Abstract
Currently, cloning efficiency in pigs is very low. Donor cell type and number of cloned embryos transferred to an individual surrogate are two major factors that affect the successful rate of somatic cell nuclear transfer (SCNT) in pigs. This study aimed to compare the influence of different donor fibroblast cell types and different transferred embryo numbers on recipients' pregnancy rate and delivery rate, the average number of total clones born, clones born alive and clones born healthy per litter, and the birth rate of healthy clones (=total number of healthy cloned piglets born /total number of transferred cloned embryos). Three types of donor fibroblasts were tested in large-scale production of cloned pigs, including fetal fibroblasts (FFBs) from four genetically similar Western swine breeds of Pietrain (P), Duroc (D), Landrace (L), and Yorkshire (Y), which are referred to as P,D,LY-FFBs, adult fibroblasts (AFBs) from the same four breeds, which are designated P,D,L,Y-AFBs, and AFBs from a Chinese pig breed of Laiwu (LW), which is referred to as LW-AFBs. Within each donor fibroblast cell type group, five transferred cloned embryo number groups were tested. In each embryo number group, 150–199, 200–249, 250–299, 300–349, or 350–450 cloned embryos were transferred to each individual recipient sow. For the entire experiment, 92,005 cloned embryos were generated from nearly 115,000 matured oocytes and transferred to 328 recipients; in total, 488 cloned piglets were produced. The results showed that the mean clones born healthy per litter resulted from transfer of embryos cloned from LW-AFBs (2.53±0.34) was similar with that associated with P,D,L,Y-FFBs (2.72±0.29), but was significantly higher than that resulted from P,D,L,Y-AFBs (1.47±0.18). Use of LW-AFBs as donor cells for SCNT resulted in a significantly higher pregnancy rate (72.00% vs. 59.30% and 48.11%) and delivery rate (60.00% vs. 45.93% and 35.85%) for cloned embryo recipients, and a significantly higher birth rate of healthy clones (0.5009% vs. 0.3362% and 0.2433%) than that resulting from P,D,L,Y-AFBs and P,D,L,Y-FFBs. This suggests that using LW-AFBs as donor cells results in a higher cloning efficiency in pigs, compared with the other two donor fibroblast cell types. The birth rate of healthy clones was significantly improved when the number of transferred cloned embryos was increased from 150–199 to 200–450 per recipient. However, increase of the number of transferred embryos from 200–249 to 250–450 per surrogate did not change the birth rate of healthy clones. This suggests that transfer of excessive (250–450) cloned embryos to an individual surrogate is not necessary for increasing the cloning efficiency in pigs, and the relatively optimal number of reconstructed embryos transferred to individual recipient is 200–249. Furthermore, our results indicated that the numbers of total born clones, clones born alive, and clones born healthy per litter have a significantly high positive correlation with each other. The present study provides useful information for improving SCNT efficiency in pigs.
Introduction
Pigs are not only important livestock but also valuable animal models for biomedical as well as biological research. Success in production of cloned pigs from somatic cells was first reported almost at the same time in 2000 by three independent groups (Betthauser et al., 2000; Onishi et al., 2000; Polejaeva et al., 2000). Since then, the somatic cell nuclear transfer (SCNT) technique has been used to amplify superior pigs or has been applied in combination with genetic modifications to generate different types of transgenic or knockout pigs (Klymiuk et al., 2010; Prather et al., 2008; Schmidt et al., 2010; Vajta and Callesen 2012). Even though the pig SCNT procedure has been developed for over 10 years and a large number of cloned pigs have been produced, this approach is still inefficient. The cloning efficiency (number of live born cloned piglets/number of reconstructed embryos) is usually around 1–3% (Whitworth and Prather 2010; Yang et al., 2007; Zhao et al., 2010). This low success rate is considered to be the major problem that limits extensive application of the SCNT technique in pigs. Thus, improvement of the efficiency of this technique will facilitate its application in the swine industry as well as in biomedical research. Because the efficiency of SCNT is affected by factors such as donor cell type and number of transferred cloned embryos per recipient, optimization of these conditions may significantly increase the ability to generate cloned animals. However, so far, very few studies that compare the impact of different donor cell types and different transferred embryo numbers on pig cloning efficiency have been reported.
In an effort to apply the cloning technique to large-scale multiplication of excellent boars in the swine industry, we used approximately 115,000 matured porcine oocytes to produce 92,005 cloned embryos during 2009–2011. Following the transfer of these reconstructed embryos into surrogates, we eventually obtained 488 cloned male piglets with potentially superior genetics. The donor fibroblasts that were used to produce all these cloned male piglets could be classified into three types. The first two types of donor fibroblast include fetal fibroblasts (FFBs) and adult fibroblasts (AFBs) from four genetically related Western fast-growing pig breeds—Pietrain (P), Duroc (D), Landrace (L), and Yorkshire (Y)(Fang and Andersson 2006; Megens et al., 2007), which are referred to as P,D,L,Y-FFBs and P,D,L,Y-AFBs, respectively. The third type of donor fibroblast is the AFB from a slow-growing Chinese native pig breed, Laiwu (LW), which is designated as LW-AFBs. The embryo transfer methods that were used for pig cloning in the present study can also be divided, according to the number of embryos transferred to per recipient, into five different groups. In each group, an individual surrogate received 150–199, 200–249, 250–299, 300–349, or 350–450 two-cell stage cloned embryos. Here, we present the data obtained from our large pig cloning work and report the influence of donor fibroblast cell type and number of transferred cloned embryos per recipient on pig SCNT efficiency,
Materials and Methods
Donor cell isolation, ovary collection, and oocyte maturation
The experimental protocol for this study was approved by the South China Agricultural University's institutional animal care and use committee. Three types of fibroblasts were used as donor cells for SCNT. The first donor cell type was FFBs from 25-day-old male fetuses of P, D, L, or Y pigs. These fetuses potentially had superior genetics because their parents had excellent production performance. The second donor cell type was AFBs from the ears of 1-year-old, genetically selected superior boars of P, D, L, or Y pigs. The third donor cell type was AFBs from the ears of 1-year-old superior LW boars. FFBs and AFBs were isolated as described by Deng et al. (2011). Isolated fibroblasts were frozen in liquid nitrogen. Before SCNT, fibroblasts were thawed and cultured for 8–10 days in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS) at 39°C in a humidified atmosphere of 5% CO2 and 95% air. Cultured cells at passages three to eight were used for SCNT. All porcine ovaries used in this study were collected from a same local slaughterhouse.
Cumulus–oocyte complexes (COCs) were aspirated from the ovaries and matured in vitro for 42–44 h following the protocol described by Deng et al. (2011). Matured COCs were freed from cumulus cells by repeated pipetting in 0.1% hyaluronidase. Matured oocytes with the first polar body were selected for cloning.
Somatic cell nuclear transfer
The zona pellucida of a cumulus-free oocyte was held with a holding micropipette and a fine glass needle was used to make a slit near the first polar body. The first polar body and adjacent cytoplasm, presumably containing all the chromosomes, were extruded by squeezing with the same needle. A single fibroblast cell with a smooth surface was microinjected into the perivitelline space of the oocytes through the same slit. The oocyte–donor cell complexes were cultured in PZM3 medium at 39°C for 1.5 h and then activated to fuse by two successive DC pulses at 1.2 kv/cm for 30 μsec using an electrofusion instrument (model CF-150/B, BLS company, Budapest, Hungary).
Embryo transfer and pregnancy diagnosis
The reconstructed embryos were cultured in PZM3 medium at 39°C for 20 h. Usually at this time point, 50% of the cloned embryos are at the two-cell stage. The embryos were then loaded into a tube and kept in a portable incubator (Minitube) during transportation to the recipient farm. Estrus-synchronized LY (Landrace♂×Yorkshire♀) or YL (Yorkshire♂×Landrace♀) hybrid sows in parity 2–5 were used as embryo recipients. Within 12 h after recipient sows showed signs of estrus with a standing response to boars, they were anesthetized with ketamine and xylazine for induction and 3% isoflurane for maintenance. One oviduct was exposed by surgery. The cloned embryos were put directly into the oviduct of the recipient using a syringe. The pregnancy status of the recipient sows was monitored using an ultrasound equipped with a convex transducer at 1 month after embryo transfer.
Delivery of cloned piglets
If spontaneous farrowing did not occur until gestation day 116, the recipients were injected with a prostaglandin analog (200 μg/recipient), and after about 24 h they delivered vaginally under supervision or with assistance. The newborn cloned piglets were weighed and examined clinically. The numbers of total born clones, clones born alive, and clones born healthy per litter were recorded at birth. Healthy piglets were defined as live, newborn, normal clones that were heavier than 0.8 kg and did not show any defects, such as macroglossia, cryptorchidism, ligament contracture, cleft palate, and testes hypertrophy.
Statistical analysis
For analysis of pregnancy rate and delivery rate of recipients, and birth rate of healthy cloned piglets, data were subjected to the GENMOD Procedure of SAS 9.2 program (SAS Institute, Cary, NC). For analysis of the average number of total born clones, clones born alive, and clones born healthy per litter, data were subjected to the GLM Procedure of SAS 9.2 program (SAS Institute, Cary, NC). Values were present as mean±standard error of mean (SEM). Significant difference of means between two different groups was determined at p<0.05.
Results
Effects of donor cell type and transferred cloned embryo number on pregnancy rate and delivery rate of recipients
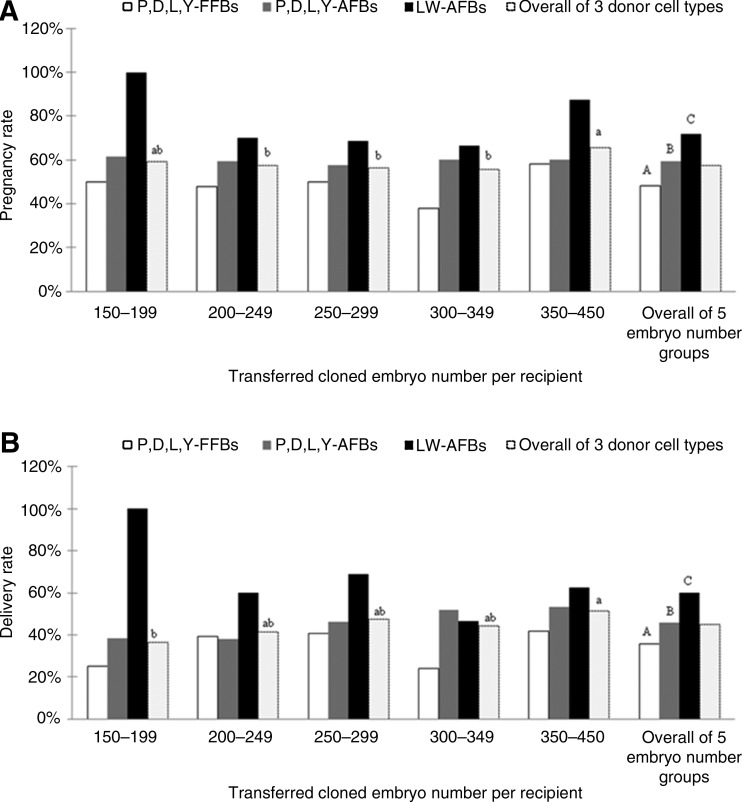
The pregnancy rate at 1 month after embryo transfer and the farrowing rate of recipients were calculated and shown in Figure 1. The overall pregnancy rate and delivery rate of surrogates received with LW-AFBs–derived SCNT embryos were significantly higher than those of surrogates transferred with embryos cloned from P,D,L,Y-FFBs, which were also significantly higher than that of recipient sows transferred with P,D,L,Y-AFBs–derived embryos (pregnancy rate, 72.00% vs. 59.30% vs. 48.11%; delivery rate, 60.00% vs. 45.93% vs. 35.85%). However, surrogates that received embryos cloned from different donor fibroblasts did not show a significant difference in pregnancy rate and delivery rate if they received the same number of cloned embryos. Similarly, when using the same type of fibroblasts as donor cells for SCNT, even surrogates that received different numbers of embryos exhibited no significant difference in pregnancy rate as well as farrowing rate. Furthermore, when the number of transferred embryos reached 350–450 per recipient female, the resulting overall pregnancy rate of the three donor cell types was not different from the 150–199 group (65.71% vs. 59.09%, p=0.2417), yet the overall delivery rate of the three donor cell types was significantly different from the 150–199 embryo number group (51.43% vs. 36.3%, p=0.0274).
FIG. 1.
Effects of donor cell type and number of cloned embryos transferred to individual surrogate on pregnancy rate (A) and delivery rate (B) of surrogates. Pregnancy rate was calculated by total number of pregnant recipients/total number of recipients (see the corresponding numbers in Table 1); delivery rate was calculated by total number of farrowed recipients/total number of recipients (see the corresponding numbers in Table 1). Values within the same embryo number group (including the overall of 5 embryo number groups) labeled with different capital letters differ at p<0.05, labeled with a same capital letter are not different from each other (p>0.05), and with no label of any letter are not different from each other (p>0.05). Values within the same donor cell type group (including the overall of 3 donor cell type groups) labeled with different small letters differ at p<0.05, labeled with a same small letter are not different from each other (p>0.05), and with no label of any letter are not different from each other (p>0.05).
Effects of donor cell type and transferred cloned embryo number on the mean number of born clones per litter
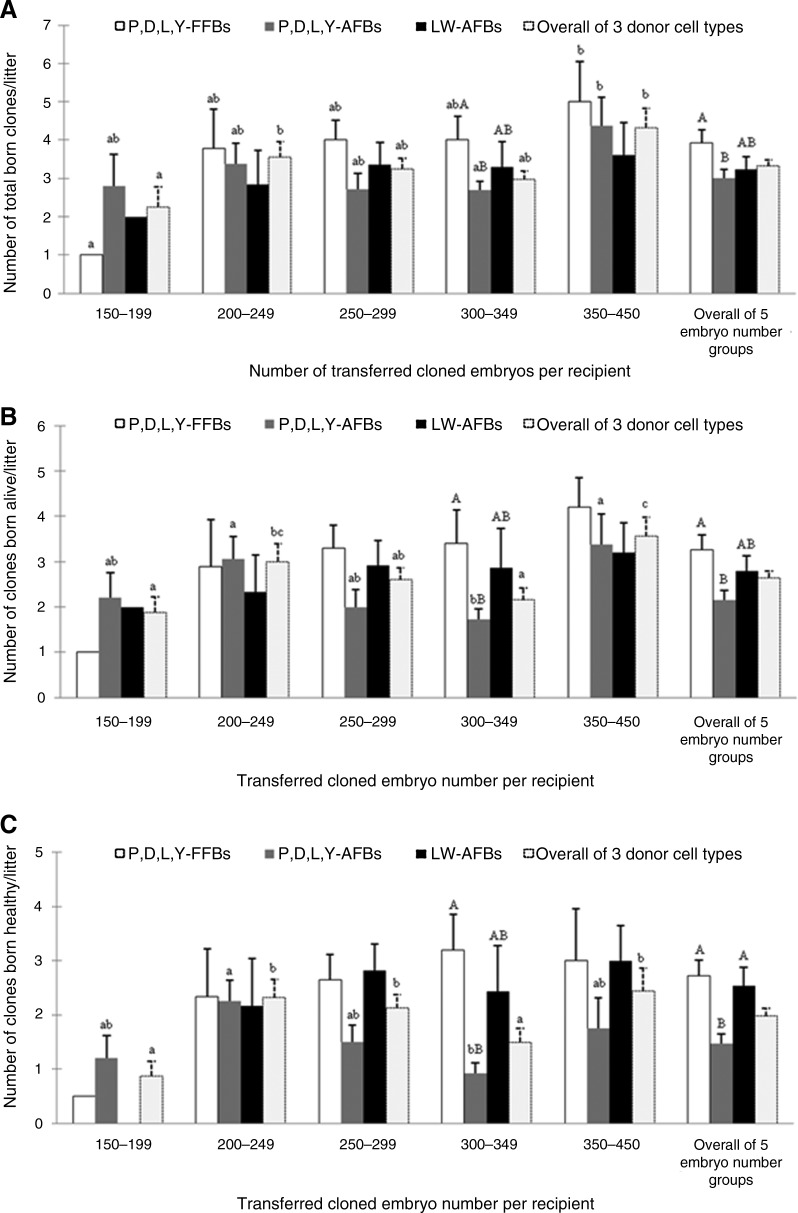
The average number of total clones born/litter, clones born alive/litter and clones born healthy/litter were analyzed and are shown in Figure 2. With the increase in numbers of P,D,L,Y-FFB– or LW-AFB–derived embryos transferred to individual recipients, it seems there was an increased trend in the mean number of total born clones, clones born alive, and clones born healthy per litter. Nevertheless, this trend was not observed when using P,D,L,Y-AFBs as donor cells for production of cloned piglets. When transferring the same number of 150–199, 200–249, 250–299, or 350–450 embryos into surrogates, donor cell type did not affect the average number of total born clones, clones born alive, and clones born healthy per litter. However, the mean total born clones/litter, clones born alive/litter, and clones born healthy/litter resulting from transfer of 300–349 P,D,L,Y-FFB–derived embryos were significantly higher than that resulted from transfer of the same number of P,D,L,Y-AFB–derived embryos (total born clones per litter, 4.0±0.63 vs. 2.69±0.23; clones born alive per litter, 3.4±0.75 vs. 1.73±0.23; clones born healthy per litter, 3.20±0.66 vs. 0.92±0.2).
FIG. 2.
Effects of donor cell type and number of cloned embryos transferred to individual surrogate on mean total born clones/litter (A), mean clones born alive/litter (B), and mean clones born healthy/litter (C). The farrowing data of all 147 recipients were used for calculating the mean total born clones/litter, mean clones born alive/litter, and mean clones born healthy/litter. Values within the same embryo number group (including the overall of 5 embryo number groups) labeled with different capital letters differ at p<0.05, labeled with a same capital letter are not different from each other (p>0.05), and with no label of any letter are not different from each other (p>0.05). Values within the same donor cell type group (including the overall of 3 donor cell type groups) labeled with different small letters differ at p<0.05, labeled with a same small letter are not different from each other (p>0.05), and with no label of any letter are not different from each other (p>0.05).
Recipients transferred with embryos cloned from P,D,L,Y-FFBs delivered significantly more total born clones/litter, clones born alive/litter, and clones born healthy/litter than those farrowed by surrogates that received P,D,L,Y-AFB–derived embryos (total born clones/litter, 3.92±0.35 vs. 3.01±0.22; clones born alive/litter, 3.26±0.33 vs. 2.15±0.21; clones born healthy/litter, 2.72±0.35 vs. 1.47±0.18). Using LW-AFBs as nuclei donor for SCNT only tended to increase the mean number of total born clones per litter and the mean number of clones born alive per litter, but significantly increased the mean number of clones born healthy per litter, as compared to that resulting from using P,D,L,Y-AFBs as donor cells for cloning (mean number of clones born healthy per litter, 2.53±0.34 vs. 1.47±0.18). The average total born clones/litter, clones born alive/litter, and clones born healthy/litter of overall of three donor cell types changed in a similar pattern with the increase of transferred embryo number. They all exhibited a significant increase when the number of embryos transferred to per recipient increased from 150–199 to 200–249, but tended to be lower or significantly decreased with the number of transferred embryos increased to 300–349, and then increased again when the embryo number transferred reached 350–450.
Effects of donor cell type and transferred cloned embryo number on the birth rate of healthy clones
The birth rate of healthy clones was calculated and is summarized in Table 1. When using the same type of fibroblasts as donor cells for SCNT, the number of transferred embryos to an individual foster mother has no significant impact on the birth rate of healthy clones. Similarly, donor cell type did not significantly affect the birth rate of healthy cloned piglets if surrogates received the same number of embryos. When the data were pooled from all embryo number groups, the birth rate of healthy clones derived from LW-AFBs (0.5009%=76/15,172) was significantly higher, as compared with P,D,L,Y-AFBs (0.2433%=116/47,682) and P,D, L,Y-FFBs (0.3362%=44/13,485). If only considering the effect of transferred embryo number, transfer of 150–199 embryos to an individual recipient resulted in a significantly lower birth rate of healthy clones than that resulting from transferring 200–249, 250–299, 300–349, or 350–450 embryos to individual surrogates.
Table 1.
Effects of Donor Cell Type and Number of Cloned Embryos Transferred to Individual Recipient on the Birth Rate of Healthy Cloned Piglets
| Donor cell types | No. of transferred embryos per recipient | Total no. of transferred embryos | Total no. of recipients | Total no. of pregnant recipients | Total no. of farrowed recipients | Total no. of clones born health | Clones born healthy/transferred embryos |
|---|---|---|---|---|---|---|---|
| P,D,L,Y-FFBs | 150–199 | 1,496 | 8 | 4 | 2 | 1 | 0.0668% |
| 200–249 | 5,128 | 23 | 11 | 9 | 21 | 0.4095% | |
| 250–299 | 11,506 | 42 | 21 | 17 | 45 | 0.3911% | |
| 300–349 | 6,682 | 21 | 8 | 5 | 16 | 0.2394% | |
| 350–450 | 4,339 | 12 | 7 | 5 | 15 | 0.3457% | |
| Overall of 5 embryo number groups | 29,151 | 106 | 51 | 38 | 98 | 0.3362%a | |
| P,D,L,Y-AFBs | 150–199 | 2,243 | 13 | 8 | 5 | 6 | 0.2675% |
| 200–249 | 9,537 | 42 | 25 | 16 | 36 | 0.3775% | |
| 250–299 | 14,164 | 52 | 30 | 24 | 36 | 0.2542% | |
| 300–349 | 16,017 | 50 | 30 | 26 | 24 | 0.1498% | |
| 350–450 | 5,721 | 15 | 9 | 8 | 14 | 0.2447% | |
| Overall of 5 embryo number groups | 47,682 | 172 | 102 | 79 | 116 | 0.2433%a | |
| LW-AFBs | 150–199 | 195 | 1 | 1 | 1 | 0 | 0 |
| 200–249 | 2,325 | 10 | 7 | 6 | 13 | 0.5591% | |
| 250–299 | 4,406 | 16 | 11 | 11 | 31 | 0.7036% | |
| 300–349 | 4,821 | 15 | 10 | 7 | 17 | 0.3526% | |
| 350–450 | 3,425 | 8 | 7 | 5 | 15 | 0.4380% | |
| Overall of 5 embryo number groups | 15,172 | 50 | 36 | 30 | 76 | 0.5009%b | |
| Overall of 3 donor cell types | 150–199 | 3,934 | 22 | 13 | 8 | 7 | 0.1779%a |
| 200–249 | 16,990 | 75 | 43 | 31 | 72 | 0.4238%b | |
| 250–299 | 30,076 | 110 | 62 | 52 | 111 | 0.3691%b | |
| 300–349 | 27,520 | 86 | 48 | 38 | 57 | 0.2071%ab | |
| 350–450 | 13,485 | 35 | 23 | 18 | 44 | 0.3263%b | |
| Overall of 5 embryo number groups | 92,005 | 328 | 189 | 147 | 291 | 0.3163% |
Values within the same embryo number group (including the overall of 5 embryo number groups) labeled with different capital letters differ at p<0.05, labeled with the same capital letter are not different from each other (p>0.05), and with no label are not different from each other (p>0.05). Values within the same donor cell type group (including the overall of 3 donor cell type groups) labeled with different small letters differ at p<0.05, labeled with the same small letter are not different from each other (p>0.05), and with no label are not different from each other (p>0.05).
Correlation analyses
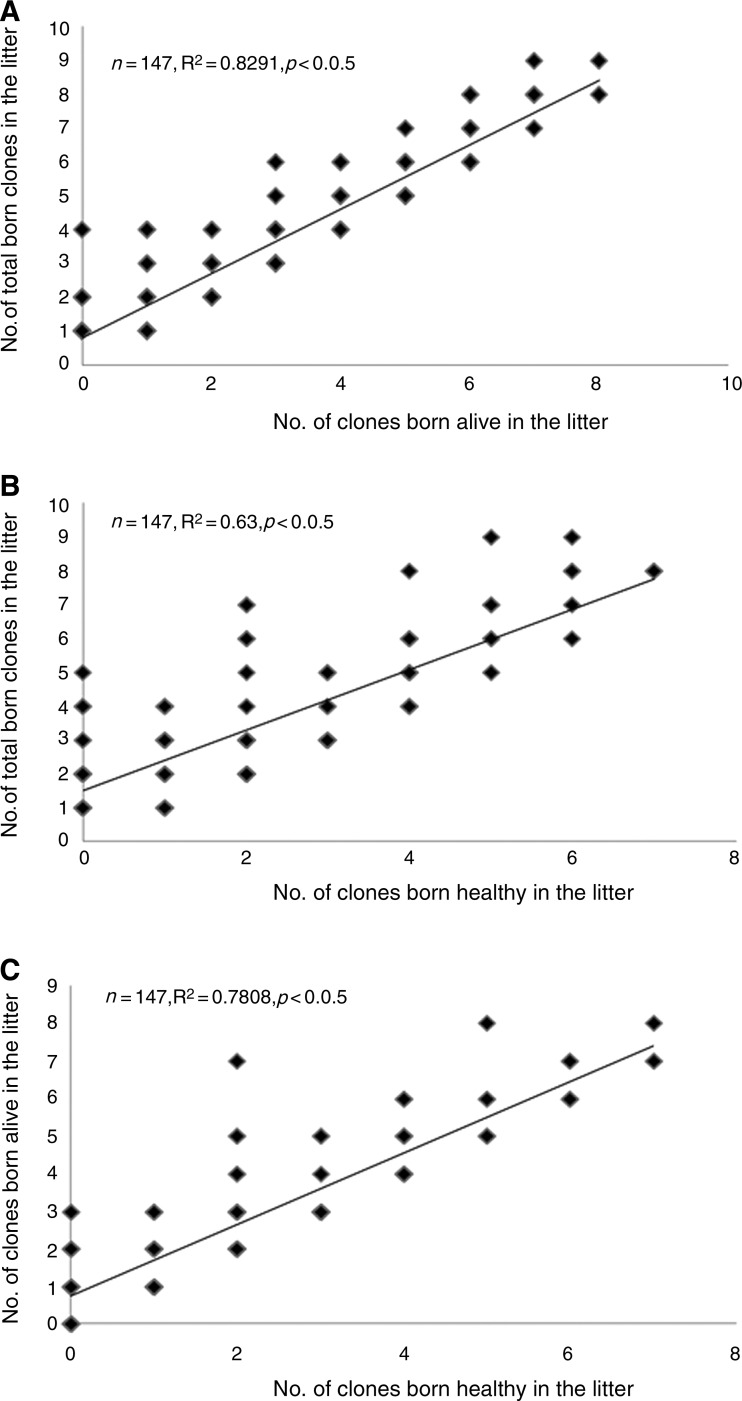
Significantly high positive correlations were found between the number of total born clones and number of clones born alive, between the number of total born clones and number of clones born healthy, and between the number of clones born alive and number of clones born healthy in the litter (Fig. 3). We also used the data of 147 recipients that gave birth to cloned piglets to analyze the relationship between the number of embryos transferred to recipients and the number of total born clones, or the number of clones born alive, or the number of clones born healthy in the litter, but no significant correlation was found between them (data was not shown).
FIG. 3.
Correlation between the number of total born clones and number of clones born alive (A), between the number of total born clones and number of clones born healthy (B), and between the number of clones born alive and number of clones born healthy (C) in the litter.
Discussion
Donor cell type and the number of transferred cloned embryos per recipient are two major factors that influence the success rate of cloning. In this study, we investigated the effect of these two factors on the efficiency of pig cloning. Our results showed that transfer of LW-AFB–derived cloned embryos resulted in a significantly higher pregnancy rate and delivery rate than those resulting from transfer of P,D,L,Y-FFBs and P,D,L,Y-AFB–derived cloned embryos. This suggests that embryos generated from fibroblasts of Chinese local LW pigs rather than Western pig breeds allow their surrogate mothers to establish pregnancy and maintain pregnancy to term more easily. However, this seems contrary to the finding that pregnancy rate and delivery rate were significantly increased if the transferred cloned embryos and their recipient are of the same breed (Koo et al., 2009; Schmidt et al., 2010). In the present study, LY or YL hybrid sows were used as recipients, which are genetically more similar with cloned embryos generated from P,D,L,Y-FFBs and P,D,L,Y-AFBs. However, we observed a higher pregnancy rate and delivery rate in LY and YL surrogates that received embryos cloned from LW-AFBs that were more genetically different. The pregnancy rate and delivery rate observed with P,D,L,Y-FFBs-based SCNT was significantly lower than that associated with P,D,L,Y-AFB– and LW-AFB–based cloning, implying that use of AFBs instead of FFBs as donor cells for SCNT increased the pregnancy rate as well as the farrowing rate of recipients. This is favorable for applying the SCNT technique to amplify superior genetics in the swine industry, where AFBs from genetically proven excellent boars are the preferred donor cells for cloning.
In our study, the number of transferred cloned embryos did not seem to have much influence on recipients' pregnancy rate and delivery rate, since surrogates that received 150–349 embryos exhibited a similar pregnancy rate and farrowing rate. In pigs, a signal from three or more embryos is required to maintain pregnancy (King et al., 2002). The pregnancy rate and delivery rate of surrogate females receiving cloned embryos is usually lower than that resulting from transfer of unmanipulated embryos (Hornen et al., 2007; Kurome et al., 2008; Lagutina et al., 2007; Park et al., 2010). The major possible reason for this is that the low developmental ability of cloned embryos significantly reduces their signaling to the recipient mother after embryo transfer, and thus they are unable to cause the recipients establish pregnancy or maintain pregnancy to term. This negative effect could be minimized by increasing the number of transferred cloned embryos to an individual surrogate. Schmidt et al. (2010) showed that when the number of transferred cloned blastocysts selected with normal morphology was increased from <60 to 60–120 per recipient, the surrogates' pregnancy rate and delivery rate were significantly elevated. However, in our study, the cloned embryos were unselected and transferred at around two-cell stage. Therefore, even if we increased the transferred embryo number from 150–199 to 300–349 per recipient, this still may not be enough to significantly increase the survived cloned embryo number after transfer, and thereby does not change the recipients' pregnancy rate and farrowing rate.
Among the three types of donor fibroblasts tested in this study, P,D,L,Y-FFBs were associated with the lowest recipient pregnancy rate and delivery rate. However, these fibroblasts were also associated with the largest mean number of total born clones per litter, the largest mean number of clones born alive per litter, and the largest mean number of clones born healthy per litter. The reason for this is unknown. Moreover, transfer of 350–450 cloned embryos tended to increase recipients' pregnancy rate and delivery rate, as well as the mean number of total born clones, clones born alive, and clones born healthy per litter.
In the swine industry, producers are more interested in efficient production of healthy superior cloned pigs. Therefore, we analyzed the effects of donor fibroblast cell type and transferred cloned embryo number on the birth rate of healthy clones. Our results demonstrated that using LW-AFBs as donor cells for SCNT significantly increased the birth rate of healthy clones, indicating LW-AFBs may have a higher reprogramming efficiency than P,D,L,Y-FFBs and P,D,L,Y-AFBs. In pig cloning, the number of early-stage cloned embryos transferred to an individual surrogate is typically less than 200 (Betthauser et al., 2000; Boquest et al., 2002; Hao et al., 2009; Onishi et al., 2000; Polejaeva et al., 2000; Waghmare et al., 2011). However, our results showed that transfer of more than 200 cloned embryos at approximately the two-cell stage to an individual surrogate increased the birth rate of healthy clones. More interestingly, transfer of 200–249, 250–299, 300–349, or 350–450 cloned embryos to individual recipients caused no significant change in the birth rate of healthy clones. This suggests that transfer of excessive numbers of cloned embryos to an individual recipient was not necessary for enhancing the cloning efficiency in pigs. Therefore, transfer of 200–249 cloned embryos at approximately the two-cell stage to an individual recipient should be considered in pig SCNT.
In conclusion, this study demonstrated that among the three types of tested donor fibroblasts, LW-AFBs were associated with the highest birth rate of healthy cloned piglets. Increase of the number of transferred cloned embryos (at around the two-cell stage) from 150–199 to 200–450 per recipient sow significantly enhanced the birth rate of healthy clones. However, transfer of excessive (250–450) cloned embryos to an individual surrogate was unnecessary for increasing cloning efficiency, and the relatively optimal number of cloned embryos transferred to individual recipient at approximately the two-cell stage was 200–249. In addition, the number of total born clones, number of clones born alive, and number of clones born healthy in the litter significantly and positively correlated with each other.
Acknowledgments
This study was supported by a grant from the National High Technology Research and Development Program of China (863 Program, grant number 2011AA100304) and a grant from the National Basic Research Program of China (973 Program, grant number 2011CB944202). We thank Dr. Hao Zhang and Fei Tang for their help in preparation of the manuscript.
Author Disclosure Statement
The authors state that there are no conflicts of interest and they have received no payment for preparation of this manuscript.
References
- Betthauser J. Forsberg E. Augenstein M. Childs L. Eilertsen K. Enos J. Forsythe T. Golueke P. Jurgella G. Koppang R., et al. Production of cloned pigs from in vitro systems. Nat. Biotech. 2000;18:1055–1059. doi: 10.1038/80242. [DOI] [PubMed] [Google Scholar]
- Boquest A.C. Grupen C.G. Harrison S.J. McIlfatrick S.M. Ashman R.J. d'Apice A.J.F. Nottle M.B. Production of cloned pigs from cultured fetal fibroblast cells. Biol. Reprod. 2002;66:1283–1287. doi: 10.1095/biolreprod66.5.1283. [DOI] [PubMed] [Google Scholar]
- Deng W. Yang D. Zhao B. Ouyang Z. Song J. Fan N. Liu Z. Zhao Y. Wu Q. Nashun B., et al. Use of the 2A peptide for generation of multi-transgenic pigs through a single round of nuclear transfer. PLoS ONE. 2011;6:e19986. doi: 10.1371/journal.pone.0019986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M. Andersson L. Mitochondrial diversity in European and Chinese pigs is consistent with population expansions that occurred prior to domestication. Proc. R. Soc. B Biol. Sci. 2006;273:1803–1810. doi: 10.1098/rspb.2006.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y. Wax D. Zhong Z. Murphy C. Ross J.W. Rieke A. Samuel M. Spate L. Dyce P. Li J., et al. Porcine skin-derived stem cells can serve as donor cells for nuclear transfer. Cloning Stem Cells. 2009;11:101–110. doi: 10.1089/clo.2008.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornen N. Kues W.A. Carnwath J.W. Lucas-Hahn A. Petersen B. Hassel P. Niemann H. Production of viable pigs from fetal somatic stem cells. Cloning Stem Cells. 2007;9:364–373. doi: 10.1089/clo.2006.0009. [DOI] [PubMed] [Google Scholar]
- King T. Dobrinsky Zhu J. Finlayson H. Bosma W. Harkness L. Ritchie W. Travers A. McCorquodale C. Day B., et al. Embryo development and establishment of pregnancy after embryo transfer in pigs: Coping with limitations in the availability of viable embryos. Reproduction. 2002;123:507–515. [PubMed] [Google Scholar]
- Klymiuk N. Aigner B. Brem G. Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol. Reprod. Dev. 2010;77:209–221. doi: 10.1002/mrd.21127. [DOI] [PubMed] [Google Scholar]
- Koo O.J. Park H.J. Kwon D.K. Kang J.T. Jang G. Lee B.C. Effect of recipient breed on delivery rate of cloned miniature pig. Zygote. 2009;17:203–207. doi: 10.1017/S0967199409005267. [DOI] [PubMed] [Google Scholar]
- Kurome M. Ishikawa T. Tomii R. Ueno S. Shimada A. Yazawa H. Nagashima H. Production of transgenic and non-transgenic clones in miniature pigs by somatic cell nuclear transfer. J. Reprod. Dev. 2008;54:156–163. doi: 10.1262/jrd.19165. [DOI] [PubMed] [Google Scholar]
- Lagutina I. Lazzari G. Duchi R. Turini P. Tessaro I. Brunetti D. Colleoni S. Crotti G. Galli C. Comparative aspects of somatic cell nuclear transfer with conventional and zona-free method in cattle, horse, pig and sheep. Theriogenology. 2007;67:90–98. doi: 10.1016/j.theriogenology.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Megens H.-J. Crooijmans R.P.M.A. Cristobal M.S. Hui X. Li N. Groenen M.A.M. Biodiversity of pig breeds from China and Europe estimated from pooled DNA samples: Differences in microsatellite variation between two areas of domestication. Genet. Select. Evol. 2007;40:103–128. doi: 10.1186/1297-9686-40-1-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi A. Iwamoto M. Akita T. Mikawa S. Takeda K. Awata T. Hanada H. Perry A.C. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000;289:1188–1190. doi: 10.1126/science.289.5482.1188. [DOI] [PubMed] [Google Scholar]
- Park H.J. Koo O.J. Kwon D.K. Kang J.T. Jang G. Lee B.C. Effect of roscovitine-treated donor cells on development of porcine cloned embryos. Reprod. Domest. Anim. 2010;45:1082–1088. doi: 10.1111/j.1439-0531.2009.01499.x. [DOI] [PubMed] [Google Scholar]
- Polejaeva I.A. Chen S.H. Vaught T.D. Page R.L. Mullins J. Ball S. Dai Y. Boone J. Walker S. Ayares D.L., et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- Prather R.S. Shen M. Dai Y. Genetically modified pigs for medicine and agriculture. Biotechnol. Genet. Eng. Rev. 2008;25:245–265. doi: 10.7313/upo9781904761679.011. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Kragh P.M. Li J. Du Y. Lin L. Liu Y. Bogh I.B. Winther K.D. Vajta G. Callesen H. Pregnancies and piglets from large white sow recipients after two transfer methods of cloned and transgenic embryos of different pig breeds. Theriogenology. 2010;74:1233–1240. doi: 10.1016/j.theriogenology.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Vajta G. Callesen H. Establishment of an efficient somatic cell nuclear transfer system for production of transgenic pigs. Theriogenology. 2012;77:1263–1274. doi: 10.1016/j.theriogenology.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Waghmare S.K. Estrada J. Reyes L. Li P. Ivary B. Sidner R.A. Burlak C. Tector A.J. Gene targeting and cloning in pigs using fetal liver derived cells. J. Surg. Res. 2011;171:e223–229. doi: 10.1016/j.jss.2011.07.051. [DOI] [PubMed] [Google Scholar]
- Whitworth K.M. Prather R.S. Somatic cell nuclear transfer efficiency: How can it be improved through nuclear remodeling and reprogramming? Mol. Reprod. Dev. 2010;77:1001–1015. doi: 10.1002/mrd.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. Smith S.L. Tian X.C. Lewin H.A. Renard J.P. Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- Zhao J. Whyte J. Prather R.S. Effect of epigenetic regulation during swine embryogenesis and on cloning by nuclear transfer. Cell Tissue Res. 2010;341:13–21. doi: 10.1007/s00441-010-1000-x. [DOI] [PubMed] [Google Scholar]