Background: Vibrio cholerae, the etiologic agent of cholera, secretes outer membrane vesicles (OMVs) that are internalized into host cells.
Results: OMVs activate an inflammatory response in intestinal epithelial cells (ECs) via a NOD1-dependent pathway that activates dendritic cells (DCs) and promotes T cell polarization toward Th2/Th17 responses.
Conclusion: OMVs stimulate EC-DC cross-talk in generating an inflammatory response.
Significance: Findings are important for the development of efficient vaccine strategies with OMVs.
Keywords: Cellular Immune Response, Chemokines, Cytokine, Inflammation, Vesicles, Outer Membrane Vesicles, Vibrio cholerae, Dendritic Cells, Epithelial Cells, Immune Response
Abstract
Like other Gram-negative pathogens, Vibrio cholerae, the causative agent of the diarrheal disease cholera, secretes outer membrane vesicles (OMVs). OMVs are complex entities composed of a subset of envelope lipid and protein components and play a role in the delivery of effector molecules to host cells. We previously showed that V. cholerae O395 cells secrete OMVs that are internalized by host cells, but their role in pathogenesis has not been well elucidated. In the present study, we evaluated the interaction of OMVs with intestinal epithelial cells. These vesicles induced expression of proinflammatory cytokines such as IL-8 and GM-CSF and chemokines such as CCL2, CCL20, and thymic stromal lymphopoietin in epithelial cells through activation of MAPK and NF-κB pathways in NOD1-dependent manner. Epithelial cells stimulated with OMVs activated dendritic cells (DCs) in a direct co-culture system. Activated DCs expressed high levels of co-stimulatory molecules; released inflammatory cytokines IL-1β, IL-6, TNF-α, and IL-23 and chemokines CCL22 and CCL17; and subsequently primed CD4+ T cells leading to IL-4, IL-13, and IL-17 expression. These results suggest that V. cholerae O395 OMVs modulate the epithelial proinflammatory response and activate DCs, which promote T cell polarization toward an inflammatory Th2/Th17 response.
Introduction
Vibrio cholerae is a Gram-negative bacterium that causes the diarrheal disease cholera in humans. This organism colonizes human small intestine and produces a potent enterotoxin called cholera toxin, which is primarily responsible for the diarrheal syndromes (1). Cholera is considered a prototypical noninflammatory toxigenic diarrhea. However, acute V. cholerae O1 infection triggers mucosal innate immune responses with infiltration of neutrophils, degranulation of mast cells and eosinophils, and expression of proinflammatory cytokines (2). In addition, in vivo studies suggest significant up-regulation of proinflammatory molecules and inflammatory cells in the duodenal tissue of cholera patients (3). At the molecular level, the pathogenesis of cholera is a multifactorial process and involves several genes encoding virulence factors that aid the pathogen in its colonization, coordinated expression of virulence factors, and toxin action (4). Previously our laboratory has reported that in V. cholerae several bacterial factors are involved in up-regulation of cytokines that recruit different immune cells at the mucosal surface and trigger an inflammatory response (5, 6). We further characterized this strain and found that it produced outer membrane vesicles (OMVs)3 (7). OMVs produced by a wide variety of Gram-negative bacteria are spherical nanostructures (20–250 nm) and contain not only components of the outer membrane such as LPS, outer membrane proteins, and phospholipids but also periplasmic proteins and peptidoglycan given that OMVs can entrap parts of the underlying bacterial periplasm (8).Thus, OMVs are heterogeneous complexes of pathogen-associated molecular patterns (PAMPs) such as LPS, peptidoglycan, flagellin, and CpG DNA as well as other outer membrane proteins, virulence factors, or immunomodulatory compounds that are important for pathogenesis (9). Several studies have demonstrated that OMVs play a role as protective transport vesicles, delivering toxins, enzymes, and DNA to eukaryotic cells (8). Furthermore, different studies have provided evidence that OMVs have a potential role to trigger the innate inflammatory response. OMVs of Helicobacter pylori and Pseudomonas aeruginosa have been shown to elicit IL-8 production by epithelial cells (10, 11).
The pathogen recognition receptors (PRRs) in the host recognize a vast array of PAMPs, and the recognition of Gram-negative bacteria by PRRs is mediated primarily by Toll-like receptor (TLRs) (12). It has been reported that V. cholerae induces inflammatory response recognition through TLR5 (6) and TLR4 signaling pathways (13). In addition to TLRs that recognize bacterial ligands at the plasma membrane and luminal side of intracellular vesicles, a family of proteins called nucleotide-binding oligomerization domain (NOD), sense bacterial peptidoglycan (PG) in the cytosol. Upon recognition of its bacterial ligand, NOD induces activation of mitogen-activated protein kinase and NF-κB signaling pathways that are involved in cellular responses against bacteria by releasing cytokines and chemokines that recruit and activate immune inflammatory cells (14). It was recently revealed that OMVs from H. pylori, P. aeruginosa, and Neisseria gonorrhea harbor PG with NOD1 agonist activity (15). Thus, we speculated that this intracellular NOD receptor might play an important role in recognition of O395 OMV to induce an inflammatory response.
The intestinal dendritic cells (DCs) are located throughout intestinal lamina propria as immature cells, and DCs are recruited to the microbial site of infection by chemokines and induce an inflammatory response (16). Epithelial cells (ECs) were found to actively influence DC functions in bacterial handling across epithelial monolayers (17). Several reports have shown that bacteria have the ability to elicit a characteristic cytokine response in an in vitro co-culture model of epithelial cell and dendritic cell interaction (18). Upon activation by inflammatory mediators across EC monolayers, DCs undergo maturation, which is associated with high surface expression of co-stimulatory molecules as well as different secreted immunomodulatory cytokines that drives the naïve T cells into Th1 or Th2 cells. In addition to Th1 or Th2 cells, DCs have also been implicated in the promotion of Th17 cells. Th17 cells are a distinct population of CD4+ T cells that secrete IL-17 and have been associated with chronic inflammatory conditions such as rheumatoid arthritis (19) and multiple sclerosis (20). In addition, IL-17 proinflammatory function leading to IL-8 stimulation raises the possibility that IL-17 may play a role during bacterial infections (21). IL-23, a heterodimeric cytokine that consists of a p19 subunit specific for IL-23 and the p40 subunit of IL-12 (22), has been shown to play a significant role in the maintenance of Th17 cells (23). Recently, we have reported that V. cholerae stimulated EC-DC cross-talk and induced CD4+ T cells to differentiate into inflammatory Th2 cells (24). Several reports have been published about the inflammatory response of V. cholerae O395 strains, but it is not known how O395 OMVs interact with the host. Therefore, it is of interest to evaluate whether OMVs can modulate DC function through the epithelial barrier, which is important for the activation of T cells.
We previously reported that cholera toxin is associated with V. cholerae OMVs, which are internalized into intestinal epithelial cells (7). In this study, we demonstrated that OMVs induce an inflammatory response in intestinal epithelial cells in a NOD1-dependent manner and produce different cytokines and chemokines that recruit dendritic cells, leading subsequently to a predominant Th2 and Th17 response.
EXPERIMENTAL PROCEDURES
Bacterial Strain and Growth Conditions
The streptomycin-resistant V. cholerae O395 (O1 classical, Ogawa, cholera toxin-positive) strain used in the present study was maintained at −70 °C in Luria-Bertani medium containing 20% (v/v) glycerol and grown in LB medium with 1 mg/ml streptomycin (Sigma-Aldrich) at 37 °C.
Epithelial Cell Culture
Int407, HEK293, and HT29 cells (National Center for Cell Sciences, Pune, India) were grown in high glucose Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) containing penicillin/streptomycin and gentamycin in the presence of 5% CO2 at 37 °C. Cells were seeded and maintained in T-25 tissue culture flasks (Falcon, BD Biosciences). Medium was removed from confluent cell layers, cells were washed with PBS, fresh medium without antibiotic was added, and cells were incubated with V. cholerae O395 OMV (50 μg/ml) or with 50 μg/ml iE-DAP (γ-d-glutamyl-meso-diaminopimelic acid; InvivoGen) as a cognate elicitor of NOD1 for the stipulated time periods. Cells designated as non-infected controls were also replenished with fresh medium.
Isolation of Outer Membrane Vesicles
The vesicles were isolated from late exponential phase (8 h) V. cholerae cultures using the procedure described earlier (7). Briefly, cells from a 1-liter culture were harvested by centrifugation (4500 × g, 15 min, 4 °C), and the supernatant was filtered through 0.45-μm-pore size membrane (Millipore) to remove residual cells. An aliquot of the filtrate was tested for the presence of viable V. cholerae cells on LB agar. In all cases, no colonies were detected. Protease inhibitor mixture (Sigma-Aldrich) was then added to the filtrate to prevent protein degradation, and the filtrate was kept at 4 °C. Vesicles were recovered by ultracentrifugation (140,000 × g, 4 h, 4 °C) using a Sorvall T-865 rotor, washed with 0.1 m phosphate-buffered saline (PBS), and suspended in PBS. The protein concentration was determined using Bradford reagent (Bio-Rad). OMVs were stored at −80 °C and used within a week.
Cytotoxicity Assay by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT)
Int407, HEK293, and HT29 cells were seeded in 96-well plates and cultured as described above until confluence. Cells were then incubated with various concentrations of OMV and incubated in the presence of 5% CO2 at 37 °C for 48 h. Medium was removed, and the cells were washed with PBS followed by addition of fresh medium without antibiotic. For the MTT (Sigma) assay, MTT reagent was added to each well of a 96-well plate containing treated or untreated cells and incubated at 37 °C in 5% CO2 for 3 h. The reaction was stopped with the addition of the solubilization reagent DMSO (Sigma-Aldrich). The absorbance of the samples was then measured with an ELISA plate reader (EMax molecular filter plate) at 550 nm.
RNA Extraction and cDNA Preparation
Total RNA was harvested from cells using an Rneasy Mini kit (Qiagen Inc., Valencia, CA). For cDNA preparation, 5 μg of RNA was treated with RNase-free DNase (Invitrogen) in a 10-μl volume according to the manufacturer's protocol. For the reverse transcription reaction, 2 μl of DNase-treated RNA was reverse transcribed using the SuperScriptTM First-Strand Synthesis System (Invitrogen) with 0.50 μg of oligo(dT)12–18 in a total volume of 20 μl. The cDNA synthesis was done at 42 °C for 50 min following the manufacturer's instructions.
Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
In semiquantitative RT-PCRs, about 2 μl of cDNA was PCR-amplified in a 30-μl reaction volume containing 10 mm Tris-HCl (pH 8.3), 50 mm KCl, 1.5–2.5 mm MgCl2, a 0.26 mm concentration of each dNTP, and 25 pmol of each primer as described earlier (5). The annealing temperature and product size of all the genes are listed in Table 1. For each cytokine and chemokine gene, the primers were designed using Primer3 software. To determine the cytokine and chemokine gene expression, reactions were heat-denatured for 5 min at 95 °C and then amplified with 35 PCR cycles, each comprising successive incubations at 95 °C for 30 s and annealing at 52–72 °C (given for each respective gene in Table 1) for 1 min and at 72 °C for 30 s. A further extension step was done at 72 °C for 7 min. PCRs were normalized by analysis of expression of the gene encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Amplicons were identified by ethidium bromide staining of agarose gels.
TABLE 1.
Primer sequences used in RT-PCR for the genes mentioned below
| Gene (protein) | Primers | Product size | Annealing temperature |
|---|---|---|---|
| bp | °C | ||
| IL8 (IL-8) | F 5′-ATGACTTCCAAGCTGGCCGTGGCT-3 | 289 | 60 |
| R 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ | |||
| CSF2 (GM-CSF) | F 5′-ACACTGCTGAGATGAATGAAACAGTAG-3′ | 286 | 55 |
| R 5′-TGGACTGGCTCCCAGCAGTCAAAGGGGATG-3′ | |||
| CCL2 (CCL2) | F 5′-CCCCAGTCACCTGCTGTTAT-3′ | 173 | 55 |
| R 5′-TGGAATCCTGAACCCACTTC-3′ | |||
| CCL20 (CCL20) | F 5′-GCAAGCAACTTTGACTGCT-3′ | 150 | 54 |
| R 5′-ATTTGCGCACACAGACAACT-3′ | |||
| TSLP (TSLP) | F 5′-CCCAGGCTATTCGGAAACTCAG-3′ | 116 | 52 |
| R 5′-CGCCACAATCCTTGTAATTGTG-3′ | |||
| CCL3 (CCL3) | F 5′-TGCAACCAGTTCTCTGCATC-3′ | 198 | 55 |
| R 5′-TTTCTGGACCCACTCCTCAC-3′ | |||
| CCL4 (CCL4) | F 5′-AAGCTCTGCGTGACTGTCC-3′ | 211 | 56 |
| R 5′-GCTTGCTTCTTTTGGTTTGG-3′ | |||
| CCL19 (CCL19) | F 5′-ATCCCTGGGTACATCGTGAG-3′ | 172 | 56 |
| R 5′-GCTTCATCTTGGCTGAGGTC-3′ | |||
| IL6 (IL-6) | F 5′-CCTTCCAAAGATGGCTGAAA-3′ | 230 | 60 |
| R 5′-CAGGGGTGGTTATTGCATCT-3′ | |||
| IL1B (IL-1β) | F 5′-AAACAGATGAAGTGCTCCTTCCAGG-3′ | 388 | 60 |
| R 5′-TGGAGAACACCACTTGTTGCTCCA-3′ | |||
| TNFA (TNF-α) | F 5′-CGGGACGTGGAGCTGGCCGAGGAG-3′ | 355 | 72 |
| R 5′-CACCAGCTGGTTATCTCTCAGCTC-3′ | |||
| CCL17 (CCL17) | F 5′-CTTCTCTGCAGCACATCCAC-3′ | 163 | 57 |
| R 5′-CTGCCCTGCACAGTTACAAA-3′ | |||
| CCL22 (CCL22) | F 5′-ACTGCACTCCTGGTTGTCCT-3′ | 217 | 52 |
| R 5′-CGGCACAGATCTCCTTATCC-3′ | |||
| IL12B (IL-12p40) | F 5′-ATGTCGTAGAATTGGATTGGTATCCG-3′ | 358 | 65 |
| R 5′-GTACTGATTGTCGTCAGCCACCAGC-3′ | |||
| IL23A (IL-12p19) | F 5′-TTCCCCATATCCAGTGTGGAG-3′ | 151 | 55 |
| R 5′-TCAGGGAGCAGAGAAGGCTC-3′ | |||
| IL12A (IL-12p35) | F 5′-TTCACCACTCCCAAAACCTGC-3′ | 225 | 58 |
| R 5′-GAGGCCAGGCAACTCCCATTA G-3′ | |||
| IL17A (IL-17) | F 5′-ACCAATCCCAAAAGGTCCTC-3′ | 280 | 58 |
| R 5′-GGGGACAGAGTTCATGTGGT-3′ | |||
| NOD1 (NOD1) | F 5′-TCCAAAGCCAAACAGAAACTC-3′ | 180 | 60 |
| R 5′-CAGCATCCAGATGAACGTG-3′ | |||
| NOD2 (NOD2) | F 5′-GAAGTACATCCGCACCGAG-3′ | 174 | 60 |
| R 5′-GACACCATCCATCCATGAGAAGACAG-3′ | |||
| GAPDH (GAPDH) | F 5′-ATGGGGAAGGTGAAGGTCGG-3′ | 450 | 55 |
| R 5′-GGATGCTAAGCAGTTGGT-3′ |
Small Interfering RNA (siRNA) Knockdown
Gene silencing of NOD1 by siRNA was performed by transfection of siRNA (Santa Cruz Biotechnology) according to the manufacturer's instructions using Lipofectamine 2000 (Invitrogen). HEK293 cells were seeded in 24-well plates at 24 h prior to siRNA transfection. At 72 h after siRNA treatment, cells were stimulated as indicated.
Reporter Assays
For transient transfection, HEK293 cells were seeded at a density of 70% without fetal bovine serum and antibiotics. The following day, cells were transfected with 1 μg of plasmid DNA containing NF-κB-driven (nuclear factor) luciferase reporter gene (Stratagene) using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. At 48 h post-transfection, cells were stimulated for 3 h with O395 OMV. The results are expressed as NF-κB-luciferase units. The cells were then lysed in luciferase lysis buffer (Promega, Madison, WI), and luminescence was measured as relative luciferase units in a luminometer (Junior LB9505, Berthold, Germany).
Generation of DCs
Peripheral blood mononuclear cells were isolated by density gradient centrifugation through Ficoll-Hypaque. Cells were washed twice with sterile PBS and transferred to 24-well tissue culture plates coated with human IgG at a concentration of 3–5 × 105cells/ml. Following 2 h of incubation, non-adherent cells were flushed away by extensive washing with PBS. To obtain immature DCs, adherent monocytes were incubated in RPMI 1640 medium containing 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; BD Pharmingen) and 20 ng/ml IL-4 (BD Pharmingen) at 37 °C in 5% CO2 for 6 days. Morphology and surface phenotype of monocyte-derived DCs were monitored by microscopy and flow cytometry.
Dendritic Cell/Epithelial Cell Co-culture
Direct System
Epithelial cell monolayers of HT29 cells (50 μl; 3–4 × 105 cells) were seeded in the Transwell filter inserts (3-μm-diameter pores; Millipore), which were placed in 24-well plates containing 1 ml of medium. Monolayers were maintained for 5–7 days (changing medium every other day) until a transepithelial resistance reading of 300 ohms/cm2 (measured using a Millicell ERS volt meter; Millipore) was achieved. Filters were turned upside down, and DCs (4 × 105) were seeded on the filter facing the basolateral membrane of epithelial cells for 4 h to let the cells attach to the filter. Filters were then turned upside down again into 24-well plates. At this point, the cells were kept in antibiotic-free medium. The Transwell insert filters were either left unstimulated or stimulated directly with OMV. Following 12 h of incubation, medium was changed to one containing antibiotics. DCs and culture supernatants were collected after 24 h from the bottom chamber. DCs were detached from filters by gentle centrifugation and analyzed by cytofluorometry for surface activation markers. Cytokines were measured in culture supernatants by enzyme-linked immunosorbent assay.
Indirect System
Medium from EC monolayers was removed, and cells were washed with PBS and kept in fresh medium without antibiotic. Some wells were stimulated with O395 OMV from the apical surface (top chamber), and the rest were left unstimulated as controls. After 12 h, OMVs were washed out, and the medium was changed to one containing antibiotics in all the wells including controls. Culture supernatants were collected after 12 h from the lower chamber (facing the basolateral membrane) and used to activate DCs. DCs were incubated for 24 h in the culture supernatant and analyzed phenotypically for expression of surface activation markers. Analysis of cytokines released by DCs was performed by measuring culture supernatants before and after DC incubation.
Western Blot Analysis
OMV-incubated or unincubated epithelial cells were lysed by lysis buffer (100 μl; 1.5 m Tris-HCl (pH 6.8), 10% SDS, 10% glycerol, and 1% bromphenol blue). Before loading, samples were boiled (100 °C,10 min)with β-mercaptoethanol (Sigma-Aldrich), and then samples were subjected to SDS-PAGE with prestained protein molecular weight markers (10 μl; Genei, India), semidry electrotransferred (Bio-Rad) onto a nitrocellulose membrane, and blocked with 5% BSA. The primary antibodies used were rabbit phospho-p44/42 (ERK1/2) MAPK monoclonal antibody, p44/p42 MAPK antibody, phospho-p38 antibody, p38 antibody, phospho-SAPK/JNK, and IκBα (1:1000 dilution; Cell Signaling Technology) and mouse β-actin (1:5000 dilution; Sigma) in TBS-Tween 20 buffer containing 5% BSA. Then the membrane was washed with TBS-Tween 20 followed by incubation with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Genei) or rabbit anti-mouse immunoglobulin G (Genei) at 1:2000 dilutions. The alkaline phosphatase-positive bands were visualized in a developing solution (1× 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Genei), 1.5 mm Tris-HCl (pH 8.8), and water) in the dark at room temperature for 10 min.
Flow Cytometry
DCs were harvested and washed three times in cold FACS buffer (PBS, 3% FCS, and 0.02% NaN3) and then stained with appropriate antibodies (anti-CD80, -HLA-DR, and -CD83) for 30 min on ice in the dark. After proper washing, cells were fixed in 4% paraformaldehyde and analyzed by flow cytometry with appropriate isotype control antibodies (FACSCalibur, BD Biosciences).
DC-T Cell Co-cultures
DCs conditioned with O395 OMV-stimulated or unstimulated EC supernatant were collected after 24 h and washed twice to remove any cytokine. Allogenic CD4+CD45RA+ naïve T cells were isolated from peripheral blood mononuclear cells (MACS isolation system, Miltenyi Biotec, Auburn, CA). DCs were then co-cultured with 5 × 104 freshly purified T cells in round bottomed 96-well culture plates in a ratio of 1:5 (DC/T cell) in the presence or absence of neutralizing antibody IL-23p19 (10 μg/ml; eBioscience). After 5 days of culture, cells and supernatants were collected for RNA extraction and cytokine analyses, respectively.
ELISA
IL-8, GM-CSF, CCL2, CCL20, thymic stromal lymphopoietin (TSLP), IL-6, IL-1β, IFN-γ, IL-13, IL-4, TNF-α, and IL-17 concentrations were determined by commercially available ELISA kits (R&D Systems).
Statistical Analysis
The data from RT-PCR, ELISA, and Western blot were recorded as means ± S.D. from at least three different experiments. Comparison between two groups was analyzed by Student's t test. Differences were considered significant at p ≤ 0.05. The semiquantitative RT-PCR and Western blots were quantified by using the image analysis software ImageJ. The integrated density of each band was normalized to the corresponding GAPDH band in the case of RT-PCR. In the case of Western blot, the integrated density of each band was normalized to the corresponding β-actin or control antibody (unphosphorylated).
RESULTS
Proinflammatory Response Is Induced by V. cholerae O395 OMV in NOD1-dependent Manner
Recent work in our laboratory has shown that cholera toxin is associated with V. cholerae O395 OMVs, which are internalized into intestinal epithelial cells (7).The ability of OMVs to internalize into intestinal epithelial cells suggests direct interaction with the host cells. To further elucidate the mechanism of OMV-host interaction, we evaluated the inflammatory response of epithelial cells to V. cholerae O395 OMVs.
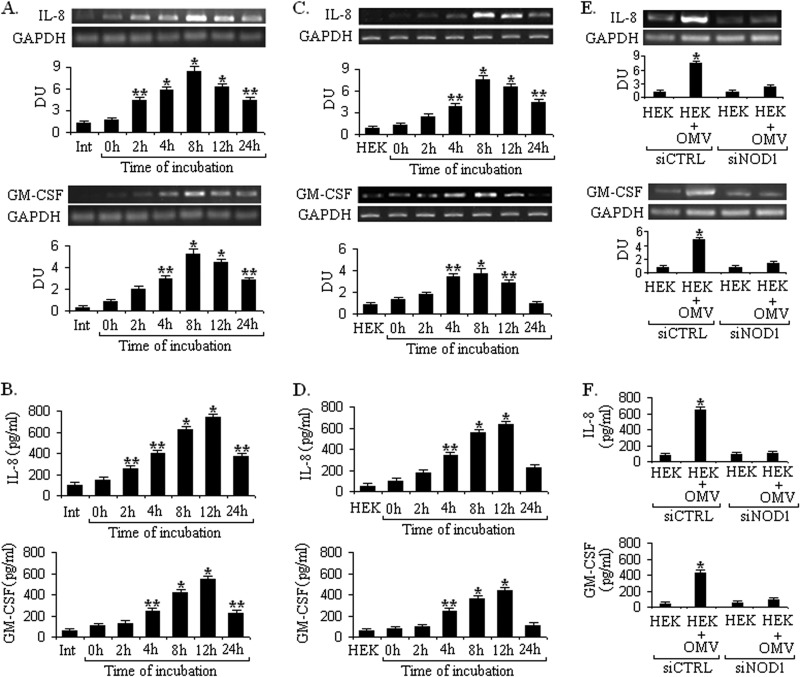
To investigate the inflammatory response, OMVs were isolated from V. cholerae O395, and nontoxic effective doses of OMVs were determined by the MTT assay in Int407 and HEK293 epithelial cell lines (supplemental Fig. S1i). OMVs (50 μg/ml) were co-incubated with the intestinal epithelial cell line Int407 for different time points (0, 2, 4, 6, 8, 12, and 24 h); subsequently the expression of cytokines was assayed by RT-PCR and ELISA. Int407 cells were chosen as a well recognized model for host-V. cholerae interaction specifically for studying the inflammatory response (5). It was observed that OMVs significantly induced the expression of IL-8, a chemoattractant g neutrophils, and proinflammatory GM-CSF, a strong chemoattractant for neutrophils and eosinophils, as compared with nonstimulated control cells, and mRNA expression of both IL-8 and GM-CSF reached a maximum at 8 h and then declined at later time points (Fig. 1A). To assess whether the mRNA level of these cytokines correlated with protein levels, we studied the protein expression from the same set of experiments by ELISA. Protein expression of both IL-8 and GM-CSF reached a maximum after12 h (Fig. 1B), suggesting that OMVs are strong inducers of the inflammatory response in epithelial cells. Similar results were also observed using HEK293 epithelial cells (Fig. 1, C and D).
FIGURE 1.
V. cholerae OMV-induced proinflammatory response relies on NOD1. Int407 and HEK293 cells were stimulated with or without V. cholerae O395 OMV. The kinetics (0, 2, 4, 8,12, and 24 h) of IL8 and GM-CSF expression and secretion in Int407 and HEK293 cells were analyzed by RT-PCR (A and C) and ELISA (B and D), respectively, following O395 OMV stimulation. To check NOD1-dependent cytokine expression, HEK293 cells transfected (72 h) with control (CTRL) and NOD1 siRNAs were either left unstimulated (control) or stimulated with OMV. IL-8 and GM-CSF cytokine production was analyzed at mRNA and protein levels by RT-PCR (E) and ELISA (F), respectively. Densitometric quantitation in densitometry units (DU) for each cytokine is shown below the representative agarose gel sections after normalization to GAPDH. Data represent mean and S.D. (error bars) of three independent experiments performed under similar conditions. **, p ≤ 0.05 and *, p ≤ 0.001 compared with cells without OMV.
OMVs are associated with a mixture of different PAMPs such as LPS and peptidoglycan, and these have been shown to elicit the host immune response to pathogenic bacteria (15, 25). The expression of TLR4 and TLR2 is restricted in the intestinal epithelial cells (26), and recent evidence suggests that peptidoglycan is sensed by intracellularly localized nod-like receptor family proteins. NOD1 and NOD2 are well characterized PG sensors in the cytosolic compartment that induce the innate immune response. Thus, we evaluated NOD1 and NOD2 expression in HEK293 epithelial cells during stimulation with OMV (supplemental Fig. S1ii). HEK293 cells were chosen as this epithelial cell line is easy to transfect and does not express most PRRs such as TLRs (27, 28). It was observed that cytosolic PRR NOD1 was significantly up-regulated in HEK293 epithelial cells during OMV stimulation. In contrast, the expression of another PRR, NOD2, was constitutive in nature and not up-regulated upon OMV treatment (supplemental Fig. S1ii). Therefore, we sought to investigate the production of proinflammatory cytokines in response to O395 OMV stimulation in a NOD1-dependent manner. To confirm the critical involvement of NOD1 in epithelial cell activation by O395 OMVs, we used NOD1-specific siRNA to inhibit the expression of endogenous NOD1.Treatment with siRNA to NOD1 markedly down-regulated the proinflammatory cytokines IL-8 and GM-CSF at the mRNA (Fig. 1E) and protein levels (Fig. 1F) in contrast to cells transfected with control siRNA, indicating that NOD1 is necessary to induce the innate immune response by O395 OMV. To check the functional activity of NOD1, HEK293 cells were incubated with NOD1 cognate elicitor iE-DAP, and IL-8 and GM-CSF expression was measured at the protein level (supplemental Fig. S1iii, A). This IL-8 and GM-CSF response was reduced by siRNA treatment, similar to the profile seen after infection with V. cholerae OMV (supplemental Fig. S1iii, B), showing that NOD1 function is inhibited by specific siRNA knockdown of NOD1 transcripts. Collectively these data indicate that O395 OMV induce the proinflammatory response in a NOD1-dependent manner.
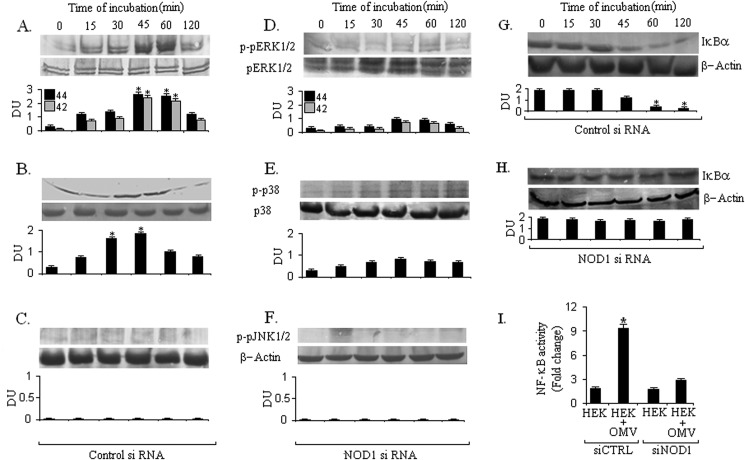
V. cholerae OMVs Activate MAPK and NF-κB Pathways in Epithelial Cells in NOD1-dependent Manner
Previous studies have reported that rapid MAPK activation occurs within intestinal epithelial cells in response to V. cholerae stimulation (29). Therefore, we evaluated whether V. cholerae O395 OMV could induce NOD1-dependent MAPK phosphorylation. To clarify the association of NOD1 with MAPK activation during OMV stimulation, HEK293 cells were transfected with either a control siRNA or a NOD1-targeting siRNA and then stimulated with O395 OMV. We performed Western blot analysis of cell lysate with antibodies specific for the phosphorylated and nonphosphorylated forms of ERK1/2, p38, and JNK1/2 for different time points. Our results demonstrated that in control siRNA-treated cells ERK1/2 and p38 were maximally phosphorylated at 45 and 30 min, respectively, during OMV stimulation (Fig. 2, A and B). In contrast, JNK1/2 showed weak activation or no phosphorylation in HEK293 cells during OMV stimulation (Fig. 2C). However, parallel stimulation of siNOD1 cells resulted in significantly reduced ERK1/2 and p38 phosphorylation at those time points, and no change was observed for JNK1/2 phosphorylation (Fig. 2, D–F). Thus, our results strongly suggest an involvement of NOD1 in V. cholerae O395 OMV-induced activation of the MAPK signaling pathway. To determine whether V. cholerae OMVs activate NF-κB in human epithelial cells in a NOD1-dependent manner, we analyzed the levels of IκBα by immunoblot. In the canonical NF-κB activation pathway, nuclear translocation of NFκB is preceded by phosphorylation and subsequent degradation of IκBα. Stimulation of epithelial cells with OMV caused significant degradation of IκBα at 60 min and later time points leading to NF-κB activation. Degradation of IκBα was not observed when cells were transfected with NOD1 siRNA (Fig. 2, G and H). To further confirm the activation of the NF-κB pathway, we measured NF-κB-dependent promoter activity by using a luciferase reporter plasmid in HEK293 cells transfected with control siRNA or with NOD1 siRNA and stimulated with OMV. The reporter assay for NF-κB showed that the luciferase activity increased in response to OMV stimulation in control siRNA-treated cells as compared with NOD1 siRNA-treated cells (Fig. 2I). Thus, these data confirm our finding that the NF-κB pathway as well as the MAPK pathway is activated by OMV in a NOD1-dependent manner.
FIGURE 2.
NOD1 is required for MAPK and NF-κB activation during V. cholerae OMV stimulation. HEK293 cells transfected (72 h) with control (CTRL) and NOD1 siRNA were left unstimulated or stimulated with V. cholerae OMV for 0, 15, 30, 45, 60, and 120 min; samples were separated by SDS-PAGE; and proteins were blotted on PVDF membrane. The blots were probed with anti-phospho-ERK1/2 (A and D), anti-phospho-p38 (B and E), anti-phospho-JNK1/2 (C and F), and IκBα (G and H). Normalization of the phospho-ERK1/2 (pERK1/2), phospho-p38 (p-p38), phospho-JNK1/2 (pJNK1/2), and IκBα was carried out with non-phosphorylated ERK1/2, p38, and β-actin, respectively. These experiments were performed thrice, and the Western blots show representative data from a single experiment. Densitometric analysis was performed with all three independent experiments. DU represents the ratio of densitometry units. Error bars represent S.D. *, p ≤ 0.001 compared with cells without OMV. To check NF-κB-luciferase reporter activity, HEK293 cells were transfected (72 h) with control and NOD1 siRNAs and then transfected with NF-κB-luciferase reporter plasmid as described under “Experimental Procedures.” After an additional 24 -h incubation, the co-transfected cells were stimulated with O395 OMV for 3 h. Luciferase activity was expressed as relative NF-κB activity (I). *, p ≤ 0.001 compared with control siRNA-treated cells.
V. cholerae O395 OMVs Recruit and Activate Dendritic Cells to Induce a Distinct Profile of Cytokine and Chemokine Secretion
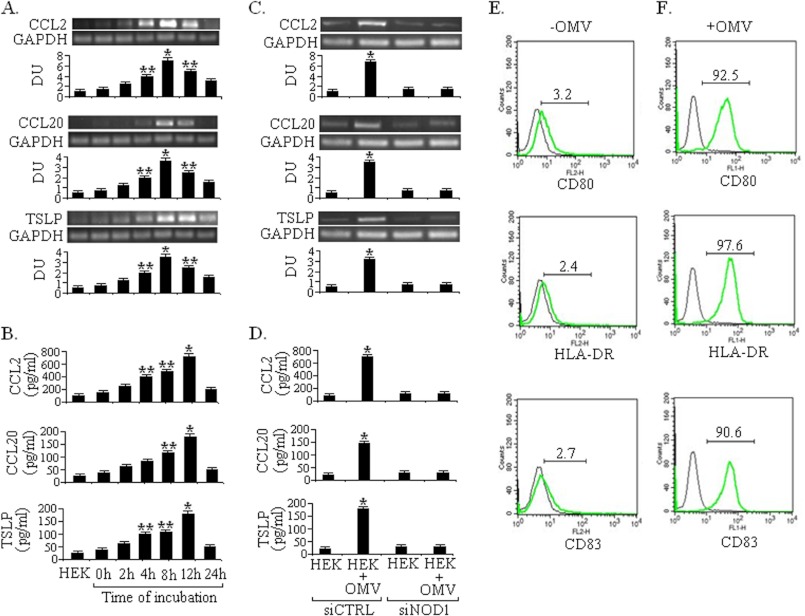
Previously we have demonstrated that TSLP, CCL2, and CCL20 released from epithelial cells in response to V. cholerae infection actively influence dendritic cells in initiating the inflammatory response (24). Therefore, we examined the potential contribution of V. cholerae OMVs in stimulating EC-DC cross-talk. First, we examined chemokine expression in HEK293 cells during O395 OMV stimulation as chemokines recruit immune cells such as neutrophils, monocytes, natural killer cells, and DCs to sites of infection and are constitutively expressed or released during inflammation. Different chemokines such as CCL2 and monocyte chemoattractant protein 1 that attract monocytes, CCL20 that recruits immature DCs, and TSLP that activates DCs were up-regulated at both mRNA and protein levels, respectively, during OMV stimulation (Fig. 3, A and B). Expression of these chemokines was abrogated when cells were transfected with NOD1 siRNA (Fig. 3, C and D). The expression of some other inflammatory chemokines such as CCL3 (macrophage inflammatory protein MIP-1α), CCL4 (MIP-1β), and CCL19 (MIP-3β) was not observed. Similar proinflammatory chemokine profiles were observed at the protein level in HEK293 cells after stimulation with iE-DAP (supplemental Fig. S1iii, C and D).We further examined the chemokine mRNA expression in HT29 cells, which can form a polarized monolayer in vitro, stimulated with V. cholerae O395 OMVs. Nontoxic effective doses of OMVs were determined by MTT assay in the HT29 cell line (supplemental Fig. S1i). As in HEK293 cells, CCL2, CCL20, and TSLP expression was up-regulated during OMV stimulation (50 μg/ml) of HT-29 cells (data not shown).
FIGURE 3.
V. cholerae OMV activates EC to produce chemokines in NOD1-dependent manner and OMV-exposed ECs activate DCs. HEK293 cell lines were cultured and incubated with V. cholerae O395 OMV. Total RNA was extracted, and mRNA expression of CCL2, CCL20, and TSLP chemokines was analyzed by RT-PCR (A) for different time points (0, 2, 4, 8, 12, and 24 h) with and without OMV incubation. Under similar conditions, secretion of CCL2, CCL20, and TSLP was evaluated by sandwich ELISA (B). To check NOD1-dependent chemokine expression, HEK293 cells transfected (72 h) with control (CTRL) or NOD1 siRNAs were left unstimulated or stimulated with OMV. The above mentioned chemokines were analyzed at mRNA and protein levels by RT-PCR (C) and ELISA (D), respectively. Densitometric quantitations in densitometry units (DU) for each of the chemokines are shown below the representative agarose gel sections after normalization to GAPDH. Data represent mean and S.D. (error bars) of three independent experiments performed under similar conditions. **, p ≤ 0.05 and *, p ≤ 0.001 compared with cells without OMV incubation. Shown is flow cytometric determination of surface phenotypes of DCs after staining with isotype-matched control mAbs (black lines) or with the mAbs indicated (green lines). Cell surface expression of CD80, HLA-DR, and CD83 on unstimulated (control) DCs (E) or cells that had been stimulated for 24 h with ECs induced with OMV (F) was assessed. The numbers in each histogram correspond to the mean fluorescence intensity of mAb staining. The figure represents one of three independent experiments.
We next tested whether these inflammatory mediators recruit and activate dendrite cells because these antigen-presenting cells are present in close contact with intestinal epithelial cells and play an active role in bacterial handling across mucosal surfaces (17, 18). We evaluated the quality of DC preparation by microscopy (data not shown) and staining with fluorescently labeled DC-specific cell surface markers such as HLA-DR, CD80, and CD83 (Fig. 3E). In the direct co-culture system, the upper Transwell chamber or the apical side of the filter was seeded with HT29 ECs, and the basolateral side of ECs was seeded with DCs and incubated for 4 h. The apical side of the EC monolayer was then stimulated with O395 OMVs for 12 h. Then DCs were detached, and the expression of cell surface co-stimulatory molecules HLA-DR, CD80, and CD83 was measured with flow cytometry. Compared with control DCs, OMV exposed EC-DC co-culture showed increased expression of all three surface molecules as indicated by increased fluorescence intensity of DCs (Fig. 3F), suggesting DC activation. In the case of indirect co-culture, DC activation markers were comparable when incubated with O395 OMV-stimulated or unstimulated EC supernatants.
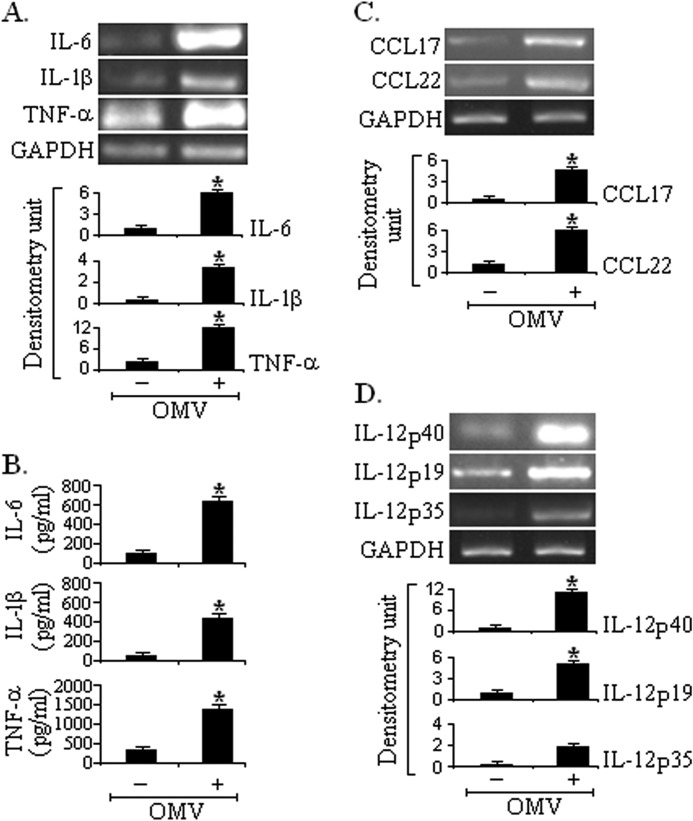
Activated DCs produce proinflammatory cytokines and chemokines, which have potential effects on CD4+ T cell polarization toward either Th1, Th2, or Th17 cells. In the direct co-culture system, proinflammatory cytokines IL-1β, IL-6, and TNF-α were up-regulated at mRNA (Fig. 4A) and protein levels (Fig. 4B). The OMV-stimulated EC-DC co-culture showed enhanced production of the mRNA for the chemokines CCL17 (also known as thymus- and activation-regulated chemokine) and CCL22 (also known as macrophage-derived chemokine) (Fig. 4C). Thymus- and activation-regulated chemokine and macrophage-derived chemokine preferentially attract Th2 cells.
FIGURE 4.
OMV-exposed ECs modulate the production of cytokines and chemokines from DCs. Monocyte-derived DCs were conditioned with only HT29 ECs or OMV-exposed ECs. After 24 h of incubation, cytokine production (IL-6, IL-1β, and TNF-α) was measured at mRNA and protein levels by RT-PCR (A) and ELISA (B), respectively. CCL17 and CCL22 mRNA expression was evaluated by RT-PCR (C) in OMV-exposed EC-DC direct co-culture. Under similar conditions, mRNA expression of IL-12/IL-23 p40, IL-23p19, and IL-12p35 was determined by RT-PCR (D). Densitometric quantitations in densitometry units for each cytokine and chemokine are shown below the representative agarose gel sections after normalization to GAPDH. Data represent mean and S.D. (error bars) of three independent experiments performed under similar conditions. *, p ≤ 0.001compared with DCs conditioned with only ECs.
Next we evaluated whether DCs conditioned with ECs stimulated with OMVs produce IL-23 or not because it has been reported that IL-23 positively regulates IL-17 production by promoting Th17 cell population, survival, and expansion. We analyzed IL-23/IL-12 mRNA expression from DCs conditioned with ECs exposed to V. cholerae O395 OMVs. As shown in Fig. 4D, significantly increased expression of IL-23/p19 and IL-12/IL-23p40 mRNA but not of IL-12/p35 was observed in OMV-stimulated EC-DC co-culture. Collectively, these data show that OMV stimulation via the intestinal epithelial barrier stimulates an increase in the expression of DC surface co-stimulatory molecules, which are indicators of DC maturation and induce a proinflammatory response by secreting different cytokines and chemokines that activate Th2/Th17 pathways.
V. cholerae O395 OMV-exposed EC-activated DCs Skew T Cell Priming to a Th2/Th17 Response
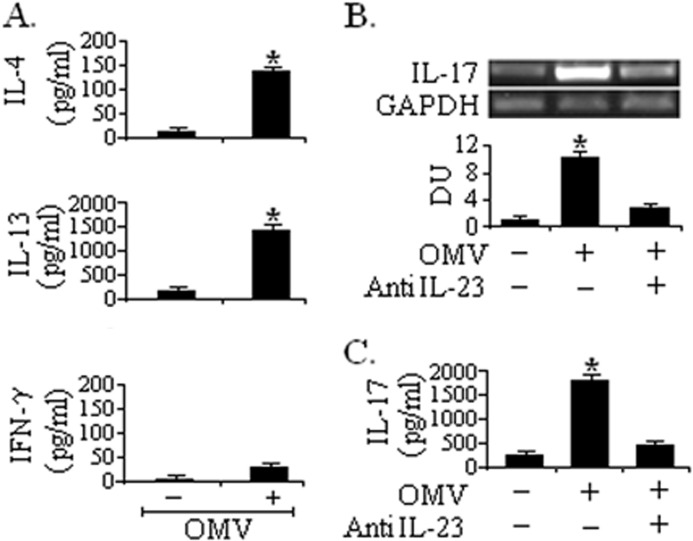
To further confirm whether different inflammatory mediators (CCL17 and CCL22) and IL-23 production can influence Th2/Th17 pathways, we purified naïve CD4+CD45RA+ T cells from peripheral blood and then cultured them with DCs conditioned with only ECs or OMV-exposed ECs for 5 days. An ELISA study showed that stimulation of DCs with OMV-challenged ECs suppressed the IFN-γ release from the naïve T cell population and resulted in an increased release of IL-4 and IL-13 compared with DCs stimulated with ECs alone (Fig. 5A). Under the same condition, IL-17 expression was confirmed by RT-PCR and ELISA. Detection of RNA following 24 h co-culture showed that OMV-stimulated EC-conditioned DCs induced significant levels of IL-17 expression (Fig. 5B) in CD4+ T cells compared with unstimulated DCs. ELISA results confirmed the secretion of IL-17 at the protein level (Fig. 5C). The expression was detectable at 5 days.
FIGURE 5.
Cytokine production by CD4+ T cells co-cultured with DCs. Monocyte-derived DCs were conditioned with only ECs or OMV-exposed ECs. After 24 h of incubation, DCs were co-cultured with naïve CD4+ T cells for 5 days, and production of T cell-derived cytokine IL-4, IL-13, and IFN-γ secretion was determined by ELISA (A). DCs were co-cultured with naïve CD4+ T cells in the presence or absence of neutralizing antibody IL-23p19, and IL-17 expression at mRNA and protein levels were evaluated by RT-PCR (B) and ELISA (C), respectively. Densitometric quantitations in densitometry units (DU) for IL-17 cytokine are shown below the representative agarose gel sections after normalization to GAPDH. Data represent mean and S.D. (error bars) of three independent experiments performed under similar conditions. *, p ≤ 0.001 compared with T cells incubated with DCs conditioned with only ECs.
To study the influence of IL-23 cytokine on IL-17 expression in this system, we co-cultured OMV-stimulated EC-conditioned DCs with CD4+ T cells in the presence of IL-23-neutralizing antibodies. It was observed that anti-IL-23p19 caused a significant decrease in IL-17 production at both RNA and protein levels (Fig. 5, B and C).
Collectively these data demonstrated that DCs conditioned with ECs exposed to O395 OMV could cause a shift to a clear Th2 and Th17 response in the naïve T cell populations and that IL-23 is the most potent cytokine involved in V. cholerae OMV-mediated IL-17 production.
DISCUSSION
The present study demonstrates that OMVs from V. cholerae O395 induce an innate immune response in intestinal epithelial cells by activating signaling cascades that culminate in the secretion of proinflammatory mediators including a DC-attracting chemokine via a NOD1-mediated pathway. Our results also suggest that DCs conditioned by V. cholerae OMV-treated epithelial cells express a high level of co-stimulatory molecules and produce proinflammatory cytokines and chemokines that ultimately promote the Th2/Th17 inflammatory response.
Intestinal epithelial cells recognize and respond to an array of bacterial products such as LPS, lipoprotein, PG, and flagellin commonly referred to as PAMPs (30). We have reported previously that V. cholerae flagellin, the structural component of flagellum, elicits a strong inflammatory response in intestinal epithelial cell via TLR5 (6). Apart from the TLR-mediated pathway, other pathogens such as Shigella flexneri, Escherichia coli, Listeria monocytogenes, and H. pylori trigger a proinflammatory response in epithelial cells via a NOD1-mediated pathway (31–33). NOD1 acts as an intracellular receptor responsible for the recognition of Gram-negative bacterial PG (14) and initiates an innate immune response. OMVs released by Gram-negative bacteria contain different PAMPs like PG, lipoproteins, etc. and can deliver PG to host cytosolic NOD1 (15). Therefore, OMVs represent a new bacterial factor by which both invasive and noninvasive bacteria initiate inflammatory processes in host cells. Our data suggest that V. cholerae OMVs also represent a mechanism to deliver bacterial PG to the host cytosol. Stimulation of intestinal epithelial cells with OMVs induces a significant inflammatory response as evidenced by production of cytokines and chemokines that diminished when cells were transfected with siRNA to NOD1. It has been reported that H. pylori-, P. aeruginosa-, and N. gonorrhea-derived OMVs also deliver PG to the cytosol and induce activation of NOD1 upon contact with the host (15). NOD2 does not seem to be critically involved in epithelial cell activation by OMV because RT-PCR analysis showed very little NOD2 up-regulation in intestinal epithelial cells upon stimulation with O395 OMV.
NOD1 has been reported to be an important regulator of innate immune responses to bacterial pathogens or their secreted products like OMVs (33). Although research has mainly focused on the role of NOD1 in activating the NF-κB-mediated proinflammatory response, in this study, we showed for the first time that NOD1 is critical not only for NF-κB activation but also for the activation of mitogen-activated protein kinase during stimulation with V. cholerae OMVs. We observed that V. cholerae O395 OMVs induced ERK1/2 and p38 phosphorylation at 45 and 30 min, respectively, but that JNK1/2 was not phosphorylated at any of the time points. In addition, ERK1/2 and p38 phosphorylation was diminished when cells were transfected with NOD1 siRNA. Additionally we showed that NOD1 is required for NF-κB activation as siRNA to NOD1 inhibited IκBα degradation as well as abrogated luciferase activity of NF-κB during V. cholerae OMV stimulation. Incidentally the transcription of proinflammatory genes is induced by the activation of NF-κB and MAPK pathways in epithelial cells following V. cholerae infection (5, 6).
Chemokines are the major mediators of DC migration (34). We explored the possibility that intestinal epithelial cell-derived chemokines during OMV stimulation could affect immature dendritic cell migration and their subsequent maturation. In an attempt to examine the role of V. cholerae O395 OMVs in DC activation in a biologically relevant setting, we used an in vitro co-culture system that essentially consisted of HT29 cells differentiated into apical and basolateral sides co-cultured with human blood-derived DCs in the presence of OMVs. The co-culture system allows identification of the contribution of EC-derived factors or bacterial products in the activation of DCs. Two possible scenarios were implemented. In one situation, DCs were incubated with supernatants of OMV-stimulated ECs (indirect co-culture), whereas in the other, the interaction of DCs with ECs and OMVs was studied directly across the monolayer of ECs (direct co-culture). DC activation was indicated by an increased number of cells expressing high levels of the activation markers HLA-DR, CD80, and CD83. Interestingly, unlike V. cholerae (24), DCs are better activated by direct EC-DC-OMV co-culture compared with indirect co-culture (by conditioning of EC supernatant). OMVs internalized by ECs (7) mediate a better response in direct cell-cell interaction.
Upon activation by signals released from the microorganisms or from infected tissues, human myeloid DCs undergo maturation into T cell-stimulatory effector DCs. Various evidence indicates that the nature of cytokine secretion by DCs dictates the polarization of Th cell responses toward Th1, Th2, or Th17. High IL-23 and IL-1β favor IL-17 production from Th cells (35), whereas high IL-12p70 favors IFN-γ-producing Th1 cells, and DCs that do not produce IL-12 drive the development of IL-4-producing Th2 cells (36). IL-23, a member of the IL-12 family participating in a variety of inflammatory disorders, has been proposed to trigger IL-17 production through the induction of a novel subset of CD4+ Th17 cells. It is reported that IL-23 enhances antigen-induced activation of both Th2 cells and Th17 cells during the effector phase of allergic airway inflammation and thereby increases Th2 cell-mediated eosinophil recruitment and Th17 cell-mediated neutrophil recruitment into the airways (37). Our data indicate that IL-23-specific p19 mRNA was up-regulated as compared with IL-12-specific p35 subunit in the DCs conditioned with ECs exposed to OMV. Other proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokines (CCL17 and CCL22) that attract Th2-type CD4+ T cells were also detected from DCs conditioned with ECs challenged with OMV.
IL-1β is a central orchestrator of immunity against various classes of pathogens and a key trigger of inflammatory diseases (38). IL-23, especially in synergy with IL-1β, plays an essential role in the induction or expansion of Th17 cells. OMV-exposed EC-stimulated DCs co-cultured with naïve CD4+ T cells sustained their differentiation toward Th17 cells as well as IL-4- and IL-13-producing Th2 cells. Our study shows that V. cholerae OMV-stimulated EC-DC co-culture subsequently activates CD4+ T cells and induces IL-17 production at both mRNA and protein levels, and IL-4 and IL-13 production at the protein level thus activates Th2/Th17 pathways. IL-17 production was abrogated in the presence of neutralizing IL-23p19 antibody at both mRNA and protein levels. These data indicate that IL-23 is essential in the regulation of IL-17 production in response to V. cholerae OMV infection. IL-17 induces the expression of a variety of cytokines and chemokines such as IL-6, IL-8, and GM-CSF from epithelial cells, vascular endothelial cells, and fibroblasts (39, 40), resulting in the induction of inflammation. Therefore, further study is needed to dissect the role of Th17 cells in the V. cholerae OMV-associated inflammatory response.
Collectively these data indicate that OMVs elicit a strong immunomodulatory response by the host apart from the whole bacterium. This study represents the first report linking the OMV of an enteric pathogen with DC activation and cytokine production through intestinal epithelial cells in a NOD1-dependent manner. Furthermore, NOD1 activation has been linked to the induction of adaptive immune responses (41). Our study shows that OMV-activated DCs prime CD4+ T cells, which promote Th2/Th17 pathways. Understanding the mechanisms of O395 OMV-mediated signaling in innate and adaptive immunity could provide new therapeutic approaches for preventing V. cholerae OMV-induced inflammation and new prospects in mucosal vaccination.
Supplementary Material
This work was supported in part by the Council of Scientific and Industrial Research (CSIR), Government of India.

This article contains supplemental Fig. S1.
- OMV
- outer membrane vesicle
- EC
- epithelial cell
- DC
- dendritic cell
- TSLP
- thymic stromal lymphopoietin
- Th
- T helper
- PAMP
- pathogen-associated molecular pattern
- PRR
- pathogen recognition receptor
- TLR
- Toll-like receptor
- NOD
- nucleotide-binding oligomerization domain
- PG
- peptidoglycan
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MIP
- macrophage inflammatory protein.
REFERENCES
- 1. Nelson E. J., Harris J. B., Morris J. G., Jr., Calderwood S. B., Camilli A. (2009) Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 7, 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qadri F., Bhuiyan T. R., Dutta K. K., Raqib R., Alam M. S., Alam N. H., Svennerholm A. M., Mathan M. M. (2004) Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut 53, 62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qadri F., Raqib R., Ahmed F., Rahman T., Wenneras C., Das S. K., Alam N. H., Mathan M. M., Svennerholm A. M. (2002) Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9, 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sánchez J., Holmgren J. (2005) Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea. Curr. Opin. Immunol. 17, 388–398 [DOI] [PubMed] [Google Scholar]
- 5. Bandyopadhaya A., Sarkar M., Chaudhuri K. (2007) Human intestinal epithelial cell cytokine mRNA responses mediated by NF-κB are modulated by the motility and adhesion process of Vibrio cholerae. Int. J. Biochem. Cell Biol. 39, 1863–1876 [DOI] [PubMed] [Google Scholar]
- 6. Bandyopadhaya A., Sarkar M., Chaudhuri K. (2008) IL-1β expression in Int407 is induced by flagellin of Vibrio cholerae through TLR5 mediated pathway. Microb. Pathog. 44, 524–536 [DOI] [PubMed] [Google Scholar]
- 7. Chatterjee D., Chaudhuri K. (2011) Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 585, 1357–1362 [DOI] [PubMed] [Google Scholar]
- 8. Ellis T. N., Kuehn M. J. (2010) Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kulp A., Kuehn M. J. (2010) Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ismail S., Hampton M. B., Keenan J. I. (2003) Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect. Immun. 71, 5670–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bauman S. J., Kuehn M. J. (2006) Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8, 2400–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeda K., Kaisho T., Akira S. (2003) Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 [DOI] [PubMed] [Google Scholar]
- 13. Matson J. S., Yoo H. J., Hakansson K., Dirita V. J. (2010) Polymyxin B resistance in El Tor Vibrio cholerae requires lipid acylation catalyzed by MsbB. J. Bacteriol. 192, 2044–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fritz J. H., Ferrero R. L., Philpott D. J., Girardin S. E. (2006) Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 7, 1250–1257 [DOI] [PubMed] [Google Scholar]
- 15. Kaparakis M., Turnbull L., Carneiro L., Firth S., Coleman H. A., Parkington H. C., Le Bourhis L., Karrar A., Viala J., Mak J., Hutton M. L., Davies J. K., Crack P. J., Hertzog P. J., Philpott D. J., Girardin S. E., Whitchurch C. B., Ferrero R. L. (2010) Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 12, 372–385 [DOI] [PubMed] [Google Scholar]
- 16. Coombes J. L., Powrie F. (2008) Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 8, 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rimoldi M., Chieppa M., Larghi P., Vulcano M., Allavena P., Rescigno M. (2005) Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood 106, 2818–2826 [DOI] [PubMed] [Google Scholar]
- 18. Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G. M., Nespoli A., Viale G., Allavena P., Rescigno M. (2005) Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6, 507–514 [DOI] [PubMed] [Google Scholar]
- 19. Chabaud M., Durand J. M., Buchs N., Fossiez F., Page G., Frappart L., Miossec P. (1999) Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42, 963–970 [DOI] [PubMed] [Google Scholar]
- 20. Kurasawa K., Hirose K., Sano H., Endo H., Shinkai H., Nawata Y., Takabayashi K., Iwamoto I. (2000) Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 43, 2455–2463 [DOI] [PubMed] [Google Scholar]
- 21. Luzza F., Parrello T., Monteleone G., Sebkova L., Romano M., Zarrilli R., Imeneo M., Pallone F. (2000) Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J. Immunol. 165, 5332–5337 [DOI] [PubMed] [Google Scholar]
- 22. Bettelli E., Kuchroo V. K. (2005) IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J. Exp. Med. 201, 169–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKenzie B. S., Kastelein R. A., Cua D. J. (2006) Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27, 17–23 [DOI] [PubMed] [Google Scholar]
- 24. Bhowmick S., Chatterjee D., Chaudhuri K. (2012) Human epithelial cells stimulated with Vibrio cholerae produce thymic stromal lymphopoietin and promote dendritic cell-mediated inflammatory Th2 response. Int. J. Biochem. Cell Biol. 44, 1779–1790 [DOI] [PubMed] [Google Scholar]
- 25. Ellis T. N., Leiman S. A., Kuehn M. J. (2010) Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 78, 3822–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naumann M. (2000) Nuclear factor-κB activation and innate immune response in microbial pathogen infection. Biochem. Pharmacol. 60, 1109–1114 [DOI] [PubMed] [Google Scholar]
- 27. Maeda S., Akanuma M., Mitsuno Y., Hirata Y., Ogura K., Yoshida H., Shiratori Y., Omata M. (2001) Distinct mechanism of Helicobacter pylori-mediated NF-κB activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 276, 44856–44864 [DOI] [PubMed] [Google Scholar]
- 28. Girardin S. E., Boneca I. G., Carneiro L. A., Antignac A., Jéhanno M., Viala J., Tedin K., Taha M. K., Labigne A., Zähringer U., Coyle A. J., DiStefano P. S., Bertin J., Sansonetti P. J., Philpott D. J. (2003) Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300, 1584–1587 [DOI] [PubMed] [Google Scholar]
- 29. Bandyopadhaya A., Das D., Chaudhuri K. (2009) Involvement of intracellular signaling cascades in inflammatory responses in human intestinal epithelial cells following Vibrio cholerae infection. Mol. Immunol. 46, 1129–1139 [DOI] [PubMed] [Google Scholar]
- 30. Sansonetti P. J. (2004) War and peace at mucosal surfaces. Nat. Rev. Immunol. 4, 953–964 [DOI] [PubMed] [Google Scholar]
- 31. Girardin S. E., Tournebize R., Mavris M., Page A. L., Li X., Stark G. R., Bertin J., DiStefano P. S., Yaniv M., Sansonetti P. J., Philpott D. J. (2001) CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2, 736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Opitz B., Püschel A., Beermann W., Hocke A. C., Förster S., Schmeck B., van Laak V., Chakraborty T., Suttorp N., Hippenstiel S. (2006) Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J. Immunol. 176, 484–490 [DOI] [PubMed] [Google Scholar]
- 33. Allison C. C., Kufer T. A., Kremmer E., Kaparakis M., Ferrero R. L. (2009) Helicobacter pylori induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J. Immunol. 183, 8099–8109 [DOI] [PubMed] [Google Scholar]
- 34. Gunn M. D. (2003) Chemokine mediated control of dendritic cell migration and function. Semin. Immunol. 15, 271–276 [DOI] [PubMed] [Google Scholar]
- 35. Wilson N. J., Boniface K., Chan J. R., McKenzie B. S., Blumenschein W. M., Mattson J. D., Basham B., Smith K., Chen T., Morel F., Lecron J. C., Kastelein R. A., Cua D. J., McClanahan T. K., Bowman E. P., de Waal Malefyt R. (2007) Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957 [DOI] [PubMed] [Google Scholar]
- 36. de Jong E. C., Vieira P. L., Kalinski P., Schuitemaker J. H., Tanaka Y., Wierenga E. A., Yazdanbakhsh M., Kapsenberg M. L. (2002) Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 37. Wakashin H., Hirose K., Maezawa Y., Kagami S., Suto A., Watanabe N., Saito Y., Hatano M., Tokuhisa T., Iwakura Y., Puccetti P., Iwamoto I., Nakajima H. (2008) IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 178, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 38. Church L. D., Cook G. P., McDermott M. F. (2008) Primer: inflammasomes and interleukin 1β in inflammatory disorders. Nat. Clin. Pract. Rheumatol. 4, 34–42 [DOI] [PubMed] [Google Scholar]
- 39. Kawaguchi M., Adachi M., Oda N., Kokubu F., Huang S. K. (2004) IL-17 cytokine family. J. Allergy Clin. Immunol. 114, 1265–1273 [DOI] [PubMed] [Google Scholar]
- 40. Moseley T. A., Haudenschild D. R., Rose L., Reddi A. H. (2003) Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 [DOI] [PubMed] [Google Scholar]
- 41. Fritz J. H., Le Bourhis L., Sellge G., Magalhaes J. G., Fsihi H., Kufer T. A., Collins C., Viala J., Ferrero R. L., Girardin S. E., Philpott D. J. (2007) Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity 26, 445–459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.