Background: β-Cells regulate α-cells via paracrine mechanisms.
Results: A GABA shunt defect impairs glucose suppression of glucagon secretion in diabetic human islets. Glucagon secretion is inhibited by γ-hydroxybutyrate produced by β-cells but is stimulated by glycine via plasma membrane receptors.
Conclusion: γ-Hydroxybutyrate and glycine serve as counterbalancing receptor-based regulators of glucagon secretion.
Significance: Amino acids and their metabolites are central regulators of α-cell function.
Keywords: β-Cell, Diabetes, Glucose Metabolism, Glycine Receptors, Insulin Secretion, GABA Shunt, γ-Hydroxybutyrate, Glucagon Secretion
Abstract
Paracrine signaling between pancreatic islet β-cells and α-cells has been proposed to play a role in regulating glucagon responses to elevated glucose and hypoglycemia. To examine this possibility in human islets, we used a metabolomic approach to trace the responses of amino acids and other potential neurotransmitters to stimulation with [U-13C]glucose in both normal individuals and type 2 diabetics. Islets from type 2 diabetics uniformly showed decreased glucose stimulation of insulin secretion and respiratory rate but demonstrated two different patterns of glucagon responses to glucose: one group responded normally to suppression of glucagon by glucose, but the second group was non-responsive. The non-responsive group showed evidence of suppressed islet GABA levels and of GABA shunt activity. In further studies with normal human islets, we found that γ-hydroxybutyrate (GHB), a potent inhibitory neurotransmitter, is generated in β-cells by an extension of the GABA shunt during glucose stimulation and interacts with α-cell GHB receptors, thus mediating the suppressive effect of glucose on glucagon release. We also identified glycine, acting via α-cell glycine receptors, as the predominant amino acid stimulator of glucagon release. The results suggest that glycine and GHB provide a counterbalancing receptor-based mechanism for controlling α-cell secretory responses to metabolic fuels.
Introduction
Since the 1960s, the bihormonal regulation of fuel metabolism by insulin and glucagon has been recognized as central to the control of glucose homeostasis in diabetes and normal humans (1–5). Glucose ingestion stimulates pancreatic islets to release insulin while simultaneously suppressing glucagon secretion, whereas secretion of both hormones is stimulated by protein meals (6). Although the pathways regulating β-cell insulin secretion are relatively well understood, the control of α-cell glucagon secretion remains an enigma (7). Although a direct action of glucose is not to be discounted, this alone cannot explain how glucose suppresses glucagon secretion (7–10). For example, previous studies have shown that α-cells have to be systemically insulinized for glucose to suppress glucagon secretion in the isolated perfused pancreas of the streptozotocin diabetic rat (11) and that glucagon levels in the fed state are increased by knock-out of α-cell insulin receptors (12). The insulin requirement for glucose to be able to suppress glucagon may partly reflect a critical permissive, “endocrine” effect of insulin on α-cell function. In contrast, the acute decrease in glucagon release during glucose-stimulated insulin secretion (GSIS)2 has been suggested to involve “paracrine” signals from β-cells acting to suppress α-cell release of glucagon. Proposed paracrine signals include direct inhibitory effects of stimulated insulin exocytosis from β-cells and/or indirect effects of hormone release by molecules co-secreted with insulin, such as zinc or purine nucleotides (7–9, 13–16). Although several groups of investigators have produced evidence supporting one or the other of these mechanisms, one difficulty with these proposals is the observation that the glucose threshold for glucagon suppression is much lower than the threshold for insulin release (about 1 versus 5 mm) (6, 17, 18). This raises the possibility that other factors generated even at subthreshold levels of glucose for GSIS might play a role in the communication between β-cells and α-cells during GSIS.
Among the other potential paracrine mediators of glucose-induced suppression of glucagon that have been suggested is γ-aminobutyric acid (GABA) (15), an inhibitory neurotransmitter produced by decarboxylation of glutamate. GABA production is the first step of the GABA shunt, which operates in β-cells but not α-cells (19). The three steps of the GABA shunt include generation of GABA via glutamate decarboxylase (GAD), conversion of GABA to succinate semialdehyde (SSA) by GABA transaminase, and finally entry into the tricarboxylic acid (TCA) cycle as succinate following oxidation by SSA dehydrogenase (19). Although there has been much effort in testing the hypothesis that GABA might be involved in glucose-induced suppression of glucagon, studies addressing this issue have remained inconclusive (20). Nevertheless, marked changes in GABA and other amino acids have been observed in β-cells during GSIS that might be of relevance to paracrine regulation of glucagon secretion (21, 22) because some of these amino acids or their derivatives are known to have important neurotransmitter functions.
Our laboratories have been studying mechanisms of insulin and glucagon regulation by amino acids in physiological conditions and in genetic disorders of hyperinsulinemic hypoglycemia and diabetes mellitus using islets isolated from mouse models of these disorders to trace the flux of amino acids and glucose during a wide range of functional states (21, 23–25). In the present investigations, we applied these methods and the concepts that were developed in the course of previous studies to examine insulin and glucagon secretion in human islets isolated from normal and type 2 diabetic (T2D) pancreases to explore the possibility that human tissues might be uniquely suited to address this important question. The results do indeed suggest an alternative mechanism for the glucose suppression of islet glucagon secretion. This novel mechanism involves the production and release by β-cells of a known inhibitory neurotransmitter, γ-hydroxybutyrate (GHB), that is generated in a branch of the β-cell GABA shunt during glucose stimulation and that as the present studies show inhibits glucagon secretion.
EXPERIMENTAL PROCEDURES
Human Islet Isolation and Culture
Human islets were isolated in the University of Pennsylvania Islet Transplantation Center according to protocols described previously (26, 27). Assignment to normal or type 2 diabetic group was based on the donor's clinical diagnosis and history. Pancreatic islets were isolated using a modification of the Ricordi method (26, 27). After culture in CMRL 1066 medium supplemented with 5% human serum albumin and 5 mm glucose at 23 °C for 3 days, islets were then cultured for an additional 3 days in RPMI 1640 medium with 10 mm glucose at 37 °C (25, 28). Donor information is presented in supplemental Table 1.
Studies with [U-13C]Glucose
Methods for metabolomic studies tracing the flow of stable isotopes in islet intermediary metabolism were described previously (21). In brief, batches of 500 islets were preincubated with Krebs-Ringer bicarbonate buffer with 0.25% BSA for 60 min and then incubated for 120 min with a 4.0 mm physiological mixture of amino acids and 300 μm NH4Cl as a control or with an additional 5 or 25 mm [U-13C]glucose (Cambridge Isotope Laboratories, Inc., Andover, MA). The composition of the physiological mixture of 19 amino acids was described previously (21).
Intracellular Amino Acid Profiles and Detailed 13C Enrichments
Intracellular levels of amino acids were measured by HPLC. GC/MS measurements of 13C isotopic enrichment of amino acids were performed on a Hewlett Packard 5970 Mass Selective Detector and/or 5971 Mass Selective Detector as described previously (21).
GHB Identification and Quantitation
The analysis of GHB was carried out using the Agilent Technologies 6890N/5973 GC/MS Selective Detector system. Electron ionization at 70 eV with an HP-5MS column cross-linked with 5% phenylmethylsiloxane was used. Data acquisition was performed in the selected ion monitoring mode. GHB-d6 was used as internal standard to quantify both GHB and its 13C enrichment. The ion intensities of m/z 239 and 233 were monitored for the quantitative assessment of GHB (29, 30).
Cytosolic Calcium Level Recordings
Cytosolic calcium levels were measured as reported previously (23). In brief, whole islets or individual islet cells obtained by trypsinization of intact isolated islets according to a published procedure (23) were perifused in Krebs-Ringer bicarbonate buffer with 0.25% BSA at a flow rate of 1 ml/min. [Ca2+]i was measured by dual wavelength fluorescence microscopy using a Zeiss AxioVision system as described previously (23).
Islet Perifusion, Batch Incubation, and Determination of Insulin and Glucagon
Islet perifusion and batch incubation methods were described previously (28). After 60-min preincubation, batches of 100 islets were incubated for another 60 min with different treatments. Insulin and glucagon were measured in the incubation supernatants using a radioimmunoassay available at the RIA Core in the Diabetes Center of the University of Pennsylvania Perelman School of Medicine.
Measurement of the Rate of Oxygen Consumption
Oxygen consumption rates as a function of glucose were measured with a Clark electrode in a water-jacketed glass reaction vessel holding 0.16 ml of stirred, air-saturated modified Hanks' buffer at 37 °C (31). After 10 min of stabilization of substrate-free medium or medium containing 3 mm glucose, a suspension of 200 islets was loaded into the vessel, and base-line respiration was measured for 5 min. Then 5 μl of buffer containing glucose was injected into the vessel through a capillary in the ground glass stopper to establish a final glucose concentration of 25 mm for the second phase of respirometry (also about 5 min). Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (final concentration, 5 μm), which serves as a test of the degree of coupling of oxidative phosphorylation and efficiency of ATP synthesis, was finally added to the vessel in a third phase of the test. Recordings of oxygen tension thus usually extended over a total period of 15 min.
Studies in Mouse Islets
Green fluorescent protein (GFP) transgenic mice with β-cell-specific expression driven by the mouse insulin promoter were purchased from The Jackson Laboratory (32). Islets were isolated by collagenase digestion and then dispersed to single cells via trypsinization. β-Cells were then purified by FACS. For glucagon and insulin secretion studies, islets were isolated from normal mice (B6/129/F1). After 3 days of culture in RPMI 1640 medium with 10 mm glucose, batches of 35 islets were preincubated with glucose-free Krebs-Ringer bicarbonate buffer with 0.25% BSA for 30 min in a 96-well plate. Islets were then incubated with different treatments for another 60 min. Supernatants were collected for insulin and glucagon measurements.
Western Blot and mRNA Analysis
Monoclonal anti-human glycine receptor antibody (Novus Biologicals, Littleton, CO) was used as the primary antibody, and goat anti-mouse HRP (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the secondary antibody. A total of 20 μg of protein from normal human islets was used for Western blotting. Total RNA from human islets was isolated from cultured islets as described above using the Trizol (Invitrogen) method. Mouse islet and β-cell RNA was extracted from freshly isolated islets or β-cells purified by FACS. The reverse transcription reaction and quantitative real time PCR (Applied Biosystems SYBR Green Master Mix kit) were used to explore the expression of selected genes critical for the metabolic and signaling pathways studied here and were performed as described previously (21). Data were calculated using GAPDH as an internal reference. The sequences of primers required for this purpose are indicated in supplemental Table 2.
Materials
Chemicals were from Sigma-Aldrich except when stated otherwise.
Calculations and Statistical Analyses
Glucose-derived 13C enrichment of amino acids was expressed as mole percent enrichment, which is the mole fraction percent of analyte containing 13C atoms above natural abundance as described previously (21, 24). All data are presented as mean ± S.E. Student's t tests were done when two groups were compared. Analysis of variance (one-way analysis of variance, GraphPad Prism) was used followed by the Bonferroni test when multiple groups were compared. Differences were considered significant when p < 0.05.
RESULTS
Insulin and Glucagon Secretion of Normal and Type 2 Diabetic Human Islets and Correlations with Intracellular GABA
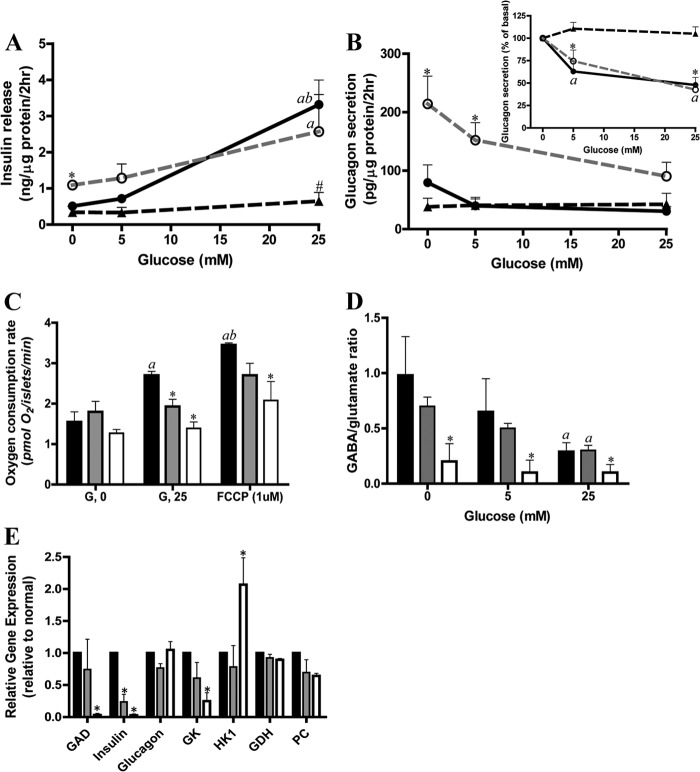
Insulin and glucagon secretion was studied in isolated islets from five non-diabetic organ donors and six donors with type 2 diabetes. As shown in Fig. 1A, in the presence of a 4.0 mm physiological amino acid mixture (AAM), 25 mm glucose stimulated a 6-fold increase in insulin secretion in normal islets, whereas there was little insulin response to 5 mm glucose. These concentrations of glucose were selected because we have previously found that 5 mm glucose is the threshold and 25 mm is the maximum for insulin secretion and glucose oxidation in human islets (33). Similar threshold and maximum concentrations of glucose were also found in normal mouse islets in more detailed studies of GSIS as shown in supplemental Fig. 1A. In contrast, islets from the six cases of T2D all showed a marked impairment of GSIS in agreement with the results of numerous studies using diabetic islets (34, 35). In contrast to the lack of effect on insulin secretion, 5 mm glucose effectively inhibited glucagon secretion by 40% in normal islets (Fig. 1B), consistent with previous reports that the threshold for glucose suppression of glucagon secretion is lower than the threshold for GSIS (17, 18). A similar phenomenon was also observed in normal mouse islets (supplemental Fig. 1). 25 mm glucose inhibited glucagon secretion only a little more than that observed at 5 mm glucose, i.e. by about an additional 10%. The T2D islets showed two different patterns of α-cell responsiveness to glucose: in three cases, inhibition of glucagon secretion by glucose was comparable with that of non-diabetic controls (T2Ds with glucose-responsive α-cells (T2D-αGR)), whereas the other three T2D cases failed to show suppression of glucagon release (T2Ds with glucose-unresponsive α-cells (T2D-αNGR)). It is noteworthy that the basal insulin secretion and glucagon secretion in response to 4.0 mm AAM was significantly higher in the T2D-αGR group compared with normal human islets and with the T2D-αNGR group. These data (the lower glucose threshold for glucagon suppression versus insulin stimulation in normal islets and the impairment in insulin stimulation but not of glucagon suppression in some T2D islets) provide a unique opportunity to investigate which factors might be critical in mediating glucose suppression of glucagon secretion in human islets and for testing the possibility that relatively high basal but poor glucose-stimulated insulin secretion in the T2D-αGR group is required for glucose suppression of glucagon release (8).
FIGURE 1.
T2D human islets have impaired insulin secretion and oxygen consumption and different glucose-mediated glucagon suppression and GABA shunt. A shows insulin secretion in response to glucose in the presence of 4.0 mm AAM in normal (filled circles with black line; n = 5) and T2D islets (T2D-αGR, open circles with dashed gray line; T2D-αNGR, triangles with dashed black line; n = 3 for each). Versus T2D-αNGR and normal, * indicates p < 0.05; versus T2D-αGR and normal, # indicates p < 0.05; 25 mm glucose versus 0 mm glucose, a indicates p < 0.05; 25 mm glucose versus 5 mm glucose, b indicates p < 0.05. B shows glucagon secretion (inset shows the percent changes). Versus T2D-αNGR and normal, * indicates p < 0.05; versus 0 mm glucose, a indicates p < 0.05. C shows the glucose stimulation of islet oxygen consumption in normal and T2D islets (normal, black-filled bars (n = 5); T2D-αGR, gray-filled bars; T2D-αNGR, open bars (n = 3 for each); also shown in D and E). Versus 0 mm glucose (G, 0), a indicates p < 0.01; versus 25 mm glucose (G, 25), b indicates p < 0.01; versus normal, * indicates p < 0.05. D shows GABA/glutamate ratios. Versus normal, * indicates p < 0.05; versus 0 mm glucose, a indicates p < 0.05. E shows expression data for selected genes detected by quantitative PCR. Versus normal, * indicates p < 0.05. Data are presented as mean ± S.E. (error bars). GK, glucokinase; PC, pyruvate carboxylase; GDH, glutamate dehydrogenase; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone.
Because the generation and release of GABA by β-cells has been suggested to play a role in glucose-mediated inhibition of glucagon secretion (15), we further examined the changes in levels of GABA and other islet amino acids and their 13C enrichments during incubation with [U-13C]glucose at 5 and 25 mm in the presence of 4 mm AAM and 0.3 mm ammonia. As shown in Table 1, in normal islets, 25 mm glucose as expected lowered aspartate concentrations (21), reflecting increased oxaloacetate consumption indicative of augmented TCA cycle flux. Of importance for the present study, we observed that glucose lowered GABA levels by 50% in normal human islets, similar to our previous findings in mouse islets (21). In the subgroup of T2D-αGR islets (which have normal glucagon suppression by glucose), the amino acid profile and responses to glucose were similar to those of normal human islets. In contrast, in the subgroup of T2D-αNGR islets (in which glucagon secretion was unresponsive to glucose), the amino acid profile was dramatically altered with large accumulation of alanine, glutamate, glutamine, glycine, and serine (a near tripling of the sum of this group of amino acids), indicating a generalized defect of amino acid metabolism. In contrast to this generalized elevation of amino acids, the levels of GABA were reduced by nearly 80% in T2D-αNGR islets compared with controls and barely responded to high glucose. The similarity of the amino acid profiles in T2D-αGR islets and normal islets further supports the validity of considering the two groups of T2D islets as distinct.
TABLE 1.
Intracellular amino acid (nmol/mg of islet protein) responses to glucose in normal and T2D islets
Data are presented as mean ± S.E. G 0, G 5, and G 25 represent 0, 5, and 25 mm glucose, respectively.
| G 0 |
G 5 |
G 25 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 5) | T2D-αGR (n = 3) | T2D-αNGR (n = 3) | Controls (n = 5) | T2D-αGR (n = 3) | T2D-αNGR (n = 3) | Controls (n = 5) | T2D-αGR (n = 3) | T2D-αNGR (n = 3) | |
| Alanine | 9 ± 2 | 10 ± 2 | 31 ± 11a | 12 ± 3 | 14 ± 3 | 46 ± 22 | 15 ± 3 | 12 ± 1 | 44 ± 21 |
| Arginine | 26 ± 4 | 25 ± 6 | 26 ± 3 | 25 ± 5 | 27 ± 5 | 27 ± 6 | 26 ± 3 | 24 ± 1 | 24 ± 5 |
| Aspartate | 58 ± 6 | 73 ± 13 | 57 ± 6 | 43 ± 2 | 57 ± 12 | 34 ± 3b | 36 ± 4c | 43 ± 2 | 29 ± 0c |
| GABA | 57 ± 8 | 63 ± 15 | 12 ± 7a,d | 44 ± 7 | 53 ± 10 | 11 ± 6a,d | 27 ± 5b | 37 ± 5 | 8 ± 4d |
| Glutamate | 66 ± 12 | 83 ± 10 | 167 ± 73 | 88 ± 21 | 114 ± 30 | 187 ± 85 | 93 ± 15 | 108 ± 2 | 182 ± 80 |
| Glutamine | 6 ± 2 | 4 ± 1 | 27 ± 12 | 8 ± 2 | 9 ± 2 | 35 ± 18 | 9 ± 2 | 8 ± 1 | 33 ± 18 |
| Glycine | 13 ± 4 | 20 ± 3 | 31 ± 5a | 14 ± 4 | 21 ± 4 | 35 ± 6a | 14 ± 3 | 17 ± 10 | 28 ± 5 |
| Isoleucine | 6 ± 0 | 8 ± 1 | 7 ± 2 | 5 ± 0 | 7 ± 1 | 7 ± 2 | 5 ± 0 | 6 ± 1 | 7 ± 1 |
| Leucine | 3 ± 1 | 2 ± 1 | 6 ± 1 | 3 ± 1 | 2 ± 1 | 6 ± 2 | 2 ± 1 | 3 ± 0 | 6 ± 2 |
| Serine | 10 ± 2 | 10 ± 1 | 33 ± 11a | 12 ± 3 | 13 ± 3 | 36 ± 14 | 13 ± 3 | 11 ± 1 | 34 ± 14 |
| Sum | 268 ± 22 | 298 ± 48 | 399 ± 118 | 280 ± 30 | 313 ± 68 | 421 ± 145 | 264 ± 35 | 265 ± 5 | 395 ± 141 |
a Versus controls, p < 0.05.
b Versus 0 mm glucose, p < 0.05.
c Versus 0 mm glucose, p < 0.01.
d Versus T2D-αGR, p < 0.05.
The energetics of T2D-αGR and T2D-αNGR islets were profoundly compromised as demonstrated by a total lack of effect of high glucose on the oxygen consumption rate (Fig. 1C), paralleling the similarity of the two groups in showing defective GSIS. Comparable results were obtained in studies including 3 mm glucose in the medium during phase 1 of the test (not shown). These observations support the view that a defect in β-cell mitochondrial function is a major cause of impaired GSIS in T2D (Fig. 1, compare A and C) (36). This observation also suggests that glucose suppression of α-cell function does not seem to require that oxidative glucose metabolism of β-cells is fully intact (Fig. 1, compare B and C).
T2D-αNGR Islets Have an Impaired GABA Shunt
The 13C isotopic enrichment of intracellular amino acids was determined by GC/MS to evaluate the metabolic flux responses to glucose in normal and T2D islets (Table 2). In normal human islets, high glucose increased [13C]alanine, which along with the trend toward increased levels of alanine reflected augmented flux through glycolysis. It requires emphasis that M + 2 of [13C]alanine was about 4-fold higher than M + 3, a phenomenon that was also observed previously in mouse islets (21). A possible interpretation is that human islets have high rates of pyruvate cycling similar to mouse islets (21). This interpretation is supported by the expression of all three isoforms of malic enzyme (1, 2, and 3) in normal human islets (shown in supplemental Fig. 2). However, given the limited experiments performed here, the precise mechanism of the high rate of dilution of the M + 3 pool of alanine remains to be established. T2D islets exhibited similar increases in the ratios of [13C]alanine M + 2 to M + 3 even in the T2D-αNGR group. The 13C enrichment of alanine was elevated in the T2D-αNGR subgroup, suggesting that mitochondrial damage (perhaps at the pyruvate dehydrogenase or pyruvate carboxylase step) was more pronounced than in the T2D-αGR group, resulting in the accumulation and increased labeling of pyruvate (37). The “aspartate switch” (21), i.e. the lowering of aspartate by high glucose, was comparable in control islets and those from type 2 diabetics. However, oxaloacetate turnover as reflected in decreased 13C enrichment of aspartate seemed to be lowest in T2D-αNGR islets. In general, the T2D-αGR islets had similar changes of 13C enrichment of amino acids as observed in controls.
TABLE 2.
13C enrichments (mole percent enrichment) of intracellular amino acids in normal and T2D human islets
Data are presented as mean ± S.E. G 5 and G 25 represent 5 and 25 mm glucose, respectively.
| G 5 |
G 25 |
|||||
|---|---|---|---|---|---|---|
| Controls (n = 5) | T2D-αGR (n = 3) | T2D-αNGR (n = 3) | Controls (n = 5) | T2D-αGR (n = 3) | T2D-αNGR (n = 3) | |
| Alanine | ||||||
| M + 2 | 5 ± 1 | 8 ± 4 | 20 ± 8 | 11 ± 1a | 11 ± 4 | 23 ± 9 |
| M + 3 | 1 ± 0.1b | 3 ± 1 | 7 ± 3c | 3 ± 0.3d,e | 2 ± 1 | 8 ± 3 |
| Sum | 6 ± 1 | 11 ± 5 | 27 ± 11 | 14 ± 2a | 14 ± 4 | 31 ± 12 |
| Aspartate | ||||||
| M + 2 | 8 ± 1 | 8 ± 2 | 5 ± 3 | 10 ± 1 | 8 ± 2 | 7 ± 3 |
| M + 3 | 11 ± 2 | 12 ± 4 | 7 ± 4 | 20 ± 2a,e | 18 ± 4 | 9 ± 4 |
| M + 4 | 8 ± 2 | 8 ± 3 | 4 ± 3 | 16 ± 2a,b | 15 ± 3 | 7 ± 3 |
| Sum | 27 ± 5 | 28 ± 8 | 16 ± 9 | 46 ± 5a | 40 ± 9 | 23 ± 11 |
| GABA | ||||||
| M + 2 | 12 ± 2 | 13 ± 5 | 7 ± 3 | 16 ± 1a | 13 ± 3 | 7 ± 4 |
| M + 3 | 8 ± 2 | 9 ± 4 | 5 ± 3 | 14 ± 2a | 10 ± 2 | 6 ± 3 |
| M + 4 | 6 ± 2 | 8 ± 4 | 3 ± 2 | 23 ± 2d,e | 17 ± 4 | 7 ± 4c |
| Sum | 26 ± 5 | 30 ± 13 | 15 ± 8 | 54 ± 4d | 41 ± 9 | 20 ± 10c |
| Glutamate | ||||||
| M + 2 | 13 ± 2 | 13 ± 3 | 10 ± 3 | 15 ± 1 | 14 ± 1 | 13 ± 2 |
| M + 3 | 13 ± 2 | 13 ± 4 | 13 ± 2 | 17 ± 1 | 16 ± 2 | 14 ± 2 |
| M + 4 | 13 ± 3 | 15 ± 5 | 13 ± 2 | 23 ± 2a,e | 23 ± 3 | 15 ± 3 |
| M + 5 | 9 ± 2 | 10 ± 4 | 10 ± 4 | 19 ± 3a | 22 ± 3 | 13 ± 6 |
| Sum | 47 ± 9 | 51 ± 14 | 45 ± 7 | 75 ± 7a | 75 ± 8 | 56 ± 9 |
| Glutamine | ||||||
| M + 2 | 3 ± 1 | 1 ± 0 | 4 ± 2 | 4 ± 2 | 2 ± 1 | 5 ± 1 |
| M + 3 | 2 ± 1 | 4 ± 1 | 2 ± 1 | 5 ± 1 | 7 ± 2 | 3 ± 1 |
| M + 4 | 2 ± 0 | 4 ± 1 | 1 ± 1 | 3 ± 1 | 6 ± 2 | 2 ± 0 |
| M + 5 | 1 ± 0 | 2 ± 1 | 1 ± 0 | 2 ± 0 | 3 ± 1 | 1 ± 0 |
| Sum | 8 ± 1 | 10 ± 3 | 9 ± 4 | 14 ± 3 | 18 ± 5 | 11 ± 1 |
a Versus 5 mm glucose, p < 0.05.
b Versus M + 2, p < 0.05.
c Versus controls, p < 0.05.
d Versus 5 mm glucose, p < 0.01.
e Versus M + 2, p < 0.01.
In contrast to the unaltered metabolomic data in T2D-αGR islets, the T2D-αNGR islets had low 13C enrichment of GABA in addition to very low GABA levels as shown in Table 1. In normal islets, 25 mm glucose increased 13C enrichment of GABA by about 100%, which together with the fall in GABA concentrations suggests that glucose metabolism greatly enhanced GABA shunt flux and GABA turnover. In normal islets, as shown in Fig. 1D, the GABA/glutamate ratio was greatly decreased at high glucose levels, indicating a crossover at the GAD step that could be explained by a combination of increased glutamate production upstream and increased GABA turnover downstream. Whereas T2D-αGR islets had a near normally functioning GABA shunt compared with control islets, the T2D-αNGR islets had a severe GABA shunt impairment with basal GABA/glutamate ratios only 20% of normal, no further decrease of this ratio by high glucose (Fig. 1D), and barely any evidence of glucose-stimulated turnover (Table 2). The decreased GABA/glutamate ratio in T2D-αNGR islets suggests a block at the GAD step. Results presented in Fig. 1E do indeed show that T2D-αNGR islets have a severe GAD gene expression defect. The expression of the insulin and glucokinase genes is also reduced, whereas the glucagon, glutamate dehydrogenase, and pyruvate carboxylase gene expressions are normal. It is noted that low GAD gene expression was also found previously in sulfonylurea receptor 1 knock-out (SUR1−/−) mouse islets, which are characterized by chronic β-cell depolarization and elevation of intracellular calcium, and was consistent with the amino acid profiles of these islets showing accumulation of glutamate and alanine but depletion of GABA (21). [U-13C]Glucose oxidation and 13C fluxes into the intracellular amino acid pool in the normal mouse islets shown in supplemental Fig. 1 was similar to those in human islets observed here.
Human Islets Express the GHB Loop of the GABA Shunt
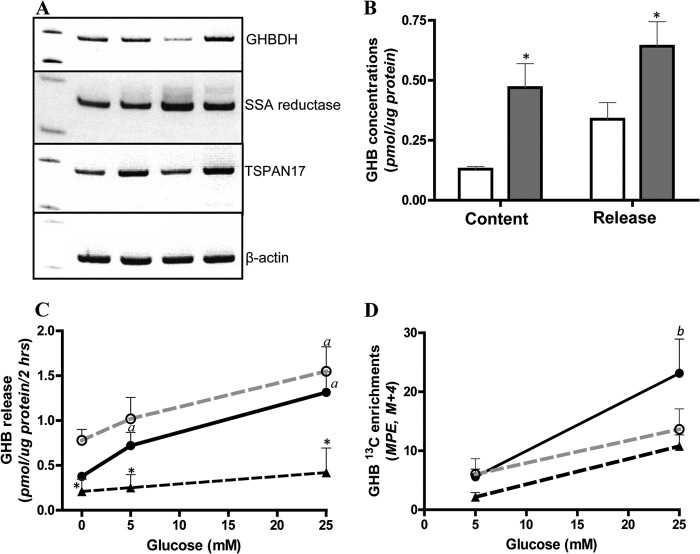
These metabolic and functional data from normal islets and islets from the two distinct subgroups of T2D suggest that the GABA shunt pathway could be mediating the effect of glucose in suppressing glucagon secretion. The association of impaired glucose-mediated suppression of glucagon release with impaired GABA shunt activity in T2D-αNGR islets suggested the possibility of a causal link between the two phenomena, such as a deficiency of GABA per se or a deficiency of some downstream metabolite of GABA. As a consequence of GABA metabolism, SSA produced via GABA transaminase enters the TCA cycle through SSA dehydrogenase. In the central nervous system, a fraction of SSA is diverted to GHB via the NADPH-dependent SSA reductase (38). GHB, a potent inhibitory neurotransmitter, is then converted back to SSA via the NAD-dependent GHB dehydrogenase (GHBDH) to form the “GHB loop” (39–41). As shown in Fig. 2A, studies with normal human islets from four separate pancreas donors showed that SSA reductase, GHBDH, and TSPAN-17, which has been suggested to function as a GHB receptor gene in brain (42, 43), are clearly expressed. Studies in three other cases of normal human islets (Fig. 2B) showed that normal human islets produce and release GHB in response to glucose: 10 mm glucose stimulated a 3-fold increase of islet GHB content and a 2-fold increase of GHB released into the incubation medium. In the normal human islets, as shown in Fig. 2C, glucose at 5 mm stimulated a significant increase of GHB release, and 25 mm augmented this response. Medium from the earlier studies of T2D-αGR islets showed a pattern of GHB release in response to glucose similar to that of normal islets, whereas the medium from the studies of T2D-αNGR islets, which had impaired glucagon suppression by glucose, showed only low basal GHB release and failure to increase GHB release in response to glucose stimulation. The increase in GHB 13C isotopic enrichment during glucose stimulation (Fig. 2D) indicated that GHB carbon was derived from glucose. These data suggested that GHB, derived from increased GABA shunt activity, might be a critical factor mediating glucose suppression of glucagon secretion in both normal and T2D-αGR islets and that the lack of GHB production might explain the lack of glucagon suppression by glucose in T2D-αNGR islets.
FIGURE 2.
Human islets express GHB loop and produce GHB in response to glucose stimulation. A shows gene expression detected by RT-PCR of the enzymes of the GHB loop including SSA reductase, GHB dehydrogenase, and the putative GHB receptor (TSPAN-17) in four different preparations of normal human islets. B shows GHB production and release in batch-incubated normal human islets. After 60-min preincubation, batches of 500 islets were incubated with 0 (open bars) or 10 mm glucose (filled bars) for another 60 min, and GHB was determined in islet homogenates and the incubation supernatants. Versus 0 mm glucose, * indicates p < 0.05 (n = 3). C and D show GHB release and its [13C]GHB enrichment from experiments with [U-13C]glucose (compare with results in Tables 1 and 2). Normal, filled circles with black line (n = 5); T2D-αGR, open circles with dashed gray line (n = 3); T2D-αNGR, triangles with dashed black line (n = 3). Versus T2D-αGR, * indicates p < 0.05; versus 0 mm glucose, a indicates p < 0.05; versus 5 mm glucose, b indicates p < 0.05. Data are presented as mean ± S.E. (error bars). MPE, mole percent enrichment.
A Role of the GABA Shunt and GHB in Glucose-mediated Suppression of Glucagon Secretion in Normal Human Islets
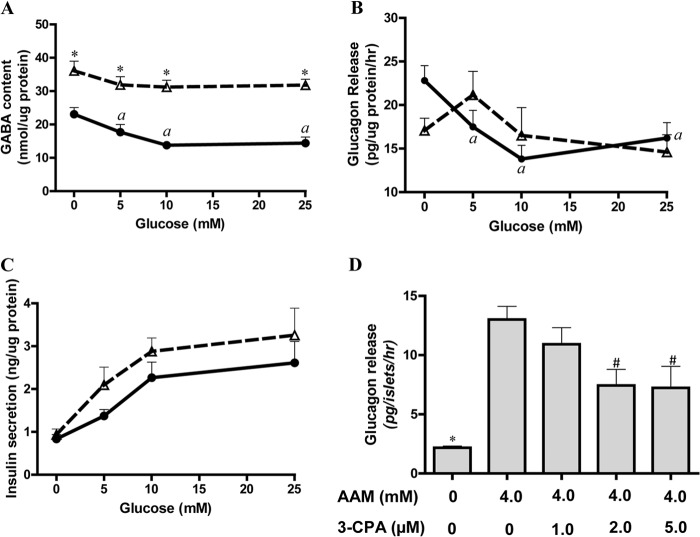
To test whether the GABA shunt could mediate glucose suppression of glucagon secretion in normal human islets, vigabatrin, a specific inhibitor of GABA transaminase (21, 44), was used to block the conversion of GABA to SSA. As shown in Fig. 3A and in supplemental Table 3, in normal human islets in the absence of exogenous amino acids, inhibition of GABA transaminase by vigabatrin caused a near doubling of intracellular GABA and eliminated the effect of glucose to lower GABA, providing further evidence that glucose metabolism increases flux through the GABA shunt. As shown in Fig. 3B, blockade of the GABA shunt by vigabatrin resulted in a loss of inhibitory control of glucagon secretion by glucose in normal human islets, although vigabatrin had no effect on glucose-mediated insulin secretion (Fig. 3C), suggesting that glucose-stimulated secretion of insulin is unlikely to mediate glucose suppression of glucagon release. This observation favors the hypothesis that events associated with increased flux through the GABA shunt or a distal metabolite of GABA (GHB) may mediate glucose suppression of glucagon secretion independently of insulin secretion or zinc release.
FIGURE 3.
GHB produced via GABA shunt mediates glucose suppression of glucagon secretion. A–C show GABA levels and glucagon and insulin secretion in normal islets in the absence (filled circles with solid line) or in the presence of 1.55 mm vigabatrin (open triangles with dashed line). A, islet intracellular GABA levels; B, glucagon secretion; C, insulin secretion. Versus untreated islets, * indicates p < 0.01; versus 0 mm glucose, a indicates p < 0.05; n = 4. D shows glucagon secretion stimulated by an amino acid mixture in batch-incubated normal human islets and inhibition of amino acid-stimulated glucagon secretion by the GHB agonist 3-CPA. After 60-min preincubation, batches of 50 islets were incubated with different treatments for another 60 min. Versus AAM stimulation, * indicates p < 0.01, and # indicates p < 0.05; n = 4. Data are presented as mean ± S.E. (error bars).
Because islets can produce GHB, we examined whether the GHB analog 3-chloropropanoic acid (3-CPA), which is a specific agonist of the GHB receptor but does not activate the GABA receptor (45), might inhibit glucagon release stimulated by amino acids. As shown in Fig. 3D, 3-CPA inhibited amino acid-stimulated glucagon secretion in four different batches of normal human islets with maximum inhibition at 2 μm. 3-CPA at concentrations of 2, 5, 10, and 20 μm had no effect on 10 mm glucose-stimulated insulin secretion or cytosolic calcium influx (data not shown), suggesting that at these low concentrations 3-CPA is unlikely to have an inhibitory effect on glucose oxidation. At higher concentrations, 3-CPA may have an inhibitory effect on pyruvate dehydrogenase (46). However, at the low concentration of 2 μm, 3-CPA is likely to have an α-cell-specific inhibition. This provides further evidence that GHB, produced during glucose oxidation and released from β-cells, binds to its specific receptor on α-cells and inhibits glucagon secretion.
Glycine Stimulates Glucagon Secretion via the Strychnine-sensitive Glycine Receptor
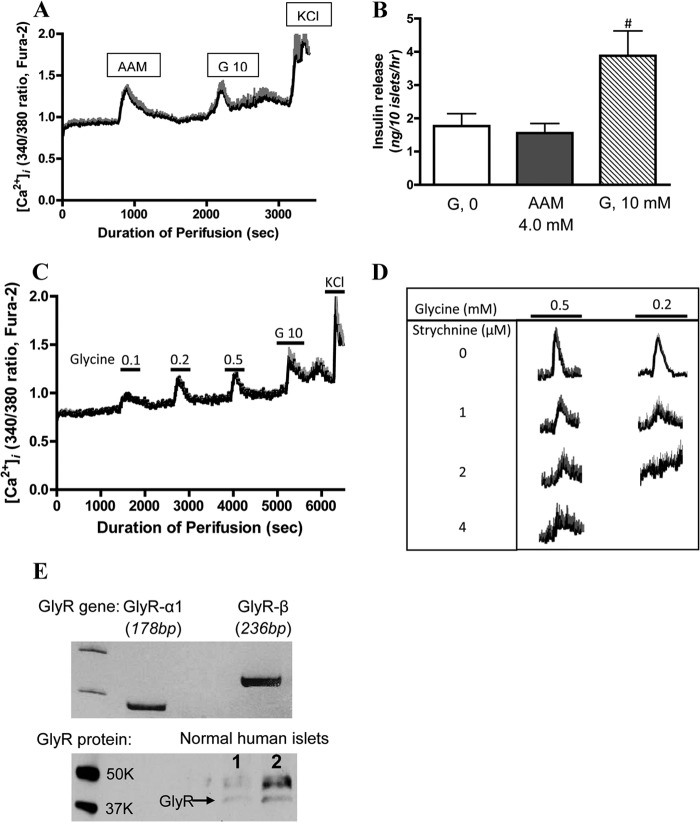
The evidence presented above suggests that GHB causes receptor-mediated glucose suppression of glucagon secretion stimulated by amino acids. To further expand the understanding of α-cell regulation, we explored whether the amino acid stimulation of glucagon secretion might also be attributable to receptor-mediated effects of certain amino acid transmitters (e.g. glutamate, aspartate, or glycine) or whether it is due to a general metabolic amino acid effect analogous to the mechanisms explaining glucose stimulation of β-cells. To examine this question, cytosolic calcium ([Ca2+]i) responses to individual amino acids were examined in normal human islets. As shown in Fig. 4A, normal human islets displayed calcium responses to both 4 mm AAM and to 10 mm glucose. As previously established, 4 mm AAM failed to stimulate insulin secretion (Fig. 4B) but stimulated a 4-fold increase of glucagon secretion (Fig. 3D), indicating that the calcium signal observed during amino acid stimulation most likely arose from α-cells. In contrast, glucose stimulated insulin but not glucagon secretion, indicating that the islet calcium response to glucose was most likely generated by β-cells. Thus, we were able to use measurements of calcium responses to amino acids versus glucose to distinguish α-cell from β-cell responses in intact human islets. Upon screening of individual amino acids, glycine was discovered to be the best candidate amino acid in the mixture causing the increased [Ca2+]i in a dose-dependent manner (Fig. 4C). Glutamate, which has previously been suggested to be a glucagon secretagogue (47, 48), elicited no response at 0.1 mm (data not shown).
FIGURE 4.
Glycine stimulates cytosolic calcium influx and glucagon secretion via strychnine-sensitive glycine receptor in normal human islets. Cytosolic calcium ([Ca2+]i) levels were measured by dual wavelength fluorescence microscopy using Fura-2 as the calcium indicator. Data are presented as a black line (mean values), and S.E. values (error bars) are given in gray. A shows [Ca2+]i responses to a 4.0 mm amino acid mixture and 10 mm glucose (G 10) (n = 5). B shows insulin secretion from batch-incubated normal human islets. Versus 0 mm glucose and amino acid mixture, # indicates p < 0.05; n = 4. C shows effects of different concentrations of glycine on [Ca2+]i (n = 4). D shows dose-dependent strychnine inhibition of [Ca2+]i stimulated by 0.2 or 0.5 mm glycine (n = 4). E shows gene expression (upper panel) detected by RT-PCR of glycine receptors α1 and β. Results are representative for comparable results from three separate preparations of normal human islets. The lower panel of E shows the presence of glycine receptor protein detected by Western blotting in two batches of normal human islets. Data are presented as mean ± S.E. (error bars).
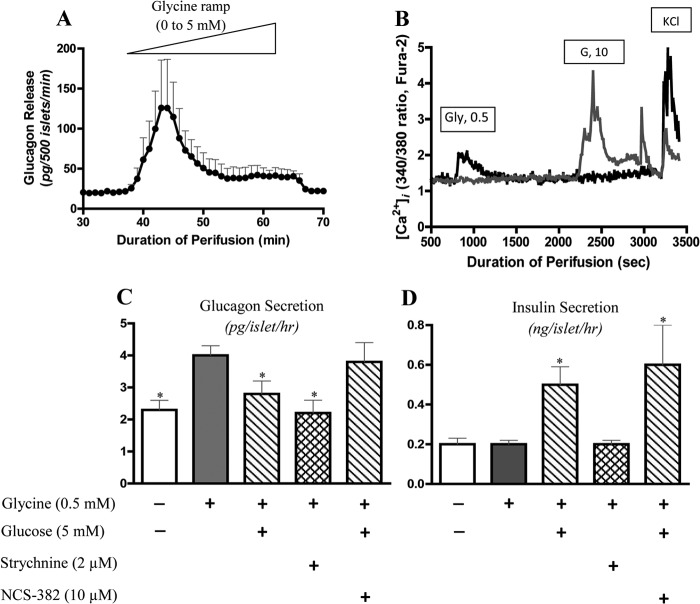
To test the hypothesis that the effect of glycine on α-cell [Ca2+]i might be due to its well established role as a neurotransmitter, the specific glycine receptor (GlyR) blocker, strychnine, was used (49). As shown in Fig. 4D, the glycine effect on [Ca2+]i in normal human islets was indeed blocked by strychnine with an ED50 of 1–2 μm. As shown in Fig. 4E, normal human islets expressed mRNA for GlyR, both α1 and β (Fig. 4E, upper panel), and GlyR protein was detectable in two cases of normal human islets (Fig. 4E, lower panel). To directly test the effect of glycine on glucagon release, three separate isolates of normal human islets were perifused with a glycine ramp. As shown in Fig. 5A, the glycine ramp stimulated glucagon secretion with a threshold of 0.3–0.5 mm and a maximum response at 1.2 mm, which is similar to the thresholds for the islet [Ca2+]i response. To confirm that the calcium response to glycine was derived from α-cells, [Ca2+]i was measured in dispersed single islet cells. As shown in Fig. 5B, individual islet cells showed differential responses to glycine and glucose: cells that responded to glycine but not glucose were likely α-cells, whereas cells responding to glucose but not glycine were likely β-cells. As shown in Fig. 5C, in normal human islets, 0.5 mm glycine stimulated glucagon secretion, and this effect was blocked by 2 μm strychnine as well as by 5 mm glucose. The inhibitory regulation of glucagon secretion by glucose was reversed by the GHB receptor antagonist NCS-382 (50). As shown in Fig. 5D, unlike glucose, glycine had no effect on insulin secretion in batch-incubated normal human islets. Similarly, neither NCS-382 nor strychnine affected the insulin responses to glucose. Additional data in control human islets showed that strychnine had no impact on 10 mm glucose-stimulated insulin secretion (10 mm glucose, 7.0 ± 2.0 ng/50 islets/h; 10 mm glucose plus 2 μm strychnine, 6.3 ± 1.7 ng/50 islets/h; n = 3, p > 0.05). These results demonstrate that the α-cell neurotransmitter receptors for glycine and GHB play a dominant role in the stimulatory and inhibitory islet regulation of glucagon secretion.
FIGURE 5.
Glycine stimulates glucagon secretion and [Ca2+]i influx, and this effect is inhibited by glucose and strychnine. A shows glucagon secretion from perifused normal human islets in response to stimulation by a glycine ramp (0–5 mm over a period of 30 min; n = 3). B shows different [Ca2+]i responses to glycine and glucose stimulation and potassium chloride (KCl) in single dispersed human islet cells (data are representative for four separate experiments with similar results). The cell indicated by the black line is sensitive to glycine stimulation and is likely the α-cell; the cell indicated by the gray line is sensitive to glucose stimulation and is likely the β-cell. [Ca2+]i levels were measured by dual wavelength fluorescence microscopy using Fura-2 as the calcium indicator. C and D show glucagon and insulin secretion in response to glycine stimulation and the effects of glucose, strychnine, and the GHB receptor antagonist NCS-382 in batch-incubated normal human islets. After 60-min preincubation, batches of 50 islets were then incubated with different treatments as indicated in the figure for another 60 min. Versus 0.5 mm glycine, * indicates p < 0.05; n = 4. Data are presented as mean ± S.E. (error bars). G, glucose.
The Expression of Genes of the GHB Loop and Receptors for GHB and Glycine in Purified Mouse β-Cells
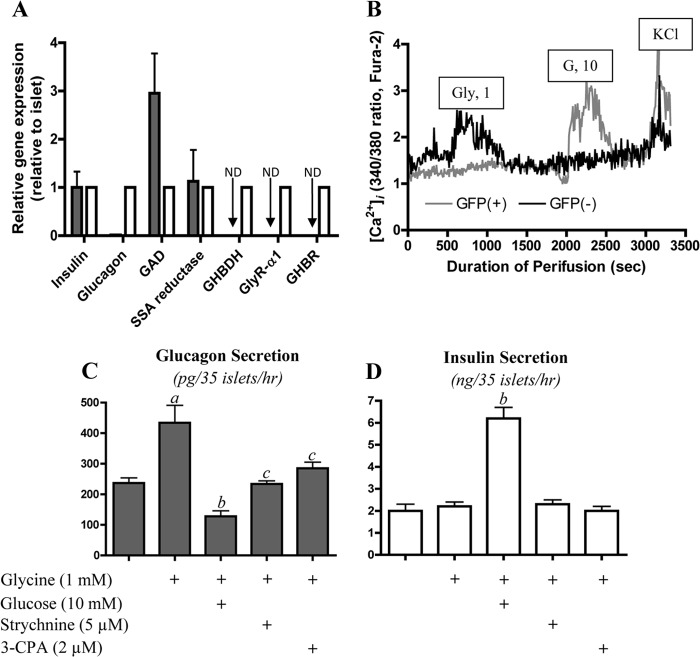
To investigate the cellular distribution of the GHB loop pathway and the receptors for GHB and glycine in the islet organ, GFP transgenic mice in which GFP was specifically expressed in β-cells via the mouse insulin promoter were used to purify β-cells by FACS (32). Insulin and glucagon gene expression, detected by quantitative PCR, was used to evaluate the purity of sorted β-cells. As shown in Fig. 6A, compared with glucagon gene expression in intact islets, purified β-cells expressed less than 1% of glucagon mRNA of the whole islet, indicating high purity of the sorted β-cells. Gene expression of the GHB receptor (TSPAN-17) and of the GlyR α1 was not detected by quantitative PCR in purified β-cells in contrast to their clear expression in intact mouse islets, suggesting that GHB receptor and GlyR are present in non-β-cells, likely in α-cells. Of significance is the finding that the key enzymes of the GHB loop, SSA reductase and GHBDH, are expressed in different cell types. SSA reductase, like the GAD gene, was highly expressed in both purified β-cells and intact islets, whereas GHBDH was undetectable in β-cells but clearly expressed in whole islets. Because β-cells lack the enzyme for converting GHB back to SSA for degradation, we speculate that the production and release of GHB by β-cells is greatly facilitated. In contrast, α-cells express GHBDH, which could help regulate GHB effects by reducing it to SSA, thus limiting its actions as a mediator. To validate the gene expression profiles, we measured [Ca2+]i of single islet cells from GFP transgenic mice. As shown in Fig. 6B, GFP-positive cells (β-cells) were not sensitive to 1 mm glycine stimulation but showed a clear response to 10 mm glucose. In contrast, GFP-negative cells (α-cells) were sensitive to glycine stimulation but not to glucose. These data support the concept that the GlyR is α-cell-specific.
FIGURE 6.
GHBDH and receptors of glycine and GHB are α-cell-specific in mouse islets. A shows relative gene expression of insulin, glucagon, GAD, SSA reductase, GHBDH, and receptors of GHB and glycine in intact mouse islets and purified β-cells from GFP transgenic mice with β-cell-specific expression (open bars, whole islets; filled bars, purified β-cells). GAPDH was used as a reference gene. ND, not detectable; n = 3. B shows the calcium response to glycine and glucose stimulation in a single GFP-positive or -negative islet cell from GFP transgenic mice (data are representative for three separate experiments with similar results). [Ca2+]i levels were measured by dual wavelength fluorescence microscopy using Fura-2 as the calcium indicator. C and D show glucagon and insulin secretion in isolated batch-incubated normal mouse islets. Versus glucose-free basal conditions, a indicates p < 0.05; versus 1 mm glycine stimulation, b indicates p < 0.01, and c indicates p < 0.05; n = 5. Data are presented as mean ± S.E. (error bars). GHBR, GHB receptor; G, glucose.
In light of the gene expression data from purified mouse β-cells, it was logical to also test the gene expression of the GHB loop and of the receptors for GHB and glycine in normal and T2D human islets used in our earlier studies. As shown in supplemental Fig. 3, expression of SSA reductase was decreased about 50% in the T2D-αGR and 60% in the T2D-αNGR groups of human islets. Assuming SSA reductase to be β-cell-specific (Fig. 6A), its reduced expression may reflect the 40–50% reduction of β-cell mass, which has been previously reported in T2D (51). By comparison with the modest reduction of mRNA expression of SSA reductase, the much greater reduction in expression of insulin, GAD, and glucokinase indicates that the impaired expression of these genes is not simply the consequence of loss of β-cell mass. Expression of GHBDH and GHB receptor mRNA showed a trend toward decreased levels in T2D islets, whereas GlyR expression appeared to be unaltered.
The phenomenon of GHB inhibition and of glycine stimulation of glucagon secretion was also examined in isolated, cultured normal mouse islets. As shown in Fig. 6C, 1 mm glycine doubled glucagon secretion without stimulation of insulin release (Fig. 6D). In mouse islets, 10 mm glucose strongly inhibited glycine-stimulated glucagon secretion while stimulating a marked increase in insulin secretion. Glycine stimulation of glucagon secretion by α-cells was mediated via its receptor because the GlyR antagonist, strychnine, prevented glycine-induced glucagon secretion. The GHB receptor agonist 3-CPA also inhibited glycine-stimulated glucagon secretion in normal mouse islets. Thus, regulation of α-cells by glycine and GHB in normal mouse islets is indistinguishable from that observed in human islets.
DISCUSSION
These studies of glucose control of glucagon secretion in human islets indicate that the neurotransmitter GHB plays an important role in transmitting an inhibitory paracrine signal from β-cells to α-cells during exposure to glucose. GHB is produced as a consequence of the increase in GABA shunt activity in β-cells that accompanies the increased flux of glucose carbons into TCA cycle pathways during GSIS. Evidence for involvement of GHB in paracrine control of α-cells was first detected in islets from type 2 diabetic donors that were non-responsive to glucose suppression of glucagon release because of chronic depletion of GABA shunt enzymes and metabolites. Studies in normal human islets demonstrated increased production and release of GHB during glucose stimulation and showed that inhibition of the GABA shunt pathway to GHB by the GABA transaminase inhibitor vigabatrin blocked glucose suppression of glucagon release. In normal human islets, glucagon secretion was directly inhibited by activation of the GHB receptor with 3-CPA and increased by blocking the GHB receptor with NCS-382. Additional studies in normal human islets identified glycine as an important specific amino acid stimulator of glucagon release, suggesting that glycine may serve as a counterbalance to the inhibitory effect of GHB, thus permitting glucagon secretion to rise in response to hypoglycemia. Gene expression profiles of purified mouse β-cells and intact islets demonstrated that GHB was specifically produced in β-cells. The presence of SSA reductase but absence of GHBDH in β-cells ensures that GHB production and release are specific to β-cells. In contrast, GHBDH and the receptor for GHB are expressed in α-cells, suggesting that α-cells cannot only sense GHB but also may remove GHB by an unknown uptake mechanism and by GHB degradation through GHBDH.
The identification of GHB as an inhibitory mediator of islet glucagon responses to glucose was not anticipated at the outset of these investigations, although it was clear that the mechanisms by which glucose suppresses and hypoglycemia stimulates glucagon release are controversial. Wollheim and co-workers (7) recently summarized evidence that α-cells are controlled in a paracrine/endocrine fashion by factors released from β-cells during GSIS that suppress glucagon release. The list of candidate factors includes insulin itself, zinc co-secreted with insulin from insulin granules, GABA, glutamate, somatostatin, ghrelin, GLP-1, and glucagon. The greatest attention has been focused on insulin and zinc. However, as noted in the Introduction, because of the differences observed in glucose concentration thresholds for suppression of glucagon versus stimulation of insulin, other factors may contribute. Our studies in normal human islets also confirmed that there was a discrepancy between the levels of glucose that suppress glucagon and stimulate insulin in normal human islets. Even more discrepant with insulin or zinc being significant regulators of glucagon secretion was our finding that islets from a subset of type 2 diabetic humans showed a normal suppression of glucagon by glucose despite having impairment of insulin secretion. Although we cannot rule out the possibility that a permissive effect of basal insulin secretion in normal or T2D islets is required for glucose-mediated glucagon suppression, it is unlikely that a surge of insulin or zinc release via a paracrine mechanism mediates acute glucose suppression of glucagon secretion. The data presented here strongly support the notion that a metabolic signal generated during glucose oxidation in β-cells controls glucagon secretion from α-cells and that GHB is likely the signal.
Our experiments did not specifically address all of the various paracrine factors proposed as modulators of α-cell glucagon secretion; however, some of the observations are pertinent to the possible paracrine roles of insulin, zinc, and GABA. With regard to insulin or co-secreted zinc, in addition to the discrepancies in glucose thresholds for glucagon versus insulin release noted above, it is worth emphasizing that when vigabatrin was used to block GABA transamination and the production of SSA as substrate for GHB synthesis glucose was no longer able to suppress glucagon secretion even though the release of insulin was not affected. This provides evidence that intraislet release of insulin granules does not directly suppress glucagon. On the other hand, Kulkarni and co-workers (12) have reported that mice with α-cell-specific insulin receptor knock-out have elevated basal glucagon secretion and a blunted glucagon response to fasting hypoglycemia, supporting the concept that intraislet insulin signaling plays some role in regulating α-cell function in both normo- and hypoglycemic conditions. These observations are not necessarily contradictory to our findings because the well established requirement for insulin in controlling α-cell response to glucose may reflect a “permissive” endocrine effect as opposed to a paracrine effect of insulin. It should also be considered that intraislet insulin signaling may be critical for maintaining glucose suppression of α-cell glucagon secretion but that the low threshold for glucose suppression of glucagon secretion may indicate that such suppression operates without an acute surge of a paracrine insulin signal. Studies by Robertson and co-workers (52) have suggested that zinc might exert its inhibitory effect on glucagon release via α-cell KATP channels and that this could explain the lack of a glucagon response to hypoglycemia observed in SUR1−/− mice. Our observation that glycine stimulates glucagon release suggests an alternative target for the action of zinc because zinc has been shown to be a potent inhibitor of the glycine receptor (53, 54). In addition, the poor glucagon responses of SUR1−/− islets to hypoglycemia noted by Robertson and co-workers (52) might reflect the fact that these islets have very low GABA levels and GABA shunt activity due to decreased expression of GAD as we have reported previously (21). Thus, SUR1−/− islets may resemble the subset of human T2D islets in our study that were not responsive to glucose suppression of glucagon release due to decreased expression of GAD and impaired stores of GABA. As noted in the review by Wollheim and co-workers (7), although GABA can be released in response to glucose, there are observations that argue against β-cell GABA per se having a primary role in glucose suppression of glucagon release (22); this argument is supported by our observation that vigabatrin blockade of GABA transaminase led to an increase in islet GABA but prevented glucose from inhibiting glucagon secretion. The precursor of GABA, glutamate, also appears to be an unlikely candidate for paracrine regulation of α-cells because recent studies by Feldmann et al. (55) indicate that the release of glutamate from β-cells is not a regulated process but occurs merely by reverse flux via the plasma membrane glutamate transporter.
Although it has long been recognized that amino acids are important stimulators of both insulin and glucagon secretion by pancreatic islets (6, 17, 56), the specific mechanisms involved have only recently become apparent primarily with regard to β-cells. As we have reported (28), leucine plays a key role in amino acid-stimulated insulin secretion by allosterically activating glutamate dehydrogenase to increase oxidation of glutamate derived from glutamine and other amino acids. Amino acids, particularly glutamine, can also potentiate GSIS at steps distal to the elevation of cytosolic calcium possibly at the level of GLP-1 receptor signaling (25, 57). In the present experiments with human islets, we found that glycine, acting through plasma membrane glycine receptors, appeared to be the most effective among the amino acids in activating α-cell glucagon release without stimulating insulin secretion. This observation is consistent with in vivo studies in human subjects showing that oral or intravenous administration of glycine stimulates a rise in plasma glucagon (but not insulin) levels (58, 59). Because the threshold for glycine stimulation of glucagon release was similar to ambient plasma concentrations (∼0.2 mm), it is reasonable to consider that blood glycine plays a role in basal rates of glucagon secretion. In this way, glucose stimulation of islets suppresses basal glucagon release via the increased production and release of GHB from β-cells, whereas hypoglycemia by lowering the production of GHB may relieve the inhibitory influence of β-cells and permit ambient glycine to stimulate glucagon release and thus restore plasma glucose levels to normal. The glycine-based stimulation of glucagon release during hypoglycemia probably operates in synergy with adrenergic stimulation of the α-cell.
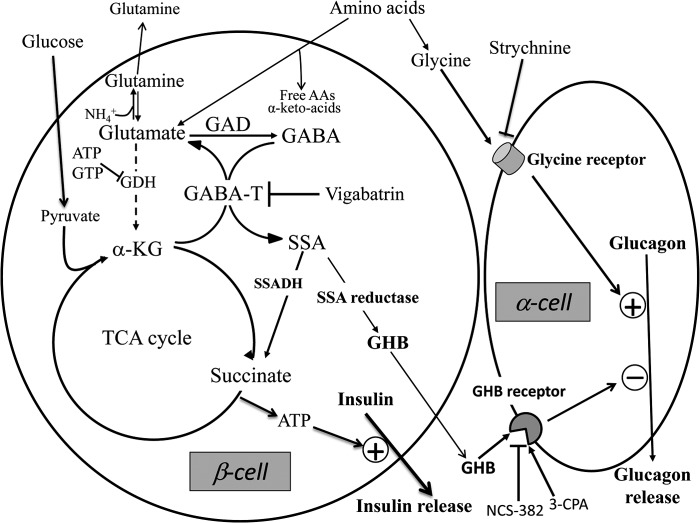
It should be noted that the stimulation of GHB production as a side reaction of the GABA shunt during GSIS parallels other predictable changes in β-cell amino acid pools as they respond to increased substrate flux during glucose stimulation. For example, GSIS is associated with elevations of islet alanine, reflecting increased production of pyruvate; decreased aspartate, reflecting increased utilization of oxaloacetate to form citrate with acetyl-CoA; and increased glutamate, reflecting increased α-ketoglutarate production and transamination with both GABA and aspartate. As shown in Fig. 7, the pathway of glucose carbons flowing into GHB provides a relatively direct mechanism for coordinating glucose sensing between β-cells and α-cells as a functional unit. The flow of glucose carbons into glycolysis and the TCA cycle generates an increase in the ATP/ADP ratio to initiate the process of insulin release; simultaneously, the expansion of TCA cycle substrates leads to the generation of GHB, which can transmit an inhibitory paracrine signal to adjacent α-cells and decrease glucagon secretion to suppress hepatic glucose production. In contrast to glucose stimulation, which selectively elevates insulin and decreases glucagon release, amino acid stimulation of the islet organ can promote insulin release via oxidation through GDH while simultaneously stimulating glucagon release via the glycine receptor to stimulate hepatic glucose production and prevent the development of hypoglycemia.
FIGURE 7.
Opposite effects of glycine and GHB on glucagon secretion and the metabolic interaction between β- and α-cells. α-Cells are stimulated by glycine via its receptor and are inhibited by GHB produced by β-cells also via its receptor. During glucose stimulation of insulin secretion, increased generation of α-ketoglutarate (α-KG) from the TCA cycle supports enhanced flux through the GABA shunt, which leads to increased production of GHB. GHB is generated from SSA via SSA reductase. Vigabatrin acts as a GABA transaminase (GABA-T) inhibitor. Strychnine inhibits the glycine receptor. 3-CPA is a GHB agonist, and NCS-382 is a GHB antagonist. ATP and GTP inhibit glutamate dehydrogenase (GDH). SSADH, SSA dehydrogenase; AAs, amino acids.
GHB was introduced as a sedative drug in the 1960s and later found to be an endogenous inhibitory brain neurotransmitter produced from GABA and released from presynaptic vesicles (60, 61). At low 1–4 μm concentrations, it acts on specific high affinity G-protein-coupled GHB receptors that have not been fully defined. At pharmacologic levels seen with recreational drug use, GHB also has effects mediated via GABAB receptors (62). Therefore, it is possible that the effect of β-cell GHB production on glucagon secretion involves the GABA as well as the GHB receptors. Our results indicate that the pathway for GHB synthesis is present in β-cells and that α-cells appear to express a putative GHB receptor, consistent with the observation that glucagon secretion responds to agents, such as 3-CPA, that act specifically on GHB but not GABA receptors (45). Although a role for GHB in islets has not previously been described, it has been suggested that patients with SSA dehydrogenase deficiency who have markedly elevated GHB levels may have an increased risk of hypoglycemia (63). Hypoglycemia has also been observed in patients admitted for intoxication with recreational use of GHB.3 These observations might be consistent with GHB suppression of glucagon release.
In summary, the present study provides a new explanation of how glucose can suppress glucagon secretion and how amino acids can stimulate glucagon secretion. Glucose oxidation increases the flux rate of the GABA shunt, resulting in increased production of GHB, and the augmented GHB release from β-cells causes inhibition of α-cells via the GHB receptor. A contribution of the GABA receptor to the GHB inhibition of α-cells cannot be excluded. Glycine serves as a receptor-mediated stimulator of glucagon secretion explaining in part at least fuel stimulation of α-cells. These newly proposed mechanisms may provide potential drug targets to suppress hyperglucagonemia in T2D patients.
Supplementary Material
Acknowledgments
We gratefully acknowledge the important contribution to this study by the team of the Human Islet Resource Center at University of Pennsylvania, especially Zaw Min, Yanping Luo, Seyed Ziaie, Yanjing Li, and Kumar Vivek. This study was approved by the Institutional Review Board at the University of Pennsylvania (805393).
This work was supported, in whole or in part, by National Institutes of Health Grants U01DK089529 (to C. L., co-principal investigator), DK53012 (to C. A. S.), DK22122 (to F. M. M.), DK53761 (to I. N.), HD26979 (to M. Y.), U42-RR-016600 and U01 DK070430 (to A. N.), 10028044 from the NIDDK/Beckman Research Center (City of Hope) Integrated Islet Distribution Program, and DK19525 (to the Radioimmunoassay and Islet Cores of the Diabetes Research Center of the University of Pennsylvania, Perelman School of Medicine). This work was also supported by American Diabetes Association Grant 7-11-BS-34 (to N. D.). This work was presented in part at the 2009 and the 2011 annual meetings of the American Diabetes Association.

This article contains supplemental Figs. 1–3 and Tables 1–3.
H. Brunengraber, personal communication.
- GSIS
- glucose-stimulated insulin secretion
- GHB
- γ-hydroxybutyrate
- GAD
- glutamate decarboxylase
- SSA
- succinate semialdehyde
- 3-CPA
- 3-chloropropanoic acid
- AAM
- amino acid mixture
- GlyR
- glycine receptor
- T2D
- type 2 diabetic
- GHBDH
- GHB dehydrogenase
- M + n
- containing n 13C-enriched atoms.
REFERENCES
- 1. Unger R. H., Eisentraut A. M., McCall M. S., Madison L. L. (1962) Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J. Clin. Investig. 41, 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Felig P., Wahren J., Sherwin R., Hendler R. (1976) Insulin, glucagon, and somatostatin in normal physiology and diabetes mellitus. Diabetes 25, 1091–1099 [DOI] [PubMed] [Google Scholar]
- 3. Sherwin R., Wahren J., Felig P. (1976) Evanescent effects of hypo- and hyperglucagonemia on blood glucose homeostasis. Metabolism 25, 1381–1383 [DOI] [PubMed] [Google Scholar]
- 4. Fisher M., Sherwin R. S., Hendler R., Felig P. (1976) Kinetics of glucagon in man: effects of starvation. Proc. Natl. Acad. Sci. U.S.A. 73, 1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. (1970) Studies of pancreatic α cell function in normal and diabetic subjects. J. Clin. Investig. 49, 837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pagliara A. S., Stillings S. N., Hover B., Martin D. M., Matschinsky F. M. (1974) Glucose modulation of amino acid-induced glucagon and insulin release in the isolated perfused rat pancreas. J. Clin. Investig. 54, 819–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gromada J., Franklin I., Wollheim C. B. (2007) α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 28, 84–116 [DOI] [PubMed] [Google Scholar]
- 8. Braaten J. T., Faloona G. R., Unger R. H. (1974) The effect of insulin on the α-cell response to hyperglycemia in long-standing alloxan diabetes. J. Clin. Investig. 53, 1017–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Marchand S. J., Piston D. W. (2010) Glucose suppression of glucagon secretion: metabolic and calcium responses from α-cells in intact mouse pancreatic islets. J. Biol. Chem. 285, 14389–14398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matschinsky F. M., Rujanavech C., Pagliara A., Norfleet W. T. (1980) Adaptations of α2- and β-cells of rat and mouse pancreatic islets to starvation, to refeeding after starvation, and to obesity. J. Clin. Investig. 65, 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matschinsky F. M., Pagliara A. S., Hover B. A., Pace C. S., Ferrendelli J. A., Williams A. (1976) Hormone secretion and glucose metabolism in islets of Langerhans of the isolated perfused pancreas from normal and streptozotocin diabetic rats. J. Biol. Chem. 251, 6053–6061 [PubMed] [Google Scholar]
- 12. Kawamori D., Kurpad A. J., Hu J., Liew C. W., Shih J. L., Ford E. L., Herrera P. L., Polonsky K. S., McGuinness O. P., Kulkarni R. N. (2009) Insulin signaling in α cells modulates glucagon secretion in vivo. Cell Metab. 9, 350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishihara H., Maechler P., Gjinovci A., Herrera P. L., Wollheim C. B. (2003) Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat. Cell Biol. 5, 330–335 [DOI] [PubMed] [Google Scholar]
- 14. Zhou H., Zhang T., Harmon J. S., Bryan J., Robertson R. P. (2007) Zinc, not insulin, regulates the rat α-cell response to hypoglycemia in vivo. Diabetes 56, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 15. Wendt A., Birnir B., Buschard K., Gromada J., Salehi A., Sewing S., Rorsman P., Braun M. (2004) Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53, 1038–1045 [DOI] [PubMed] [Google Scholar]
- 16. Walker J. N., Ramracheya R., Zhang Q., Johnson P. R., Braun M., Rorsman P. (2011) Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes. Metab. 13, Suppl. 1, 95–105 [DOI] [PubMed] [Google Scholar]
- 17. Matschinsky F., Bedoya F. (1994) in Endocrinology (DeGroot L. J., ed) pp. 1290–1303, W. B. Saunders, Philadelphia [Google Scholar]
- 18. Ravier M. A., Rutter G. A. (2005) Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 54, 1789–1797 [DOI] [PubMed] [Google Scholar]
- 19. Sorenson R. L., Garry D. G., Brelje T. C. (1991) Structural and functional considerations of GABA in islets of Langerhans. β-Cells and nerves. Diabetes 40, 1365–1374 [DOI] [PubMed] [Google Scholar]
- 20. Winnock F., Ling Z., De Proft R., Dejonghe S., Schuit F., Gorus F., Pipeleers D. (2002) Correlation between GABA release from rat islet β-cells and their metabolic state. Am. J. Physiol. Endocrinol. Metab. 282, E937–E942 [DOI] [PubMed] [Google Scholar]
- 21. Li C., Nissim I., Chen P., Buettger C., Najafi H., Daikhin Y., Nissim I., Collins H. W., Yudkoff M., Stanley C. A., Matschinsky F. M. (2008) Elimination of KATP channels in mouse islets results in elevated [U-13C]glucose metabolism, glutaminolysis, and pyruvate cycling but a decreased γ-aminobutyric acid shunt. J. Biol. Chem. 283, 17238–17249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang C., Kerckhofs K., Van de Casteele M., Smolders I., Pipeleers D., Ling Z. (2006) Glucose inhibits GABA release by pancreatic β-cells through an increase in GABA shunt activity. Am. J. Physiol. Endocrinol. Metab. 290, E494–E499 [DOI] [PubMed] [Google Scholar]
- 23. Li C., Chen P., Palladino A., Narayan S., Russell L. K., Sayed S., Xiong G., Chen J., Stokes D., Butt Y. M., Jones P. M., Collins H. W., Cohen N. A., Cohen A. S., Nissim I., Smith T. J., Strauss A. W., Matschinsky F. M., Bennett M. J., Stanley C. A. (2010) Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J. Biol. Chem. 285, 31806–31818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C., Matter A., Kelly A., Petty T. J., Najafi H., MacMullen C., Daikhin Y., Nissim I., Lazarow A., Kwagh J., Collins H. W., Hsu B. Y., Nissim I., Yudkoff M., Matschinsky F. M., Stanley C. A. (2006) Effects of a GTP-insensitive mutation of glutamate dehydrogenase on insulin secretion in transgenic mice. J. Biol. Chem. 281, 15064–15072 [DOI] [PubMed] [Google Scholar]
- 25. Li C., Buettger C., Kwagh J., Matter A., Daikhin Y., Nissim I. B., Collins H. W., Yudkoff M., Stanley C. A., Matschinsky F. M. (2004) A signaling role of glutamine in insulin secretion. J. Biol. Chem. 279, 13393–13401 [DOI] [PubMed] [Google Scholar]
- 26. Ricordi C., Lacy P. E., Scharp D. W. (1989) Automated islet isolation from human pancreas. Diabetes 38, Suppl. 1, 140–142 [DOI] [PubMed] [Google Scholar]
- 27. Deng S., Vatamaniuk M., Lian M. M., Doliba N., Wang J., Bell E., Wolf B., Raper S., Matschinsky F. M., Markmann J. F. (2003) Insulin gene transfer enhances the function of human islet grafts. Diabetologia 46, 386–393 [DOI] [PubMed] [Google Scholar]
- 28. Li C., Najafi H., Daikhin Y., Nissim I. B., Collins H. W., Yudkoff M., Matschinsky F. M., Stanley C. A. (2003) Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J. Biol. Chem. 278, 2853–2858 [DOI] [PubMed] [Google Scholar]
- 29. Mercer J. W., Oldfield L. S., Hoffman K. N., Shakleya D. M., Bell S. C. (2007) Comparative analysis of γ-hydroxybutyrate and γ-hydroxyvalerate using GC/MS and HPLC. J. Forensic Sci. 52, 383–388 [DOI] [PubMed] [Google Scholar]
- 30. Richard D., Ling B., Authier N., Faict T. W., Eschalier A., Coudoré F. (2005) GC/MS profiling of γ-hydroxybutyrate and precursors in various animal tissues using automatic solid-phase extraction. Preliminary investigations of its potential interest in postmortem interval determination. Anal. Chem. 77, 1354–1360 [DOI] [PubMed] [Google Scholar]
- 31. Mehrvar M., Abdi M. (2004) Recent developments, characteristics, and potential applications of electrochemical biosensors. Anal. Sci. 20, 1113–1126 [DOI] [PubMed] [Google Scholar]
- 32. Hara M., Wang X., Kawamura T., Bindokas V. P., Dizon R. F., Alcoser S. Y., Magnuson M. A., Bell G. I. (2003) Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 284, E177–E183 [DOI] [PubMed] [Google Scholar]
- 33. Doliba N. M., Fenner D., Zelent B., Bass J., Sarabu R., Matschinsky F. M. (2012) Repair of diverse diabetic defects of β-cells in man and mouse by pharmacological glucokinase activation. Diabetes Obes. Metab. 14, Suppl. 3, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Doliba N. M., Qin W., Najafi H., Liu C., Buettger C. W., Sotiris J., Collins H. W., Li C., Stanley C. A., Wilson D. F., Grimsby J., Sarabu R., Naji A., Matschinsky F. M. (2012) Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am. J. Physiol. Endocrinol. Metab. 302, E87–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deng S., Vatamaniuk M., Huang X., Doliba N., Lian M. M., Frank A., Velidedeoglu E., Desai N. M., Koeberlein B., Wolf B., Barker C. F., Naji A., Matschinsky F. M., Markmann J. F. (2004) Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53, 624–632 [DOI] [PubMed] [Google Scholar]
- 36. Maechler P., Wollheim C. B. (2001) Mitochondrial function in normal and diabetic β-cells. Nature 414, 807–812 [DOI] [PubMed] [Google Scholar]
- 37. MacDonald M. J., Longacre M. J., Stoker S. W., Kendrick M., Thonpho A., Brown L. J., Hasan N. M., Jitrapakdee S., Fukao T., Hanson M. S., Fernandez L. A., Odorico J. (2011) Differences between human and rodent pancreatic islets: low pyruvate carboxylase, ATP citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J. Biol. Chem. 286, 18383–18396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaufman E. E., Nelson T., Goochee C., Sokoloff L. (1979) Purification and characterization of an NADP+-linked alcohol oxido-reductase which catalyzes the interconversion of γ-hydroxybutyrate and succinic semialdehyde. J. Neurochem. 32, 699–712 [DOI] [PubMed] [Google Scholar]
- 39. Bessman S. P., Fishbein W. N. (1963) γ-Hydroxybutyrate, a normal brain metabolite. Nature 200, 1207–1208 [DOI] [PubMed] [Google Scholar]
- 40. Gibson K. M., Hoffmann G. F., Hodson A. K., Bottiglieri T., Jakobs C. (1998) 4-Hydroxybutyric acid and the clinical phenotype of succinic semialdehyde dehydrogenase deficiency, an inborn error of GABA metabolism. Neuropediatrics 29, 14–22 [DOI] [PubMed] [Google Scholar]
- 41. Kaufman E. E., Relkin N., Nelson T. (1983) Regulation and properties of an NADP+ oxidoreductase which functions as a γ-hydroxybutyrate dehydrogenase. J. Neurochem. 40, 1639–1646 [DOI] [PubMed] [Google Scholar]
- 42. Andriamampandry C., Taleb O., Viry S., Muller C., Humbert J. P., Gobaille S., Aunis D., Maitre M. (2003) Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator γ-hydroxybutyrate (GHB). FASEB J. 17, 1691–1693 [DOI] [PubMed] [Google Scholar]
- 43. Andriamampandry C., Taleb O., Kemmel V., Humbert J. P., Aunis D., Maitre M. (2007) Cloning and functional characterization of a γ-hydroxybutyrate receptor identified in the human brain. FASEB J. 21, 885–895 [DOI] [PubMed] [Google Scholar]
- 44. Jung M. J., Lippert B., Metcalf B. W., Böhlen P., Schechter P. J. (1977) γ-Vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: effects on brain GABA metabolism in mice. J. Neurochem. 29, 797–802 [DOI] [PubMed] [Google Scholar]
- 45. Macias A. T., Hernandez R. J., Mehta A. K., MacKerell A. D., Jr., Ticku M. K., Coop A. (2004) 3-Chloropropanoic acid (UMB66): a ligand for the γ-hydroxybutyric acid receptor lacking a 4-hydroxyl group. Bioorg. Med. Chem. 12, 1643–1647 [DOI] [PubMed] [Google Scholar]
- 46. Whitehouse S., Cooper R. H., Randle P. J. (1974) Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem. J. 141, 761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uehara S., Muroyama A., Echigo N., Morimoto R., Otsuka M., Yatsushiro S., Moriyama Y. (2004) Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by α-cells of islet of Langerhans. Diabetes 53, 998–1006 [DOI] [PubMed] [Google Scholar]
- 48. Bertrand G., Gross R., Puech R., Loubatières-Mariani M. M., Bockaert J. (1993) Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur. J. Pharmacol. 237, 45–50 [DOI] [PubMed] [Google Scholar]
- 49. Young A. B., Snyder S. H. (1973) Strychnine binding associated with glycine receptors of the central nervous system. Proc. Natl. Acad. Sci. U.S.A. 70, 2832–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmidt C., Gobaille S., Hechler V., Schmitt M., Bourguignon J. J., Maitre M. (1991) Anti-sedative and anti-cataleptic properties of NCS-382, a γ-hydroxybutyrate receptor antagonist. Eur. J. Pharmacol. 203, 393–397 [DOI] [PubMed] [Google Scholar]
- 51. Rahier J., Guiot Y., Goebbels R. M., Sempoux C., Henquin J. C. (2008) Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10, Suppl. 4, 32–42 [DOI] [PubMed] [Google Scholar]
- 52. Slucca M., Harmon J. S., Oseid E. A., Bryan J., Robertson R. P. (2010) ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes 59, 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bloomenthal A. B., Goldwater E., Pritchett D. B., Harrison N. L. (1994) Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+. Mol. Pharmacol. 46, 1156–1159 [PubMed] [Google Scholar]
- 54. Nevin S. T., Cromer B. A., Haddrill J. L., Morton C. J., Parker M. W., Lynch J. W. (2003) Insights into the structural basis for zinc inhibition of the glycine receptor. J. Biol. Chem. 278, 28985–28992 [DOI] [PubMed] [Google Scholar]
- 55. Feldmann N., del Rio R. M., Gjinovci A., Tamarit-Rodriguez J., Wollheim C. B., Wiederkehr A. (2011) Reduction of plasma membrane glutamate transport potentiates insulin but not glucagon secretion in pancreatic islet cells. Mol. Cell. Endocrinol. 338, 46–57 [DOI] [PubMed] [Google Scholar]
- 56. Pagliara A. S., Stillings S. N., Haymond M. W., Hover B. A., Matschinsky F. M. (1975) Insulin and glucose as modulators of the amino acid-induced glucagon release in the isolated pancreas of alloxan and streptozotocin diabetic rats. J. Clin. Investig. 55, 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. De León D. D., Li C., Delson M. I., Matschinsky F. M., Stanley C. A., Stoffers D. A. (2008) Exendin-(9–39) corrects fasting hypoglycemia in SUR-1−/− mice by lowering cAMP in pancreatic β-cells and inhibiting insulin secretion. J. Biol. Chem. 283, 25786–25793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gannon M. C., Nuttall J. A., Nuttall F. Q. (2002) The metabolic response to ingested glycine. Am. J. Clin. Nutr. 76, 1302–1307 [DOI] [PubMed] [Google Scholar]
- 59. Müller W. A., Aoki T. T., Cahill G. F., Jr. (1975) Effect of alanine and glycine on glucagon secretion in postabsorptive and fasting obese man. J. Clin. Endocrinol Metab. 40, 418–425 [DOI] [PubMed] [Google Scholar]
- 60. Nicholson K. L., Balster R. L. (2001) GHB: a new and novel drug of abuse. Drug Alcohol Depend. 63, 1–22 [DOI] [PubMed] [Google Scholar]
- 61. Crunelli V., Emri Z., Leresche N. (2006) Unravelling the brain targets of γ-hydroxybutyric acid. Curr. Opin. Pharmacol. 6, 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mathivet P., Bernasconi R., De Barry J., Marescaux C., Bittiger H. (1997) Binding characteristics of γ-hydroxybutyric acid as a weak but selective GABAB receptor agonist. Eur. J. Pharmacol. 321, 67–75 [DOI] [PubMed] [Google Scholar]
- 63. Pearl P. L., Novotny E. J., Acosta M. T., Jakobs C., Gibson K. M. (2003) Succinic semialdehyde dehydrogenase deficiency in children and adults. Ann. Neurol. 54, Suppl. 6, S73–S80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.