Background: The pathway(s) of light-independent H2 production in green algae are yet unknown.
Results: Pyruvate:ferredoxin oxidoreductase PFR1 and [Fe-Fe]-hydrogenase HYDA1 of Chlamydomonas can be coupled for pyruvate-dependent H2 production.
Conclusion: H2 production by green algae in the dark is similar to bacterial PFOR-dependent fermentation.
Significance: Understanding the fermentation metabolism of green algae allows insights into plastid bioenergetic pathways.
Keywords: Algae, Chlamydomonas, Hydrogenase, Iron-Sulfur Protein, Metabolism, Fermentation, Hydrogen, Pyruvate:Ferredoxin Oxidoreductase
Abstract
In anaerobiosis, the green alga Chlamydomonas reinhardtii evolves molecular hydrogen (H2) as one of several fermentation products. H2 is generated mostly by the [Fe-Fe]-hydrogenase HYDA1, which uses plant type ferredoxin PETF/FDX1 (PETF) as an electron donor. Dark fermentation of the alga is mainly of the mixed acid type, because formate, ethanol, and acetate are generated by a pyruvate:formate lyase pathway similar to Escherichia coli. However, C. reinhardtii also possesses the pyruvate:ferredoxin oxidoreductase PFR1, which, like pyruvate:formate lyase and HYDA1, is localized in the chloroplast. PFR1 has long been suggested to be responsible for the low but significant H2 accumulation in the dark because the catalytic mechanism of pyruvate:ferredoxin oxidoreductase involves the reduction of ferredoxin. With the aim of proving the biochemical feasibility of the postulated reaction, we have heterologously expressed the PFR1 gene in E. coli. Purified recombinant PFR1 is able to transfer electrons from pyruvate to HYDA1, using the ferredoxins PETF and FDX2 as electron carriers. The high reactivity of PFR1 toward oxaloacetate indicates that in vivo, fermentation might also be coupled to an anaerobically active glyoxylate cycle. Our results suggest that C. reinhardtii employs a clostridial type H2 production pathway in the dark, especially because C. reinhardtii PFR1 was also able to allow H2 evolution in reaction mixtures containing Clostridium acetobutylicum 2[4Fe-4S]-ferredoxin and [Fe-Fe]-hydrogenase HYDA.
Introduction
Chlamydomonas reinhardtii is a photoautotrophic eukaryote that is equipped with a repertoire of fermentative enzymes allowing the cells to perform a mixed acid type fermentation (1–4). Of special interest for biotechnological applications is the capability of the cells to generate molecular hydrogen (H2) in the absence of oxygen (O2) (5). H2 is generated by a highly efficient hydrogenase of the [Fe-Fe] type, HYDA1 (6–8), which, despite its extreme sensitivity toward O2 (9–11), is located in the chloroplast (6). The natural electron donor of HYDA1 is the photosynthetic ferredoxin PETF (7, 12), and the highest rates of H2 evolution are observed in the light (4, 13). However, the cells have to be adapted to dark anaerobic conditions to induce hydrogenase activity (7, 14). Upon the shift to illumination, H2 production is only transient, because the hydrogenase enzymes are inactivated by photosynthetically generated O2 (15), and assimilatory photosynthetic electron sinks, the Calvin cycle above all, are reactivated (16, 17). However, a sustained H2 metabolism in illuminated C. reinhardtii cells is induced by sulfur deprivation (18). Sulfur deficiency results in a strongly reduced photosystem 2 activity, mainly because of photo-damage and inadequate recycling of the D1 core subunit of photosystem 2 (19–21). Hence, incubated in sealed flasks, sulfur-deficient algae establish hypoxic conditions despite illumination, because O2 evolution rates drop below respiratory O2 consumption (18). Anerobiosis elicits hydrogenase gene expression (22, 23) and allows the O2-intolerant enzyme to be active. However, only the diminution of assimilatory electron sinks caused by cessation of cell division (18, 21) allows sustained and relatively high H2 evolution rates (17). Electrons for photosynthetic H2 production originate from residual photosystem 2 activity (24–26) but also from nonphotochemical plastoquinone reduction via plastidic NAD(P)H:plastoquinone oxidoreductase NDA2 (27–29). Electrons for this photosystem 2-independent, so-called indirect pathway result from oxidative starch and possibly protein degradation (18, 24, 26). It is generally accepted that in sulfur-deprived C. reinhardtii cells, H2 generation serves as an alternative electron sink, allowing photosynthetic electron transport and thus energy generation to continue while preventing an over-reduction of the photosynthetic machinery (18, 25).
H2 production in nutrient-deficient green algae, however, is not the only pathway allowing the cells to maintain an energy and redox balance. Rather, the cells accumulate formate and ethanol simultaneously (30–32). The excretion of nongaseous fermentation products prevails in anaerobic Chlamydomonas cells in the dark (4). The generation of formate, ethanol, and acetate in a ratio of 2:1:1 in dark-incubated algae resembles mixed acid fermentation of prokaryotes like the enterobacterium Escherichia coli (33). The presence of a pyruvate:formate lyase (PFL1), catalyzing the thioclastic cleavage of pyruvate to formate and acetyl-CoA in the eukaryotic alga, was therefore proposed (34) and genetically and biochemically proven (2, 30, 35). Because C. reinhardtii does not contain genes for a formate-hydrogen lyase complex that is responsible for formate-dependent H2 generation in fermenting E. coli (33), light-independent H2 production in the green alga was proposed to result from pyruvate:ferredoxin oxidoreductase (PFOR)2 activity (36). A Chlamydomonas PFOR (PFR1) was identified on the genetic (2) and protein levels, and the protein is located in the chloroplast of the cells (37, 38). PFOR enzymes oxidatively decarboxylate pyruvate to yield acetyl-CoA and CO2, simultaneously transferring electrons to flavodoxin or ferredoxin (39). Thereby, ferredoxin reduction by plastidic C. reinhardtii PFR1 might couple fermentative pyruvate catabolism to H2 generation in a way typical for strict anaerobic bacteria of the genus Clostridium (40–43).
In this study we show that C. reinhardtii PFR1 heterologously produced in E. coli is active in pyruvate- and oxaloacetate-dependent methyl viologen reduction. Moreover, PFR1 enables methyl viologen- or ferredoxin-dependent H2 production by isolated Chlamydomonas HYDA1, which proves that the long postulated pathway of dark H2 generation in the green alga is biochemically possible.
EXPERIMENTAL PROCEDURES
Organisms and Growth Conditions
E. coli strain DH5α MCR (Novagen) was used for cloning procedures. Heterologous expression of C. reinhardtii PFR1 and ferredoxin encoding cDNAs was done in E. coli BL21 (DE3) ΔiscR (44, 45). E. coli strains were grown according to standard procedures as described before (30). Clostridium acetobutylicum ATCC 824 was used for heterologous synthesis of C. reinhardtii HYDA1 and homologous expression of C. acetobutylicum [Fe-Fe]-hydrogenase HYDA and ferredoxin CAC0303.
C. reinhardtii strain CC124 (137c, mt− nit1− nit2−) was grown in Tris acetate-phosphate (TAP) medium (46) on a shaker with bottom-up illumination of 100 μmol of photons·m−2·s−1 at 20 °C. For determination of in vivo H2 production rates in the light or in the dark, C. reinhardtii cultures were grown until they reached a chlorophyll (Chl) content of 15 μg·ml−1, harvested by mild centrifugation (2,000 × g, 3 min, 20 °C), and resuspended in fresh TAP medium to reach a final Chl concentration of 110 μg·ml−1. The cell suspension was transferred to a shaded flask and purged with nitrogen gas for 4 h. In vitro hydrogenase activity was determined as described before (47) to ensure the anaerobic induction. Afterward, 2-ml aliquots of the cell culture were withdrawn using a syringe and transferred to sealed and O2-free head space bottles. Half of the withdrawn 2-ml aliquots were incubated in the dark and half in the light (100 μmol of photons·m−2·s−1). The H2 amount in the head space was analyzed after various time points of incubation by gas chromatography as reported previously (35).
Cloning of C. reinhardtii PFR1 cDNA
Total RNA of anaerobic algal cells was isolated according to Philipps et al. 2011 (35), and cDNA was synthesized after DNase digestion (Turbo DNAfree kit; Ambion) using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. The PFR1 coding region was amplified from this cDNA with hot start Pfu DNA polymerase (Stratagene) using oligonucleotides 5′-TTGGATCCCCCGCCGCTGCTGGCCGCGCCACCAACG-3′ and 5′-TAGATATCACGTCCTTCTATTCTAGAGTGGCCGCCGCAGCCGCTCT-3′. The bold letters indicate restriction sites for BamHI and EcoRV used for cloning. The forward oligonucleotide was generated in a way that the protein encoded by the cDNA would lack the putative chloroplast target sequence indicated by the first VXA amino acid motif typical for cleavage of Chlamydomonas chloroplast targeting sequences (48). The sequence was ligated with vector pASK-IBA37 (IBA GmbH) via BamHI and EcoRV restriction sites. This resulted in construct pIBA37_PFR1, allowing a tightly regulated expression via the anhydrotetracycline inducible tet promoter and in recombinant PFR1 enzyme equipped with an N-terminal His6 tag.
Heterologous Production and Purification of His-tagged Recombinant PFR1 and Other Proteins
E. coli BL21 (DE3) ΔiscR (44) was transformed with vector pIBA37_PFR1 by electroporation (49). Precultures were grown shaking in 200 ml of LB medium at 37 °C overnight and used to inoculate four 2-liter flasks, each containing 500 ml of Vogel-Bonner minimal medium (50) with 100 μg·ml−1 ampicillin, 0.2 μm of the O2 indicator resazurin, and 50 μm thiamine hydrochloride. As soon as the cultures had reached an A600 of 0.6 after growth at 37 °C, 5 g of glucose·l−1 was added to the medium, and the cell suspensions were transferred to a 2-liter bottle. Expression of the PFR1 cDNA was induced by adding 0.2 μg·ml−1 anhydrotetracycline. The flasks were sealed air tight and incubated at 8 °C overnight. Then the suspension was transferred to centrifugation flasks in an anaerobic tent (Toepfer LabSystems), and the cells were harvested by centrifugation (20 min at 7,500 × g, 4 °C). All further steps were also conducted under strictly anaerobic conditions (1% H2, 99% N2) in the anaerobic tent. The E. coli cell pellet was resuspended in O2-free buffer (100 mm Tris-HCl, pH 8.0, 10% (v/v) glycerol), and the cells were lysed by sonication (five times for 30 s; output, 25; Branson Sonifier 250). Cell debris was removed by centrifugation (60 min, 200,000 × g, 4 °C). The soluble fraction was filtered through sterile filters (pore size, 0.2 μm; Sarstedt AG & Co.) and loaded on a 4-ml gravity flow nickel-nitrilotriacetic acid fast flow column pre-equilibrated with 100 mm Tris-HCl, pH 8.0, 10 mm imidazole, and 0.5 mm thiamine pyrophosphate (TPP). Removal of unspecifically binding proteins was obtained by washing the column with the above mentioned buffer containing increased concentrations of imidazole (40-ml steps with 10 and 20 mm imidazole). His-tagged PFR1 was eluted in 1.2-ml steps using 10 ml of buffer containing 100 mm imidazole. Protein concentration of the main fraction was determined spectrophotometrically (NanoDrop; Peqlab) at λ = 280 nm, and this protein solution was used for further analysis. The size and purity of the eluted protein fractions were analyzed by denaturating SDS-PAGE according to standard techniques (51). PFR1 activity tests were conducted immediately after purification.
Heterologous production of C. reinhardtii [Fe-Fe]-hydrogenase HYDA1, as well as homologous production of HYDA in C. acetobutylicum and subsequent purification via Strep-tag II was done as described before (52, 53). Recombinant Chlamydomonas [2Fe2S]-ferredoxins PETF and FDX5 were obtained as published previously (12, 54). C. reinhardtii FDX2 was produced accordingly by amplifying the FDX2 coding region without the N-terminal transit peptide encoding sequence using oligonucleotides 5′-ATGGTAGGTCTCAGCGCCACTTTAAGGTCACGTTTAAGACC-3′ and 5′-ATGGTAGGTCTCATATCAGAGCTTGGACTCCTGGTCGGT-3′ (BsaI restriction sites are indicated by bold letters). The C. acetobutylicum ferredoxin CAC0303 encoding region was amplified from the E. coli expression vector pET21c0303 (55) using hot start Pfu DNA polymerase (Stratagene) with oligonucleotides 5′-GAAGGATCCGCATATAAAATAACAGACGCTTGTG-3′ and 5′-AAGCGGCGCCACGGAGCTCGAATTCT-3′. The bold letters mark the BamHI and EheI restriction sites used for ligation with the clostridial expression vector pThydACR1-C-tag after excision of its insert (52). All further steps (transformation and cultivation of C. acetobutylicum, as well as protein purification) were done as described before (53).
PFR1 Activity Assays Using Methyl Viologen as Artificial Electron Acceptor
The enzymatic activity of recombinant C. reinhardtii PFR1 was determined by following the reduction of methyl viologen spectrophotometrically at λ = 604 nm (56) using a 96-well plate reader (Beckmann, Paradigm 1113) operated in an anaerobic tent and connected to a PC running multimode analysis software. The molar extinction coefficient of methyl viologen used was ϵ604 = 13.6 mm−1 cm−1 (57). The standard reaction mixture contained 1.4 μm recombinant PFR1, 100 mm Tris-HCl, pH 8.0, 10 mm sodium pyruvate, 2 mm sodium CoA, 5 mm TPP, 10 mm methyl viologen, and 16 mm dithioerythritol in a final volume of 100 μl. The reaction was started by adding PFR1 and conducted at room temperature. Absorbance was measured every 30 s until saturation was reached. The value obtained after 6 min, which was in the late linear phase, was used for determining activity. Kinetics were performed varying the concentration of one substrate while keeping the concentrations of all other substrates constant and saturating. The Km and Vmax values were determined in each reaction and calculated using GraphPad Prism® software.
In Vitro Reconstitution of PFR1-coupled H2 Production
For analyzing the H2 producing capacity of [Fe-Fe]-hydrogenases upon electron delivery by PFR1-catalyzed ferredoxin reduction, recombinant enzymes and proteins were mixed in various combinations. The standard reaction mixture contained 0.7 μm recombinant PFR1, 40 μm ferredoxin, 0.01 μm hydrogenase, 10 mm sodium pyruvate, 2 mm sodium CoA, 5 mm TPP, and 16 mm dithioerythritol in 200 μl of 100 mm potassium-phosphate buffer, pH 6.8. The reactions were carried out in sealed 2-ml reaction vessels. Before incubation, the reaction mixtures were purged with argon for 3 min to reset the system. After incubation for 30 min at 37 °C in a shaking water bath, 400 μl of the head space were injected in a gas chromatograph (GC-2010 (Shimadzu), equipped with a PLOT fused silica coating molsieve column (5 Å, 10 m × 0.32 mm) from Varian) to determine the H2 concentration.
RESULTS
Recombinant C. reinhardtii PFR1 Has Typical PFOR Activity
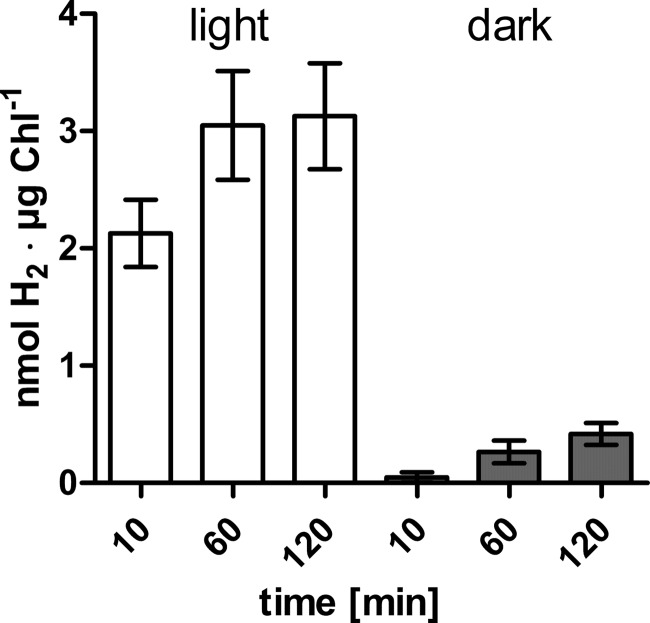
As described in the introduction, in vivo H2 production in C. reinhardtii is higher in the light, because electrons are provided by photosynthetic activity. We compared the light-dependent and -independent H2 evolution rates of anaerobically adapted Chlamydomonas cell suspensions in a setup moderately modified from those reported before (as in Ref. 35, for example) (Fig. 1). In cells transferred from anaerobic conditions in the dark to illumination, H2 accumulated to 2.13 nmol of H2·μg Chl−1 within the first 10 min, whereas cells kept in the dark produced only 0.05 nmol of H2·μg Chl−1 in the same time period (Fig. 1). In the following 50 min, the cells exposed to light produced additional 0.92 nmol of H2·μg Chl−1 (plus 43.2%) and shaded cells generated 0.22 nmol of H2·μg Chl−1 (plus 437.5%). In both illuminated and dark-incubated C. reinhardtii cell suspensions, H2 generation rates slowed down in the following hour, because the former evolved 0.078 nmol of H2·μg Chl−1 and the latter evolved 0.152 nmol of H2·μg Chl−1 (Fig. 1). To analyze whether the low but significant H2 production in dark-adapted algae might be driven by pyruvate oxidation via PFOR activity, Chlamydomonas PFR1 was heterologously produced.
FIGURE 1.
In vivo H2 evolution rates of C. reinhardtii cultures in the light or in the dark. Concentrated cell suspensions were flushed with nitrogen for 4 h until they had reached an in vitro hydrogenase activity of 109 ± 18 nmol of H2·μg Chl−1·h−1. Then culture aliquots were withdrawn, transferred to gas tight head space bottles, and incubated in the light (white bars) or the dark (gray bars) until the indicated time points before determining the H2 concentration of the head space by gas chromatography. The results shown are the mean values from three independent experiments carried out as technical duplicates. The error bars indicate the standard deviation.
The annotated gene models of the C. reinhardtii PFR1 gene have changed considerably from the first Chlamydomonas genome version to the most recent annotation on Phytozome v8.0, C. reinhardtii v5.3. Although most parts of the primary sequences are the same in the newest gene models (Cre11.g473950.t1.1 and g1910.t2 on Phytozome v8.0, C. reinhardtii v4.3 and v5.3, respectively, and au5.g2553_t1 and SKA_Chlre2_kg.scaffold_62000019 on JGIv4), a region starting at position 940 in the Cre11.g473950.t1.1 protein model is highly variable. We aligned all available PFR1 models, as well as the sequence translated from the PFR1 cDNA amplified in this study with bacterial enzymes and concluded that the cDNA and protein sequences, respectively, obtained here are correct (i.e., amino acids AKKWVLFCARLLTQ starting at position 940 in Cre11.g473950.t1.1 are actually missing). Therefore, we used the protein sequence deduced from our cDNA for the alignment shown in Fig. 2. The alignments revealed that the C. reinhardtii PFR1 polypeptide sequence contains all sequence motifs known to be essential for PFOR enzyme activity (marked in Fig. 2). It features an N-terminal conserved 2-oxoacid-binding site (YPITP) (58), a C-terminal TPP-binding site (59), and three [4Fe-4S]-cluster-binding signatures, two of which are typical for 2[4Fe-4S]-ferredoxins and one of which is atypical (60, 61) (Fig. 2). The comparisons with bacterial enzymes revealed that the C. reinhardtii PFR1 sequence contains an N-terminal extension that is not homologous to other PFOR proteins. Because PFR1 was shown to be localized in the chloroplast (37), we assumed that the first VXA amino acid motif (starting at position 24 of Cre11.g473950.t1.1) might represent a chloroplast targeting sequence cleavage site (48). Therefore, we excluded the respective region encoding these first 24 residues from the cDNA used for heterologous production of PFR1.
FIGURE 2.
Stacked polypeptide alignment of pyruvate:ferredoxin oxidoreductase primary sequences. Eight PFOR sequences were used for an alignment using ClustalW2 and WebLogo 3 (89, 90). These sequences were C. reinhardtii PFR1 derived from the cDNA obtained in this study, Volvox carteri f. nagariensis (Phytozome v8.0, V. carteri Vocar20008508m), Chlorella variabilis (GenBankTM EFN55341.1), C. acetobutylicum PFO (GenBankTM NP_348846.1), Clostridium pasteurianum (GenBankTM AAD55756.1), Desulfovibrio africanus POR (GenBankTM CAA70873.1), E. coli YdbK (GenBankTM YP_002999180.1), and Synechococcus sp. PCC.7002 NifJ (GenBankTM ACA99434.1). The conserved YPITP substrate-binding site (58), as well as three [4Fe-4S]-cluster coordinating motifs and the region homologous to TPP-binding (59, 91) sites, are underlined. Note that the third [4Fe-4S]-cluster-binding site is atypical and consists of the CXXC motif at positions 942–945 and two separated cysteines at positions 970 and 1221 (60, 61). The first amino acid of recombinant PFR1 is marked by an asterisk.
After heterologous expression of the truncated PFR1 cDNA in E. coli and subsequent purification of the His-tagged protein via nickel-nitrilotriacetic acid chromatography, a protein of the expected size (144 kDa) could be eluted. Activity assays using pyruvate and CoA as substrates and methyl viologen as artificial electron acceptor resulted in a specific activity of 0.45 ± 0.01 units·mg−1 (1 unit was defined as the conversion of 1 μmol of pyruvate or CoA and the reduction of 2 μmol of methyl viologen, respectively, per minute) (Fig. 3). The Km values obtained for methyl viologen, CoA, and pyruvate in this assay were 2.3, 0.7, and 1.7 mm, respectively (Fig. 3).
FIGURE 3.
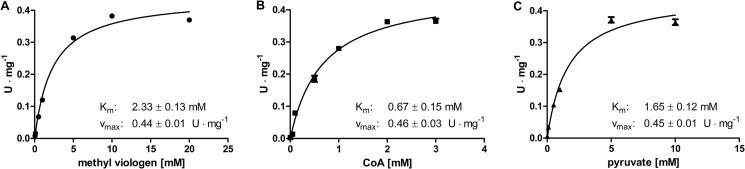
Kinetic parameters of recombinant C. reinhardtii PFR1 heterologously produced in E. coli and purified via His-tag affinity chromatography. Enzymatic activity was determined following the reduction of methyl viologen spectrophotometrically in 100-μl reaction mixtures containing 1.4 μm PFR1, 5 mm TPP, 16 mm dithioerythritol in 100 mm Tris-HCl, pH 8. The Km values of the individual substrates were determined in the presence of 2 mm CoA and 10 mm pyruvate (A, methyl viologen), 10 mm methyl viologen and 10 mm pyruvate (B, CoA), or 2 mm CoA and 10 mm methyl viologen (C, pyruvate). Each kinetic was analyzed from two independent PFR1 preparations as technical duplicates. The Km and Vmax values were calculated using GraphPad Prism® software. The graphs show the mean values, and the error bars indicate the standard deviation.
Chlamydomonas PFR1 Allows Pyruvate-dependent H2 Production
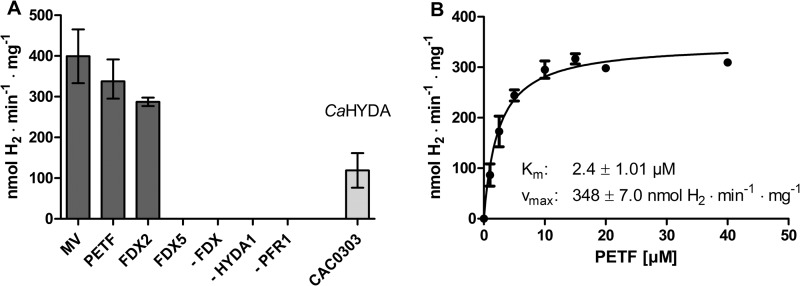
To analyze whether PFR1-catalyzed pyruvate oxidation would allow H2 production by C. reinhardtii HYDA1, reconstitution assays were performed in which various combinations of electron carriers (methyl viologen, and ferredoxins) were mixed. The combination of recombinant PFR1 and C. reinhardtii HYDA1 in the presence of pyruvate, CoA, and methyl viologen as artificial electron carrier resulted in a H2 production rate of 400 ± 66 nmol of H2·min−1·mg PFR1−1, which was only slightly higher than the rate obtained with Chlamydomonas ferredoxin PETF (Fig. 4A; 338 ± 32 nmol of H2·min−1·mg PFR1−1). The Km value of PFR1 for PETF was determined as 2.4 ± 0.34 μm (Fig. 4B), which is considerably lower than the Km value of HYDA1 for PETF (20–30 μm) (7, 12). We also examined whether two further ferredoxins, FDX2 and FDX5, would allow PFR1-dependent H2 evolution. FDX2 (62) and especially FDX5 transcripts and FDX5 protein (54, 63, 64) have been shown to accumulate in anaerobic Chlamydomonas cells, which makes them candidates for being involved in pathways specific for anaerobiosis. Furthermore, both ferredoxin isoforms are localized in the plastid (54, 62) together with HYDA1 and PFR1. Using FDX2 as electron carrier, a PFR1-dependent H2 evolution rate of 287 ± 15 nmol of H2·min−1·mg PFR1−1 could be observed, whereas no activity was determined using FDX5 (Fig. 4A). Notably, recombinant C. reinhardtii PFR1 also allowed H2 generation of C. acetobutylicum HYDA in the presence of the clostridial ferredoxin CAC0303 in a rate of 118 ± 35 nmol of H2·min−1·mg PFR1−1 (Fig. 4A).
FIGURE 4.
H2 generation in reconstitution assays of recombinant C. reinhardtii PFR1 and [Fe-Fe]-hydrogenases. A, each reaction contained PFR1 (0.7 μm) and 0.01 μm HYDA1 of C. reinhardtii (except one reaction, which contained C. acetobutylicum ferredoxin (CAC0303) and [Fe-Fe]-hydrogenase HYDA, indicated by the label CaHYDA), 10 mm pyruvate, and 2 mm CoA in 100 mm potassium phosphate buffer, pH 6.8. Electron carriers were applied as indicated below the x axis (10 mm methyl viologen (MV), 40 μm of the Chlamydomonas ferredoxins PETF, FDX2, or FDX5, or 40 μm clostridial ferredoxin CAC0303). The reaction mixtures were incubated for 30 min at 37 °C before analyzing the amount of H2 in the gas phase. As controls, reaction mixtures were analyzed that lacked one of the enzymes or proteins indicated by a dash. B, the dependence of PFR1-coupled H2 generation on PETF concentration was determined using C. reinhardtii HYDA1 in reaction mixtures as described for A and the indicated concentrations of PETF. The values and standard deviations shown in all of the experiments are from three independent PFR1 preparations, and the H2 production rate was related to mg of PFR1 enzyme. The error bars indicate the standard deviation.
Using the amount of recombinant C. reinhardtii HYDA1 enzyme as a basis, the PFR1- and methyl viologen-mediated H2 production rates (80 ± 13 μmol of H2·min−1·mg HYDA1−1) were only 16% of those determined with sodium diothionite-reduced methyl viologen (516 ± 42 μmol of H2·min−1·mg HYDA1−1; Table 1). However, in the presence of PETF as the electron carrier, the reaction driven by 0.7 μm PFR1 reached 42% of the reaction in which sodium diothionite served as chemical electron donor (68 ± 7 versus 160 ± 17 μmol of H2·min−1·mg HYDA1−1; Table 1). In this reaction mixture, 0.7 μm PFR1 was close to saturation because the rates obtained using 0.35 μm PFR1 were 61 ± 9, and those in assays containing 0.9 μm PFR1 were 72 ± 8 μmol of H2·min−1·mg HYDA1−1.
TABLE 1.
H2 evolution rates of C. reinhardtii HYDA1 using electrons provided by sodium dithionite (NaDT) or pyruvate oxidation via PFR1 related to the amounts of hydrogenase protein
All of the reactions contained 100 mm potassium phosphate buffer and 0.01 μm C. reinhardtii HYDA1. PFR1-containing reactions included 10 mm pyruvate and 2 mm CoA. The values shown were derived from three independent PFR1 and HYDA1 preparations ± standard deviation.
| Reaction components | H2·min−1·mg HYDA1−1 |
|---|---|
| μmol | |
| 100 mm NaDT, 10 mm methyl viologen | 516 ± 42 |
| 50 mm NaDT, 40 μm PETF | 160 ± 17 |
| 0.7 μm PFR1, 10 mm methyl viologen | 80 ± 13 |
| 0.7 μm PFR1, 40 μm PETF | 68 ± 7 |
PFR1 Is Able to Use Oxaloacetate, but Not α-Ketoglutarate as a Substrate
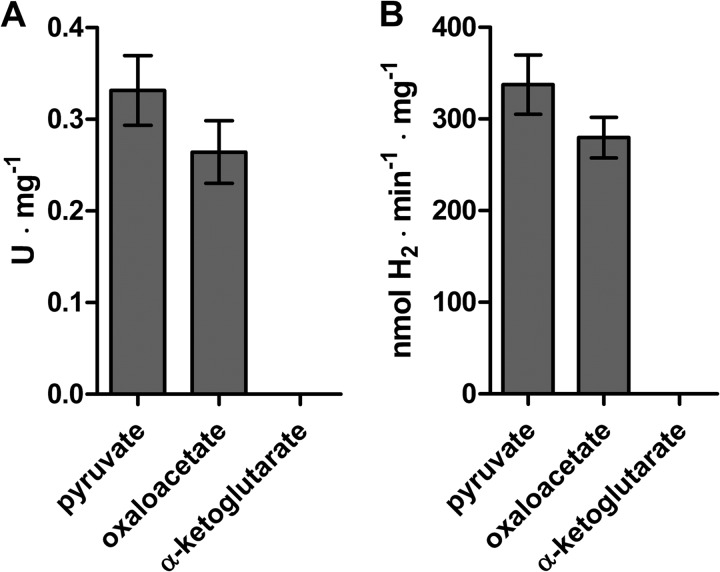
Some bacterial PFOR enzymes have been reported to be able to oxidize various substrates such as 2-oxoacid:ferredoxin oxidoreductase from Sulfolobus species strain 7 or Sulfolobus solfataricus P1 (58, 65), whereas others such as PFOR from E. coli can only oxidize pyruvate (66). We examined the methyl viologen reducing activity of C. reinhardtii PFR1 in the presence of oxaloacetate and α-ketoglutarate. When oxaloacetate was included in the reaction mixture, a specific activity of 0.26 ± 0.03 U·mg−1 was observed, but no methyl viologen reduction could be detected using α-ketoglutarate (Fig. 5A). In a reconstitution assay using the same substrates but using the electron carrier PETF and the [Fe-Fe]-hydrogenase HYDA1 to examine substrate-dependent H2 evolution, oxaloacetate was almost as suitable as pyruvate, whereas no H2 production could be observed in the presence of α-ketoglutarate (Fig. 5B).
FIGURE 5.
Substrate specificity of C. reinhardtii PFR1. The capacity of PFR1 to reduce methyl viologen (A) and to drive H2 evolution by C. reinhardtii HYDA1 (B) using oxaloacetate or α-ketoglutarate was examined. The reaction mixtures contained 10 mm pyruvate, oxaloacetate, or α-ketoglutarate and 2 mm CoA in 100 mm potassium phosphate buffer, pH 6.8, and additionally 1.4 μm PFR1 and 10 mm methyl viologen (A) or 0.7 μm PFR1, 40 μm PETF, and 0.01 μm HYDA1 (B). A, methyl viologen reduction was determined spectrophotometrically. B, H2 evolution rates were determined by gas chromatography as described in the legend of Fig. 4. The results shown are the means and standard deviations from two independent experiments carried out as technical duplicates.
DISCUSSION
C. reinhardtii has long been known for its complex mixed acid fermentative metabolism, which has more similarities to fermentation of bacteria or strictly anaerobic protists than to plant or animal anaerobic pathways (1, 2, 4, 34). In addition to PFL1, which is mainly known from enterobacteria such as E. coli (33), a cDNA encoding pyruvate:ferredoxin oxidoreductase was identified in C. reinhardtii (2) and shown to accumulate in anaerobic algal cells (30, 63). PFR1 was proposed to be involved in dark H2 production by C. reinhardtii (36, 63) in analogy to fermentative H2 production in clostridiae (40, 42, 67) or anaerobic hydrogenosome containing protists (68, 69). This model was supported by the phenotype of a Chlamydomonas pfl1 mutant strain, which showed higher yields of ethanol, CO2, and H2 generation in the dark (35). The fermentative pattern of the mutant was interpreted in a way that the absence of PFL1 would result in higher pyruvate supply to PFR1, which reduces ferredoxin upon oxidative decarboxylation of pyruvate to acetyl-CoA and CO2. In contrast, further allelic pfl1 mutants reported recently do not exhibit higher dark H2 generation in a different experimental setup (70). Other metabolic pathways providing electrons for H2 generation in the dark were also discussed, such as ferredoxin reduction by FNR in analogy to the pathways allowing sulfate assimilation in the roots of higher plants (71).
In this study we show that PFR1-driven H2 production is biochemically plausible, because recombinant C. reinhardtii PFR1 is able to allow H2 generation by isolated [Fe-Fe]-hydrogenases. Although these results cannot prove that this reaction occurs in living Chlamydomonas cells, they indicate that the PFR1-dependent pyruvate to H2 pathway can be operable in vivo (Fig. 6). The biochemical properties of the purified PFR1 protein are similar to PFOR enzymes isolated and characterized from other organisms regarding pyruvate- and CoA-dependent methyl viologen reduction (72, 73). The Km values for pyruvate and methyl viologen were in the range of the Km values determined for other PFOR enzymes, whereas the Km for CoA was higher (74–76). Also, the specific activity of our PFR1 preparations were at the lower range when compared with other PFOR enzymes (74, 77, 78). This might support the physiological data obtained so far, which all speak for PFL1 being the major fermentative enzyme in C. reinhardtii wild type cultures (4, 30, 35). However, we cannot exclude that the protein solution contained inactive PFR1 enzymes. We observed a marked instability of the enzyme, despite its isolation and examination under strictly anaerobic conditions sufficient for the analysis of the extremely O2-sensitive [Fe-Fe]-hydrogenase of C. reinhardtii (9–11). Instability of isolated PFOR enzymes has been reported before (79), and besides destruction by O2 (73, 80), loss of the TPP factor has been proposed to be one reason for this phenomenon (75).
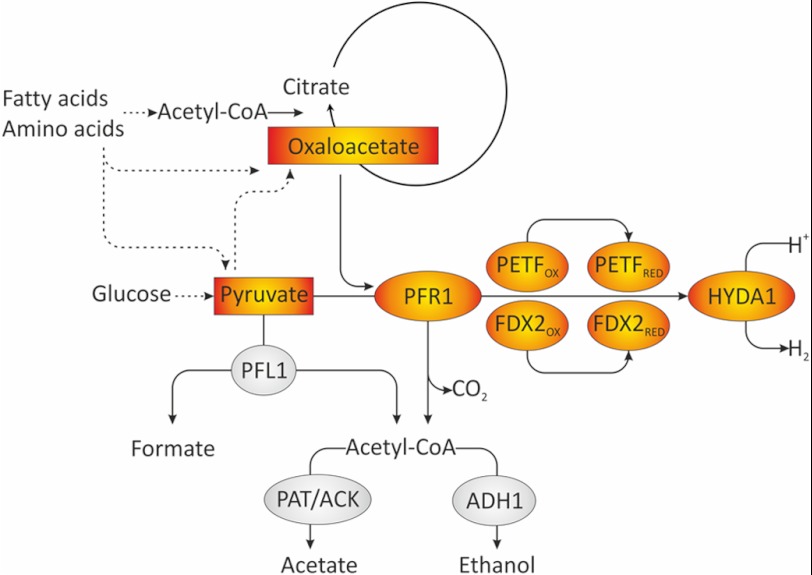
FIGURE 6.
Model of fermentative pathways involved in dark anaerobic H2 production in C. reinhardtii. In the wild type, PFL1 is the major fermentative enzyme in short term anaerobiosis and cleaves pyruvate into formate and acetyl-CoA. The latter can be reduced to ethanol via bifunctional acetaldehyde-alcohol dehydrogenase (ADH1) (92) or converted to acetate via phosphotransacetylase and acetate kinase (PAT/ACK). In addition to PFL1, PFR1 is capable of pyruvate oxidation. In a pfl1 mutant or in long term anaerobiosis, pyruvate oxidation might be preferably catalyzed by PFR1. PFR1 transfers electrons to ferredoxins and thereby allows H2 generation via the [Fe-Fe]-hydrogenase. In addition to pyruvate, PFR1 is able to use oxaloacetate as a substrate. This might link the oxidation of other substrates such as fatty acids or amino acids to fermentative H2 production, possibly via an anaerobically operating glyoxylate cycle or parts thereof.
In reaction mixtures combining PFR1 with C. reinhardtii HYDA1 and ferredoxin PETF, which is the most suitable redox partner for HYDA1 known so far (12), a pyruvate-dependent H2 production could be observed, showing that the postulated reaction is possible. Notably, PFR1-driven H2 generation via PETF was only 2.5-fold lower than H2 generation using sodium dithionite-reduced PETF as an electron donor for HYDA1. This indicates that the capacity of PFR1-coupled H2 production is quite high, despite the low specific activity of PFR1. We assume that both the low Km value of PFR1 for PETF and the high specific activity of HYDA1 contribute to the high efficiency of the coupled system. The low rates of in vivo H2 evolution in dark-incubated algal cells are therefore probably limited by PFR1 substrate supply rather than by PFR1 activity.
PFR1-mediated H2 production by HYDA1 was also possible using FDX2 as an electron carrier (Fig. 6), and the rates obtained were only moderately lower than with PETF. Notably, FDX2 lacks one phenylalanine residue that, in PETF, is essential for proper interaction with HYDA1 (12, 81). The capability of HYDA1 to generate H2 using FDX2 as an electron donor in the PFR1-driven system might therefore indicate a different interaction mechanism. FDX2 was suggested to be specifically involved in nitrite reduction. The protein can hardly be detected in C. reinhardtii cells incubated in ammonium-containing medium but accumulates in cells growing on nitrate and allows a higher catalytic activity of Chlamydomonas nitrite reductase than PETF (62). However, because FDX2 was also as efficient as PETF for FNR catalytic activity and even better regarding affinity (62), FDX2 might in general be used for reactions that can also employ PETF.
The results obtained with FDX2 indicated that the electron transfer reaction coupling PFR1 and HYDA1 might be different from the other electron delivering reactions analyzed so far. Therefore we analyzed whether FDX5 was able to shuttle electrons between PFR1 and HYDA1, although FDX5 is unable to drive H2 generation activity upon artificial reduction (54). The FDX5 gene is strongly induced upon anaerobiosis (54, 63) but also in copper-deficient C. reinhardtii cells (62, 64, 82). FDX5 is regulated by CRR1 (the copper response regulator 1) under both conditions (64). Notably, it was shown recently that the HYDA1 gene is also activated by the absence of copper (82) and partially regulated by CRR1 (83). Although a connection between FDX5 and HYDA1 might be suggested from these findings, the data presented here confirm that a direct metabolic interaction does not take place.
PFOR-mediated H2 generation is central to anaerobic energy generation in several strict anaerobes such as amitochondriates (69) and clostridiae (67). Notably, C. reinhardtii was able to allow C. acetobutylicum HYDA activity in the presence of clostridial 2[4Fe-4S]-ferredoxin. This indicates that PFR1 kept the basic features of the evolutionary old PFOR protein (68, 84), despite the fact that C. reinhardtii and clostridial PFOR sequences were calculated to be evolutionary distant (85). A similar promiscuity regarding redox partners was observed for other PFOR enzymes, such as Clostridium thermoaceticum (74) and Rhodobacter capsulatus PFORs (86).
Recombinant C. reinhardtii PFR1 had methyl viologen reducing and H2 driving activity also in the presence of oxaloacetate as substrate, whereas α-ketoglutarate did not result in measurable activity. Both metabolites are intermediates of the TCA cycle, whereas the branch via α-ketoglutarate and succinyl-CoA is absent in the glyoxylate cycle. An anaerobically operating glyoxylate cycle is active in the alga (87), and the oxidation of malate was suggested to contribute to H2 photoproduction (88). Pyruvate and oxaloacetate are furthermore end products of the catabolism of several amino acids. It might be assumed that oxaloacetate degradation by PFR1 supports the anaerobic operation of the glyoxylate cycle or parts thereof (Fig. 6). The physiological role of PFR1 in fermenting C. reinhardtii cells might therefore become important during long term fermentation. A 2:1:1 ratio of formate:ethanol:acetate production observed in algae after 4–6 h of anaerobiosis (4, 34, 35) is typical for pyruvate:formate lyase activity. However, formate production only prevails in the first hours of anaerobiosis, whereas ethanol and especially CO2 generation rates increase thereafter, simultaneously to a slowdown of starch degradation (34). Although speculative, a scenario might be envisioned in which PFL1 is mostly responsible for short term fermentation using starch and glucose, respectively, as substrate. In long term anaerobiosis, PFR1 activity would allow Chlamydomonas to utilize acetyl-CoA derived from fatty acids and end products of amino acid catabolism as energy sources (Fig. 6). Furthermore, the coupling to the hydrogenase is a means to dispose of electrons via the nontoxic and highly diffusible H2 molecule.
Acknowledgments
We are thankful for the gift of pET21c0303 from Laurence Girbal (Laboratoire d'Ingénierie des Systèmes Biologiques et des Procédés, Toulouse, France). We also thank Annika Brünje and Simone Schmidt for technical support during cloning of CAC0303 and FDX2.
This work was supported by grants from the Deutsches Zentrum für Luft- und Raumfahrt (ModuLES) and the Volkswagen Foundation (LigH2t) (to T. H.).
- PFOR
- pyruvate:ferredoxin oxidoreductase
- Chl
- chlorophyll
- TPP
- thiamine pyrophosphate
- PETF
- ferredoxin PETF/FDX1.
REFERENCES
- 1. Hemschemeier A., Happe T. (2005) The exceptional photofermentative hydrogen metabolism of the green alga Chlamydomonas reinhardtii. Biochem. Soc. Trans. 33, 39–41 [DOI] [PubMed] [Google Scholar]
- 2. Atteia A., van Lis R., Gelius-Dietrich G., Adrait A., Garin J., Joyard J., Rolland N., Martin W. (2006) Pyruvate formate-lyase and a novel route of eukaryotic ATP synthesis in Chlamydomonas mitochondria. J. Biol. Chem. 281, 9909–9918 [DOI] [PubMed] [Google Scholar]
- 3. Atteia A., van Lis R., Tielens A. G., Martin W. F. (2013) Anaerobic energy metabolism in unicellular photosynthetic eukaryotes. Biochim. Biophys. Acta 1827, 210–223 [DOI] [PubMed] [Google Scholar]
- 4. Gfeller R. P., Gibbs M. (1984) Fermentative metabolism of Chlamydomonas reinhardtii. I. Analysis of fermentative products from starch in dark and light. Plant Physiol. 75, 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemschemeier A., Happe T. (2011) Alternative photosynthetic electron transport pathways during anaerobiosis in the green alga Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1807, 919–926 [DOI] [PubMed] [Google Scholar]
- 6. Happe T., Mosler B., Naber J. D. (1994) Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 222, 769–774 [DOI] [PubMed] [Google Scholar]
- 7. Happe T., Naber J. D. (1993) Isolation, characterization and N-terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 214, 475–481 [DOI] [PubMed] [Google Scholar]
- 8. Kamp C., Silakov A., Winkler M., Reijerse E. J., Lubitz W., Happe T. (2008) Isolation and first EPR characterization of the [FeFe]-hydrogenases from green algae. Biochim. Biophys. Acta 1777, 410–416 [DOI] [PubMed] [Google Scholar]
- 9. Goldet G., Brandmayr C., Stripp S. T., Happe T., Cavazza C., Fontecilla-Camps J. C., Armstrong F. A. (2009) Electrochemical kinetic investigations of the reactions of [FeFe]-hydrogenases with carbon monoxide and oxygen. Comparing the importance of gas tunnels and active-site electronic/redox effects. J. Am. Chem. Soc. 131, 14979–14989 [DOI] [PubMed] [Google Scholar]
- 10. Lambertz C., Leidel N., Havelius K. G., Noth J., Chernev P., Winkler M., Happe T., Haumann M. (2011) O2 reactions at the six-iron active site (H-cluster) in [FeFe]-hydrogenase. J. Biol. Chem. 286, 40614–40623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stripp S. T., Goldet G., Brandmayr C., Sanganas O., Vincent K. A., Haumann M., Armstrong F. A., Happe T. (2009) How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc. Natl. Acad. Sci. U.S.A. 106, 17331–17336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winkler M., Kuhlgert S., Hippler M., Happe T. (2009) Characterization of the key step for light-driven hydrogen evolution in green algae. J. Biol. Chem. 284, 36620–36627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stuart T. S., Gaffron H. (1972) The mechanism of hydrogen photoproduction by several algae. Planta 106, 101–112 [DOI] [PubMed] [Google Scholar]
- 14. Gaffron H., Rubin J. (1942) Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 26, 219–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghirardi M. L., Togasaki R. K., Seibert M. (1997) Oxygen sensitivity of algal H2-production. Appl. Biochem. Biotechnol. 63–65, 141–151 [DOI] [PubMed] [Google Scholar]
- 16. Cinco R. M., MacInnis J. M., Greenbaum E. (1993) The role of carbon dioxide in light-activated hydrogen production by Chlamydomonas reinhardtii. Photosynth. Res. 38, 27–33 [DOI] [PubMed] [Google Scholar]
- 17. Rühle T., Hemschemeier A., Melis A., Happe T. (2008) A novel screening protocol for the isolation of hydrogen producing Chlamydomonas reinhardtii strains. BMC Plant Biol. 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melis A., Zhang L., Forestier M., Ghirardi M. L., Seibert M. (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 122, 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wykoff D. D., Davies J. P., Melis A., Grossman A. R. (1998) The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol. 117, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H. C., Newton A. J., Melis A. (2005) Role of SulP, a nuclear-encoded chloroplast sulfate permease, in sulfate transport and H2 evolution in Chlamydomonas reinhardtii. Photosynth. Res. 84, 289–296 [DOI] [PubMed] [Google Scholar]
- 21. Davies J. P., Yildiz F. H., Grossman A. (1996) Sac1, a putative regulator that is critical for survival of Chlamydomonas reinhardtii during sulfur deprivation. EMBO J. 15, 2150–2159 [PMC free article] [PubMed] [Google Scholar]
- 22. Happe T., Kaminski A. (2002) Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 269, 1022–1032 [DOI] [PubMed] [Google Scholar]
- 23. Forestier M., King P., Zhang L., Posewitz M., Schwarzer S., Happe T., Ghirardi M. L., Seibert M. (2003) Expression of two [Fe]-hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions. Eur. J. Biochem. 270, 2750–2758 [DOI] [PubMed] [Google Scholar]
- 24. Fouchard S., Hemschemeier A., Caruana A., Pruvost J., Legrand J., Happe T., Peltier G., Cournac L. (2005) Autotrophic and mixotrophic hydrogen photoproduction in sulfur-deprived Chlamydomonas cells. Appl. Environ. Microbiol. 71, 6199–6205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hemschemeier A., Fouchard S., Cournac L., Peltier G., Happe T. (2008) Hydrogen production by Chlamydomonas reinhardtii. An elaborate interplay of electron sources and sinks. Planta 227, 397–407 [DOI] [PubMed] [Google Scholar]
- 26. Chochois V., Dauvillée D., Beyly A., Tolleter D., Cuiné S., Timpano H., Ball S., Cournac L., Peltier G. (2009) Hydrogen production in Chlamydomonas. Photosystem II-dependent and -independent pathways differ in their requirement for starch metabolism. Plant Physiol. 151, 631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desplats C., Mus F., Cuiné S., Billon E., Cournac L., Peltier G. (2009) Characterization of Nda2, a plastoquinone-reducing type II NAD(P)H dehydrogenase in Chlamydomonas chloroplasts. J. Biol. Chem. 284, 4148–4157 [DOI] [PubMed] [Google Scholar]
- 28. Jans F., Mignolet E., Houyoux P. A., Cardol P., Ghysels B., Cuiné S., Cournac L., Peltier G., Remacle C., Franck F. (2008) A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc. Natl. Acad. Sci. U.S.A. 105, 20546–20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mignolet E., Lecler R., Ghysels B., Remacle C., Franck F. (2012) Function of the chloroplastic NAD(P)H dehydrogenase Nda2 for H2 photoproduction in sulphur-deprived Chlamydomonas reinhardtii. J. Biotechnol. 162, 81–88 [DOI] [PubMed] [Google Scholar]
- 30. Hemschemeier A., Jacobs J., Happe T. (2008) Biochemical and physiological characterization of the pyruvate formate-lyase Pfl1 of Chlamydomonas reinhardtii, a typically bacterial enzyme in a eukaryotic alga. Eukaryot. Cell 7, 518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Philipps G., Happe T., Hemschemeier A. (2012) Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta 235, 729–745 [DOI] [PubMed] [Google Scholar]
- 32. Winkler M., Heil B., Heil B., Happe T. (2002) Isolation and molecular characterization of the [Fe]-hydrogenase from the unicellular green alga Chlorella fusca. Biochim. Biophys. Acta 1576, 330–334 [DOI] [PubMed] [Google Scholar]
- 33. Sawers R. G. (2005) Formate and its role in hydrogen production in Escherichia coli. Biochem. Soc. Trans. 33, 42–46 [DOI] [PubMed] [Google Scholar]
- 34. Kreuzberg K. (1984) Starch fermentation via a formate producing pathway in Chlamydomonas reinhardii, Chlorogonium elongatum and Chlorella fusca. Physiol. Plant. 61, 87–94 [Google Scholar]
- 35. Philipps G., Krawietz D., Hemschemeier A., Happe T. (2011) A pyruvate formate lyase-deficient Chlamydomonas reinhardtii strain provides evidence for a link between fermentation and hydrogen production in green algae. Plant J. 66, 330–340 [DOI] [PubMed] [Google Scholar]
- 36. Grossman A. R., Catalanotti C., Yang W., Dubini A., Magneschi L., Subramanian V., Posewitz M. C., Seibert M. (2011) Multiple facets of anoxic metabolism and hydrogen production in the unicellular green alga Chlamydomonas reinhardtii. New Phytol. 190, 279–288 [DOI] [PubMed] [Google Scholar]
- 37. Terashima M., Specht M., Naumann B., Hippler M. (2010) Characterizing the anaerobic response of Chlamydomonas reinhardtii by quantitative proteomics. Mol. Cell. Proteomics 9, 1514–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Terashima M., Specht M., Hippler M. (2011) The chloroplast proteome. A survey from the Chlamydomonas reinhardtii perspective with a focus on distinctive features. Curr. Genet. 57, 151–168 [DOI] [PubMed] [Google Scholar]
- 39. Ragsdale S. W. (2003) Pyruvate ferredoxin oxidoreductase and its radical intermediate. Chem. Rev. 103, 2333–2346 [DOI] [PubMed] [Google Scholar]
- 40. Carere C. R., Kalia V., Sparling R., Cicek N., Levin D. B. (2008) Pyruvate catabolism and hydrogen synthesis pathway genes of Clostridium thermocellum ATCC 27405. Indian J. Microbiol. 48, 252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim B. H., Bellows P., Datta R., Zeikus J. G. (1984) Control of carbon and electron flow in Clostridium acetobutylicum fermentations. Utilization of carbon monoxide to inhibit hydrogen production and to enhance butanol yields. Appl. Environ. Microbiol. 48, 764–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calusinska M., Happe T., Joris B., Wilmotte A. (2010) The surprising diversity of clostridial hydrogenases. A comparative genomic perspective. Microbiology 156, 1575–1588 [DOI] [PubMed] [Google Scholar]
- 43. Demuez M., Cournac L., Guerrini O., Soucaille P., Girbal L. (2007) Complete activity profile of Clostridium acetobutylicum [FeFe]-hydrogenase and kinetic parameters for endogenous redox partners. FEMS Microbiol. Lett. 275, 113–121 [DOI] [PubMed] [Google Scholar]
- 44. Akhtar M. K., Jones P. R. (2008) Deletion of iscR stimulates recombinant clostridial Fe-Fe hydrogenase activity and H2-accumulation in Escherichia coli BL21(DE3). Appl. Microbiol. Biotechnol. 78, 853–862 [DOI] [PubMed] [Google Scholar]
- 45. Schwartz C. J., Giel J. L., Patschkowski T., Luther C., Ruzicka F. J., Beinert H., Kiley P. J. (2001) IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc. Natl. Acad. Sci. U.S.A. 98, 14895–14900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harris E. H. (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use, Academic Press, San Diego: [DOI] [PubMed] [Google Scholar]
- 47. Hemschemeier A., Melis A., Happe T. (2009) Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res. 102, 523–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Franzén L. G., Rochaix J. D., von Heijne G. (1990) Chloroplast transit peptides from the green alga Chlamydomonas reinhardtii share features with both mitochondrial and higher plant chloroplast presequences. FEBS Lett. 260, 165–168 [DOI] [PubMed] [Google Scholar]
- 49. Sambrook J., Russell D. W. (2006) Transformation of E. coli by Electroporation. CSH Protoc. 2006, pii. [DOI] [PubMed] [Google Scholar]
- 50. Vogel H. J., Bonner D. M. (1956) Acetylornithinase of Escherichia coli. Partial purification and some properties. J. Biol. Chem. 218, 97–106 [PubMed] [Google Scholar]
- 51. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 52. Girbal L., von Abendroth G., Winkler M., Benton P. M., Meynial-Salles I., Croux C., Peters J. W., Happe T., Soucaille P. (2005) Homologous and heterologous overexpression in Clostridium acetobutylicum and characterization of purified clostridial and algal Fe-only hydrogenases with high specific activities. Appl. Environ. Microbiol. 71, 2777–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. von Abendroth G., Stripp S., Silakov A., Croux C., Soucaille P., Girbal L., Happe T. (2008) Optimized over-expression of [FeFe] hydrogenases with high specific activity in Clostridium acetobutylicum. Int. J. Hydrogen Energy 33, 6076–6081 [Google Scholar]
- 54. Jacobs J., Pudollek S., Hemschemeier A., Happe T. (2009) A novel, anaerobically induced ferredoxin in Chlamydomonas reinhardtii. FEBS Lett. 583, 325–329 [DOI] [PubMed] [Google Scholar]
- 55. Guerrini O., Burlat B., Léger C., Guigliarelli B., Soucaille P., Girbal L. (2008) Characterization of two 2[4Fe4S] ferredoxins from Clostridium acetobutylicum. Curr. Microbiol. 56, 261–267 [DOI] [PubMed] [Google Scholar]
- 56. Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. (1977) Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J. Bacteriol. 132, 604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mayhew S. G. (1978) The redox potential of dithionite and SO2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur. J. Biochem. 85, 535–547 [DOI] [PubMed] [Google Scholar]
- 58. Fukuda E., Kino H., Matsuzawa H., Wakagi T. (2001) Role of a highly conserved YPITP motif in 2-oxoacid:ferredoxin oxidoreductase. Heterologous expression of the gene from Sulfolobus sp. strain 7, and characterization of the recombinant and variant enzymes. Eur. J. Biochem. 268, 5639–5646 [DOI] [PubMed] [Google Scholar]
- 59. Hawkins C. F., Borges A., Perham R. N. (1989) A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 255, 77–82 [DOI] [PubMed] [Google Scholar]
- 60. Chabrière E., Charon M. H., Volbeda A., Pieulle L., Hatchikian E. C., Fontecilla-Camps J. C. (1999) Crystal structures of the key anaerobic enzyme pyruvate:ferredoxin oxidoreductase, free and in complex with pyruvate. Nat. Struct. Biol. 6, 182–190 [DOI] [PubMed] [Google Scholar]
- 61. Pieulle L., Magro V., Hatchikian E. C. (1997) Isolation and analysis of the gene encoding the pyruvate-ferredoxin oxidoreductase of Desulfovibrio africanus, production of the recombinant enzyme in Escherichia coli, and effect of carboxy-terminal deletions on its stability. J. Bacteriol. 179, 5684–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Terauchi A. M., Lu S. F., Zaffagnini M., Tappa S., Hirasawa M., Tripathy J. N., Knaff D. B., Farmer P. J., Lemaire S. D., Hase T., Merchant S. S. (2009) Pattern of expression and substrate specificity of chloroplast ferredoxins from Chlamydomonas reinhardtii. J. Biol. Chem. 284, 25867–25878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mus F., Dubini A., Seibert M., Posewitz M. C., Grossman A. R. (2007) Anaerobic acclimation in Chlamydomonas reinhardtii. Anoxic gene expression, hydrogenase induction, and metabolic pathways. J. Biol. Chem. 282, 25475–25486 [DOI] [PubMed] [Google Scholar]
- 64. Lambertz C., Hemschemeier A., Happe T. (2010) Anaerobic expression of the ferredoxin-encoding FDX5 gene of Chlamydomonas reinhardtii is regulated by the Crr1 transcription factor. Eukaryot. Cell 9, 1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park Y. J., Yoo C. B., Choi S. Y., Lee H. B. (2006) Purifications and characterizations of a ferredoxin and its related 2-oxoacid:ferredoxin oxidoreductase from the hyperthermophilic archaeon, Sulfolobus solfataricus P1. J. Biochem. Mol. Biol. 39, 46–54 [DOI] [PubMed] [Google Scholar]
- 66. Blaschkowski H. P., Neuer G., Ludwig-Festl M., Knappe J. (1982) Routes of flavodoxin and ferredoxin reduction in Escherichia coli. CoA-acylating pyruvate:flavodoxin and NADPH: flavodoxin oxidoreductases participating in the activation of pyruvate formate-lyase. Eur. J. Biochem. 123, 563–569 [PubMed] [Google Scholar]
- 67. Carpenter C. E., Reddy D. S., Cornforth D. P. (1987) Inactivation of clostridial ferredoxin and pyruvate-ferredoxin oxidoreductase by sodium nitrite. Appl. Environ. Microbiol. 53, 549–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Horner D. S., Hirt R. P., Embley T. M. (1999) A single eubacterial origin of eukaryotic pyruvate:ferredoxin oxidoreductase genes. Implications for the evolution of anaerobic eukaryotes. Mol. Biol. Evol. 16, 1280–1291 [DOI] [PubMed] [Google Scholar]
- 69. Hackstein J. H., Akhmanova A., Boxma B., Harhangi H. R., Voncken F. G. (1999) Hydrogenosomes. Eukaryotic adaptations to anaerobic environments. Trends Microbiol. 7, 441–447 [DOI] [PubMed] [Google Scholar]
- 70. Catalanotti C., Dubini A., Subramanian V., Yang W., Magneschi L., Mus F., Seibert M., Posewitz M. C., Grossman A. R. (2012) Altered fermentative metabolism in Chlamydomonas reinhardtii mutants lacking pyruvate formate lyase and both pyruvate formate lyase and alcohol dehydrogenase. Plant Cell 24, 692–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yonekura-Sakakibara K., Onda Y., Ashikari T., Tanaka Y., Kusumi T., Hase T. (2000) Analysis of reductant supply systems for ferredoxin-dependent sulfite reductase in photosynthetic and nonphotosynthetic organs of maize. Plant Physiol. 122, 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pieulle L., Guigliarelli B., Asso M., Dole F., Bernadac A., Hatchikian E. C. (1995) Isolation and characterization of the pyruvate-ferredoxin oxidoreductase from the sulfate-reducing bacterium Desulfovibrio africanus. Biochim. Biophys. Acta 1250, 49–59 [DOI] [PubMed] [Google Scholar]
- 73. Pineda E., Encalada R., Rodríguez-Zavala J. S., Olivos-García A., Moreno-Sánchez R., Saavedra E. (2010) Pyruvate:ferredoxin oxidoreductase and bifunctional aldehyde-alcohol dehydrogenase are essential for energy metabolism under oxidative stress in Entamoeba histolytica. FEBS J. 277, 3382–3395 [DOI] [PubMed] [Google Scholar]
- 74. Furdui C., Ragsdale S. W. (2000) The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood-Ljungdahl pathway. J. Biol. Chem. 275, 28494–28499 [DOI] [PubMed] [Google Scholar]
- 75. Uyeda K., Rabinowitz J. C. (1971) Pyruvate-ferredoxin oxidoreductase. 3. Purification and properties of the enzyme. J. Biol. Chem. 246, 3111–3119 [PubMed] [Google Scholar]
- 76. Menon S., Ragsdale S. W. (1997) Mechanism of the Clostridium thermoaceticum pyruvate:ferredoxin oxidoreductase. Evidence for the common catalytic intermediacy of the hydroxyethylthiamine pyropyrosphate radical. Biochemistry 36, 8484–8494 [DOI] [PubMed] [Google Scholar]
- 77. Lin W. C., Yang Y. L., Whitman W. B. (2003) The anabolic pyruvate oxidoreductase from Methanococcus maripaludis. Arch. Microbiol. 179, 444–456 [DOI] [PubMed] [Google Scholar]
- 78. Blamey J. M., Adams M. W. (1993) Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim. Biophys. Acta 1161, 19–27 [DOI] [PubMed] [Google Scholar]
- 79. Meinecke B., Bertram J., Gottschalk G. (1989) Purification and characterization of the pyruvate-ferredoxin oxidoreductase from Clostridium acetobutylicum. Arch. Microbiol. 152, 244–250 [DOI] [PubMed] [Google Scholar]
- 80. Cavazza C., Contreras-Martel C., Pieulle L., Chabrière E., Hatchikian E. C., Fontecilla-Camps J. C. (2006) Flexibility of thiamine diphosphate revealed by kinetic crystallographic studies of the reaction of pyruvate-ferredoxin oxidoreductase with pyruvate. Structure 14, 217–224 [DOI] [PubMed] [Google Scholar]
- 81. Winkler M., Hemschemeier A., Jacobs J., Stripp S., Happe T. (2010) Multiple ferredoxin isoforms in Chlamydomonas reinhardtii. Their role under stress conditions and biotechnological implications. Eur. J. Cell Biol. 89, 998–1004 [DOI] [PubMed] [Google Scholar]
- 82. Castruita M., Casero D., Karpowicz S. J., Kropat J., Vieler A., Hsieh S. I., Yan W., Cokus S., Loo J. A., Benning C., Pellegrini M., Merchant S. S. (2011) Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell 23, 1273–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pape M., Lambertz C., Happe T., Hemschemeier A. (2012) Differential expression of the Chlamydomonas [FeFe]-hydrogenase-encoding HYDA1 gene is regulated by the copper response regulator 1. Plant Physiol. 159, 1700–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Embley T. M., van der Giezen M., Horner D. S., Dyal P. L., Bell S., Foster P. G. (2003) Hydrogenosomes, mitochondria and early eukaryotic evolution. IUBMB Life 55, 387–395 [DOI] [PubMed] [Google Scholar]
- 85. Hug L. A., Stechmann A., Roger A. J. (2010) Phylogenetic distributions and histories of proteins involved in anaerobic pyruvate metabolism in eukaryotes. Mol. Biol. Evol. 27, 311–324 [DOI] [PubMed] [Google Scholar]
- 86. Yakunin A. F., Hallenbeck P. C. (1998) Purification and characterization of pyruvate oxidoreductase from the photosynthetic bacterium Rhodobacter capsulatus. Biochim. Biophys. Acta 1409, 39–49 [DOI] [PubMed] [Google Scholar]
- 87. Gibbs M., Gfeller R. P., Chen C. (1986) Fermentative metabolism of Chlamydomonas reinhardii. III. Photoassimilation of acetate. Plant Physiol. 82, 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Willeford K. O., Gibbs M. (1989) Localization of the enzymes involved in the photoevolution of H2 from acetate in Chlamydomonas reinhardtii. Plant Physiol. 90, 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 90. Crooks G. E., Hon G., Chandonia J. M., Brenner S. E. (2004) WebLogo. A sequence logo generator. Genome Res. 14, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Muller Y. A., Lindqvist Y., Furey W., Schulz G. E., Jordan F., Schneider G. (1993) A thiamin diphosphate binding fold revealed by comparison of the crystal structures of transketolase, pyruvate oxidase and pyruvate decarboxylase. Structure 1, 95–103 [DOI] [PubMed] [Google Scholar]
- 92. Magneschi L., Catalanotti C., Subramanian V., Dubini A., Yang W., Mus F., Posewitz M. C., Seibert M., Perata P., Grossman A. R. (2012) A mutant in the ADH1 gene of Chlamydomonas reinhardtii elicits metabolic restructuring during anaerobiosis. Plant Physiol. 158, 1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]