Background: MRP4 is an endogenous transporter of cyclic nucleotides that can regulate cell migration. The role of MRP4 in fibroblast migration is unknown.
Results: MRP4-deficient fibroblasts migrate faster and have a moderately higher level of intracellular cyclic nucleotides.
Conclusion: Inhibition of MRP4 increases fibroblast migration via alteration of intracellular cyclic nucleotide levels.
Significance: Inhibition of MRP4 facilitates wound repair.
Keywords: ABC Transporter, Cyclic Nucleotides, Fibroblast, Migration, Multidrug Transporters, MRP4
Abstract
It has long been known that cyclic nucleotides and cyclic nucleotide-dependent signaling molecules control cell migration. However, the concept that it is not just the absence or presence of cyclic nucleotides, but a highly coordinated balance between these molecules that regulates cell migration, is new and revolutionary. In this study, we used multidrug resistance protein 4 (MRP4)-expressing cell lines and MRP4 knock-out mice as model systems and wound healing assays as the experimental system to explore this unique and emerging concept. MRP4, a member of a large family of ATP binding cassette transporter proteins, localizes to the plasma membrane and functions as a nucleotide efflux transporter and thus plays a role in the regulation of intracellular cyclic nucleotide levels. Here, we demonstrate that mouse embryonic fibroblasts (MEFs) isolated from Mrp4−/− mice have higher intracellular cyclic nucleotide levels and migrate faster compared with MEFs from Mrp4+/+ mice. Using FRET-based cAMP and cGMP sensors, we show that inhibition of MRP4 with MK571 increases both cAMP and cGMP levels, which results in increased migration. In contrast to these moderate increases in cAMP and cGMP levels seen in the absence of MRP4, a robust increase in cAMP levels was observed following treatment with forskolin and isobutylmethylxanthine, which decreases fibroblast migration. In response to externally added cell-permeant cyclic nucleotides (cpt-cAMP and cpt-cGMP), MEF migration appears to be biphasic. Altogether, our studies provide the first experimental evidence supporting the novel concept that balance between cyclic nucleotides is critical for cell migration.
Introduction
Multidrug resistance protein 4 (MRP4/ABCC4)2 is a membrane protein belonging to the C subfamily of ATP-binding cassette transporters. MRP4 is identified as an endogenous transporter of cyclic nucleotides and utilizes the energy of ATP hydrolysis to translocate the substrates across the membrane (1, 2). As an endogenous efflux transporter of cyclic nucleotides, MRP4 can regulate the intracellular cAMP and cGMP concentrations together with the different isoforms of phosphodiesterase. Thus, MRP4 can control various cyclic nucleotide-dependent signaling pathways. The role of cyclic nucleotides and their downstream signaling molecules in regulation of different types of cell migration is well established (3, 4). Earlier, some studies showed the two nucleotides had an opposite effect; and, according to the “Yin-Yang” hypothesis (4), cAMP inhibits, whereas cGMP stimulates, cell migration. But the actual role of cAMP and cGMP in cell migration is still controversial. Some recent studies have revealed the importance of cAMP and cAMP-dependent protein kinase A (PKA) for localized cell protrusion and efficient cell migration (5, 6). The leading edge of a migrating cell accumulates more PKA compared with the rest of the cell body, and this compartmentalized activation of PKA is an important early step in directional cell migration. PKA has been found to both facilitate and inhibit actin cytoskeleton dynamics and cell migration because of the diversity of the substrate for PKA. But inhibition of PKA activity as well as interference with PKA anchoring leads to a defective pseudopod formation followed by an attenuated cell migration in a Rac GTPase-dependent fashion (7). Paradoxically, elevation of cAMP inhibits lamellipodia formation in mouse embryonic fibroblast cells (MEFs) and mouse breast tumor cells by acting downstream of small GTPase Rac (8). In contrast, cGMP-dependent protein kinase (PKG) activation decreases phosphorylation of the actin-severing protein cofilin (Ser-3) and hence helps in generating actin monomer for polymerization of new filaments in the cell protrusions. Conversely, in hypoxic conditions, adhesion and migration of vascular smooth muscle cells increase due to down-regulation of PKG (9). Cells that overexpress vasodilator-stimulated phosphoprotein, a major substrate of PKG, have a higher rate of protrusion formation but have a slower cell migration rate. PKG-dependent phosphorylation of vasodilator-stimulated phosphoprotein can relieve this overall negative effect on cell migration (10, 11). The Rac GTPase and p21-activated kinase-dependent feedback mechanism is essential for repetitive behavior of cell migration, and cGMP positively regulates the activity of Rac and p21-activated kinase and thus stimulates the process of cell migration. PKA and PKG can also exert differential effects on contractility and tension transmission in migrating cells via phosphorylation and activation of both myosin light chain kinase and myosin phosphatase (10). Thus, cyclic nucleotides and cyclic nucleotide-dependent kinases are involved in and regulate different signaling events that compose cell migration. Highly regulated PKA and PKG activities and therefore a balanced intracellular cyclic nucleotide concentration are essential for proper cell movement.
In our studies, we hypothesize that it is not just the overall increase or decrease in intracellular cyclic nucleotide level but rather a tight balance between cAMP and cGMP that is essential for the process of cell migration. To test our hypothesis and to understand the dichotomous role of cyclic nucleotides in cell migration, we used MEFs from Mrp4+/+ and Mrp4−/− mice and NIH 3T3 cells for migration assays. We found that (a) MRP4 is an intracellular cAMP/cGMP transporter in these fibroblasts and has a profound effect on intracellular cyclic nucleotide dynamics; (b) cyclic nucleotides have a biphasic effect on fibroblast migration; and (c) a highly regulated intracellular cAMP/cGMP concentration is important for fine-tuned fibroblast migration.
EXPERIMENTAL PROCEDURES
Reagents
MRP4 inhibitor MK571 was obtained from Cayman Chemical (Ann Arbor, MI). Forskolin was obtained from Tocris (Ellisville, MO). IBMX, cpt-cAMP, and cpt-cGMP were purchased from Sigma-Aldrich. Zaprinast was purchased from Enzo Life Sciences (Farmingdale, NY).
Cell Cultures
MEF cells and NIH 3T3 cells were cultured in DMEM (Invitrogen) media containing 10% FBS and 1% penicillin/streptomycin and maintained in 5% CO2 incubator at 37 °C. Lipofectamine LTX reagent was used to transfect the Mrp4+/+ and Mrp4−/− primary MEFs with 1 μg of total SV40 genomic DNA. One day post transfection the cells were serially diluted (1000 to 10 cells per well) and plated in flat bottom 96-well plates. SV40 immortalized clones were picked from the lower dilutions wells where the media turned into yellow. Lipofectamine 2000 (Invitrogen) was used for all transient transfections, and MRP4-overexpressing cell lines were generated by using lentivirus vector and selected by puromycin (2 μg/ml).
Animals
Mrp4−/− and Mrp4+/+ (C57BL/6) mice (12) were provided by Dr. John Schuetz (St. Jude Children's Research Hospital, Memphis, TN).
Skin Explant Outgrowth Assay
Full thickness dorsal skin biopsies (5 mm) were excised from 8 weeks old MRP4−/− and wild-type mice after removing the hair and were fixed in fibronectin-coated six-well plates, placing the dermal side down. The skin explants were cultured in 2 ml of DMEM media containing 10% FBS and 1% penicillin/streptomycin and maintained in a 5% CO2 incubator at 37 °C for 5 days (13, 14). Representative images of 100× magnification of the cellular outgrowth from each explant were taken, and the proximal distance of cellular migration was measured by NIH Image J software.
High Content Microscopy
Cells were grown for 24 h in a fibronectin-coated 96-well Essen BioScience ImageLock microplate in a standard CO2 incubator. Wounds were made precisely by the 96-pin Wound-Maker provided with the IncuCyteTM (15). After washing thoroughly with PBS to remove the detached cells, the cells were placed in the IncuCyteTM with appropriate media. The wound images were automatically acquired by IncuCyteTM (Essen Bioscience) from the incubator at 1-h intervals. The kinetics of the relative wound density (RWD) was analyzed by IncuCyteTM software.
Wound Healing Assay
Cell migration was measured according to the previously described method (16). In brief, the confluent monolayers of fibroblast cells were wounded by scraping with a pipette tip across the monolayer. Cells were washed with PBS and incubated with appropriate media. Images of 100× magnification were taken at the initial time of wounding and then at 6 h or 10 h post-wounding using a cooled electron microscope (EM)-CCD camera (Hamamatsu; Bridgewater; Denver, CO). The cell migration was analyzed by Image J software and represented as a percentage of initial wound length.
Immunoblotting
For detection of MRP4 in fibroblast cells using Western blotting, the cells were lysed in lysis buffer (1× PBS, containing 0.2% Triton X-100 and protease inhibitors: 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, and 1 μg/ml aprotinin). The lysate was centrifuged at 16,000 × g for 10 min at 4 °C, and the clear supernatant was mixed with 5× Laemmli sample buffer (containing 2.5% β-mercaptoethanol), denatured, subjected to SDS-PAGE on 4–15% gel (Bio-Rad), transferred to a PVDF membrane, and the membranes were then incubated with specific antibodies. We used anti-MRP4 antibody (rabbit polyclonal antibody) (12) and anti-β-actin antibody (mouse) that was purchased from Sigma-Aldrich. The protein bands were detected by chemiluminescence using ECLTM Western blotting detection reagents from GE Healthcare. We loaded 50 μg of total protein per lane.
Fluorescence Resonance Energy Transfer Microscopy
CFP-EPAC-YFP was a generous gift from Dr. Kees Jalink (Division of Cell Biology, The Netherlands Cancer Institute, Amsterdam, The Netherlands), and Cygnet 2.1 was a generous gift from Dr. Wolfgang R Dostmann (Department of Pharmacology, University of Vermont, Burlington, VT). Cells were transiently transfected with CFP-EPAC-YFP (cAMP sensor) (17) or Cygnet 2.1 (cGMP sensor) (18) and were grown in 35-mm glass-bottomed dishes (MatTek) for 24–48 h; cells were then washed with Hanks Balanced Salt Solution and mounted on an Olympus microscopy system for FRET imaging. Images were recorded with a cooled CCD camera Hamamatsu ORCA285 (Hamamatsu, Japan) mounted on the Olympus microscope IX51 (U-Plan Fluorite 60 × 1.25 NA oil-immersion objective), and the system was controlled by SlideBook software (version 4.1, Intelligent Imaging Innovations; Denver, CO) with ratio and FRET modules used to obtain and analyze the images. Excitation light was provided by a 300-watt Xenon lamp and attenuated with a Neutral Density filter with 50% light transmission. Images were captured using a JP4 CFP/YFP filter set (Chroma; Brattleboro, VT), including a 430/25-nm excitation filter, a double dichroic beam splitter, and two emission filters (470/30-nm for CFP and 535/30-nm for FRET) alternated by a filter-changer Lambda 10-3 (Sutter Instruments; Novato, CA). Time-lapse images were acquired with 100 ms of exposure time, and 1-min intervals. Multiple regions of interest on the cell were selected after background subtraction for quantitative data analysis (4–6 cells per condition were averaged). The emission ratio images (CFP/FRET) were obtained at different time points as described previously (19). The representative pseudocolor cell images of 600× magnification were shown to highlight the changes in the ratio of CFP/FRET fluorescence intensity.
Cyclic AMP and cGMP Measurement
cAMP and cGMP were measured by specific competitive immunoassay according to the manufacturer's instructions (Enzo Life Sciences; complete ELISA kit for cAMP and cGMP). The Fluostar Omega (BMG Labtech) microplate reader was used to measure the optical density at 405 nm.
Statistical Analysis
Student's t test (two-tailed) was performed to compare the mean values of different groups; p values < 0.05 were considered to be significant. All of the results are represented as mean ± S.E., with n equaling the number of experiments.
RESULTS
MRP4-deficient Mice Fibroblast Cells Migrate Rapidly and Heal Wounds Faster
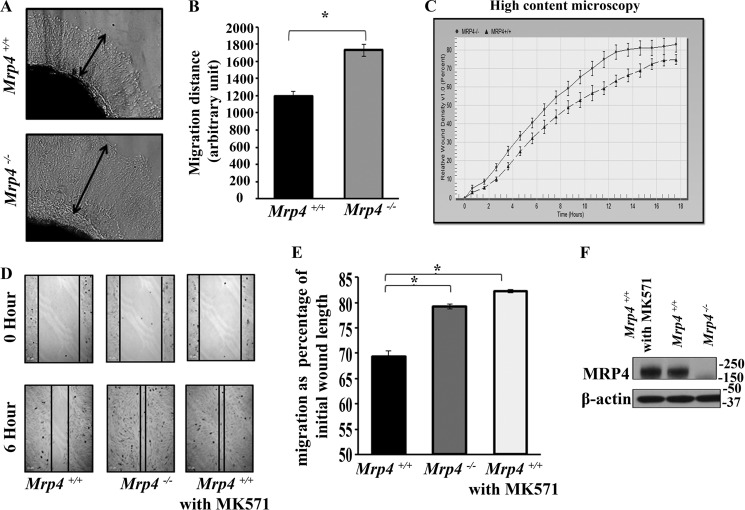
Cyclic nucleotides and cyclic nucleotide-dependent kinases are important for different hallmark steps of cell migration (5, 10). Among the endogenous substrates, MRP4 has been reported to have high affinity for cAMP and cGMP (10, 20). Hence, MRP4 can regulate the intracellular cyclic nucleotide level; and MRP4-deficient cells have altered cyclic nucleotides levels, leading to modified cAMP/cGMP-mediated signaling pathways, including cell migration. Here, we study the role of MRP4 in cell migration using the ex vivo skin explant outgrowth assay. We isolated the skin from Mrp4−/− and Mrp4+/+ mice and then made biopsy patches. They were grown in fibronectin-coated wells for 4 days as described under “Experimental Procedures.” The fibroblast cells migrated out from the skin patches after 2 days. The representative images of cellular outgrowth from skin explants at day 4 post-culture are shown in Fig. 1A. Coating with fibronectin (10 μg/ml) helped the fibroblasts to migrate properly. An extensive halo of cells appeared around Mrp4−/− and wild type mice skin explants. But Mrp4−/− cells migrated 40% more rapidly than the wild type (Fig. 1B).
FIGURE 1.
MRP4-deficient fibroblast cells can migrate faster. Biopsy patches (5 mm) isolated from skin of Mrp4−/− mice or wild type mice were cultured with appropriate media in fibronectin-coated (10 μg/ml PBS) six-well dishes. A, the halo of fibroblast cells came out from the skin explant after 2 days, and the images with 100× magnification represent the 4th day post-seeding. B, the proximal distance of the cellular outgrowth from the explant is represented in the bar graph. C, the MEFs isolated from Mrp4−/− or Mrp4+/+ mice were grown on fibronectin-coated 96-well Essen BioScience ImageLock dishes, and the wounds were made precisely using the 96-pin Wound-Maker provided with the IncuCyteTM. The kinetics of the RWD was analyzed using IncuCyteTM software. D, the conventional wound healing assay was performed on the Mrp4−/− or Mrp4+/+or MK571-treated Mrp4+/+ MEFs, and images were taken with 100× magnification at the 0 and 6th hour. E, the initial and final wound lengths were calculated, and cell migrations as a percentage of initial wound length are represented in the bar graph. F, the immune blots data show the expression of MRP4 in Mrp4−/−, Mrp4+/+, and MK571-treated Mrp4+/+ MEFs. All data represent at least three independent experiments (n = 3). *, p < 0.05.
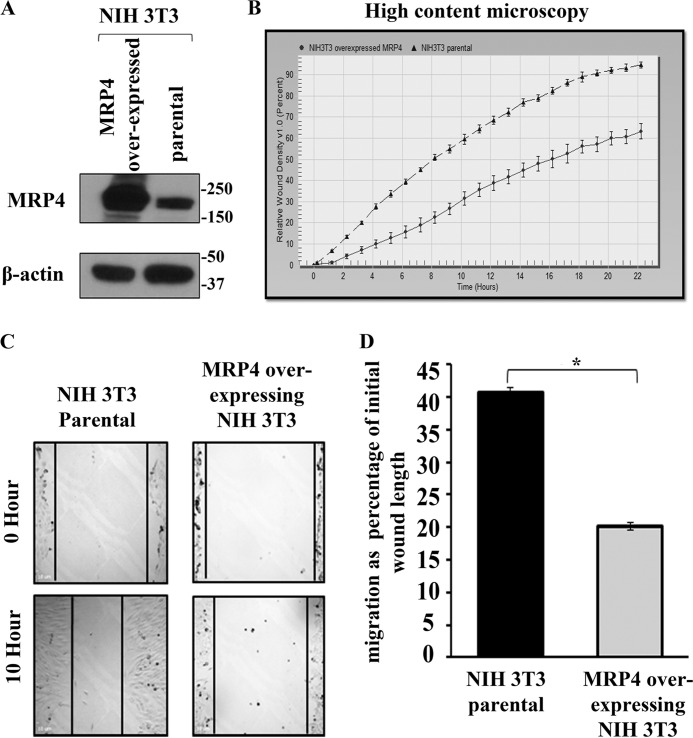
For in vitro wound healing assays, we used two most commonly used fibroblast cell types: 1) MEFs and 2) NIH 3T3. We generated MEFs from wild type and Mrp4−/− mice (21). Using a high-content microscopic technique, we investigated the regulation of fibroblast migration by MRP4 in vitro. The precise wounds were made in the confluent monolayer of MEFs isolated from either Mrp4−/− or Mrp4+/+ mice, and images were taken at 1-h intervals for 18 h using the IncuCyteTM live cell imaging system. The RWD, the spatial cell density in the wound area relative to the spatial cell density outside the wound area, was measured at every time point. The RWD parameter has the advantage of being self-normalized for changes in cell density due to cell proliferation outside the wound area. The kinetics of the RWD is depicted in Fig. 1C. The MRP4-deficient MEFs were found to have faster RWD kinetics than Mrp4+/+ MEFs. These data were supported by the conventional in vitro wound healing assays in which cell migration was represented as a percentage of initial wound length. Cell migration was measured for Mrp4−/−, Mrp4+/+, and MRP4 inhibitor MK571-treated Mrp4+/+ MEFs. MK571-treated MEFs had a similar migration rate as Mrp4−/− MEFs, and both migrated significantly faster than the wild type MEFs (Fig. 1, D and 1E). MK571 did not alter the MRP4 expression in MEFs (Fig. 1F) but antagonized the function of MRP4. All of these data indicated that MRP4 has an inhibitory effect on MEF migration. To further investigate the effect of MRP4 on fibroblast cell migration, we used the in vitro wound healing assay with control and MRP4-overexpressing NIH 3T3 cell lines. The MRP4-overexpressing stable cell line had a higher level of functionally active MRP4 compared with the control NIH 3T3 cell line (Fig. 2A). The high-content microscopy studies revealed slower RWD kinetics for NIH 3T3 cells overexpressing MRP4 compared with the control cells (Fig. 2B). In conventional wound healing experiments, the MRP4 overexpression also showed an inhibitory effect on cell migration (Fig. 2, C and D). These data suggest that MRP4 plays a key role in fibroblast migration. Given that we and others have already shown that MRP4 has an effect on intracellular cyclic nucleotide dynamics (19, 22), and cAMP and cGMP are involved in many hallmark steps of cell migration (6, 10), we rationalized that the MRP4 mediated regulation of intracellular cyclic nucleotides levels is important in this aspect.
FIGURE 2.
MRP4 overexpression decreases fibroblast cells migration. A, MRP4-overexpressing stable NIH 3T3 cells had a higher expression of MRP4 compared with the NIH 3T3 parental cells. B, the MRP4-overexpressing and parental NIH 3T3 cells were grown on fibronectin-coated 96-well Essen BioScience ImageLock dishes, and the wounds were made precisely using the 96-pin Wound-Maker provided with the IncuCyteTM. The kinetics of the RWD was analyzed using IncuCyteTM software. C, the conventional wound healing assay was performed on the MRP4-overexpressing or parental NIH 3T3 cells, and images were taken with 100× magnification at the 0 and 10th hour. D, the initial and final wound lengths were calculated, and cell migrations as a percentage of initial wound length are represented in the bar graph. All data represent at least three independent experiments (n = 3). *, p < 0.05.
Inhibition of MRP4 Augments the Intracellular Cyclic Nucleotide Levels in Fibroblast Cells
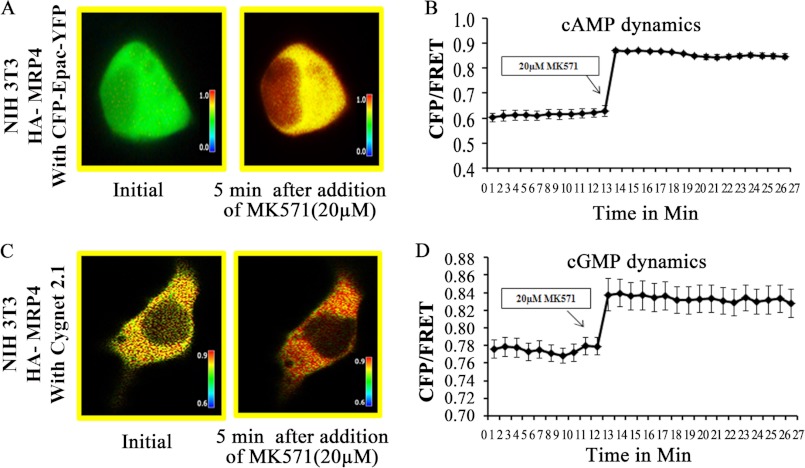
It has been reported that MRP4 is an endogenous efflux transporter of cyclic nucleotides and thereby regulates the intracellular cyclic nucleotide dynamics (1). To investigate the effect of MRP4 inhibition on intracellular cAMP and cGMP dynamics in live fibroblast cells, we used FRET microscopy. We transiently transfected the MRP4-overexpressing NIH 3T3 cells either with cAMP sensor CFP-EPAC-YFP (23) or with cGMP sensor, Cygnet 2.1 (18). Using these unimolecular FRET-based sensors, we were able to monitor the intracellular cAMP and cGMP dynamics with a very high temporal and spatial resolution. Application of 20 μm (19) of the MRP4 inhibitor MK571 induced an increase in the intracellular cAMP level represented by the CFP/FRET emission ratio (Fig. 3, A and B) in fibroblast cells. Similarly, the cGMP level was also increased by the addition of 20 μm MK571 (Fig. 3, C and D). For both of the cases, the effect was rather rapid (within 30 s). These data confirmed that inhibition of MRP4 is responsible for the increases in both intracellular cAMP and cGMP levels. The effect was more profound, however, on the cAMP level compared with the cGMP level, possibly indicating a higher affinity of MRP4 for cAMP.
FIGURE 3.
Inhibition of MRP4 augments intracellular cAMP and cGMP levels. MRP4-overexpressing NIH 3T3 cells were transfected with a FRET-based cAMP sensor CFP-EPAC-YFP or cGMP sensor Cygnet 2.1. A, the pseudocolor images of CFP/FRET with 600× magnification before and 5 min after addition of MK571 (20 μm) for cells expressing cAMP sensor are shown. B, the line graph indicates the intracellular cAMP dynamics before and after addition of MK571 as a change in CFP/FRET ratio. C, the pseudocolor images of CFP/FRET with 600× magnification before and 5 min after addition of MK571 (20 μm) for cells expressing cGMP sensor are shown. D, the line graph indicates intracellular cGMP dynamics before and after addition of MK571 as a change in CFP/FRET ratio. Images in each panel were captured from the same field of view. The colored bar shows the magnitude of the emission ratio. All data represent at least three independent experiments (n = 3). *, p < 0.05.
Increased Accumulation of Intracellular Cyclic Nucleotides in Mrp4−/− MEF Cells
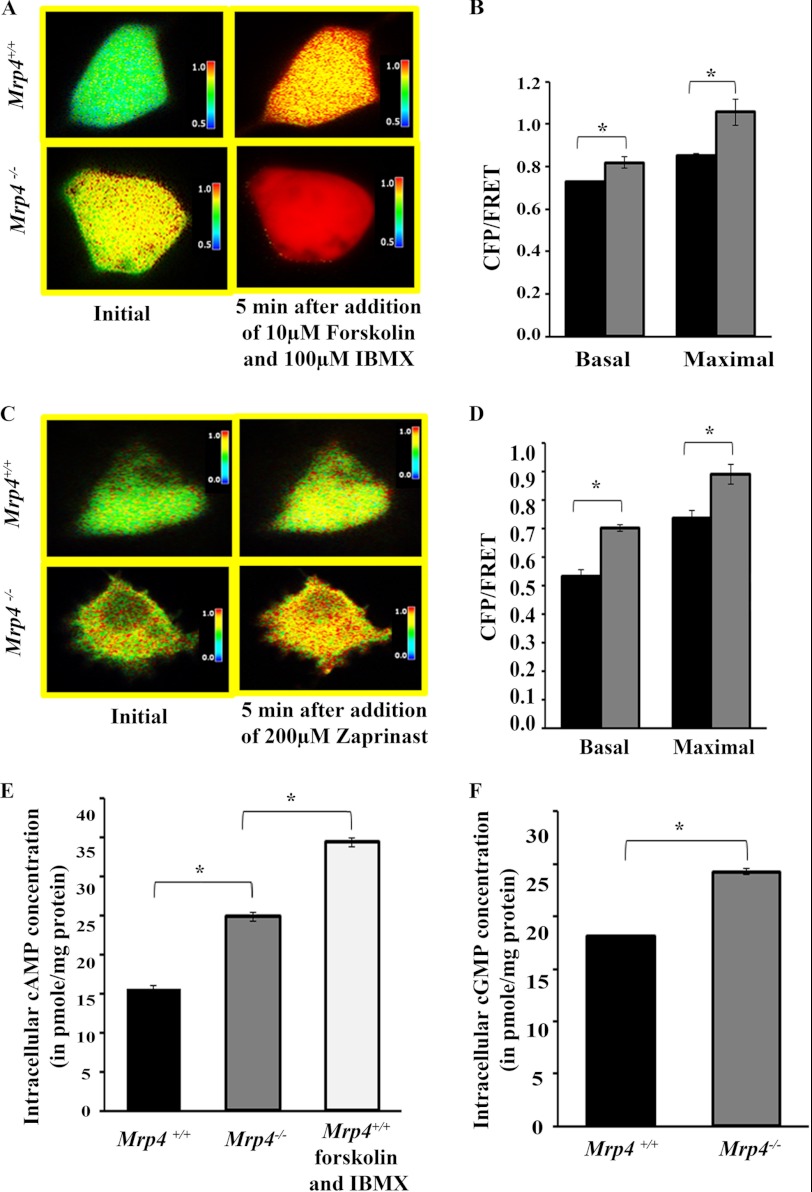
MRP4 as an efflux transporter of cyclic nucleotides is capable of regulating the intracellular cAMP/cGMP level. In the absence of MRP4, there should be a diminished cyclic nucleotides extrusion rate and increased intracellular cAMP/cGMP levels. We used a highly sensitive FRET-based cAMP sensor (CFP-EPAC-YFP) and cGMP sensor (Cygnet 2.1) for monitoring the intracellular cyclic nucleotide levels in Mrp4−/− and Mrp4+/+ MEFs. In the absence of MRP4, the MEFs showed a higher basal level of intracellular cAMP and cGMP (Fig. 4, A and C) compared with the MEFs that endogenously express MRP4. When we treated the cAMP sensor-transfected cells with 10 μm forskolin, a global elevator of cAMP, and 100 μm IBMX, a nonspecific phosphodiesterase inhibitor, the rise in cAMP level was higher in Mrp4−/− MEFs compared with the Mrp4+/+ MEFs. We also noticed that the effect of forskolin and IBMX on cAMP level was more prominent compared with the absence of MRP4 (Fig. 4, A and B). After addition of zaprinast, a specific phosphodiesterase 5 inhibitor, to the cGMP sensor-transfected cells, intracellular cGMP level increased more in Mrp4−/− cells than in Mrp4+/+ MEFs (Fig. 4, C and D). There was, however, a transient increase in cGMP level after addition of zaprinast, whereas a sustained increase in cAMP level was obtained after addition of forskolin and IBMX. Using specific competitive immunoassays for cAMP and cGMP, we further found the higher intracellular cAMP and cGMP levels in MRP4-deficient MEFs (Fig. 4, E and F). When we treated the Mrp4+/+ MEFs with 10 μm forskolin and 100 μm IBMX for 10 min, the intracellular cAMP level increased to a higher extent compared with the Mrp4−/− MEFs. The data suggest that the intracellular cAMP level can be moderately regulated by MRP4, whereas forskolin and IBMX can strongly elevate the cAMP level inside the cells.
FIGURE 4.
MRP4-deficient MEFs have moderately higher levels of intracellular nucleotides. Mrp4−/− MEFs or Mrp4+/+ MEFs were transfected with a FRET-based cAMP sensor CFP-EPAC-YFP or cGMP sensor Cygnet 2.1. A, the pseudocolor images of CFP/FRET with 600× magnification before and 5 min after addition of forskolin (10 μm) and IBMX (100 μm) for cells expressing cAMP sensor are shown. B, the bar graphs represent the CFP/FRET ratio in basal condition and after treatment in MEFs from Mrp4+/+ (black bars) or Mrp4−/− (gray bars). C, the pseudocolor images of CFP/FRET with 600× magnification before and 5 min after addition of zaprinast (200 μm) for cells expressing cGMP sensor are shown. D, the bar graphs represent the CFP/FRET ratio in basal condition and after treatment in MEFs from Mrp4+/+ (black bars) or Mrp4−/− (gray bars). Images in each panel were captured from the same field of view. The colored bar shows the magnitude of the emission ratio. E, the intracellular cAMP levels in Mrp4−/−, Mrp4+/+, and forskolin (10 μm) and IBMX (100 μm) treated Mrp4+/+ MEFs were measured using competitive immunoassay. F, in similar experiments, cGMP levels were also measured for Mrp4−/− and Mrp4+/+ MEFs. All data represent at least three independent experiments (n = 3). *, p < 0.05.
Migration of MEFs Is Attenuated in the Presence of Excessive cAMP
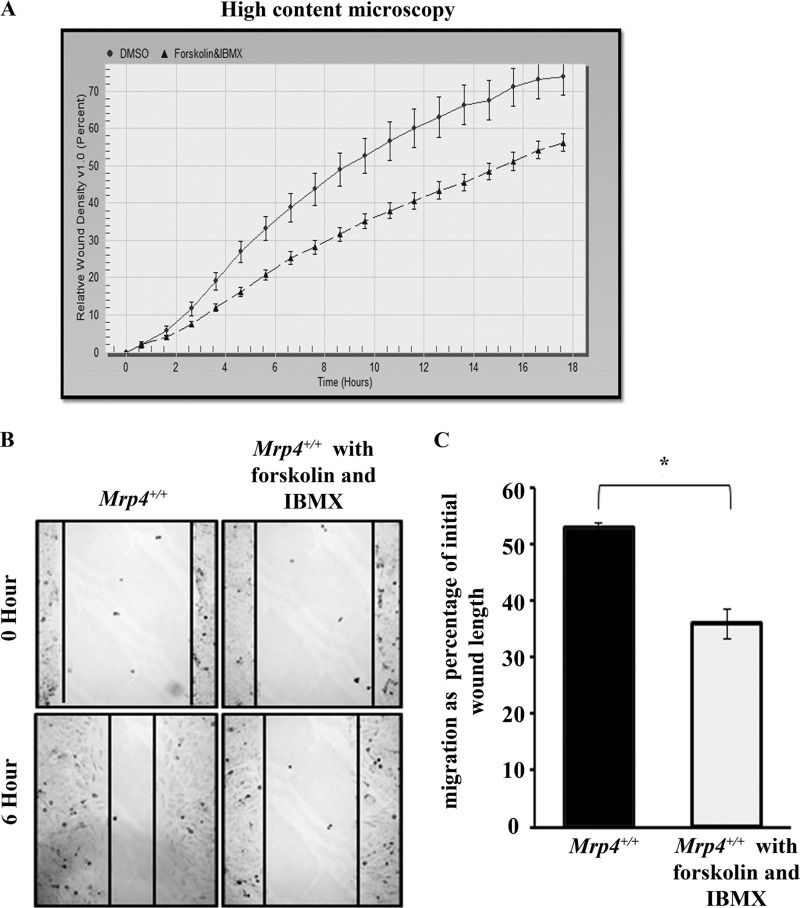
We observed that the inhibition of the cyclic nucleotide transporter MRP4 led to accumulation of more cAMP inside the cells and resulted in an increased migration rate. As the importance of cAMP in directional migration is well established (5), the global cAMP-inducing agent forskolin and the nonspecific phosphodiesterase inhibitor IBMX should regulate cell migration. The strong elevation with forskolin and reduced degradation with IBMX lead to a robust increase in intracellular cAMP level. So, we next tried to find the effect of forskolin and IBMX treatment on MEF migration. The high content microscopy studies revealed that the RWD kinetics is faster for control MEFs compared with the forskolin- and IBMX-treated MEFs (Fig. 5A). A similar result was found with the conventional wound healing experiments, which showed significantly slower cell migration after forskolin and IBMX treatment (Fig. 5, B and C). However, using the CFP-PKA regulatory subunit and YFP-PKA catalytic subunit as FRET-based cAMP sensor, we found inhibition of MRP4 increased the cAMP level near the leading edge of a migrating fibroblast, whereas forskolin or IBMX treatment caused a robust increase in the cAMP level throughout the cell (supplemental Movie S1). Thus, forskolin and IBMX, which strongly elevate the intracellular cAMP level, had an inhibitory effect on MEF cell migration; whereas inhibition of MRP4, which moderately increased the cAMP level inside the cell, stimulated cell migration.
FIGURE 5.
Forskolin and IBMX inhibit MEF migration by strongly elevating intracellular cAMP levels. A, dimethyl sulfoxide (DMSO)-treated or forskolin (10 μm) and IBMX (100 μm) treated Mrp4+/+ MEFs were grown on fibronectin-coated 96-well Essen BioScience ImageLock dishes, and the wounds were made precisely using the 96-pin Wound-Maker provided with the IncuCyteTM. The kinetics of the RWD was analyzed by IncuCyteTM software. B, the conventional wound healing assay was done with dimethyl sulfoxide- or forskolin (10 μm) and IBMX (100 μm) treated Mrp4+/+ MEFs, and images were taken with 100× magnification at the 0th and 6th hour. C, the initial and final wound lengths were calculated, and cell migrations as a percentage of initial wound length are represented in the bar graph. All data represent at least three independent experiments (n = 3). *, p < 0.05.
Cyclic Nucleotides Show a Biphasic Effect on MEF Cell Migration
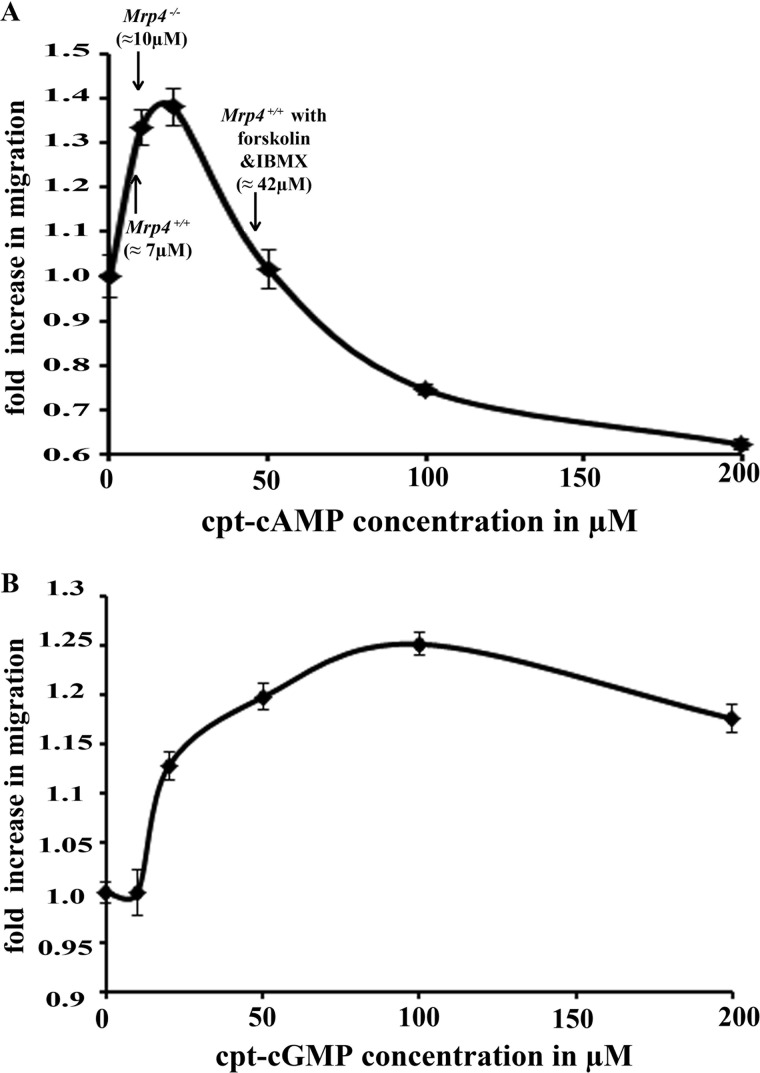
The effect of MRP4 inhibition on fibroblast migration was found to be opposite to the effect of forskolin and IBMX treatment. This paradoxical effect of cAMP on cell migration compelled us to investigate whether cyclic nucleotides regulate cell migration in a concentration-dependent manner. We treated the MEFs with different concentrations of cell-permeable cpt-cAMP and cpt-cGMP (0, 10, 20, 50, 100, and 200 μm). With conventional in vitro wound healing assays, we found both cAMP and cGMP had a biphasic effect on cell migration. Up to 20 μm cAMP can stimulate cell migration in a dose-dependent fashion; but after that, it began to decrease the migration rate (Fig. 6A). We further noticed that the intracellular concentration of cAMP for Mrp4+/+ MEFs was ≈ 7 μm and for Mrp4−/− MEFs ≈ 10 μm, whereas prolonged treatment with 10 μm forskolin and 100 μm IBMX (>30 min) strongly increased the concentration up to ≈ 42 μm (Fig. 6A). Where the absence of MRP4 increased the intracellular cAMP concentration to a level at which cAMP exerted its stimulating effect on migration, extended treatment with forskolin or IBMX further raised the concentration; and, as a consequence, cell migration was inhibited. cGMP had a similar biphasic effect, but the concentration range for stimulation was broader, and the effect was milder than with cAMP. It positively regulated cell migration up to 100 μm and then exerted the inhibitory effect (Fig. 6B). Altogether, these data suggest that a moderate elevation of cyclic nucleotides stimulates cell migration but that a further increase leads to inhibition.
FIGURE 6.
cAMP and cGMP have a biphasic effect on fibroblast cell migration. The conventional wound healing assay was done with Mrp4+/+ MEFs using different concentrations (0, 10, 20, 50, 100, and 200 μm) of cell-permeable cpt-cAMP (A) or cpt-cGMP (B). Images were taken with 100× magnification at the 0 and 6th hour. The initial and final wound lengths were calculated, and the fold increases in cell migration rates were represented. A, the intracellular cAMP concentration were measured by competitive immunoassay for Mrp4−/−, Mrp4+/+, and forskolin (10 μm) and IBMX (100 μm) treated Mrp4+/+ MEFs. The intracellular cAMP levels corresponding to those cells are indicated by arrows. All data represent at least three independent experiments (n = 3).
DISCUSSION
Our study demonstrates that inhibition of MRP4 moderately increases the intracellular cyclic nucleotide level and stimulates fibroblast cell migration. MRP4 was first identified as an efflux transporter of a nucleoside-based antiviral drug in a human T-lymphoid cell line (12). Besides having a role in conferring drug resistance, MRP4 was also found to regulate quite diverse physiological phenomena via alteration of the intracellular concentration of its different endogenous substrates (24). However, among the endogenous substrates, MRP4 has been reported to have the high affinity for cAMP (Km = 45 μm) and cGMP (Km = 10 μm), and these two cyclic nucleotides are very important signaling molecules involved in many physiological and pathological events. These elegant but simple secondary messengers regulate different complex cellular phenomena, mostly via the activation of their corresponding kinases (25, 26). Cell migration is a highly integrated multistep process that plays a central role in embryonic development, tissue repair, cancer, and many other physiological and pathological events (27, 28). These secondary messengers play important roles in the signaling pathways at various stages of the cell migration process, either directly or by activating their corresponding kinases. The PKA activity increases at the leading edge of a migrating cell in a Rac GTPase-dependent manner and is important for maintaining cell polarity and pseudopod formation (6). Conversely, cAMP has been reported to inhibit Rac-induced lamellipodia formation (8). Similarly, PKG has been shown to be down regulated during vascular smooth muscle cell migration (9), whereas PKG is also important for maintaining the repetitive behavior of migration by phosphorylating vasodilator-stimulated phosphoprotein (10). The complex and equivocal effects of cAMP and cGMP on cell migration ensure a tightly regulated and balanced intracellular cyclic nucleotide level for fine-tuned cell migration.
Our ex vivo skin explant outgrowth study as well as the high-content microscopy and conventional wound healing data showed significantly increased cell migration for MRP4-deficient fibroblast cells, suggesting an important role of MRP4 in the regulation of fibroblast migration. In similar experiments, inhibition of MRP4 significantly increased cell migration, whereas overexpression of MRP4 reduced fibroblast motility suggesting a negative effect of MRP4 on fibroblast cell migration. To understand the mechanism, we focused on different endogenous substrates for MRP4 that are important in this aspect. As MRP4 has high affinity for cAMP and cGMP and these 2-s messengers were already reported to be involved in different hallmark steps in cell migration, we tried to monitor the effect of MRP4 inhibition on intracellular cyclic nucleotide levels. Using FRET-based sensors for cAMP and cGMP, we found that MRP4 inhibition by MK571 increased both cAMP and cGMP levels inside the cells and that the effect was stronger for cAMP compared with cGMP, indicating the higher affinity of MRP4 for cAMP. Using the same sensor, we found that Mrp4−/− MEFs had higher basal levels of cAMP and cGMP. The intracellular cyclic nucleotide level is controlled by phosphodiesterase-mediated hydrolysis and by the process involving active efflux transport from the cell. The phosphodiesterase 5 inhibitor zaprinast prevents breakdown of cGMP and thus increases the intracellular cGMP level. In the absence of MRP4, the extrusion of cGMP from the cell was also reduced, and there was more accumulation of intracellular cGMP; therefore, the zaprinast-induced elevation of cGMP is greater in Mrp4−/− MEFs. Forskolin, which elevates the cAMP level by stimulating adenylate cyclase, and the nonspecific phosphodiesterase inhibitor IBMX cause a huge and prolonged increase in the intracellular cAMP level. The MRP4-deficient cells accumulated more cAMP than Mrp4+/+ MEFs upon treatment with forskolin and IBMX because of less efflux transportation of cAMP. We found that the sustained and robust increase in cAMP level evoked by forskolin and IBMX inhibited fibroblast migration, whereas a stimulatory effect was shown for a moderate increase in cAMP level, mostly near the leading edge of a migrating fibroblast, by the inhibition of MRP4. Thus, MRP4 inhibition may facilitate fibroblast cell polarization, which is a hallmark step for directional migration. In wound healing studies with cell-permeable cyclic nucleotides, cpt-cAMP in concentrations up to 20 μm enhanced the cell migration in a concentration-dependent manner; after that, the effect was inhibitory. The intracellular cAMP level of Mrp4−/− MEFs was moderately higher than that of Mrp4+/+ MEFs (∼1.5 fold), and it fell within the stimulatory range (1–20 μm). Prolonged treatment (>30 min) with forskolin and IBMX, however, increased the level further (∼6.5 fold), at which point the effect of cAMP was no longer stimulatory, but instead inhibitory to cell migration. This explains the opposing effects of forskolin, IBMX treatment, and MRP4 inhibition on cell migration. A similar but milder biphasic effect was also shown for cpt-cGMP, which stimulated cell migration when used in concentrations up to 100 μm. Hence, balanced and highly controlled intracellular cyclic nucleotides levels are implicated as important in fibroblast cell migration.
Similar to our findings, the knockdown of MRP4 has already been reported to enhance human retinal vascular endothelial cell migration (29). However, some other studies with dendritic cells (24) and arterial smooth muscle cells (22) showed a stimulatory effect of MRP4 on cell migration. This dichotomy of MRP4 as an effector may be related to the ambiguous role of cyclic nucleotides in cell migration and can be explained by the tissue-specific expression level of MRP4 and its interactome. Variation in MRP4-dependent regulation of intracellular cyclic nucleotide levels and differential effects of cyclic nucleotides on different types of cell migration could be the major issue. The other endogenous substrates of MRP4 may also be involved in this process of regulation (30). Taken together, our findings suggest that MRP4 can negatively regulate fibroblast cell migration, at least in part, in a cyclic nucleotide-dependent manner. Our observation that inhibition of MRP4 increases fibroblast cell migration warrants the use of MRP4 inhibitor as a new and potent wound healing agent. During the use of MRP4 inhibitor to reduce chemotherapy resistance, the indirect effect on cancer metastasis should be taken into consideration. Further detailed studies are required to examine the role of MRP4 in different types of cancer metastasis. However, together with enhanced wound repair, augmentation of intracellular cAMP by inhibition of MRP4 can be a good strategy to reduce fibroblast to myofibroblast transformation (31, 32) for preventing unwanted tissue fibrosis.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-DK080834, RO1-DK093045 (to A. P. N.), and RO1-GM60904 (to J. D. S).

This article contains supplemental Movie 1.
- MRP4
- multidrug resistance protein 4
- MEF
- mouse embryonic fibroblast
- IBMX
- isobutylmethylxanthine
- PKA
- cAMP-dependent protein kinase A
- PKG
- cGMP-dependent protein kinase
- RWD
- relative wound density.
REFERENCES
- 1. Russel F. G., Koenderink J. B., Masereeuw R. (2008) Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signaling molecules. Trends Pharmacol. Sci. 29, 200–207 [DOI] [PubMed] [Google Scholar]
- 2. Schinkel A. H., Jonker J. W. (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 55, 3–29 [DOI] [PubMed] [Google Scholar]
- 3. Elferink J. G., de Koster B. M. (1993) The effect of cyclic GMP and cyclic AMP on migration by electroporated human neutrophils. Eur. J. Pharmacol. 246, 157–161 [DOI] [PubMed] [Google Scholar]
- 4. Elferink J. G., VanUffelen B. E. (1996) The role of cyclic nucleotides in neutrophil migration. Gen. Pharmacol. 27, 387–393 [DOI] [PubMed] [Google Scholar]
- 5. Howe A. K. (2004) Regulation of actin-based cell migration by cAMP/PKA. Biochim. Biophys. Acta 1692, 159–174 [DOI] [PubMed] [Google Scholar]
- 6. Lim C. J., Kain K. H., Tkachenko E., Goldfinger L. E., Gutierrez E., Allen M. D., Groisman A., Zhang J., Ginsberg M. H. (2008) Integrin-mediated protein kinase A activation at the leading edge of migrating cells. Mol. Biol. Cell 19, 4930–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howe A. K., Baldor L. C., Hogan B. P. (2005) Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc. Natl. Acad. Sci. U.S.A. 102, 14320–14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L., Zhang J. J., Huang X. Y. (2008) cAMP inhibits cell migration by interfering with Rac-induced lamellipodium formation. J. Biol. Chem. 283, 13799–13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Negash S., Narasimhan S. R., Zhou W., Liu J., Wei F. L., Tian J., Raj J. U. (2009) Role of cGMP-dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia. Am. J. Physiol. Heart Circ. Physiol. 297, H304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo D., Tan Y. C., Wang D., Madhusoodanan K. S., Zheng Y., Maack T., Zhang J. J., Huang X. Y. (2007) A Rac-cGMP signaling pathway. Cell 128, 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krause M., Dent E. W., Bear J. E., Loureiro J. J., Gertler F. B. (2003) Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 19, 541–564 [DOI] [PubMed] [Google Scholar]
- 12. Schuetz J. D., Connelly M. C., Sun D., Paibir S. G., Flynn P. M., Srinivas R. V., Kumar A., Fridland A. (1999) MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat. Med. 5, 1048–1051 [DOI] [PubMed] [Google Scholar]
- 13. Kopecki Z., Arkell R., Powell B. C., Cowin A. J. (2009) Flightless I regulates hemidesmosome formation and integrin-mediated cellular adhesion and migration during wound repair. J. Invest. Dermatol. 129, 2031–2045 [DOI] [PubMed] [Google Scholar]
- 14. Mazzalupo S., Wawersik M. J., Coulombe P. A. (2002) An ex vivo assay to assess the potential of skin keratinocytes for wound epithelialization. J. Invest. Dermatol. 118, 866–870 [DOI] [PubMed] [Google Scholar]
- 15. Liu L., Wang Y. D., Wu J., Cui J., Chen T. (2012) Carnitine palmitoyltransferase 1A (CPT1A): a transcriptional target of PAX3-FKHR and mediates PAX3-FKHR-dependent motility in alveolar rhabdomyosarcoma cells. BMC Cancer 12, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghosh M. C., Makena P. S., Gorantla V., Sinclair S. E., Waters C. M. (2012) CXCR4 regulates migration of lung alveolar epithelial cells through activation of Rac1 and matrix metalloproteinase-2. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ponsioen B., Zhao J., Riedl J., Zwartkruis F., van der Krogt G., Zaccolo M., Moolenaar W. H., Bos J. L., Jalink K. (2004) Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Reports 5, 1176–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Honda A., Adams S. R., Sawyer C. L., Lev-Ram V., Tsien R. Y., Dostmann W. R. (2001) Spatiotemporal dynamics of guanosine 3′,5′-cyclic monophosphate revealed by a genetically encoded, fluorescent indicator. Proc. Natl. Acad. Sci. U.S.A. 98, 2437–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C., Krishnamurthy P. C., Penmatsa H., Marrs K. L., Wang X. Q., Zaccolo M., Jalink K., Li M., Nelson D. J., Schuetz J. D., Naren A. P. (2007) Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131, 940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sellers Z. M., Naren A. P., Xiang Y., Best P. M. (2012) MRP4 and CFTR in the regulation of cAMP and β-adrenergic contraction in cardiac myocytes. Eur. J. Pharmacol. 681, 80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suetsugu S., Yamazaki D., Kurisu S., Takenawa T. (2003) Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell 5, 595–609 [DOI] [PubMed] [Google Scholar]
- 22. Hara Y., Sassi Y., Guibert C., Gambaryan N., Dorfmüller P., Eddahibi S., Lompré A. M., Humbert M., Hulot J. S. (2011) Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J. Clin. Invest. 121, 2888–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Penmatsa H., Zhang W., Yarlagadda S., Li C., Conoley V. G., Yue J., Bahouth S. W., Buddington R. K., Zhang G., Nelson D. J., Sonecha M. D., Manganiello V., Wine J. J., Naren A. P. (2010) Compartmentalized cyclic adenosine 3′,5′-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol. Biol. Cell 21, 1097–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van de Ven R., Scheffer G. L., Reurs A. W., Lindenberg J. J., Oerlemans R., Jansen G., Gillet J. P., Glasgow J. N., Pereboev A., Curiel D. T., Scheper R. J., de Gruijl T. D. (2008) A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood 112, 2353–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott J. D. (1991) Cyclic nucleotide-dependent protein kinases. Pharmacol. Ther. 50, 123–145 [DOI] [PubMed] [Google Scholar]
- 26. Copsel S., Garcia C., Diez F., Vermeulem M., Baldi A., Bianciotti L. G., Russel F. G., Shayo C., Davio C. (2011) Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J. Biol. Chem. 286, 6979–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration: integrating signals from front to back. Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 28. Lauffenburger D. A., Horwitz A. F. (1996) Cell migration: a physically integrated molecular process. Cell 84, 359–369 [DOI] [PubMed] [Google Scholar]
- 29. Tagami M., Kusuhara S., Imai H., Uemura A., Honda S., Tsukahara Y., Negi A. (2010) MRP4 knockdown enhances migration, suppresses apoptosis, and produces aggregated morphology in human retinal vascular endothelial cells. Biochem. Biophys. Res. Commun. 400, 593–598 [DOI] [PubMed] [Google Scholar]
- 30. Lin Z. P., Zhu Y. L., Johnson D. R., Rice K. P., Nottoli T., Hains B. C., McGrath J., Waxman S. G., Sartorelli A. C. (2008) Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Mol. Pharmacol. 73, 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swaney J. S., Roth D. M., Olson E. R., Naugle J. E., Meszaros J. G., Insel P. A. (2005) Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 102, 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camelliti P., Borg T. K., Kohl P. (2005) Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 65, 40–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.