Background: The constitutively active kinase GSK3β is a negative regulator of thrombin-stimulated platelet function.
Results: Interfering with PKCα and Akt blocked thrombin-mediated GSK3α/β phosphorylation and reduced platelet function.
Conclusion: PKCα and Akt phosphorylate GSK3α/β resulting in reduced GSK3α/β activity and increased thrombin-mediated platelet function.
Significance: This study shows a novel mechanism by which GSK3α/β is regulated and contributes to platelet function.
Keywords: Akt PKB, Glycogen Synthase Kinase 3, PI 3-Kinase (PI3K), Protein Kinase C (PKC), Protein Phosphorylation, Thrombosis, Platelets
Abstract
Glycogen synthase kinase-3 is a Ser/Thr kinase, tonically active in resting cells but inhibited by phosphorylation of an N-terminal Ser residue (Ser21 in GSK3α and Ser9 in GSK3β) in response to varied external stimuli. Recent work suggests that GSK3 functions as a negative regulator of platelet function, but how GSK3 is regulated in platelets has not been examined in detail. Here, we show that early thrombin-mediated GSK3 phosphorylation (0–30 s) was blocked by PKC inhibitors and largely absent in platelets from PKCα knock-out mice. In contrast, late (2–5 min) GSK3 phosphorylation was dependent on the PI3K/Akt pathway. Similarly, early thrombin-mediated inhibition of GSK3 activity was blocked in PKCα knock-out platelets, whereas the Akt inhibitor MK2206 reduced late thrombin-mediated GSK3 inhibition and largely prevented GSK3 inhibition in PKCα knock-out platelets. More importantly, GSK3 phosphorylation contributes to platelet function as knock-in mice where GSK3α Ser21 and GSK3β Ser9 were mutated to Ala showed a significant reduction in PAR4-mediated platelet aggregation, fibrinogen binding, and P-selectin expression, whereas the GSK3 inhibitor CHIR99021 enhanced these responses. Together, these results demonstrate that PKCα and Akt modulate platelet function by phosphorylating and inhibiting GSK3α/β, thereby relieving the negative effect of GSK3α/β on thrombin-mediated platelet activation.
Introduction
When a blood vessel becomes damaged, platelets quickly adhere to the newly exposed extracellular matrix and become activated through GPVI signaling, resulting in the exposure of fibrinogen binding sites on integrin αIIbβ3 and granule secretion. A subset of platelets will also expose phosphatidylserine on the outer membrane, nucleating the formation of the prothrombinase complex and subsequent thrombin generation. Thrombin is a potent platelet agonist that cleaves the protease-activated receptors (PARs)3 PAR1 and PAR4 on the platelet surface thereby further activating and recruiting platelets to the site of injury. The PAR receptors are G protein-coupled receptors, which, upon cleavage of the receptor, activate the phospholipase C pathway, leading to an increase in intracellular Ca2+ and activation of conventional protein kinase C (PKC) isoforms. Of these, the PKC isoforms PKCα and PKCβ play nonredundant roles in granule secretion and thrombus formation (1–3).
In addition to activation of the phospholipase C pathway, many platelet agonists also activate class I phosphatidylinositol 3 (PI3)-kinases, which phosphorylate PI(4,5)P2 in the membrane leading to an increase in the signaling molecule PI(3,4,5)P3. Human and mouse platelets express all class I PI3K isoforms (PI3Kα, PI3Kβ, PI3Kδ, and PI3Kγ), and the use of genetic models and isoform-specific inhibitors has revealed that PI3Kβ plays a predominant role in platelet function, in particular, downstream of GPVI and integrin αIIbβ3 (4–6). In addition to a role for PI3K in agonist-stimulated platelet function, it has recently become apparent that several platelet primers, such as TPO, IGF-1, and GAS6, also mediate their potentiating effect on platelet function through a PI3K-dependent pathway (7–9). One of the most studied pathways downstream of PI3K is Akt (aka protein kinase B), a Ser/Thr kinase involved in cell proliferation, survival, and metabolism (10). Activation of PI3K results in an increase in PI(3,4,5)P3, which recruits the PH domain-containing kinases PDK1 and Akt to the membrane where PDK1 phosphorylates Akt on Thr308 in the T-loop. Akt is subsequently phosphorylated by mTORC2 on Ser473, resulting in maximal Akt activity (11). Studies on various Akt transgenic mice suggest that Akt activation contributes to integrin activation, granule secretion, and thrombosis (12–14). How Akt regulates platelet function is still an active area of research.
Although numerous substrates for Akt have been described, only a few have definitively been identified in human platelets so far; the proline-rich Akt substrate of 40 kDa (PRAS40) (11) and the Ser/Thr kinase glycogen synthase kinase 3 (GSK3) (11, 12, 15). Although PDE3A was initially reported to be an Akt substrate (16), the availability of improved reagents allowed us to demonstrate that PDE3A activity is regulated mainly by PKC in human platelets (17). In addition, eNOS has been proposed to be a direct Akt substrate (18), although whether eNOS is even present in platelets is a matter of considerable debate (19, 20). GSK3 was the first Akt substrate to be identified by its ability to phosphorylate and inhibit the activity of glycogen synthase (21). GSK3 exists in two isoforms α and β, of which GSK3β is likely to be expressed predominantly in platelets (15, 22). A recent study suggested that GSK3β negatively regulates platelet function as the small molecule GSK3 inhibitor SB216763 potently enhanced PAR1-mediated aggregation, whereas GSK3β+/− mice showed increased PAR4-mediated aggregation and in vivo thrombosis (22). It has been challenging, however, to demonstrate a direct link among Akt, GSK3α/β phosphorylation, and changes in platelet function. Indeed, GSK3 is a promiscuous substrate, which can be phosphorylated by several kinases including PKC (23, 24), PKA (25), p90RSK (26, 27), and Akt (10). The aim of the present study was therefore to elucidate the role of GSK3α and GSKβ phosphorylation in platelet function and identify the signaling pathways involved. Using genetic and pharmacological approaches, we present intriguing evidence that both PKCα and Akt phosphorylate and inhibit GSK3, thereby promoting thrombin-mediated integrin αIIbβ3 activation and granule secretion.
EXPERIMENTAL PROCEDURES
Mice
All animal studies were approved by local research ethics, and mice were bred for this purpose under a UKHome Office project license. GSK3αS21A/S21A/βS9A/S9A (GSK3 KI), control wild-type GSK3α/β+/+/+/+ (GSK3 WT), PKCα−/− (PKCα KO), and control wild-type PKCα+/+ (PKCα WT) animals were generated, bred, and genotyped as described previously (28–30). GSK3αS21A/S21A/βS9A/S9A (GSK3 KI) were kindly provided by Dario Alessi, MRC Phosphorylation Unit, Dundee and the PKCα−/− (PKCα KO) by Professor Jeff Molkentin, Cincinnati Children's Hospital, Cincinnati, OH.
Reagents
pSer473 Akt, pThr308 Akt, Akt2 (L79BZ), Akt3(62A8), Akt3 (L47B1), Akt3 (pAb), pSer9 GSK3β, pSer21/9 GSK3α/β, GSK3β, pThr246 PRAS40, PKC phospho-motif (used for analysis of pleckstrin phosphorylation), pThr202/Tyr204 ERK, pThr180/Tyr182 p38, integrin β3 and PKCα antibodies were from Cell Signaling Technologies (New England Biolabs). Akt1 (B-1), P-Selectin (M-20) and PAR4 (C-20) antibodies were from Santa Cruz (Insight Biotechnology, Wembley, UK). The Akt1 rabbit mAb (AW24) was from Millipore. Akt2 antiserum 1.1 raised against amino acids 453–470 of murine Akt2 in rabbits was kindly provided by Dick Denton and Kelly Moule (School of Biochemistry, University of Bristol). PE-labeled anti-mouse P-selectin was from Emfret Analytics (Wurzberg, Germany). Human brain tissue lysate was from Abcam (Cambridge, UK). PAR1-activating peptide (SFLLRN-NH2) was from Bachem (Weil am Rhein, Germany). RPRAATF, RRAAEELDSRAGS(P)PQL, and PAR4-activating peptide (AYPGKF-NH2) were synthesized by Graham Bloomberg (University of Bristol). CHIR99021 was from Merck Chemicals. MK2206 was from Selleck Chemicals (Stratech, Newmarket, UK). Bisindolylmaleimide IX (BIM) was from Tocris (Bristol, UK). Chronolume was from Chrono-log Corporation (Labmedics, Manchester, UK). Microcystin-LR was from Axxora (Nottingham, UK). [γ-32P]ATP was from PerkinElmer Life Sciences. Enhanced chemiluminescent detection reagents were from GE Healthcare. Peroxidase-conjugated secondary antibodies were from Jackson Immunoresearch. NuPAGE SDS-PAGE sample buffer was from Invitrogen. All other reagents were from Sigma unless otherwise indicated.
Platelet Isolation
Blood was obtained with approval from the local Research Ethics Committee of the University of Bristol from healthy drug-free volunteers, who gave full informed consent in accordance with the Declaration of Helsinki. Mouse blood was drawn by cardiac puncture under terminal anesthesia. Washed human and mouse platelets were isolated as described previously (31). Platelets were resuspended at 4 × 108/ml in modified HEPES-Tyrode buffer (145 mm NaCl, 3 mm KCl, 0.5 mm Na2HPO4, 1 mm MgSO4, 10 mm HEPES, pH 7.2, 0.1% (w/v) d-glucose, 0.02 unit/ml apyrase, and 10 μm indomethacin).
Platelet Extraction
Platelets were treated with vehicle (0.2% dimethyl sulfoxide) or compound for 15 min, stimulated as indicated, and lysed directly in 4× NuPAGE sample buffer (whole cell lysate). Alternatively, platelets were extracted with an equal volume of ice-cold (i) radioimmunoprecipitation assay buffer (50 mm HEPES, pH 7.4, 400 mm NaCl, 2 mm EDTA, 2% (v/v) IGEPAL CA-630, 1% (w/v) sodium deoxycholate, 0.2% (w/v) SDS, 40 mm sodium β-glycerophosphate, 20 mm sodium pyrophosphate, 2 mm benzamidine, 2 μm microcystin-LR, 10 mm sodium orthovanadate, and 2 μg/ml each pepstatin, antipain, and leupeptin) for immunoprecipitation of Akt or (ii) Triton X-100 extraction buffer (50 mm HEPES, pH 7.4, 2% (v/v) Triton X-100, 2 mm EDTA, 40 mm sodium β-glycerophosphate, 20 mm sodium pyrophosphate, 2 mm benzamidine, 20% (v/v) glycerol, 10 mm sodium orthovanadate, 2 μm microcystin-LR, and 2 μg/ml each pepstatin, antipain, and leupeptin) for activity assays.
Immunoprecipitation of Akt
Akt1, Akt2, or Akt3 was immunoprecipitated from radioimmunoprecipitation assay lysates by incubation with anti-Akt1 (B1), anti-Akt2 (antiserum 1.1), or anti-Akt3 (L47B1) for 2 h at 4 °C. Immune complexes were washed with extraction buffer prior to elution with 2× NuPAGE sample buffer.
Immunoblotting
Proteins were resolved by electrophoresis on 8% bis-tris gels as described previously (17). Samples were transferred to PVDF membrane, blocked with blocking buffer (Sigma), and subjected to immunoblotting.
Akt and GSK3 Activity Assay
Akt and GSK3 activity were measured as described previously (33). Briefly, Akt1, Akt2, or Akt3 was immunoprecipitated from platelet lysates by incubation with anti-Akt1 (B1), anti-Akt2 (antiserum 1.1), or anti-Akt3 (L47B1). Immune complexes were washed and resuspended in assay buffer (20 mm HEPES, pH 7.4, 10 mm MgCl2, 20 mm sodium β-glycerophosphate, 1 mm EGTA, and 1 mm DTT) in the presence of 50 μm ATP (2 μCi of [γ-32P]ATP), 0.5 mg/ml RPRAATF peptide, and 2.5 μm PKA inhibitor (IP-20). GSK3 activity in cell lysates was measured in assay buffer in the presence of 50 μm ATP (2 μCi of [γ-32P]ATP), 0.71 mg/ml RRAAEELDSRAGS(P)PQL, and 2.5 μm PKA inhibitor (IP-20). Assays were terminated after a 30-min incubation at 30 °C by spotting onto P81 phosphocellulose filter paper. Filters were washed with 0.75% orthophosphoric acid before measuring the incorporation of [γ-32P]ATP by Cherenkov counting. Akt activity results are expressed as pmol of phosphate incorporated per 108 platelets. For GSK3 activity LiCl (50 mm) was used to distinguish and correct for nonspecific activity; therefore, activity is expressed as a percentage of basal LiCl inhibitable activity.
Platelet Aggregation and Dense Granule Secretion
Washed platelets (2 × 108/ml) were incubated with vehicle (0.2% dimethyl sulfoxide) or the indicated compounds for 15 min. Agonist-stimulated aggregation and dense granule secretion were simultaneously monitored using a luciferin/luciferase reagent (Chronolume) in a Chronolog 590–2A aggregometer 37 °C under stirring conditions.
Integrin αIIbβ3 Activation and α-Granule Secretion
Integrin αIIbβ3 activation was assessed using fibrinogen labeled with fluorescein as described previously (34). α-Granule secretion was assessed as P-selectin exposure with PE-labeled anti-P-selectin. Platelets (2 × 107/ml) were incubated with vehicle (0.2% dimethyl sulfoxide) or the indicated compounds for 15 min before stimulation in the presence of 0.2 mg/ml FITC-fibrinogen and PE-labeled anti-P-selectin for 10 min. Nonspecific binding of FITC-fibrinogen was determined in the presence of 1 mm EDTA. Samples were fixed with 1% paraformaldehyde for 30 min and analyzed by flow cytometry on a BD LSR II (BD Biosciences).
Statistics
Data were analyzed and fitted using GraphPad Prism 4.02 software. All data are presented as the mean ± S.E. of at least three independent observations. Concentration-response curves were fitted with a four-parameter logistic equation. Differences in best-fit parameters between data sets were determined by F test where the null hypothesis states that both data sets can be fitted using the same parameters. Data presented with statistical analysis were tested using either a paired Student's t test (aggregation/ATP secretion data) or two-way analysis of variance with Bonferroni multiple comparison post hoc test (quantification blots/GSK3 activity data).
RESULTS
Temporal Correlation among PAR1-mediated GSK3, Akt Thr308, and Pleckstrin Phosphorylation
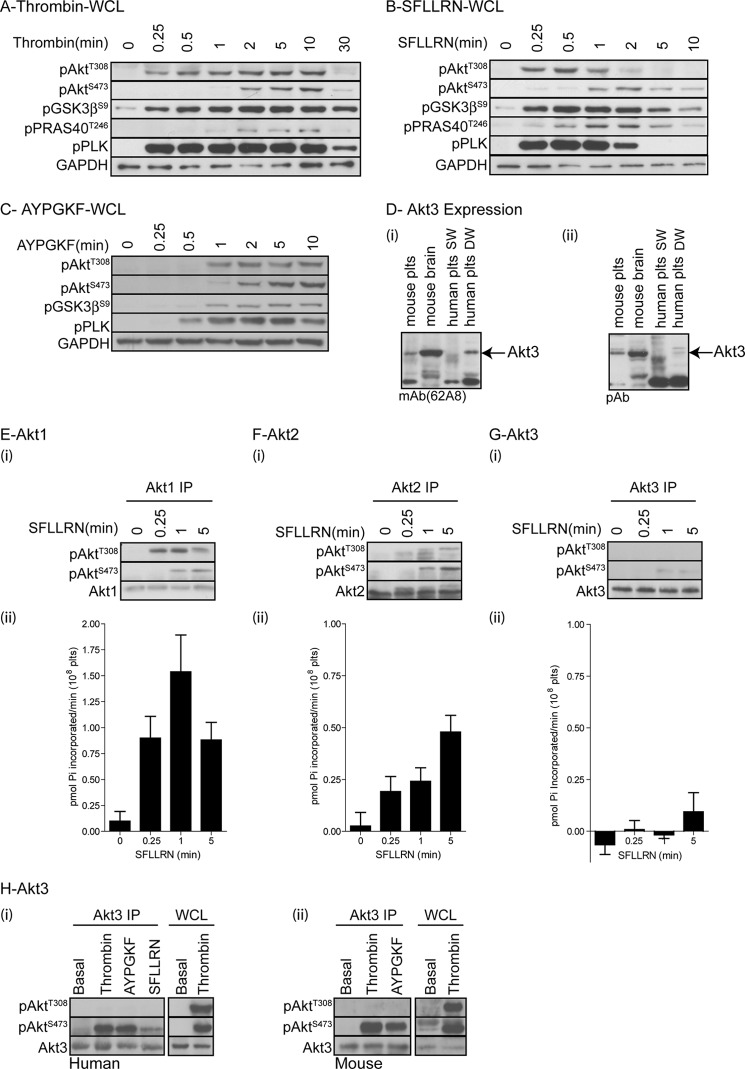
To obtain insight into the phosphorylation and regulation of GSK3 downstream of PAR receptors, we initially performed time course studies with thrombin, the PAR1 agonist peptide SFLLRN, and the PAR4 agonist peptide AYPGKF. Fig. 1, A and B, demonstrates that GSK3β becomes rapidly phosphorylated upon stimulation with thrombin and SFLLRN, respectively, whereas it is more delayed in response to AYPGKF (Fig. 1C). Interestingly, thrombin/PAR1-mediated GSK3β phosphorylation correlated with the onset of phosphorylation of Akt Thr308 and the PKC substrate pleckstrin. In contrast, Akt Ser473 phosphorylation was more delayed (Fig. 1, A and B) and correlated closely with phosphorylation of the Akt substrate PRAS40. These data are in agreement with our previous findings that Akt Ser473 phosphorylation is essential for Akt2 activity and phosphorylation of PRAS40 but dispensable for Akt1 activity and phosphorylation of GSK3β (11). Interestingly, the kinetic differences in Akt, GSK3, and pleckstrin phosphorylation between PAR1/thrombin and PAR4 suggests that PAR1 is responsible for the rapid Akt Thr308, GSK3, and pleckstrin phosphorylation by thrombin in human platelets. This is supported by the finding that the PAR1 antagonist SCH79797, prevented early thrombin-mediated Akt Thr308, GSK3, and pleckstrin phosphorylation (data not shown).
FIGURE 1.
Temporal phosphorylation of different Akt isoforms, GSK3, PRAS40, and pleckstrin in human platelets. A–D, washed human platelets were stimulated with 0.2 unit/ml thrombin (A), 5 μm SFLLRN (B), or 100 μm AYPGKF (C) for the indicated time period before extraction in NuPAGE sample buffer and immune blotting with the indicated antibodies (A–C). In addition, mouse brain lysate (positive control) and mouse and human platelet (SW, single washed; DW, double washed) lysates were immunoblotted for Akt3 using two different antibodies (mAb62A8 (i) and pAb (ii) (D). E–G, alternatively, platelets were stimulated with 5 μm SFLLRN for the indicated time period and extracted in Nonidet P-40 extraction buffer followed by immunoprecipitation (IP) of Akt1 (E), Akt2 (F), and Akt3 (G). Akt phosphorylation was analyzed by immunoblotting with the indicated antibodies (i), and in vitro Akt activity was analyzed using the peptide substrate RPRAATF (ii). Bar graphs (ii) show activity expressed as pmol of Pi incorporated per minute for 108 platelets (mean ± S.E. (error bars), n = 3). H, human (i) and mouse (ii) platelets were stimulated with thrombin (0.2 unit/ml), AYPGKF (100 nm), and SFLLRN (5 μm) for 5 min and extracted in Nonidet P-40 followed by immunoprecipitation of Akt3. Akt3 immunoprecipitates (Akt3 IP) and whole cell lysates (WCL) were immunoblotting with the indicated antibodies. Results (A–H) are representative of at least three independent experiments.
In addition to expressing Akt1 and Akt2, a recent study suggested that human and mouse platelets also express Akt3 (14). Here, we confirm these findings and show expression of Akt3 in both human and mouse platelets using two different anti-Akt3 antibodies (Fig. 1D). Note that Akt3 is picked up in double washed, but not single washed, human platelets, suggesting that residual albumin may interfere with antibody recognition. Mouse brain extract has been used as a positive control (Fig. 1D). To directly correlate Akt isoform phosphorylation status with kinase activity, we subsequently immunoprecipitated Akt1, Akt2, and Akt3 isoforms followed by immunoblotting and in vitro kinase activity assays. Fig. 1E shows that Akt1 is rapidly and transiently phosphorylated on Thr308 in response to SFLLRN, whereas Ser473 phosphorylation is more delayed. In vitro kinase studies demonstrated that Akt1 activity correlated with the rapid phosphorylation of Thr308. In contrast, Akt2 activation was slower, correlating with concomitant phosphorylation of Thr308 and Ser473 (Fig. 1F, numbering of Akt1 phosphorylation sites is used for simplicity; these correspond to Thr309 and Ser474 in Akt2). Note that Akt2 Ser473 phosphorylation results in a band shift, demonstrating that maximal phosphorylation of Akt2 occurs at the later time point (5 min), where a single band was detected in the pThr308 blot. In contrast to Akt1 and Akt2, we were unable to detect Akt3 Thr308 phosphorylation and only weak Akt3 Ser473 phosphorylation in response to the PAR1 peptide, with no concurrent increase in Akt3 activity (Fig. 1G). Similar results were found using an alternative Akt3 antibody (62A8) for immunoprecipitation (data not shown), demonstrating that Akt3 is not activated under these conditions. As Akt3 has previously been implicated in thrombin-stimulated GSK3β phosphorylation in mouse platelets (14), we performed similar experiments using thrombin and the PAR4 peptide AYPGKF on human and mouse platelets. Although thrombin and AYPGKF induced Ser473 phosphorylation in both human and mouse platelets, we were unable to detect any Akt3 Thr308 phosphorylation, suggesting that Akt3 is unlikely to be involved in thrombin-mediated GSK3α/β phosphorylation.
Akt-dependent and Akt-independent Phosphorylation of GSK3β
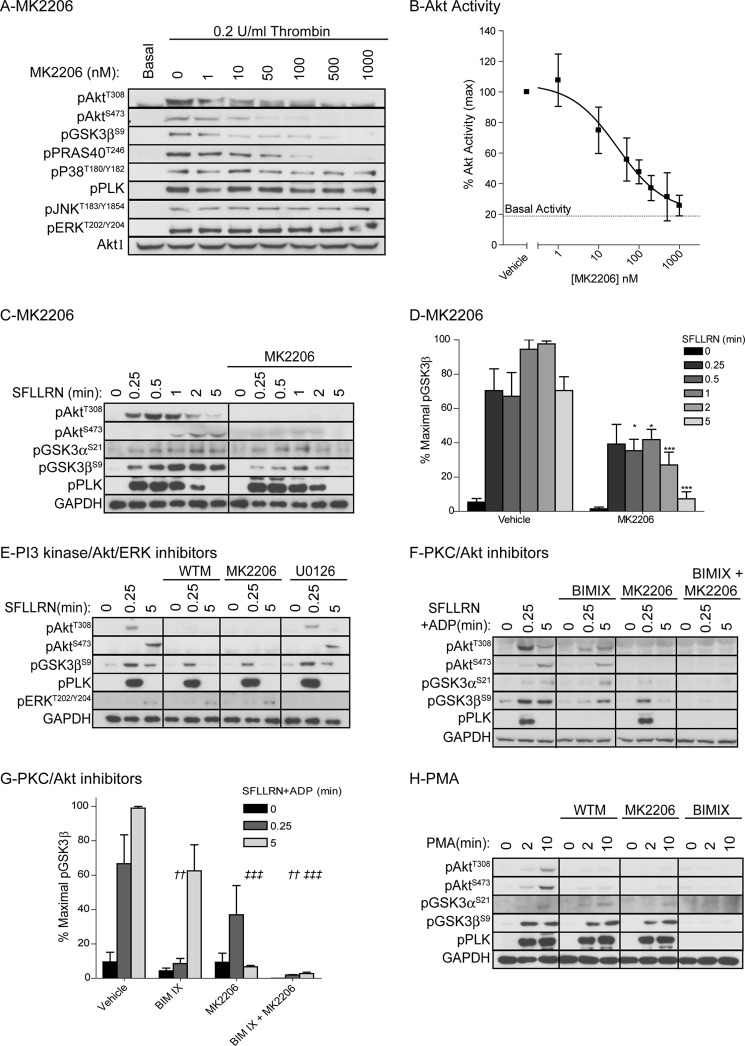
The SFLLRN time course studies suggested that Akt1 might be involved in GSK3 phosphorylation at early time points, whereas both Akt1 and Akt2 may be involved at later time points. Because Akt isoform-specific inhibitors are not available, we investigated the role of Akt in GSK3 phosphorylation by incubating platelets with the allosteric Akt inhibitor MK2206. This compound dose-dependently inhibited thrombin-stimulated Akt phosphorylation and activity with maximal inhibition at 500 nm (Fig. 2, A and B). MK2206 completely blocked GSK3α/β phosphorylation after a 5-min stimulation with SFLLRN (Fig. 2, C and D), which is in agreement with our previous studies using the related compound Akti-1/2 (35, 36). Interestingly, GSK3α/β phosphorylation was only partially reduced 15 s to 2 min after SFLLRN stimulation (Fig. 2, C and D), under conditions where Akt phosphorylation was absent (Fig. 2C). Similar results were found using the PI3K inhibitor wortmannin (Fig. 2E, 15 s), showing that early SFLLRN-mediated GSK3α/β phosphorylation is partially independent of the PI3K/Akt pathway.
FIGURE 2.
Role for PKC and Akt in PAR1-mediated GSK3 phosphorylation in human platelets. A, washed human platelets were incubated with the indicated concentration of MK2206 for 10 min followed by stimulation with 0.2 unit/ml thrombin for 5 min. Platelets were extracted in NuPAGE sample buffer and immunoblotted with the indicated antibodies . B, alternatively, Akt1 was immunoprecipitated and in vitro kinase activity determined using the peptide substrate RPRAATF. In vitro kinase activity is expressed as a percentage of Akt activity in the presence of vehicle (B, mean ± S.E. (error bars), n = 3). C–H, washed human platelets were incubated with vehicle, 1 μm MK2206 (C–H), 100 nm wortmannin (WT; E and H), 10 μm U0126 (E) or 1 μm BIMIX (F–H) for 10 min before stimulation with 5 μm SFLLRN (C–E), 5 μm SFLLRN+10 μm ADP (F and G), or 100 nm PMA (H) for the indicated time period. Platelets were extracted in NuPAGE sample buffer followed by immunoblotting with the indicated antibodies (C, E, F, and H). Results (A, C, E, F, and H) are representative of at least three independent experiments. The bar graphs (D and G) depict densitometric analysis of GSK3β phosphorylation of the data shown in C and F, respectively. Data (mean ± S.E.) are expressed as the percentage of maximal GSK3 phosphorylation induced by SFLLRN (D, n = 5) and SFLLRN+ADP (G, n = 3). Star notation (D: *, p < 0.05; ***, p < 0.001) indicates a significant difference between vehicle and MK2206-treated samples at the matching time point (two-way analysis of variance with Bonferroni post test). Dagger notation (G: ††, p < 0.01) indicates a significant difference compared with 0.25-min SFLLRN+ADP treatment in the presence of vehicle, whereas double dagger notation (‡‡‡: p < 0.001) indicates a significant difference compared with 5-min SFLLRN+ADP treatment in the presence of vehicle (two-way analysis of variance with Bonferroni post test).
Role of PKCα in Early GSK3α/β Phosphorylation
Alternative candidates to phosphorylate GSK3 independently of PI3K/Akt are PKC (23, 24), PKA (25), and p90RSK (26, 27). Of these, PKA and p90RSK are unlikely to be involved because PKA is not activated in platelets under these conditions, and blocking p90RSK activity using an inhibitor of the upstream kinase MEK (U0126) had no significant effect on GSK3β phosphorylation, despite blocking ERK phosphorylation (Fig. 2E). To examine whether PKC is involved in early GSK3α and GSK3β phosphorylation, we treated platelets with the PKC inhibitor bisindolylmaleimide IX (BIMIX). Because PKC inhibition blocks secretion of ADP, an important co-factor in PAR-mediated Akt activation (37), platelets were stimulated with SFLLRN in the presence of ADP. BIMIX blocked SFLLRN-mediated GSK3α/β phosphorylation 15 s after stimulation and blocked residual phosphorylation in the presence of MK2206 (Fig. 2, F and G), suggesting that PKC contributes to early GSK3α/β phosphorylation.
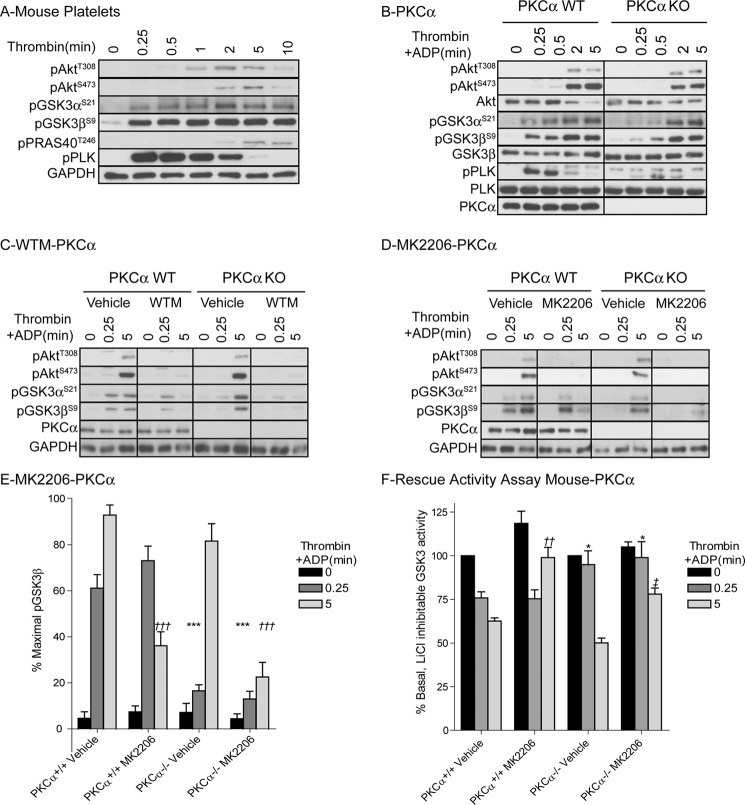
The ability of PKC to phosphorylate GSK3α/β was further confirmed in experiments using the PKC activator phorbol 12-myristate-13-acetate (PMA). PMA robustly stimulated pleckstrin and GSK3α/β phosphorylation in a PKC-dependent, PI3K/Akt-independent, manner (Fig. 2H). One of the candidate PKC isoforms that plays an important role in platelet activation and granule secretion is PKCα (1). We next evaluated whether GSK3α/β phosphorylation is affected in platelets from PKCα knock-out mice. Fig. 3A shows the phosphorylation events in mouse platelets in response to thrombin. Interestingly, GSK3α/β phosphorylation is clearly detectable within 15 s of thrombin stimulation, whereas Akt Thr308 and Ser473 phosphorylation are more delayed. PKCα-deficient platelets (Fig. 3B) show a significant delay in GSK3α/β phosphorylation, demonstrating that PKCα contributes to early GSK3α/β phosphorylation. In contrast, wortmannin (Fig. 3C) and MK2206 (Fig. 3, D and E) have no effect on early GSK3α/β phosphorylation, but strongly reduce later GSK3α/β phosphorylation in mouse platelets. The residual GSK3α/β phosphorylation present in platelets from PKCα KO animals is blocked by wortmannin and MK2206, confirming that both PKCα and PI3K /Akt contribute to thrombin-stimulated GSK3α/β phosphorylation (Fig. 3, C–E).
FIGURE 3.
Thrombin-stimulated GSK3 phosphorylation and inhibition of GSK3α/β activity is delayed in platelets from PKCα KO mice. Washed PKCα+/+ (PKCα WT, A–F) and PKCα−/− (PKCα KO, B–F) mouse platelets were stimulated with 0.2 unit/ml thrombin (A) or 0.2 unit/ml thrombin+10 μm ADP (B–F) for the indicated times. Platelets were extracted in NuPAGE sample buffer followed by immunoblotting with the indicated antibodies (A–D). Results (A–D) are representative of at least three independent experiments. The bar graph (E) depicts densitometric analysis of GSK3β phosphorylation of the data shown in D. Data (mean ± S.E. (error bars), n = 5) are expressed as the percentage of maximal GSK3 phosphorylation induced by thrombin+ADP in PKCα WT mouse platelets. Star notation (E: ***, p < 0.001) indicates a significant difference compared with 0.25-min thrombin+ADP treatment in vehicle-treated PKCα WT platelets whereas dagger notation (E: †††, p < 0.001) indicates a significant difference compared with 5-min thrombin+ADP treatment in vehicle-treated PKCα WT platelets. Alternatively, platelets were extracted in Nonidet P-40 (F) followed by analysis of in vitro GSK3 activity using the substrate peptide RRAAEELDSRAGS(P)PQL. In vitro kinase activity is expressed as a percentage of GSK3 activity obtained in the absence of thrombin and ADP (mean ± S.E., n = 5). Star notation (F: *, p < 0.05) indicates a significant difference compared with 0.25-min thrombin+ADP treatment in vehicle-treated PKCα WT platelets; dagger notation (††, p < 0.01) indicates a significant difference compared with 5-min thrombin+ADP treatment in vehicle-treated PKCα WT platelets, whereas double dagger notation (‡, p < 0.01) indicates a significant difference compared with 5-min thrombin+ADP treatment in vehicle-treated PKCα KO platelets.
Thrombin-mediated Inhibition of GSK3α/β Activity Is Dependent on PKCα and Akt
To evaluate the effect of phosphorylation on GSK3α/β activity; we performed in vitro GSK3α/β activity assays on platelet extracts. Thrombin stimulation resulted in a time-dependent reduction in GSK3α/β activity (Fig. 3F). The Akt inhibitor MK2206 strongly reduced thrombin-mediated GSK3α/β inhibition at the later (5 min) time point, whereas PKCα KO platelets showed impaired thrombin-mediated GSK3α/β inhibition at the early (15 s) time point. Furthermore, MK2206 largely prevented thrombin-mediated inhibition of GSK3α/β activity in PKCα KO platelets. Together these results demonstrate that phosphorylation and inhibition of GSK3α/β in platelets are dependent mainly on PKCα and Akt.
Phosphorylation of GSK3α/β by Akt and PKCα Contributes to Platelet Function
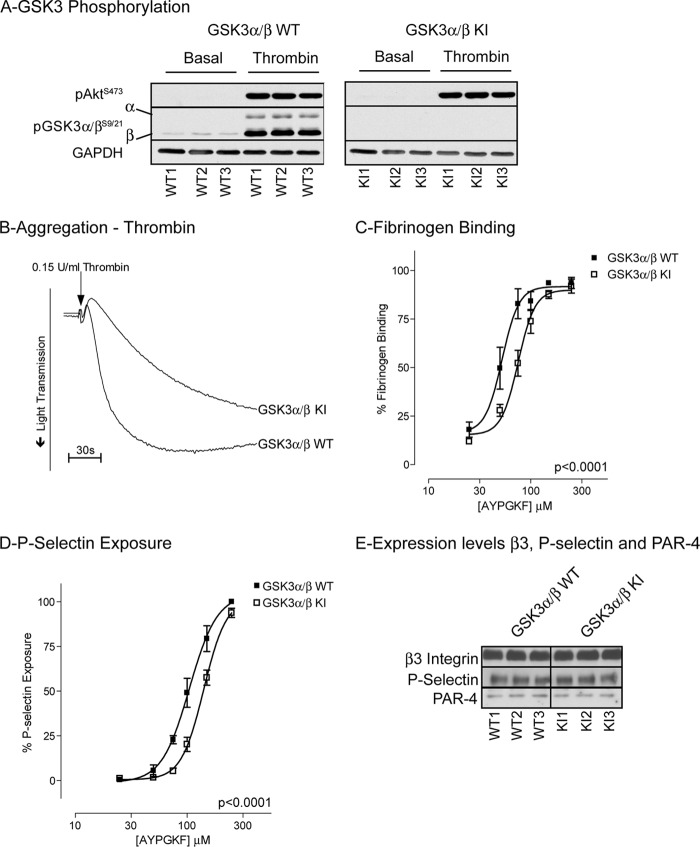
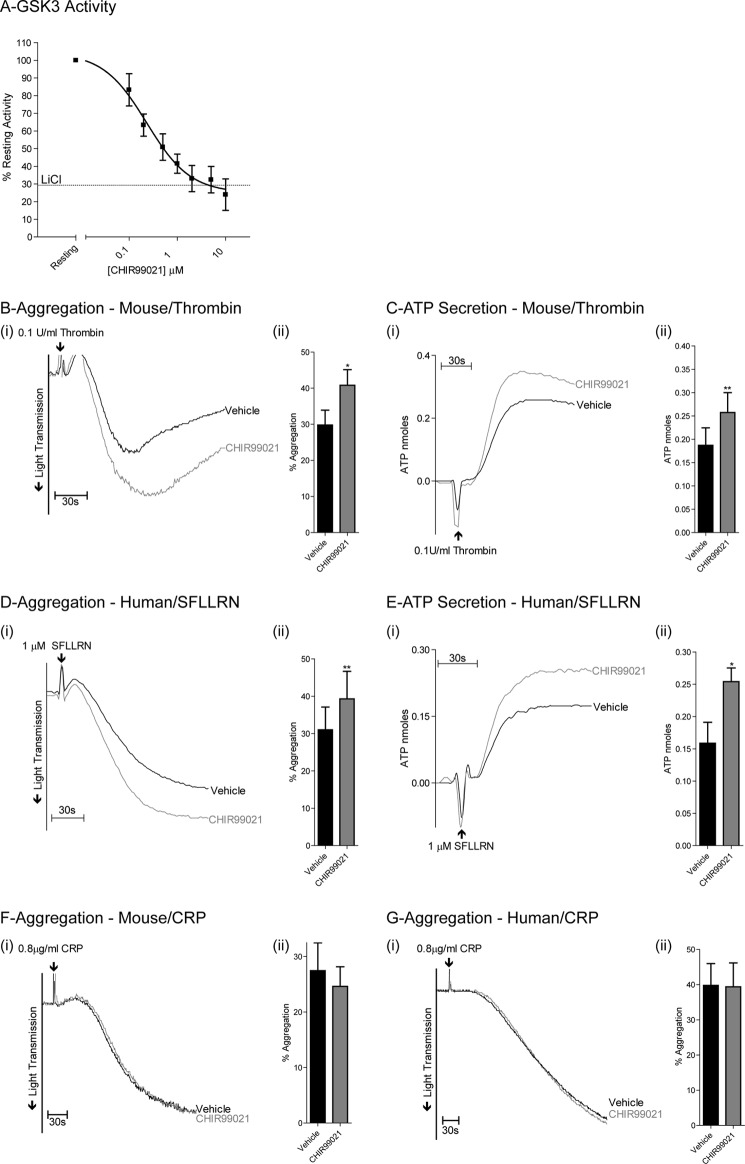
To directly link phosphorylation of GSK3 to platelet function, we performed functional studies on platelets from knock-in (KI) mice where the inhibitory Ser phosphorylation site on GSK3α (Ser21) and GSK3β (Ser9) were mutated to Ala. Western blotting confirmed that thrombin-stimulated phosphorylation of GSK3α and GSK3β is absent in platelets derived from GSK3 KI mice (Fig. 4A). Thrombin-stimulated aggregation of GSK3 KI platelets was significantly reduced compared with wild type (Fig. 4B). Furthermore, the concentration-response curve for PAR4-mediated fibrinogen binding was shifted to the right (Fig. 4C). Similar results were found for P-selectin expression, a marker for α-granule secretion (Fig. 4D). The reduction in functional responses was not due to reduced expression of key pathway components, as levels of integrin β3, P-selectin and PAR4 were normal in GSK3 KI mice (Fig. 4E). These results together demonstrate that phosphorylation of GSK3 contributes to platelet activation by thrombin. To confirm that inhibition of GSK3α/β indeed results in increased platelet function, we performed additional experiments with the GSK3α/β inhibitor CHIR99021 (also named CT-99021), which has improved specificity over compounds used previously in platelets (36, 38). CHIR99021 dose-dependently inhibited GSK3α/β activity platelets (Fig. 5A), which was associated with significantly increased aggregation (Fig. 5, B and D) and ATP secretion (Fig. 5, C and E) of murine and human platelets, respectively, confirming a negative role for GSK3α/β in thrombin-mediated platelet function. Interestingly, platelet aggregation in response to collagen-related peptide was unchanged in the presence of CHIR99021 (Fig. 5, F and G).
FIGURE 4.
Reduced platelet aggregation, fibrinogen binding, and P-selectin exposure in GSK3α/β KI platelets. A, washed GSK3α+/+/β+/+ (GSK3α/β WT) and GSK3 αS21A/S21A/βS9A/S9A (GSK3α/β KI) platelets were stimulated with 0.15 unit/ml thrombin for 5 min before extraction in NuPAGE sample buffer and immunoblot analysis with the indicated antibodies. B, platelets were stimulated with 0.15 unit/ml thrombin under stirring conditions and aggregation monitored for 5 min. C and D, a representative aggregation curve is presented for three independent experiments. Washed platelets were incubated with the indicated concentrations of PAR4 agonist AYPGKF-NH2 for 15 min in the presence of FITC-fibrinogen (C) or PE-anti-P-selectin Ab (D). Samples were fixed in 4% paraformaldehyde and analyzed by flow cytometry (C and D). Results are expressed as mean ± S.E. (error bars; n = 6) of percentage of maximal fibrinogen binding to GSK3α/β WT platelets. C and D, significance testing using GraphPad Prism 4.0 determined that different curve fittings were required for GSK3α/β WT and GSK3α/β KI data. E, washed GSK3α/β WT and GSK3α/β KI platelets were lysed in NuPAGE sample buffer and receptor expression levels determined by immunoblotting with the indicated antibodies.
FIGURE 5.
Inhibiting GSK3α/β activity in mouse and human platelets increases thrombin-mediated platelet aggregation and ATP secretion. A, washed human platelets were incubated with the indicated concentrations of the GSK3α/β inhibitor CHIR99021 for 10 min, extracted in Nonidet P-40, and in vitro GSK3α/β activity was analyzed using the substrate peptide RRAAEELDSRAGS(P)PQL. In vitro kinase activity is expressed as a percentage of GSK3α/β activity obtained in the absence of CHIR99021 (A, mean ± S.E. (error bars) n = 3). (i), washed mouse (B, C, and F) and human (C, D, and G) platelets were stimulated with 0.1 unit/ml thrombin (B and C), 1 μm SFLLRN (D and E), or 0.8 μg/ml collagen-related peptide (CRP) (F and G). Aggregation (B, D, F, and G) and ATP secretion (C and E) were subsequently recorded for 5 min. Traces are representative of four independent experiments. (ii), the bar graphs (B–G) indicate the average increase in aggregation and ATP secretion (nmoles, mean ± S.E., n = 4). *, p < 0.05; **, p < 0.01 (Student's paired t test).
DISCUSSION
In this study we investigated the role and regulation of GSK3α/β phosphorylation in platelet function by using a combination of genetic and pharmacological approaches. We demonstrate for the first time that GSK3α/β is phosphorylated and regulated by two distinct kinases, PKCα and Akt, and that this phosphorylation event contributes to PAR-mediated platelet activation. The role of the kinases shows differential temporal kinetics with PKCα supporting early, rapid GSK3α/β phosphorylation and Akt taking over at later time points.
Pharmacological inhibitor studies showed that early PAR1-mediated GSK3α/β phosphorylation was only mildly affected by the PI3K inhibitor wortmannin and the Akt inhibitor MK2206, suggesting that another kinase may be involved. Previous literature suggests that PKC (23, 24), PKA (25), and p90RSK (26, 27), of which PKC and p90RSK are activated upon platelet activation, can also contribute to GSK3 phosphorylation under certain conditions. Blocking p90RSK activation using a MEK inhibitor, however, had no effect on GSK3α/β phosphorylation, ruling out a role for p90RSK. In contrast, early GSK3α/β phosphorylation was blocked by the PKC inhibitor BIMIX, suggesting that PKC, rather than Akt, is involved in GSK3α/β phosphorylation. This was furthermore confirmed by the ability of the PKC activator PMA to stimulate GSK3α/β phosphorylation in a PKC-dependent, Akt-independent manner. Interestingly, there was a clear temporal discrepancy between GSK3α/β phosphorylation (<15 s) and Akt (Thr308 and Ser473) phosphorylation (1–2 min) in mouse platelets, further supporting the hypothesis that an alternative kinase is involved in early GSK3α/β phosphorylation. Indeed, this kinase is likely to be PKCα, as early thrombin-mediated GSK3α/β phosphorylation was absent in PKCα−/− mouse platelets. These findings are supported by a recent study by Li et al. (22), reporting that thrombin-mediated GSK3β phosphorylation in platelets is partly PI3K-independent.
The lack of Akt isoform-specific inhibitors makes it challenging to determine which Akt isoforms are involved in GSK3 phosphorylation in human platelets. Previous studies by our group and others have reported the phosphorylation and activation of Akt1 and Akt2 isoforms in human platelets (11, 12, 39). In addition, a recent study suggested that the Akt3 isoform is also expressed in human and mouse platelets and significantly contributes to GSK3β phosphorylation and thrombin-stimulated platelet function (14). In the present study, we therefore analyzed the temporal activation of all three Akt isoforms to determine their potential contribution to GSK3α/β phosphorylation in human platelets. Although we confirmed that Akt3 was expressed in both human and mouse platelets, we were unable to detect Akt3 Thr308 phosphorylation and Akt3 activity in human platelets in response to the PAR1 peptide SFLLRN, the PAR4 peptide AYPGKF and thrombin, suggesting that Akt3 is unlikely to contribute to GSK3α/β phosphorylation under these conditions.
In contrast, Akt1 was very rapidly and transiently activated by SFLLRN, whereas Akt2 activation was more delayed and maximal 5 min after stimulation. These results raised the possibility that Akt1 contributes to early (15 s) GSK3α/β phosphorylation, whereas Akt2 may be involved in later (5 min) GSK3α/β phosphorylation. However, both Akt1 and Akt2 are active at the later time point, and a contribution of either isoform to GSK3α/β phosphorylation can therefore not be ruled out. Indeed, maximal Akt1 activity detected in human platelets was approximately three times higher than maximal Akt2 activity, a finding that corresponds to the relative Akt mRNA expression in human platelets (39). Our results using the PAR1 peptide SFLLRN also revealed that Thr308 phosphorylation detected in total lysate is very rapid and correlates closely to Akt1, but not Akt2 phosphorylation, suggesting that Akt1 is the main isoform detected in total lysate.
Interestingly, there was also a clear difference in the kinetics of thrombin-stimulated Akt Thr308 phosphorylation between mouse and human platelets. In human platelets, Thr308 was very rapidly phosphorylated (<15 s), whereas in mouse platelets this was much more delayed (>1–2 min). We found that an antagonist of PAR1 (a receptor not expressed on mouse platelets) blocked early thrombin-stimulated Akt Thr308 and pleckstrin phosphorylation in human platelets (data not shown), suggesting that PAR1 mediates the rapid thrombin-mediated activation of Akt1 and PKC in human platelets. This is in agreement with our finding that Akt Thr308 and pleckstrin phosphorylation in response to the PAR4 peptide AYPGKF was delayed in human platelets. The early PKC-mediated GSK3 phosphorylation is human platelets is therefore dependent on PAR1, whereas in mouse platelets it is dependent on PAR4. This reflects the relative importance of these receptors in thrombin-mediated platelet activation in human versus mouse platelets.
Like human platelets, murine platelets also express all three Akt isoforms. Studies on platelets from Akt1+/−Akt2−/− mice and Akt3−/− showed a mild reduction in thrombin-stimulated GSK3β phosphorylation in the absence of these isoforms, suggesting any of the isoforms can contribute to GSK3β phosphorylation (14, 22). The relatively small effect of Akt isoform deficiency on GSK3β phosphorylation may therefore be explained by partial redundancy between the Akt isoforms, as well as contribution of PKCα to early GSK3β phosphorylation. Interestingly, a recent study on neutrophils also described a role for phospholipase C/PKC and PI3K/Akt pathway in GSK3α/β phosphorylation (40), suggesting that dual regulation of GSK3α/β by PKC and Akt may be a more general mechanism in hematopoietic cells.
In many cell types, phosphorylation of GSK3 on Ser21 (GSK3α) and Ser9 (GSK3β) results in a reduction in GSK3 activity of approximately 20–50%. Here, we confirmed that thrombin stimulation of platelets results in a temporal reduction in GSK3α/β activity in wild type murine platelets. Early (15 s) inhibition, but not late (5 min) inhibition of GSK3α/β activity was completely absent in PKCα-deficient platelets, confirming a role for PKCα in regulating early GSK3α/β phosphorylation and activity. In contrast, late thrombin-mediated inhibition of GSK3α/β was largely prevented by the Akt inhibitor MK2206 in both wild type and PKCα-deficient platelets, supporting a role for Akt in late inhibition of GSK3α/β activity. There was some residual inhibition in GSK3α/β activity in the presence of the Akt inhibitor, suggesting that regulation by an alternative kinase cannot be ruled out completely.
Platelets from GSK3 KI mice bearing nonphosphorylatable alanine at Ser21/Ser9 of GSK3α/β exhibited significantly reduced aggregation, fibrinogen binding, and P-selectin exposure, demonstrating for the first time that GSK3α/β phosphorylation contributes to thrombin-mediated platelet function. As aggregation and secretion are rapid platelet responses that happen in the time frame of PKC-mediated GSK3 phosphorylation, Akt-mediated GSK3 phosphorylation in mouse platelets may be of less functional significance. However, we cannot rule out the possibility that Akt-mediated GSK3 regulation contributes to consolidation of platelet aggregation and thrombus formation.
The GSK3 inhibitor CHIR99021 increased thrombin/PAR-mediated aggregation in mouse and human platelets, showing that a reduction in GSK3 activity supports thrombin-mediated platelet function, a finding in agreement with previous reports (14, 22). Interestingly, we found no effect of CHIR99021 on collagen-related peptide-mediated platelet aggregation, suggesting that the role of GSK3 may be limited to thrombin or G protein-mediated platelet function. In contrast to our results, two reports described an inhibitory effect of GSK3 inhibitors on collagen/collagen-related peptide-mediated aggregation (14, 15). This discrepancy may be explained by the higher specificity of the compound used in our study (36, 38).
It is at present unknown how GSK3 may regulate platelet function in response to thrombin. There are nearly 100 proposed GSK3 substrates described in literature, but for many of these substrates, in vivo phosphorylation by GSK3 and its effect on substrate function have not been confirmed (32). Indeed, no GSK3 substrates have thus far been positively identified in platelets. Future identification of GSK3 substrates may provide more insight in how GSK3 may negatively regulate thrombin-mediated platelet function. In conclusion, our data demonstrate that PKCα and Akt promote thrombin-stimulated platelet function by phosphorylating and inhibiting GSK3α/β, thereby increasing platelet function.
Acknowledgments
We thank the healthy blood donors within the Medical Sciences Building, University of Bristol, for generous donations; Elizabeth Aitken, Dr. Chris Williams, and Dr. Matthew Harper for expert technical support; and Professors Dario Alessi, Medical Research Council Phosphorylation Unit, Dundee, and Jeff Molkentin, Cincinnati Children's Hospital, Cincinnati, OH, for providing the GSK3αS21A/S21A/βS9A/S9A (GSK3 KI) and PKCα−/− (PKCα KO) mice, respectively.
This work was supported by British Heart Foundation Grants PG/08/056, PG/09/059, PG/10/100, RG/10/006, and FS/11/62.
- PAR
- protease-activated receptor
- bis-tris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- GSK3
- glycogen synthase kinase 3
- PE
- phycoerythrin
- PMA
- phorbol 12-myristate-13-acetate.
REFERENCES
- 1. Konopatskaya O., Gilio K., Harper M. T., Zhao Y., Cosemans J. M., Karim Z. A., Whiteheart S. W., Molkentin J. D., Verkade P., Watson S. P., Heemskerk J. W., Poole A. W. (2009) PKCα regulates platelet granule secretion and thrombus formation in mice. J. Clin. Invest. 119, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Konopatskaya O., Poole A. W. (2010) Protein kinase Cα: disease regulator and therapeutic target. Trends Pharmacol. Sci. 31, 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilio K., Harper M. T., Cosemans J. M., Konopatskaya O., Munnix I. C., Prinzen L., Leitges M., Liu Q., Molkentin J. D., Heemskerk J. W., Poole A. W. (2010) Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. J. Biol. Chem. 285, 23410–23419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia A., Kim S., Bhavaraju K., Schoenwaelder S. M., Kunapuli S. P. (2010) Role of phosphoinositide 3-kinase β in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem. J. 429, 369–377 [DOI] [PubMed] [Google Scholar]
- 5. Martin V., Guillermet-Guibert J., Chicanne G., Cabou C., Jandrot-Perrus M., Plantavid M., Vanhaesebroeck B., Payrastre B., Gratacap M. P. (2010) Deletion of the p110β isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood 115, 2008–2013 [DOI] [PubMed] [Google Scholar]
- 6. Canobbio I., Stefanini L., Cipolla L., Ciraolo E., Gruppi C., Balduini C., Hirsch E., Torti M. (2009) Genetic evidence for a predominant role of PI3Kβ catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood 114, 2193–2196 [DOI] [PubMed] [Google Scholar]
- 7. Pasquet J. M., Gross B. S., Gratacap M. P., Quek L., Pasquet S., Payrastre B., van Willigen G., Mountford J. C., Watson S. P. (2000) Thrombopoietin potentiates collagen receptor signaling in platelets through a phosphatidylinositol 3-kinase-dependent pathway. Blood 95, 3429–3434 [PubMed] [Google Scholar]
- 8. Cosemans J. M., Van Kruchten R., Olieslagers S., Schurgers L. J., Verheyen F. K., Munnix I. C., Waltenberger J., Angelillo-Scherrer A., Hoylaerts M. F., Carmeliet P., Heemskerk J. W. (2010) Potentiating role of Gas6 and Tyro3, Axl, and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J. Thromb. Haemost. 8, 1797–1808 [DOI] [PubMed] [Google Scholar]
- 9. Hers I. (2007) Insulin-like growth factor-1 potentiates platelet activation via the IRS/PI3Kα pathway. Blood 110, 4243–4252 [DOI] [PubMed] [Google Scholar]
- 10. Hers I., Vincent E. E., Tavaré J. M. (2011) Akt signalling in health and disease. Cell. Signal. 23, 1515–1527 [DOI] [PubMed] [Google Scholar]
- 11. Moore S. J., Hunter R. W., Hers I. (2011) mTORC2-mediated Akt Ser473 phosphorylation is not required for Akt1 activity in human platelets. J. Biol. Chem. 286, 24533–24560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woulfe D., Jiang H., Morgans A., Monks R., Birnbaum M., Brass L. F. (2004) Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J. Clin. Invest. 113, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J., De S., Damron D. S., Chen W. S., Hay N., Byzova T. V. (2004) Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood 104, 1703–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Brien K. A., Stojanovic-Terpo A., Hay N., Du X. (2011) An important role for Akt3 in platelet activation and thrombosis. Blood 118, 4215–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barry F. A., Graham G. J., Fry M. J., Gibbins J. M. (2003) Regulation of glycogen synthase kinase 3 in human platelets: a possible role in platelet function? FEBS Lett. 553, 173–178 [DOI] [PubMed] [Google Scholar]
- 16. Zhang W., Colman R. W. (2007) Thrombin regulates intracellular cyclic AMP concentration in human platelets through phosphorylation/activation of phosphodiesterase 3A. Blood 110, 1475–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunter R. W., Mackintosh C., Hers I. (2009) Protein kinase C-mediated phosphorylation and activation of PDE3A regulate cAMP levels in human platelets. J. Biol. Chem. 284, 12339–12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stojanovic A., Marjanovic J. A., Brovkovych V. M., Peng X., Hay N., Skidgel R. A., Du X. (2006) A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J. Biol. Chem. 281, 16333–16339 [DOI] [PubMed] [Google Scholar]
- 19. Gambaryan S., Kobsar A., Hartmann S., Birschmann I., Kuhlencordt P. J., Müller-Esterl W., Lohmann S. M., Walter U. (2008) NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J. Thromb. Haemost. 6, 1376–1384 [DOI] [PubMed] [Google Scholar]
- 20. Rowley J. W., Oler A. J., Tolley N. D., Hunter B. N., Low E. N., Nix D. A., Yost C. C., Zimmerman G. A., Weyrich A. S. (2011) Genome wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 118, e101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 22. Li D., August S., Woulfe D. S. (2008) GSK3β is a negative regulator of platelet function and thrombosis. Blood 111, 3522–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goode N., Hughes K., Woodgett J. R., Parker P. J. (1992) Differential regulation of glycogen synthase kinase-3β by protein kinase C isotypes. J. Biol. Chem. 267, 16878–16882 [PubMed] [Google Scholar]
- 24. Cook D., Fry M. J., Hughes K., Sumathipala R., Woodgett J. R., Dale T. C. (1996) Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 15, 4526–4536 [PMC free article] [PubMed] [Google Scholar]
- 25. Fang X., Yu S. X., Lu Y., Bast R. C., Jr., Woodgett J. R., Mills G. B. (2000) Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. U.S.A. 97, 11960–11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Mesquita D. D., Zhan Q., Crossley L., Badwey J. A. (2001) p90-RSK and Akt may promote rapid phosphorylation/inactivation of glycogen synthase kinase 3 in chemoattractant-stimulated neutrophils. FEBS Lett. 502, 84–88 [DOI] [PubMed] [Google Scholar]
- 27. Saito Y., Vandenheede J. R., Cohen P. (1994) The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem. J. 303, 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braz J. C., Gregory K., Pathak A., Zhao W., Sahin B., Klevitsky R., Kimball T. F., Lorenz J. N., Nairn A. C., Liggett S. B., Bodi I., Wang S., Schwartz A., Lakatta E. G., DePaoli-Roach A. A., Robbins J., Hewett T. E., Bibb J. A., Westfall M. V., Kranias E. G., Molkentin J. D. (2004) PKC-α regulates cardiac contractility and propensity toward heart failure. Nat. Med. 10, 248–254 [DOI] [PubMed] [Google Scholar]
- 29. Harper M. T., Molkentin J. D., Poole A. W. (2010) Protein kinase Cα enhances sodium-calcium exchange during store-operated calcium entry in mouse platelets. Cell Calcium 48, 333–340 [DOI] [PubMed] [Google Scholar]
- 30. McManus E. J., Sakamoto K., Armit L. J., Ronaldson L., Shpiro N., Marquez R., Alessi D. R. (2005) Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 24, 1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore S. F., Hunter R. W., Hers I. (2011) mTORC2 protein-mediated protein kinase B (Akt) serine 473 phosphorylation is not required for Akt1 activity in human platelets. J. Biol. Chem. 286, 24553–24560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sutherland C. (2011) What are the bona fide GSK3 substrates? Int. J. Alzheimers Dis. 2011, 505607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hers I., Tavaré J. M., Denton R. M. (1999) The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro 31-8220) are potent inhibitors of glycogen synthase kinase-3 activity. FEBS Lett. 460, 433–436 [DOI] [PubMed] [Google Scholar]
- 34. Xia Z., Wong T., Liu Q., Kasirer-Friede A., Brown E., Frojmovic M. M. (1996) Optimally functional fluorescein isothiocyanate-labelled fibrinogen for quantitative studies of binding to activated platelets and platelet aggregation. Br. J. Haematol. 93, 204–214 [DOI] [PubMed] [Google Scholar]
- 35. Hunter R. W., Harper M. T., Hers I. (2008) The PKB inhibitor Akti-1/2 potentiates PAR1-mediated platelet function independently of its ability to block PKB. J. Thromb. Haemost. 6, 1923–1932 [DOI] [PubMed] [Google Scholar]
- 36. Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007) The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim S., Jin J., Kunapuli S. P. (2004) Akt activation in platelets depends on Gi signaling pathways. J. Biol. Chem. 279, 4186–4195 [DOI] [PubMed] [Google Scholar]
- 38. Tighe A., Ray-Sinha A., Staples O. D., Taylor S. S. (2007) GSK-3 inhibitors induce chromosome instability. BMC Cell Biol. 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kroner C., Eybrechts K., Akkerman J. W. (2000) Dual regulation of platelet protein kinase B. J. Biol. Chem. 275, 27790–27798 [DOI] [PubMed] [Google Scholar]
- 40. Tang W., Zhang Y., Xu W., Harden T. K., Sondek J., Sun L., Li L., Wu D. (2011) A phospholipase Cβ/PI3Kγ-GSK3 signaling pathway regulates cofilin phosphatase slingshot2 and neutrophil polarization and chemotaxis. Dev. Cell 21, 1038–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]