Background: The in vivo function of the adenocarcinoma-associated gene, Agr2, is not known in the stomach.
Results: Agr2 KO mice displayed hyperplasia and defective cell maturation in the stomach.
Conclusion: Agr2 expression promotes cell lineage differentiation and results in a negative feedback for cell proliferation.
Significance: Agr2 functions in regulating homeostasis between differentiating and proliferating cells in the stomach.
Keywords: Cancer Biology, Cell Differentiation, Cell Proliferation, Development, Gastric Cancer, Gene Knockout, Intestinal Epithelium, Stem Cells
Abstract
Recent studies of epithelial tissues have revealed the presence of tissue-specific stem cells that are able to establish multiple cell lineages within an organ. The stem cells give rise to progenitors that replicate before differentiating into specific cell lineages. The mechanism by which homeostasis is established between proliferating stem or progenitor cells and terminally differentiated cells is unclear. This study demonstrates that Agr2 expression by mucous neck cells in the stomach promotes the differentiation of multiple cell lineages while also inhibiting the proliferation of stem or progenitor cells. When Agr2 expression is absent, gastric mucous neck cells increased in number as does the number of proliferating cells. Agr2 expression loss also resulted in the decline of terminally differentiated cells, which was supplanted by cells that exhibited nuclear SOX9 labeling. Sox9 expression has been associated with progenitor and stem cells. Similar effects of the Agr2 null on cell proliferation in the intestine were also observed. Agr2 consequently serves to maintain the balance between proliferating and differentiated epithelial cells.
Introduction
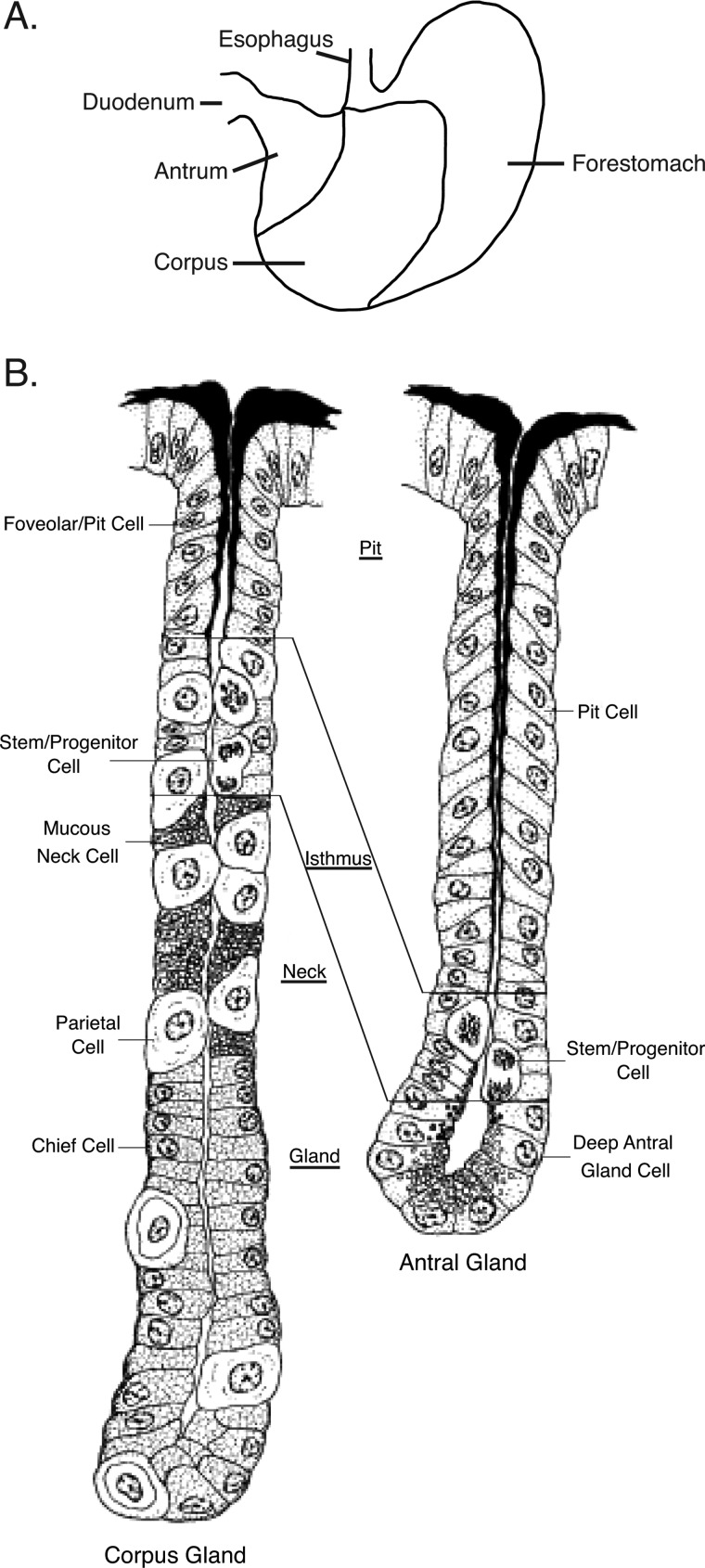
The stomach functions to store, mechanically breakdown, and enzymatically digest food. It secretes acid that inactivates microbes and produces specialized secretory products such as mucins, pepsinogen, intrinsic factor, gastrin, and ghrelin. An assortment of cell types are specifically distributed throughout the organ to fulfill these multiple functions (1). The proximal third of the murine stomach, the forestomach (Fig. 1A), is lined by a stratified squamous epithelium and serves to store food (1). The lower two-thirds of the stomach represents the glandular stomach, which is divided into two sections, the corpus at the proximal end and the antrum at the distal end (Fig. 1A). The corpus encompasses gastric glands that contain different cell types and are divided into regions extending from the stomach lumen to the gland base. The pit layer contains pit cells (also known as foveolar cells) that line the superior aspect of each gastric gland and secretes mucin (Fig. 1B). Below the pit layer is the isthmus, where putative stem and progenitor cells that give rise to all gastric cell lineages reside (2–6). Differentiating cells from the isthmus migrate superiorly and inferiorly within the gastric gland to their final destinations. Below the isthmus is the neck layer containing mucous neck cells, which are precursors for the chief cells situated at the gland base that secrete pepsinogen and intrinsic factor (7–9). Mucous neck cells specifically express trefoil factor 2 (TFF2) and a secreted protein that binds the lectin Griffonia simplicifolia II (GSII)2 (10–13). Acid-secreting parietal cells are distributed throughout the gastric gland below the pit layer, and the chief cells reside at the gland base. The distal half of the glandular stomach is the antrum, which contains mucous glands (Fig. 1B). Proliferating cells allegedly representing stem and progenitor cells in the antrum reside in the isthmus region just above the base of the antral gland. Mucin-secreting cells reside superiorly and inferiorly to the antral isthmus. Throughout the glandular stomach are isolated enteroendocrine cells that secrete a variety of products such as gastrin, ghrelin, somatostatin, and gastric inhibitory peptide.
FIGURE 1.
Schematic of murine stomach anatomy and histology. A, gross anatomy of the murine stomach is shown. The corpus and antrum comprise the glandular stomach. B, shown is a normal glandular structure showing the distribution of cell lineages in the corpus and antral glands. The figure is adapted from Lee (58). The material is reproduced with permission of John Wiley & Sons, Inc., New York.
Stem cells within the isthmus of the corpus and antrum contribute to all cell lineages within the glandular stomach. The means by which homeostasis is maintained between terminally differentiated cells and stem or progenitor cells are not known. In this study, the phenotype produced by an Agr2 null mouse provides insights into its role in maintaining homeostasis.
Anterior gradient 2 (Agr2) is a gene that is widely expressed in human adenocarcinomas of the breast, lung, colon, pancreas, esophagus, ovary, prostate, and stomach (14–22). Agr2 is highly conserved among vertebrates from amphibians to humans. The Xenopus laevis homologue of Agr2, XAG2, participates in the development of the cement gland and forebrain (23, 24). Agr2 expression in salamanders serves an important role in nerve-dependent limb regeneration (25). Several studies have also demonstrated that Agr2 supports many of the transformed properties of adenocarcinoma cell lines (26, 27). Potential mechanisms include activation of the Hippo signaling pathway through the co-activator Yap1 and the induced expression of the EGF receptor ligand amphiregulin (28).
The present study explored the in vivo function of Agr2 by generating a null mouse. The resultant mouse expressed a phenotype that was most pronounced in the stomach and was consistent with a role for Agr2 in regulating cell proliferation, differentiation, and homeostasis among the different cell lineages in the mouse glandular stomach.
EXPERIMENTAL PROCEDURES
Generation of the Agr2 Null Mouse Model
The mouse, B6129S5-Agr2tm1Lex, in which exons 2 to 5 were flanked by LoxP sites, was originally generated using 129SvEvBrd-derived embryonic stem cells and bred on a 129/SvEv-C57BL/6 background. The mouse was obtained from Lexicon Pharmaceuticals (catalog no. LEXKO-2300). Exon 2 contains the start codon for AGR2 protein. Agr2 null mice were produced by breeding the B6129S5-Agr2tm1Lex mouse with another that constitutively expresses Cre recombinase, TgCMV-Cre (B6.C-Tg(CMV-Cre)1Cgn/J, (The Jackson Laboratory, Bar Harbor, ME). Homozygous Agr2−/− mice (Agr2 KO) were generated by breeding heterozygous Agr2LoxP/WT;TgCMV-Cre. Female homozygous Agr2−/− bred poorly, which necessitated the heterozygous breeding.
A conditional Agr2 null (Agr2 KO) mouse in which excision of the floxed Agr2 exons was achieved in adult mice after tamoxifen administration was generated by breeding the conditional B6129S5-Agr2tm1Lex mice with a CreERT2 mouse (Gt(ROSA)26Sortm1(cre/ERT2)Tyj (The Jackson Laboratory). Tamoxifen was administered to 8-week Agr2LoxP/LoxP;CMV-CreERT2 mice by intraperitoneal injection at a dose of 75 mg/kg body weight for 5 consecutive days. The mice were then kept for an additional 21 days without tamoxifen before they were sacrificed. Controls consisted of Agr2LoxP/LoxP and Agr2+/+ mice treated with tamoxifen in a similar manner. It should be noted that tamoxifen has been described to induce parietal cell apoptosis and an increase in gastric proliferation that is reversible after cessation of the drug (29). Experiments were performed that determined that proliferation returns to wild-type levels by 21 days after the last tamoxifen administration.
The care and use of animals was performed under the auspices of Stanford's Institutional Animal Care and Use Committee as approved under Stanford University's Animal Welfare Assurance (A3213-01).
Antibodies and Probes for Immunohistochemistry
Antibodies were kindly provided by the following individuals: anti-TFF2 by Lars Thim (Novo Nordisk A/S, Maløv, Denmark) (30); anti-gastric intrinsic factor by David Alpers (Washington University, St. Louis, MO); anti-ATP4A by Michael Caplan (Yale University, New Haven, CT). Other antibodies employed included: anti-AGR2 (Imgenex, San Diego, CA); anti-Ki-67 (catalog #M7249, DAKO, Carpinteria, CA ); anti-SOX9 (EMD Millipore, Billerica, MA); anti-MUC5AC (catalogue #MS-145-P0, Thermo Fisher Scientific, Kalamazoo, MI). The lectin, GSII, was obtained from Vector Laboratories, Inc. (Burlingame, CA).
Labeling of proliferating cells was achieved by the intraperitoneal injection of the nucleotide analog, 5-ethynyl-2′-deoxyuridine (EdU) at a dose of 10 μg/g of mouse body weight 2 h before sacrificing the mice (31). Visualization of the incorporated EdU was achieved with the Click-iT® EdU Alexa Fluor® 488 Imaging kit (Invitrogen).
Immunohistochemistry
Slides were deparaffinized by immersing in xylene twice for 5 min each and hydrated by immersing for 2 min each in a series of 100, 80, and 50% ethanol and finally in distilled H2O. Slides for histological analysis were stained with hematoxylin and eosin by standard methods, with generally 3–4 sections reviewed per specimen. For immunofluorescence or immunohistochemistry, antigen retrieval was performed in a pressure cooker set to 118 °C for 3 min and removed at 90 °C in antigen unmasking solution (DAKO) followed by equilibration at room temperature for 1 h. Endogenous peroxidase activity was then blocked with freshly made 1.5% H2O2 for 30 min followed by washing in PBS (pH 7.4). The slides were placed in 5% serum blocking solution (goat, horse, or rabbit serum as appropriate) for 30 min to block nonspecific binding of antibody to the tissue. The sections were incubated with primary antibody diluted in 2% serum overnight at 4 °C. The dilutions used for each primary antibody used were AGR2 (1:250), synaptophysin (1:500), TFF2 (1:1000), gastric intrinsic factor (1:100), MUC5AC (1:500), Sox9 (1:1000), and Ki-67 (1:50). The respective secondary antibodies were used at a dilution of 1:300. Staining was visualized using Vectastain ABC kit (Vector Laboratories) for immunohistochemistry. Slides were counterstained with Gill No. 3 hematoxylin. Immunofluorescence slides were mounted with Vectashield mounting media containing DAPI (Vector Laboratories). Labeling with biotinylated GSII (Vector Laboratories) was performed at 10 μg/ml after blocking for 30 min with Carbo-free solution (Vector Laboratories).
DNA Microarray
Total RNA was extracted from fresh whole mouse stomachs using TRIzol reagent (Invitrogen). The RNA was reverse-transcribed, labeled, and hybridized to mouse oligonucleotide DNA microarrays (MouseRef-8 v2 Expression BeadChip, Illumina, San Diego, CA). Each array was normalized using the GenePattern software suite Version 3.3.3 (32, 33). The microarray data were deposited in the NCBI GEO database with accession number GSE40062.
RNA Extraction and qRT-PCR
Total RNA was isolated from tissues using TRIzol® reagent (Invitrogen). The glandular stomach was divided into the corpus and antrum, and 3 μg of total RNA from each was used to make cDNA. First-strand cDNAs were synthesized using Superscript II reverse transcriptase (Invitrogen) with random hexamer primers. Quantitative RT-PCR reactions were performed using IQ SYBR Green Supermix and the iCycler iQTM detection system (Bio-Rad). The primers used include: Actb, 5-GGCTGTATTCCCCTCCATCG-3, 5-CCAGTTGGTAACAATGCCATGT-3; Atp4a, 5-ATCTGCCTCATTGCCTTTGCCATC-3, 5-GTGACCACAACCACAGCAATGAGT-3; Atp4b, 5-ACTACTGTTGGAACCCGGACACT-3, 5-ATAGATGCACAAGGCAAAGAGCCC-3; Chga, 5-TGCTGAAGGAACTTCAAGACCTGG-3, 5-ATCCTCAAAGCTGCTGTGTTGCTG-3; Chgb, 5-CCAAGTCCAGTGTTCCAACGATCA-3, 5-CTGGGTCTCTTAGCAACCGTACTT-3; Gast, 5-TGGAACAGCGCCAGTTCAACAA-3, 5-TTCTTCTTCCTCCATTCGTGGCCT-3; Ghrl, 5-AGGAATCCAAGAAGCCACCAGCTA-3, 5-ATGCCAACATCGAAGGGAGCATTG-3; Gif, 5-CAGCATCCTGATTGCCATGAACCT-3, 5-ACACTTTACTTCCAGGGTCTCTGC-3; Herpud1, 5-ACCGCAGTTGGAGTGTGAGT-3, 5-TGATCCAACAGCAGCTTCCCAGAA-3; Hspa5, 5-AGACTGCTGAGGCGTATTTGGGAA-3, 5-AGCATCTTTGGTTGCTTGTCGCTG-3; Muc5ac, 5-TGGAAGGCAGTACACAGTACATGG-3, 5-TGGAAGGCAGTACACAGTACATGG-3; Pgc, 5-AAACCGGCATCATGAAGTGGATGG-3, 5-TTGTTCCTTCATGGTCTCCCGGAT-3; Reg1, 5-AGGAAGCTGAAGAAGACCTGCCAT-3, 5-AGAACTGACACCAGGTAGCCTGAA-3, Reg3b, 5-GCTCAATAGCGCTGAGGCTTCATT-3, 5-CTTGACAAGCTGCCACAGAAAGCA-3; Reg3g, 5-TGCCTATGGCTCCTATTGCTATGC-3, 5-CCACTGAGCACAGACACAAGATGT-3; Sox9, 5-CCACATTCCTCCTCCGGCAT-3, 5-TCGCTTCAGATCAACTTTGCCAGC-3; Sst, 5-GCTCTGCATCGTCCTGGCTTT-3, 5-AGTACTTGGCCAGTTCCTGTTTCC-3; Tff2, 5-ACCTGATCTTTGAAGTGCCCTGGT-3, 5-AAACTTTCTTCTGGCTTGCAGCTCCC-3.
RESULTS
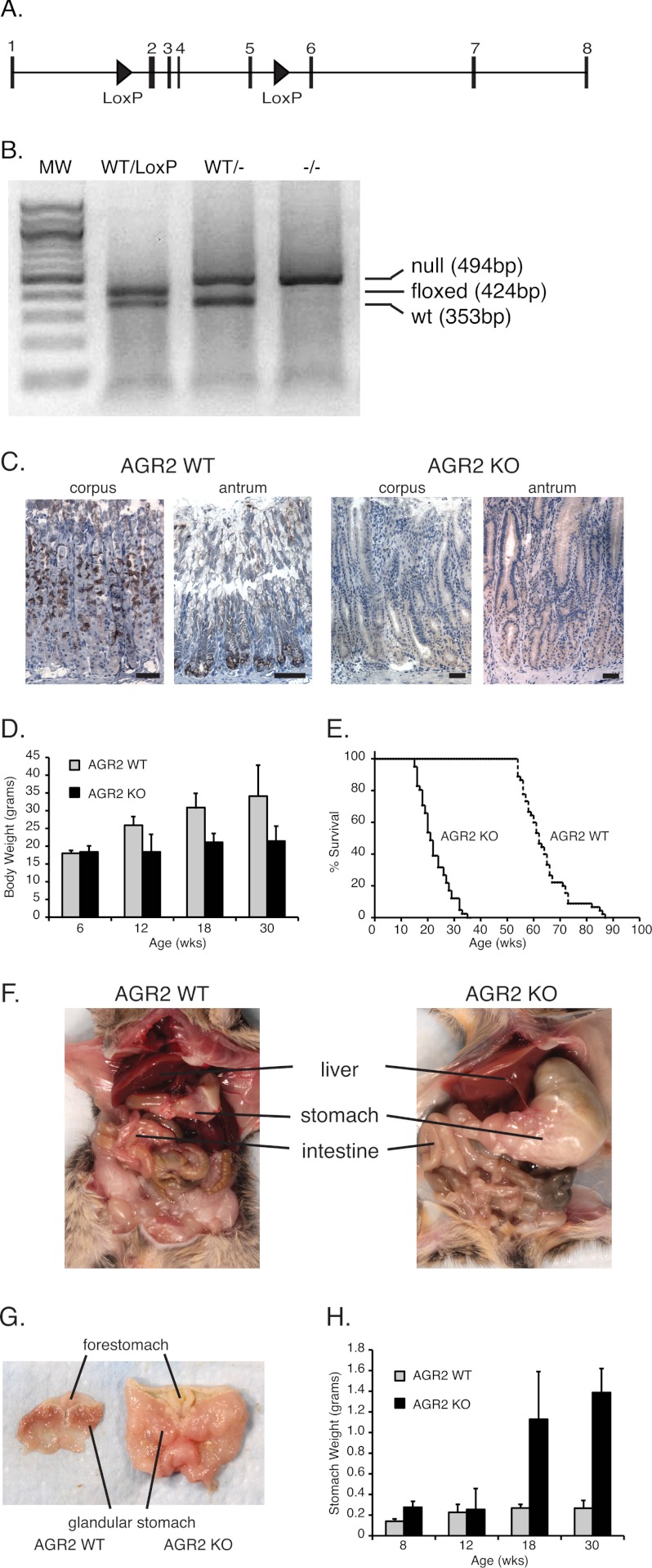
Agr2 KO Mice Exhibited Enlarged Stomachs and Die Prematurely
A conditional Agr2LoxP/LoxP mouse with LoxP sites flanking exons 2–5 of the Agr2 gene was generated to explore the gene function in vivo (Fig. 2A). Mating the Agr2LoxP/LoxP mouse with a mouse that constitutively expresses Cre recombinase in all cells resulted in AGR2−/− (Agr2 KO) progeny (Fig. 2B). Homozygous Agr2−/− mice were viable at birth and appeared to undergo normal development. Immunohistochemistry and qRT-PCR revealed no evidence of Agr2 expression in the stomach or any other tissue (Fig. 2C). After 12 weeks of age, however, the weight of the Agr2 KO mice began to lag behind their wild-type (WT) controls (Fig. 2D), which was associated with signs of progressive morbidity that necessitated euthanasia (Fig. 2E). Autopsies revealed massively enlarged stomachs with large amounts of retained food (Fig. 2F). The presentation was consistent with gastric outlet obstruction due to pronounced mucosal hyperplasia in the antrum, which precluded the transport of food into the intestine. In addition to weight loss, other signs of poor nutrition included the loss of fat pads, hypoalbuminemia, and anemia.
FIGURE 2.
Agr2 KO mice phenotype. A, shown is a schematic of the Agr2 gene on chromosome 12 that encompasses 11,176 base pairs. The LoxP sites (triangles) flank exons 2 and 5. The ATG start site is in exon 2. B, PCR genotyping of Agr2WT/LoxP, Agr2WT/−, and Agr2−/− (null) is shown. C, immunohistochemistry of AGR2 expression in the corpus and antrum of Agr2 WT and Agr2 KO mice is shown. The black scale bar represents 20 μm. D, shown is average body weight at different ages for Agr2 WT (n = 29) versus Agr2 KO (n = 16). Error bars represent 1 ± S.D. E, shown is a survival curve for Agr2 WT (solid line; n = 88) versus Agr2 KO (dashed line; n = 37). F, shown is a representative image of the abdominal cavity for each mouse genotype at 26 weeks. G, shown are representative images of the stomach for each genotype. H, shown is stomach weight at different ages for Agr2 WT (n = 29) versus Agr2 KO (n = 16). Error bars represent ± 1 S.D. Differences in survival, body weight, and stomach weight are significant with p < 0.0001 for all pairs.
The entire glandular epithelium, including the corpus and antrum, was grossly thickened (Fig. 2G). Eighteen-week-old Agr2 KO mouse stomachs averaged 5 times heavier than their wild-type littermates (Fig. 2H). The small and large intestine also appeared larger than the wild-type controls (Fig. 2F). Thus Agr2 loss resulted in reduced body weight, enlarged stomachs, thickening of the glandular mucosa, and premature death due to intestinal obstruction.
Agr2 Expression in the Wild-type Murine Stomach
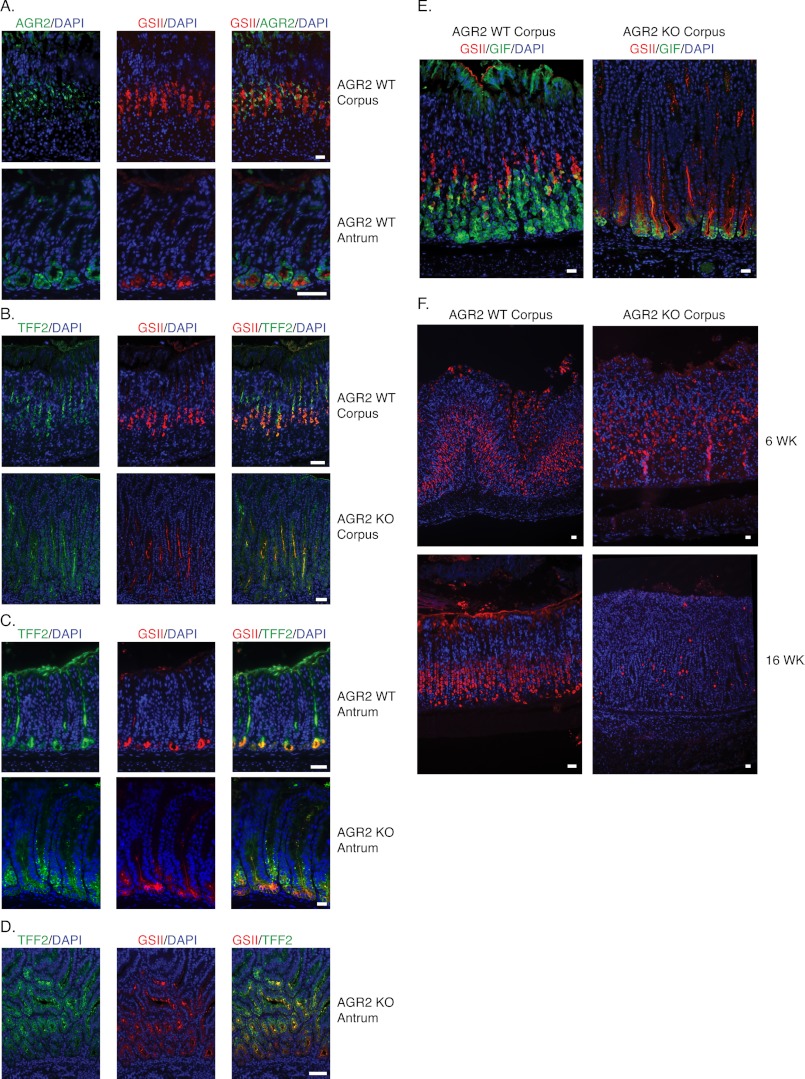
Immunohistochemistry of the adult Agr2 WT murine stomach revealed AGR2 protein expression in the corpus neck and the base of antral glands (Fig. 2C; see Fig. 4A). Within the wild-type glands, AGR2-positive cells were co-labeled with anti-TFF2 antisera and the lectin, GSII, both of which are established markers for mucous neck cells (see Fig. 4, A and B) (10, 12, 34). Cells at the base of the mucous glands in the antrum were also positive for AGR2, GSII, and TFF2 in Agr2 WT mice (Figs. 4, A and C). Agr2, Tff2, and GSII are thus expressed in mucous neck cells and deep antral gland cells in the Agr2 WT glandular stomach. Agr2 expression was not detected in any other cell type in the stomach.
FIGURE 4.
Mucous neck, chief, and pit cell markers in Agr2 WT and Agr2 KO mice. A, shown is Agr2 WT gastric corpus and antrum labeled with anti-AGR2 antisera (green) and the mucous neck cell marker, GSII lectin (red). B and C, shown is double-labeling with anti-TFF2 antisera (green) and GSII lectin (red) of Agr2 WT and Agr2 KO mice in the corpus (B) and antrum (C). D, shown is an area of pronounced antral hyperplasia. Data were obtained from 6-week-old mice (A–C) and from a 16-week-old Agr2 KO mouse (D). E, shown are 3-week-old Agr2 WT and Agr2 KO corpus labeled with anti-gastric intrinsic factor (green) and GSII lectin (red). F, shown is labeling of Agr2 WT and Agr2 KO corpus at 6 and 16 weeks with anti-ATP4A antisera that binds a H+:K+-ATPase subunit specifically expressed by parietal cells. Nuclei are stained blue with DAPI. All scale bars represent 25 μm.
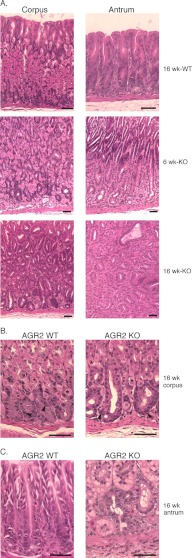
Agr2 KO Develops Hyperplasia in the Glandular Stomach
The histology of Agr2 KO stomachs at 6 and 16 weeks revealed progressive mucosal hyperplasia of the corpus and antrum (Fig. 3). There was no evidence of tumor formation or extension of the hyperplasia through the muscular layers or serosa.
FIGURE 3.
Gastric Histology of Agr2 WT and Agr2 KO mice. A, corpus and antrum of Agr2 WT mice at 16 weeks and Agr2 KO mice at 6 and 16 weeks are shown. The brackets at 6 weeks mark the expanding zone of clear cells in the antrum above the lamina propria. B, shown is high magnification of the corpus gland base containing chief and parietal cells in Agr2 WT and Agr2 KO mice. Representative parietal cells are marked with asterisks, and chief cells are marked with arrowheads. C, high magnification of the antral gland base is shown. The black scale bars represent 50 μm.
Review by a pathologist revealed no evidence of inflammatory infiltrates in the mucosa. There was mild inflammatory infiltration of the submucosa and muscular layers that was determined to be an unlikely cause of the changes within the glandular epithelium.
Expansion of Tff2- and GSII-expressing Mucous Neck Cells
Cell lineages are normally established and appropriately located in the murine stomach by 3 weeks, and by 6 weeks the mucosa has achieved its full thickness (5, 6, 12). Stomachs from 6-week-old mice were labeled with GSII lectin and anti-TFF2 antisera to evaluate the effects of Agr2 loss on mucous neck cells. Agr2 KO mice at 6 weeks displayed a significant increase in the number of TFF2- and GSII-labeled cells in the corpus and antrum compared with Agr2 WT aged-matched controls (Fig. 4, B and C). In the corpus, TFF2/GSII-labeled cells extended beyond their normal location in the neck to the gland base (Fig. 4B). In the antrum, TFF2/GSII-labeled cells extended beyond their normal location at the gland base toward the lumen (Fig. 4C), which was most significant in areas of significant hyperplasia (Fig. 4D). Agr2 loss thus results in prominent expansion of TFF2/GSII-positive cells.
Maturation Defect of Chief Cells and Loss of Parietal Cells in Agr2 KO Mice
The gastric glands are poorly developed in the murine stomach at birth (12). At this stage the stomach is essentially a tube with invaginations representing the developing gastric glands, and gastric intrinsic factor (Gif), a marker for chief cells, is co-expressed with the mucous neck cell marker, GSII, at the bottom of the developing gland. As the gastric glands develop the two markers segregate into different cells. By 3 week, GSII and GIF are segregated into two different cell types that have located to their respective locations in the neck and gland base, respectively (Fig. 4E). In contrast, 3-week-old Agr2 KO mice displayed co-localization of GSII and GIF at the gland base, which was also observed for Agr2 KO mice at the ages of 6, 12, and 24 weeks (data not shown), indicating that the chief cells never mature. Immunofluorescence intensity also revealed decreased GIF expression by chief cells in the Agr2 KO mice (Fig. 4E). Quantitative RT-PCR of gastric corpus mRNA for Gif revealed a 6.7-fold decrease in Agr2 KO mice compared with Agr2 WT controls (Table 1).
TABLE 1.
qRT-PCR of stomach RNA
RNA was extracted at 6 and 16 weeks of age from the corpus and antrum of Agr2 WT and Agr2 KO mice. The results are expressed as a ratio of the KO to the WT. − signifies no significant expression from which to assess a ratio.
| Gene | qRT-PCR of RNA from the corpus and antrum (null/WT) |
|||
|---|---|---|---|---|
| 6 Weeks old |
16 Weeks old |
|||
| Corpus | Antrum | Corpus | Antrum | |
| Pitt cell | ||||
| MUC5AC | 1.04 | 1.41 | 0.11 | 0.20 |
| Parietal cell | ||||
| ATP4A | 0.71 | − | 0.049 | − |
| ATP4B | 0.95 | − | 0.042 | − |
| Chief cell | ||||
| PGC | 1.32 | − | 0.33 | − |
| GIF | 0.87 | − | 0.15 | − |
| Mucous neck cell | ||||
| TFF2 | 0.97 | 0.66 | 6.28 | 2.64 |
| Enteroendocrine | ||||
| CHGA | 1.10 | 0.81 | 0.13 | 0.13 |
| CHGB | 1.11 | 0.31 | 0.37 | 0.37 |
| GAST | − | 0.29 | − | 0.84 |
| GHRL | 0.84 | − | 0.32 | − |
| SST | 1.41 | 2.07 | 0.23 | 0.07 |
| ER stress | ||||
| HERPUD1 | 1.05 | 2.10 | 1.46 | 0.65 |
| HSPA5 | 0.98 | 1.62 | 1.80 | 1.15 |
| Growth factors | ||||
| REG1 | 0.54 | 0.54 | 36.76 | 100.43 |
| REG3B | 0.49 | 0.73 | 66.26 | 207.94 |
| REG3G | 0.90 | 0.44 | 71.01 | 247.28 |
| Miscellaneous | ||||
| SOX9 | 1.54 | 1.25 | 3.61 | 1.57 |
Although parietal cells appeared to be abundant at 6 weeks of age, their numbers declined by 16 weeks in Agr2 KO mice as determined by histology and immunohistochemistry (Figs. 3 and 4F). Quantitative RT-PCR of a 16-week-old Agr2 KO mouse stomach confirmed a greater than 20-fold decrease in transcript levels for the H+:K+-ATPase subunits Atp4A and Atp4B, which are specifically expressed by parietal cells (Table 1).
Decreased Expression of Pit and Enteroendocrine Cell Markers
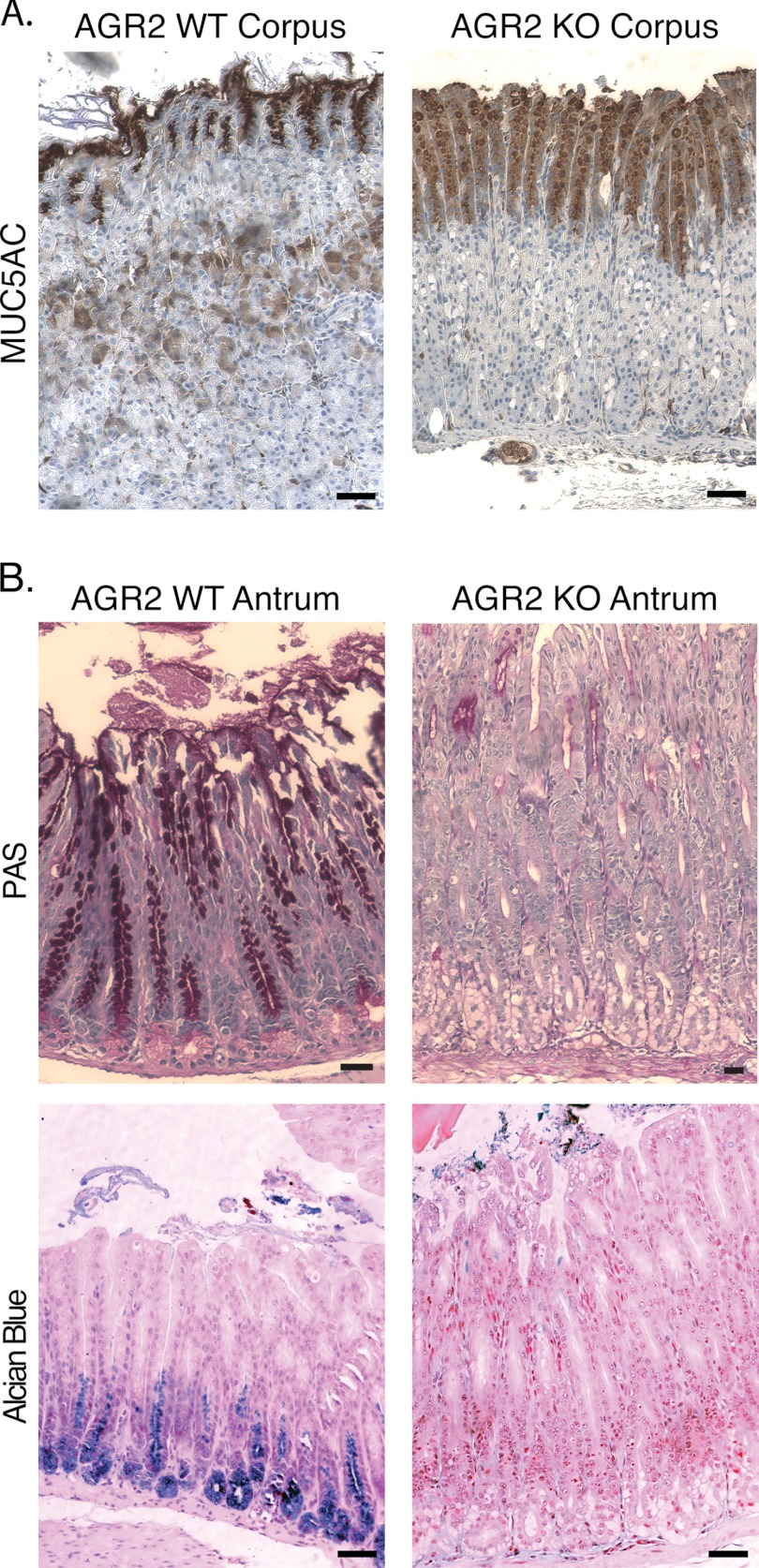
Pit cells in the glandular stomach are responsible for mucin secretion, which can be detected with antibodies against the secretory mucin MUC5AC. Agr2 KO mice displayed decreased MUC5AC staining intensity compared with Agr2 WT mice in the corpus (Fig. 5A). The total number of MUC5AC-positive cells, however, increased in the Agr2 KO. Quantitative RT-PCR of 16-week-old Agr2 KO mice detected a 5- and 9-fold decrease in Muc5ac transcript levels in the corpus and antrum, respectively (Table 1). In the antrum, intense periodic acid-Schiff staining, which is a characteristic of neutral mucins such as MUC5AC, was observed above the isthmus in the antrum of Agr2 WT mice. In contrast, periodic acid-Schiff staining of cells in the Agr2 KO antrum decreased dramatically, but again the number of positively labeled cells increased. Cells at the base of antral glands in Agr2 WT mice stained with Alcian blue, which is characteristic of acidic mucins. Alcian blue staining was absent in the Agr2 KO mice (Fig. 5B).
FIGURE 5.
Mucus Declines in Agr2 KO mice. A, shown is MUC5AC immunohistochemistry of the corpus in Agr2 KO and WT mice. B, shown is staining in the antrum for neutral (periodic acid-Schiff (PAS), top row) and acid (Alcian blue, bottom row) mucins in Agr2 KO and WT mice. All scale bars represent 50 μm.
Enteroendocrine cells were affected in 16-week-old Agr2 KO mice as qRT-PCR of gastric RNA detected a 3- and 5-fold decrease in the transcript levels for ghrelin and somatostatin, respectively. Similar decreases in transcripts levels were detected for the general endocrine biomarkers chromogranin A (Chga, 8-fold) and chromogranin B (Chgb, 2.7-fold) (Table 1). Despite the low numbers of parietal cells, which normally induces an increase in gastrin synthesis, gastrin transcript levels in the 16-week-old Agr2 KO mice antrum were 20% lower than Agr2 WT controls (Table 1). No significant changes were observed in pit or enteroendocrine markers in 6-week-old Agr2 KO mice (Table 1). In summary, pit cell and enteroendocrine cell lineage marker expression declines with age.
Increased Cell Proliferation in Agr2 KO Mice
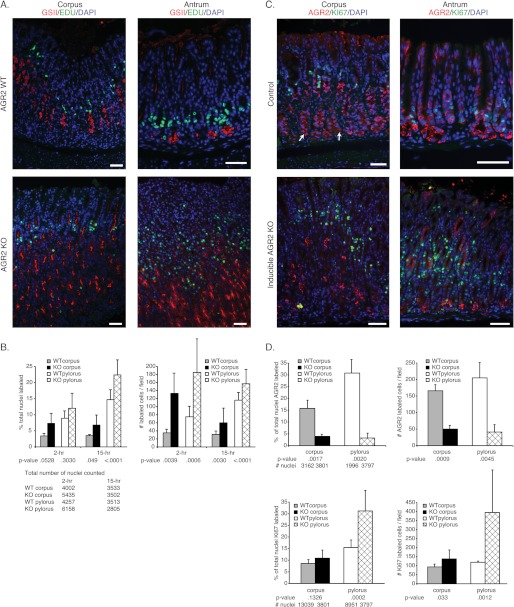
In view of the glandular hyperplasia observed in the stomach, an assessment of cell proliferation and apoptosis was performed. TUNEL assays of Agr2 WT and Agr2 KO mice revealed no apoptosis in the stomachs of either group (data not shown). Two different assays were used to assess cell proliferation. One approach utilized the injection of a nucleotide analog, EdU followed by its detection with immunofluorescence (31). A second approach utilized Ki-67 antisera, which is a biomarker for actively cycling cells (35). Consistent with previous studies (2), EdU-labeled cells were detected in the isthmus of the Agr2 WT corpus and antrum (Fig. 6A). Agr2 KO mice displayed a 3.8- and 2.5-fold increase in EdU-labeled cells in the corpus and antrum, respectively, compared with Agr2 WT mice (Fig. 6B).
FIGURE 6.
Determination of cell proliferation in constitutive and inducible Agr2 KO mice. A, shown is labeling of the corpus (left) and antrum (right) of Agr2 WT (top panel) and Agr2 KO (bottom panel) mice with GSII lectin (red) and EdU (green) for proliferation. Tissue was harvested 2 and 15 h after EdU administration. Note that the bulk of EdU labeling is above the GSII layer, which is consistent with the isthmus layer. B, shown is quantitation of proliferating cells 2 and 15 h post-EdU labeling by counting EdU labeled cells as a percentage of the total number of nuclei (left graph). The total nuclei number is listed below the chart. The absolute number of EdU labeled cells per microscopic field (∼590 μm of mucosal length/field) was also plotted (right graph). C, shown is labeling of the corpus (left) and antrum (right) for AGR2 (red) and the proliferation marker Ki-67 (green) of 8-week-old tamoxifen-treated Agr2LoxP/LoxP (top) and Agr2LoxP/LoxP:CMV-CreERT2 (inducible KO, bottom) mice. The white arrows indicate representative AGR2-positive cells at the gland base. D, shown is quantitation of AGR2 (top graphs)- and Ki-67 (bottom graphs)-labeled cells in the inducible Agr2 KO mouse as a percentage of the total nuclei number (left graphs) or the absolute number of labeled cells per field (right graphs). The total number of nuclei counted is listed below the left graph. Error bars for all charts represent ± 1 S.D. Nuclei are stained blue with DAPI in all the immunofluorescence images. All scale bars represent 50 μm.
Induced Agr2 Loss in Adult Mice Also Affects Cell Proliferation
Embryonic loss of Agr2 in the constitutive Agr2 KO mouse could potentially affect development to produce the findings described. Therefore, 8-week-old adult Agr2LoxP/LoxP;CMV-CreERT2 mice were injected with a 5-day course of tamoxifen to induce Cre recombinase activity and sacrificed 21 days after the last dose. Agr2 KO mice exhibited a 3- and 5-fold decrease in AGR2-labeled cells in the corpus and antrum, respectively, compared with the control Agr2LoxP/LoxP mice that were also treated with tamoxifen in a similar manner (Fig. 6, C and D). Immunohistochemistry for Ki-67-labeled nuclei revealed a 1.5- and 3.3-fold increase in cell proliferation in the corpus and antrum, respectively, compared with the control. The loss of Agr2 expression in adult mice thus results in increased cell proliferation in the corpus and antrum of the glandular stomach.
A recently reported adverse effect of tamoxifen is reversible parietal cell loss, metaplasia of chief cells, and increased cell proliferation (29). The tamoxifen-induced effects reverted to base line 21 days after a single dose of tamoxifen. The metaplasia previously described was characterized by TFF2 and GSII labeling at the gastric gland base, similar to what was observed in the Agr2 KO. Agr2 WT controls that possessed a floxed Agr2 but without Cre recombinase activity were evaluated for AGR2 protein expression and proliferation with immunohistochemistry. As demonstrated in the previous aforementioned study, cell proliferation returned to base line 21 days after tamoxifen administration. Similar to the previous study, parietal cell numbers returned, but there was still residual GSII and TFF2 labeling at the gland base (data not shown). Labeling for AGR2 protein was also detected at the gland base in addition to the neck region (Fig. 6C).
A Fraction of GSII Labeled Cells Are Proliferating in Agr2 KO Mice
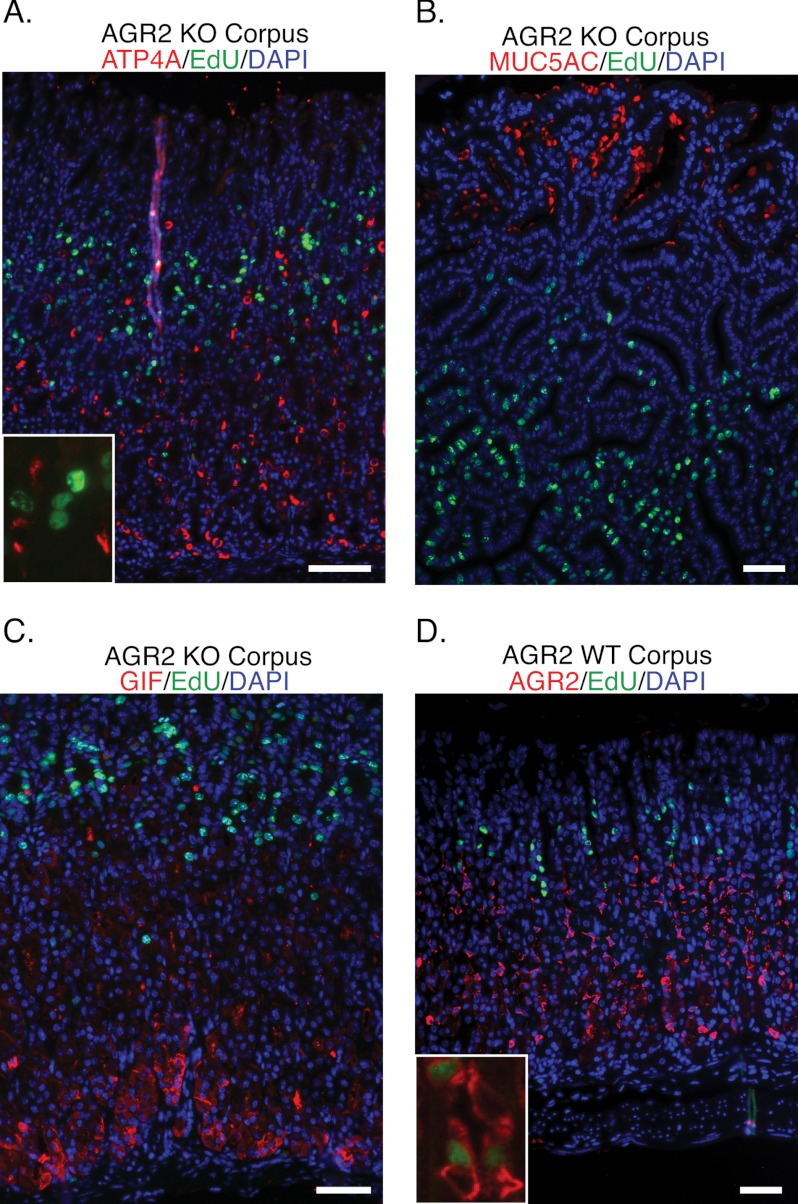
Proliferating cells labeled for 2 h with EdU were evaluated for co-expression of ATP4A (parietal cells), MUC5AC (pit cells), and GIF (chief cells) in the Agr2 KO mice (Fig. 7, A–C). None of the proliferating cells was found to express markers consistent with the differentiated cell types. In contrast, 37 and 24% of proliferating EdU-labeled cells in the corpus and antrum, respectively, co-labeled with the mucous neck cell marker GSII in Agr2 WT mice, which increased to 53 and 43% in Agr2 KO mice (Fig. 6A). An assessment of proliferating AGR2-expressing cells in Agr2 WT mice was determined using double immunofluorescence for AGR2 and EdU (Fig. 7D). After a 2-h labeling with EdU of Agr2 WT mice, 16 and 14% of the EdU-positive cells were also positive for AGR2 in the corpus and antrum, respectively. In summary, parietal, pit, and chief cell cells were not proliferating. There is a fraction of proliferating GSII-positive cells that may account for the TFF2/GSII expanded cell population in the Agr2 KO.
FIGURE 7.
Proliferating cells in Agr2 KO do not label with differentiated lineage markers. Agr2 KO mice (A–C) were sacrificed 2 h after injection with EdU (green) and labeled for the following cell markers: A, ATP4A, a parietal cell marker (red). The inset shows a magnified image of a representative EdU labeled nuclei and ATP4A labeled cells. B, MUC5AC, a pit cell marker (red). C, GIF, gastric intrinsic factor, a chief cell marker (red). D, AGR2, expressed by mucous neck cells in Agr2 WT (red) and EdU (green). The inset shows a magnified representative image of cells labeled for both AGR2 and EdU. Nuclei are stained blue with DAPI in all images. All scale bars represent 50 μm.
Sox9 Is Co-expressed by TFF2/GSII-labeled Cells in Agr2 WT and Agr2 KO Mice
Recent studies have suggested increased Sox9 expression in mucous neck cells, gastric intestinal metaplasia, and gastric adenocarcinoma in humans (36). Sox9 has been associated with stem cells and pluripotent, mitotically active progenitor cells in a wide variety of organs that include the pancreas, intestine, and liver (37, 38). Other studies have shown Sox9 to be necessary for initiating differentiation (39, 40).
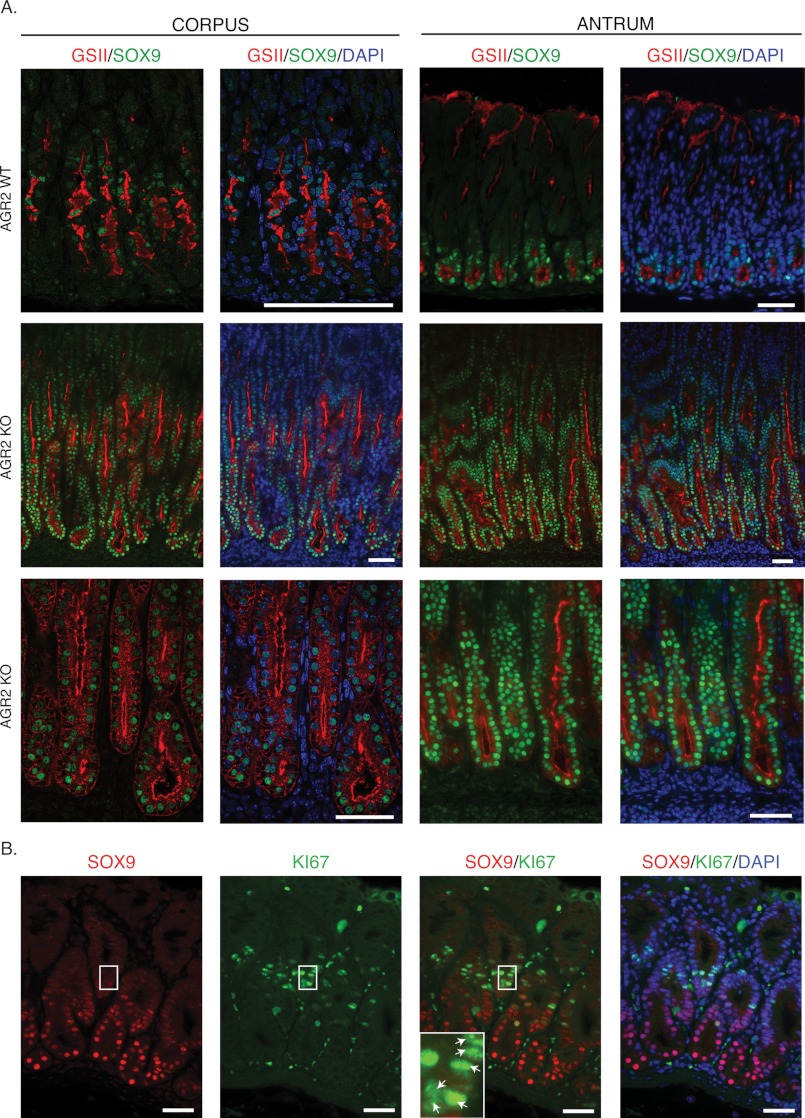
Nuclear SOX9 protein in Agr2 WT mice was detected by immunohistochemistry in the isthmus of the gastric corpus and the base of the antral glands (Fig. 8A). SOX9 co-localized with GSII-labeled cells in the Agr2 WT. In the Agr2 KO, SOX9- and GSII-co-labeled cells increased dramatically in both the corpus and antrum (Fig. 8A). Quantitative RT-PCR revealed a 3.6- and 1.6-fold increase in Sox9 transcript levels in the corpus and antrum, respectively (Table 1). A small fraction of nuclear-labeled SOX9 cells was also co-labeled with the proliferation antigen, Ki-67 (Fig. 8B). Agr2 WT mice thus express SOX9 in mucous neck cells and deep antral gland cells. In the absence of AGR2, there is an expansion of SOX9-positive cells that is also GSII-positive.
FIGURE 8.
Nuclear SOX9 positive cell numbers increase in the Agr2 KO and is co-expressed with GSII. A, 3-week-old Agr2 WT (top row) and Agr2 KO (middle and bottom row) mice were labeled with anti-SOX9 antisera (green) and GSII lectin (red) and DAPI stain for the nucleus (blue). The bottom row represents a magnified image showing co-labeling of SOX9 and GSII. The Agr2 WT corpus is shown at higher magnification because the nuclear SOX9 signal was much less intense than the antrum. B, Agr2 KO antrum labeled with antisera for SOX9 (red) and Ki-67 (green) is shown. The inset contains a magnified view of the area within the white rectangle showing double-labeled nuclei with Ki-67 and SOX9 (arrows). Proliferating SOX9+ cells generally label less intensely. The nuclei are stained with DAPI in all images. The scale bars represent 50 μm.
The Small and Large Intestine Also Exhibit Enhanced Proliferation and SOX9 Expression in the Agr2 KO
Gross inspection of the small intestines revealed that they were enlarged (Fig. 2), which was verified by their increased weight in older mice. No significant difference in small intestine weight was observed at less than 12 weeks of age. For Agr2 KO mice greater than 24 weeks of age, the mean weight was 2-fold higher when compared with WT (1.9 versus 1.1, p value = 4.6 × 10−9) or heterozygotes (1.9 versus 1.1, p value = 4.8 × 10−7) (Fig. 9B).
FIGURE 9.
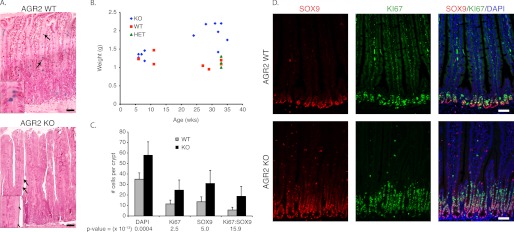
The small intestine of Agr2 KO mice exhibit decreased mucin and increased weight, cell proliferation, and nuclear SOX9. A, hematoxylin and eosin stained the small intestine of Agr2 WT and KO mice. Mucus-secreting goblet cells are stained with Alcian blue. The arrows highlight Alcian blue-stained goblet cells, and a magnified view is displayed in the inset. B, shown is a scatter plot of mouse age versus small intestine weight of Agr2 KO (KO), WT (WT), and heterozygotes (HET). C, shown is the average number of SOX9- and Ki-67-labeled cells per crypt (WT, n = 41 crypts; KO, n = 51). p values ( × 10−13) between WT and KO are listed below each measured label. Error bars represent ± 1 S.D. D, double-labeled immunofluorescence for SOX9 (red) and Ki-67 (green) is shown. The nuclei are stained blue with DAPI. The scale bar in all images represents 50 μm.
Two previous studies have reported the generation of AGR2 null mice whose major phenotype was reduced mucin production in intestinal goblet cells (41, 42). Immunohistochemical analysis of the Agr2 KO mouse intestine described in this study also revealed decreased mucin production by the small intestine (Fig. 9A). Alcian-blue staining goblet cells were present, but the signal intensity in each cell was dramatically reduced.
Further analysis of the histology revealed no signs of abnormal inflammation in the Agr2 KO intestines. There were no signs of infiltration by inflammatory cells into the mucosa, submucosa, or muscular layers.
Similar to the previous analysis of the stomach, nuclear SOX9 expression and cell proliferation was also determined in the intestines (Fig. 9D). The increase in DAPI-, Ki-67-, and SOX9-labeled nuclei per crypt was 1.7-, 2.1-, and 2.3-fold, respectively, in Agr2 KO compared with Agr2 WT mice (Fig. 9C). Ki-67 labeling was largely absent from the intestinal crypt bottoms where stem cells arise but enhanced in a crypt region usually associated with progenitor cells. Similar to the stomach, nuclear-labeled SOX9 cells were largely distinct from the proliferating cells, although the number of Ki-67- and SOX9-co-labeled cells increased by 3.3-fold per crypt in the Agr2 KO.
Endoplasmic Reticulum Stress Response Genes Are Not Induced in Agr2 KO Mice
AGR2 protein resides and functions in the endoplasmic reticulum (ER) (43). A previous study of Agr2 null mice detected enhanced expression of genes associated with the ER stress response (42). Examination of the ER stress response genes Hspa5 and Herpud1 by qRT-PCR in Agr2 WT and KO mouse stomachs revealed no significant change (Table 1). As a positive control, ER stress was induced with tunicamycin in the mouse lung adenocarcinoma cell line, 394T4, which resulted in a 21- and 25-fold increase in Hspa5 and Herpud1 transcripts, respectively.
Analysis of Gene Expression with DNA Microarrays
RNA derived from the whole stomach of Agr2 WT and Agr2 KO mice were analyzed with respect to gene expression using DNA microarrays. For selected genes, confirmation was obtained by qRT-PCR (Table 1). Gene expression analysis revealed 858 genes that showed at least a 3-fold or more change in gene expression in the Agr2 KO versus the Agr2 WT stomach (GEO accession GSE40062). Many of the 486 genes whose expression declined were associated with the differentiated features of specific cell lineages as previously described. In contrast, 372 genes were enhanced 3-fold or more in the Agr2 KO, which included transcripts for members of the Reg family of genes, Reg1, Reg3A, and Reg3G (Table 1).
DISCUSSION
The in vivo function for Agr2 was explored using both constitutive and inducible conditional Agr2 null mice. Constitutive Agr2 loss resulted in a consistent phenotype featuring hyperplasia of the glandular stomach and premature death that was presumed secondary to gastric outlet obstruction. Histologic analysis of the Agr2 KO mouse revealed an expansion in the number of cells expressing the mucous neck cell markers TFF2/GSII in the corpus and antrum. The same TFF2/GSII-positive cells were found to express AGR2 in the stomach of Agr2 WT mice, indicating that Agr2 expression regulates the number of mucous neck cells and deep antral gland cells.
In addition to the expansion of TFF2/GSII positive cells, the maturation of mucous neck cells to chief cells was perturbed. Agr2 KO mice displayed persistent labeling by GSII lectin of chief cells at the bottom of the gland base, a feature usually lost with postnatal development of the gastric glands (5, 6, 12, 44).
The increase in TFF2/GSII-labeled cells observed in this study is similar to previous findings in which parietal cells were destroyed using drugs or toxins or through infection with Helicobacter felis (44–47). These models also expressed signs of incomplete maturation or metaplasia of chief cells characterized by TFF2/GSII labeling of cells in the gastric gland base and a decrease in intrinsic factor expression. Although not directly targeted, the number of parietal cells in the Agr2 KO mice also a exhibited substantial decline with age compared with the Agr2 WT mice. The constellation of features that include incomplete maturation of chief cells, loss of parietal cells, and expansion of TFF2/GSII-positive cells in the Agr2 KO mice were similar to those previously described for a preneoplastic entity known as spasmolytic protein expressing metaplasia (SPEM) (47–49). Additional evidence that Agr2 may contribute to the development of SPEM was obtained from the tamoxifen-treated Agr2LoxP/LoxP controls (Fig. 6C). Tamoxifen is known to induce a reversible metaplasia similar to SPEM. The control Agr2LoxP/LoxP mice expressed AGR2 in the same metaplastic cells that expressed GSII in the gastric gland base, thus supporting a potential role for Agr2 in the development of SPEM. Although the observed hyperplasia in the Agr2 KO mouse did not express signs of overt transformation with the formation of metastatic tumors, the features exhibited by the Agr2 KO support a role for Agr2 in the development of neoplasia.
The absence of Agr2 expression resulted in a significant increase in cell proliferation. Increased cell proliferation was observed in both the stomach and intestinal tract and thus reflects what is likely a universal function for Agr2. Most proliferating cells were located in the gastric isthmus region and were unlabeled by any specific lineage marker, suggesting they may be stem cells or early progenitors. Because a definitive marker for stem cells in the gastric corpus has yet to be identified, the hypothesis is inferred from previous studies that have implicated the isthmus as the source of gastric stem cells. Cell proliferation in the intestine was greatest in the mid-crypt, which is most consistent with the proliferation of progenitor cells. The results support a model in which Agr2 expression results in negative feedback for cell proliferation. The results observed with the inducible Agr2 KO in adult mice established that Agr2 also regulates cell proliferation in the mature adult mice.
The results concerning cell proliferation may be viewed as contrary to previously published data in which Agr2 enhanced the growth and cell division of adenocarcinoma cell lines. Previous work demonstrated that Agr2 expression activates the Hippo and EGF signaling pathways in lung and esophageal adenocarcinoma cell lines (28). These results may be reconciled if there are different stimuli for cell division among stem cells and progenitors. In the wild-type animal, Agr2 expression may promote cell division in a post-stem cell compartment while at the same time providing a negative feedback for an earlier compartment such as stem cells or early progenitors.
Agr2 exhibits other characteristics consistent with a role in a post-stem cell compartment, including the maturation of cell lineages. Gastric chief cells in the Agr2 KO never fully develop. In the intestine, mucin-secreting goblet cells are present, but the mucin content is markedly decreased. The findings were similar to a recent study focused on intestinal goblet cell development in zebrafish, which concluded that Agr2 serves a role in terminal differentiation (50). Similar to the present study, reduced zebrafish Agr2 expression resulted in decreased mucin production without an actual change in goblet cell numbers.
The Agr2 KO progressively expressed lower transcript levels for the products of enteroendocrine cells and mucin-secreting pit cells in the postnatal period. In addition, parietal cells also declined in numbers as the mouse aged. The result suggests that Agr2 loss results in incomplete maturation of chief cells and compromises the persistence or maturation of other differentiated cell lineages. Previous studies have demonstrated that disruption of one lineage can affect the homeostasis and maintenance of other lineages in the stomach (44–47). Another potential cause for the delayed effects on parietal-, enteroendocrine-, and mucin-secreting cells may be the postnatal development of the gastric glands. Complete development as characterized by attainment of full thickness of the gastric glands is not achieved until 6 weeks of age in the mouse. The full impact of the Agr2 null may not be achieved until 6 weeks when the gastric glands are fully developed and Agr2 may be fully functional in the newly established mucous neck cells. How Agr2 affects other cell lineages remains unclear and is a focus of current investigations.
The decline in intestinal goblet cell mucin observed in this study was similar to two previous publications that also generated Agr2 null mice. One study by Park et al. (41) attributed the decrease in intestinal mucus production to a role for Agr2 in MUC2 folding as supported by its in vitro binding to AGR2 protein (42).
In contrast to the study by Zhao et al. (42), this study and that of Park et al. (41) did not result in an increase in inflammation or ER stress response. It should be noted that the mice in the study by Park et al. (41) were housed in a gnotobiotic facility. A recent zebrafish study also found no evidence of an increased ER stress response with the reduction in Agr2 expression (50), a result shared by the Agr2 KO mice in this study.
Clearly the most profound difference between this study and the two previously published Agr2 null mice were the effects observed in the stomach. No abnormalities were previously reported in the stomach. The reasons for the differences are unclear, although all three studies generated the Agr2 null mice using different targeting constructs, promoters for the expression of Cre recombinase, and husbandry conditions. Similar to this study, Park et al. (41) did report weight loss by the Agr2 null mice beginning at 16 weeks, which were followed to 21 weeks. In contrast, the Agr2 KO mice in this study were followed for up to 36 weeks, and significant morbidity requiring euthanasia was encountered at 26 weeks.
The DNA microarray analysis supported a significant regulatory role for Agr2, which revealed changes in expression of 3-fold or more for >800 genes in the Agr2 KO. Previous work determined that AGR2 protein functions from within the endoplasmic reticulum (43), which may mediate the assembly or folding of yet to be defined regulatory proteins that reside or are in transit through the ER. Among the genes identified by the DNA microarrays were members of the Reg family of genes, which have been previously implicated in promoting gastric mucosal growth. Enhanced Reg1 expression has been implicated in gastric regeneration and the expansion of progenitor cells in the neck zone (51, 52) and in human preneoplastic and neoplastic lesions (53, 54).
Agr2 loss results in incomplete maturation of chief cells and a decline in other differentiated cell lineages. The persistence of immature cells that do not fully mature is also supported by the expansion of nuclear SOX9-labeled cells in the Agr2 KO mice. Previous work has shown that Sox9 can deter maturation and may serve to maintain the pluripotent state of stem or progenitor cells (38). Recent studies have also shown that Sox9 is expressed in proliferating stem cells (55), which provides insight into the significant fraction of proliferating cells that are SOX9-positive in this study (Figs. 8 and 9). Furthermore, mucous neck cells and deep antral gland cells in the normal glandular stomach expressed AGR2 and SOX9, and the absence of Agr2 expression results in the expansion of GSII/SOX9-labeled cells, some of which are actively proliferating (Fig. 4A). The co-expression with Sox9 indicates that Agr2 is expressed in cells that may represent an immature progenitor before terminal differentiation and is consistent with prior studies (9). The data also indicate that Agr2 may affect the fate of cells that do not ultimately express the gene.
Prior studies describing a Sox9 null mutation that was specifically induced in the intestine shared many features with that of the Agr2 KO mouse described in the present study (56). The absence of intestinal Sox9 expression constrained the maturation of goblet cells and led to an increase in intestinal cell proliferation, hyperplasia, and dysplasia. The phenotypes of the Agr2 and Sox9 null mutants indicate that the expression of either gene results in negative feedback inhibition of cell proliferation. Considering the significant increase in nuclear SOX9 observed in the Agr2 KO stomach and intestine, Agr2 may function downstream of Sox9. In fact, a recently published gene expression profile demonstrated that induced expression of Sox9 in HT-29 cells by transfection significantly enhanced Agr2 expression (57).
This study supports a role for Agr2 in regulating cell homeostasis at the level of stem/early progenitor cell proliferation, which is operational in adult mice. Agr2 expression influences cell lineage maturation. Agr2 is also expressed in metaplasia, and disrupted Agr2 expression results in many features consistent with a preneoplastic process for stomach cancer.
Acknowledgments
We appreciate Aiwen Dong and Christine Cartwright for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant DK063624 (to A. W. L.).
The microarray data were deposited in the NCBI GEO database with accession number GSE40062.
- GSII
- G. simplicifolia II lectin
- EdU
- 5-ethynyl-2′-deoxyuridine
- GIF
- gastric intrinsic factor
- ER
- endoplasmic reticulum
- SPEM
- spasmolytic protein expressing metaplasia
- qRT
- quantitative real-time
- GIF
- gastric intrinsic factor.
REFERENCES
- 1. Lee E. R., Trasler J., Dwivedi S., Leblond C. P. (1982) Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am. J. Anat. 164, 187–207 [DOI] [PubMed] [Google Scholar]
- 2. Karam S. M., Leblond C. P. (1993) Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat. Rec. 236, 259–279 [DOI] [PubMed] [Google Scholar]
- 3. Bjerknes M., Cheng H. (2002) Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G767–G777 [DOI] [PubMed] [Google Scholar]
- 4. Mills J. C., Shivdasani R. A. (2011) Gastric epithelial stem cells. Gastroenterology 140, 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keeley T. M., Samuelson L. C. (2010) Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am. J. Physiol. Gastrointest Liver Physiol. 299, G1241–G1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karam S. M., Li Q., Gordon J. I. (1997) Gastric epithelial morphogenesis in normal and transgenic mice. Am. J. Physiol. 272, G1209–G1220 [DOI] [PubMed] [Google Scholar]
- 7. Ramsey V. G., Doherty J. M., Chen C. C., Stappenbeck T. S., Konieczny S. F., Mills J. C. (2007) The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134, 211–222 [DOI] [PubMed] [Google Scholar]
- 8. Kataoka K., Takeoka Y., Furihata C. (1990) Immunocytochemical study of pepsinogen 1-producing cells in the fundic mucosa of the stomach in developing mice. Cell Tissue Res. 261, 211–217 [DOI] [PubMed] [Google Scholar]
- 9. Quante M., Marrache F., Goldenring J. R., Wang T. C. (2010) TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology 139, 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeffrey G. P., Oates P. S., Wang T. C., Babyatsky M. W., Brand S. J. (1994) Spasmolytic polypeptide. A trefoil peptide secreted by rat gastric mucous cells. Gastroenterology 106, 336–345 [DOI] [PubMed] [Google Scholar]
- 11. Ebisu S., Goldstein I. J. (1978) Bandeiraea simplicifolia lectin II. Methods Enzymol. 50, 350–354 [DOI] [PubMed] [Google Scholar]
- 12. Ihida K., Suganuma T., Tsuyama S., Murata F. (1988) Glycoconjugate histochemistry of the rat fundic gland using Griffonia simplicifolia agglutinin-II during the development. Am. J. Anat. 182, 250–256 [DOI] [PubMed] [Google Scholar]
- 13. Oinuma T., Kawano J., Suganuma T. (1991) Glycoconjugate histochemistry of Xenopus laevis fundic gland with special reference to mucous neck cells during development. Anat. Rec. 230, 502–512 [DOI] [PubMed] [Google Scholar]
- 14. Hao Y., Triadafilopoulos G., Sahbaie P., Young H. S., Omary M. B., Lowe A. W. (2006) Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology 131, 925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lowe A. W., Olsen M., Hao Y., Lee S. P., Taek Lee K., Chen X., van de Rijn M., Brown P. O. (2007) Gene expression patterns in pancreatic tumors, cells, and tissues. PLoS ONE 2, e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson D. A., Weigel R. J. (1998) hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG- 2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem. Biophys. Res. Commun. 251, 111–116 [DOI] [PubMed] [Google Scholar]
- 17. Zhang J. S., Gong A., Cheville J. C., Smith D. I., Young C. Y. (2005) AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer 43, 249–259 [DOI] [PubMed] [Google Scholar]
- 18. Fritzsche F. R., Dahl E., Dankof A., Burkhardt M., Pahl S., Petersen I., Dietel M., Kristiansen G. (2007) Expression of AGR2 in non small cell lung cancer. Histology and Histopathology 22, 703–708 [DOI] [PubMed] [Google Scholar]
- 19. Zhu H., Lam D. C., Han K. C., Tin V. P., Suen W. S., Wang E., Lam W. K., Cai W. W., Chung L. P., Wong M. P. (2007) High resolution analysis of genomic aberrations by metaphase and array comparative genomic hybridization identifies candidate tumour genes in lung cancer cell lines. Cancer Lett. 245, 303–314 [DOI] [PubMed] [Google Scholar]
- 20. Edgell T. A., Barraclough D. L., Rajic A., Dhulia J., Lewis K. J., Armes J. E., Barraclough R., Rudland P. S., Rice G. E., Autelitano D. J. (2010) Increased plasma concentrations of anterior gradient 2 protein are positively associated with ovarian cancer. Clin. Sci. 118, 717–725 [DOI] [PubMed] [Google Scholar]
- 21. Lee S., Bang S., Song K., Lee I. (2006) Differential expression in normal-adenoma-carcinoma sequence suggests complex molecular carcinogenesis in colon. Oncol. Rep. 16, 747–754 [PubMed] [Google Scholar]
- 22. Bai Z., Ye Y., Liang B., Xu F., Zhang H., Zhang Y., Peng J., Shen D., Cui Z., Zhang Z., Wang S. (2011) Proteomics-based identification of a group of apoptosis-related proteins and biomarkers in gastric cancer. Int. J. Oncol. 38, 375–383 [DOI] [PubMed] [Google Scholar]
- 23. Aberger F., Weidinger G., Grunz H., Richter K. (1998) Anterior specification of embryonic ectoderm. The role of the Xenopus cement gland-specific gene XAG-2. Mech. Dev. 72, 115–130 [DOI] [PubMed] [Google Scholar]
- 24. Sive H. L., Hattori K., Weintraub H. (1989) Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell 58, 171–180 [DOI] [PubMed] [Google Scholar]
- 25. Kumar A., Godwin J. W., Gates P. B., Garza-Garcia A. A., Brockes J. P. (2007) Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318, 772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu D., Rudland P. S., Sibson D. R., Platt-Higgins A., Barraclough R. (2005) Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 65, 3796–3805 [DOI] [PubMed] [Google Scholar]
- 27. Wang Z., Hao Y., Lowe A. W. (2008) The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 68, 492–497 [DOI] [PubMed] [Google Scholar]
- 28. Dong A., Gupta A., Pai R. K., Tun M., Lowe A. W. (2011) The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway coactivator YAP1 activation. J. Biol. Chem. 286, 18301–18310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huh W. J., Khurana S. S., Geahlen J. H., Kohli K., Waller R. A., Mills J. C. (2012) Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142, 21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madsen J., Nielsen O., Tornøe I., Thim L., Holmskov U. (2007) Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 55, 505–513 [DOI] [PubMed] [Google Scholar]
- 31. Salic A., Mitchison T. J. (2008) A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 105, 2415–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Workman C., Jensen L. J., Jarmer H., Berka R., Gautier L., Nielser H. B., Saxild H. H., Nielsen C., Brunak S., Knudsen S. (2002) A new non-linear normalization method for reducing variability in DNA microarray experiments. Genome Biol. 3, research0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reich M., Liefeld T., Gould J., Lerner J., Tamayo P., Mesirov J. P. (2006) GenePattern 2.0. Nat. Genet. 38, 500–501 [DOI] [PubMed] [Google Scholar]
- 34. Lefebvre O., Wolf C., Kédinger M., Chenard M. P., Tomasetto C., Chambon P., Rio M. C. (1993) The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J. Cell Biol. 122, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133, 1710–1715 [PubMed] [Google Scholar]
- 36. Sashikawa Kimura M., Mutoh H., Sugano K. (2011) SOX9 is expressed in normal stomach, intestinal metaplasia, and gastric carcinoma in humans. J. Gastroenterol. 46, 1292–1299 [DOI] [PubMed] [Google Scholar]
- 37. Furuyama K., Kawaguchi Y., Akiyama H., Horiguchi M., Kodama S., Kuhara T., Hosokawa S., Elbahrawy A., Soeda T., Koizumi M., Masui T., Kawaguchi M., Takaori K., Doi R., Nishi E., Kakinoki R., Deng J. M., Behringer R. R., Nakamura T., Uemoto S. (2011) Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas. and intestine. Nat. Genet. 43, 34–41 [DOI] [PubMed] [Google Scholar]
- 38. Seymour P. A., Freude K. K., Tran M. N., Mayes E. E., Jensen J., Kist R., Scherer G., Sander M. (2007) SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. U.S.A. 104, 1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vidal V. P., Chaboissier M. C., Lützkendorf S., Cotsarelis G., Mill P., Hui C. C., Ortonne N., Ortonne J. P., Schedl A. (2005) Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15, 1340–1351 [DOI] [PubMed] [Google Scholar]
- 40. Nowak J. A., Polak L., Pasolli H. A., Fuchs E. (2008) Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3, 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park S. W., Zhen G., Verhaeghe C., Nakagami Y., Nguyenvu L. T., Barczak A. J., Killeen N., Erle D. J. (2009) The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. U.S.A. 106, 6950–6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao F., Edwards R., Dizon D., Afrasiabi K., Mastroianni J. R., Geyfman M., Ouellette A. J., Andersen B., Lipkin S. M. (2010) Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev. Biol. 338, 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gupta A., Dong A., Lowe A. W. (2012) AGR2 gene function requires a unique endoplasmic reticulum localization motif. J. Biol. Chem. 287, 4773–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nozaki K., Ogawa M., Williams J. A., Lafleur B. J., Ng V., Drapkin R. I., Mills J. C., Konieczny S. F., Nomura S., Goldenring J. R. (2008) A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 134, 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nam K. T., Lee H. J., Sousa J. F., Weis V. G., O'Neal R. L., Finke P. E., Romero-Gallo J., Shi G., Mills J. C., Peek R. M., Jr., Konieczny S. F., Goldenring J. R. (2010) Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139, 2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Q., Karam S. M., Gordon J. I. (1996) Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J. Biol. Chem. 271, 3671–3676 [PubMed] [Google Scholar]
- 47. Nomura S., Yamaguchi H., Ogawa M., Wang T. C., Lee J. R., Goldenring J. R. (2005) Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G362–G375 [DOI] [PubMed] [Google Scholar]
- 48. Goldenring J. R., Nam K. T., Wang T. C., Mills J. C., Wright N. A. (2010) Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia. Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 138, 2207–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmidt P. H., Lee J. R., Joshi V., Playford R. J., Poulsom R., Wright N. A., Goldenring J. R. (1999) Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab. Invest. 79, 639–646 [PMC free article] [PubMed] [Google Scholar]
- 50. Chen Y. C., Lu Y. F., Li I. C., Hwang S. P. (2012) Zebrafish agr2 is required for terminal differentiation of intestinal goblet cells. PLoS ONE 7, e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fukuhara H., Kadowaki Y., Ose T., Monowar A., Imaoka H., Ishihara S., Takasawa S., Kinoshita Y. (2010) In vivo evidence for the role of RegI in gastric regeneration. Transgenic overexpression of RegI accelerates the healing of experimental gastric ulcers. Lab. Invest. 90, 556–565 [DOI] [PubMed] [Google Scholar]
- 52. Miyaoka Y., Kadowaki Y., Ishihara S., Ose T., Fukuhara H., Kazumori H., Takasawa S., Okamoto H., Chiba T., Kinoshita Y. (2004) Transgenic overexpression of Reg protein caused gastric cell proliferation and differentiation along parietal cell and chief cell lineages. Oncogene 23, 3572–3579 [DOI] [PubMed] [Google Scholar]
- 53. Yamaoka T., Yoshino K., Yamada T., Idehara C., Hoque M. O., Moritani M., Yoshimoto K., Hata J., Itakura M. (2000) Diabetes and tumor formation in transgenic mice expressing Reg I. Biochem. Biophys. Res. Commun. 278, 368–376 [DOI] [PubMed] [Google Scholar]
- 54. Steele I. A., Dimaline R., Pritchard D. M., Peek R. M., Jr., Wang T. C., Dockray G. J., Varro A. (2007) Helicobacter and gastrin stimulate Reg1 expression in gastric epithelial cells through distinct promoter elements. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G347–G354 [DOI] [PubMed] [Google Scholar]
- 55. Formeister E. J., Sionas A. L., Lorance D. K., Barkley C. L., Lee G. H., Magness S. T. (2009) Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1108–G1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mori-Akiyama Y., van den Born M., van Es J. H., Hamilton S. R., Adams H. P., Zhang J., Clevers H., de Crombrugghe B. (2007) SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 133, 539–546 [DOI] [PubMed] [Google Scholar]
- 57. Zalzali H., Naudin C., Bastide P., Quittau-Prévostel C., Yaghi C., Poulat F., Jay P., Blache P. (2008) CEACAM1, a SOX9 direct transcriptional target identified in the colon epithelium. Oncogene 27, 7131–7138 [DOI] [PubMed] [Google Scholar]
- 58. Lee E. R. (1985) Dynamic histology of the antral epithelium in the mouse stomach. I. Architecture of antral units. Am. J. Anat. 172, 187–204 [DOI] [PubMed] [Google Scholar]