyme. J. Cell Biol. 196, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phu L., Izrael-Tomasevic A., Matsumoto M. L., Bustos D., Dynek J. N., Fedorova A. V., Bakalarski C. E., Arnott D., Deshayes K., Dixit V. M., Kelley R. F., Vucic D., Kirkpatrick D. S. (2011) Improved quantitative mass spectrometry methods for characterizing complex ubiquitin signals. Mol. Cell. Proteomics 10, M110.003756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. (1995) Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. U.S.A. 92, 3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newton K., Matsumoto M. L., Wertz I. E., Kirkpatrick D. S., Lill J. R., Tan J., Dugger D., Gordon N., Sidhu S. S., Fellouse F. A., Komuves L., French D. M., Ferrando R. E., Lam C., Compaan D., Yu C., Bosanac I., Hymowitz S. G., Kelley R. F., Dixit V. M. (2008) Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134, 668–678 [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto M. L., Wickliffe K. E., Dong K. C., Yu C., Bosanac I., Bustos D., Phu L., Kirkpatrick D. S., Hymowitz S. G., Rape M., Kelley R. F., Dixit V. M. (2010) K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol. Cell 39, 477–484 [DOI] [PubMed] [Google Scholar]
- 30. Dynek J. N., Goncharov T., Dueber E. C., Fedorova A. V., Izrael-Tomasevic A., Phu L., Helgason E., Fairbrother W. J., Deshayes K., Kirkpatrick D. S., Vucic D. (2010) c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 29, 4198–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nijman S. M., Luna-Vargas M. P., Velds A., Brummelkamp T. R., Dirac A. M., Sixma T. K., Bernards R. (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123, 773–786 [DOI] [PubMed] [Google Scholar]
- 32. Guzzo R. M., Sevinc S., Salih M., Tuana B. S. (2004) A novel isoform of sarcolemmal membrane-associated protein (SLMAP) is a component of the microtubule organizing centre. J. Cell Sci. 117, 2271–2281 [DOI] [PubMed] [Google Scholar]
- 33. Goudreault M., D'Ambrosio L. M., Kean M. J., Mullin M. J., Larsen B. G., Sanchez A., Chaudhry S., Chen G. I., Sicheri F., Nesvizhskii A. I., Aebersold R., Raught B., Gingras A. C. (2009) A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteomics 8, 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bai S. W., Herrera-Abreu M. T., Rohn J. L., Racine V., Tajadura V., Suryavanshi N., Bechtel S., Wiemann S., Baum B., Ridley A. J. (2011) Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. 9, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sowa M. E., Bennett E. J., Gygi S. P., Harper J. W. (2009) Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zohn I. E., Anderson K. V., Niswander L. (2007) The Hectd1 ubiquitin ligase is required for development of the head mesenchyme and neural tube closure. Dev. Biol. 306, 208–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Komander D., Reyes-Turcu F., Licchesi J. D., Odenwaelder P., Wilkinson K. D., Barford D. (2009) Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 10, 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Licchesi J. D., Mieszczanek J., Mevissen T. E., Rutherford T. J., Akutsu M., Virdee S., El Oualid F., Chin J. W., Ovaa H., Bienz M., Komander D. (2012) An ankyrin-repeat ubiquitin-binding domain determines TRABID's specificity for atypical ubiquitin chains. Nat. Struct. Mol. Biol. 19, 62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bremm A., Freund S. M., Komander D. (2010) Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat. Struct. Mol. Biol. 17, 939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z. J. (2000) Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 [DOI] [PubMed] [Google Scholar]
- 41. Breitman M., Zilberberg A., Caspi M., Rosin-Arbesfeld R. (2008) The armadillo repeat domain of the APC tumor suppressor protein interacts with Striatin family members. Biochim. Biophys. Acta 1783, 1792–1802 [DOI] [PubMed] [Google Scholar]
- 42. Näthke I. S., Adams C. L., Polakis P., Sellin J. H., Nelson W. J. (1996) The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 134, 165–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wigley W. C., Fabunmi R. P., Lee M. G., Marino C. R., Muallem S., DeMartino G. N., Thomas P. J. (1999) Dynamic association of proteasomal machinery with the centrosome. J. Cell Biol. 145, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi T., Bao J., Wang N. X., Zheng J., Wu D. (2012) Identification of small molecule TRABID deubiquitinase inhibitors by computation-based virtual screen. BMC Chem. Biol. 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spink K. E., Polakis P., Weis W. I. (2000) Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 19, 2270–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Castets F., Rakitina T., Gaillard S., Moqrich A., Mattei M. G., Monneron A. (2000) Zinedin, SG2NA, and striatin are calmodulin-binding, WD repeat proteins principally expressed in the brain. J. Biol. Chem. 275, 19970–19977 [DOI] [PubMed] [Google Scholar]
- 47. Pashkova N., Gakhar L., Winistorfer S. C., Yu L., Ramaswamy S., Piper R. C. (2010) WD40 repeat propellers define a ubiquitin-binding domain that regulates turnover of F box proteins. Mol. Cell 40, 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cohn M. A., Kowal P., Yang K., Haas W., Huang T. T., Gygi S. P., D'Andrea A. D. (2007) A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol. Cell 28, 786–797 [DOI] [PubMed] [Google Scholar]
- 49. Cohn M. A., Kee Y., Haas W., Gygi S. P., D'Andrea A. D. (2009) UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J. Biol. Chem. 284, 5343–5351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kee Y., Yang K., Cohn M. A., Haas W., Gygi S. P., D'Andrea A. D. (2010) WDR20 regulates activity of the USP12 x UAF1 deubiquitinating enzyme complex. J. Biol. Chem. 285, 11252–11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gerdes J. M., Liu Y., Zaghloul N. A., Leitch C. C., Lawson S. S., Kato M., Beachy P. A., Beales P. L., DeMartino G. N., Fisher S., Badano J. L., Katsanis N. (2007) Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat. Genet. 39, 1350–1360 [DOI] [PubMed] [Google Scholar]
- 52. Corbit K. C., Shyer A. E., Dowdle W. E., Gaulden J., Singla V., Chen M. H., Chuang P. T., Reiter J. F. (2008) Kif3a constrains β-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 10, 70–76 [DOI] [PubMed] [Google Scholar]
- 53. Kean M. J., Ceccarelli D. F., Goudreault M., Sanches M., Tate S., Larsen B., Gibson L. C., Derry W. B., Scott I. C., Pelletier L., Baillie G. S., Sicheri F., Gingras A. C. (2011) Structure-function analysis of core STRIPAK Proteins. A signaling complex implicated in Golgi polarization. J. Biol. Chem. 286, 25065–25075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ribeiro P. S., Josué F., Wepf A., Wehr M. C., Rinner O., Kelly G., Tapon N., Gstaiger M. (2010) Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 39, 521–534 [DOI] [PubMed] [Google Scholar]
- 55. Jho E. H., Zhang T., Domon C., Joo C. K., Freund J. N., Costantini F. (2002) Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]