Background: Sex determining factor SRY possesses dual nuclear import mechanisms, with its CaM-dependent mechanism being poorly understood.
Results: hsc70 enhances CaM-dependent nuclear accumulation of SRY through its ability to bind the SRY·CaM complex, dependent on Ca2+.
Conclusion: hsc70 is a key mediator of the CaM-dependent nuclear transport mechanism of SRY.
Significance: hsc70 may mediate nuclear transport of other developmentally important transcription factors.
Keywords: Calcium, Calmodulin, Heat Shock Protein, Nuclear Translocation, Nuclear Transport, SRY, hsc70
Abstract
We recently showed that the developmentally important family of SOX (SRY (sex determining region on the Y chromosome)-related high mobility group (HMG) box) proteins require the calcium-binding protein calmodulin (CaM) for optimal nuclear accumulation, with clinical mutations in SRY that specifically impair nuclear accumulation via this pathway resulting in XY sex reversal. However, the mechanism by which CaM facilitates nuclear accumulation is unknown. Here, we show, for the first time, that the 70-kDa heat shock cognate protein hsc70 plays a key role in CaM-dependent nuclear import of SRY. Using a reconstituted nuclear import assay, we show that antibodies to hsc70 significantly reduce nuclear accumulation of wild type SRY and mutant derivatives thereof that retain CaM-dependent nuclear import, with an increased rate of nuclear accumulation upon addition of both CaM and hsc70, in contrast to an SRY mutant derivative with impaired CaM binding. siRNA knockdown of hsc70 in intact cells showed similar results, indicating clear dependence upon hsc70 for CaM-dependent nuclear import. Analysis using the technique of fluorescence recovery after photobleaching indicated that hsc70 is required for the maximal rate of SRY nuclear import in living cells but has no impact upon SRY nuclear retention/nuclear dynamics. Finally, we demonstrate direct binding of hsc70 to the SRY·CaM complex, with immunoprecipitation experiments from cell extracts showing association of hsc70 with wild type SRY, but not with a mutant derivative with impaired CaM binding, dependent on Ca2+. Our novel findings strongly implicate hsc70 in CaM-dependent nuclear import of SRY.
Introduction
The SOX (SRY (sex determining region on the Y chromosome)-related high mobility group (HMG)3 box) family of chromatin remodeling factors play key roles in development (1), which is strongly dependent on nuclear localization efficiency (2). SRY plays a key role in mammalian sex determination within the nucleus, with 15% of all XY sex-reversed patients presenting with mutations in the Sry gene (2). Although many mutations in SRY impair its ability to bind and bend DNA, other mutations that map to either of the nuclear localization signals of SRY (NLS; see Fig. 1, bottom panel), which flank its HMG box domain (2) have been shown to impair the nuclear targeting of SRY specifically. Of these, the C-terminal β-NLS mediates nuclear transport conventionally through binding the nuclear transport molecule importin (Imp) β1, whereas the second N-terminal CaM-NLS facilitates nuclear transport through direct binding to the calcium binding protein CaM (3). The dual nuclear import mechanisms of SRY contribute approximately equally to the overall nuclear accumulation of SRY (2–5) and are independently functional with Ca2+ as a switch between the two (3).
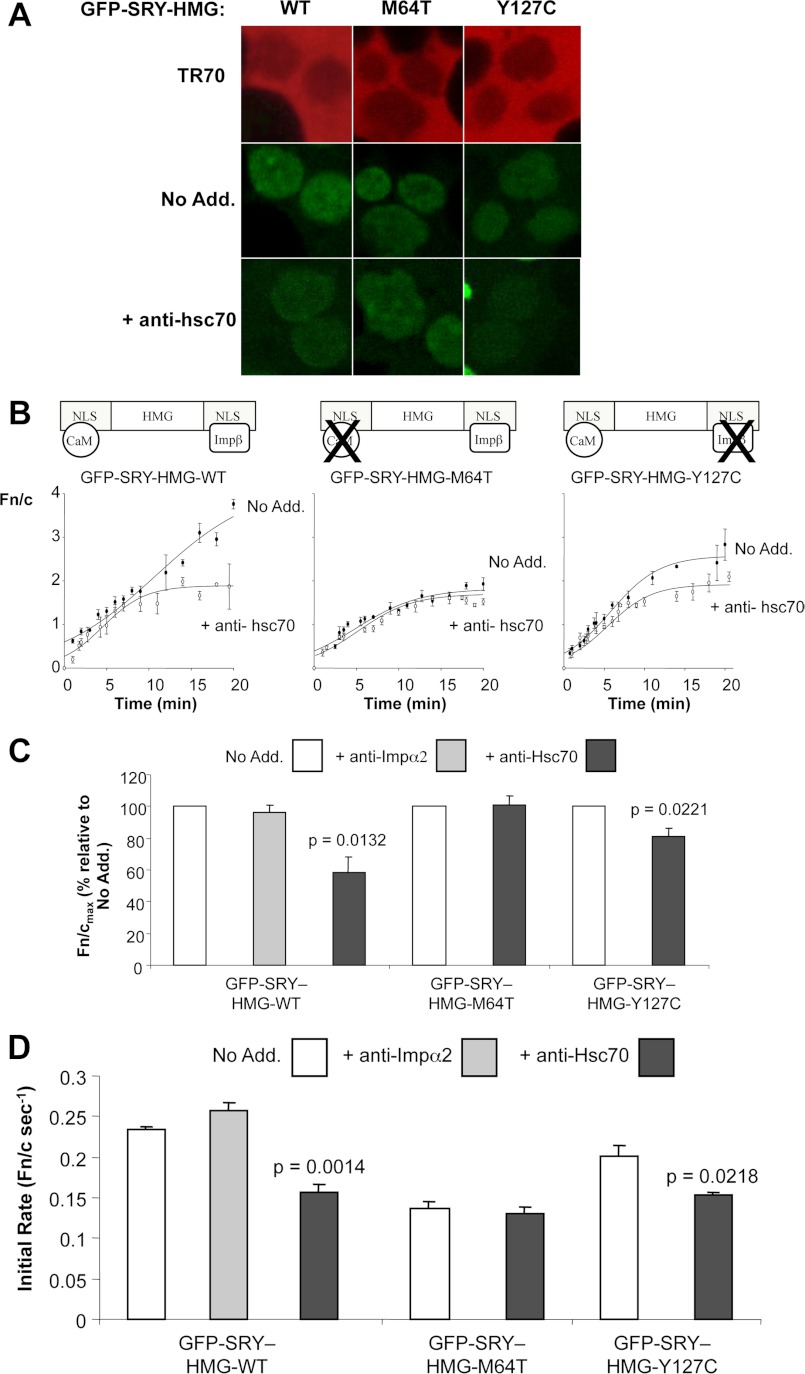
FIGURE 1.
CaM-NLS-dependent nuclear import of SRY requires hsc70. Nuclear import of GFP-SRY-HMG-wild type (WT) and its mutant derivatives (see schematics in B depicting effects of the mutations) was reconstituted in vitro in the absence or presence of specific antibodies to hsc70 (anti-hsc70) and Impα2 (anti-Impα2), as indicated. A, CLSM images of cells after 18 min are shown; exclusion of TR70 demonstrates nuclear integrity. B, CLSM images such as those shown in A were analyzed using ImageJ software to determine the nuclear to cytoplasmic ratio (Fn/c). Results represent the mean ± S.E. from a single typical experiment, with each data point representing more than three separate measurements of Fn and Fc above background. Curves were fitted (3 parameter sigmoidal) using SigmaPlot software. Schematic representations of the SRY HMG (high mobility group) domain, the CaM-binding and the Impβ1 binding NLS, with effects of sex-reversing mutations indicated, are shown above. C, pooled data (mean ± S.E., n > 2) for the percentage maximal nuclear accumulation, Fn/cmax, in the presence and absence of indicated antibodies. D, pooled data (mean ± S.E., n > 2) for the initial rate of nuclear accumulation (Fn/c s−1) in the presence and absence of indicate antibodies. p values are indicated where there were significant differences compared with in the absence of the indicated antibodies. No Add., no addition.
We recently showed that the CaM antagonist calmidazolium chloride reduces nuclear accumulation of various SOX proteins, including SRY, SOX-2, SOX-9, and SOX-10, as well as the nucleosomal binding protein HMGN1 (2). Intriguingly, the yeast protein Nhp6Ap also possesses a CaM-dependent, Ran-independent mode of nuclear transport (6), implying a general role for CaM in the nuclear transport of HMG proteins. However, little is known of the mechanism by which CaM facilitates nuclear translocation.
The 70-kDa heat shock cognate protein hsc70 has been previously shown to be involved in modulating nuclear transport, with a role in facilitating the subcellular localization of members of the Imp family (7), as well as in regulating the nuclear transport of proteins such as the temperature sensitive p53 mutant p53Val-135 (8), Simian virus 40 large tumor antigen (T-ag) (9) and nucleoplasmin (10). hsc70 is a constitutively expressed member of the hsp70 family of chaperone proteins, found in both the nuclear and cytoplasmic compartments, known to play a role in protein folding and translocation of proteins across the endoplasmic reticulum and mitochondrial membranes (11, 12).
Here, we assess the role of hsc70 in CaM-dependent nuclear import of SRY for the first time. We find that direct binding of hsc70 to SRY·CaM dependent on Ca2+, is required for CaM-dependent nuclear import of SRY, presumably to facilitate passage through the nuclear pore.
EXPERIMENTAL PROCEDURES
Plasmid Construction
Plasmids encoding the GFP-SRY full length and HMG wild type and mutant derivatives of the CaM-NLS (M64T and R76P) and the β-NLS (Y127C and R133W) for bacterial and mammalian expression, as well as GFP-TRF1, GFP-T-ag-NLS (amino acids 111–135), and GFP alone, have all been described (3, 13, 14).
Cell Culture, Transfection, and Imaging
HTC rat hepatoma tissue culture and HeLa human cervical cancer cell lines were cultured in a 5% CO2-humidified atmosphere at 37 °C, in Dulbecco's modified Eagle's medium (DMEM; ICN Biomedicals, Costa Mesa, CA), supplemented with 10% heat-inactivated fetal calf serum (FCS; CSL, Ltd., Parkville, Victoria, Australia), 1 mm l-glutamine, 1 mm penicillin/streptomycin, and 20 mm Hepes.
HeLa cells were transfected using siPORTTM NeoFXTM (Ambion) or LipofectamineTM 2000 (Invitrogen) for siRNA (100 nm hsc70 Stealth siRNA duplexes (Invitrogen) with the sense sequence 5′-UAAUUCUAAGUACAUUGAGACCAGC-3′) (7) or plasmid DNA expression constructs respectively, according to the manufacturer's specifications. Cells were imaged live by confocal laser scanning microscopy (CLSM, Nikon C1) 20h post-transfection and quantitative analysis of digitised images performed using Image J (National Institutes of Health, Bethesda, MD) for the nuclear (Fn) and cytoplasmic (Fc) fluorescence subsequent to subtraction of autofluorescence to derive the nuclear to cytoplasmic ratio (Fn/c) as described previously (3, 15).
Bacterial Expression of Recombinant Proteins
His6-GFP-SRY-HMG wild type and mutant derivatives were expressed and purified as described previously (2). GFP alone, purified as described previously (14, 16) was provided by Kylie Wagstaff, and CaM-GST (3) was provided by Jade Forwood.
Native Polyacrylamide Gel Electrophoresis
Briefly, His6-GFP-SRY-HMG-wild type was incubated in the presence and absence of hsc70 (Stressgen Biotechnologies, Victoria, BC, Canada) and/or CaM-GST with 2 μm CaCl2 added, for 20 min at room temperature, prior to native gel electrophoresis and fluorimaging (Typhoon Trio fluorimager, GE Healthcare) as described previously (2).
In Vitro Nuclear Transport Assays
Mechanically perforated HTC cells were used to assess nuclear import of GFP-SRY-HMG wild type and mutant derivatives in conjunction with CLSM (MRC-500; Bio-Rad, Richmond, CA) as described previously (3). Experiments were carried out in 5 μl containing 10 μm GFP-fusion protein, a 70-kDa Texas red dextran to assess nuclear integrity, untreated rabbit reticulocyte lysate (45 μg/μl; Promega, Madison, WI), and an ATP regenerating system (0.125 g/ml creatine phosphokinase; 30 mm phosphocreatine; 2 mm ATP), with or without antibodies specific for hsc70 (Stressgen Biotechnologies) or Impα (BD Transduction Laboratories), or 1.5 μm hsc70 (Stressgen Biotechnologies) and/or CaM (Calbiochem, La Jolla, CA) purified protein. Image analysis was performed using NIH ImageJ software as described above. Data were fitted to a sigmoidal curve and the Fn/cmax and initial rates (from the first linear portion of the recovery curve) were determined using Sigmaplot software, as described previously (17–19).
Immunoprecipitation/Immunoblotting
Immunoprecipitation was performed to assess association of hsc70 with SRY using GFP-TRAP® (Chromotek, Martinsried, Germany), as per the manufacturer's instructions, using 500 μg of rabbit reticulocyte lysate and 7 μg of recombinant GFP-fusion protein (as indicated) and 10 μl of equilibrated GFP-TRAP® beads. Proteins dissociated from the beads were then subjected to SDS-PAGE (12% gel) electrophoresis and transferred to polyvinylidene difluoride membrane that was then probed with anti-hsc70 and then anti-GFP antibodies followed by goat anti-rat IgG-HRP (Millipore, Bedford, MA) or goat anti-mouse IgG-HRP (Millipore) before development using the Western Lighting Chemiluminescence reagent (PerkinElmer Life Sciences). Assays to confirm the functionality of siRNA to hsc70 (100 nm) were similarly performed, with intact HeLa cells lysed 72 h post-treatment (2). Densitometric analysis was performed on digitized images of immunoblots using NIH ImageJ public domain software (3).
Fluorescence Recovery after Photobleaching (FRAP)
FRAP was performed as described previously (19). Following siRNA treatment, HeLa cells were transiently transfected and imaged live on a heated stage using an Olympus Fluoview 1000 microscope and an 100× oil immersion lens (Nikon) 20 h post-transfection. Images were collected prior to photobleaching using 3% total laser power with excitation at 488 nm, scanning at a rate of 12.5 μs/pixel. Bleaching was performed in an area corresponding to ∼50% of the nucleus, scanning the area 40 times at a rate of 10 μs/pixel at 100% laser power. Cells were imaged immediately post-bleaching with images acquired at 20-s intervals over a period of 280 s to monitor fluorescence recovery, using settings prior to photobleaching. Image analysis was performed using ImageJ as described above. Results were expressed as the fractional recovery of Fn/c (Fn/c of respective time points divided by prebleach value), and data were fitted exponentially according to the formula y = a(1−e−bx) as described (17–20) to determine the maximal recovery and half-time (t½). The initial rate was determined using results for the Fn/c between 0 and 100 s post-bleaching. For analysis of intranuclear mobility of SRY, results were expressed as the fractional recovery of the bleached area (Fnbleach) relative to prebleach (Fnnon-bleach), and data were fitted exponentially as above (19).
RESULTS
hsc70 Is Necessary for CaM-dependent Nuclear Import of SRY
We previously reported CaM-dependent nuclear import for SRY, as well as a number of other related SOX proteins, independent of the classical mode of nuclear transport through Imps (2, 3). The heat shock cognate protein hsc70 has been previously reported to play a role in modulating the nuclear import of proteins such as SV40-T-ag and nucleoplasmin (9, 10), as well as being a CaM-binding protein (21, 22) able to associate with nucleoporins (7). As a first step in establishing the potential role of hsc70 in CaM-dependent SRY nuclear import, we employed an established system of SRY nuclear transport reconstituted in vitro in mechanically perforated HTC cells (see “Experimental Procedures” (3)). We have previously used this system to demonstrate that CaM-dependent nuclear accumulation of SRY is active, requires ATP hydrolysis, and nucleoporin (nuclear pore) function, but does not require Ran (3). We also used the system to demonstrate that CaM dependence of nuclear import does not apply to a number of other proteins, including the Impβ1-recognized telomere repeat factor binding protein (TRF)-1 (3). As described previously, wild type SRY in the form of the bacterially expressed GFP-SRY-HMG fusion protein accumulated strongly within intact nuclei (those excluding Texas Red dextran; see Fig. 1A, top panel), but significantly, showed reduced nuclear accumulation upon addition of anti-hsc70 antibody (Fig. 1A). Quantitative analysis (see “Experimental Procedures” and supplemental Fig. 1) to determine the extent of nuclear accumulation expressed in terms of the nuclear to cytoplasmic ratio (Fn/c), confirmed this observation, revealing a significant (p = 0.01) 40% reduction in the maximal level of SRY nuclear accumulation in the presence of anti-hsc70 antibody (Fig. 1, B and C); because Impβ1-dependent transport accounts for ∼50% of SRY nuclear accumulation in this system (2–5), this equates to close to complete inhibition of CaM-dependent nuclear import of SRY. No such changes to SRY nuclear import were observed in the presence of antibodies to importin α2 (see Fig. 1C), as described previously (3), indicating that the results observed in the presence of anti-hsc70 were specific for SRY, supporting the idea of a role for hsc70 in SRY nuclear import.
Mutant derivatives of GFP-SRY HMG with inactive CaM- or β-NLS, previously shown to retain only specific Impβ1- or CaM-dependent nuclear transport respectively (see Fig. 1B) (3), were similarly analyzed (see Fig. 1). Significant reductions in nuclear accumulation were observed for both the CaM (R76P,M64T)- and β (Y127C)-NLS mutant derivatives compared with wild type SRY in the absence of anti-hsc70 antibody (see Figs. 1 and 2) as described previously (3). In the case of the CaM-NLS mutant (M64T), which lacks CaM binding, no change was observed upon addition of anti-hsc70 antibody, but significantly (p = 0.02) reduced (∼20%) nuclear accumulation was observed for the β-NLS mutant (Y127C), which possesses wild type CaM binding (see Fig. 1). Significant (p < 0.05) reductions in the initial rate of nuclear accumulation were also observed for the wild type SRY (Fn/c s−1 of 0.23) and β-NLS mutant (Y127C; Fn/c s−1 of 0.20), in the presence of anti-hsc70 antibody (Fn/c s−1 of 0.16 and 0.15, respectively), with no such changes observed for the CaM-NLS (M64T) mutant (see Fig. 1D). These results clearly indicate that hsc70 contributes to CaM- rather than Impβ1-dependent nuclear import of SRY, modulating both the rate and maximal level of CaM-dependent nuclear accumulation.
FIGURE 2.
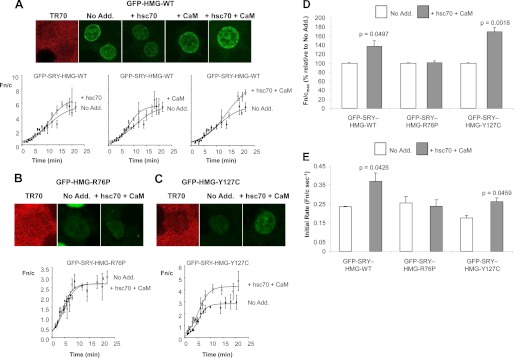
SRY nuclear import is enhanced by hsc70 and CaM. Nuclear import of GFP-SRY-HMG-wild type (WT) and its mutant derivatives was reconstituted in vitro as per the legend to Fig. 1, with or without (No Add.) 1.5 μm hsc70 and/or CaM. CLSM images of cells at 18–20 min are shown (top panels), with TR70 indicating intact nuclei, for GFP-SRY-HMG-WT (A), the CaM-NLS (B), and β-NLS (C) mutant derivatives, with quantitative analysis of such images (bottom panels) as per the legend to Fig. 1. D and E, pooled data (mean ± S.E., n = 3) for percentage maximal nuclear accumulation (Fn/cmax) (D) and initial rate of nuclear accumulation (E) determined as per the legend to Fig. 1, in the presence and absence of the various additions for the indicated GFP-SRY-HMG derivatives. p values are shown where there were significant differences compared with in the absence of the addition of CaM and hsc70 proteins.
hsc70 and CaM Enhance SRY Nuclear Accumulation through Binding of hsc70 to the SRY·CaM Complex
To confirm the contribution of hsc70 to SRY nuclear import, the effect of adding purified hsc70 and/or CaM to the transport assay was assessed. No significant changes to wild type SRY nuclear import were observed in the presence of purified hsc70 or CaM alone (Fig. 2, A and D), in stark contrast to in the presence of both CaM and hsc70, where significantly (p < 0.05) ∼40% increased nuclear accumulation of SRY was observed (see Fig. 2, A and D). No enhancement of nuclear accumulation was observed for the CaM-NLS mutant (R76P) in the presence of hsc70 and CaM (see Fig. 2, B and D), whereas significantly (p < 0.002) ∼70% increased maximal nuclear accumulation was observed for the β-NLS mutant (Y127C) derivative. Significant increases (p < 0.05) in the initial rate of transport were also observed for the wild type protein (Fn/c s−1 of 0.23) and β-NLS mutant (Y127C; Fn/c s−1 of 0.18) derivative in the presence of both hsc70 and CaM (Fn/c s−1 of 0.39 and 0.26, respectively), with no such changes observed for the CaM-NLS mutant (R76P) derivative. These results clearly imply that in combination with CaM, hsc70 enhances SRY nuclear import dependent on the CaM-NLS.
Hsp70 has been previously shown to bind to CaM in a Ca2+-dependent manner (21). The ability of hsc70 to bind to SRY and the SRY·CaM complex was assessed using native PAGE. A marked shift in mobility was observed for the wild type GFP-SRY-HMG protein in the presence of CaM as described previously (3), indicating complex formation (Fig. 3A), with no shift observed for SRY in the presence of hsc70 alone, indicating lack of complexation between hsc70 and SRY. Intriguingly, in the presence of hsc70 and CaM, a supershifted band was observed distant from that of the SRY·CaM complex. The clear implication is that hsc70 can bind to SRY in the presence of CaM in a trimeric complex.
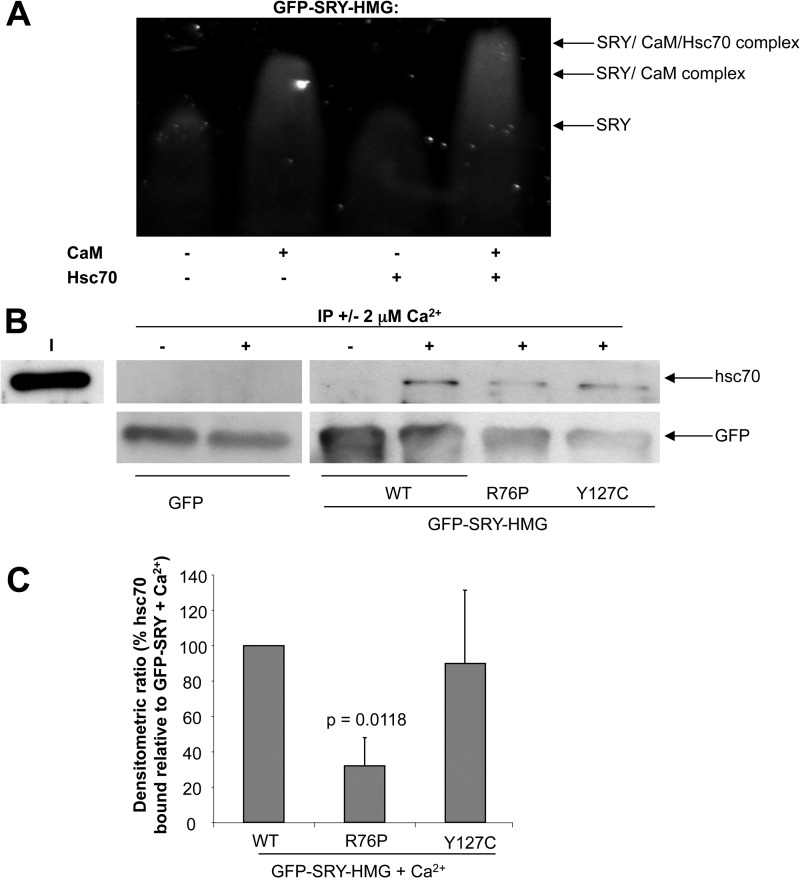
FIGURE 3.
hsc70 specifically binds the SRY/CaM complex in Ca2+-dependent fashion. A, native PAGE gel mobility shift assay (see “Experimental Procedures”) for hsc70 and CaM binding to GFP-SRY-HMG (wild type) was performed, where GFP-SRY-HMG (1 μm) was incubated in the absence and presence of hsc70 (1 μm) and/or CaM (1 μm) in the presence of Ca2+ (2 mm) for 20 min at room temperature as indicated, and then fluorimaging was performed subsequent to gel electrophoresis. Results are shown for a single typical assay from a series of three similar experiments, with SRY·CaM and SRY·CaM·hsc70 complex formation indicated on the right. B, immunoprecipitation using GFP-TRAPTM resin was performed as described under “Experimental Procedures,” with immunoblots probed with anti-GFP or anti-hsc70 antibodies to detect binding of GFP or GFP-SRY-HMG wild type and NLS mutant derivatives in the absence and presence of 2 μm Ca2+ as indicated to hsc70. I, input. C, densitometric analysis for immunoprecipitation assays was performed on images such as those in B for hsc70 binding to the GFP-SRY-HMG and NLS mutant derivatives in the presence of Ca2+, as indicated. Results represent the mean ± S.E. (n = 3) of the percentage hsc70 bound relative to GFP-SRY in the presence of Ca2+, with p values shown where there were significant differences compared with wild type.
CaM binding to SRY is known to be Ca2+-dependent (3). To extend the observations, immunoprecipitation assays using GFP-TRAPTM were performed to assess association of hsc70 to GFP-SRY and its mutant derivatives. hsc70 was pulled down with wild type SRY in the presence of 2 μm Ca2+ but not with the GFP control protein (see Fig. 3B). Only low levels of association of hsc70 with SRY were observed in the absence of Ca2+. As expected, significantly (p < 0.02), ∼70% reduced binding was observed of hsc70 to the CaM-NLS mutant derivative (R76P), in contrast to the β-NLS mutant derivative (Y127C) retaining wild type CaM binding (see Fig. 3C), which showed hsc70 binding comparable with that of wild type SRY. Taken together, these results indicate that hsc70 binds to SRY·CaM dependent upon Ca2+ and that this is required for enhanced CaM-dependent nuclear import of SRY.
hsc70 Is Required for Optimal CaM-dependent Nuclear Import of SRY in Living Cells
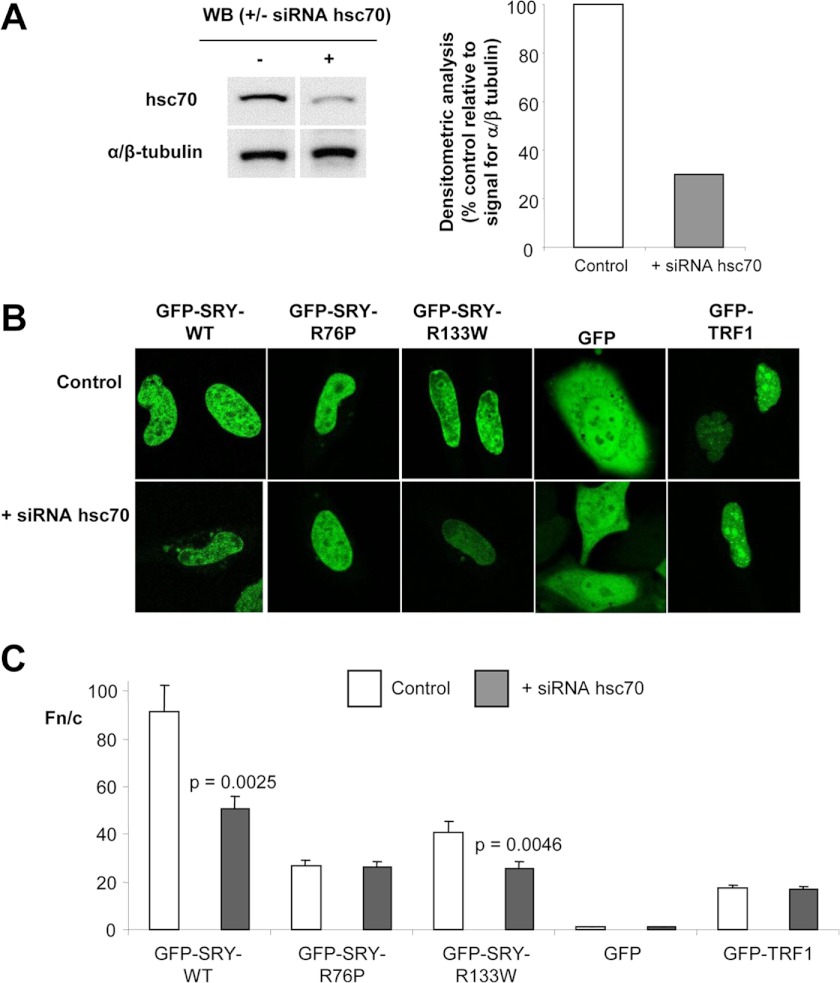
To confirm that the above results have relevance to intact cell systems, HeLa cells were treated with hsc70-specific small interfering RNA duplexes (hsc70 siRNA) to knock down endogenous hsc70 protein expression prior to transfection to express full-length SRY-GFP-fusion proteins. Treatment led to an ∼65% reduction in hsc70 protein levels at 72 h compared with the untransfected control cells (Fig. 4A). In contrast to wild type full-length SRY, which showed exclusively nuclear localization as described previously (2), the protein showed increased cytoplasmic localization in the presence of siRNA to hsc70 (see Fig. 4B). Quantitative analysis (Fig. 4C) indicated significantly (p < 0.003) ∼40% reduced nuclear accumulation of SRY upon knockdown of hsc70 expression. Reduced nuclear accumulation was observed for the NLS mutants compared with wild type in the absence of siRNA to hsc70 as described previously (2). Although no reduction to nuclear accumulation was observed for the CaM-NLS mutant (R76P) possessing intact Imp-dependent but impaired CaM-dependent nuclear import upon knockdown of hsc70, a significant (p = 0.0046) ∼40% reduction was observed for the β-NLS mutant (R133W) that possesses intact CaM-dependent but impaired Impβ1-dependent nuclear import in the presence of siRNA to hsc70. The control proteins (Fig. 4, B and C), GFP alone, and TRF1, imported into the nucleus through the specific action of Impβ1, showed no changes to subcellular localization in the presence of siRNA to hsc70, indicating that reduction of hsc70 protein levels specifically inhibits SRY nuclear import and does not impact upon other nuclear transport pathways. Taken together, these results indicate that hsc70 is required for CaM-dependent nuclear import of SRY in intact cells.
FIGURE 4.
siRNA against hsc70 inhibits CaM-dependent nuclear import of SRY. A, immunoblot analysis (left panel) for the protein levels of hsc70 of HeLa cells treated with hsc70-specific small interfering RNA duplexes (hsc70 siRNA) 72 h post-treatment and probed with anti-hsc70 and anti-α/β tubulin antibody. Densitometric analysis (right panel) of images such as those in the left panel was performed to quantify the relative amount of hsc70 protein in the absence and presence of siRNA to hsc70 standardized relative to α/β-tubulin in whole cell extracts. Results are for a single typical experiment from a series of three similar experiments. B, HeLa cells were transiently transfected to express the indicated GFP-fusion proteins, 48 h post-treatment with siRNA to hsc70, and imaged live 20 h post-transfection by CLSM. C, results for the quantitative analysis, whereby CLSM images such as those in A were analyzed using ImageJ software for the nuclear to cytoplasmic ratio (Fn/c; fold extent of nuclear accumulation). Results are for the mean ± S.E. (n > 44) of a single typical experiment from a series of three similar experiments; p values are indicated where the Fn/c values are significantly different in the presence of hsc70 siRNA. WB, Western blot.
The role of hsc70 in enhancing SRY nuclear import in living cells was assessed using the established FRAP technique (18). Briefly, an area corresponding to 50% of the nucleus of cells transfected to express full length GFP-SRY fusion protein in the absence or presence of hsc70 siRNA was photobleached, and the return of nuclear fluorescence from the translocation of unbleached, cytoplasmic fluorescent protein into the nucleus monitored by CLSM for up to 280 s (see Fig. 5A and Table 1; also see “Experimental Procedures”).
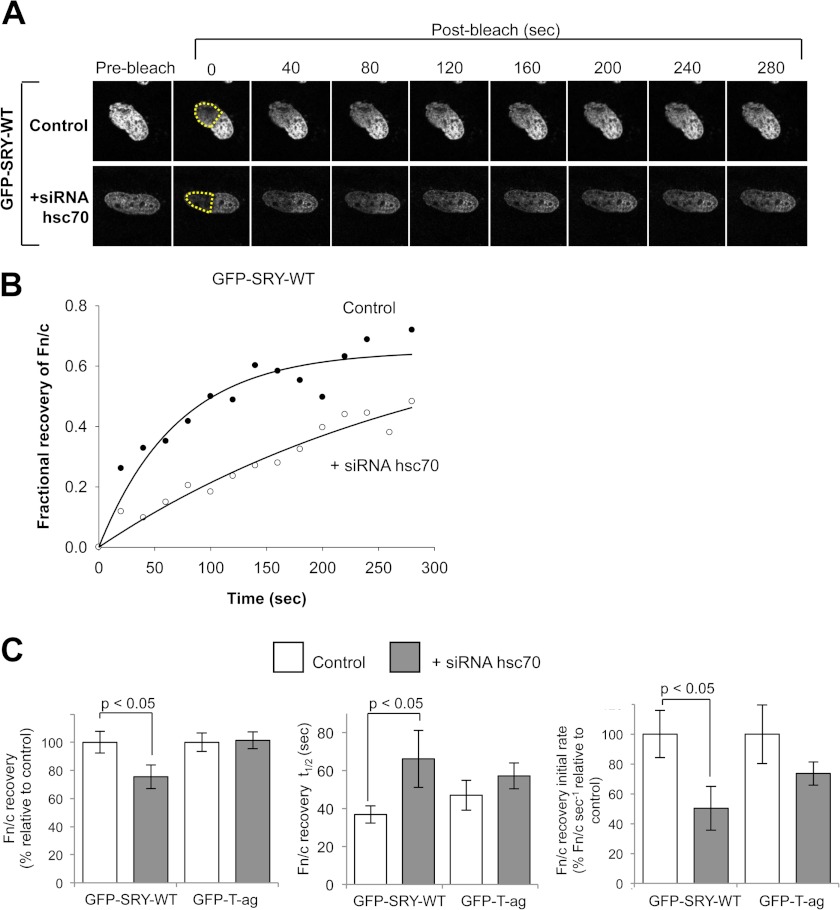
FIGURE 5.
Efficient nuclear import of SRY is dependent on hsc70 expression. A, representative CLSM images of the return of nuclear fluorescence after photobleaching in HeLa cells transfected to express full-length GFP-SRY- wild type (WT) in the presence or absence of siRNA to hsc70 over time (s) after photobleaching (post-bleach). Yellow dotted lines indicate regions of the nucleus photobleached. B, images such as those in A were quantified for the recovery over time of the nuclear to cytoplasmic fluorescence ratio (Fn/c) after photobleaching, expressed in terms of fractional recovery of respective time points divided by the initial (prebleach) value. Results shown are for a representative cell. C, pooled data for the mean ± S.E. (n > 13) for the percentage maximal recovery (left panel; normalized to 0 s post-bleach), time (s) taken to reach half maximal recovery (t½) (middle panel) and initial rate of recovery (right panel) in the presence and absence of siRNA to hsc70 for GFP-SRY-WT and GFP-T-ag NLS. p values are indicated where the values are significantly different in the presence of hsc70 siRNA.
TABLE 1.
Pooled data for nuclear transport kinetics in vivo as determined in FRAP experiments
Pooled data (mean ± S.E.) from assays performed as described in Fig. 5. Fract. recovery is the maximal recovery of fluorescence, Init. Rate is the initial rate of recovery (Fn/c), and t½ indicates the time taken to reach half maximal fluorescence recovery.
| Sample | Kinetic parameters for nuclear transport |
|||
|---|---|---|---|---|
| Fract. recovery | Init. rate | t½ | n | |
| Fn/c s−1 | s | |||
| GFP-SRY control | 0.63 ± 0.05 | 0.10 ± 0.02 | 36.8 ± 4.5 | 23 |
| GFP-SRY + siRNA hsc70 | 0.48 ± 0.05a | 0.05 ± 0.01a | 66.2 ± 15.0a | 18 |
| GFP-T-ag control | 0.40 ± 0.03 | 0.009 ± 0.002 | 47.0 ± 7.9 | 22 |
| GFP-T-ag + siRNA hsc70 | 0.41 ± 0.02 | 0.007 ± 0.001 | 57.2 ± 6.8 | 28 |
a p < 0.05 for significant differences compared with control (Student's t test, performed using GraphPad Prism software (version 5)).
The fractional recovery of the nuclear to cytoplasmic fluorescence ratio (Fn/c) was calculated by expressing the postbleach Fn/c value at each time point relative to the initial (prebleach) fluorescence value; this also enabled the determination of the time taken to achieve 50% recovery (t½) (see Fig. 5, B and C). Upon the addition of siRNA to hsc70, a significant (p < 0.05) reduction of ∼25% was observed for Fn/c recovery of full-length wild type GFP-SRY compared with untreated cells. Consistent with this, the t½ of the Fn/c recovery was significantly (p < 0.05) increased by almost 2-fold in the presence of siRNA to hsc70 compared with the control (t½ of ∼66 and 36 s, respectively; see Table 1). The hsc70 siRNA treatment also resulted in a significant (p < 0.05) 50% reduction in the initial rate of recovery compared with untreated cells (see Fig. 5C and Table 1). No significant differences were observed for the Impα/β-recognized nuclear import cargo GFP-T-ag NLS in either extent or rate of recovery in the absence or presence of siRNA to hsc70 (see Fig. 5C and Table 1), emphasizing the specificity of the effects; hsc70 is clearly required for optimal nuclear import of SRY, its knockdown reducing the rate of SRY nuclear import, as well as the extent of maximal accumulation.
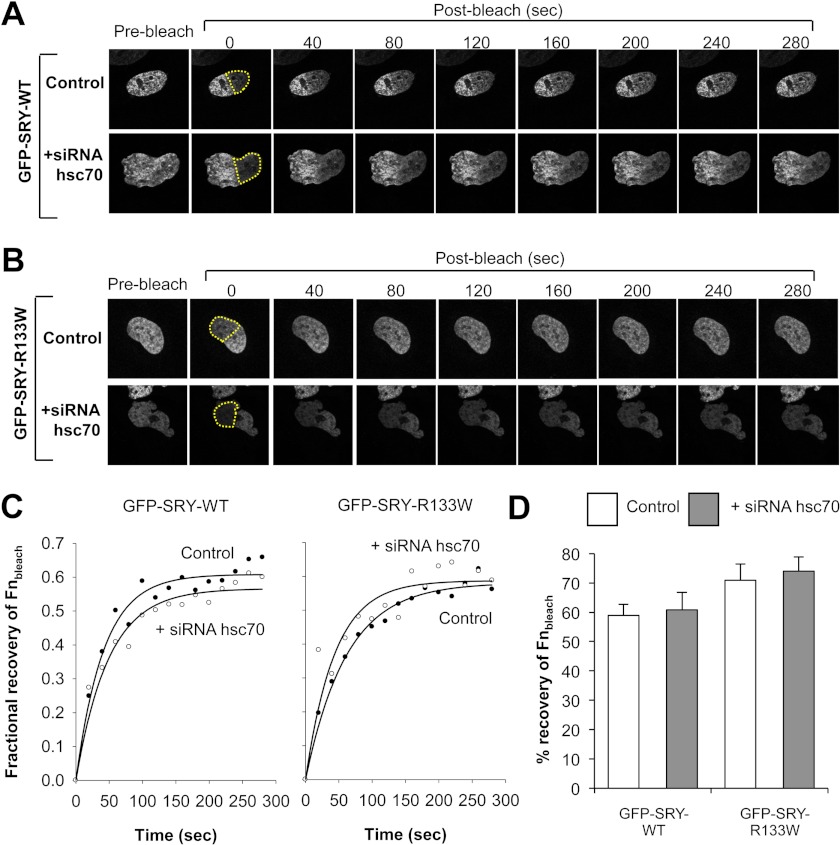
Retention of SRY in the Nucleus Is Independent of hsc70
The FRAP technique was also used to assess the effect of siRNA knockdown of hsc70 on the intranuclear mobility of SRY (19), whereby the fluorescence recovery of a bleached area (Fnbleach) relative to a non-bleached (Fnnon-bleach) area of the nucleus was monitored for cells expressing either full length wild type SRY or a mutant retaining only the CaM-dependent nuclear import pathway (R133W), in the absence and presence of hsc70 siRNA (Fig. 6, A and B). Results were quantified by calculating the ratio of Fnbleach at each time point to a prebleach value for Fnnon-bleach (see Fig. 6, C and D). In all cases, the fractional recovery of fluorescence was ∼60–70%, indicating that ∼30–40% of nuclear SRY is relatively immobile in the nucleus through binding to nuclear components (e.g. DNA). Importantly, no significant difference in wild type or β-NLS mutant derivative (R133W) intranuclear mobility was observed in the absence or presence of hsc70 siRNA (see Fig. 6, C and D), indicating that hsc70 does not contribute to SRY nuclear accumulation by regulating nuclear retention/nuclear dynamics. Rather, the main role of hsc70 appears to be in facilitating SRY nuclear import.
FIGURE 6.
Retention of SRY in the nucleus is independent of hsc70. A and B, representative CLSM images of recovery of nuclear fluorescence after photobleaching in HeLa cells transiently transfected to express full-length GFP-SRY-wild type (WT; A) and GFP-SRY-R133W (B) treated with or without siRNA to hsc70 over time (s), after photobleaching (post-bleach). The yellow dotted line indicates the bleached area (∼50% of nucleus). C, quantification of the recovery over time of nuclear fluorescence after photobleaching (Fnbleach, expressed in terms of fractional recovery of respective time points relative to the Fnnon-bleach prebleach value). Results shown are for a representative cell. D, pooled data for the mean ± S.E. (n = 3) percentage maximal recovery of Fnbleach, normalized to 0 s post-bleach.
DISCUSSION
This study implicates hsc70 for the first time as playing a role in CaM-dependent nuclear import of SRY. It functions through binding directly to the SRY·CaM complex, as confirmed by native PAGE and immunoprecipitation experiments showing association of hsc70 with wild type SRY, as well as a β-NLS mutant derivative thereof, in the presence of Ca2+ but not with a CaM-NLS SRY mutant. That hsc70 plays a key role in SRY nuclear import is indicated by the fact that antibodies against hsc70 in vitro and siRNA against hsc70 in living intact cells significantly reduce the rate and maximal extent of nuclear import of wild type SRY and a β-NLS SRY mutant but not a CaM-NLS SRY mutant derivative. There were no discernible effects of hsc70 knockdown on nuclear accumulation conferred by the T-ag NLS, which is independent of CaM. Finally, the addition of hsc70, together with CaM, significantly enhanced the rate and maximal extent of nuclear accumulation of wild type SRY, as well as of the β-NLS mutant, with no effect for the CaM-NLS mutant derivative. Notably, nuclear retention of SRY is not affected by siRNA knockdown of hsc70 as demonstrated using the FRAP technique in live cells; thus, hsc70 facilitates nuclear import rather than nuclear retention of SRY.
Previous reports have implicated a role for hsc70 in regulating nuclear protein transport (8–10). Although hsc70 itself can shuttle between the nuclear and cytoplasmic compartments, the mechanism is poorly understood (7). The related hsp70 protein has also been implicated in nuclear protein import (23), with yeast hsp70 implicated in complex formation of Impβ (Srp1) with NLS-containing cargo (24). hsc70 has also been implicated in translocation of the papillomavirus minor capsid protein L2 to promyelocytic leukemia protein nuclear bodies (25).
Although in vitro binding studies support the notion that hsp70 may bind directly to NLS (24), it is clear that hsc70 does not bind directly to SRY. Instead, hsc70 only binds to SRY bound to CaM, presumably through CaM itself, consistent with previous studies showing direct binding of hsp70 to Ca2+/CaM (21). This study clearly implicates a role for hsc70 in mediating CaM-dependent nuclear transport of SRY where CaM is the adaptor enabling hsc70 to be linked to SRY. As CaM-dependent nuclear import of SRY is independent of Ran and Imps, it is clear that hsc70 does not act to facilitate SRY nuclear import by binding to Imps. Hsp70 has been previously shown to be involved in the NPC translocation step of transport in yeast (24), with hsc70 shown to associate with nucleoporins (7). The hsp70 family member hsp72 has been reported to facilitate movement across the nuclear membrane (26), whereas hsp90 has also been implicated in mediating nuclear translocation of glucocorticoid receptor through interaction with nucleoporin 62 and Impβ1 (27, 28). Because CaM-NLS-dependent nuclear import of SRY is known to be dependent on nucleoporin interactions (3), it can be postulated that hsc70 binds to the SRY·CaM·Ca2+ complex via CaM to promote translocation of SRY through the nuclear pore by mediating interactions with the constituent nucleoporins. In the nucleus, binding of SRY to its specific DNA targets results in the release of CaM and thus hsc70 (2, 29). The extent to which the role of hsc70 in nuclear transport of proteins other than SRY may relate to CaM-dependent nuclear import is a focus of future work in this laboratory.
Supplementary Material
Acknowledgments
We are indebted to Kylie Wagstaff and Jade Forwood for GFP and CaM-GST protein, respectively, and to Cassandra David for tissue culture.
This work was supported by Australian Research Council Grant COE348239 and National Health and Medical Research Council (Australia) Grant APP1002486.

This article contains supplemental Figs. 1 and 2.
- HMG
- high mobility group
- CaM
- calmodulin
- NLS
- nuclear localization signal
- Imp
- importin
- T-ag
- SV40 large T-antigen
- TRF1
- telomeric repeat binding factor 1
- FRAP
- fluorescence recovery after photobleaching
- Fn
- nuclear fluorescence
- CLSM
- confocal laser scanning microscopy.
REFERENCES
- 1. Lefebvre V., Dumitriu B., Penzo-Méndez A., Han Y., Pallavi B. (2007) Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int. J. Biochem. Cell Biol. 39, 2195–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaur G., Delluc-Clavieres A., Poon I. K., Forwood J. K., Glover D. J., Jans D. A. (2010) Calmodulin-dependent nuclear import of HMG-box family nuclear factors: importance of the role of SRY in sex reversal. Biochem. J. 430, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaur G., Jans D. A. (2011) Dual nuclear import mechanisms of sex determining factor SRY: intracellular Ca2+ as a switch. FASEB J. 25, 665–675 [DOI] [PubMed] [Google Scholar]
- 4. Forwood J. K., Harley V., Jans D. A. (2001) The C-terminal nuclear localization signal of the sex-determining region Y (SRY) high mobility group domain mediates nuclear import through importin β 1. J. Biol. Chem. 276, 46575–46582 [DOI] [PubMed] [Google Scholar]
- 5. Harley V. R., Layfield S., Mitchell C. L., Forwood J. K., John A. P., Briggs L. J., McDowall S. G., Jans D. A. (2003) Defective importin β recognition and nuclear import of the sex-determining factor SRY are associated with XY sex-reversing mutations. Proc. Natl. Acad. Sci. U.S.A. 100, 7045–7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanover J. A., Love D. C., DeAngelis N., O'Kane M. E., Lima-Miranda R., Schulz T., Yen Y. M., Johnson R. C., Prinz W. A. (2007) The High Mobility Group Box Transcription Factor Nhp6Ap enters the nucleus by a calmodulin-dependent, Ran-independent pathway. J. Biol. Chem. 282, 33743–33751 [DOI] [PubMed] [Google Scholar]
- 7. Kose S., Furuta M., Koike M., Yoneda Y., Imamoto N. (2005) The 70-kD heat shock cognate protein (hsc70) facilitates the nuclear export of the import receptors. J. Cell Biol. 171, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akakura S., Yoshida M., Yoneda Y., Horinouchi S. (2001) A role for Hsc70 in regulating nucleocytoplasmic transport of a temperature-sensitive p53 (p53Val-135). J. Biol. Chem. 276, 14649–14657 [DOI] [PubMed] [Google Scholar]
- 9. Yang J., DeFranco D. B. (1994) Differential roles of heat shock protein 70 in the in vitro nuclear import of glucocorticoid receptor and simian virus 40 large tumor antigen. Mol. Cell. Biol. 14, 5088–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imamoto N., Matsuoka Y., Kurihara T., Kohno K., Miyagi M., Sakiyama F., Okada Y., Tsunasawa S., Yoneda Y. (1992) Antibodies against 70-kD heat shock cognate protein inhibit mediated nuclear import of karyophilic proteins. J. Cell Biol. 119, 1047–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young J. C., Barral J. M., Ulrich Hartl F. (2003) More than folding: localized functions of cytosolic chaperones. Trends Biochem. Sci. 28, 541–547 [DOI] [PubMed] [Google Scholar]
- 12. Fan A. C., Young J. C. (2011) Function of cytosolic chaperones in Tom70-mediated mitochondrial import. Protein Pept. Lett. 18, 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forwood J. K., Jans D. A. (2002) Nuclear import pathway of the telomere elongation suppressor TRF1: inhibition by importin α. Biochemistry 41, 9333–9340 [DOI] [PubMed] [Google Scholar]
- 14. Baliga B. C., Colussi P. A., Read S. H., Dias M. M., Jans D. A., Kumar S. (2003) Role of prodomain in importin-mediated nuclear localization and activation of caspase-2. J. Biol. Chem. 278, 4899–4905 [DOI] [PubMed] [Google Scholar]
- 15. Argentaro A., Sim H., Kelly S., Preiss S., Clayton A., Jans D. A., Harley V. R. (2003) A SOX9 defect of calmodulin-dependent nuclear import in campomelic dysplasia/autosomal sex reversal. J. Biol. Chem. 278, 33839–33847 [DOI] [PubMed] [Google Scholar]
- 16. Lixin R., Efthymiadis A., Henderson B., Jans D. A. (2001) Novel properties of the nucleolar targeting signal of human angiogenin. Biochem. Biophys. Res. Commun. 284, 185–193 [DOI] [PubMed] [Google Scholar]
- 17. Roth D. M., Moseley G. W., Pouton C. W., Jans D. A. (2011) Mechanism of microtubule-facilitated “fast track” nuclear import. J. Biol. Chem. 286, 14335–14351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roth D. M., Harper I., Pouton C. W., Jans D. A. (2009) Modulation of nucleocytoplasmic trafficking by retention in cytoplasm or nucleus. J. Cell. Biochem. 107, 1160–1167 [DOI] [PubMed] [Google Scholar]
- 19. Lam M. H., Thomas R. J., Loveland K. L., Schilders S., Gu M., Martin T. J., Gillespie M. T., Jans D. A. (2002) Nuclear transport of parathyroid hormone (PTH)-related protein is dependent on microtubules. Mol. Endocrinol. 16, 390–401 [DOI] [PubMed] [Google Scholar]
- 20. Lam M. H., Henderson B., Gillespie M. T., Jans D. A. (2001) Dynamics of leptomycin B-sensitive nucleocytoplasmic flux of parathyroid hormone-related protein. Traffic 2, 812–819 [DOI] [PubMed] [Google Scholar]
- 21. Stevenson M. A., Calderwood S. K. (1990) Members of the 70-kilodalton heat shock protein family contain a highly conserved calmodulin-binding domain. Mol. Cell. Biol. 10, 1234–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moriya M., Ochiai M., Yuasa H. J., Suzuki N., Yazawa M. (2004) Identification of Ca2+-dependent calmodulin-binding proteins in rat spermatogenic cells as complexes of the heat-shock proteins. Mol. Reprod. Dev. 69, 316–324 [DOI] [PubMed] [Google Scholar]
- 23. Shi Y., Thomas J. O. (1992) The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol. Cell. Biol. 12, 2186–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shulga N., Roberts P., Gu Z., Spitz L., Tabb M. M., Nomura M., Goldfarb D. S. (1996) In vivo nuclear transport kinetics in Saccharomyces cerevisiae: a role for heat shock protein 70 during targeting and translocation. J. Cell Biol. 135, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Florin L., Becker K. A., Sapp C., Lambert C., Sirma H., Müller M., Streeck R. E., Sapp M. (2004) Nuclear translocation of papillomavirus minor capsid protein L2 requires Hsc70. J. Virol. 78, 5546–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou Y., Mao H., Li S., Cao S., Li Z., Zhuang S., Fan J., Dong X., Borkan S. C., Wang Y., Yu X. (2010) HSP72 inhibits Smad3 activation and nuclear translocation in renal epithelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 21, 598–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Echeverría P. C., Mazaira G., Erlejman A., Gomez-Sanchez C., Piwien Pilipuk G., Galigniana M. D. (2009) Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin β. Mol. Cell. Biol. 29, 4788–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galigniana M. D., Echeverría P. C., Erlejman A. G., Piwien-Pilipuk G. (2010) Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus 1, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harley V. R., Lovell-Badge R., Goodfellow P. N., Hextall P. J. (1996) The HMG box of SRY is a calmodulin binding domain. FEBS Lett. 391, 24–28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.