Background: Delivery of the vesicle into the pre-fusion state during tethering is not understood.
Results: Interactions between the COG complex, golgins and Rabs were mapped. Two ends of the golgin TMF both bind COG and different Rabs, the middle binds the target membrane.
Conclusion: COG may reel the vesicle into docking along the golgin.
Significance: Mechanistic link between tethering complex and coiled tether established.
Keywords: Golgi, Membrane Trafficking, Protein-Protein Interactions, Rab Proteins, Vesicles, COG Complex, golgin, Vesicle Tethering
Abstract
Protein sorting between eukaryotic compartments requires vesicular transport, wherein tethering provides the first contact between vesicle and target membranes. Here we map and start to functionally analyze the interaction network of the conserved oligomeric Golgi (COG) complex that mediates retrograde tethering at the Golgi. The interactions of COG subunits with members of transport factor families assign the individual subunits as specific interaction hubs. Functional analysis of selected interactions suggests a mechanistic tethering model. We find that the COG complex interacts with two different Rabs in addition to each end of the golgin “TATA element modulatory factor” (TMF). This allows COG to potentially bridge the distance between the distal end of the golgin and the target membrane thereby promoting tighter docking. Concurrently we show that the central portion of TMF can bind to Golgi membranes that are liberated of their COPI cover. This latter interaction could serve to bring vesicle and target membranes into close apposition prior to fusion. A target selection mechanism, in which a hetero-oligomeric tethering factor organizes Rabs and coiled transport factors to enable protein sorting specificity, could be applicable to vesicle targeting throughout eukaryotic cells.
Introduction
Vesicular transport sorts proteins between organelles, thereby ensuring their distinct biochemical compositions. This requires that vesicles carrying different cargoes are delivered to the appropriate target compartment. Vesicle tethering is believed to play a critical role in determining targeting specificity (1, 2). Rather than a physical attachment per se, vesicle tethering is defined as the collection of protein interaction steps that a vesicle undergoes leading to SNARE-mediated membrane fusion (3). Tethering factors, either extended coiled-coil dimers or multisubunit hetero-oligomers, are the organizers of vesicle targeting. Whereas the coiled-coil dimers (known as golgins at the Golgi) supposedly capture the vesicle while it is distant from its target (4–6), multisubunit complexes are thought to coordinate the protein interactions of tethering (7, 8).
Vesicle transport within the Golgi is a particularly useful system for studying the targeting specificity and tethering of vesicles. The mammalian Golgi typically consists of four to eight cisternae, each of these containing a unique set of modifying enzymes. Most of the transport through the Golgi is believed to occur by cisternal maturation (9), which means that secretory proteins always remain within the cisternae, while resident enzymes are recycled via retrograde vesicles (10). This sorting model implies that several distinct types of retrograde coat protein I (COPI)4 vesicles with differing enzyme content generate the differential enzyme distribution of the Golgi. Indeed, such vesicles have been shown to use different members of the golgin family for tethering (2).
Tethering factors have been shown to interact with Rab GTPases (4, 11), a protein family responsible for the specificity of membrane organization (12). For example, the golgins GM130 and p115 synergistically interact with Rab1, whereby the GM130-Rab1 binding enhances the p115-Rab1 interaction (13). These interactions are necessary for the tethering of COPI vesicles at the cis-Golgi (14) and likely involve an induced conformational change of GM130 (15). Moreover, the C-terminal coil of the golgin TMF has been shown to interact with Rab6, this interaction being necessary for Golgi localization of this golgin (16, 17). Many golgins bind more than one Rab in Drosophila (18) as well as mammals (19–21), suggesting either a combinatorial Rab-code for golgin-assisted vesicle targeting and/or the need for multiple Rabs for moving vesicles through the mesh of golgins covering the organelle (6). The questions remain: what are the molecular details of these golgin-Rab interactions during tethering, and how do interactions between Rabs and golgins lead to a specific vesicle targeting reaction?
The central coordinator for retrograde vesicle tethering at the Golgi is the multisubunit conserved oligomeric Golgi (COG) complex (8, 22). Indeed, mutations in the COG complex cause glycosylation defects in both model organisms and patients (23–25). COG localization has no cis-trans preference (26), implying that it coordinates vesicle targeting at every cisterna. Yet, lobe A (subunits 1–4) is necessary for maintaining proteins in the early/medial Golgi, and lobe B (subunits 5–8) is needed primarily for the targeting of late-Golgi processing enzymes (27–31). Given that the only protein motifs in COG are predicted coiled coils (32), a plausible molecular function for COG is to act as a protein interaction hub. Indeed, the yeast ortholog was found in a direct physical interaction with the Rab Ypt1p and the vesicle coat COPI (33), whereas mammalian COG has been found to interact with the SNAREs syntaxin5 and 6 (34, 35), the SM protein Sly1 (36), several Rabs (37), and the golgins p115 and golgin-84 (38, 39). Development of a general model for COG mediated tethering is hampered by the lack of knowledge about the full set of protein interactions that the complex is involved in. We therefore determined the map of interactions between the complex and other proteins implicated in vesicle tethering. A number of Rabs, golgins, and subunits of the COPI coat combinatorially interact with different COG subunits. Upon further functional dissection of a subset of these interactions we discovered a network between COG, the Rabs1 and 6, and different regions of the golgin TMF. Since we also find that TMF binds to Golgi membranes cleared of COPI, and its COG interacting regions dominantly inhibit protein glycosylation, we propose a model in which interactions between a tethering complex and a coiled coil tether could jointly reel in the vesicle in preparation for fusion. By using a subset of the available combinatorial interactions this proposed mechanism suggests how vesicle targeting specificity could guide protein sorting within the cell.
EXPERIMENTAL PROCEDURES
Antibodies
For immunoblotting affinity-purified anti-Cog4 (1:300), anti-His-HRP (1:10000, Sigma), anti-GST (1:1000, Invitrogen monoclonal), anti-Cog3 (1:2000, 40, monoclonal), affinity-purified anti-TMF (1:500, Lowe laboratory), anti-HA.11 (1:500, Covance monoclonal), anti-GFP (1:2000, Invitrogen monoclonal), and anti-myc (1:1000, Cell Signaling monoclonal) were used. The anti-Cog4 antibodies (Oka et al., 27) were affinity purified against recombinant insect cell produced His6-Cog4 (Richardson et al., 43). The anti TMF antibodies were raised against GST-tagged head and tail regions in sheep, and affinity purified against these same proteins. Serum was pre-cleared against GST before affinity purification against immunogen. Anti-HA 3F10 (Roche) or anti-GFP (Invitrogen) was used for immunoprecipitation. For immunofluorescence mouse monoclonal anti-GalNAcT2 (1:5, tissue culture supernatant, kind gift from H. Clausen), anti-GFP (1:500, Invitrogen polyclonal), anti-Rab6 (1:50, Santa Cruz Biotechnology, C19), anti-βCOP (1:3000, MAD, T.E. Kreis laboratory) were used.
Yeast Two-hybrid Assays
COG subunits, golgins, and Rabs were expressed as Gal4 binding or activation domain fusion proteins from pGAD/pGBD/pGBDU plamids (66). Rab binding domain fusion proteins contained mutations to lock the protein in the active (T) or inactive (D) conformation (67). Pairs of fusion constructs were introduced into yeast (genotype: trp1–901 leu2–3,112 ura3–52 his3–200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ) by mating of haploid single transformants (Fig. 1A) or pairwise co-transformation (Figs. 1B and 2 A and C). Cells were subsequently scored for growth on selective media, typically following 1 week of incubation at 30 °C or 2 weeks at 14 °C. For COPI subunits the pACT2 vector was used.
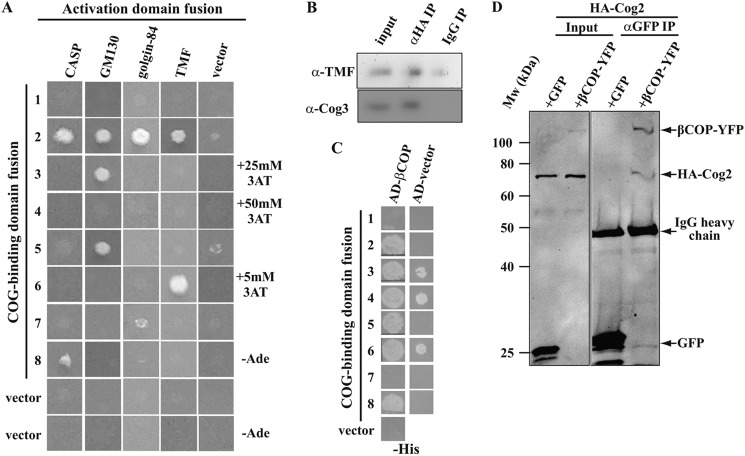
FIGURE 1.
COG shows combinatorial interactions with multiple Rabs. A, Y2H assay with all eight COG subunits and 14 mammalian Rab-GTPases on media lacking histidine (−His). Full-length COG subunit prey constructs were tested against Rab bait fusions locked in the active (T) or inactive (D) conformation. B, summary of yeast two-hybrid interactions found between Cog4, 5, 6, and 57 T-locked human Rabs. Rabs19b and 45 show auto-activation. Growth on −His-Ade media at 14 °C and/or 30 °C is shown as a positive result (red). C and D, pull-down assay between wild type GST-Rabs loaded with the indicated nucleotide and partially purified bovine COG complex at 8 mg/ml (C) or purified Cog4-His (D). Lower panels show the amount of Rab proteins eluted from the beads. Eluted COG was probed with an anti-Cog4 antibody (C) or anti-His antibody (D). E, mitochondrial co-targeting of COG subunits and Rabs. mCherryActA tagged Cog5 (first column) Cog6 or mCherryActA by itself (second column), which are all targeted to mitochondria, were co-expressed with GTP-locked tagged Rabs in Hela cells. Cells were stained with the Golgi marker giantin and anti-myc for Rab2 before analysis using confocal microscopy. Scale bar 10 μm.
FIGURE 2.
COG-golgin and COG-COPI interactions. A, Y2H interactions between COG subunit bait constructs and golgin prey constructs on −His or, where indicated −Ade media. To suppress self-activation 3-amino triazole (3AT) was added at the indicated concentrations to −His media. B, co-immunoprecipitation of the golgin TMF and the COG complex from HEK293 cells stably expressing HA-tagged Cog1 transiently transfected with GFP-TMF. Immunoprecipitation was with 3F10 anti-HA antibodies (αHA IP) or the same amount of nonspecific IgG (IgG IP). The precipitates were probed with anti-TMF (top row) or anti-Cog3 (bottom row). Input is 0.1% of the precipitate for TMF and 3% for Cog3. C, yeast two hybrid interactions between COG subunit bait constructs and βCOP or vector only prey constructs. All other COPI subunits gave negative Y2H results with COG subunits. D, co-immunoprecipitation of co-transfected HA-Cog2 and βCOP-YFP or GFP from Hela cells using anti-GFP antibodies. Inputs and precipitates were probed with anti-GFP and anti-HA.11 at the same time. Input is 10%.
GST Pull-down Experiments
GST-Rab or GST-TMF fragment constructs were expressed in the Escherichia coli BL21strain, induced at A600 = 0.8 with 0.5 mm IPTG followed by overnight incubation at 18 °C. Rab30 lacked the C-terminal 19 amino acids to aid solubility (PDB structure 2EW1). After harvesting, cells were lysed in GST-lysis buffer (20 mm Hepes, 200 mm KCl, 2 mm MgCl2, 1 mm DTT, pH 7.4 with additional protease inhibitors) and the lysate cleared by centrifugation (15,000 rpm for 15 min). Glutathione-agarose (Clontech) equilibrated with GST-lysis buffer was added and rotated for 1 h at 4 °C. Beads were pre-blocked with 0.1% BSA for COG binding experiments. The beads were washed with GST-lysis buffer before pull-down experiments.
For GST-Rabs, beads were washed with nucleotide buffer (20 mm Hepes, 200 mm KCl, 5 mm MgCl2, 1 mm DTT) with 1 mm nucleotide and 10 mm EDTA, and incubated overnight at 4 °C. The beads were then incubated for 30 min in the same buffer lacking EDTA. Nucleotide loading was confirmed by reverse phase HPLC analysis. 60 μg of recombinant His6-Cog4 purified from insect cells (Richardson et al., 43) or 8 mg COG-containing sample purified from bovine brain by ammonium sulfate precipitation and butyl Sepharose chromatography (Ungar et al., 22) were added. For GST-TMF fragments, the beads were incubated with either His6-Rab1(T), His6-Rab1(D), or partially purified COG. His6-Rab1a was purified in GTP or GDP locked mutant forms from E. coli using Ni2+-chelate chromatography, dialyzed into GST-lysis buffer, then incubated with 1 mm of the appropriate nucleotide and 5 mm EDTA for 1.5 h. Subsequently 10 mm MgCl2 and 0.5 mm of nucleotide were added, and following 30 min of incubation the Rab sample mixed with TMF-fragment containing beads.
For all GST pull-downs, after 1 h of incubation at 4 °C with rotation and washing with nucleotide buffer, bound proteins were eluted into SDS-PAGE buffer. All samples were analyzed using SDS-PAGE followed by immunoblotting using the Immobilon HRP substrate (Millipore) and the Syngene GeneGenius ECL imaging system.
Immunoprecipitation
GFP-TMF expression plasmids were transfected into HEK-293 cells stably expressing HA-tagged Cog1 using the GeneCellin transfection reagent (BioCellChallenge). For COG-COPI co-immunoprecipitations YFP-βCOP and HA-Cog2 (Sohda et al., 2007) were co-transfected into HeLa cells. After 36–48 h, cells were washed with IP buffer (20 mm Hepes, 110 mm KOAc, 1 mm MgCl2, pH 7.4) then scraped into 1 ml of IP lysis buffer (IP buffer with 1% Triton X-100). All steps were performed at room temperature. The cell slurry was rotated for 30 min prior to 30 min of centrifugation. The lysate obtained was incubated with 1.5 μl anti-HA 3F10 (0.1 μg/μl) or the equivalent amount of IgG for 1 h, then 10 μl of pre-equilibrated HiTrap Protein G beads (GE Healthcare) were added for a further 25 min. Beads were washed four times with buffer (IP buffer + 0.1% Triton X-100) and the bound proteins eluted with SDS-PAGE buffer, followed by immunoblot analysis.
Immunofluorescence and Lectin Staining
Hela cells grown on coverslips were fixed 72 h after transfection for lectin or 16–30 h after transfection for immunofluorescence staining. BFA treatment was performed for 5 min using 5 μg/ml BFA immediately prior to fixation. Cells washed with PBS were fixed using MES-buffered ice cold methanol (90% methanol, 10 mm MES, 0.1 mm EDTA, 0.1 mm MgCl2, pH 6.9) for 5 min at −20 °C before washing with PBS. For lectin-staining cells were blocked for 1 h in P-L (PBS with 0.1% BSA) and incubated with 10 μg/ml GNL-FITC (Vector labs) in P-L for 1 h. For immunofluorescence cells were blocked in P-B buffer (PBS with 0.5% (w/v) saponin, 2% (w/v) BSA, 2% (v/v) goat serum) for 1 h and incubated with primary antibody in P-B for 1 h, washed in PBS and then incubated with Alexa-488 and/or 568 labeled secondary antibodies (1:400, Invitrogen) in P-B for 1 h. All coverslips were washed with PBS, and water before mounting for microscopy. Lectin-stained cells were imaged with a Leica IRB epifluorescence microscope, other imaging was done using confocal microscopy on an Olympus BX60 upright microscope. For Rab6 RNAi a SMARTpool oligo mix (Dharmacon) was transfected using oligofectamine. For mitochondrial localization experiments fluorescently tagged chimeras of full-length COG proteins were generated using pmCherry-C1-ActA. HeLa cells were co-transfected with COG and Rab constructs, fixed 20 h after transfection and imaged with the 63× oil 1.4 NA objective of a LSM510 Zeiss Laser inverted microscope outfitted with confocal optics.
RESULTS
COG-Rab Interactions
COG is a large hetero-oligomeric complex whose tethering function in the cell likely involves a series of protein interactions. The main aim of our study was to generate a full interaction map of COG that can later form the basis for functional dissection of retrograde vesicle tethering at the Golgi. Rab GTPases are involved in all steps of vesicle transport, and several tethering complexes (including COG) have been shown to interact with Rabs (33, 37, 41). Using a directed yeast two-hybrid (Y2H) approach we tested the interactions between COG subunits and 14 Rab-GTPases. 10 interactions involving three COG subunits (4, 5, and 6) and seven Rabs (1a, 2a, 4a, 6a, 14, 30, 36) were found of the 184 possible combinations (Fig. 1A). All observed interactions were with Rabs locked in the active conformation (T) as opposed to the inactive (D) conformation.
Encouraged by these initial results, we extended our survey to test a more complete set of 57 constitutively active mammalian Rabs (42) against the three COG subunits found to interact with Rabs in the initial assay (Fig. 1B). This experiment used increased stringency of detection, and revealed additional interactions with Rab1b, 6b, 10, 39, and 41. Combining the results, and rejecting interactions that were positive in one but negative in the other experiment, we identify 8 interactions: Cog4 with Rab30; Cog5 with Rab2a and 39; and Cog6 with Rab1a, 1b, 6a, 6b, and 41. Except for the previously reported Rab1a, 6a and 30 interactions (37) all these interactions are novel.
To validate our Y2H findings, we tested the ability of GST-tagged wild type Rabs to pull down partially purified bovine brain COG complex (22). Rab1a, 2a, 6a, and 30 loaded with either GDP or GTP were tested; GTP-loaded endosomal Rab5a was included as a negative control (Fig. 1C). Corroborating the high stringency Y2H results (Fig. 1B), active (GTP-loaded) Rab1a, 6a, and 30 displayed an increased affinity for COG in comparison to the inactive (GDP-loaded) forms (Fig. 1C). Binding of COG to GST-Rab2 was not observed (data not shown), suggesting that this interaction is not sufficiently long-lived for an in vitro pull-down experiment. Finally, two Rabs, 4a and 14, that interacted with Cog6 only in the less stringent Y2H experiment (Fig. 1A) were tested; one of them, GTP-Rab4a, was able to pull down significant quantities of the COG complex (Fig. 1C). Although Rab4a has not been implicated in Golgi-transport, these results suggest that this Rab might in fact be a COG interaction partner.
To investigate COG subunit interactions in isolation we used recombinant Cog4-His6 (to-date the only mammalian COG subunit that has been purified in a well-folded form (43)). Immobilized GST-Rab1a and GST-Rab30 both bound Cog4 in their active forms (Fig. 1D), whereas GST-Rab2 did not. Cog4 also bound to inactive Rab30 as previously reported (37). In contrast to the previous report though, binding to the GTP form (normalized to the amount of Rab30 on the beads) was still superior (Fig. 1D). Thus the pull-down experiments confirm the nucleotide-state specific binding of Rabs to the COG complex, indicating that COG is a bona fide effector of several Golgi Rabs.
As an additional test for the Cog5 and Cog6 interacting Rabs, for which isolated COG subunits were not available for pull-downs, in vivo relocalization experiments were carried out. We made use of an assay that moves proteins tagged with the Listeria derived ActA sequence (and their interaction partners) to mitochondria (44). This assay was recently used to relocalize COG-interacting SNARE proteins to mitochondria (45). When co-transfected with GFP-tagged Rabs, ActA-tagged Cog6 relocalized Rab1a, Rab4a, and Rab6a, whereas ActA-tagged Cog5 relocalized Rab2a to mitochondria (Fig. 1E). The relocation of these Rabs was observed only for the GTP-locked versions (Fig. 1E and data not shown).
In summary, interactions were found between COG subunits and five different Rabs: Rab1a, 2a, 4a, 6a, and 30, using at least two independent methods. In addition, robust Y2H interactions of COG subunits with four additional Rabs, Rab1b, 6b, 39, and 41, were observed. This demonstrates that COG is an effector for several different Rabs, most of them Golgi-associated (46–54), and is consistent with the suggestion that the COG complex acts in multiple vesicle tethering reactions at the Golgi.
COG Subunit Interactions with Golgins and the Vesicle Coat
A third class of proteins central to tethering at the Golgi are the golgins. COG has been shown to interact directly with two golgins, p115 and golgin-84 (38, 39). Given the interactions with a number of Rabs, we investigated whether additional golgins also interact directly with COG. Three representative golgins were chosen: GM130, acting at the cis-Golgi (55); CASP, a partner of golgin-84 that functions at the medial Golgi (2, 56); and TMF, a golgin with reported trans-Golgi functions but a localization throughout the Golgi stack (16, 17). The Y2H method was again used to test for interactions between these golgins and COG subunits (Fig. 2A). As shown previously by co-immunoprecipitation, golgin-84 interacts with Cog7 (39). Yet a stronger interaction was detected with Cog2 (Fig. 2A) that has been reported to interact with the golgin p115 (38). Indeed, the Cog2 subunit interacted with all tested golgins (Fig. 2A). These interactions are not promiscuous, since Cog2 did not interact with any of the Rabs tested (Fig. 1A). Cog2 is not a generic coiled coil-binding protein either, since it failed to bind most other COG subunits (31) that have a strong propensity to form coiled coils close to their N termini (32). The tested golgins also interact with other COG subunits; namely, CASP with Cog8, GM130 with Cog3 and 5, and TMF with Cog6 (Fig. 2A).
All of the tested golgins, except for TMF, have previously been co-immunoprecipitated with COG (34, 38, 39). GFP-TMF was therefore tested for co-immunoprecipitation with COG from HEK293 cells stably expressing HA-tagged Cog1 that can complement the absence of endogenous Cog1 (26), and was expressed at close to endogenous levels (27 and data not shown). The anti-HA precipitation recovers the full COG complex from these cells, as shown by probing for Cog3 (Fig. 2B, lower panel) that is only present in cells as part of the full eight subunit complex (22). Anti-HA antibodies specifically co-precipitated GFP-TMF with COG (Fig. 2B, upper panel). Thus, all tested golgins that interact with COG subunits by Y2H also co-immunoprecipitate with the complex.
A further important player in intra-Golgi vesicle transport is the COPI protein complex (57, 58) that has been shown to interact with COG in yeast (33). To test whether this interaction is conserved in mammalian cells, we examined the pairwise interactions of COG and all COPI subunits using Y2H (Fig. 2C). Using high-stringency selection, we found robust interactions between βCOP and Cog2, 5, and 8. None of the other COPI subunits interacted with COG (data not shown). The COG-COPI interaction was confirmed by co-immunoprecipitation of HA-tagged Cog2 using anti-GFP antibodies following co-transfection with YFP-βCOP (Fig. 2D).
Mapping the COG/TMF Interactions
Interactions between COG and golgins have been observed before (38, 39), yet it is not known how these interactions support vesicle tethering. Golgins have been suggested to tether vesicles that are distant from the target. We thus wondered whether a golgin-COG interaction could help bring the golgin-tethered vesicles into closer proximity with the target membrane. TMF, which requires its C-terminal coils for binding to the Golgi via Rab6 (16; see also Fig. 4A), was chosen to map the COG binding site(s) along its length. Utilizing Y2H with TMF fragments whose boundaries were based on coiled-coil predictions (59, Fig. 3A), we found that both Cog2 and Cog6 interacted with the two C-terminal TMF-coils (Fig. 3B, Cterm fragment). Although this is the same region previously mapped as the Rab6 binding site (16), we in this study were able to narrow down this Rab binding site to the terminal coil of the golgin (Fig. 3B, Cterm-C fragment). The COG binding site therefore could overlap but is not identical to the Rab6 binding site on the TMF C terminus.
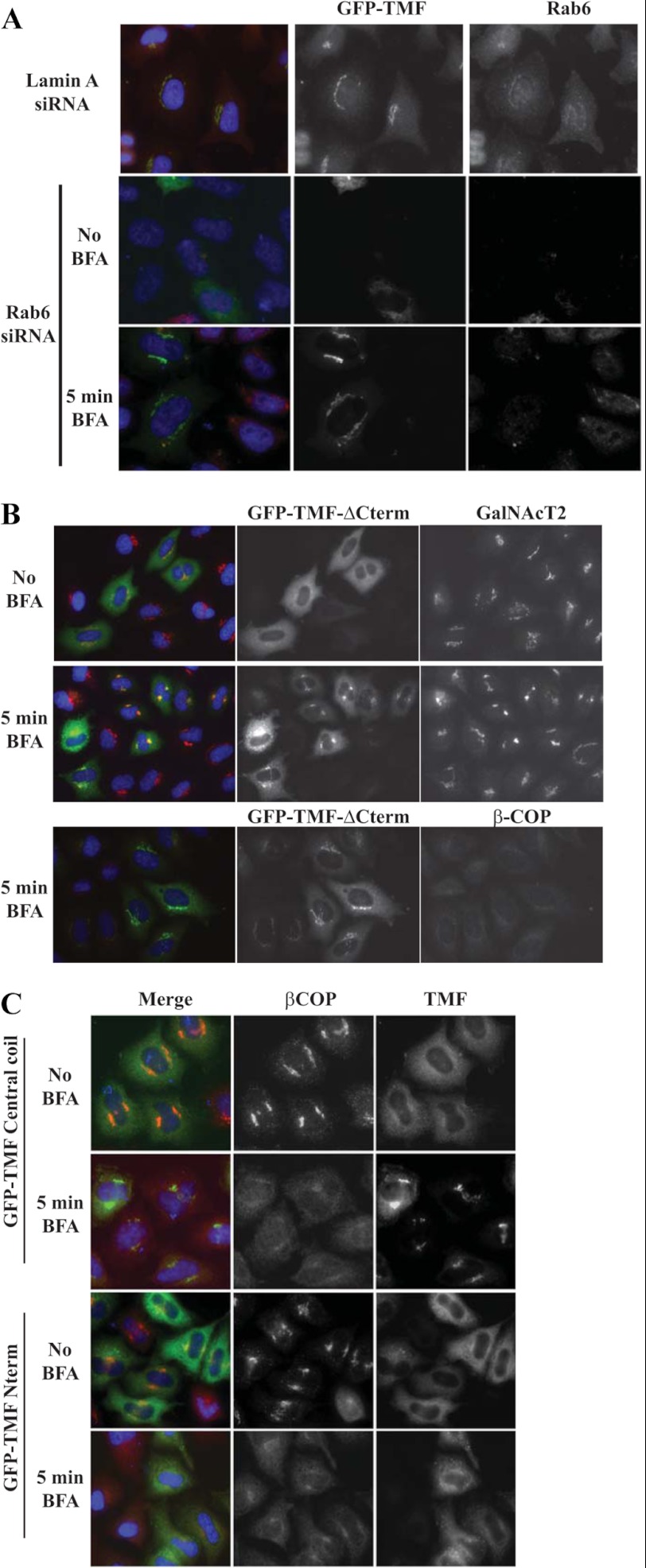
FIGURE 4.
The TMF central coil can bind a cryptic site on the Golgi under the COPI coat. A, Hela cells transfected with full-length GFP-TMF and Rab6 siRNA (bottom rows) or lamin A siRNA (top row) were stained for Rab6 (red) and GFP (green) before (middle) or after (bottom) 5 min BFA treatment. B, Hela cells transfected with GFP-TMF ΔCterm and treated with BFA as indicated were stained for GFP (green) and either GalNAcT2 (red, top) or βCOP (red, bottom). C, Hela cells transfected with GFP-TMF central coil (top) or GFP-TMF Nterm (bottom), and treated with BFA as indicated, were stained for GFP (green) and βCOP (red). The central coil fragment used in these experiments has slightly different boundaries, it encompasses amino acids 440–781.
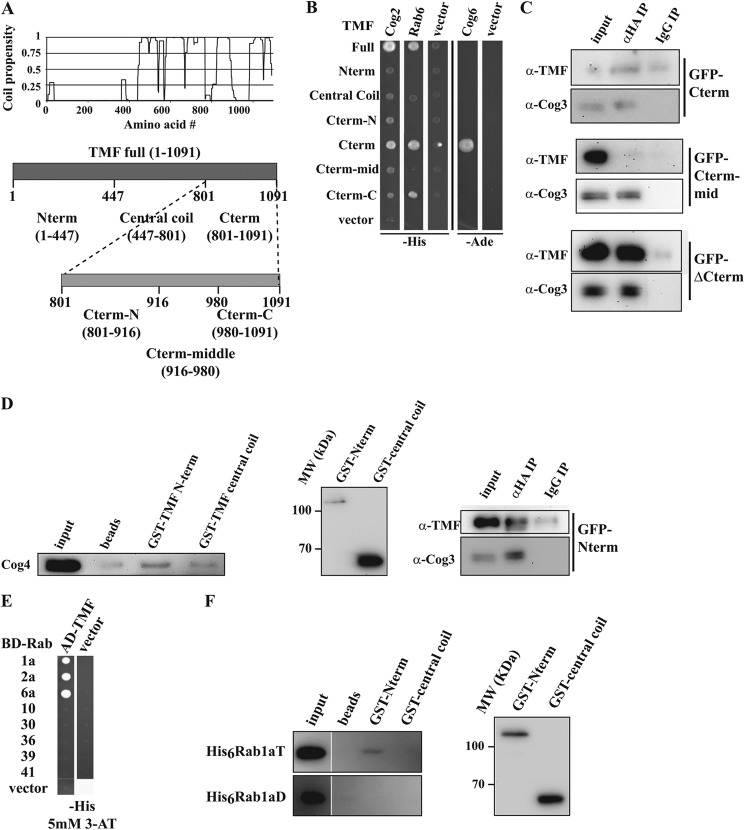
FIGURE 3.
Mapping of COG interaction sites on TMF. A, coil propensity of TMF (16, 59), and the derived constructs representing various regions of the golgin. B, Y2H interactions of TMF fragment prey constructs with Cog2, Cog6, and Rab6 baits. C, co-immunoprecipitation of TMF fragments with the COG complex. Experiments as in Fig. 2B using the GFP tagged TMF constructs Cterm, Cterm-mid, and ΔCterm expressed in Cog1HA cells. Inputs are 0.1% of precipitate for TMF and 3% for Cog3. D, probing the interaction between COG and TMF Nterm. Partially purified COG (as in Fig. 1C) was pulled down with GST tagged Nterm and central coil fragments. COG was probed by anti-Cog4 immunoreactivity (first column). The amounts of used TMF fragments probed with anti-GST are shown in the middle panel. GFP-TMF N-term expressed in Cog1HA cells was immunoprecipitated as in C (bottom panel). E, yeast two-hybrid interactions between full-length TMF prey fusions and active (T) Rabs. F, GST-TMF fragments were used to pull-down recombinant His-tagged Rab1a as in D. GDP and GTP locked mutants of Rab1a loaded with the appropriate nucleotide were used. The Rab1aT and Rab1aD images are from the same blot exposed for the same time, but have been rearranged in the figure for more clarity. TMF fragment levels are shown on the right as in D.
To confirm the interaction between the TMF C-terminal region and COG by co-immunoprecipitation, Cog1HA cells transfected with GFP-tagged TMF truncation constructs (Fig. 3A) were used. As expected from the Y2H results, the Cterm but not the Cterm-mid fragment co-precipitated with COG using HA-specific antibodies (Fig. 3C, top and middle panels). Surprisingly, TMF lacking the C-terminal region, termed ΔCterm, very efficiently co-precipitated with COG as well (Fig. 3C, bottom panel). This was not due to heterodimerization with endogenous full-length TMF, since endogenous TMF was not detected in the precipitate (data not shown). It is unlikely that the lack of a positive Y2H signal with the N-terminal fragments (Nterm and central coil) of TMF is due to the lack of interaction strength, since the efficiency of the ΔCterm co-IP is better than the one with the Cterm fragment. The absence of Y2H signal could instead be due to the requirement of multiple COG subunits or a further endogenous factor.
The ΔCterm TMF construct consists of a predicted coiled coil (central coil) and a globular head domain (Nterm) (Fig. 3A). To further define the region involved in COG binding, we pulled down partially purified COG complex using recombinant GST-Nterm and -central coil fragments (Fig. 3D, left panel). Whereas the central coil fragment did not pull COG down significantly above the levels observed with beads only, the Nterm fragment did. Indeed, the TMF Nterm fragment also efficiently co-immunoprecipitated with COG from Cog1HA cells (Fig. 3D, right panel), demonstrating that this interaction exists in vivo. The COG complex thus has two separate interactions with the golgin TMF, one binding site being in the C-terminal, the other in the N-terminal region of the golgin.
Many golgins have been shown before to interact with multiple Rabs (18, 20, 21). We therefore asked whether, besides Rab6, TMF also interacted with other COG-partner Rabs, and found that both Rab1a and Rab2a interacted with full TMF by Y2H (Fig. 3E). Neither Rab, however, interacted with the TMF fragments used in Fig. 3B (data not shown); therefore, the binding of His-tagged Rab1a to GST-tagged TMF fragments was tested. Nterm-TMF bound specifically to the active form of Rab1 (Fig. 3F, top). The two opposing ends of TMF thus not only bind COG, but also two distinct Rabs.
Multiple Regions of TMF Can Bind to the Golgi
Previous work has shown that TMF is recruited to the Golgi via Rab6 (16, 17). In line with this, we find that depletion of Rab6 by RNAi results in loss of Golgi association of TMF (Fig. 4A, middle row). To investigate the possibility that TMF may associate with other Golgi factors in addition to Rab6, we performed the same experiment but this time incubated the cells with BFA for 5 min to remove the COPI coat covering the Golgi rims (57) prior to fixation. Under these conditions, strikingly, we observe that TMF is efficiently recruited to the Golgi even when Rab6 is depleted (Fig. 4A, bottom row). This result indicates an additional binding partner at the Golgi membrane, and strongly suggests that binding to this additional factor is masked by the COPI coat. Note that recruitment of TMF in the presence of Rab6 is unaffected by BFA treatment or βCOP depletion (data not shown), suggesting that Rab6 binding is the dominant recruitment mechanism when the COPI coat is unperturbed. In addition, neither this short BFA treatment, nor depletion of Rab6 perturbs COG localization to the Golgi (data not shown), in line with the multiple contacts COG makes with Golgi localized proteins.
To identify the domain of TMF that binds to this new putative binding partner, we first analyzed recruitment of the ΔCterm TMF truncation mutant lacking the C-terminal Rab6 binding site. Again, this construct was efficiently recruited to the Golgi membrane after BFA treatment that removed COPI from the Golgi as expected (Fig. 4B). This result indicates that binding is via a region distinct from the C terminus. Further mapping indicated that the central coil region was sufficient to mediate Golgi recruitment under conditions where COPI had been removed by a short BFA treatment (Fig. 4C). This implies that during vesicle tethering TMF can bind to Golgi membranes via its central coil domain once the coat is locally cleared off the Golgi rim.
Overexpression of TMF Fragments Leads to Changes in Glycosylation
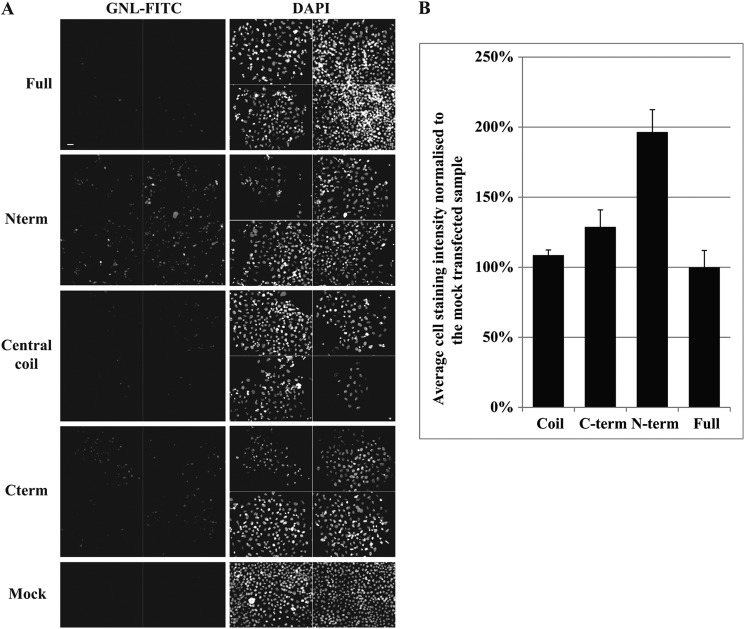
After finding an in vivo role for the central coil fragment of TMF in Golgi targeting we wondered whether the protein interactions of the N- and C-terminal regions identified had in vivo relevance too. We reasoned that overexpression of these TMF fragments could act in a dominant fashion, since these overproduced fragments may saturate their cognate binding partners. Therefore, TMF fragments were overexpressed and glycosylation, as a readout of Golgi function, examined in HeLa cells. We used the lectin GNL that shows increased affinity to HeLa cells depleted of COG4 (43) by binding to terminal α1–3 mannose (60). This sugar is generally presented on incompletely processed high-mannose and hybrid glycans. Expression of full-length TMF or the central coil domain did not significantly increase GNL staining compared with mock-transfected HeLa cells (Fig. 5). However, overexpression of the Cterm and especially the Nterm regions, which interact with COG and Rab6 or Rab1 respectively, significantly increased GNL affinity (Fig. 5). This suggests that the COG-Rab-TMF interactions may play a critical role in the vesicular sorting of enzymes responsible for eliminating the GNL epitope.
FIGURE 5.
Overexpression of TMF fragments disrupts glycosylation. A, Hela cells transfected with an inert plasmid (Mock) or with GFP-tagged full-length (full), central coil, Cterm or Nterm constructs of TMF were stained with GNL-FITC (left column) and DAPI (right column) 72 h after transfection. Transfection efficiency was 50–80% as verified prior to the methanol fixation that destroys GFP fluorescence. Four separate fields of view (two for mock) are shown for each condition. Scale bar 200 μm. B, quantification of the images in A.
DISCUSSION
The COG complex functions as the CATCHR family tethering complex for retrograde intra-Golgi vesicle transport (22, 24, 61). In this study, we have begun the molecular dissection of COG-mediated vesicle tethering by defining the protein interaction network of COG, and starting to dissect part of it. It is important to consider that a variety of different vesicles will be subject to COG-mediated tethering while being directed to different destinations within the Golgi. Previous work suggests that COG facilitates all retrograde intra-Golgi-targeting reactions, since it localizes to every cisterna (26), and mediates the localization of enzymes in different Golgi regions (28–30, 40). Therefore COG is likely to require a large number of interactions to guide all the distinct tethering reactions. As an example, although TMF null mutant mice and COG lobe B mutant fruit flies are both male sterile due to a spermatogenesis defect (62–64), the defect during spermatogenesis occurs much earlier in this process for the COG mutants, possibly because some of the COG-mediated steps that do not require TMF are more critical for spermatogenesis than the one involving TMF.
Physiological Importance of COG Interactions
COG binding is in all cases stronger to the GTP-bound Rab, as expected for a true effector (33, 41). Even more compellingly, although we tested the full range of human Rabs for interaction with COG, of those that we identify as COG interactors, all but Rab4a have been assigned a function at the Golgi (12). Although previous work assigns a recycling endosomal function to Rab4a, the combination of our Y2H, GST pull-down, and mitochondrial relocation results strongly implicate COG as a Rab4a effector. Future work will need to clarify the physiological context of this particular COG-Rab interaction. It is important to note that although Rab1 has predominantly been regarded to function in anterograde transport, we presume that its interaction with COG fulfills an additional retrograde transport function. The specificity of the interactions is particularly highlighted by the fact that Cog2 shows interactions with all tested golgins; yet none of the Rabs or SNAREs tested showed interactions with Cog2. In contrast, Cog4 and Cog6, which show interactions with many Rabs, only interact with one golgin (Figs. 1, 2, and 6A).
FIGURE 6.
Model of COG interactions and COG-mediated vesicle tethering via TMF and the Rabs 1 and 6. A, an interaction map of COG with Rabs, golgins and SNAREs highlighting how subunits form hubs for these transport factor families. Only the interactions of these factors with their respective hub-subunits are shown for clarity. βCOP is added to show its hub-promiscuity, lobe A and B of the complex are represented as dashed red and blue ovals, respectively. B, proposed molecular mechanism of COG mediated tethering. For details see the text. All details are drawn to scale. The TMF and COG drawings shapes are inspired by the EM structures of the golgin p115 (5) and lobe A of yeast COG (65).
The resulting interaction diagram (Fig. 6A) lends itself to some generalization that, for the first time, may be used to assign functions to different COG subunits. As such, interaction hubs within COG can be outlined for the different classes of transport factor partners (Fig. 6A), including the interactions between SNAREs and COG subunits (45). We stress that the assignment of these hubs is based solely on interaction data, and will undoubtedly be refined by future mechanistic experiments. All golgins tested so far, including p115 (38), interact with Cog2 that in turn does not interact with Rabs or SNAREs, therefore we term this the golgin hub. Rabs in contrast, interact with Cog4, Cog5, and Cog6. Since only one SNARE (34) but no golgins interact with Cog4, and one golgin but no SNAREs with Cog5, we call Cog4 and 5 Rab hubs. The hub for SNAREs is Cog8 (45) since the only other relevant interactor here is the golgin CASP. This leaves Cog6 to serve as a hub for both Rabs and SNAREs. The vesicle coat component βCOP can bind to the hubs of all interaction partner families by interacting with Cog2, 5, and 8. A possible reason could be the need for redundancy. While the interactions of COG with other types of transport factors are combinatorial, it is quite likely that only a subset of those factors will interact with the complex during any one tethering event. However, the COG-COPI interaction is necessary for all tethering reactions, independently of the set of Rab, golgin, and SNARE partners in question. Thus alternative COG-COPI binding sites could be necessitated by the incompatibility of a single site with all possible COG-Rab, COG-golgin, and COG-SNARE interactions.
The Relationship between the Interaction Map and COG Lobe A/Lobe B Phenotypes
Defects in the two halves of COG, lobes A (Cog1–4) and B (Cog5–8), result in different phenotypes. Lobe A defects have more severe consequences than lobe B defects (24, 30). One suggested explanation for these differences has been that lobe A is important for early/medial, whereas lobe B for late Golgi enzyme targeting (29, 40). An alternative suggestion has been that lobe B has an enzyme targeting role, whereas lobe A plays a role in Golgi organization (28). Both of these earlier suggestions are compatible with the found interaction set. The hub for golgins is the lobe A subunit Cog2. Golgin interactions could well determine the Golgi integrity phenotype associated with lobe A defects, since golgins have been strongly associated with Golgi integrity (4). Alternatively, the one Rab hub in lobe A (Cog4) could be responsible for the targeting of some early Golgi enzyme subsets through the Rabs 1, 30, and/or 41, while the remaining COG partners could determine lobe B phenotypes, mainly affecting the targeting of late Golgi enzymes.
Model of Vesicle Tethering at the Golgi
The observed COG-TMF-Rab interactions, together with the BFA-induced Golgi recruitment of TMF fragments, prompt us to propose a tentative molecular model for COG-mediated tethering (Fig. 6B).
TMF is known to attach to the Golgi by its C-terminal tail binding to Rab6 (Fig. 4, A and B and Ref. 17). Rab1, in contrast, binds TMF at the opposite end (Fig. 3F), and thus could facilitate the binding of an approaching vesicle to TMF. The fact that a significant fraction of COG has been localized to vesicles by EM (26) supports an initial interaction of COG with the approaching vesicle, this may require the observed COG-βCOP binding. Long range tethering could then be completed by binding of both Rab1 and COG to the Nterm region of TMF (Fig. 6B, panel 1). Approach of the vesicle to the target could be further facilitated by COG binding to Rab6 and the TMF-Cterm, while still bound to the opposite end of the golgin, thereby restricting the golgin into a bent conformation (Fig. 6B, panel 2). EM images of purified bovine COG show that the most splayed out conformations of the complex can reach at least 80 nm (22), a length sufficient to bridge the head and tail of a partially bent golgin. Further interaction studies involving all four proteins together, as well as structural studies will be necessary to validate such a proposal. However, since the COG subunits that bind Rab6 and the Cterm of TMF are different from the ones that bind Rab1, such an arrangement is compatible with our current knowledge.
The binding of COG to the membrane proximal region of TMF would also bring the complex into proximity of the COPI coat present on the cisternal rim (57, 58), providing a further occasion for COG-βCOP binding. Once TMF is close enough the central coil region can bind to a factor that is normally concealed by the COPI coat covering the Golgi (Fig. 6B, panel 3). The short BFA treatment that moves the TMF central coil to the Golgi (Fig. 4C), has likely revealed this binding site by removing COPI, although the effect of BFA on other Arf1-mediated processes could be involved here too. The tight TMF binding could thus complete docking.
It is clear that our interaction data are but static representations of the proposed mechanistic model. Yet it is intriguing that both the Nterm and Cterm regions of TMF, which we suggest to participate in critical binding events, perturb glycan processing in a dominant fashion (Fig. 5). In contrast, the central coil region, which binds a cryptic site that is secluded until the machinery is primed, is ineffective in the same assay.
Our data and the model presented above link hetero-oligomeric and coiled-coil tethers. These protein families have been shown to interact, yet this is to our knowledge the first molecular model for their combined mode of action. In this model vesicle and target membranes could be marked by combinatorial sets of Rabs, and thus targeting could be achieved through specific golgins that have combinatorial Rab interactions themselves (18). CATCHR family complexes acting at other compartments (e.g. exocyst, Dsl1 complex) could use a similar mechanism to reel a vesicle into close proximity of the target membrane, albeit their protein partners would certainly be different.
Acknowledgments
We thank Frederick Hughson for his support in the initial phase of this work, to Francis Barr (Oxford) for Rab plasmids, Henrik Clausen (Copenhagen) for antibodies and Heather Bruce for help with plasmid construction. The Technology Facility at York's Department of Biology helped with imaging and HPLC analysis.
This work was supported, in whole or in part, by a National Institutes of Health Grant 1R01GM083144, (to V. L.), BBSRC Grant BB/F006993/1/ and a Marie Curie Grant 201098 (to D. U.), a MRC Senior Fellowship to ML (G117/494), and a DFG grant (Excellence Cluster “Inflammation at Interfaces”) (to R. D.).
- COPI
- coat protein I
- COG
- conserved oligomeric Golgi
- 3AT
- 3-amino triazole
- Y2H
- yeast two hybrid
- BFA
- Brefeldin A
- TMF
- TATA element modulatory factor
- CATCHR
- complexes associated with tethering containing helical rods.
REFERENCES
- 1. Ungar D., Oka T., Krieger M., Hughson F. M. (2006) Retrograde transport on the COG railway. Trends Cell Biol. 16, 113–120 [DOI] [PubMed] [Google Scholar]
- 2. Malsam J., Satoh A., Pelletier L., Warren G. (2005) Golgin tethers define subpopulations of COPI vesicles. Science 307, 1095–1098 [DOI] [PubMed] [Google Scholar]
- 3. Waters M. G., Pfeffer S. R. (1999) Membrane tethering in intracellular transport. Curr. Opin. Cell Biol. 11, 453–459 [DOI] [PubMed] [Google Scholar]
- 4. Ramirez I. B., Lowe M. (2009) Golgins and GRASPs: holding the Golgi together. Semin. Cell Dev. Biol. 20, 770–779 [DOI] [PubMed] [Google Scholar]
- 5. Sapperstein S. K., Walter D. M., Grosvenor A. R., Heuser J. E., Waters M. G. (1995) p115 is a general vesicular transport factor related to the yeast ER-Golgi transport factor Uso1p. Proc. Natl. Acad. Sci. U.S.A. 92, 522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munro S. (2011) The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb. Perspect. Biol. 3, a005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu I. M., Hughson F. M. (2010) Tethering factors as organizers of intracellular vesicular traffic. Annu. Rev. Cell Dev. Biol. 26, 137–156 [DOI] [PubMed] [Google Scholar]
- 8. Miller V. J., Ungar D. (2012) Re'COG'nition at the Golgi. Traffic 13, 891–897 [DOI] [PubMed] [Google Scholar]
- 9. Emr S., Glick B. S., Linstedt A. D., Lippincott-Schwartz J., Luini A., Malhotra V., Marsh B. J., Nakano A., Pfeffer S. R., Rabouille C., Rothman J. E., Warren G., Wieland F. T. (2009) Journeys through the Golgi–taking stock in a new era. J. Cell Biol. 187, 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glick B. S., Elston T., Oster G. (1997) A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett. 414, 177–181 [DOI] [PubMed] [Google Scholar]
- 11. Ungar D., Hughson F. M. (2003) SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 19, 493–517 [DOI] [PubMed] [Google Scholar]
- 12. Hutagalung A. H., Novick P. J. (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beard M., Satoh A., Shorter J., Warren G. (2005) A cryptic Rab1-binding site in the p115 tethering protein. J. Biol. Chem. 280, 25840–25848 [DOI] [PubMed] [Google Scholar]
- 14. Allan B. B., Moyer B. D., Balch W. E. (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444–448 [DOI] [PubMed] [Google Scholar]
- 15. Diao A., Frost L., Morohashi Y., Lowe M. (2008) Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. J. Biol. Chem. 283, 6957–6967 [DOI] [PubMed] [Google Scholar]
- 16. Fridmann-Sirkis Y., Siniossoglou S., Pelham H. R. (2004) TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol. 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamane J., Kubo A., Nakayama K., Yuba-Kubo A., Katsuno T., Tsukita S., Tsukita S. (2007) Functional involvement of TMF/ARA160 in Rab6-dependent retrograde membrane traffic. Exp. Cell Res. 313, 3472–3485 [DOI] [PubMed] [Google Scholar]
- 18. Sinka R., Gillingham A. K., Kondylis V., Munro S. (2008) Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J. Cell Biol. 183, 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Short B., Preisinger C., Körner R., Kopajtich R., Byron O., Barr F. A. (2001) A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J. Cell Biol. 155, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayes G. L., Brown F. C., Haas A. K., Nottingham R. M., Barr F. A., Pfeffer S. R. (2009) Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol. Biol. Cell 20, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosing M., Ossendorf E., Rak A., Barnekow A. (2007) Giantin interacts with both the small GTPase Rab6 and Rab1. Exp. Cell Res. 313, 2318–2325 [DOI] [PubMed] [Google Scholar]
- 22. Ungar D., Oka T., Brittle E. E., Vasile E., Lupashin V. V., Chatterton J. E., Heuser J. E., Krieger M., Waters M. G. (2002) Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. J. Cell Biol. 157, 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kingsley D. M., Kozarsky K. F., Segal M., Krieger M. (1986) Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid-linked carbohydrate chains. J. Cell Biol. 102, 1576–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whyte J. R., Munro S. (2001) The Sec34/35 Golgi Transport Complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 1, 527–537 [DOI] [PubMed] [Google Scholar]
- 25. Wu X., Steet R. A., Bohorov O., Bakker J., Newell J., Krieger M., Spaapen L., Kornfeld S., Freeze H. H. (2004) Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat. Med. 10, 518–523 [DOI] [PubMed] [Google Scholar]
- 26. Vasile E., Oka T., Ericsson M., Nakamura N., Krieger M. (2006) IntraGolgi distribution of the Conserved Oligomeric Golgi (COG) complex. Exp. Cell Res. 312, 3132–3141 [DOI] [PubMed] [Google Scholar]
- 27. Oka T., Ungar D., Hughson F. M., Krieger M. (2004) The COG and COPI complexes interact to control the abundance of GEARs, a subset of Golgi integral membrane proteins. Mol. Biol. Cell 15, 2423–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peanne R., Legrand D., Duvet S., Mir A. M., Matthijs G., Rohrer J., Foulquier F. (2011) Differential effects of lobe A and lobe B of the Conserved Oligomeric Golgi complex on the stability of {β}1,4-galactosyltransferase 1 and {α}2,6-sialyltransferase 1. Glycobiology 21, 864–876 [DOI] [PubMed] [Google Scholar]
- 29. Shestakova A., Zolov S., Lupashin V. (2006) COG Complex-Mediated Recycling of Golgi Glycosyltransferases is Essential for Normal Protein Glycosylation. Traffic 7, 191–204 [DOI] [PubMed] [Google Scholar]
- 30. Oka T., Vasile E., Penman M., Novina C. D., Dykxhoorn D. M., Ungar D., Hughson F. M., Krieger M. (2005) Genetic analysis of the subunit organization and function of the COG complex: Studies of Cog5 and Cog7 deficient mammalian cells. J. Biol. Chem. 280, 32736–32745 [DOI] [PubMed] [Google Scholar]
- 31. Ungar D., Oka T., Vasile E., Krieger M., Hughson F. M. (2005) Subunit map of the Conserved Oligomeric Golgi complex. J. Biol. Chem. 280, 32729–32735 [DOI] [PubMed] [Google Scholar]
- 32. Whyte J. R., Munro S. (2002) Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627–2637 [DOI] [PubMed] [Google Scholar]
- 33. Suvorova E. S., Duden R., Lupashin V. V. (2002) The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J. Cell Biol. 157, 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shestakova A., Suvorova E., Pavliv O., Khaidakova G., Lupashin V. (2007) Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J. Cell Biol. 179, 1179–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laufman O., Hong W., Lev S. (2011) The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport. J. Cell Biol. 194, 459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laufman O., Kedan A., Hong W., Lev S. (2009) Direct interaction between the COG complex and the SM protein, Sly1, is required for Golgi SNARE pairing. EMBO J. 28, 2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukuda M., Kanno E., Ishibashi K., Itoh T. (2008) Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol. Cell Proteomics 7, 1031–1042 [DOI] [PubMed] [Google Scholar]
- 38. Sohda M., Misumi Y., Yoshimura S., Nakamura N., Fusano T., Ogata S., Sakisaka S., Ikehara Y. (2007) The Interaction of Two Tethering Factors, p115 and COG complex, is Required for Golgi Integrity. Traffic 8, 270–284 [DOI] [PubMed] [Google Scholar]
- 39. Sohda M., Misumi Y., Yamamoto A., Nakamura N., Ogata S., Sakisaka S., Hirose S., Ikehara Y., Oda K. (2010) Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic 11, 1552–1566 [DOI] [PubMed] [Google Scholar]
- 40. Pokrovskaya I. D., Willett R., Smith R. D., Morelle W., Kudlyk T., Lupashin V. V. (2011) COG complex specifically regulates the maintenance of Golgi glycosylation machinery. Glycobiology 21, 1554–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guo W., Roth D., Walch-Solimena C., Novick P. (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haas A. K., Yoshimura S., Stephens D. J., Preisinger C., Fuchs E., Barr F. A. (2007) Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J. Cell Sci. 120, 2997–3010 [DOI] [PubMed] [Google Scholar]
- 43. Richardson B. C., Smith R. D., Ungar D., Nakamura A., Jeffrey P. D., Lupashin V. V., Hughson F. M. (2009) Structural basis for a human glycosylation disorder caused, in part, by mutation of the COG4 gene. Proc. Natl. Acad. Sci. U.S.A. 106, 13329–13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sengupta D., Truschel S., Bachert C., Linstedt A. D. (2009) Organelle tethering by a homotypic PDZ interaction underlies formation of the Golgi membrane network. J. Cell Biol. 186, 41–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willett R., Kudlyk T., Pokrovskaya I., Ungar D., Duden R., Lupashin V. (2012) COG complex forms spatial landmarks for distinct SNARE complexes. Nat. Commun., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Plutner H., Cox A. D., Pind S., Khosravi-Far R., Bourne J. R., Schwaninger R., Der C. J., Balch W. E. (1991) Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J. Cell Biol. 115, 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saraste J., Lahtinen U., Goud B. (1995) Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway. J. Cell Sci. 108, 1541–1552 [DOI] [PubMed] [Google Scholar]
- 48. Ali B. R., Wasmeier C., Lamoreux L., Strom M., Seabra M. C. (2004) Multiple regions contribute to membrane targeting of Rab GTPases. J. Cell Sci. 117, 6401–6412 [DOI] [PubMed] [Google Scholar]
- 49. Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. (1991) The small GTP-binding protein rab4 is associated with early endosomes. Proc. Natl. Acad. Sci. U.S.A. 88, 6313–6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Opdam F. J., Echard A., Croes H. J., van den Hurk J. A., van de Vorstenbosch R. A., Ginsel L. A., Goud B., Fransen J. A. (2000) The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J. Cell Sci. 113, 2725–2735 [DOI] [PubMed] [Google Scholar]
- 51. Goud B., Zahraoui A., Tavitian A., Saraste J. (1990) Small GTP-binding protein associated with Golgi cisternae. Nature 345, 553–556 [DOI] [PubMed] [Google Scholar]
- 52. Kelly E. E., Giordano F., Horgan C. P., Jollivet F., Raposo G., McCaffrey M. W. (2012) Rab30 is required for the morphological integrity of the Golgi apparatus. Biol. Cell 104, 84–101 [DOI] [PubMed] [Google Scholar]
- 53. Chen T., Han Y., Yang M., Zhang W., Li N., Wan T., Guo J., Cao X. (2003) Rab39, a novel Golgi-associated Rab GTPase from human dendritic cells involved in cellular endocytosis. Biochem. Biophys. Res. Commun. 303, 1114–1120 [DOI] [PubMed] [Google Scholar]
- 54. Haas A. K., Fuchs E., Kopajtich R., Barr F. A. (2005) A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 7, 887–893 [DOI] [PubMed] [Google Scholar]
- 55. Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T. E., Warren G. (1995) Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131, 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gillingham A. K., Pfeifer A. C., Munro S. (2002) CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin. Mol. Biol. Cell 13, 3761–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. (1991) Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell 64, 649–665 [DOI] [PubMed] [Google Scholar]
- 58. Beck R., Rawet M., Ravet M., Wieland F. T., Cassel D. (2009) The COPI system: molecular mechanisms and function. FEBS Lett. 583, 2701–2709 [DOI] [PubMed] [Google Scholar]
- 59. Lupas A., Van Dyke M., Stock J. (1991) Predicting coiled coils from protein sequences. Science 252, 1162–1164 [DOI] [PubMed] [Google Scholar]
- 60. Shibuya N., Goldstein I. J., Van Damme E. J., Peumans W. J. (1988) Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J. Biol. Chem. 263, 728–734 [PubMed] [Google Scholar]
- 61. Hughson F. M., Reinisch K. M. (2010) Structure and mechanism in membrane trafficking. Curr. Opin. Cell Biol. 22, 454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lerer-Goldshtein T., Bel S., Shpungin S., Pery E., Motro B., Goldstein R. S., Bar-Sheshet S. I., Breitbart H., Nir U. (2010) TMF/ARA160: A key regulator of sperm development. Dev. Biol. 348, 12–21 [DOI] [PubMed] [Google Scholar]
- 63. Farkas R. M., Giansanti M. G., Gatti M., Fuller M. T. (2003) The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Mol. Biol. Cell 14, 190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Belloni G., Sechi S., Riparbelli M. G., Fuller M. T., Callaini G., Giansanti M. G. (2012) Mutations in Cog7 affect Golgi structure, meiotic cytokinesis and sperm development during Drosophila spermatogenesis. J. Cell Sci. doi: 10.1242/jcs. 108878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lees J. A., Yip C. K., Walz T., Hughson F. M. (2010) Molecular organization of the COG vesicle tethering complex. Nat. Struct. Mol. Biol. 17, 1292–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. James P., Halladay J., Craig E. A. (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li G., Stahl P. D. (1993) Structure-function relationship of the small GTPase rab5. J. Biol. Chem. 268, 24475–24480 [PubMed] [Google Scholar]